Coral reef community responses vary but bioerosion increases under natural ocean acidification.
Keywords: coral reef, ocean acidification, community composition, calcification, bioerosion
Abstract
Ocean acidification threatens the survival of coral reef ecosystems worldwide. The negative effects of ocean acidification observed in many laboratory experiments have been seen in studies of naturally low-pH reefs, with little evidence to date for adaptation. Recently, we reported initial data suggesting that low-pH coral communities of the Palau Rock Islands appear healthy despite the extreme conditions in which they live. Here, we build on that observation with a comprehensive statistical analysis of benthic communities across Palau’s natural acidification gradient. Our analysis revealed a shift in coral community composition but no impact of acidification on coral richness, coralline algae abundance, macroalgae cover, coral calcification, or skeletal density. However, coral bioerosion increased 11-fold as pH decreased from the barrier reefs to the Rock Island bays. Indeed, a comparison of the naturally low-pH coral reef systems studied so far revealed increased bioerosion to be the only consistent feature among them, as responses varied across other indices of ecosystem health. Our results imply that whereas community responses may vary, escalation of coral reef bioerosion and acceleration of a shift from net accreting to net eroding reef structures will likely be a global signature of ocean acidification.
INTRODUCTION
Shifts in ocean chemistry are likely occurring more rapidly now than in the past 300 million years (1). Excess CO2 released from fossil fuel emissions and deforestation is absorbed by the surface oceans, driving down seawater pH and calcium carbonate (CaCO3) saturation state (Ω), a process termed ocean acidification (OA) (2). Coral reefs are considered especially vulnerable to OA. Reefs are made of CaCO3 produced by calcifying organisms, including corals and coralline algae, and laboratory experiments have shown that biogenic calcification is slowed and its destruction is accelerated at levels of OA projected for the end of this century (2, 3). Some experiments have raised key questions regarding the potential for coral reef organisms to adapt to OA or for covarying environmental factors, such as light, water flow, and nutrient availability, to modulate the impacts of OA (4–8). However, most studies of naturally low-pH reefs, including CO2 vents in Papua New Guinea (PNG) and Japan, freshwater seeps in Mexico, and upwelling regions of the eastern tropical Pacific, have yielded no evidence to support either scenario (9, 10).
In our previous report (11), we presented our initial data showing that coral communities of the Palau Rock Islands, formed by a labyrinthine maze of uplifted karst, appear healthy despite the relatively extreme pH conditions in which they live. As water flows from the open ocean over the barrier reefs and into the Rock Island bays, its carbonate system chemistry is altered by a combination of biological and hydrographic processes that elevate pCO2 and drive down pH and Ω, a natural form of acidification (11). The long residence time of seawater within the Rock Islands exacerbates this process, and as a result, the benthic communities in Palau’s most acidified reefs live in conditions with pH and Ω levels equivalent to those predicted for the western tropical Pacific open ocean by 2100 (Fig. 1) (12). Recent continuous pH data collected in situ over multiple, consecutive diel cycles (Fig. 1, inset) reveal that whereas natural acidification decreases the mean pH within the Rock Islands, the diel range in pH is maintained across Palau’s natural OA gradient. The downward shift in pH and Ω without change in amplitude or frequency of variability contrasts the extreme and highly variable conditions at CO2 vent sites and freshwater seeps (13, 14), and is more consistent with the predicted nature of the progression of OA in the marine environment (12).
Fig. 1. Natural acidification gradient across Palau mirrors projected anthropogenic CO2-driven changes in ocean chemistry.
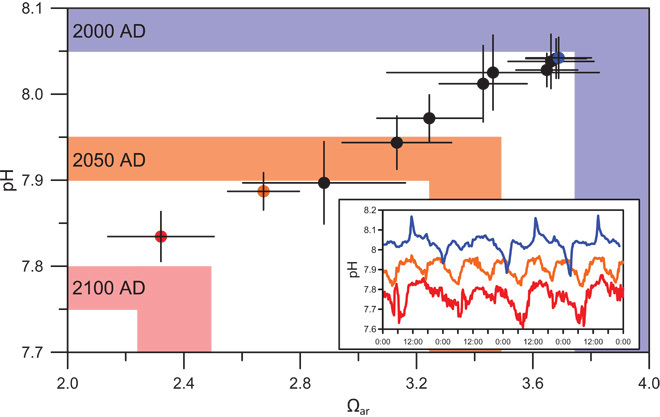
Shown are the mean (±1 SD) dawn-to-dusk pH and Ωar for our 11 reef study sites and diurnal pH variability at three of those sites over 4 days (inset) (fig. S1: sites 1, 2, and 10). Shaded regions indicate the range of western tropical Pacific open ocean pH and Ωar levels in 2000 (blue) and open ocean values predicted for 2050 (orange) and 2100 (red) (12, 52). Colored points correspond to pH time series in inset.
Here, we assess coral reef benthic community structure and key ecosystem processes across a natural gradient in seawater pH and Ω in Palau. We evaluate these response variables against a comprehensive characterization of carbon chemistry to investigate whether measureable changes in benthic community structure; coral community composition; declines in skeletal extension, density, and calcification; and/or increases in the prevalence and rates of bioerosion seen in laboratory experiments and analog sites can be detected and attributed to OA in Palau. Finally, we compare our data with those collected at other naturally low-pH reefs to identify response variables common across all sites, independent of the mechanism of acidification, biogeography, frequencies of variability, and presence of other environmental factors that may exacerbate or mask the OA impact.
