Abstract
Objective
To define the inflammatory cell infiltrate preceding fibrosis in a laryngotracheal stenosis (LTS) murine model.
Study Design
Prospective controlled murine study.
Setting
Tertiary care hospital in a research university.
Subjects and Methods
Chemomechanical injury mice (n=44) sustained bleomycin-coated wire-brush injury to the laryngotracheal complex while mechanical injury controls (n=42) underwent PBS-coated wire-brush injury. Mock surgery controls (n=34) underwent anterior transcervical tracheal exposure only. Inflammatory and fibrosis protein and gene expression was assessed in each condition. Immunohistochemistry served as a secondary outcome.
Results
In chemomechanical injury mice, there was an up-regulation of: Collagen I (p<0.0001, p<0.0001), Tgf-β (p=0.0023, p=0.0008), and elastin (p<0.0001, p<0.0001) on Day 7, acute inflammatory gene: Il1β (p=0.0027, p=0.0008) on Day 1, and macrophage gene: CD11b (p=0.0026, p=0.0033) on Day 1 versus mechanical and mock controls respectively. M1 marker iNOS expression decreased (p=0.0014) while M2 marker arg1 (p=0.0002) increased on Day 7 compared to mechanical controls. Flow cytometry demonstrated increased macrophages (p=0.0058, day 4) and M1 macrophages (p=0.0148, day 4, p=0.0343, day 7, p=0.0229, day 10) compared to mock controls. There were similarities between chemomechanical and mechanical injury mice with an increase in M2 macrophages at day 10 (p=0.0196).
Conclusions
The mouse model demonstrated increased macrophages involved with the development of LTS. Macrophage immunophenotype suggested that dysregulated M2 macrophages have a role in abnormal laryngotracheal wound healing in both species. These results support this animal model as a representation for human disease. Furthermore, this data delineates inflammatory cells and signaling pathways in LTS that may potentially be modulated to lessen fibroblast proliferation and collagen deposition.
Keywords: trachea, laryngotracheal stenosis, subglottic stenosis, mouse model, airway epithelial injury
Introduction
Laryngotracheal stenosis (LTS) is a poorly understood fibroproliferative disease that is characterized by epithelial injury, inflammatory cell infiltrate, and cytokine-mediated fibroblast proliferation with subsequent collagen deposition.1 The three most frequent causes are iatrogenic, idiopathic, and autoimmune disease.2 While the underlying cause may be different, it ultimately results in triggering an abnormal wound healing response. In LTS, the initial inflammatory phase consists of neutrophil, leukocyte, and macrophage infiltration with minimal fibrosis. This transitions to a pathologic fibroproliferative phase, which leads to active tissue remodeling and the formation of permanent scar tissue.
Recently a number of authors have focused on dysregulated immune cells as possible effector cells for the airway scar tissue.3, 4 Animal models suggest that inflammatory cells and their mediators may be responsible for laryngotracheal fibrosis in humans. A recent ex-situ mouse model showed evidence of lymphocytes mediating the formation of tracheal granulation tissue.3 These results, and others, demonstrated a significant thickening in the subepithelial connective tissue, or lamina propria, with fibroblasts, angiogenesis, and a predominately lymphocytic infiltrate.3-5 In another study, investigators demonstrated that abnormal fibroblasts potentiate the ongoing chronic immune response in scarred vocal folds.6 This suggests that signaling between immune cells and fibroblasts may contribute importantly to pathologic scar formation.
