Uniform mesoporous single-crystal TiO2 spheres with radial channels from driving orientation assembly can be used for energy storage.
Keywords: Mesoporous materials, Titania, Assembly, Dye-sensitized solar cells, Synthesis, Radial orientation, Single-crystalline, Optoelectronic
Abstract
Highly crystalline mesoporous materials with oriented configurations are in demand for high-performance energy conversion devices. We report a simple evaporation-driven oriented assembly method to synthesize three-dimensional open mesoporous TiO2 microspheres with a diameter of ~800 nm, well-controlled radially oriented hexagonal mesochannels, and crystalline anatase walls. The mesoporous TiO2 spheres have a large accessible surface area (112 m2/g), a large pore volume (0.164 cm3/g), and highly single-crystal–like anatase walls with dominant (101) exposed facets, making them ideal for conducting mesoscopic photoanode films. Dye-sensitized solar cells (DSSCs) based on the mesoporous TiO2 microspheres and commercial dye N719 have a photoelectric conversion efficiency of up to 12.1%. This evaporation-driven approach can create opportunities for tailoring the orientation of inorganic building blocks in the assembly of various mesoporous materials.
INTRODUCTION
Mesoporous semiconductors have attracted widespread interest because of their structural versatility for energy and environmental applications (1–9). Unfortunately, most of the mesoporous semiconductors have amorphous or polycrystalline pore walls that are irregularly oriented in space, adversely affecting the transport of electrons, ions, and guest molecular species in optoelectronic devices and lithium-ion batteries (10–13). This limitation could be overcome if both the orientation of mesopore channels and the nanocrystal building blocks of the channels were precisely controlled in a three-dimensional (3D) open configuration with respect to the particle surface (14–20). Although such a 3D oriented mesostructure configuration is easy to image, the actual synthesis is extremely difficult. This is because the random assembly of the surfactant micelles and inorganic oligomers spontaneously occurs as a result of the minimization of the surface energy during the assembly process (21, 22). Here, we demonstrate a simple evaporation-driven oriented assembly approach to synthesize 3D open radially oriented mesoporous TiO2 microspheres with single-crystal–like anatase pore walls that dominate (101) exposed facets in an amphiphilic triblock copolymer–containing sol-gel solution. The process starts with liquid-liquid phase separation caused by the preferential evaporation of tetrahydrofuran (THF) solvent at a low temperature of 40°C; uniform 3D mesoporous TiO2 microspheres assembled by PEO-PPO-PEO [poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide)]/titania oligomer spherical composite micelles form at the liquid-liquid phase interface. Continuous evaporation of the residual THF and hydrolyzed solvents at 80°C drives the oriented growth of both mesochannels and nanocrystal building blocks from the initially formed spherical composite micelles along the free radial and restricted tangential direction within the TiO2 microspheres. This yields 3D open, radially-oriented mesoporous TiO2 microspheres with single-crystalline–like anatase walls that dominate (101) exposed facets (Fig. 1 and figs. S1 to S5). The unique mesoporous TiO2 microspheres can be used for dye-sensitized solar cells (DSSCs) with commercial dye N719, revealing an excellent photoelectric conversion efficiency.
Fig. 1. Schematic representation of the formation process through evaporation-driven oriented assembly.
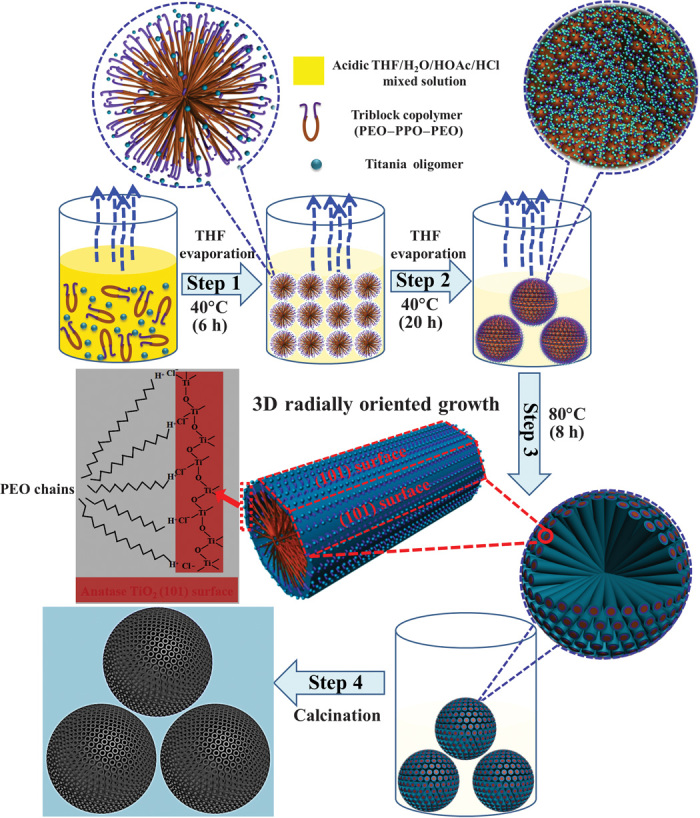
Step 1: Formation of the PEO-PPO-PEO/titania oligomer composite spherical micelles with PPO segments as a core and titania-associated PEO segments as a shell with the initial preferential evaporation of THF solvent at 40°C for 6 hours. Step 2: Aggregation of the composite spherical micelles into big spheres on the interface of the poor solvent water-rich phase, which is driven by the increasing concentration of the spherical micelles and the requirement of minimization of interface energy. Step 3: The second-step evaporation of THF and residual solvents hydrolyzed from titanium tetrabutoxide (TBOT) precursor (further treated at 80°C for 8 hours) could further drive the composite spherical micelles to fuse into cylinders, leading to continuous 3D radially-oriented growth of cylindrical micelles and TiO2 nanoentities. Step 4: The 3D open radially oriented mesoporous TiO2 microspheres with single-crystal–like anatase walls that dominate (101) facets are obtained by removing the triblock copolymer templates after calcination in air at 400°C for 2 hours.
