Abstract
Background and Aim
Previous studies have demonstrated that coffee consumption may be inversely correlated with hepatic fibrosis and cirrhosis. However, the reported results have been inconsistent. To summarize previous evidences quantitatively, a meta-analysis was performed.
Methods
The Medline, Web of Science, and Embase databases (from inception to June 2015) were searched to identify relevant trials that evaluated the effects of coffee consumption on hepatic fibrosis or cirrhosis. Odds ratios (ORs) of advanced hepatic fibrosis or cirrhosis for low or moderate, high, and any coffee consumption versus no consumption were pooled. Two cups per day was used as the cut-off level between low or moderate and high consumption.
Results
Sixteen studies were included, involving 3034 coffee consumers and 132076 people who do not consume coffee. The pooled results of the meta-analysis indicated that coffee consumers were less likely to develop cirrhosis compared with those who do not consume coffee, with a summary OR of 0.61 (95%CI: 0.45–0.84). For low or moderate coffee consumption versus no consumption, the pooled OR of hepatic cirrhosis was 0.66 (95%CI: 0.47–0.92). High coffee consumption could also significantly reduce the risk for hepatic cirrhosis when compared with no coffee consumption (OR = 0.53, 95%CI: 0.42–0.68). The effect of coffee consumption on hepatic fibrosis was summarized as well. The pooled OR of advanced hepatic fibrosis for coffee consumption versus no consumption was 0.73 (95%CI: 0.58–0.92). The protective effect of coffee on hepatic fibrosis and cirrhosis was also identified in subgroup meta-analyses of patients with alcoholic liver disease and chronic hepatitis C virus (HCV) infection.
Conclusion
Coffee consumption can significantly reduce the risk for hepatic fibrosis and cirrhosis.
Introduction
Hepatic fibrosis and cirrhosis are the major causes of mortality and morbidity in patients with chronic liver disease (CLD), and are a global health burden [1, 2]. Persistent liver injury caused by CLD can induce scar formation and healing, which consequently lead to fibrosis and cirrhosis [3, 4]. The 5-year cumulative incidence of decompensation in cirrhotic patients is approximately 20%. Those patients with decompensated cirrhosis only have a 5-year survival rate of 14–35% [5]. Worse still, the risk for hepatocellular carcinoma greatly increases after the development of fibrosis and cirrhosis.
Controlling CLD is the most important approach to prevent hepatic fibrosis. However, dietary factors may also have protective effects on liver diseases [6, 7]. Coffee intake has been found to be inversely correlated with serum levels of alanine aminotransferase (ALT) and gamma-glutamyltransferase (GGT), both of which are markers of liver injury and indicators of hepatic fibrosis [8–10]. Moreover, coffee is able to prevent hepatocellular carcinoma development that is mainly caused by hepatic fibrosis and cirrhosis [11, 12]. Recently, some studies have evaluated the association of coffee consumption with hepatic fibrosis and cirrhosis [13–15]. However, the reported results have been inconsistent, and no comprehensive meta-analyses have been conducted previously.
Meta-analysis is an important method to summarize the results from multiple studies quantitatively as well as to generate a final conclusion, especially when the results are inconsistent [16]. The goal of the present meta-analysis was to summarize the effects of low or moderate, high, and any coffee consumption on hepatic fibrosis and cirrhosis, compared with no consumption, in a quantitative manner.
Methods
Search strategy and study selection
A literature search was performed by two reviewers (FL and WXW). Medline, Web of Science, and Embase were searched from inception to June 2015, using the following combination of text words and Mesh terms: “beverage” or “caffeine” or “coffee”, “risk”, combined with “liver” or “hepatic cirrhosis” or “liver cirrhosis” or “hepatic fibrosis” or “liver fibrosis”. All epidemiological trials that evaluated the effects of coffee consumption on hepatic fibrosis or cirrhosis were included. Relevant reference lists were also screened to identify additional potential studies. Furthermore, manual searches and correspondence with authors were performed when necessary.
Studies were included if they met the following criteria: (a) cohort or case-control study, (b) coffee consumption was the exposure of interest, (c) focused on primary outcomes of advanced hepatic fibrosis or cirrhosis diagnosed according to the guidelines of the American Association for the Study of Liver Diseases (d) reported odds ratios (OR) or relative risk with relevant 95% confidence intervals (CIs) or data to calculate them. Studies that did not provide adequate information of primary outcomes or were not published in English were excluded. When multiple publications were identified from the same population, only the most complete one was selected. The Newcastle-Ottawa Scale was used to assess the quality of the included studies. Studies with total scores of more than six were considered to be of high quality [17].
Data extraction
Each included study was abstracted in duplicate and evaluated independently by FL and WXW using a data collection form. The following data were extracted: (a) study characteristics (author, year of publication, sample size, and study design), (b) details of coffee consumption, (c) types of primary outcome (advanced hepatic fibrosis or cirrhosis) and (d) measurement of association (OR or relative risk) or data to calculate them, and adjusted covariates in the analysis. Estimates adjusted for potential covariates were utilized whenever possible.
Any conflicting opinions on study selection or data extraction were resolved by consensus of the investigators. The corresponding author (HDH) also provided arbitration when necessary.
