Abstract
Recent studies into the global causes of severe diarrhea in young children have identified the protozoan parasite Cryptosporidium as the second most important diarrheal pathogen after rotavirus1–3. Diarrheal disease is estimated to be responsible for 10.5% of overall child mortality4. Cryptosporidium is also an opportunistic pathogen in the context of HIV-AIDS and organ transplantation5,6. There is no vaccine and only a single approved drug that provides no benefit for those in gravest danger, malnourished children and immunocompromised patients7,8. Cryptosporidiosis drug and vaccine development is limited by the poor tractability of the parasite, which includes lack of continuous culture, facile animal models, and molecular genetic tools3,9. Here we describe an experimental framework to genetically modify this important human pathogen. We establish and optimize transfection of C. parvum sporozoites in tissue culture. To isolate stable transgenics we develop a mouse model that delivers sporozoites directly into the intestine, a Cryptosporidium CRISPR/Cas9 system, and in vivo selection for aminoglycoside resistance. We derive reporter parasites suitable for in vitro and in vivo drug screening, and we evaluate the basis of drug susceptibility by gene knock out. We anticipate the ability to genetically engineer the parasite will be transformative for Cryptosporidium research. Genetic reporters will provide quantitative correlates for disease, cure and protection and the role of parasite genes in these processes is now open to rigorous investigation.
Cryptosporidium infection occurs through fecal oral transmission of the environmentally resilient oocyst. The oocyst shelters four sporozoites that emerge in the small intestine and invade the epithelium. While there is no tissue culture system for continuous passage, Cryptosporidium parvum development can be observed for 2–3 days by infecting human ileocecal adenocarcinoma cells (HCT-8)10. To achieve transfection sporozoites were excysted from oocysts purified from the feces of experimentally infected calves using a protocol that mimics stomach and intestinal passage11, and then electroporated prior to infection of HCT-8 cells (Fig. 1a). The transfection plasmids used here flanked a variety of reporter genes with candidate C. parvum 5′ and 3′ regulatory sequences derived from highly expressed housekeeping genes. We observed significant reporter activity 48 hours after transfection using plasmids carrying nanoluciferase (Nluc, Fig. 1b), a small ATP independent enzyme from deep sea shrimp12, but not firefly luciferase or fluorescent proteins. Nluc luminescence correlated with the number of parasites and the amount of DNA used for transfection. Luminescence was also shown to require the presence of parasite specific promoter elements and the introduction of DNA into parasites and not host cells (Fig. 1). Furthermore, reporter signal was ablated by the antiparasitic drug nitazoxanide. Transient transfection of C. parvum is inefficient (<10,000 fold when compared to the related apicomplexan Toxoplasma gondii in parallel experiments) and requires a highly sensitive reporter like Nluc to be noticeable.
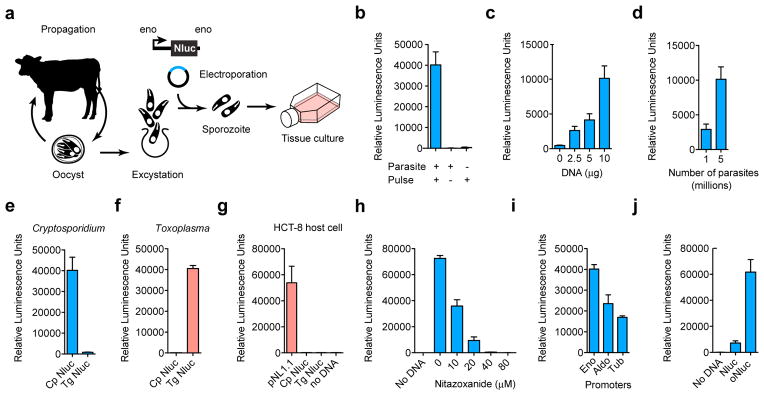
Figure 1. Transfection of Cryptosporidium parvum.
a, Schematic overview. C. parvum sporozoites were prepared from oocysts purified from infected calves and electroporated in the presence of plasmid DNA prior to infection of HCT-8 cells. Luminescence measurements (the means of 3 technical replicates, s.d. shown as error bar) of T. gondii (f), human HCT-8 cells (g), or C. parvum (all other panels, blue) transfected with nanoluciferase (Nluc) expression plasmids. C. parvum transfection requires electroporation (b) of DNA (c) into parasites (d). Transfection also requires plasmids to carry parasite specific promoter sequences (e, f, testing C. parvum and T. gondii promoters in both parasites), and is susceptible to the Cryptosporidium drug nitazoxanide (h). Lipofection of HCT-8 cells with the original Nluc plasmid pNL1.1 (Promega), but not derived parasite vectors results in luciferase activity in the host alone (g). Choice of promoter (i, Eno, enolase; Aldo, aldolase; Tub, alpha-tubulin 5′ regions, the 3′ UTR was uniformly from the enolase gene) or codon composition (j, oNluc, optimized to 35% GC) influences expression level in C. parvum. Note automatic gain adjustment of luminescence measurements, units are not comparable between panels. Independent experiments were repeated 3 times, and representative data are shown.