RESULTS
In situ synchrotron radiation small-angle x-ray scattering (SAXS) patterns of the products harvested at different intervals of time from the preparation (Fig. 2, A to E) confirm the transformation of the initial PEO-PPO-PEO/titania oligomers from spherical micelles (cubic Im m mesophase) to radial cylindrical micelles (hexagonal p6mm mesophase). After evaporation at 40°C for 6 hours, distinct diffraction arcs are observed, as shown in the 2D SAXS image (Fig. 2B), which can be indexed to a cubic mesophase (space group Imm), indicating that the spherical composite micelles were initially oriented 2D in the membrane form at the interface between the THF- and water-rich phases. Transmission electron microscopy (TEM) images (figs. S2 and S3) show that with continuous solvent evaporation at 40°C for 20 hours, the spherical composite micelles further aggregate together to form large, dense 3D spherical particles with improved cubic Imm symmetry, consistent with the appearance of high-order reflections for (220), (310), and (330) planes (Fig. 2, A and C). After the final-step evaporation of solvents at 80°C for 8 hours, the SAXS pattern of the as-made PEO-PPO-PEO/titania composite microspheres (Fig. 2, A and D) shows two intense scattering peaks with lattice spacing d100 = 13.47 nm and d110 = 7.76 nm (d100/d110 = 1: ). The second-order reflection confirmed both the periodicity and the orientation of the 3D radially aligned hexagonal cylindrical pores, suggesting structural transformation from a cubic to a hexagonal mesostructure. The wide-angle x-ray diffraction (WAXRD) patterns show that evaporation treatment at 80°C can lead to crystallization and oriented growth of anatase walls (fig. S4). After removal of the amphiphilic triblock copolymer templates by calcination at 400°C for 2 hours, the obtained mesoporous TiO2 microspheres exhibited similar SAXS peaks at higher q values with d100 = 12.12 nm and d110 = 6.98 nm (Fig. 2, A and E), indicating that the 3D hexagonal cylindrical mesostructure was retained.
Fig. 2. Microstructure characterization of the radially oriented mesoporous TiO2 microspheres.
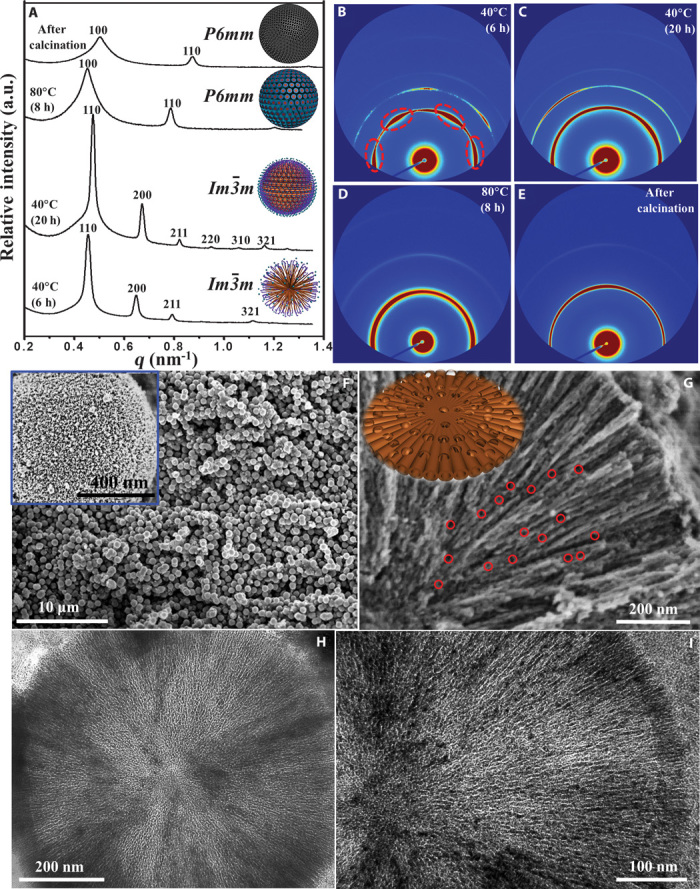
(A) In situ synchrotron radiation 1D SAXS patterns of the mesoporous TiO2 microsphere products harvested at different intervals of reaction time. Insets: Corresponding schematic representation of the four samples. a.u., arbitrary units. (B to E) 2D SAXS images of the four samples. (F) SEM image of the mesoporous TiO2 microspheres. Inset: SEM image of a single mesoporous TiO2 microsphere. (G) SEM image of a single ultramicrotomed, radially-oriented mesoporous TiO2 microsphere with a large number of interchannel pores (~5 to 15 nm in diameter, marked by red circles). Inset: Corresponding schematic representation of the structure models for the radially oriented channels with interchannel pores. (H and I) TEM images of a single ultramicrotomed, mesoporous TiO2 microsphere.