Statistical analysis
The effects of coffee consumption on hepatic fibrosis or cirrhosis were assessed by ORs combined with the corresponding 95%CIs. Summary ORs for low or moderate, high, and any coffee consumption versus no consumption were calculated. Due to the variances of coffee consumption among included studies, a coffee intake ≥ 2 cups per day was defined as high consumption. Coffee intake < 2 cups per day was defined as low or moderate consumption. Summary estimates were calculated by the fixed-effects model [18] or the random-effects model [19]. If no heterogeneity is identified among the studies, these two models will generate identical results. However, when heterogeneity is found, the 95%CI of the summary estimate calculated by the random-effects model will be wider than that calculated using the fixed-effects model [20]. The statistical significance claims of the random-effects model will also be more conservative. The heterogeneity among the included studies was evaluated by Cochran’s Q test and the I2 statistic. If the Q-test gave a p value < 0.1 to indicate significant heterogeneity [21], the summary estimates would be pooled using a random-effects model. The study weights were adjusted based on the extent of variation among the included studies when a random-effects model was used. Summary estimates were calculated as weighted averages of the estimates in the individual studies [20].
One-way sensitivity analysis was conducted to estimate the credibility of the pooled results. Galbraith plot was used to search for a potential source of heterogeneity. The outliers identified by the Galbraith plot were considered as the sources of heterogeneity [22]. Funnel plots were calculated to assess publication bias, together with Egger's regression asymmetry test, and Begg’s rank correlation test [23, 24]. All statistical analyses were performed by Stata (version 12.0). All p values were two tailed. With the exception of Cochran’s Q-test, all tests with a p value < 0.05 were considered to be statistically significant.
Results
Through database searches, 1657 potential citations were identified. After detailed evaluation and removal of duplicates, 16 studies were finally selected, including 7 case-control studies [14, 25–30] and 9 cohort studies [13, 15, 31–37] (Fig 1). In the case-control studies, previous coffee consumption of the participants was determined. In the cohort studies, coffee consumption at the study baseline was determined. Among the included studies, eight measured the associations of coffee consumption with cirrhosis development [14, 25–29, 31, 32], seven measured the associations of coffee consumption with hepatic fibrosis [13, 15, 30, 33, 34, 36, 37], and one reported on both the associations of coffee consumption with advanced hepatic fibrosis and cirrhosis [35]. Finally, 3034 coffee consumers and 132076 people who do not consume coffee were included. The study characteristics are shown in Table 1. Most of the included trials were of good quality.
Fig 1. Study selection procedure.
Table 1. Study characteristics.
| Study | Year | Country | Study design | No. of cases | No. of controls/cohort size | Outcome | Adjustments | Quality score* |
|---|---|---|---|---|---|---|---|---|
| Corrao | 1994 | Italy | case-control | 115 | 167 | cirrhosis | Smoking, alcohol consumption | 8 |
| Corrao | 2001 | Italy | case-control | 274 | 458 | cirrhosis | Education, age, HBsAg, alcohol consumption, HCV, intake of energy, carbohydrates, lipids, and proteins | 8 |
| GALLUS | 2002 | Italy | case-control | 101 | 1538 | cirrhosis | Age, sex, education, area of residence, year of interview, body mass index, diabetes, history of hepatitis, alcohol consumption, tobacco | 6 |
| Klatsky | 2006 | USA | cohort | 330 | 125580 | cirrhosis | Sex, race or ethnicity, smoking, alcohol consumption, education, body mass index | 8 |
| Freedman | 2009 | USA | cohort | 331 | 776 | cirrhosis | Age, body mass index, education, ethnicity, sex, baseline Ishak fibrosis score, total energy intake, lifetime alcohol consumption | 9 |
| Stroffolini | 2010 | Italy | case-control | 136 | 613 | cirrhosis | Alcohol consumption | 7 |
| Modi | 2010 | USA | cohort | 54 | 177 | fibrosis | Age, sex, race, body mass index, alcohol consumption | 8 |
| Ong | 2011 | Hong Kong | cohort | 216 | 1045 | fibrosis | Age, body mass index, alcohol consumption | 7 |
| Costentin | 2011 | France | cohort | 55 | 238 | fibrosis | Not specified | 8 |
| Anty | 2012 | France | cohort | 68 | 195 | fibrosis | AST, homeostatic model assessment-insulin resistance, metabolic syndrome, presence of nonalcoholic steatohepatitis | 7 |
| Walton | 2013 | UK | case-control | 95 | 191 | cirrhosis | Age, alcohol consumption | 6 |
| Triantos | 2013 | UK | case-control | 240 | 391 | cirrhosis | Age, gender, smoking, alcohol consumption | 6 |
| Machado | 2014 | Brazil | cohort | 64 | 136 | fibrosis | Not specified | 7 |
| El-Serag | 2014 | USA | cohort | 355 | 597 | fibrosis | Demographic, clinical, and other dietary variables | 6 |
| Bambha | 2014 | USA | cohort | 258 | 782 | both | Age, smoking, diabetes | 6 |
| Khalaf | 2015 | USA | case-control | 342 | 568 | fibrosis | Age, alcohol use, body mass index, metabolic syndrome | 8 |
*: Study quality was assessed using the Newcastle-Ottawa Scale (score of 0–9). AST, aspartate transaminase; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus.