Inflammation’s role in fibrosis is better established in the lower airway and other organ systems. It is initiated by lymphocyte–macrophage interactions that produce cytokines, growth factors, and proteolytic enzymes. These factors stimulate extracellular matrix deposition with persistent tissue remodeling and destruction of normal architecture.7 CD4+ T-cell dependent pathways are key regulators of fibrosis of the lung, kidney and skin.8-12 Similar to T-helper (Th1 and Th2) lymphocytes, macrophages have been recognized as having distinct states of activation, with polarization driven by T-cell phenotype and associated cytokines. Classically activated macrophages (CAM or M1) are activated by Th1 cytokines, such as IFNγ, while alternatively activated macrophages (AAM or M2) are induced by Th2 cytokines, including IL-4 and IL-13. M1 polarization is considered proinflammatory, mediating host defense against bacteria, viruses, and other microorganisms, while the M2 phenotype is classically thought to have anti-inflammatory functions and regulate wound healing. However, sustained M2 polarization has also been implicated in pathologic inflammation and fibrosis.8, 13 Specific to the larynx, other investigators have demonstrated that AAM and fibroblast interaction promotes inflammation associated with vocal fold scarring.6
Our laboratory developed an in vivo in situ murine model of LTS with using a bleomycin-coated wire brush to create chemical and mechanical injury to the trachea which resulted in pathologic wound healing and fibrosis at 3 weeks.4 In contrast PBS coated wire brush injury demonstrated initial injury and lamina propria thickening that was consistent with a physiologic wound healing response appreciated by 3 weeks.4 Bleomycin acted as an accelerant to create lasting injury in the mouse model. Use of this model provides a platform for identification and modulation of specific immune mechanisms to reduce the development of airway fibrosis. We designed a controlled animal study using immunohistochemistry, gene expression and protein analysis to answer the question: Are macrophages, specifically M2 macrophages, key immune effector cells in the development of acquired laryngotracheal stenosis?
Methods
Experimental Design
This study was approved by the Johns Hopkins University Animal Care and Use Committee (MO12M354). The experimental design was a 14-day prospective controlled in-vivo in-situ animal. One-hundred-and-twenty C57BL/6 (Charles River Laboratory, Germantown, MD) mice were used in this study. Outside of planned euthanasia time points, the experimental group (n=44) had 10 deaths, control group #1 (n=42) had 8 deaths, and control group #2 (n=34) had 1 death. Mouse laryngotracheal complexes were chemomechanically injured with a bleomycin-coated wire brush. Mice were sacrificed at 1, 7, and 14 days for gene expression and 4, 7, and 10 days for protein expression. The primary outcome measures were gene expression and protein expression with secondary outcomes of immunohistochemistry (IHC). Results from the experimental group (chemomechanical injury) were compared with 2 control groups: 1) mechanical injury: mice that underwent tracheal injury with a saline-coated wire brush, and 2) mock surgery: mice that underwent a tracheal cut down with no internal tracheal injury.
RNA Extraction and Gene Expression Analysis
Tracheal tissue from mouse specimens was homogenized in a Bullet Blender Homogenizer (Next Advance Inc., Averill Park, NY). RNA was extracted with the RNeasy Mini Kit (Qiagen, Valencia, CA), and cDNA was synthesized with the iScript™ cDNA synthesis kit (BioRad, Hercules, CA). Quantitative real-time PCR (qRT-PCR) was carried out on the StepOnePlus Real Time PCR System (Life Technologies, Carlsbad, CA) and monitored with the Power SYBR ® Green PCR Mastermix (Life Technologies). cDNA samples (n=3, 1 μL for a total volume of 25 μL per reaction) were analyzed for each gene of interest. The level of expression of each target gene was calculated as 2(−ddCt) as previously described.14 The denaturation step temperature was 95°C for 15 seconds, and annealing and extension temperature step was 60°C for 60 seconds for all primers. Error was calculated as the standard error of the mean of ddCt.15
Flow Cytometry for Protein Analysis
Fresh dissected mouse tracheas specimens were rinsed with PBS and placed in dissociation enzyme solution (Liberase 100 μg/ml, Roche 5401127001, DNASE I 40 μg/ml, Roche 10104159001 in DMEM) at 37 °C for 45 minutes. Extra tracheal tissue was dissected and the remaining trachea was minced. A second enzymatic dissociation at 37 °C, for 60 minutes was performed to generate a single cell solution. After incubation to block nonspecific antibody binding, conjugated antibody staining was performed for 45 min at room temperature. Cells were centrifuged and washed twice with cold PBS and fixed with 4% paraformaldehyde. The stained cells were analyzed on a LSR II flow cytometer (BD Biosciences, San Jose, CA). Data analysis was performed with FlowJo software (FlowJo, LLC Ashland, OR). Cell surface protein was expressed as the increase in mean fluorescence intensity over background or percentage of positive staining cells.