Scanning electron microscopy (SEM) and TEM images (Fig. 2, F to I) show that the products consist of uniform spheres with a diameter of ~800 nm after calcination. The cylindrical open pores exposed on the rough surface of the microspheres can be observed in the inset of Fig. 2F. The center-to-center distance between two adjacent cylindrical mesopore channels was on average ~11.8 nm, and the pore size was estimated to be ~5.5 nm. Close inspection of the mesoporous TiO2 microspheres (Fig. 2G) revealed that the close-packed cylindrical mesochannels were arranged radially from the center to the surface. A large number of interchannel pores with diameters of ~5 to 15 nm were observed on the wall surface of cylindrical mesopore channels (marked by red circles in Fig. 2G). Such a pore arrangement model can be further discerned from high-angle annular dark-field scanning TEM (HAADF-STEM) images of a single microsphere (fig. S6). The cylindrical pore size can be enlarged to 5 to 10 nm when the final evaporation temperature increases from 80° to 120°C (fig. S7). To confirm the internal structure, we examined the ultramicrotomed sections of a single mesoporous TiO2 microsphere using TEM technique. The results revealed that the cylindrical mesopores were distributed radially from the center to the surface along the radial and restricted tangential direction, and all the mesopores were regularly arranged and highly open (Fig. 2, H and I, and fig. S8). Considering a spherically symmetric model of the mesoporous TiO2 microspheres, some of the cylindrical pores deviated slightly from the hexagonal symmetry and branched into two hexagonal channels in between (23).
High-resolution TEM (HRTEM) images and selected-area electron diffraction (SAED) patterns further reveal that the radially arranged mesopore walls have a single-crystal–like anatase characteristic (Fig. 3 and figs. S9 and S10). The lattice fringe distance perpendicular to the pore walls is ~0.238 nm, which corresponds to the interplanar distance of the (004) atomic plane in anatase, indicating that the mesopore walls grow with a preferred [001] orientation (Fig. 3, A and B, and fig. S10A). The interfacial angle between two adjacent faces is ~68.3° (Fig. 3B and fig. S10C), which is the same as the theoretical value for the angle between the (004) and (101) planes of anatase (24), suggesting that the (101) plane is the dominant exposed facet along the pore walls. A fast Fourier transform–filtered TEM image (fig. S10D) and SAED patterns (Fig. 3C and fig. S9, A to D) show slightly elongated diffraction spots and a slightly distorted hexagonal symmetry. Such distorted cylindrical pore channels can cause some lattice distortion between the boundaries of the crystalline domains when they are assembled in a radial and restricted tangential direction to form single-crystal–like TiO2 pore frameworks (18, 25). The WAXRD pattern (Fig. 3D) confirms the highly crystalline anatase phase (space group I41/amd) of the radially oriented mesoporous TiO2 microspheres. Nitrogen adsorption-desorption isotherms showed characteristic type IV curves with two distinguishable capillary condensation steps (Fig. 3E). A distinct capillary condensation step at P/P0 = 0.40 to 0.7 reflected uniform cylindrical mesopores resulting from the radial primary mesopore channels of the TiO2 microspheres. Moreover, a hysteresis loop at a higher pressure (P/P0 = 0.90 to 0.99) reflected the interchannel mesopores from the structural defects, consistent with the results from the HRTEM and HAADF-STEM images. The pore size distribution calculated using the Barrett-Joyner-Halenda (BJH) model (inset of Fig. 3E) showed two sets of pores. The primary pore size is centered at 5.7 nm, and the secondary pore size is 10 to 30 nm, which agrees well with that estimated from the SEM and TEM images. The Brunner-Emmet-Teller (BET) surface area and pore volume were calculated to be as high as 112 m2/g and 0.164 cm3/g, respectively. The x-ray photoelectron spectroscopy (XPS) results for the mesoporous TiO2 microspheres indicate the Ti4+ oxidation state (Fig. 3F) and the presence of lattice oxygen, O-Ti4+ and Ti-OH (Fig. 3G). The O-Ti4+ and massive Ti-OH groups on the surface can act as binding dye and work as charge transfer channels (26).
Fig. 3. Single-crystal pore wall characterizations.