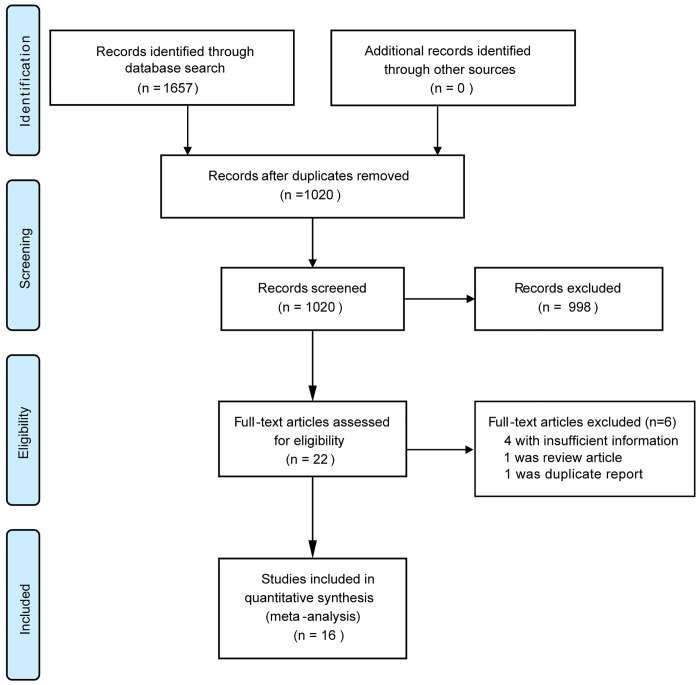
Seven included studies reported the association of coffee consumption with hepatic cirrhosis, compared with no coffee consumption [14, 25, 26, 29, 31, 32, 35]. All of the studies identified that coffee intake could reduce the risk for cirrhosis, yet only three of them had results that reached statistical significance [14, 25, 29]. Significant heterogeneity among the studies was found (I2 = 72.1%; Q-test, p < 0.01). The pooled results of the meta-analysis indicated that coffee consumers were less likely to develop cirrhosis compared with those who do not consume coffee, with a summary OR of 0.61 (95%CI: 0.45–0.84, random-effects model, Fig 2A). The Galbraith plot found that a study from the USA showing no significant association [32] and a study from Italy reporting a much stronger association [14] mainly contributed to the heterogeneity (S1 Fig). A sensitivity analysis indicated that no single study significantly influenced the pooled estimate (S2 Fig). After removing the two studies identified as outliers in the Galbraith plot, heterogeneity was found to be significantly reduced, yet the pooled estimates were still stable (OR = 0.66, 95%CI: 0.52–0.83; Table 2). Subgroup analysis was performed according to the study quality. The pooled estimates were similar between low- and high-quality studies, with OR values of 0.64 (95%CI: 0.44–0.91) and 0.60 (95%CI: 0.36–0.99), respectively.
Fig 2. Pooled odds ratios (ORs) of hepatic cirrhosis.
(A) Coffee consumption could significantly reduce the risk for hepatic cirrhosis, compared with no consumption. (B) Low or moderate coffee consumption could significantly reduce the risk for hepatic cirrhosis, compared with no consumption. Note: coffee consumption a: 0–100 mg/day; b: 101–200 mg/day; c: 201–300 mg/day; d: <1 cup/day; e: 1–2 cups/day.
Table 2. Sensitivity analysis for meta-analysis of coffee consumption vs no consumption on cirrhosis development.
| Meta-analysis | Omitted Study | No. of Included Study | OR | 95% CI | P-het* | I2 |
|---|---|---|---|---|---|---|
| Overall | 0 | 7 | 0.61 (0.45–0.84) | 0.447–0.841 | p<0.01 | 72.10% |
| Sensitivity analysis 1 | Freedman | 6 | 0.56 (0.40–0.78) | 0.403–0.777 | 0.02 | 62.80% |
| Sensitivity analysis 2 | Corrao 2001 | 6 | 0.72 (0.57–0.90) | 0.569–0.904 | 0.125 | 42.00% |
| Sensitivity analysis 3 | Corrao 2001 and Freedman | 5 | 0.66 (0.52–0.83) | 0.524–0.828 | 0.34 | 11.50% |
* P value of heterogenity.
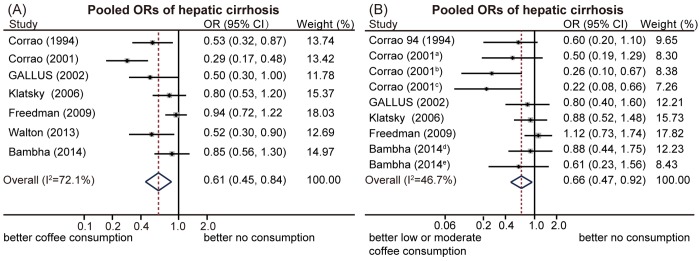
Nine subgroups in six included studies [14, 25, 26, 31, 32, 35] were reported about the associations of low or moderate coffee consumption with cirrhosis, compared with no coffee consumption. The pooled OR was 0.66 (95%CI: 0.47–0.92, random-effects model, Fig 2B), with significant heterogeneity identified among the included studies (I2 = 46.7%; Q-test, p = 0.059). Study-specific and overall estimates of cirrhosis for high coffee consumption compared to no consumption is shown in Fig 3. The pooled OR was 0.53 (95%CI: 0.42–0.68, fixed-effects model), based on nine subgroups in eight included studies [14, 25–28, 31, 32, 35]. No significant heterogeneity was found (I2 = 2.8%; Q-test, p = 0.441).