Immunohistochemistry
A laryngotracheal complex from day 7 and 14 were fixed in 10% formalin for 24 hours and then each specimen was embedded in paraffin. Slides were made from 5 micrometer (μm) thick sections cut through in an axial plane, which were then deparaffinized and stained with conjugated rat anti-mouse F4/80 (AbD Serotec, Raleigh NC) antibody for 30 minutes. After rinsing, slides were mounted with Prolong ® antifade reagent and incubated with DAPI (Cell Signaling Technology, Danvers, MA). Images were obtained on a confocal microscope (LSM 510, Zeiss, Germany).
Statistical Analysis
Results for murine data were expressed as averages ± standard error of the mean. Statistical analysis comparing bleomycin-induced LTS and control groups was performed using Prism (Graph Pad, San Diego, CA) with a 2-factor analysis of variance followed by a Tukey multiple comparison test post-hoc to examine differences between groups. Statistical significance was assessed at an alpha level of p < 0.05.
Results
Upregulation of fibrosis, acute inflammatory, and macrophage gene expression in chemomechanical injury mice
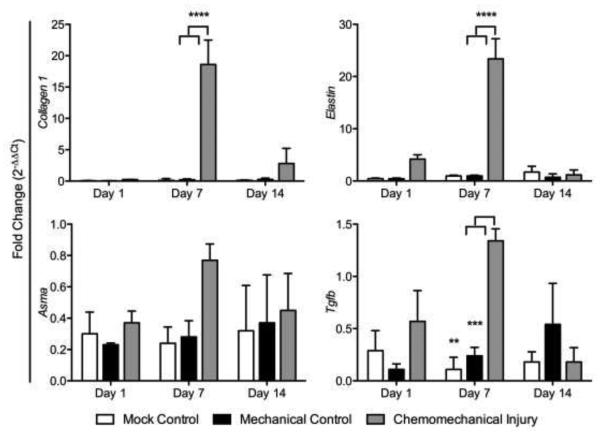
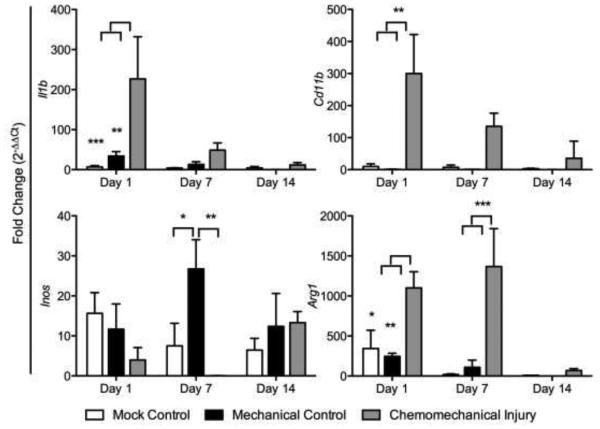
Fibrosis gene expression showed elevated collagen I, Tgf-β, elastin, and αSMA in the chemomechanical injury group (bleomycin and wire brush injury) compared to that in the mechanical injury (wire brush injury only) and mock controls (tracheal cut down but no brush injury) with the exception of Tgf-β and elastin on day 14 (Figure 1). Gene expression peaked on day 7 in collagen I (p<0.0001, p<0.0001), Tgf-β (p=0.0023, p=0.0008), and elastin (p<0.0001, p<0.0001) when compared to mechanical and mock controls respectively. The acute inflammatory response in the experimental group was significantly greater for Il1β (p=0.0027, p=0.0008) versus mechanical and mock controls on day 1; trended down at day 7 and normalized to mock control levels by day 14 (Figure 2a). Assessing gene expression for macrophage markers, there was a significant upregulation seen at day 1 for the mouse macrophage marker CD11b, in the chemomechanical injury group (p=0.0026, p=0.0033) versus mechanical and mock controls on day 1, respectively (Figure 2b). The M1 marker iNOS was significantly less (p=0.0014) than the mechanical injury control at day 7, otherwise it was not significantly different from mechanical injury or mock controls; while the M2 marker Arg1 was significantly higher than mechanical and mock controls at days 1 (p=0.0089, p=0.0207) and 7 (p=0.0002, p=0.0001).
Figure 1.
Mouse LTS (red columns) fibrosis-related gene expression peaked at 7 days (red) compared to that in the mechanical injury (blue) and mock (green) controls.
Figure 2.
Chemomechanical injury demonstrates an increase in acute inflammation markers Tnfa and IL1b, macrophages marker Cd11b, and M2 marker Arg1.
