Ixodes scapularis, commonly known as the blacklegged tick, is responsible for transmitting Lyme disease (caused by Borrelia burgdorferi), the most common vector-borne disease in the United States (Centers for Disease Control and Prevention 2014). The blacklegged tick can also transmit Anaplasma phagocytophilum (the etiologic agent of human granulocytic anaplasmosis), Babesia microti (the causative agent of babesiosis), Borrelia miyamotoi (a relapsing fever Borrelia), and deer tick virus. In the northeastern U.S., the highest risk of exposure to the blacklegged tick is likely peridomestic, due to fragmented forest landscapes and other land-use characteristics, as well as the intrusion of humans into prime habitat for blacklegged ticks and their hosts (Falco and Fish 1988, Maupin et al. 1991, Nicholson and Mather 1996, Brownstein et al. 2005). Despite this, most reports of tick abundance and infection rates focus primarily on ticks collected from public lands and forested research sites (Aliota et al. 2014, Barbour et al. 2009, Diuk-Wasser et al. 2012, Hersh et al. 2014, Keesing et al. 2014).
We collected ticks from residential properties in Lyme disease-endemic areas and determined infection rates for nymphal I. scapularis as part of a two-year, multi-site tickborne disease intervention study involving the Centers for Disease Control and Prevention (CDC) and the Emerging Infections Programs in Connecticut (CT), Maryland (MD) and New York (NY). Here, we present tick densities and infection rates for B. burgdorferi, A. phagocytophilum, and B. microti from nymphal I. scapularis, reflecting peridomestic exposure to these pathogens.
After providing informed consent, heads of households in select endemic areas of Fairfield, Litchfield and New Haven Counties, CT; Baltimore, Carroll, Harford and Howard Counties, MD; and Dutchess County, NY, were enrolled in a randomized controlled trial to determine whether a single springtime application of pesticide reduced the incidence of human tickborne disease. Eligible properties were at least a half-acre in size, as that is considered a reasonable surrogate for the presence of tick habitat (Maupin et al. 1991). Enrolled properties were randomized for treatment with either pesticide or water placebo. Tick habitat on randomly selected properties from both groups was drag-sampled for host-seeking nymphal blacklegged ticks during peak nymphal activity in May through July in 2011 and 2012, using methods described previously (Maupin et al. 1991, Mather et al. 1996). Briefly, a 1 m2 flannel cloth was dragged over leaf litter, lawn and vegetation found on residential perimeters. Each area was sampled only once; up to forty 30 s drags were conducted for a total of up to 20 min per property. At a small number of properties in Maryland and New York that were drag-sampled, a half meter flag was used in addition to the drags (Rulison 2013) to collect ticks in particularly dense vegetation, using the same timed-sampling protocol. Nymphal tick density (the number of I. scapularis nymphs collected per hour) was calculated for placebo properties only and averaged for each year by study site (CT, MD and NY) and overall (across all sampled placebo properties).
Ticks were pooled by property in 95% ethanol and sent to the CDC Division of Vector-Borne Diseases in Fort Collins, Colorado for testing. Ticks collected in CT were identified at Western CT State University prior to shipment, whereas ticks from MD and NY were identified at the CDC. I. scapularis nymphs were tested individually for B. burgdorferi, A. phagocytophilum, and B. microti using real-time multiplex polymerase chain reaction (PCR) (Hojgaard et al. 2014). This assay was also used to identify other Borrelia genospecies by comparing PCR cycle threshold values (Ct values) for two specific Borrelia targets (fliD and gB31). Pathogen infection rates were calculated for all tested nymphs, regardless of treatment group, by study site and year. Difference in B. burgdorferi infection rates was assessed using a Z-test for proportions.
In CT, to systematically characterize I. scapularis nymphal emergence and activity (phenology), ticks were sampled weekly from three forested Fairfield County nature preserves using the same timed-sampling protocol. All three sites were located centrally to the CT residential study sites and also within 96 km of the Dutchess County, NY, study area. The phenology sampling locations were maple and oak dominated forests with low-lying barberry. Nymphal I. scapularis ticks collected at phenology sites were counted and replaced after each 30 s drag, and the average nymphal density was calculated. Nymphal ticks were replaced at the phenology sites so that tick abundance would not be affected by frequent sampling. Study approval was obtained by the Institutional Review Boards of the CDC, the state health departments in CT, MD and NY, Western CT State University, and Yale University.