Fig 3. Pooled odds ratios (ORs) of hepatic cirrhosis.
High coffee consumption significantly reduced the risk for hepatic cirrhosis, compared with no consumption. Note: a: 2 cups/day; b: ≥ 3 cups/day.
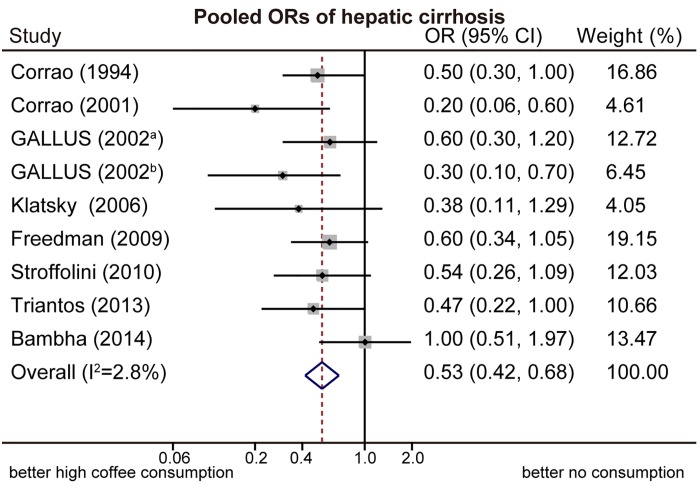
Estimated ORs of advanced hepatic fibrosis for coffee consumption compared with no consumption are shown in Fig 4. The pooled results of eight studies [13, 15, 30, 33–37] showed that coffee consumers were less likely to develop fibrosis, compared with those who do not consume coffee (OR = 0.73, 95%CI: 0.58–0.92, random-effects model). Significant heterogeneity was observed among these studies (I2 = 80.9%, p < 0.01). A sensitivity analysis showed the robustness of the pooled results and identified a cohort study from Hong Kong [34] as the major source of heterogeneity because no significant association with a high weight percent was found.
Fig 4. Pooled odds ratios (ORs) of advanced hepatic fibrosis.
Coffee consumption significantly reduced the risk for advanced hepatic fibrosis, compared with no consumption.
The study-specific ORs and relevant CIs of cirrhosis or advanced hepatic fibrosis for coffee consumption compared with no consumption are presented in Table 3, stratified by the presence of hepatitis and alcohol drinking. Five studies [14, 25–27, 31] compared the effects of coffee consumption with no coffee consumption on cirrhosis development in alcohol drinking population, which rendered a summary OR of 0.49 (95%CI: 0.36–0.67; I2 = 43.2%, Q-test, p = 0.038; random-effects model). Moreover, five studies [15, 30, 33, 36, 37] compared the effects of coffee consumption with no coffee consumption on advanced hepatic fibrosis in patients infected with hepatitis C virus (HCV). The pooled OR was 0.65 (95%CI: 0.49–0.87; I2 = 46.3%, Q-test, p = 0.061; random-effects model). These resulsts suggested that coffee consumption has hepatoprotective effecf on hepatic fibrosis and cirrhosis in these two population.
Table 3. Study-specific odds ratios (ORs) and pooled ORs, with 95%CIs, for coffee consumption and hepatic fibrosis/cirrhosis, classified by strata of chronic liver disease and alcohol consumption.