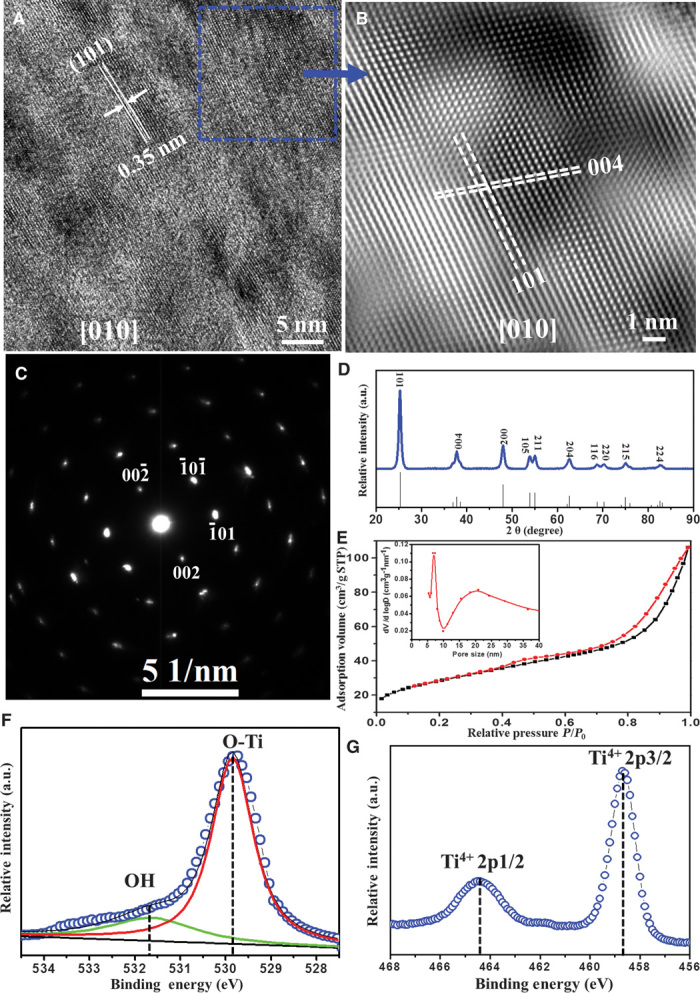
(A) HRTEM images taken from the area of the cylindrical mesopore bundles of an ultramicrotomed, mesoporous TiO2 microsphere with [010] incidence, perpendicular to the mesopore channels. (B) A fast Fourier transform–filtered TEM image recorded from the dotted square area in (A). (C) The SAED pattern taken from the cylindrical pore bundles region with [010] incidence. (D) The WAXRD pattern of the mesoporous TiO2 microspheres, compared to the standard anatase (space group I41/amd, JCPDS card no. 21-1272). (E) Nitrogen adsorption-desorption isotherms; inset: pore size distributions of the mesoporous TiO2 microspheres with two sets of pores. The primary pore size is centered at 5.7 nm, and the secondary pore size at 10 to 30 nm. (F and G) XPS core-level spectra of Ti2p and O1s, respectively, for the mesoporous TiO2 microspheres.
The loading capacity of the commercial dye N719 in the mesoporous TiO2 microspheres was ~2.13 × 10−7 mol cm−2, about twice those of the frequently used commercial Degussa TiO2 P25 (1.12 × 10−7 mol cm−2) and conventional mesoporous TiO2 bulk (1.45 × 10−7 mol cm−2). Such high dye loading could be attributed to the large accessible surface area and the single-crystal–like anatase walls with dominant (101) exposed facets. The anatase (101) facet here is believed to be the best surface for loading N719 dye because the distance between two binding carboxylate groups of N719 dye matches well with the distance between Ti atoms on anatase (101) facets (20). In addition, the electrode films based on the mesoporous TiO2 microspheres have high light-scattering capacity in the visible and near-infrared (NIR) regions (400 to 800 nm) (Fig. 4A), indicating that the incident sunlight can be significantly scattered within the film.
Fig. 4. Photovoltaic device characterization.
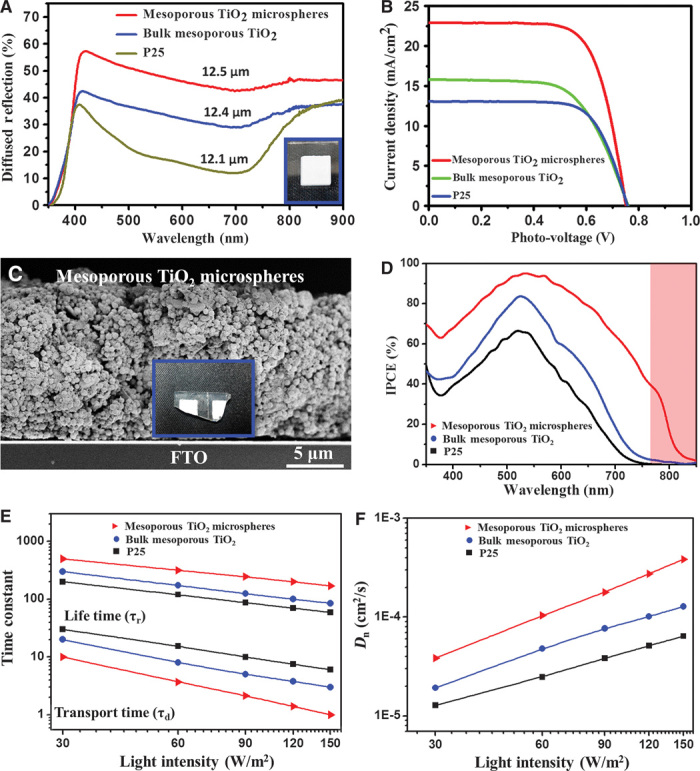
(A) Diffuse reflectance spectra of TiO2 films with a thickness of about 12 μm. Inset: Photograph of the 3D open, radially-oriented mesoporous TiO2 microsphere-based film. (B) J-V curves of DSSCs fabricated from the three TiO2 samples with N719 dye under AM 1.5G simulated sunlight with a power density of 100 mW cm−2. (C) A cross-sectional SEM image of a DSSC composed of 3D open mesoporous TiO2 microspheres. Inset: Photograph of the sliced films for SEM. (D) IPCE spectra of the DSSCs based on the radially oriented mesoporous TiO2 spheres with a uniform size of 800 nm, conventional mesoporous TiO2 bulk, and commercial Degussa TiO2 P25. The pink marked shadow region shows that the photo-response region is extended over 800 nm for the single-crystal–like mesoporous TiO2 microspheres. (E) Electron transport time and electron lifetime. (F) Electron diffusion coefficient (Dn) for the mesoporous TiO2 microspheres.