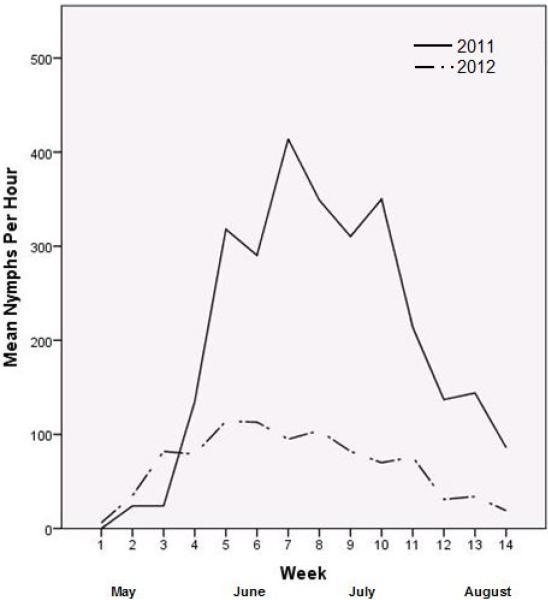
Ixodes scapularis nymphs were the most common tick and life stage collected at all sites and in both years, accounting for 864 (91%) of 952 total ticks collected from 267 properties. Dermacentor variabilis adults were the second most common tick (n=69), collected both years in all sites. Six I. scapularis adults were collected in 2011 in CT. Other ticks collected include Amblyomma americanum (one adult and two nymphs in MD and one nymph in CT, all in 2012), Haemaphysalis leporispalustris (six nymphs in MD in 2012), and Ixodes dentatus nymphs (one in MD and two in NY in 2012). The average I. scapularis nymphal rate across all sampled placebo properties was 27.4 nymphs/hour (range 0-174, standard deviation 37.0) in 2011 and 7.6 nymphs/hour (range 0-60, standard deviation 12.7) in 2012 (Table 1). In 2011 at the CT phenology sites, peak nymphal activity was detected the second week of June with an average of 414 nymphs/hour (range 0-981, standard deviation 239.01) (Figure 1). In 2012, peak activity was detected the first week of June with an average of 113 nymphs/hour (range 0-276, standard deviation 87.06).
Table 1.
Ixodes scapularis nymphal densities and infection rates by study site and year, Connecticut, Maryland, and New York, 2011-2012.
| 2011 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I. scapularis nymphal densities1 | I. scapularis nymphal pathogen infection rates2 | I. scapularis nymphal pathogen coinfection rates | ||||||||||||
| Site | Collection Dates |
Number of properties |
Avg nymphs per hr |
Std dev nymphs per hr |
Number of properties |
Number nymphs tested |
B. burg infection rate |
A. phag infection rate |
B. micro infection rate |
Number B. burg B. micro coinfected |
B. burg B. micro coinf. rate |
Proportion B. burg B. micro of all B. micro |
Proportion B. burg B. micro of all B. burg |
B. burg A. phag coinf. rate |
| CT | 6/15-7/20 | 25 | 46.1 | 45.7 | 50 | 439 | 16.4% | 3.0% | 5.9% | 11 | 2.5% | 42.3% | 15.3% | 0.0% |
| MD | 6/20-7/1 | 13 | 22.0 | 32.2 | 29 | 68 | 20.6% | 1.5% | 0.0% | 0 | 0.0% | n/a | n/a | 0.0% |
| NY | 6/21-7/28 | 29 | 13.6 | 21.8 | 58 | 168 | 23.2% | 4.8% | 7.1% | 8 | 4.8% | 66.7% | 20.5% | 0.0% |
| All sites | 67 | 27.4 | 37.0 | 137 | 675 | 18.5% | 19 | |||||||
| 2012 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I. scapularis nymphal densities1 | I. scapularis nymphal pathogen infection rates2 | I. scapularis nymphal pathogen coinfection rates | ||||||||||||
| Site | Collection Dates |
Number of properties |
Avg nymphs per hr |
Std dev nymphs per hr |
Number of properties |
Number nymphs tested |
B. burg infection rate |
A. phag infection rate |
B. micro infection rate |
Number B. burg B. micro coinfected |
B. burg B. micro coinf. rate |
Proportion B. burg B. micro of all B. micro |
Proportion B. burg B. micro of all B. burg |
B. burg A. phag coinf. rate |
| CT | 5/23-6/22 | 32 | 5.1 | 8.1 | 64 | 75 | 9.3% | 4.0% | 0.0% | 0 | 0.0% | n/a | n/a | 1.3% |
| MD | 5/16-6/7 | 9 | 10.2 | 12.8 | 20 | 56 | 17.9% | 0.0% | 0.0% | 0 | 0.0% | n/a | n/a | 0.0% |