| Study | Year | Strata of covariate | Coffee consumption | OR | |
|---|---|---|---|---|---|
| Hepatic cirrhosis | |||||
| Corrao | 1994 | Alcohol | 1–100 g/day | Consumption vs. no consumption | 0.54 (0.26–1.13) |
| ≥ 100 g/day | Consumption vs. no consumption | 0.58 (0.14–2.43) | |||
| Corrao | 2001 | Alcohol | 1–25 g/day | ≤ 1 vs. no consumption | 0.69 (0.27–1.79) |
| >1 vs. no consumption | 0.52 (0.22–1.25) | ||||
| 26–50 g/day | ≤ 1 vs. no consumption | 1.25 (0.45–3.49) | |||
| >1 vs. no consumption | 0.53 (0.21–1.35) | ||||
| ≥ 51 g/day | ≤ 1 vs. no consumption | 0.22 (0.08–0.66) | |||
| >1 vs. no consumption | 0.2 (0.07–0.54) | ||||
| GALLUS | 2002 | Alcohol | < 3 drinks/day | ≥ 2 vs. <2 cups/day | 0.2 (0.1–0.6) |
| ≥ 3 drinks/day | ≥ 2 vs. <2 cups/day | 0.8 (0.4–1.6) | |||
| Klatsky | 2006 | Alcohol | drinking | <1 vs. no or seldom consumption | 0.7 (0.4–1.1) |
| 1–3 vs. no or seldom consumption | 0.6 (0.4–0.8) | ||||
| ≥ 4 vs. no or seldom consumption | 0.2 (0.1–0.4) | ||||
| Freedman | 2009 | HCV | 0–1 vs. no consumption | 1.12 (0.73–1.74) | |
| 1–3 vs. no consumption | 1.03 (0.68–1.54) | ||||
| ≥ 3 vs. no consumption | 0.6 (0.34–1.05) | ||||
| Stroffolini | 2010 | Alcohol | 1–3 drinks/day | >2 vs. 0–2 cups/day | 0.36 (0.1–1.28) |
| >3 drinks/day | >2 vs. 0–2 cups/day | 0.62 (0.25–1.55) | |||
| Triantos | 2013 | CLD | >2 vs. no consumption | 0.47 (0.22–1) | |
| Walton | 2013 | CLD | Consumption vs. no consumption | 0.52 (0.3–0.9) | |
| Bambha | 2014 | NAFLD | <1 vs. no consumption | 0.88 (0.44–1.75) | |
| 1–2 vs. no consumption | 0.61 (0.24–1.56) | ||||
| ≥ 2 vs. no consumption | 1 (0.51–1.97) | ||||
| Pooled estimate of alcohol drinking population | Consumption vs. no consumption | 0.49 (0.36–0.67) | |||
| Hepatic fibrosis | |||||
| Modi | 2010 | HCV | 43–125 mg/day vs. seldom consumption | 0.96 (0.27–3.4) | |
| 125–345 mg/day vs. seldom consumption | 1.63 (0.82–3.3) | ||||
| 345–1028 mg/day vs. seldom consumption | 0.51 (0.27–0.98) | ||||
| Costentin | 2011 | HCV | 1.5–3 vs. <1.5 cups/day | 0.23 (0.08–0.63) | |
| 3–5 vs. 1.5 cups/day | 0.57 (0.26–1.27) | ||||
| > 5 vs. 1.5 cups/day | 0.65 (0.29–1.44) | ||||
| Ong | 2011 | HBV | Consumption vs. no consumption | 1 (0.99–1) | |
| Anty | 2012 | NAFLD | Consumption vs. no consumption | 0.75 (0.58–0.98) | |
| Machado | 2014 | HCV | Consumption vs. no consumption | 0.16 (0.03–0.8) | |
| El-Serag | 2014 | HCV | Consumption vs. no consumption | 0.69 (0.5–0.96) | |
| Khalaf | 2015 | HCV | Low consumption vs. no consumption | 0.71 (0.53–0.95) | |
| Pooled estimate of HCV population | Moderate/high vs. no/low consumption | 0.65 (0.49–0.87) | |||
CLD, chronic liver disease; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease.
Discussion
Coffee consumption might reduce the risks for hepatic fibrosis and cirrhosis. However, previous studies have reported inconsistent results and failed to provide a conclusive recommendation [14, 25–29, 31, 32, 35]. In the present meta-analysis, the pooled estimates demonstrated significantly reduced risks for hepatic cirrhosis and advanced fibrosis in coffee consumers. More importantly, the included studies that reported hepatoprotective effects of coffee were mostly from Western countries where coffee consumption is frequent [13–15, 25–29, 31–33, 35, 36].
The mechanism of the protective effects of coffee consumption on hepatic fibrosis and cirrhosis remains to be elucidated. Several studies have identified preventive effects of coffee and caffeine extracts on hepatic fibrosis in standard rodent models. For example, Shim et al. have found that in an immortalized human hepatic stellate cell (HSC) line, caffeine can reduce the risk for hepatic fibrosis by downregulating the expression of α-smooth muscle actin and procollagen type Ic as well as inducing apoptosis of HSCs [38]. Decreased liver inflammation and fibrosis were also observed in thioacetamide-treated rats used in the study by Shim et al. Moreover, Moreno et al. have demonstrated that coffee consumption reduced collagen content and the levels of the profibrogenic cytokine transforming growth factor, which consequently prevented the development of hepatic cirrhosis in a CCl4-treated rat model [39]. Another potential mechanism is that coffee intake might significantly alleviate liver inflammation, since it has been shown to have a potent antioxidant capacity by increasing the plasma concentration of glutathione, thus preventing scar formation and promoting healing [40]. Moreover, coffee consumption might prevent liver damage by reducing systemic and liver oxidative stress as well as decreasing the concentrations of proinflammatory and inflammatory cytokines in the liver [41]. As HSCs are the major effector cells in the pathogenesis of hepatic fibrosis/cirrhosis, it seems that the suppression of HSC A 2a adenosine receptors by caffeine is the primary mechanism to prevent fibrosis/cirrhosis [42].
Coffee consumption has been found to be correlated with a reduction of ALT activity in a large population-based study of CLD [43]. Moreover, it also decreased GGT levels and protected liver cells from damage [10, 44]. As ALT and GGT activity are well-established risk indicators for hepatic fibrosis and cirrhosis [45, 46], the hepatoprotective effects of coffee on hepatic fibrosis and cirrhosis might partly be modulated by the reduction of ALT and GGT.