RESULTS
Carbon system chemistry
Characterization of Palau’s carbonate chemistry environment was achieved by discrete water sampling at 11 study sites during multiple tidal cycles, seasons, and years from dawn (6:00 a.m.) to dusk (6:00 p.m.), combined with continuous, 4-day-long pH sensor deployment and water sampling to characterize diurnal variability at a subset of these sites (Fig. 1, fig. S1, and tables S1 and S2). Site average dawn-to-dusk pH and Ω of the CaCO3 mineral aragonite (Ωar) are typically within error of 24-hour mean values (15) and are thus considered representative of the full diel range in carbon system chemistry. Average pH/Ωar ranged from 8.05 (±0.04 SD)/3.7 (±0.3) at the highest-pH/Ωar barrier reef site to 7.84 (±0.03)/2.3 (±0.2) at the lowest-pH/Ωar Rock Island site, falling as low as 7.61/1.86 in the early hours of the morning. Mean pH/Ωar levels were significantly different across study sites [ANOVA (analysis of variance), F10,14 = 21.4, P < 0.001], but were not correlated with concentrations of NO2−/NO3− (r = −0.42, P = 0.19), PO43− (r = −0.36, P = 0.27), or NH4+ (r = −0.34, P = 0.31). A comparison of our mean 2011–2013 Ωar (3.68±0.05 SE) measured at site 10 (fig. S1) just offshore of Palau’s northwest barrier reef with Ωar at the same site in 1994 (3.87±0.04) and 2000 (3.80±0.02) (16, 17) shows a 0.19 decrease over 20 years (see the Supplementary Materials). This is similar to the direction and magnitude of change (−0.24) recorded at the open ocean station ALOHA over the same period (Ωar = 3.75 to Ωar = 3.51) (17), suggesting that the strong influence of local processes on Palau’s carbon system chemistry is superimposed on a steady decline in Ωar caused by air-sea exchange of anthropogenic CO2.
Benthic community cover
Benthic communities were compared across eight sites spanning the full range of carbonate chemistry using cover and composition estimates derived from five 50-m transect lines per site collected at 3-m depth (fig. S1 and tables S1 and S2). Despite the steep decline in pH and Ωar, our analysis revealed no significant change in the cover of live hard coral, macroalgae, and crustose coralline algae or in coral genera richness or diversity [Fig. 2 and table S3; generalized linear model (GLMs), P > 0.05]. Coral communities within the lowest-Ωar reef site (Ωar = 2.32) hosted the highest coral cover (>60%) and genus richness (12.6 genera transect−1) and the lowest macroalgae cover (<1%).
Fig. 2. Palau coral reef community responses to acidification.
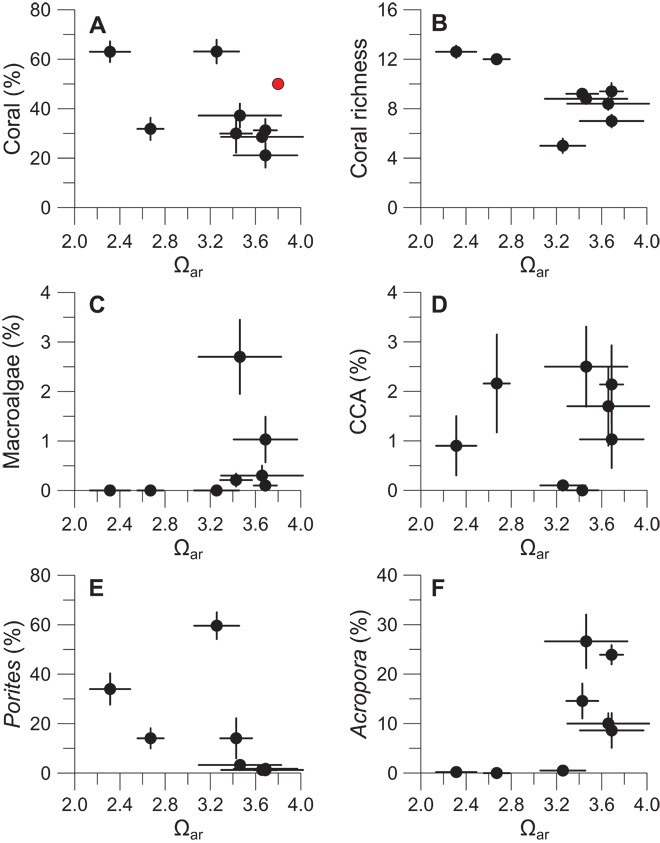
(A) Mean (±1 SE) percent coral cover per transect (n = 5 per site) from 2010 to 2012 (circles) versus site mean Ωar (±1 SD) with pre-1998 bleaching barrier coral cover (18) and estimated Ωar (16) from 1992 to 1994 (red circle). (B) Number of coral genera observed (richness). (C and D) Macroalgae (C) and crustose coralline algae (CCA) (D). (E and F) Percent cover of Porites spp. (E) and Acropora spp. (F).
Coral community composition
Whereas coral cover and richness were insensitive to differences in pH among sites, we detected a significant relationship between pH/Ωar and coral community composition as well as shifts in the presence and abundance of coral genera across sites (Fig. 3; redundancy analysis, pseudo-F7,32 = 8.80, P < 0.001). The coral compositions of the barrier sites (highest pH and Ωar) were similar to each other, defined by abundant Acropora, Montipora, and Pocillopora. Coral communities occupying the lagoonal sites (lowest pH and Ωar) were distinct from the barrier communities but were also distinct from each other: one site (Ωar = 3.24) was Porites-dominated, whereas another (Ωar = 2.67) was defined by populations of Leptastrea, Platygyra, Favites, and Favia. Our lowest-Ωar site identified to date (Ωar =2.32) uniquely hosted Pachyseris, Symphyllia, Mycedium, Lobophyllia, Plerogyra, and Merulina. In general, Porites abundance increased with decreasing pH (log-linear GLM, P < 0.05), whereas the abundances of Acropora, Montipora, and Pocillopora declined (negative binomial GLM, P < 0.01; table S4).
Fig. 3. Detrended correspondence analysis (DCA) scores for eight Palauan reef sites and coral genera.