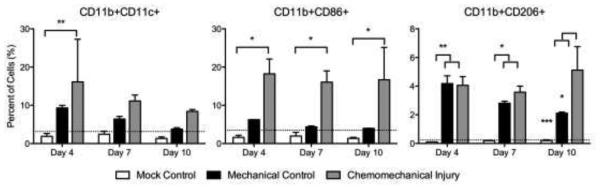
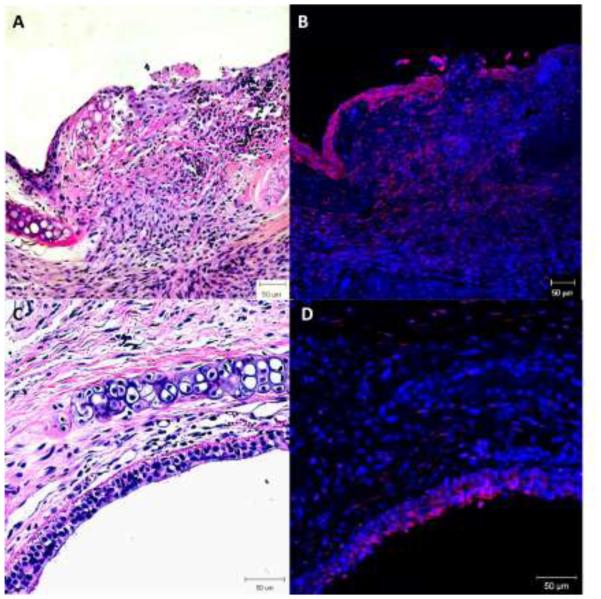
Macrophage cell surface protein expression showed significantly elevated CD11b+/CD11c+ macrophages at day 4 (p=0.0058) in the experimental group than the mock surgical control while it was higher but not significantly different from the mechanical control (Figure 3a). CD86+/CD11b (M1) cells were significantly elevated (p=0.0148, p=0.0343, p=0.0229) at days 4, 7 and 10 respectively, in the chemomechanical injury compared with the mock controls; though not significantly elevated against mechanical controls (Figure 3b). CD206+/CD11b+ (M2) cells were significantly increased for both the experimental group and mechanical injury control over mock surgery at days 4 (p=0.0041, p=0.0034) and 7 (p=0.0102, p=0.0387), with the chemomechanical injury group remaining elevated over the mock controls (p=0.0009) and significantly increasing (p=0.0196) over the mechanical control at day 10 (Figure 3c). Immunohistochemical staining (Figure 4) of chemomechanical injury mice demonstrated a high density of F4/80 macrophages (red staining in 4B & D) in the lamina propria and epithelium at day 7 with a reduced presence at day 14.
Figure 3.
CD11b+/CD11c+ macrophages increase at day 4 in the experimental group versus mock. CD11b+/CD86+ (M1) expression increases versus mock. CD11b+/CD206+ (M2) expression significantly increases on day 10, differentiating from the mechanical injury control. Dotted line=baseline expression.
Figure 4.
H&E stain shows a thickened lamina propria (A&C) with F4/80 immunohistochemical staining showing high density macrophages (red) at day 7 (B). Day 14 shows a fibrotic appearance (C) with reduced macrophage presence (D). 20x magnification.
Discussion
In this study, the “in-situ in-vivo” mouse LTS model is shown to similarly demonstrate increased macrophage presence associated with fibrosis. The chemomechanical injury model revealed an initial acute inflammatory response followed by increased macrophage gene and protein expression preceding the significant increase in fibrosis gene markers at day 7 in a bleomycin-induced LTS, which is different from mechanical injury and mock controls. Macrophage immunophenotype demonstrated a proinflammatory M1 response by flow cytometry that was greater than the mock control but not significantly different from the mechanical injury alone. Furthermore, the M1 gene iNOS was not increased. In both the chemomechanical and mechanical injury cohorts, the M2 cell surface marker CD206 was elevated at early time points compared to mock controls, with the chemomechanical injury group remaining elevated at day 10 while the mechanical controls were not different from mock controls. Consistent significant gene elevation in Arg1 expression in the experimental cohort supports the finding of an increased M2 response.