Hepatic fibrosis and cirrhosis caused by CLD are global health problems. Therefore, it is important to develop some simple approaches to prevent fibrosis and cirrhosis in patients with high risk. Through subgroup meta-analysis, we identified significant hepatoprotective effects of coffee consumption on hepatic fibrosis and cirrhosis in patients with alcoholic liver disease and chronic HCV infection, which mainly contribute to CLD in Western countries. Thus, coffee consumption seems to be an attractive lifestyle for CLD patients, as coffee intake is popular in those areas. In addition to its antifibrotic effect, coffee can reduce the risk for liver cancer [12, 47], which is the worst outcome for CLD patients.
As the present study is based on the evidences of observational studies, it is difficult to determine whether it is occasional for the inverse associations of coffee consumption with hepatic fibrosis and cirrhosi. Caffeine metabolism in CLD patients has been reported to be impaired due to liver injury [48]. Therefore, the inverse associations may partly be due to the reduction of coffee consumption caused by digestive tract discomfort. This could have rendered a spurious protective effect of coffee on hepatic fibrosis and cirrhosis. However, all the included studies used CLD patients as controls to eliminate such a possibility.
There are some limitations to the present study. First, most of the included studies were in Western countries. Thus, generalization of the current findings to other populations should be made with caution. In addition, analysis of publication bias could not be conducted due to limited studies in each pooled meta-analysis. As null results in small studies tend not to be published, the associations of coffee consumption with hepatic fibrosis and cirrhosis development may have been overestimated due to potential publication bias. Third, there was variance in consumption duration among the included studies, which might have been a potential source of heterogeneity. Therefore, the conclusion of the current study that coffee consumption can reduce the risk for hepatic fibrosis/cirrhosis might be underestimated because some differences among the study groups might not have been identified if the consumption duration was insufficient. Finally, the estimations of coffee consumption in the included trials were mainly obtained by self-reporting, which might have caused some bias. Nevertheless, this assessment has been previously shown to be valid and reproducible [49, 50].
In conclusion, the present meta-analysis suggests that coffee consumption can prevent the development of hepatic fibrosis and cirrhosis. However, further prospective studies are still needed to control the bias and confounding factors.
Supporting Information
Two studies, as the outliers, were found to be the potential source of heterogeneity.
(TIF)
No single study was found to significantly influence the pooled estimate.
(TIF)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding was provided by the National Natural Science Foundation of China (81171560), National Key Technology Support Program (2012BAI35B03), and the “Par-Eu Scholars Program" of Chongqing city. This study was also supported by the National Science and Technology Major Project of China (2008ZX10002-006, 2012ZX10002007001, 2011ZX09302005, 2012ZX09303001-001, 2012ZX10002003), the Key Project of Chongqing Science and Technology (cstc2012gg-yyjsB10007), the Chongqing Natural Science Commission Foundation (cstc2011jjA10025), the Medical Research Fund by Chongqing Municipal Health Bureau (2009-1-71), and the “Par-Eu Scholars Program" of Chongqing city. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician. 2006;74(5):756–62. . [PubMed] [Google Scholar]
- 2. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–50. . [DOI] [PubMed] [Google Scholar]
- 3. Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328(25):1828–35. 10.1056/NEJM199306243282508 . [DOI] [PubMed] [Google Scholar]
- 4. Hui AY, Friedman SL. Molecular basis of hepatic fibrosis. Expert reviews in molecular medicine. 2003;5(5):1–23. 10.1017/S1462399403005684 . [DOI] [PubMed] [Google Scholar]
- 5. Chu CM, Liaw YF. Hepatitis B virus-related cirrhosis: natural history and treatment. Semin Liver Dis. 2006;26(2):142–52. Epub 2006/05/05. 10.1055/s-2006-939752 . [DOI] [PubMed] [Google Scholar]
- 6. Larter CZ, Yeh MM, Haigh WG, Van Rooyen DM, Brooling J, Heydet D, et al. Dietary modification dampens liver inflammation and fibrosis in obesity-related fatty liver disease. Obesity. 2013;21(6):1189–99. 10.1002/oby.20123 . [DOI] [PubMed] [Google Scholar]