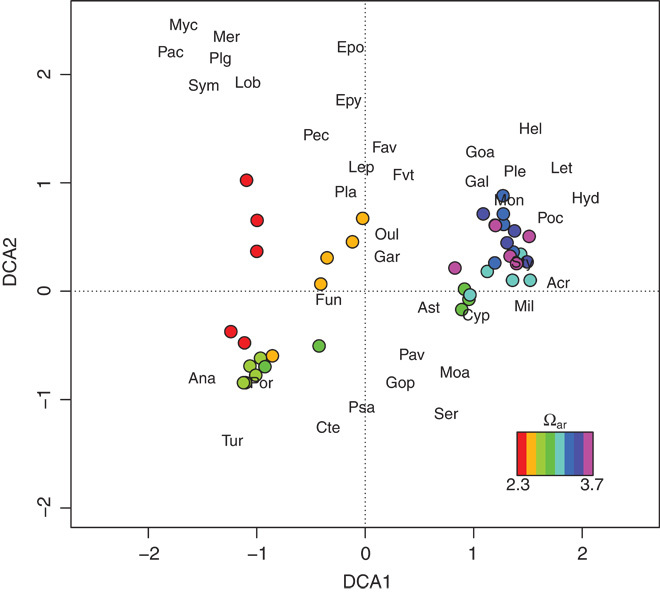
DCA scores are for each of five transects collected per reef site (circles, colored by mean Ωar for each site) and their coral genera (names abbreviated). See table S6 for genus abbreviations.
Coral skeletal growth and macrobioerosion
The skeletal extension, density, and calcification rates of two coral genera (Porites and Favia) did not exhibit statistically significant changes across the pH gradient (Fig. 4 and table S5; GLMs, P > 0.05). The presence of macrobioerosion in Porites corals increased significantly at low Ωar (logistic regression, P <0.001), and the volume percent of skeleton removed by bioeroding organisms, predominantly the bivalve Lithophaga, increased 11-fold from the highest-Ωar to the lowest-Ωar sites (log-linear GLM, P =0.03) in coral skeletons with nonzero bioerosion. We did not detect a significant relationship between skeletal density and the volume of coral skeleton eroded (log-linear GLM, P = 0.35), but the likelihood that Porites coral skeletons were bioeroded increased as skeletal density decreased (logistic regression, P < 0.001).
Fig. 4. Skeletal growth responses of two coral genera to acidification.
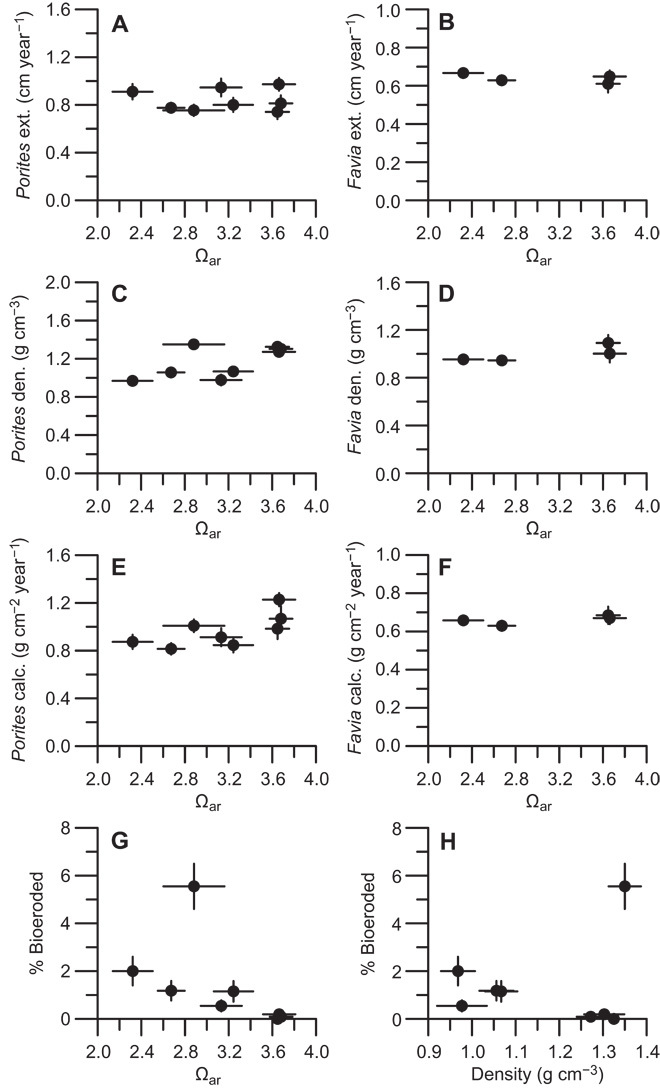
(A to H) Skeletal analyses include 2006–2010 site mean (±1 SE) extension (ext.), density (den.), and calcification rates (calc.) for Porites (A, C, and E) and Favia (B, D, and F); percent Porites skeletal volume macrobioeroded (G) versus Ωar (±1 SD); and the relationship between Porites density and skeletal volume macrobioeroded (H).
DISCUSSION
Despite the pH and Ωar conditions already at predicted end-of-century open ocean levels and pCO2 up to 720 μatm, the Rock Islands support high coral cover, richness, and diversity and very low macroalgae cover. This observation counters expectations based on some laboratory CO2 manipulation experiments and studies of other naturally low-pH reefs in which severe declines in coral richness and coralline algal cover and increases in macroalgae are signature impacts of OA (13, 14). In general, coral cover on Palau’s high-pH barrier reefs (28 to 37%) was lower than that of the low-pH bay reefs (32 to 63%), a trend likely exacerbated by a bleaching event in 1998 that caused declines in coral cover on the barrier reef but not the bays. Nevertheless, the relatively high cover and diversity in the low-pH reefs cannot be solely attributed to differential bleaching-induced mortality: the pre-1998 cover on the barrier (50% in 1992) was already lower than the current coral cover on Palau’s lowest-pH reefs (63%) (18). The skeletal extension, density, and calcification rates of Porites and Favia corals did not change significantly with declining pH and Ωar, indicating that the rates of CaCO3 production, a physiological process considered one of the most sensitive to OA, are maintained across Palau’s OA gradient. In contrast, many laboratory CO2 manipulation experiments with Porites and Favia corals have shown significant declines in calcification of these genera with declining pH/Ωar (7, 14, 19–21).