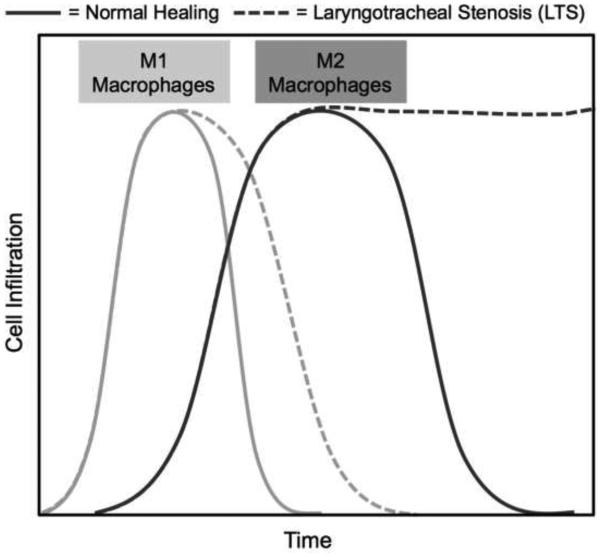
Macrophages are immune effector cells that regulate the inflammatory response, phagocytize cells and debris, and stimulate fibroproliferation.16 Macrophages and their cytokine signaling contribute to a normal wound healing response; however, dysregulated macrophages and cytokine release play a critical role in abnormal wound healing with resultant inflammation and fibrosis. Studies show macrophage immunophenotype to be associated with fibrosis in various organ systems.17-19 For example, renal and myocardial fibrosis are associated with increased M1 macrophages in some studies,17, 19 and increased M2 macrophages in others.20 The distal airway scar in interstitial pulmonary fibrosis is associated with increased M2 markers.18, 21 These seemingly contradictory results suggest that the impact of macrophage immunophenotype on pathogenic wound healing may vary depending on the context, organ system, initiating event, or temporal variation.22 Figure 5 summarizes the mouse macrophage response, showing the expected increase in M1 macrophages in both chemomechanical and mechanical injury mice. While the M2 macrophage infiltration is similar at earlier time points, the chemomechanical injury group significantly increases above the mechanical injury mice at day 10. Pathological wound healing associated with M2 macrophages has been associated with increased Arg1 expression and reduced NOS activity,23 as was also seen in this study. The proposed mechanism for this pathway is the arginase increase causing an upregulation of proline and subsequent increased fibroblast proliferation and collagen deposition.21, 24 Further investigation into macrophage immunophenotype and its effect on human LTS may reveal potential druggable targets to reverse or slow the inflammation and fibrosis.
Figure 5.
Schematic representation of macrophage immunophenotype extrapolated from bleomycin-induced laryngotracheal stenosis. Chemomechanical injury mice demonstrate a similar M1 response versus mechanical injury with a subsequent prolonged M2 response.
Current medical therapies are not successful in halting the development of laryngotracheal scar tissue. Specific immunomodulation may be more effective than current adjuvant therapies, such as steroids. Examples include TGF-β inhibitors, which have been investigated in LTS; and TNFα inhibitors, which are effective in treating other inflammatory diseases.25, 26 One advantage to the treatment of LTS over fibrosis in other organ systems, like the heart, lungs, and kidneys, is the ability to both topically apply medications combined with mechanical dilation of the area of fibrosis. The application of a topical therapy targeting abnormal macrophages or other drivers of inflammation, perhaps via a drug eluting stent, has the potential to prevent restenosis and halt the cycle of serial dilation. Furthermore, direct sustained local immunotherapy at the area of fibrosis avoids side effects associated with systemic therapy.
There are a few limitations to this study and the comparison between bleomycin-induced LTS and human LTS. The pathophysiology of mouse LTS is an acute process, accelerated by the chemotherapeutic agent, bleomycin, which may differ from patients with LTS, especially those whose fibrosis is more of a chronic process. While bleomycin-induced LTS may not exactly reproduce human LTS, bleomycin is commonly used inducer in fibrosis models. A further benefit to this mouse model is the comparison between the experimental and 2 control groups. The mock control group, with its external injury, allows for assessment of transmural injury. The mechanical injury group may be considered a physiologic wound healing group to compare with the sustained inflammation and excessive fibroproliferation seen in the chemomechanical injury group. Future studies may be used to further investigate differences between these groups and yield insight into why the majority of patients who have long-term intubation or other risk factors do not develop LTS.