To investigate the photovoltaic performance of DSSCs, we compared their current density (J)–voltage (V) characteristics with those of conventional mesoporous TiO2 bulk with randomly oriented pore channels and P25 under simulated AM 1.5 light (Fig. 4B). The DSSCs based on radially oriented mesoporous TiO2 microspheres showed an ultrahigh short-circuit photocurrent density (Jsc) of ~22.91 mA cm−2, an open-circuit voltage (Voc) of ~0.75 V, and a fill factor of ~0.71, yielding a power conversion efficiency (PCE) of ~12.1%. The DSSC performance measurements were repeated several times for 15 individual solar cells, and the obtained PCE had a close average value of ~12.1% (fig. S11), which was also confirmed by an accredited photovoltaic calibration laboratory (fig. S12), indicating a good reliability of the DSSCs. In contrast, the PCEs are ~7.6 and 6.8% for the DSSCs based on the conventional mesoporous TiO2 and P25, respectively (Table 1 and Fig. 4B).
Table 1. Photovoltaic parameters of DSSCs based on the photoanodes of radially oriented mesoporous TiO2 microspheres, bulk mesoporous TiO2, and commercial P25 after TiCl4 treatment [measured under AM 1.5 sunlight illumination (100 mW cm−2)].
The active area of the devices with a metal mask was about 0.16 cm2. FF, fill factor.
| Samples |
Voc (mV) |
Jsc (mA cm−2) |
FF (%) |
η (%) |
Adsorbed dye (×10−7 mol cm−2)* |
| Mesoporous TiO2 microspheres |
751 | 22.9 | 70.6 | 12.1 | 2.13 |
| Bulk mesoporous TiO2 | 759 | 15.8 | 63.1 | 7.6 | 1.45 |
| P25 | 734 | 12.3 | 75.0 | 6.8 | 1.12 |
*Dye-adsorbed films with an area of ~10 cm2 were used for estimating the adsorbed dye concentration. The commercial N719 dye was first desorbed into a 0.1 M NaOH solution in water and ethanol [1:1 (v/v)], and the desorbed N719 dye concentration was then measured by using an ultraviolet-visible spectrophotometer.
Figure 4C and fig. S13 show the cross-sectional SEM image of a typical mesoporous TiO2 microsphere–based film device. All the mesoporous TiO2 electrodes were sensitized with commercially available N719 dye, and the film thickness was ~14 μm. The incident photo–to–current conversion efficiency (IPCE) of the mesoporous TiO2 microspheres exceeded 60% in a broad spectral range from 300 to 700 nm, reaching its maximum of ~95% at 530 nm (Fig. 4D). The photo-response region was remarkably extended over 800 nm for the DSSCs based on the radially oriented single-crystal–like mesoporous TiO2 microspheres. Such an expanded photo-responsive behavior is attributed to the 3D open, radially-oriented mesopore configuration and large particle size (~800 nm) of the mesoporous TiO2 microspheres, which results in the pronounced light-scattering property and expanded photo-responsive ability in the NIR region. From the overlap integral with the standard global AM 1.5 solar emission spectrum, the integrated photocurrent density from the IPCE spectrum was calculated as 19.7 mA cm−2, which is 86% of the measured Jsc value (22.9 mA cm−2). The experimental Jsc error between the J-V curve and IPCE was ~14%. This is probably due to the spectral mismatch between the two simulated solar lights in the two separated instruments. The transport time (τd), electron lifetime (τr), and diffusion coefficient (Dn), calculated using intensity-modulated photocurrent spectroscopy (IMPS) and intensity-modulated photovoltage spectroscopy (IMVS) (Fig. 4, E and F), demonstrate that the electron transportation rate within the mesoporous TiO2 microspheres is more rapid than those inside the mesoporous TiO2 bulk and P25, and the charge recombination is relatively more suppressed at the mesoporous TiO2 microsphere photoanode-electrolyte interface (27, 28).
On the basis of the above characterization of the DSSCs, the high PCE of the mesoporous TiO2 microspheres was associated with the high loading capacity of N719 dye and the large accessible surface area with dominant (101) exposed facets, high rate of electron transfer through single-crystalline–like anatase walls, and pronounced light-scattering ability due to 3D open, radially-oriented mesopore configuration. The mesoporous TiO2 microspheres also demonstrated high performance in photocatalytic reactions (fig. S14).
To understand the evaporation-driven oriented assembly process, we used the cryo-SEM technique to examine the products harvested from the sol solution at different intervals of time (fig. S15). The growth of these mesoporous TiO2 microspheres was found to proceed through a phase separation and gradual transformation of Pluronic/titania composites from spherical to radial cylindrical micelles. At the first stage of the process, the preferential evaporation of THF at 40°C increased the concentration of the mixed solution (containing water, HCl, acetic acid, amphiphilic block copolymer, and titania oligomers), inducing a liquid-liquid phase separation by hydrolytic polycondensation of the TBOT precursor. The spherical PEO-PPO-PEO/titania composite micelles formed at the interface between the THF- and water-rich phases, minimizing the surface tension (fig. S15B). TEM images show that these spherical micelles have a typical core-shell structure with PEO-PPO-PEO as the core and titania oligomers as the shell (fig. S2). With continuous THF evaporation at 40°C, these small spherical micelles aggregated together to form large, dense, spherical particles, reducing interface energy; hence, the solution became turbid (fig. S15C). Finally, second-step evaporation of THF and residual solvents (such as butyl alcohol) from inside the microspheres (at 80°C) caused the PEO-PPO-PEO/titania spherical micelles to fuse into cylinders, leading to a continuous 3D radially oriented growth of cylindrical micelles (fig. S15D). This driving force was the result of reducing surface energy when the interface between the spherical composite micelles and THF solvent disappeared after complete solvent evaporation.