We observed significant changes in coral community structure that track changes in pH and Ωar. However, the compositional heterogeneity among low-pH, low-Ωar sites on Palau suggests that coral community structure under OA conditions is not deterministic, and there is no single community of acidification “winners” within Palau’s low-pH reefs. Indeed, other local environmental and/or ecological factors, including changes in wave energy, temperature, and/or light, all of which covary as pH decreases from the barrier reefs to the Rock Island bays, may play a larger role in shaping community composition. Several observations support this interpretation. First, the shift from offshore Acropora-abundant to inshore Porites-abundant communities is consistent with worldwide reef zonation patterns (22). Second, the presence and abundance of other genera (including Favia and Favites) did not change with decreasing pH. Finally, a number of branching and foliose genera (for example, Pachyseris, Anacropora, Mycedium, Merulina, and Lobophyllia) typically associated with lower wave energy and/or light levels, and not considered insensitive to pH (23), were more abundant on Palau’s lowest-pH reefs than on the high-pH barrier reefs.
We also found that coral macrobioerosion increases significantly as pH decreases in Palau. In contrast to the change in community composition, factors other than pH are unlikely to explain this trend. Across multiple studies of coral bioerosion, there is no consistent correlation between macrobioerosion rates and degrees of wave exposure or intensity of flow (24–28). Elevated nutrient concentrations have been shown to correlate with increased coral macrobioerosion (15, 29), but nutrient concentrations do not change with decreasing seawater pH or with increased bioerosion in Palau. Our hypothesis that low pH causes the observed increase in coral macrobioerosion is supported by multiple laboratory experiments that show elevated bioerosion at low pH (30–32). Furthermore, in a recent field study, pH/Ωar emerged as a consistent factor in Porites macrobioerosion on 11 Pacific reef systems (15) spanning a wide range of pH, wave energy, and flow conditions (33). There are several potential mechanisms by which OA can cause increased macrobioerosion of live corals. One hypothesis is that skeletons accreted under OA are less dense, making them easier for bioeroders to penetrate (34). Although low-pH conditions in Palau do not necessarily produce lower-density skeletons, colonies with less dense skeletons are, in general, more likely to show evidence of bioerosion. In addition, lower pH may facilitate bioerosion by increasing the efficiency of biochemical dissolution (30, 35), one of the methods Lithophaga employs to excavate coral skeletons (36).
We compared our results with those obtained from similar analyses of naturally low-pH coral reef ecosystems near volcanic CO2 vents in Milne Bay, PNG (13), submarine freshwater springs (ojos) in Puerto Morelos, Mexico (14, 37), and low-pH upwelling zones in the eastern tropical Pacific (38, 39) in an effort to identify common response variables solely attributable to changes in pH (Table 1). Despite the paucity of naturally more acidified reefs identified to date, across-site comparisons are necessary because none are perfect analogs for coral reefs under future OA. CO2 accumulation is not always the primary or only driver of low pH/Ωar, and pH variability can be extreme relative to projected future values, as well as spatially and temporally heterogeneous. Covariability among pH and other variables including nutrients, salinity, light, water flow, and/or temperature can make it difficult to attribute specific ecological changes solely to acidification. Furthermore, scales of larval connectivity and recruitment sources vary between sites and may influence the adaptive potential of coral communities.
Table 1. Diverse reef responses to natural acidification in the Palau Rock Islands, PNG CO2 vents, Mexico ojos, and eastern tropical Pacific (ETP) upwelling regions.
For each site, response variables are reported as the ratio of each variable in low/high pH sites. + indicates a significant increase in the response variable from high to low pH, and − indicates a significant decrease. n.d., no data available.
| Palau* | PNG† | Mexico‡ | ETP§ | |
| Hard coral cover | 1.9 | 1.1 | 0.5 (−) | 0.0 (−) |
| Macroalgae cover | 0.7 | 2.1 (+) | n.d. | n.d. |
| Coralline algae cover | 1.1 | 0.2 (−) | n.d. | n.d. |
| Hard coral richness | 1.6 | 0.6 (−) | 0.3 (−) | 0.2 (−) |
| Porites cover | 16.0 (+) | 2.3 (+) | 0.8 | n.d. |
| Porites extension | 1.0 | 1.1 | 1.0 | 0.6 |
| Porites density | 0.8 | 1.0 | 0.8 (−) | 0.8 (−) |
| Porites calcification | 0.8 | 1.1 | 0.7 (−) | 0.5 (−) |
| Bioerosion¶ | 11.3 (+) | 1.9 (+) | 1.8 (+) | 1.9 (+) |
*For Palau, ratios are calculated for the two lowest and the two highest Ωar reefs, and the indicated significance is for the trend across all sites (Ωar = 3.7 to 2.3).
†For PNG, ratios and trends are reported for Ωar = 3.5 to 2.9 (13).
‡Community data for Mexico are reported for Ωar > 2.5 to Ωar < 2.5 (37), and skeletal growth parameters are reported for Ωar > 2 to Ωar < 2 (14).
§For the ETP, hard coral cover, hard coral richness, and Porites extension, density, and calcification data are reported for four reef sites within the Galapagos (Ωar = 3.3 to 2.4) (39). Porites macrobioerosion rates are compared across the Galapagos, the Gulf of Panama, and Gulf of Chiriquí (Ωar = 3.5 to 2.5) (38).
¶Trends in bioerosion are estimated by the percent volume of macrobioerosion of Porites skeletal cores (Palau and Mexico), Porites bioeroder density (PNG), or bioerosion rate (ETP).