In summary, bleomycin-induced LTS demonstrated an increased presence of macrophages involved with the development of laryngotracheal fibrosis. Macrophage immunophenotype suggested that dysregulated M2 macrophages have a role in the abnormal laryngotracheal wound healing. The data presented here begins to delineate inflammatory cells and signaling pathways involved in LTS that may potentially be modulated to lessen fibroblast proliferation and collagen deposition. Further studies targeting these immune cells in the mouse model could help identify medical therapies for human clinical trials.
Acknowledgments
Funding Sources: Research reported in this publication was supported by the National Institute of Deafness and Other Communication Disorders of the National Institutes of Health under award number 1K23DC014082. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was financially supported by a Triological Society Career Development Award and The Johns Hopkins FAMRI Center of Excellence.
References
- 1.Minnigerode B, Richter HG. Pathophysiology of subglottic tracheal stenosis in childhood. Prog Pediatr Surg. 1987;21:1–7. doi: 10.1007/978-3-642-71665-2_1. [DOI] [PubMed] [Google Scholar]
- 2.Gelbard A, Francis DO, Sandulache VC, Simmons JC, Donovan DT, Ongkasuwan J. Causes and consequences of adult laryngotracheal stenosis. Laryngoscope. 2014 Oct;:7. doi: 10.1002/lary.24956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh A, Malaisrie N, Leahy KP, et al. Cellular adaptive inflammation mediates airway granulation in a murine model of subglottic stenosis. Otolaryngol Head Neck Surg. 2011 Jun;144(6):927–33. doi: 10.1177/0194599810397750. [DOI] [PubMed] [Google Scholar]
- 4.Hillel AT, Namba D, Ding D, Pandian V, Elisseeff JH, Horton MR. An in situ, in vivo murine model for the study of laryngotracheal stenosis. JAMA Otolaryngol Head Neck Surg. 2014 Oct 1;140(10):961–6. doi: 10.1001/jamaoto.2014.1663. [DOI] [PubMed] [Google Scholar]
- 5.Richter GT, Mehta D, Albert D, Elluru RG. A novel murine model for the examination of experimental subglottic stenosis. Arch Otolaryngol Head Neck Surg. 2009 Jan;135(1):45–52. doi: 10.1001/archoto.2008.516. [DOI] [PubMed] [Google Scholar]
- 6.King SN, Chen F, Jette ME, Thibeault SL. Vocal fold fibroblasts immunoregulate activated macrophage phenotype. Cytokine. 2013 Jan;61(1):228–36. doi: 10.1016/j.cyto.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004 Aug;4(8):583–94. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins SL, Chan-Li Y, Hallowell RW, Powell JD, Horton MR. Pulmonary vaccination as a novel treatment for lung fibrosis. PLoS One. 2012;7(2):e31299. doi: 10.1371/journal.pone.0031299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonian PL, Roark CL, Diaz del Valle F, et al. Regulatory role of gammadelta T cells in the recruitment of CD4+ and CD8+ T cells to lung and subsequent pulmonary fibrosis. J Immunol. 2006 Oct 1;177(7):4436–43. doi: 10.4049/jimmunol.177.7.4436. [DOI] [PubMed] [Google Scholar]
- 10.Tapmeier TT, Fearn A, Brown K, et al. Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int. 2010 Aug;78(4):351–62. doi: 10.1038/ki.2010.177. [DOI] [PubMed] [Google Scholar]
- 11.Wallace VA, Kondo S, Kono T, et al. A role for CD4+ T cells in the pathogenesis of skin fibrosis in tight skin mice. Eur J Immunol. 1994 Jun;24(6):1463–6. doi: 10.1002/eji.1830240634. [DOI] [PubMed] [Google Scholar]
- 12.Wong VW, Paterno J, Sorkin M, et al. Mechanical force prolongs acute inflammation via T-cell-dependent pathways during scar formation. FASEB J. 2011 Dec;25(12):4498–510. doi: 10.1096/fj.10-178087. [DOI] [PubMed] [Google Scholar]