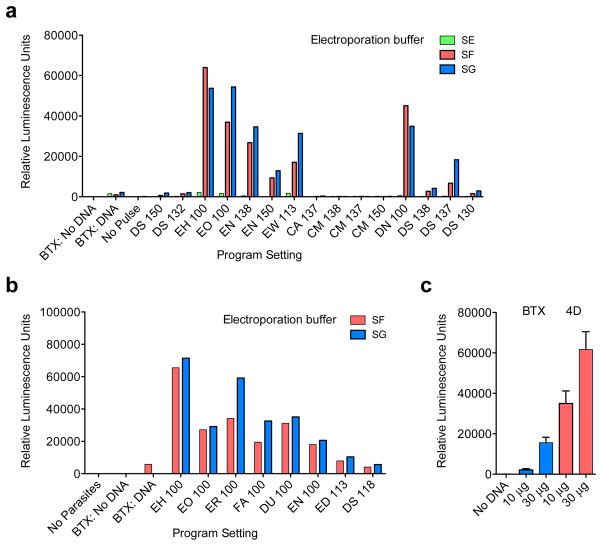
In an effort to enhance efficiency we evaluated different electroporation devices, electrical wave programs, and buffer compositions (Extended Data Fig. 1), this produced tenfold enhancement. We tested flanking sequences from different C. parvum genes and identified the enolase promoter to be strongest. The C. parvum genome is AT rich and shows significant codon bias13, we also noted preference of A over T within the first 20 codons and thus explored codon optimization and found sixfold enhancement (Fig. 1j).
To enable enrichment of transgenic parasites we next explored selection of drug resistance. The aminoglycoside antibiotic paromomycin does not cure cryptosporidiosis in people, but is effective in tissue culture (Fig. 2a) and in immunocompromised mice14. Work in other protist models has shown aminoglycoside phosphotransferases to confer resistance to paromomycin15,16. Appreciation of C. parvum drug resistance in culture is complicated by the lack of continuous growth. We thus constructed translational fusions between the Nluc reporter and the neomycin resistance marker (Neo)15 to focus our observation on the small subset of transfected parasites. Luciferase activity in parasites expressing Nluc-Neo showed reduced susceptibility to paromomycin treatment compared to Nluc alone (Fig. 2c), and thus we concluded that Nluc-Neo confers drug resistance in this transient assay.
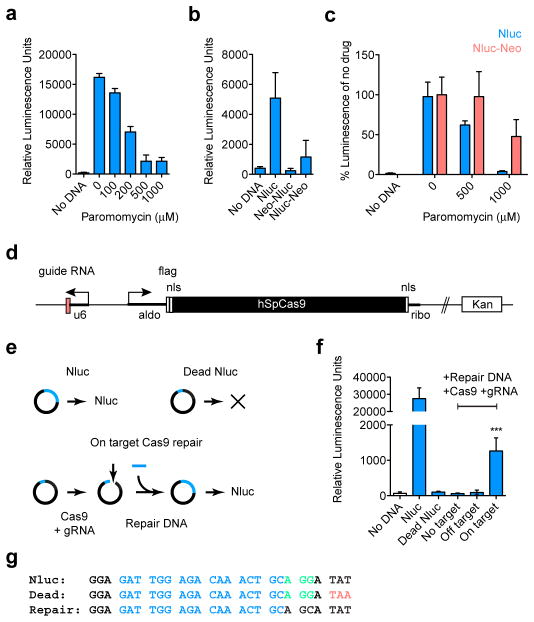
Figure 2. Luciferase assays for C. parvum drug resistance and CRISPR/Cas9 activity.
a, HCT-8 cells were infected with Nluc transfected sporozoites and grown for 2 days in presence of paromomycin. b, Translational fusions were constructed placing Neo to the N- or C-terminus of Nluc. Nluc-Neo shows luciferase activity, albeit at reduced level when compared to Nluc alone. c, C. parvum transfected with Nluc (blue) or Nluc-Neo (red) were grown in different concentrations of paromomycin. Luciferase activity for each plasmid was normalized to its drug free level. d, CRISPR/Cas9 plasmid for C. parvum (u6, newly annotated promoter CM000433:553110–553472; nls, nuclear localization signal; flag, epitope tag; ribo, ribosomal protein L13a 3′ UTR). e, Outline and g, sequences for Nluc repair assay (guide RNA target, blue; protospacer adjacent motif, green; mutagenized codon 18, red). f, Sporozoites were transfected with Nluc or a codon 18 termination mutant (Dead Nluc), note ablation of signal. In addition to the Dead Nluc plasmid, some parasites also received a 125 bp double-stranded repair DNA fragment, and the Cas9 plasmid with the indicated guide RNAs (no target, empty gRNA cassette; off target, GFP gRNA; on target, Nluc gRNA). Statistical analysis compares Dead Nluc alone with Dead Nluc and Cas9 and specific gRNA. Note significant Cas9 mediated restoration of luciferase activity (***p=0.0006, unpaired t test). n=3 technical replicates for a, b, c, and controls from f; n=6 technical replicates for On target samples in f. Error bar is s.d. and all experiments depicted here were repeated 3 times and representative data are shown.
Our genome searches indicated that Cryptosporidium species lack nonhomologous end joining DNA repair. This suggested transgene integration to be rare and to require homologous recombination17,18. Such recombination can be enhanced by long flanking regions and/or double strand breaks introduced by restriction enzymes, TALENs or CRISPR/Cas918,19. To build a C. parvum CRISPR/Cas9 system we constructed a plasmid where the C. parvum U6 RNA promoter drives a guide RNA cassette20 and the Streptococcus pyogenes Cas9 gene21 is flanked by parasite regulatory sequences (Fig. 2d). To test this system we conducted a Cas9 dependent DNA repair experiment (Fig. 2e–g). We introduced a stop codon into the Nluc reporter that ablated luciferase activity (Dead Nluc). We then targeted the dead gene with a guide RNA, and provided a short double-stranded template for repair that restores read through translation and renders the repaired gene resistant to further Cas9 cutting. When C. parvum sporozoites are cotransfected with a specific guide, luciferase activity is restored (p=0.0006, unpaired t test). No change is observed with no or off target guides.