Our across-site comparison reveals few commonalities among low-pH reefs studied to date (Table 1). Despite comparable natural gradients in pH/Ωar, trends in coral cover and richness and cover of macroalgae and coralline algae are inconsistent, with the Rock Islands unique in showing no sensitivity to pH in any of these response variables. Coral richness declined steeply with declining pH in Mexico, PNG, and the eastern tropical Pacific, but did not change in Palau. Macroalgae cover increased at low pH in PNG only. On a CO2 reef vent site in Japan (not included in Table 1), hard coral–dominated communities gave way to soft coral–dominated reef communities as pH declined from the fringing reef to the shallow back reef pools cut off from the ocean at low tide (40). This did not occur at the PNG CO2 vent site. Porites abundance increased with pH decline in Palau and PNG, but it was not affected by low pH in Mexico. Porites calcification in Palau and PNG was insensitive to decreasing pH, whereas in Mexico, the eastern tropical Pacific, and in laboratory OA experiments, Porites calcification declined with low pH (7, 19, 21, 41).
The inconsistencies in community responses to acidification across naturally low-pH reef systems, and between reefs and laboratory experiments, may be due to a number of different factors. Although ultimately producing similar average pH and Ωar conditions, distinct mechanisms of acidification at each site lead to differences in extremes and frequencies of variability. For example, whereas the maximum seawater pCO2 levels in the Palau Rock Islands (pCO2 ~720 μatm) are close to the 2100 AD projections for the open ocean, the maximum concentrations at the Yucatan (pCO2 ~5120 μatm) (14) and PNG (pCO2 ~5740 μatm) (13) sites are about seven to eight times higher. pH variability in Palau is dominated by the diurnal and tidal cycles as it will be on future reefs, whereas PNG and Mexico are characterized by high-frequency spikes associated with pulses of CO2 and groundwater discharge (13, 14, 42). Yet, the hydrographic and biological processes that lower pH/Ωar in Palau also result in low total alkalinity (TA) and dissolved inorganic carbon (DIC) conditions, which are not expected under future OA. Furthermore, in the eastern tropical Pacific, temperature and nutrient concentrations covary with pH, making it difficult to attribute patterns in reef communities solely to upwelling-driven acidification (39).
Across the handful of naturally low-pH sites studied to date, Palau appears unique in showing no obvious sensitivity to OA across a range of key ecological indices. Laboratory experiments suggest that covarying environmental factors including light, flow, nutrients, and food availability can modulate the negative impact of OA on calcification. None of these factors can explain the apparent OA tolerance of Palau’s benthic communities. Most factors that alleviate the impacts of pH in laboratory experiments are absent from Palau’s Rock Island bays, where high temperature, high shade, low flow, and low nutrient concentrations accompany OA conditions (4, 5, 8, 18) (table S1). The low-pH communities in Palau differ from other naturally low-pH sites studied to date in their relative isolation within bays and inlets and in OA conditions that are chronic and of less extreme ranges. It is possible that selective pressure has driven local, community-wide adaptation to low pH over long time scales (that is, thousands of years). In contrast, in PNG and Mexico, low-pH communities are more exposed, scales of connectivity may be larger than in low-pH areas, and the possibility that larvae are recruited to low-pH reefs from populations under less acidification pressure may preclude adaptation (11).
Increases in macrobioerosion with declining pH are the only consistent coral reef community response to natural acidification across low-pH reefs. In Palau, bioerosion increased significantly at low Ωar. The same patterns have been reported at low-pH reef sites in PNG (93% increase in bioeroder density from high pH to low pH) and Mexico (78% increase in Porites coral skeleton eroded) (13, 14). In the eastern tropical Pacific, elevated rates of reef bioerosion (87% increase from Ωar = 3.5 to Ωar = 2.5) and decreased reef cementation have also been observed in low-pH, nutrient-rich upwelling zones (38), a component of which is attributable to low pH (15). Enhanced bioerosion and lack of cementation threaten the structural integrity of corals and reef systems, increasing the impact of predation and the risk of physical destruction by storms (30, 43). Moreover, if macrobioerosion is indicative of larger-scale reef erosion and dissolution (15, 25, 44), increases in CaCO3 erosion under OA will have significant implications for the persistence of reef structures. Structural fragility is incompatible with the ability of barrier and fringing reefs to absorb and dissipate the energy associated with the constant day-to-day pounding of waves, seasonal storms, and the less frequent but more catastrophic tsunamis. Indeed, that bioerosion rates are elevated in all naturally low-pH coral reef systems studied to date suggests that the signature of 21st century OA will emerge most strongly and most universally through its impact on reef structural integrity.
MATERIALS AND METHODS
Water sample collection and analysis
Surface water samples (0 to 3 m, n = 195) for salinity, nutrients, TA, and DIC were collected at multiple time points between sunrise and sunset on 19 to 24 September 2011, 28 March to 7 April 2012, 7 to 9 December 2012, and 1 to 15 November 2013 [data for 2011–2012 previously published in (11)]. TA and DIC analyses were performed using a Versatile INstrument for the Determination of Total inorganic carbon and titration Alkalinity (VINDTA, Marianda Analytics and Data), which uses open cell potentiometric (TA) and coulometric (DIC) titrations, and standardized using certified reference materials obtained from Andrew Dickson [Scripps Institution of Oceanography (45)]. Analysis of replicate samples (n = 13) showed a mean precision of ~2 μmol kg−1 for TA and ~1 μmol kg−1 for DIC. Full CO2 system parameters were calculated from temperature, salinity, TA, and DIC using CO2SYS (46) with the constants of Mehrbach et al. (47) refit by Dickson and Millero (48). In situ pH time series were collected using a SAMI-pH sensor (Sunburst Sensors) deployed at three sites (1, 2, and 10) for 4 days in November 2013 (sites 2 and 10) and August 2014 (site 1).