- 7. White DL, Richardson PA, Al-Saadi M, Fitzgerald SJ, Green L, Amaratunge C, et al. Dietary history and physical activity and risk of advanced liver disease in veterans with chronic hepatitis C infection. Dig Dis Sci. 2011;56(6):1835–47. 10.1007/s10620-010-1505-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casiglia E, Spolaore P, Ginocchio G, Ambrosio GB. Unexpected effects of coffee consumption on liver enzymes. European journal of epidemiology. 1993;9(3):293–7. . [DOI] [PubMed] [Google Scholar]
- 9. Honjo S, Kono S, Coleman MP, Shinchi K, Sakurai Y, Todoroki I, et al. Coffee consumption and serum aminotransferases in middle-aged Japanese men. J Clin Epidemiol. 2001;54(8):823–9. . [DOI] [PubMed] [Google Scholar]
- 10. Tanaka K, Tokunaga S, Kono S, Tokudome S, Akamatsu T, Moriyama T, et al. Coffee consumption and decreased serum gamma-glutamyltransferase and aminotransferase activities among male alcohol drinkers. International journal of epidemiology. 1998;27(3):438–43. Epub 1998/08/11. . [DOI] [PubMed] [Google Scholar]
- 11. Bravi F, Bosetti C, Tavani A, Bagnardi V, Gallus S, Negri E, et al. Coffee drinking and hepatocellular carcinoma risk: a meta-analysis. Hepatology. 2007;46(2):430–5. 10.1002/hep.21708 . [DOI] [PubMed] [Google Scholar]
- 12. Bravi F, Bosetti C, Tavani A, Gallus S, La Vecchia C. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2013;11(11):1413–21 e1. 10.1016/j.cgh.2013.04.039 . [DOI] [PubMed] [Google Scholar]
- 13. Anty R, Marjoux S, Iannelli A, Patouraux S, Schneck A-S, Bonnafous S, et al. Regular coffee but not espresso drinking is protective against fibrosis in a cohort mainly composed of morbidly obese European women with NAFLD undergoing bariatric surgery. Journal of Hepatology. 2012;57(5):1090–6. 10.1016/j.jhep.2012.07.014 . [DOI] [PubMed] [Google Scholar]
- 14. Corrao G, Zambon A, Bagnardi V, D'Amicis A, Klatsky A, Collaborative SG. Coffee, caffeine, and the risk of liver cirrhosis. Annals of epidemiology. 2001;11(7):458–65. 10.1016/s1047-2797(01)00223-x . [DOI] [PubMed] [Google Scholar]
- 15. Costentin CE, Roudot-Thoraval F, Zafrani E-S, Medkour F, Pawlotsky J-M, Mallat A, et al. Association of caffeine intake and histological features of chronic hepatitis C. Journal of Hepatology. 2011;54(6):1123–9. 10.1016/j.jhep.2010.08.027 . [DOI] [PubMed] [Google Scholar]
- 16. Wang X, Liu F, Wei F, Ren H, Hu H. Efficacy and safety of pegylated interferon plus ribavirin therapy for chronic hepatitis C genotype 6: a meta-analysis. PLoS One. 2014;9(6):e100128 10.1371/journal.pone.0100128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 18. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48. . [PubMed] [Google Scholar]
- 19. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–88. . [DOI] [PubMed] [Google Scholar]
- 20. Higgins J. Green S. Cochrane handbook for systematic reviews of interventions version 5.1. 0. The Cochrane Collaboration. 2011;5(0). [Google Scholar]
- 21. Hedges LV, Pigott TD. The power of statistical tests in meta-analysis. Psychological methods. 2001;6(3):203–17. . [PubMed] [Google Scholar]
- 22. Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Statistics in medicine. 1988;7(8):889–94. . [DOI] [PubMed] [Google Scholar]
- 23. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. . [PubMed] [Google Scholar]
- 25. Corrao G, Lepore AR, Torchio P, Valenti M, Galatola G, D'Amicis A, et al. The effect of drinking coffee and smoking cigarettes on the risk of cirrhosis associated with alcohol consumption. A case-control study. Provincial Group for the Study of Chronic Liver Disease. European journal of epidemiology. 1994;10(6):657–64. Epub 1994/12/01. . [DOI] [PubMed] [Google Scholar]
- 26. Gallus S, Tavani A, Negri E, La Vecchia C. Does coffee protect against liver cirrhosis? Annals of epidemiology. 2002;12(3):202–5. . [DOI] [PubMed] [Google Scholar]
- 27. Stroffolini T, Cotticelli G, Medda E, Niosi M, Del Vecchio-Blanco C, Addolorato G, et al. Interaction of alcohol intake and cofactors on the risk of cirrhosis. Liver International. 2010;30(6):867–70. 10.1111/j.1478-3231.2010.02261.x . [DOI] [PubMed] [Google Scholar]
- 28. Triantos C, Manolakopoulos S, Smirnidis A, Vlachogiannakos J, Goulis J, Kalafateli M, et al. Is caffeine responsible for the hepatoprotective effect of coffee consumption in patients with chronic liver diseases? A multicentre study. Gastroenterology. 2013;1):S1008 . [Google Scholar]
- 29. Walton HB, Masterton GS, Hayes PC. An epidemiological study of the association of coffee with chronic liver disease. Scottish medical journal. 2013;58(4):217–22. Epub 2013/11/12. 10.1177/0036933013507869 . [DOI] [PubMed] [Google Scholar]
- 30. Khalaf N, White D, Kanwal F, Ramsey D, Mittal S, Tavakoli-Tabasi S, et al. Coffee and Caffeine Are Associated With Decreased Risk of Advanced Hepatic Fibrosis Among Patients With Hepatitis C. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2015. 10.1016/j.cgh.2015.01.030 . [DOI] [PubMed] [Google Scholar]