DISCUSSION
The oriented growth of the single-crystal–like anatase pore walls along the [001] direction is related to the solvent evaporation–driven oriented assembly growth process (24, 29, 30). The addition of a strong aqueous acid (HCl) can partially charge the initially formed anatase nanocrystals by selective adsorption of Cl− ions on the (101) planes (Fig. 1 and fig. S5) (24), which may act as a “bridge” between the nanocrystal building blocks and hydrophilic segment boundary of Pluronic F127. The STEM image and energy-dispersive x-ray spectroscopy (EDX) element mapping analyses confirm the presence of Cl− ions on the crystallographic planes of a single ultramicrotomed TiO2 microsphere (fig. S16). As confirmed by in situ synchrotron radiation SAXS (Fig. 2A), a continuous slow evaporation of the residual THF and hydrolyzed solvent (for example, n-butyl alcohol) at 80°C actually causes the initially formed spherical micelles to fuse into radially oriented cylindrical micelles. Simultaneously, this second-step evaporation from the center to the outside of the sphere particles can also cause TiO2 crystallization oriented around F127 cylindrical micelles at a low temperature of 80°C. Our results revealed that the slow evaporation and confinement of the triblock copolymer hydrophilic boundary could correct the crystallographic orientation of the nanocrystal building blocks. After calcination at 400°C for 2 hours, the oriented anatase nanocrystals can further crystallize into single-crystal–like pore walls with dominant (101) exposed facets. However, the products obtained in the same conditions without F127 are typically crackable monoliths composed of aggregates of irregular TiO2 nanocrystals (fig. S17), indicating that Pluronic F127 is an essential template in the evaporation-driven oriented growth of mesoporous TiO2 microspheres.
Furthermore, conventional mesoporous TiO2 bulk with irregularly oriented cylindrical pores and polycrystalline walls can be obtained (figs. S18 and S19) when the initial yellow sol-gel reaction solution (fig. S1A) evaporates quickly under a low-vacuum condition (~200 Pa) at 40°C, implying that control of the solvent evaporation rate is vital for the micelles and inorganic nanoentities to adapt their preferred orientation to the interface. Thus, if proper control of the solvent evaporation rate and initial soluble precursors is achieved, this general evaporation-driven oriented assembly method can easily extend the synthesis of mesoporous oriented single crystals to a range of functional semiconductors.
In summary, well-defined, uniform 3D open, mesoporous TiO2 microspheres with radially oriented mesochannels and single-crystal–like pore walls that dominate (101) exposed facets have been successfully synthesized by a novel evaporation-driven oriented assembly method. Such an unprecedented type of 3D open, mesoporous TiO2 microsphere has a large accessible surface area (112 m2/g), a large pore volume (0.164 cm3/g), highly single-crystalline–like walls, and a uniform submicrometer size. Owing to their unique features, especially dominant (101) exposed facets and large surface area, the resultant DSSCs have a high energy-conversion efficiency of up to 12.1%. Such a general evaporation-driven oriented assembly procedure results in a dramatic transformation of surfactant/inorganic oligomer micelles from spherically to radially oriented cylindrical micelles by controlling the solvent step evaporation. This evaporation-driven oriented assembly method can be easily extended to synthesize highly crystalline mesoporous materials with oriented configuration for advanced applications in sensors, photocatalysis, drug delivery, etc.
MATERIALS AND METHODS
The 3D open, radially-oriented TiO2 microspheres were synthesized by the evaporation-driven oriented assembly (EDOA) approach (see Supplementary Materials and Methods for detailed preparation of TiO2 microspheres). In summary, Pluronic F127 (PEO106PPO70PEO106, weight-average molecular weight = 12,600 g/mol), water, acetic acid, concentrated HCl (36 wt %), and TBOT were vigorously stirred in THF solvent to form a clear, golden yellow solution. Sequentially, the obtained clear solution was transferred into a volumetric flask and left in a drying oven to evaporate the THF solvent at 40°C for 8 hours, then at 80°C for another 12 hours to completely remove the solvent. Finally, the white TiO2 mesoporous powder was obtained by calcination in air at 450°C for 3 hours. The detailed methods and characterization are available in the Supplementary Materials.
Supplementary Material
Acknowledgments
We thank Y. F. Luo, G. Zhou, and H. W. Han for a useful discussion on the characterization of the DSSCs. Funding: This work is supported by the State Key Basic Research Program of China (2013CB934104 and 2012CB224805), the National Science Foundation (21210004), the Science and Technology Commission of Shanghai Municipality (08DZ2270500), the Shanghai Leading Academic Discipline Project (B108), King Abdulaziz City for Science and Technology (project no. 29-280), and Deanship of Scientific Research, King Saud University–The International Highly Cited Research Group Program (IHCRG#14-102). Y.L. also acknowledges the Interdisciplinary Outstanding Doctoral Research Funding of Fudan University (EZH2203302/001). Competing interests: The authors declare that they have no competing interests.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/4/e1500166/DC1
Materials and Methods
Fig. S1. Photographs of the mixed solution for the preparation of the mesoporous TiO2 microspheres during the evaporation of the solvent THF.
Fig. S2. TEM images with different magnifications of the sample taken from the preparation solution after the solvent evaporation at 40°C for 6 hours.
Fig. S3. STEM image and EDX elemental maps of the sample taken from the preparation solution after THF evaporation at 40°C for 6 hours.
Fig. S4. WAXRD patterns of the precipitates obtained at different solvent evaporation times without calcination.