Benthic community data collection
At each of eight sites (fig. S1: sites 1, 2, 5, 6, 7, 9, 10, and 11), five 50-m transects were laid on the reef at 3-m depth and a photograph of a 0.5 m × 0.5 m quadrat taken every meter. Photographs were analyzed using Coral Point Count with Excel extensions (49). Benthic cover of each photograph was evaluated by randomly overlaying five crosses on each image and identifying the type of cover and taxa underneath each cross, with all corals identified to the genus level, for a total of 200 points evaluated per transect and 1000 points evaluated per site. Porites corals were identified as massive species, branching species, or other. All transects were conducted in 2010, except for those from site 2, which were conducted in 2012.
Coral skeletal core collection and analysis
Coral skeletal cores were collected on SCUBA from 86 massive Porites colonies (fig. S1: sites 1, 2, 3, 4, 5, 8, 9, and 10) and 25 Favia colonies (sites 1, 2, 8, and 9) in April 2011, September 2011, March to April 2012, and November 2013. Cores were scanned using a Siemens Volume Zoom Helical Computerized Tomography (CT) scanner, and extension, density, and calcification rates were calculated using annual banding patterns visualized from three-dimensional CT images in MATLAB [detailed procedure for analyzing coral growth rates in (15)]. The 2006–2010 averages of these parameters for each coral were used to compare growth rates across reef sites. CT scan images were used to determine the proportion of the skeleton eroded (>1 mm boring diameter) by boring organisms (including bivalves, worms, and sponges), calculated as the total volume of CaCO3 removed relative to the total volume of the coral core, for each Porites coral. Boring percentage data for sites 1, 2, 7, and 8 were previously published in (15).
Statistical analysis
Statistical analyses were conducted in R (version 3.0.1) (50). To assess the health of Palau’s benthic communities, percent cover (coral, macroalgae, and crustose coralline algae) and community ecological indices (genera richness, Shannon diversity, and Shannon evenness) were calculated for each of the five transects per reef site. GLMs were used to evaluate the relationship between Ωar and the site-mean benthic cover data and ecological indices. Linear, log-linear, and polynomial models were evaluated for all response variables, but no model fits showed significant relationships between any community index and Ωar (P < 0.05). DCA on coral genera abundance data was conducted using the vegan package (51) to evaluate spatial trends in community structure across reef sites. Differences in community structure across the Ωar gradient were formally tested using redundancy analysis with a Monte Carlo permutation test (10,000 permutations). Log-linear or negative binomial count models were constructed to assess the relationship between the presence/absence and abundance of the eight most abundant genera on Palauan reefs (total abundance > 50 individuals) and Ωar. Linear models were used to evaluate the relationship between Ωar and Porites and Favia mean annual rates of extension, density, and calcification rates and Porites tissue thickness. Porites bioerosion data were fit to two separate models to evaluate changes in the presence/absence of bioerosion and volume percent of boring with changes in Ωar and skeletal density: binary presence/absence data were fit to a logistic regression model, and a log-linear model was fit to site means of all nonzero boring percent data.
Acknowledgments
We thank G. P. Lohmann, K. Pietro, A. Shalapyonok, J. Arruda, D. Ketten, D. McCorkle, E. Bonk, K. Hoering, D. Wellwood, S. Lentz, G. Mereb, A. Merep, and the staff of the Palau International Coral Reef Center (PICRC) for assistance with fieldwork and analyses. We acknowledge the invaluable contribution of J. Andrew (deceased 2013). Funding: This work was supported by an NSF Graduate Research Fellowship to H.C.B. and T.M.D., A New Wave Fellowship to H.C.B., National Science Foundation awards OCE-1220529 and OCE-1031971, The Dalio Foundation Inc., through the Dalio Explore Fund, Ray Dalio through the WHOI Access to the Sea Fund, The Tiffany & Co. Foundation and The Nature Conservancy. Author contributions: H.C.B. and A.L.C. designed the study and wrote the manuscript. H.C.B. collected and analyzed coral cores and seawater samples for carbon system chemistry and performed the statistical analyses. Y.G. provided all ecological survey data and assisted with permitting and collection of samples in Palau. V.R.S. provided guidance on the statistical design and analysis. T.M.D. developed the computer program for quantification of coral calcification and bioerosion rates. K.E.F.S. calculated changes in seawater chemistry on Palau over the last two decades. H.C.B., A.L.C., T.M.D., and K.E.F.S. participated in fieldwork, and all authors contributed to the final manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: The data reported in this paper are tabulated in the Supplementary Materials and archived with Pangea and BCO-DMO (Biological and Chemical Oceanography Data Management Office) databases. Ecological survey data are archived with PICRC (www.picrc.org).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/5/e1500328/DC1
Fig. S1. Map of study sites in Palau.
Table S1. Environmental data for Palauan reef sites.
Table S2. Mean seawater CO2 system data (TA, total DIC, pCO2, carbonate ion concentration, pH, and Ωar) for each reef site ±1 SE.
Table S3. Model estimates for benthic community cover and ecological indices.
Table S4. Model estimates for changes in the abundance of dominant Palauan coral genera across the Ωar gradient.
Table S5. Model fits for coral skeletal growth parameters.
Table S6. List of reef sites where coral genera were observed and the total number of individuals counted across all sites.