- 13.Trial J, Cieslik KA, Haudek SB, Duerrschmid C, Entman ML. Th1/M1 conversion to th2/m2 responses in models of inflammation lacking cell death stimulates maturation of monocyte precursors to fibroblasts. Front Immunol. 2013;4:287. doi: 10.3389/fimmu.2013.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillel AT, Varghese S, Petsche J, Shamblott MJ, Elisseeff JH. Embryonic germ cells are capable of adipogenic differentiation in vitro and in vivo. Tissue Eng Part A. 2009 Mar;15(3):479–86. doi: 10.1089/ten.tea.2007.0352. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001 Dec;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Haft S, Lee JY, Ghosh A, et al. Inflammatory protein expression in human subglottic stenosis tissue mirrors that in a murine model. Ann Otol Rhinol Laryngol. 2014 Jan;123(1):65–70. doi: 10.1177/0003489414521146. [DOI] [PubMed] [Google Scholar]
- 17.Jarmuz T, Roser S, Rivera H, Gal A, Roman J. Transforming growth factor-beta1, myofibroblasts, and tissue remodeling in the pathogenesis of tracheal injury: potential role of gastroesophageal reflux. Ann Otol Rhinol Laryngol. 2004 Jun;113(6):488–97. doi: 10.1177/000348940411300614. [DOI] [PubMed] [Google Scholar]
- 18.Ballinger MN, Newstead MW, Zeng X, et al. IRAK-M Promotes Alternative Macrophage Activation and Fibroproliferation in Bleomycin-Induced Lung Injury. J Immunol. 2015 Feb 15;194(4):1894–904. doi: 10.4049/jimmunol.1402377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita E, Shimizu A, Masuda Y, et al. Statin attenuates experimental anti-glomerular basement membrane glomerulonephritis together with the augmentation of alternatively activated macrophages. Am J Pathol. 2010 Sep;177(3):1143–54. doi: 10.2353/ajpath.2010.090608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibbons MA, MacKinnon AC, Ramachandran P, et al. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am J Respir Crit Care Med. 2011 Sep 1;184(5):569–81. doi: 10.1164/rccm.201010-1719OC. [DOI] [PubMed] [Google Scholar]
- 21.Hu Y, Zhang H, Lu Y, et al. Class A scavenger receptor attenuates myocardial infarction-induced cardiomyocyte necrosis through suppressing M1 macrophage subset polarization. Basic Res Cardiol. 2011 Nov;106(6):1311–28. doi: 10.1007/s00395-011-0204-x. [DOI] [PubMed] [Google Scholar]
- 22.Shivshankar P, Halade GV, Calhoun C, et al. Caveolin-1 deletion exacerbates cardiac interstitial fibrosis by promoting M2 macrophage activation in mice after myocardial infarction. J Mol Cell Cardiol. 2014 Nov;7(6):6–84. doi: 10.1016/j.yjmcc.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitowska K, Zakrzewicz D, Konigshoff M, et al. Functional role and species-specific contribution of arginases in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008 Jan;294(1):L34–45. doi: 10.1152/ajplung.00007.2007. [DOI] [PubMed] [Google Scholar]
- 24.Stoger JL, Goossens P, de Winther MP. Macrophage heterogeneity: relevance and functional implications in atherosclerosis. Curr Vasc Pharmacol. 2010 Mar;8(2):233–48. doi: 10.2174/157016110790886983. [DOI] [PubMed] [Google Scholar]
- 25.Vannella KM, Barron L, Borthwick LA, et al. Incomplete deletion of IL-4Ralpha by LysM(Cre) reveals distinct subsets of M2 macrophages controlling inflammation and fibrosis in chronic schistosomiasis. PLoS Pathog. 2014 Sep;10(9):e1004372. doi: 10.1371/journal.ppat.1004372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sousse LE, Yamamoto Y, Enkhbaatar P, et al. Acute lung injury-induced collagen deposition is associated with elevated asymmetric dimethylarginine and arginase activity. Shock. 2011 Mar;35(3):282–8. doi: 10.1097/SHK.0b013e3181fddd82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–96. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 28.Simpson CB, White S, McGuff HS. Anti-transforming growth factor beta as a treatment for laryngotracheal stenosis in a canine model. Laryngoscope. 2008 Mar;118(3):546–51. doi: 10.1097/MLG.0b013e31815daf6e. [DOI] [PubMed] [Google Scholar]