| NY | 6/6-7/6 | 24 | 10.0 | 16.9 | 46 | 39 | 23.1% | 0.0% | 15.4% | 3 | 7.7% | 50% | 33% | 0.0% |
| All sites | 65 | 7.6 | 12.7 | 130 | 170 | 15.3% | 3 | |||||||
Nymphal density was calculated for placebo properties only.
Pathogen infection rates were calculated for all tested nymphs, regardless of treatment group.
Figure 1.
Ixodes scapularis nymphal activity, Fairfield County, Connecticut, 2011-2012.
The overall nymphal infection rate for B. burgdorferi in 2011 was 18.5%, with site-specific infection rates ranging from 16.4% (CT) to 23.2% (NY). In 2012, the overall nymphal infection rate was 15.3%, with site-specific infection rates ranging from 9.3% (CT) to 23.1% (NY) (Table 1). The overall rates were not significantly different by study year at the 95% confidence level (Z=1, p=0.33). Anaplasma phagocytophilum was detected from at least one sampling location in each state, with infection rates of 1.5%-4.8% across all properties in a state. The B. microti infection rate ranged from 5.9% in CT in 2011 to 15.4% in NY in 2012. B. microti was not detected in MD nymphs. Two nymphs collected in NY in 2012 were determined to be infected with a non–Lyme Borrelia genospecies, one of which was co-infected with B. burgdorferi.
Co-infections with B. burgdorferi and B. microti were identified in 22 nymphs collected in CT and NY. One CT nymph was coinfected with B. burgdorferi and A. phagocytophilum. Overall, approximately one half to two thirds of B. microti-infected nymphs were co-infected with B. burgdorferi and approximately one quarter of B. burgdorferi-infected nymphs were co-infected with B. microti.
We report nymphal tick densities and infection rates from residential properties in endemic regions of CT, MD, and NY, reflecting actual potential for peridomestic exposure. The overall nymphal density for residential properties in 2011 was much greater than that in 2012, and this finding was echoed in the densities determined at the phenology sites in CT. Nymphal densities are known to vary by year of collection, even at the same study site, and this has been attributed to the variability of host populations (e.g., deer densities) and climatic conditions (Eisen et al. 2004). Despite the difference in nymphal densities, the overall B. burgdorferi infection rates for 2011 and 2012 were not significantly different and are consistent with previously published reports of both peridomestic and non-peridomestic infection rates (Falco and Fish 1988, Maupin et al. 1991, Barbour et al. 2009, Diuk-Wasser et al. 2012). Similarly, the infection rates of A. phagocytophilum and B. microti are consistent with previous findings (Aliota et al. 2014, Keesing et al 2014, Krause et al. 2014). Our data demonstrate not only a high variability in tick abundances and pathogen infection rates between residential properties, but also between years. Most previous findings have focused on non-residential properties, and therefore our findings increase the existing knowledge of infection rates on the fragmented landscapes of residential properties, where homeowners are most likely to encounter ticks.