Fig. S5. The proposed mechanism for oriented growth of the anatase single-crystal–like pore walls.
Fig. S6. HAADF-STEM images of a single ultramicrotomed 3D open radially oriented mesoporous TiO2 microspheres.
Fig. S7. SEM images of the products obtained after the second-step evaporation at a high temperature of 120°C.
Fig. S8. TEM images of an ultramicrotomed radially oriented mesoporous TiO2 microsphere with different integrity.
Fig. S9. SAED and HRTEM images taken from the cylindrical pore bundle regions with different orientation.
Fig. S10. HRTEM and SEAD images of the mesoporous TiO2 spheres taken from the area of the cylindrical pore bundles.
Fig. S11. Detail photovoltaic parameters of 15 individual mesoporous TiO2 microsphere-based cells.
Fig. S12. Independent certificate by the National Center of Supervision and Inspection on Solar Photovoltaic Products Quality.
Fig. S13. Cross-sectional and top-view SEM images of the photoanode film based on the uniform mesoporous TiO2 microspheres.
Fig. S14. Photocatalytic decomposition of methylene blue dye over the mesoporous TiO2 microspheres.
Fig. S15. Cryo-SEM images of the samples harvested at different intervals of solvent evaporation time.
Fig. S16. STEM image and EDX elemental maps of a single ultramicrotomed mesoporous TiO2 microsphere.
Fig. S17. SEM images of the products obtained under similar conditions in the absence of Pluronic F127.
Fig. S18. Field-emission SEM (FESEM) and TEM images of the ordered mesoporous TiO2 bulks with randomly oriented cylindrical pores.
Fig. S19. HRTEM images, nitrogen adsorption-desorption isotherms, pore size distributions, and SAXS integral curves of the randomly oriented mesoporous TiO2 bulk samples.
REFERENCES AND NOTES
- 1.Zhao D., Feng J., Huo Q., Melosh N., Fredrickson G. H., Chmelka B. F., Stucky G. D., Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279, 548–552 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Bach U., Lupo D., Comte P., Moser J., Weissörtel F., Salbeck J., Spreitzer H., Grätzel M., Solid-state dye-sensitized mesoporous TiO2 solar cells with high photon-to-electron conversion efficiencies. Nature 395, 583–585 (1998). [Google Scholar]
- 3.Suteewong T., Sai H., Hovden R., Muller D., Bradbury M. S., Gruner S. M., Wiesner U., Multicompartment mesoporous silica nanoparticles with branched shapes: An epitaxial growth mechanism. Science 340, 337–341 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang P., Zhao D., Margolese D. I., Chmelka B. F., Stucky G. D., Generalized syntheses of large-pore mesoporous metal oxides with semicrystalline frameworks. Nature 396, 152–155 (1998). [Google Scholar]
- 5.Armatas G. S., Kanatzidis M. G., Hexagonal mesoporous germanium. Science 313, 817–820 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Crossland E. J., Noel N., Sivaram V., Leijtens T., Alexander-Webber J. A., Snaith H. J., Mesoporous TiO2 single crystals delivering enhanced mobility and optoelectronic device performance. Nature 495, 215–219 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Tian B., Liu X., Tu B., Yu C., Fan J., Wang L., Xie S., Stucky G. D., Zhao D., Self-adjusted synthesis of ordered stable mesoporous minerals by acid–base pairs. Nat. Mater. 2, 159–163 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Sauvage F., Chen D., Comte P., Huang F., Heiniger L.-P., Cheng Y.-B., Caruso R. A., Graetzel M., Dye-sensitized solar cells employing a single film of mesoporous TiO2 beads achieve power conversion efficiencies over 10%. ACS Nano 4, 4420–4425 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Tian Z.-R., Tong W., Wang J.-Y., Duan N.-G., Krishnan V. V., Suib S. L., Manganese oxide mesoporous structures: Mixed-valent semiconducting catalysts. Science 276, 926–930 (1997). [Google Scholar]
- 10.Li D., Zhou H., Honma I., Design and synthesis of self-ordered mesoporous nanocomposite through controlled in-situ crystallization. Nat. Mater. 3, 65–72 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Inagaki S., Guan S., Ohsuna T., Terasaki O., An ordered mesoporous organosilica hybrid material with a crystal-like wall structure. Nature 416, 304–307 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Wu K. C.-W., Jiang X., Yamauchi Y., New trend on mesoporous films: Precise controls of one-dimensional (1D) mesochannels toward innovative applications. J. Mater. Chem. 21, 8934–8939 (2011). [Google Scholar]
- 13.Lee J., Orilall M. C., Warren S. C., Kamperman M., DiSalvo F. J., Wiesner U., Direct access to thermally stable and highly crystalline mesoporous transition-metal oxides with uniform pores. Nat. Mater. 7, 222–228 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Walcarius A., Sibottier E., Etienne M., Ghanbaja J., Electrochemically assisted self-assembly of mesoporous silica thin films. Nat. Mater. 6, 602–608 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Richman E. K., Brezesinski T., Tolbert S. H., Vertically oriented hexagonal mesoporous films formed through nanometre-scale epitaxy. Nat. Mater. 7, 712–717 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Shan F., Lu X., Zhang Q., Wu J., Wang Y., Bian F., Lu Q., Fei Z., Dyson P. J., A facile approach for controlling the orientation of one-dimensional mesochannels in mesoporous titania films. J. Am. Chem. Soc. 134, 20238–20241 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Wu C.