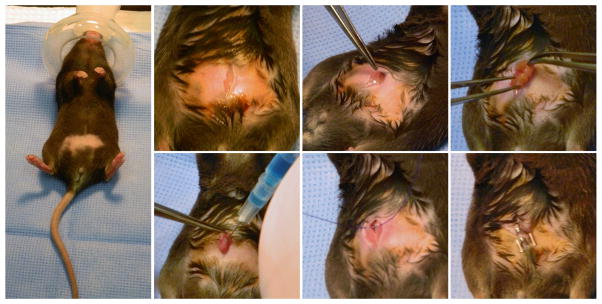
Interferon gamma knockout mice are susceptible to C. parvum infection through oral inoculation of oocysts22. However, infection with free sporozoites is less effective23, likely due to stomach passage. We developed a surgical protocol to inject transfected sporozoites directly into the small intestine to maximize infection (Extended Data Fig. 2). When mice were sacrificed 24 hours after infection luciferase activity was observed in scrapings of the intestinal epithelium. We also established an effective treatment protocol using paromomycin supplementation of the drinking water (Extended Data Fig. 3).
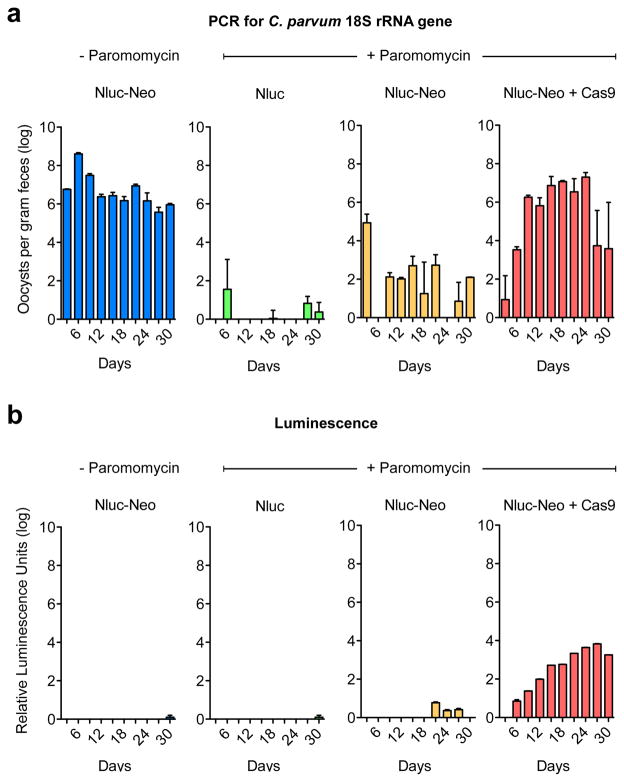
Next we infected mice by surgery with transfected sporozoites and treated with paromomycin as indicated (Fig. 3 and Extended Data Fig. 4, four mice per group). Feces were collected every three days and oocyst shedding was measured by quantitative PCR targeting the C. parvum 18S ribosomal RNA locus. Mice infected with parasites transfected with the Nluc-Neo plasmid that did not receive drug shed high numbers of oocysts and remained infected for the 30 days observed (Fig. 3b, blue). Those infected with parasites that received the Nluc plasmid (lacking the Neo gene, green) were rapidly cured by drug treatment. Those transfected with Nluc-Neo alone and drug treated were also cured (infection may persist slightly longer). In contrast, infection with parasites carrying the Nluc-Neo plasmid and the Cas9 plasmid (red, Cas9 target detailed below) rapidly rebounded to levels similar to untreated mice. Oocysts emerging from selection were purified from feces and used to infect mice that were again treated with paromomycin, wild type oocyst were used in parallel (100,000 oocysts per mouse by gavage). While paromomycin treatment cured infection with wild type parasites, transgenic parasites showed immediate robust drug resistance (Fig. 3c). When these oocysts were probed by Western blot with anti Neo antibody we detected a band consistent with an Nluc-Neo fusion protein.
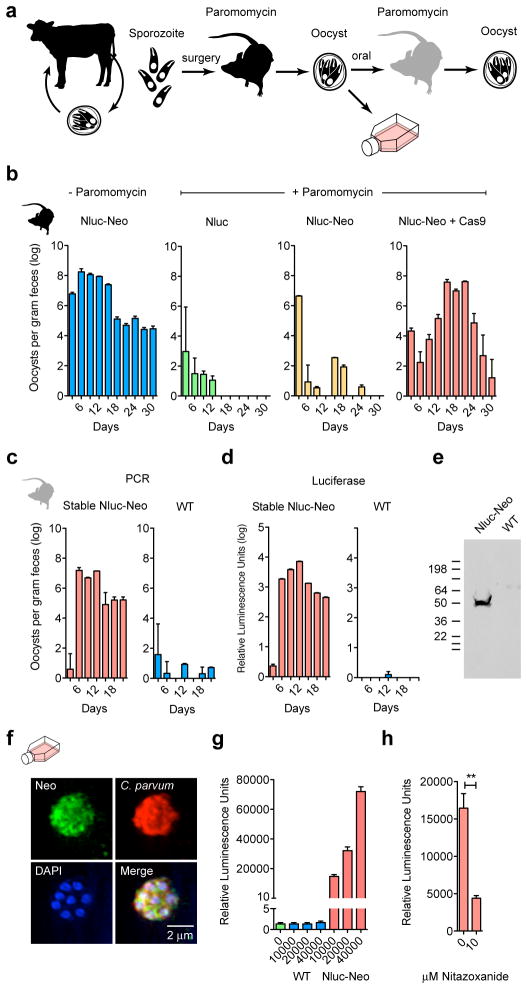
Figure 3. Mouse model for selection of stable C. parvum transgenics.