REFERENCES AND NOTES
- 1.Caldeira K., Wickett M. E., Oceanography: Anthropogenic carbon and ocean pH. Nature 425, 365 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Langdon C., Atkinson M. J., Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J. Geophys. Res. 110, C09S07 (2005). [Google Scholar]
- 3.Pandolfi J. M., Connolly S. R., Marshall D. J., Cohen A. L., Projecting coral reef futures under global warming and ocean acidification. Science 333, 418–422 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Cohen A. L., Holcomb M., Why corals care about ocean acidification: Uncovering the mechanism. Oceanography 22, 118–127 (2009). [Google Scholar]
- 5.Suggett D. J., Dong L. F., Lawson T., Lawrenz E., Torres L., Smith D. J., Light availability determines susceptibility of reef building corals to ocean acidification. Coral Reefs 32, 327–337 (2012). [Google Scholar]
- 6.Anthony K. R. N., Diaz-Pulido G., Verlinden N., Tilbrook B., Andersson A. J., Benthic buffers and boosters of ocean acidification on coral reefs. Biogeosciences 10, 4897–4909 (2013). [Google Scholar]
- 7.Drenkard E. J., Cohen A. L., McCorkle D. C., de Putron S. J., Starczak V. R., Zicht A. E., Calcification by juvenile corals under heterotrophy and elevated CO2. Coral Reefs 32, 727–735 (2013). [Google Scholar]
- 8.Comeau S., Edmunds P. J., Lantz C. A., Carpenter R. C., Water flow modulates the response of coral reef communities to ocean acidification. Sci. Rep. 4, 6681 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doney S. C., Fabry V. J., Feely R. A., Kleypas J. A., Ocean acidification: The other CO2 problem. Ann. Rev. Mar. Sci. 1, 169–192 (2009). [DOI] [PubMed] [Google Scholar]
- 10.van Hooidonk R., Maynard J. A., Manzello D., Planes S., Opposite latitudinal gradients in projected ocean acidification and bleaching impacts on coral reefs. Glob. Chang. Biol. 20, 103–112 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Shamberger K. E. F., Cohen A. L., Golbuu Y., McCorkle D. C., Lentz S. J., Barkley H., Diverse coral communities in naturally acidified waters of a western Pacific reef. Geophys. Res. Lett. 41, 499–504 (2014). [Google Scholar]
- 12.Feely R. A., Doney S. C., Cooley S. R., Ocean acidification: Present conditions and future changes in a high CO2 world. Oceanography 22, 36–47 (2009). [Google Scholar]
- 13.Fabricius K. E., Langdon C., Uthicke S., Humphrey C., Noonan S., De’ath G., Okazaki R., Muehllehner N., Glas M. S., Lough J. M., Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim Chang. 1, 165–169 (2011). [Google Scholar]
- 14.Crook E. D., Cohen A. L., Rebolledo-Vieyra M., Hernandez L., Paytan A., Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. Proc. Natl. Acad. Sci. U.S.A. 110, 11044–11049 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeCarlo T. M., Cohen A. L., Barkley H. C., Cobban Q., Young C., Shamberger K. E., Brainard R. E., Golbuu Y., Coral macrobioerosion is accelerated by ocean acidification and nutrients. Geology 43, 7–10 (2015). [Google Scholar]
- 16.Kayanne H., Hata H., Kudo S., Yamano H., Watanabe A., Ikeda Y., Nozaki K., Kato K., Negishi A., Saito H., Seasonal and bleaching-induced changes in coral reef metabolism and CO2 flux. Glob. Biogeochem. Cycles 19, GB3015 (2005). [Google Scholar]
- 17.Dore J. E., Lukas R., Sadler D. W., Church M. J., Karl D. M., Physical and biogeochemical modulation of ocean acidification in the central north Pacific. Proc. Natl. Acad. Sci. U.S.A. 106, 12235–12240 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golbuu Y., Victor S., Penland L., Idip D. Jr., Emaurois C., Okaji K., Yukihira H., Iwase A., van Woesik R., Palau’s coral reefs show differential habitat recovery following the 1998-bleaching event. Coral Reefs 26, 319–332 (2007). [Google Scholar]
- 19.DePutron S. J., McCorkle D. C., Cohen A. L., Dillon A. B., The impact of seawater saturation state and bicarbonate ion concentration on calcification by new recruits of two Atlantic corals. Coral Reefs 30, 321–328 (2010). [Google Scholar]
- 20.Albright R., Langdon C., Ocean acidification impacts multiple early life history processes of the Caribbean coral Porites astreoides. Glob. Chang. Biol. 17, 2478–2487 (2011). [Google Scholar]
- 21.Anthony K. R. N., Kline D. I., Diaz-Pulido G., Dove S., Hoegh-Guldberg O., Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. U.S.A. 105, 17442–17446 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Done T. J., Navin K. F., Shallow water benthic communities on coral reefs. Vanuatu Mar. Resour. Rep. A Biol. Surv. 10–37 (1990). [Google Scholar]
- 23.Y. I. Sorokin, Coral Reef Ecology (Springer, New York, 1995). [Google Scholar]
- 24.Osorno A., Peyrot-Clausade M., Hutchings P. A., Patterns and rates of erosion in dead Porites across the Great Barrier Reef (Australia) after 2 years and 4 years of exposure. Coral Reefs 24, 292–303 (2005). [Google Scholar]
- 25.Chazottes V., Campion-Alsumard T. L., Peyrot-Clausade M., Bioerosion rates on coral reefs: Interactions between macroborers, microborers and grazers (Moorea, French Polynesia). Palaeogeogr. Palaeoclimatol. Palaeoecol. 113, 189–198 (1995). [Google Scholar]
- 26.Tribollet A., Golubic S., Cross-shelf differences in the pattern and pace of bioerosion of experimental carbonate substrates exposed for 3 years on the northern Great Barrier Reef, Australia. Coral Reefs 24, 422–434 (2005). [Google Scholar]
- 27.Londoño-Cruz E., Cantera J., Toro-Farmer G., Orozco C., Internal bioerosion by macroborers in Pocillopora spp. in the tropical eastern Pacific. Mar. Ecol. Prog. Ser. 265, 289–295 (2003). [Google Scholar]
- 28.Perry C. T., Macroborers within coral framework at Discovery Bay, north Jamaica: Species distribution and abundance, and effects on coral preservation. Coral Reefs 17, 277–287 (1998). [Google Scholar]