- 31. Klatsky AL, Morton C, Udaltsova N, Friedman GD. Coffee, cirrhosis, and transaminase enzymes. Arch Intern Med. 2006;166(11):1190–5. Epub 2006/06/15. 10.1001/archinte.166.11.1190 . [DOI] [PubMed] [Google Scholar]
- 32. Freedman ND, Everhart JE, Lindsay KL, Ghany MG, Curto TM, Shiffman ML, et al. Coffee Intake Is Associated with Lower Rates of Liver Disease Progression in Chronic Hepatitis C. Hepatology. 2009;50(5):1360–9. 10.1002/hep.23162 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Modi AA, Feld JJ, Park Y, Kleiner DE, Everhart JE, Liang TJ, et al. Increased Caffeine Consumption Is Associated with Reduced Hepatic Fibrosis. Hepatology. 2010;51(1):201–9. 10.1002/hep.23279 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ong A, Wong VWS, Wong GLH, Chan HLY. The effect of caffeine and alcohol consumption on liver fibrosis—a study of 1045 Asian hepatitis B patients using transient elastography. Liver International. 2011;31(7):1047–53. 10.1111/j.1478-3231.2011.02555.x [DOI] [PubMed] [Google Scholar]
- 35. Bambha K, Wilson LA, Unalp A, Loomba R, Neuschwander-Tetri BA, Brunt EM, et al. Coffee consumption in NAFLD patients with lower insulin resistance is associated with lower risk of severe fibrosis. Liver International. 2014;34(8):1250–8. 10.1111/liv.12379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. El-Serag H, Kuzniarek J, Ramsey DJ, Tabasi ST, White DL, Kanwal F. Beverage intake and the risk of advanced fibrosis in HCV: Coffee, tea, or sodas? Gastroenterology. 2014;1):S129–S30. . [Google Scholar]
- 37. Machado SR, Parise ER, de Carvalho L. Coffee has hepatoprotective benefits in Brazilian patients with chronic hepatitis C even in lower daily consumption than in American and European populations. Brazilian Journal of Infectious Diseases. 2014;18(2):170–6. 10.1016/j.bjid.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shim SG, Jun DW, Kim EK, Saeed WK, Lee KN, Lee HL, et al. Caffeine attenuates liver fibrosis via defective adhesion of hepatic stellate cells in cirrhotic model. J Gastroenterol Hepatol. 2013;28(12):1877–84. 10.1111/jgh.12317 . [DOI] [PubMed] [Google Scholar]
- 39. Moreno MG, Chavez E, Aldaba-Muruato LR, Segovia J, Vergara P, Tsutsumi V, et al. Coffee prevents CCl(4)-induced liver cirrhosis in the rat. Hepatol Int. 2011;5(3):857–63. 10.1007/s12072-010-9247-6 . [DOI] [PubMed] [Google Scholar]
- 40. Esposito F, Morisco F, Verde V, Ritieni A, Alezio A, Caporaso N, et al. Moderate coffee consumption increases plasma glutathione but not homocysteine in healthy subjects. Alimentary pharmacology & therapeutics. 2003;17(4):595–601. . [DOI] [PubMed] [Google Scholar]
- 41. Vitaglione P, Morisco F, Mazzone G, Amoruso DC, Ribecco MT, Romano A, et al. Coffee reduces liver damage in a rat model of steatohepatitis: the underlying mechanisms and the role of polyphenols and melanoidins. Hepatology. 2010;52(5):1652–61. Epub 2010/11/03. 10.1002/hep.23902 . [DOI] [PubMed] [Google Scholar]
- 42. Dranoff JA, Feld JJ, Lavoie EG, Fausther M. How does coffee prevent liver fibrosis? Biological plausibility for recent epidemiological observations. Hepatology. 2014;60(2):464–7. 10.1002/hep.27032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ruhl CE, Everhart JE. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2005;128(1):24–32. . [DOI] [PubMed] [Google Scholar]
- 44. Kono S, Shinchi K, Imanishi K, Todoroki I, Hatsuse K. Coffee and serum gamma-glutamyltransferase: a study of self-defense officials in Japan. Am J Epidemiol. 1994;139(7):723–7. . [DOI] [PubMed] [Google Scholar]
- 45. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–26. 10.1053/jhep.2003.50346 . [DOI] [PubMed] [Google Scholar]
- 46. Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357(9262):1069–75. 10.1016/S0140-6736(00)04258-6 . [DOI] [PubMed] [Google Scholar]
- 47. Larsson SC, Wolk A. Coffee consumption and risk of liver cancer: a meta-analysis. Gastroenterology. 2007;132(5):1740–5. 10.1053/j.gastro.2007.03.044 . [DOI] [PubMed] [Google Scholar]
- 48. Hasegawa M, Yamada S, Hirayama C. Fasting plasma caffeine level in cirrhotic patients: relation to plasma levels of catecholamines and renin activity. Hepatology. 1989;10(6):973–7. . [DOI] [PubMed] [Google Scholar]
- 49. Tokunaga S, Hirohata T, Hirohata I. Reproducibility of dietary and other data from a self-administered questionnaire. Environmental health perspectives. 1994;102 Suppl 8:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. The American journal of clinical nutrition. 1999;69(2):243–9. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two studies, as the outliers, were found to be the potential source of heterogeneity.
(TIF)
No single study was found to significantly influence the pooled estimate.
(TIF)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.