Previous reports have suggested risk of infection as a function of tick density and tick infection prevalence (Connally et al. 2006, Nicholson and Mather 1996, Pepin et al. 2012). However, the high variability in tick abundance at a residential level, as in this study, makes predictions based on entomological factors alone complicated (Pardanani and Mather 2004). Furthermore, as properties were sampled a single time over many weeks each year, it is possible that the true picture of tick abundance at each location is not represented in our findings, given the seasonal activity of this species of tick. Our findings were potentially influenced by changing day-to-day climatic and other environmental conditions. Nonetheless, nymphal blacklegged ticks were present on approximately 60% of all control properties sampled. Given the likelihood of exposure to ticks in these endemic areas, the prevalence of pathogens found in I. scapularis nymphs suggests peridomestic risk, not only for Lyme disease, but also for anaplasmosis, babesiosis, and other emerging pathogens.
The nymphal coinfection rates that we detected are also consistent with previous reports (Swanson et al. 2006, Hersh et al. 2014) although the proportion of B. microti-infected nymphs coinfected with B. burgdorferi is striking. The coinfection rates underscore the need to educate healthcare providers to consider coinfections when patients present with tick exposure or likely tickborne disease. Our findings should also serve as a reminder to clinicians to consider emerging tickborne diseases, such as non-Lyme Borrelia infections, including B. miyamotoi, when patients with likely tick exposure but a clinical presentation (such as relapsing fever) different from common tickborne diseases.
The data reported in this paper provide additional entomological evidence for peridomestic risk for tickborne diseases in highly endemic regions. However, it is yet unclear how tick abundance and infection rates correlate with human disease outcomes. Additional investigation, including consideration of social and recreational behaviors and high-risk, high-use areas of the peridomestic environment is warranted to determine how these entomologic findings correlate with risk of human disease.
Acknowledgments
This study would not have been possible without the active involvement of Emerging Infections Program staff and partners in CT, MD and NY and at CDC. In particular, we thank Jim Meek and Julie Ray, CT Emerging Infections Program, Yale School of Public Health; Karen Thompson, Western CT State University; Ellen Stromdahl, U.S. Army Public Health Command, Entomological Sciences Program; Bryon Backenson, Gary Lukacik, Wilson Miranda, and Nadia Thomas, Emerging Infections Program, NY State Department of Health; Aimee Geissler, Gabrielle Dietrich, Rebecca Eisen, Sarah Hook, Ashley Kay, Paul Mead, and Joseph Piesman, Division of Vector-Borne Diseases, CDC. A special thank you to all those involved in tick collection who represented the agencies and programs listed above in addition to the CT Emerging Infections Program at Western CT State University and the Emerging Infections Program at the MD Department of Health and Mental Hygiene.
REFERENCES CITED
- Aliota MT, Dupuis AP, 2nd, Wilczek MP, Peters RJ, Ostfeld RS, Kramer LD. The prevalence of zoonotic tick-borne pathogens in Ixodes scapularis collected in the Hudson Valley, New York State. Vector-Borne Zoonot. Dis. 2014;14:245–250. doi: 10.1089/vbz.2013.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, Tsao JI. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am. J. Trop. Med. Hyg. 2009;81:1120–1131. doi: 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein JS, Skelly DK, Holford TR, Fish D. Forest fragmentation predicts local scale heterogeneity of Lyme disease risk. Oecologia. 2005;146:469–475. doi: 10.1007/s00442-005-0251-9. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) 2014 http://www.cdc.gov/lyme/stats/index.html.