- 29.Edinger E. N., Limmon G. V., Jompa J., Widjatmoko W., Heikoop J. M., Risk M. J., Normal coral growth rates on dying reefs: Are coral growth rates good indicators of reef health? Mar. Pollut. Bull. 40, 404–425 (2000). [Google Scholar]
- 30.Wisshak M., Schonberg C. H. L., Form A., Freiwald A., Ocean acidification accelerates reef bioerosion. PLOS One 7, e45124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tribollet A., Godinot C., Atkinson M., Langdon C., Effects of elevated pCO2 on dissolution of coral carbonates by microbial euendoliths. Glob. Biogeochem. Cycles. 23 (2009). [Google Scholar]
- 32.Reyes-Nivia C., Diaz-Pulido G., Kline D., Guldberg O.-H., Dove S., Ocean acidification and warming scenarios increase microbioerosion of coral skeletons. Glob. Chang. Biol. Biol. 19, 1919–1929 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Gove J. M., Williams G. J., mcManus M. A., Heron S. F., Sandin S. A., Vetter O. J., Foley D. G., Quantifying climatological ranges and anomalies for Pacific coral reef ecosystems. PLOS One 8, e61974 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sammarco P. P., Risk M. M., Large-scale patterns in internal bioerosion of Porites: Cross continental shelf trends on the great barrier reef. Mar. Ecol. Prog. Ser. 59, 145–156 (1990). [Google Scholar]
- 35.Kobluk D. R., Risk M. J., Rate and nature of infestation of a carbonate substratum by a boring alga. J. Exp. Mar. Biol. Ecol. 27, 107–115 (1977). [Google Scholar]
- 36.P. W. Glynn, Bioerosion and Coral Reef Growth: A Dynamic Balance, in Life and Death of Coral Reefs, C. Birkeland, Ed. (Chapman and Hall, New York, 1997), pp. 68–95. [Google Scholar]
- 37.Crook E. D., Potts D., Rebolledo-Vieyra M., Hernandez L., Paytan A., Calcifying coral abundance near low-pH springs: Implications for future ocean acidification. Coral Reefs 31, 239–245 (2011). [Google Scholar]
- 38.Manzello D. P., Kleypas J. A., Budd D. A., Eakin C. M., Glynn P. W., Langdon C., Poorly cemented coral reefs of the eastern tropical Pacific: Possible insights into reef development in a high-CO2 world. Proc. Natl. Acad. Sci. U.S.A. 105, 10450–10455 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manzello D. P., Enochs I. C., Bruckner A., Renaud P. G., Kolodziej G., Galápagos coral reef persistence after ENSO warming across an acidification gradient. 1–8 (2014). [Google Scholar]
- 40.Inoue S., Kayanne H., Yamamoto S., Kurihara H., Spatial community shift from hard to soft corals in acidified water. Nat. Clim. Chang. 3, 683–687 (2013). [Google Scholar]
- 41.Iguchi A., Ozaki S., Nakamura T., Inoue M., Tanaka Y., Suzuki A., Kawahata H., Sakai K., Effects of acidified seawater on coral calcification and symbiotic algae on the massive coral Porites australiensis. Mar. Environ. Res. 73, 32–36 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Hofmann G. E., Smith J. E., Johnson K. S., Send U., Levin L. A., Micheli F., Paytan A., Price N. N., Peterson B., Takeshita Y., Matson P. G., Crook E. D., Kroeker K. J., Gambi M. C., Rivest E. B., Frieder C. A., Yu P. C., Martz T. R., High-frequency dynamics of ocean pH: A multi-ecosystem comparison. PLOS One 6, e28983 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruggemann J., van Kessel A., van Rooij J., Breeman A., Bioerosion and sediment ingestion by the Caribbean parrotfish Scarus vetula and Sparisoma viride: Implications of fish size, feeding mode and habitat use. Mar. Ecol. Prog. Ser. 134, 59–71 (1996). [Google Scholar]
- 44.Holmes K. E., Edinger E. N., Limmon G. V., Risk M. J., Bioerosion of live massive corals and branching coral rubble on Indonesian coral reefs. Mar. Pollut. Bull. 40, 606–617 (2000). [Google Scholar]
- 45.Feely R. A., Sabine C. L., Takahashi T., Wanninkhof R., Uptake and storage of carbon dioxide in the ocean: The global CO2 survey. Oceanography 14, 18–32 (2001). [Google Scholar]
- 46.E. Lewis, D. W. R. Wallace, in ORNL/CDIAC-105 (Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, TN, 1998). [Google Scholar]
- 47.Mehrbach C., Culberso C. H., Hawley J. E., Pytkowic R., Measurement of apparent dissociation constants of carbon acid in seawater at atmospheric-pressure. Limnol. Oceanogr. 18, 897–907 (1973). [Google Scholar]
- 48.Dickson A. G., Millero F. J., A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. A 34, 1733–1743 (1987). [Google Scholar]
- 49.Kohler K. E., Gill S. M., Coral point count with excel extensions (CPCe): A visual basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 32, 1259–1269 (2006). [Google Scholar]
- 50.R Development Core Team, R: A language and environment for statistical computing (2013); www.r-project.org.
- 51.J. Oksanen, F. G. Blanchet, R. Kindt, vegan: Community ecology package (2013); http://vegan.r-forge.r-project.org/.
- 52.Denman K., Christian J. R., Steiner N., Pornter H., Nojiri Y., Potential impacts of future ocean acidification on marine ecosystems and fisheries: Current knowledge and recommendations for future research. ICES J. Mar. Sci. 68, 1019–1029 (2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/5/e1500328/DC1
Fig. S1. Map of study sites in Palau.
Table S1. Environmental data for Palauan reef sites.
Table S2. Mean seawater CO2 system data (TA, total DIC, pCO2, carbonate ion concentration, pH, and Ωar) for each reef site ±1 SE.
Table S3. Model estimates for benthic community cover and ecological indices.
Table S4. Model estimates for changes in the abundance of dominant Palauan coral genera across the Ωar gradient.
Table S5. Model fits for coral skeletal growth parameters.
Table S6. List of reef sites where coral genera were observed and the total number of individuals counted across all sites.