-W., Ohsuna T., Kuwabara M., Kuroda K., Formation of highly ordered mesoporous titania films consisting of crystalline nanopillars with inverse mesospace by structural transformation. J. Am. Chem. Soc. 128, 4544–4545 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Cölfen H., Antonietti M., Mesocrystals: Inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chem. Int. Ed. Engl. 44, 5576–5591 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Liu B., Aydil E. S., Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J. Am. Chem. Soc. 131, 3985–3990 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Adachi M., Murata Y., Takao J., Jiu J., Sakamoto M., Wang F., Highly efficient dye-sensitized solar cells with a titania thin-film electrode composed of a network structure of single-crystal-like TiO2 nanowires made by the “oriented attachment” mechanism. J. Am. Chem. Soc. 126, 14943–14949 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Teng Z., Zheng G., Dou Y., Li W., Mou C. Y., Zhang X., Asiri A. M., Zhao D., Highly ordered mesoporous silica films with perpendicular mesochannels by a simple Stöber-solution growth approach. Angew. Chem. Int. Ed. Engl. 51, 2173–2177 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Yang H., Kuperman A., Coombs N., Mamiche-Afara S., Ozin G. A., Synthesis of oriented films of mesoporous silica on mica. Nature 379, 703–705 (1996). [Google Scholar]
- 23.Pauwels B., Van Tendeloo G., Thoelen C., Van Rhijn W., Jacobs P. A., Antisense expression of a cell wall–associated protein kinase, WAK4, inhibits cell elongation and alters morphology. Adv. Mater. 13, 1317 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang H. G., Sun C. H., Qiao S. Z., Zou J., Liu G., Smith S. C., Cheng H. M., Lu G. Q., Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 453, 638–641 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Zhou L., Smyth-Boyle D., O’Brien P., A facile synthesis of uniform NH4TiOF3 mesocrystals and their conversion to TiO2 mesocrystals. J. Am. Chem. Soc. 130, 1309–1320 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Lee K. E., Gomez M A., Elouatik S, Demopoulos G P., Further understanding of the adsorption mechanism of N719 sensitizer on anatase TiO2 films for DSSC applications using vibrational spectroscopy and confocal Raman imaging. Langmuir 26, 9575–9583 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Wu W.-Q., Xu Y.-F., Rao H.-S., Su C.-Y., Kuang D.-B., Multistack integration of three-dimensional hyperbranched anatase titania architectures for high-efficiency dye-sensitized solar cells. J. Am. Chem. Soc. 136, 6437–6445 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Wang H., Nicholson P. G., Peter L., Zakeeruddin S. M., Grätzel M., Transport and interfacial transfer of electrons in dye-sensitized solar cells utilizing a Co(dbbip)2 redox shuttle. J. Phys. Chem. C 114, 14300–14306 (2010). [Google Scholar]
- 29.Jun Y.-w., Casula M. F., Sim J.-H., Kim S. Y., Cheon J., Alivisatos A. P., Surfactant-assisted elimination of a high energy facet as a means of controlling the shapes of TiO2 nanocrystals. J. Am. Chem. Soc. 125, 15981–15985 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Barnard A. S., Curtiss L. A., Prediction of TiO2 nanoparticle phase and shape transitions controlled by surface chemistry. Nano Lett. 5, 1261–1266 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/4/e1500166/DC1
Materials and Methods
Fig. S1. Photographs of the mixed solution for the preparation of the mesoporous TiO2 microspheres during the evaporation of the solvent THF.
Fig. S2. TEM images with different magnifications of the sample taken from the preparation solution after the solvent evaporation at 40°C for 6 hours.
Fig. S3. STEM image and EDX elemental maps of the sample taken from the preparation solution after THF evaporation at 40°C for 6 hours.
Fig. S4. WAXRD patterns of the precipitates obtained at different solvent evaporation times without calcination.
Fig. S5. The proposed mechanism for oriented growth of the anatase single-crystal–like pore walls.
Fig. S6. HAADF-STEM images of a single ultramicrotomed 3D open radially oriented mesoporous TiO2 microspheres.
Fig. S7. SEM images of the products obtained after the second-step evaporation at a high temperature of 120°C.
Fig. S8. TEM images of an ultramicrotomed radially oriented mesoporous TiO2 microsphere with different integrity.
Fig. S9. SAED and HRTEM images taken from the cylindrical pore bundle regions with different orientation.
Fig. S10. HRTEM and SEAD images of the mesoporous TiO2 spheres taken from the area of the cylindrical pore bundles.
Fig. S11. Detail photovoltaic parameters of 15 individual mesoporous TiO2 microsphere-based cells.
Fig. S12. Independent certificate by the National Center of Supervision and Inspection on Solar Photovoltaic Products Quality.
Fig. S13. Cross-sectional and top-view SEM images of the photoanode film based on the uniform mesoporous TiO2 microspheres.
Fig. S14. Photocatalytic decomposition of methylene blue dye over the mesoporous TiO2 microspheres.
Fig. S15. Cryo-SEM images of the samples harvested at different intervals of solvent evaporation time.
Fig. S16. STEM image and EDX elemental maps of a single ultramicrotomed mesoporous TiO2 microsphere.
Fig. S17. SEM images of the products obtained under similar conditions in the absence of Pluronic F127.
Fig. S18. Field-emission SEM (FESEM) and TEM images of the ordered mesoporous TiO2 bulks with randomly oriented cylindrical pores.
Fig. S19. HRTEM images, nitrogen adsorption-desorption isotherms, pore size distributions, and SAXS integral curves of the randomly oriented mesoporous TiO2 bulk samples.