- Connally NP, Ginsberg HS, Mather TN. Assessing peridomestic entomological factors as predictors for Lyme disease. J. Vector Ecol. 2006;31:364–370. doi: 10.3376/1081-1710(2006)31[364:apefap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Hoen AG, Cislo P, Brinkerhoff R, Hamer SA, Rowland M, Cortinas R, Vourc'h G, Melton F, Hickling GJ, Tsao JI, Bunikis J, Barbour AG, Kitron U, Piesman J, Fish D. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. Am. J. Trop. Med. Hyg. 2012;86:320–327. doi: 10.4269/ajtmh.2012.11-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, Eisen RJ, Chang C-C, Mun J, Lane RS. Acarologic risk of exposure to Borrelia burgdorferi spirochaetes: long-term evaluations in north-western California, with implications for Lyme borreliosis risk-assessment models. Med. Vet. Entomol. 2004;18:38–49. doi: 10.1111/j.1365-2915.2004.0476.x. [DOI] [PubMed] [Google Scholar]
- Falco RC, Fish D. Prevalence of Ixodes dammini near the homes of Lyme disease patients in Westchester County, New York. Am. J. Epidemiol. 1988;127:826–830. doi: 10.1093/oxfordjournals.aje.a114865. [DOI] [PubMed] [Google Scholar]
- Hersh MH, Ostfeld RS, McHenry DJ, Tibbetts M, Brunner JL, Killilea ME, LoGiudice K, Schmidt KA, Keesing F. Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS ONE. 2014;9:e99348. doi: 10.1371/journal.pone.0099348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojgaard A, Lukacik G, Piesman J. Detection of Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microti, with two different multiplex PCR assays. Ticks Tick-borne Dis. 2014;5:349–351. doi: 10.1016/j.ttbdis.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Keesing F, McHenry DJ, Hersh M, Tibbetts M, Brunner JL, Killilea M, LoGiudice K, Schmidt KA, Ostfeld RS. Prevalence of human-active and variant 1 strains of the tick-borne pathogen Anaplasma phagocytophilum in hosts and forests of eastern North America. Am. J. Trop. Med. Hyg. 2014;91:302–309. doi: 10.4269/ajtmh.13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Narasimhan S, Wormser GP, Barbour AG, Platonov AE, Brancato J, Lepore T, Dardick K, Mamula M, Rollend L, Steeves TK, Diuk-Wasser M, Usmani-Brown S, Williamson P, Sarksyan DS, Fikrig E, Fish D, Tick Borne Diseases Group Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg. Infect. Dis. 2014;20:1183–1190. doi: 10.3201/eid2007.131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather TN, Nicholson MC, Donnelly EF, Matyas BT. Entomologic index for human risk of Lyme disease. Am. J. Epidemiol. 1996;144:1066–1069. doi: 10.1093/oxfordjournals.aje.a008879. [DOI] [PubMed] [Google Scholar]
- Maupin GO, Fish D, Zultowsky J, Campos EG, Piesman J. Landscape ecology of Lyme disease in a residential area of Westchester County, NY. Am. J. Epidemiol. 1991;133:1105–1113. doi: 10.1093/oxfordjournals.aje.a115823. [DOI] [PubMed] [Google Scholar]
- Nicholson MC, Mather TN. Methods for evaluating Lyme disease risks using geographic information systems and geospatial analysis. J. Med. Entomol. 1996;33:711–720. doi: 10.1093/jmedent/33.5.711. [DOI] [PubMed] [Google Scholar]
- Pardanani N, Mather TN. Lack of spatial autocorrelation in fine-scale distributions of Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 2004;41:861–864. doi: 10.1603/0022-2585-41.5.861. [DOI] [PubMed] [Google Scholar]
- Pepin KM, Eisen RJ, Mead PS, Piesman J, Fish D, Hoen AG, Barbour AG, Hamer S, Diuk-Wasser MA. Geographic variation in the relationship between human Lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the Eastern United States. Am. J. Trop. Med. Hyg. 2012;86:1062–1071. doi: 10.4269/ajtmh.2012.11-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulison EL, Kuczaj I, Pang G, Hickling GJ, Tsao JI, Ginsberg HS. Flagging versus dragging as sampling methods for nymphal Ixodes scapularis (Acari: Ixodidae). J. Vector Ecol. 2013;38:163–167. doi: 10.1111/j.1948-7134.2013.12022.x. [DOI] [PubMed] [Google Scholar]
- Swanson SJ, Neitzel D, Reed KD, Belongia EA. Coinfections acquired from Ixodes ticks. Clin. Micro. Rev. 2006;19:708–727. doi: 10.1128/CMR.00011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]