a, Outline. Transfected sporozoites were injected into the small intestine by surgery (Extendend Data Fig. 2) and mice were treated with paromomycin. Oocysts were purified from the feces and used to infect cultures or mice by oral gavage. b, Quantitative PCR of C. parvum DNA isolated from feces of mice infected with transfected sporozoites (four mice per group) and treated as indicated. Emergence of paromomycin resistance required Nluc-Neo and a Cas9 plasmid. Upon reinfection parasites show strong drug resistance (c) and luciferase activity (d). In repeat experiments we noted that luciferase is detectable as early as 6 days after transfection in the feces of the first infected mouse (Extendended Data Fig. 4). e, Protein extracts from oocysts were analysed by SDS-PAGE and Western blot using an antibody against Neo (rabbit anti-neomycin phosphotransferase II, EMD Millipore). Predicted molecular mass of the Nluc-Neo fusion protein is 48.3 kDa. f, Immunofluorescence staining using anti Neo (mouse anti Neo, Alpha Diagnostic Inc.) and C. parvum (tryptophan synthase B) antibodies. Note multiple nuclei in DAPI stain typical for C. parvum meronts. No anti Neo staining was observed in wild type parasites. g, Luciferase assays for HCT-8 cultures infected with wild type (WT, blue) and transgenic (Nluc-Neo, red) parasites. Y-axis is split to show level of background. n=3 technical replicates, error bar is s.d., experiment done twice. h, 96-well luciferase drug assay using 1000 oocysts per well. Note significant growth inhibition on treatment with 10 μM nitazoxanide (**p=0.0036, unpaired t test). n=3 technical replicates, error bar is s.d., experiment was repeated two times and representative data are shown.
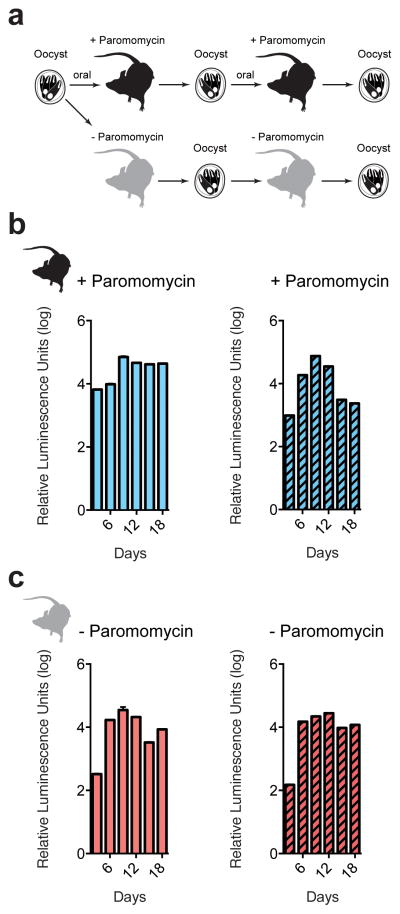
Purified oocysts were also used to infect cell cultures, and processed for immunofluorescence after 2 days. Transgenic but not wild type intracellular parasite stages showed fluorescence when probed with antibodies specific for either Neo or Nluc (Fig. 2e). These cultures also displayed strong luciferase activity not observed in wild type. This activity exceeded that previously observed in transient transfection experiments by five orders of magnitude on a per cell basis. We assessed whether these organisms may be suitable for drug screening assays by infecting 96 well plates with 1,000 oocyst per well and measured luciferase after 48h. Infected wells are clearly distinguished from uninfected (z′>0.6, n=20). Similarly, wells treated with nitazoxanide showed significant growth inhibition (p=0.0036, unpaired t test). Luciferase also provided a faster way to assess the infection state of animals. We sampled 10 mg of feces from mice diagnosed in parallel by PCR and found this assay to be sensitive and specific (Fig. 3d). We note that Nluc expression remains stable when parasites are propagated in mice in the absence of paromomycin (Extended Data Fig. 5).
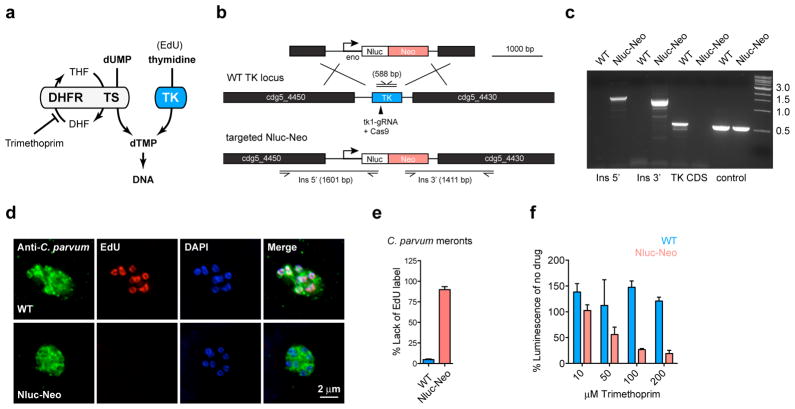
Cryptosporidium is remarkably resistant to antifolates, a mainstay of treatment against other apicomplexans, and this resistance has been attributed to differences in the target enzyme dihydrofolate reductase-thymidylate synthase (DHFR-TS)24. However, Cryptosporidium is unique among apicomplexans in that it acquired a thymidine kinase (TK) by horizontal gene transfer from bacteria25. We hypothesized that TK may also contribute to Cryptosporidium antifolate resistance by providing an alternative route to dTMP (Fig. 4a). For this reason the TK locus was targeted for insertion, allowing us to test this hypothesis by gene disruption. We mapped the locus in stable transgenic parasites by PCR using primers that link the marker genes with genomic sequences beyond the flanking regions on the targeting construct. This mapping is consistent with insertion by homologous double crossover (Fig. 4b,c). Furthermore, the TK coding sequence is no longer detectable indicating uniform loss of the gene in the selected population. We tested for DNA incorporation of the thymidine analog 5-ethynyl-2′-deoxyuridine (EdU) using click chemistry and fluorescence microscopy26. Wild type parasites grown in the presence of EdU show fluorescent nuclei. This labelling is lost in the transgenic (Fig. 4d, e) confirming loss of TK at the biochemical level. We next treated with the antifolate trimethoprim. We confirmed the previously observed resistance in wild type parasites, but noted enhanced susceptibility in the mutant (Fig. 4f). We conclude that the C. parvum TK gene is a non-essential enzyme required for the activation of thymidine, and that its presence limits the efficacy of antifolate therapy in Cryptosporidium.
Figure 4. Targeted deletion of C. parvum thymidine kinase.
a, Due to a horizontal gene transfer C. parvum has two pathways to synthesize dTMP: thymidine kinase (TK) and dihydrofolate reductase-thymidylate synthase (DHFR-TS). DHF and THF, dihydro- and tetrahydrofolic acid. b, Map of the C. parvum TKlocus, the targeting plasmid and the predicted modified locus. Primers and amplicon sizes of diagnostic PCR products are indicated. c, PCR analysis using genomic DNA from wild type (WT) and transgenic parasites (Nluc-Neo, oocysts purified from feces of infected mice shown in Fig. 3c). Primer sequences are provided in a Table S1 in the supplementary material. e, Quantification of EdU labelling experiments (meronts with 4 or more nuclei were scored, two biological repeats, n=105 each sample, error bar is s.d.), d shows representative fluorescence micrographs. Antibody to C. parvum tryptophan synthase B was used to identify parasites (green). f, Trimethoprim treatment of WT (blue) and Nluc-Neo transgenic (red) parasites. WT parasites were measured in transient transfection assays with Nluc plasmid (n=3, technical replicates, error bar is s.d.). Assay shown was conducted in the presence of 10 μM thymidine to avoid indirect host cell toxicity30 (experiments without thymidine produced indistinguishable results). Experiments were repeated 3 times and representative data are shown.
In summary, we show that significant hurdles towards genetic analysis and manipulation for cryptosporidiosis can be overcome by maximizing the efficiency of each step of the process and by focusing on in vivo propagation and selection. There is urgent need for new anti-parasitic drugs3. Cryptosporidium is not susceptible to drugs widely used against related pathogens, which reflects substantial differences in metabolism and uptake27. Luciferase reporter parasites enable phenotypic screening in culture and animals with sufficient sensitivity and specificity to warrant a comprehensive effort to discover novel compounds. Gene deletion now permits biological target validation. Genetic modification may also allow the construction of attenuated parasites as a potential oral vaccine. While infants and toddlers are highly susceptible to the disease, infection is rarely detected in older children1,3. This is consistent with infection studies in people and animals in suggesting the development of anti-parasitic and anti-disease immunity3,28,29. A better understanding of the mechanisms underlying disease and protection will be required to design and produce such a vaccine.
Methods
C. parvum reporter and drug resistance vectors
C. parvum transfection vectors were derived from plasmid pH3BG19 and modified to contain C. parvum promoter and 5′ and 3′ untranslated mRNA regions. We mined the genome and a variety of expression data sets collectively available through cryptoDB.org31 to identify genes that are highly expressed across the lifecycle. Promoters and 5′UTRs of the enolase (cgd5_1960), α-tubulin (cgd4_2860), and aldolase (cgd1_3020) genes and 3′ UTRs of enolase (51 bp), α-tubulin (97 bp), or ribosomal protein L13A (cgd5_970, UTR 211 bp) were amplified from genomic DNA by PCR (see Table S1 for a list of primer sequences and restriction sites used). Nluc was amplified from pNL1.1 (Promega Corporation, Madison, WI), firefly luciferase and different fluorescent protein genes were amplified from vectors used for T. gondii32–34. The neomycin resistance gene was amplified from plasmid pNeo415 (a kind gift of Jacek Gaertig, University of Georgia, Athens, GA) and introduced 5′ or 3′ of Nluc in a plasmid with enolase regulatory sequences. To target the TK gene, regions flanking the gene were amplified and introduced into the Nluc-Neo vector (the promoter but not the 3′ UTR were retained).
C. parvum CRISPR/Cas9 genome editing
Human codon-optimized Streptococcus pyogenes Cas9 (hSpCas9) carrying a FLAG tag and N- and C-terminal nuclear localization signals was amplified from pX33035 and introduced into the Aldolase-Nluc-ribo vector replacing the Nluc. A guide RNA cassette was synthesized containing the C. parvum U6 promoter identified by genome searches using known structural RNA sequences from P. falciparum36, two inverted BbsI restriction sites to facilitate guide cloning, a trans-activating CRISPR RNA (tracrRNA) consensus sequence and a terminator (poly T) sequence, and was introduced into the Cas9 plasmid.
To test for CRISPR/Cas9 mediated repair in vitro, we modified the codon optimized Nluc vector by introducing a premature stop codon (Y18Stop) adjacent to a guide target sequence at the beginning of the gene by site-directed mutagenesis (QuikChange II, Agilent Technologies, Santa Clara, CA). A 125 bp dsDNA oligonucleotide was synthesized that restored Y18 and disrupted the PAM-motif (G17A) of the guide RNA target thus rendering it resistant to further Cas9 cuts.
Parasite excystation and transfection
Oocyst excystation was carried out as described11 with some modification. Up to 108 C. parvum Iowa strain oocysts (Sterling Parasitology Laboratory, University of Arizona, Tucson, AZ or Bunch Grass Farm, Deary, ID) were suspended in 100 μl of 1:4 aqueous dilution of 5.25% sodium hypochlorite and incubated on ice for 5 min. Oocysts were then washed three times with ice-cold phosphate-buffered saline (PBS), suspended at 3.9×105 oocysts/ml of 0.2 mM sodium taurocholate (prepared in PBS) and incubated at 15°C (10 min) and then at 37°C (60–90 min). Emergence of sporozoites was monitored microscopically (typical efficiency 70–90%). Sporozoites were filtered through a 3 μM polycarbonate filter to remove unexcysted oocysts, washed with ice-cold PBS, and counted.
Initially we used a BTX ECM 630 device for electroporation (Harvard Apparatus, Inc., Holliston, MA). Excysted sporozoites (107) were suspended in complete cytomix buffer (120 mM KCl, 0.15 mM CaCl2, 10 mM K2HPO4/KH2PO4, pH 7.6, 25 mM HEPES, pH 7.6, 2 mM EGTA, 5 mM MgCl2, pH 7.6 supplemented with 2 mM ATP and 5 mM glutathione), mixed with plasmid DNA, and electroporated with a single 1500V pulse, resistance of 25 Ω, and a capacitance of 25 μF. To enhance transfection efficiency, we switched to using the AMAXA Nucleofactor 4D device (Lonza Cologne GmbH, Cologne, Germany). After excystation 107 sporozoites were suspended in 15 μl Lonza SF Buffer and combined with 10–50 μg DNA (prepared in Tris-EDTA, pH 8.0) at a final volume of 20 μl. The parasite-DNA mix was added to small, strip cuvettes and electroporated using program EH100. Additional electroporation conditions were explored to arrive at this protocol and those are listed in Extended Data Fig. 1.
For in vitro transfection assays, human ileocecal colorectal adenocarcinoma (HCT-8) cells (ATCC, Manassas, VA) were grown in RPMI-1640 with glutamine supplemented with 10% FBS, 1 mM sodium pyruvate, 50 U/ml penicillin, 50 μg/ml streptomycin and amphotericin B in 24, 48, or 96 well plates to 70% confluency. No effort was made to authenticate this cell line or test for mycoplasma. Prior to infection, media was replaced with DMEM with 2% FBS, 50 U/ml penicillin, 50 μg/ml streptomycin and amphotericin B, and 0.2 mM L-glutamine. For in vivo experiments electroporated sporozites were suspended in PBS and kept on ice until administered to the mice.
The T. gondii Nluc plasmid was constructed by inserting the Nluc sequence into pCTH3 vector32 and parasites were electroporated and used to infect human foreskin fibroblasts as described37. HCT-8 cells were cultured in 24 well plates until confluent, transfected with 500 ng of DNA using Lipofectamine 2000 as described by the manufacturer (Life Technologies), and assayed for Nluc activity after 48 hours.
Animal ethics statement
Animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Georgia (animal use protocol # A2012 03-028-Y3-A12).
Surgical delivery of transfected sporozites into IFN-gamma deficient mice
In preliminary experiments we noted that antibiotic removal of bacterial flora enhances susceptibility of mice. Prior to infection mice were orally treated by gavage daily for a week prior to infection with an antibiotic cocktail (3mg ampicillin, 3mg streptomycin, 0.95mg metronidazole, 3mg neomycin, and 1.5mg vancomycin in dH2O, per mouse/per day, all antibiotics purchased from Sigma). To deliver sporozoites directly to the small intestine, we developed a mouse survival surgery protocol for female C57BL/6 IFN-γ deficient mice (B6.129S7-Ifngtm1Ts/J, Jackson Laboratories, Bar Harbor, ME) aged 6–8 weeks. The abdominal area of mice was shaved with clippers. Animals were placed in isofluorane (3–5%) anesthesia induction chamber and then moved to a nosecone (1–3% isofluorane as needed) on a sterile surgical field. A sterile drape was applied over a warming pad after sterilization of the area with 70% ethanol. Respiration and response to stimulation (toe pinch) was monitored during the procedure and the vaporizer adjusted as needed. Mucous membranes and footpads were monitored for color to confirm adequate perfusion. Three betadine (Povidone-iodine) scrubs followed by a 70% ethanol wipe were applied to shaved skin prior to surgery. Ophthalmic ointment (Puralube®, Dechra Veterinary Products, Shrewsbury, UK) was applied to prevent drying of eyes. Skin was vertically incised midline of the abdominal region below the sternum with microsurgical scissors for approximately 1.5 cm followed by vertical incision of the peritoneum. Exposed jejunum/ileum was injected with 107 transfected sporozoites suspended in 200μl PBS containing sterile food coloring dye as tracer. After injection, suturing was performed to close the peritoneum. Mice were administered 0.01–0.02 ml/gram body weight of warm lactated Ringer’s solution subcutaneously after surgery. Meloxicam analgesic was also administered to the mice post surgery. At completion of procedure, the eye ointment was wiped off and the vaporizer was turned off and the mice were allowed to breathe the oxygen supply gas until they began to wake. Mice were placed in a recovery area until ambulatory and exhibiting normal respiration and were watched for 2 hours post surgery. Incision sites were monitored daily until fully healed (10–14 days). 24 hours following surgical infection, water in mouse cages was replaced with dH2O containing 16 mg/ml paromomycin, a concentration we determined to deliver a daily dose of 40 mg/kg paromomycin to each mouse (Extended Data Fig. 3). Mice were randomly assigned to groups before surgery. A sample size of 4 animals per treatment group was judged to be sufficiently large enough to draw appropriate conclusions. All mice survived surgery and were included in the results reported here. Investigators were not blinded to group allocation during the experiments.
Mouse feces collection and storage
Fecal samples were collected from mice (typically 4 mice per cage) starting three days post infection every third day for up to a month. Mice were transferred to a fresh, sterile cage for 2–3 hours, and feces from the cage were collected, pooled, and stored at 4°C.
Luciferase assay
For transient transfection experiments, electroporated sporozoites were added to 70% confluent HCT-8 culture and infection was allowed to proceed at 37°C for 48 hours. Media was removed from wells and 200 μl of lysis buffer (50mM TrisHCl, 10% glycerol, 1% Triton-X, 2mM DTT, 2mM EDTA) supplemented with NanoGlo substrate (1:50, Promega Corp., Madison, WI) was added to each well. Cells were scraped and the lysate was transferred to white 96 well plates and luminescence was measured using a Synergy H4 Hybrid Microplate Reader (BioTek Instruments, Inc., Winooski, VT). For drug assays with purified stable transgenic oocysts, the culture supernatant was collected after 48 hours from 96 well plates. An equal amount of supernatant and NanoGlo lysis buffer with substrate was combined and luminescence was measured.
For luciferase measurement from mouse fecal samples, 20 mg of feces was weighed into a 1.5 ml microcentrifuge tube and homogenized in 1 ml of lysis buffer (see above) using 10–15 glass beads (3mm) and a vortex mixer for 1 min, followed by clarification of lysate by brief centrifugation. 100 μl lysate was mixed with an equal volume of NanoGlo Luciferase Buffer (prepared with 1:50 dilution of substrate) and luminescence was measured as described.
High-throughput imaging assay for parasite and oocyst quantification in growth and drug assays
For drug assays we used either luciferase activity or a 96 well infection and imaging protocol38 using a BD Pathway instrument. Parasites and host cells were quantified using an ImageJ macro adapted from39. The ratio of parasites to host nuclei was determined for each sample image and normalized to untreated controls.
For oocyst quantification by high-throughput microscopy, we weighed collected mouse feces and diluted in PBS (5 μl/mg). Samples were incubated at 95°C for 10 minutes, vortexing every 2 minutes at high speed. Large debris was allowed to settle for 10 min, then 10 ul of the suspension were mixed with 990 μl PBS and 1 μl of FITC conjugated goat polyclonal anti Cryptosporidium antibody (GeneTex). After 1 h at RT, the sample was centrifuged at 2000xg for 15 minutes. The pellet was suspended in 200 μl PBS and transfered to a 96-well plate for microscopy. Plates were imaged using BD Pathway and oocysts were counted using an ImageJ macro. Using a standard curve (uninfected mouse feces spiked with known amounts of oocysts) oocyst counts were converted to oocysts per grams feces.
Quantification of oocyst shedding using quantitative real-time PCR (qRT-PCR)
DNA was extracted from 100 mg feces using ZR Fecal DNA MiniPrep Kit (Zymo Research Corporation, Irvine, CA) following the manufacturer’s protocol with slight modification. While in lysis buffer, the sample was freeze-thawed in liquid nitrogen five times prior to the first centrifugation step. Each sample was eluted in 50 μl water, 1μl of eluate was used for qRT-PCR along with 10 μM primers targeting Cryptosporidium 18S rRNA40 and SYBR Master Mix (Life Technologies, Carlsbad, CA) for detection. Each qRT-PCR reaction was normalized using an 8 point standard curve (fecal DNA purified from uninfected mouse feces spiked with known amounts of oocysts) for each set of samples.
Oocyst purification from mouse feces
Oocysts were purified from feces using sucrose suspension followed by a cesium chloride centrifugation41. Mouse feces were suspended in tap water, passed through a 850 μm mesh filter, followed by 250 μm mesh. This filtered suspension was mixed 1:1 with aqueous sucrose solution (specific gravity 1.33), and centrifuged at 1000xg for 5 min. Oocysts were collected from the supernatant and suspended in 0.85% saline solution. 0.5 ml of this preparation were overlayed onto 0.8 ml of 1.15 specific gravity CsCl, centrifuged for 3 min at 16,000xg. Oocysts were collected from the top ml of the sample, washed in 0.85% saline, counted with disposable counting chamber (KOVA International, Garden Grove, CA) and suspended in 2.5% potassium dichromate for storage at 4°C.
Western blotting
For Western blot analysis, oocysts from wildtype and transgenic Nlu-Neo were excysted as described above and sporozoites were lysed in SDS sample buffer. Protein extract from 107 sporozoites was loaded per lane and subjected to electrophoresis on a precast Any kD Mini-PROTEAN TGX gel (Bio-Rad, Hercules, CA) followed by transfer to 0.2 μm nitrocellulose membrane (Bio-Rad, Hercules, CA). Blots were blocked and probed with an anti-neomycin phosphotransferase II antibody (EMD Millipore, Temecula, CA) at 1:1000 dilution and goat anti-rabbit IgG (H + L)-HRP conjugate (Bio-Rad, Hercules, CA) at 1:20,000 dilution followed by detection with ECL Western Blotting Substrate (Thermo Pierce, Rockford, IL) and exposure to film. Equal loading of blots was controlled by stripping and reprobing with an antibody to αtubulin.
EdU labeling and immunofluorescence microscopy
5-ethynyl-2′-deoxyuridine (EdU) labeling was performed using the Click-iT EdU Alexa Fluor 594 Imaging Kit following the manufacturer’s instructions (Life Technologies, Carlsbad, CA). Purified stable transgenic oocysts expressing the luciferase or wildtype oocysts were inoculated into 24-well plates containing coverslips confluent with HCT-8 cells. After 24 hours, EdU was added to the media at 10 uM and left for 18 hours prior to fixation. For immunofluorescence, primary antibodies used were mouse monoclonal anti-human neomycin phosphotransferase II (NPII) (Alpha Diagnostic International Inc., San Antonio, TX), rabbit polyclonal anti-Nanoluciferase antibody (Promega Corporaton, Madison, WI), and polyclonal rabbit anti C. parvum tryptophan synthase B (TrpB, unpublished) at 1:1000, secondary antibodies were anti-mouse or anti-rabbit conjugated to Alexa488 or Alexa546 (Molecular Probes, Life Technologies, NY) at a dilution of 1:1000. DNA was visualized with DAPI (2 mg/ml). Images were collected on an Applied Precision Delta Vision inverted epifluorescence microscope at the UGA Biomedical Microscopy Core, deconvolved and adjusted for contrast using SoftWoRx software.
Statistical Methods
All bar graphs depict the mean with standard deviations shown as error bar. Unless indicated otherwise, graphed data represent three technical replicates; each experiment was repeated at least twice and representative data is shown. No statistical tests were used to predetermine sample size. Unpaired t tests were used appropriately to determine statistical significance and p-value < 0.05 was considered significant. Assumptions for statistical tests were confirmed or corrected as described. No animals were excluded from experimental measurements.
Extended Data
Extended Data Figure 1. Optimization of sporozoite transfection.
a, 10 million sporozoites prepared in either cytomix (BTX) or Lonza Buffers SE, SF, or SG (4D Nucleofection) were combined with 10 μg DNA (Eno_Nluc-GS-Nluc_Eno). Samples were electroporated using previously determined settings for BTX (1500 V, 25 Ω, 25 μF) or various program settings for 4D Nucleofection as indicated. Parasites were added to cultures of HCT-8 cells and luciferase activity was read after 48 hrs. Bars represent average of 2 technical replicates. b, Transfection was further optimized by comparing the best preliminary settings (Buffers SF and SG; programs EH 100 and EO 100) with additional pulse programs as indicated. Transfection was carried out as in a. Bars represent average of 2 technical replicates. c, Electroporation systems (BTX and 4D Nucleofection) were compared using the same number of C. parvum sporozoites and quantities of DNA using buffers and conditions optimized in a and b. Bars represent average of three technical replicates. Note about tenfold enhancement of transient transfection using 4D Nucleofection. The impact of electroporation on stable transformation cannot be assessed in this setup and may be higher. Experiments in a and b were done once for the purpose of optimization, while c was repeated three times, a single representative experiment is shown.
Extended Data Figure 2. Direct surgical injection of transfected C. parvum sporozoites into the small intestine.
Mice are shaved and anaesthetized with isofluorane (3% initially, then maintained at 1.5% for the surgery). The abdominal skin is disinfected with Betadine and a small incision is made into the peritoneum. Forceps are used to grasp the small intestine and 100ul of PBS containing 107 transfected C. parvum sporozoites is injected into the lumen. The peritoneum and the abdominal skin are each sutured with 4-0 polydioxanone and mice are injected with meloxicam (1mg/kg) subcutaneously. Each procedure takes around 15 minutes, and mice recover rapidly.
Extended Data Figure 3. Optimization of paromomycin treatment of infected mice.
a, Dosing of mice accounting for drug concentration, animal weight, and measured daily water consumption. At 16mg/ml each mouse received 40mg paromomycin daily (dotted line). b, This dose was found to be sufficient to decrease oocyst shedding in treated mice to background. By day 7 mice without paromomycin treatment shed large amounts of oocysts when compared to untreated mice. Treated mice showed no shedding above background. Oocysts were enumerated by high throughput imaging assay. Five mice were analysed individually with two technical replicates.
Extended Data Figure 4. Mouse model for selection of stable C. parvum transgenics.
Repeat of the experiment described in Fig. 3b. a, Measurement of C. parvum infection using fecal PCR. b, Luminescence measurements. Note increasing luminescence from day 6 in parasites that received resistance and Cas9 plasmids. Mice were infected in groups of 4 per cage and pooled feces was analysed for each cage (each measurement represents 3 technical replicates).
Extended Data Figure 5. C. parvum maintains stable transgene when passed serially in mice without paromomycin treatment.
a, Mice were infected orally with 100,000 transgenic oocysts. Infected mice were then treated with paromomycin (b) or left untreated (c). Oocysts were purified from fecal collections by sucrose flotation and CsCl centrifugation, and used to infect a second cohort of mice. Again, each mouse received 100,000 transgenic oocysts and mice were treated or not. Feces were tested for luminescence every 3 days. Each reading represents the pooled fecal sample from 5 mice with three technical replicates.
Supplementary Material
Acknowledgments
We thank Lisa Sharling for initial contributions and Liz Hedstrom, Jan Mead, Shipra Vaishnava, Lihua Xiao, and Yasmine Belkaid for discussion. This work was funded in part by the National Institutes of Health (R01AI112427) to BS and a pilot grant from the Centers for Disease Control and the University of Georgia Research Foundation to BS and Lihua Xiao. MJC was supported by training grant NIH T32AI060546 and BS is a Georgia Research Alliance Distinguished Investigator.
Footnotes
Competing Financial Interests
SV, CFB and BS are listed as inventors on the Patent Cooperation Treaty (PCT) application entitle Cryptosporidium transfection methods and transfected Cryptosporidium cells filed by the University of Georgia Research Foundation.
Author Contributions
SV developed transfection and luciferase assay; MCP optimized transfection and developed Cas9 system; SV, MCP, AS, CFB developed mouse infection and AS developed selection assays; CFB developed surgery; CJS and YBP constructed some of the plasmids; and MJC provided bioinformatics support. SV, MCP, AS and CFB conducted animal experiments and genotypic and phenotypic characterization. SV, MCP, AS, CFB and BS conceived of the study and BS wrote the manuscript with contributions from SV, MCP and AS.
References
- 1.Kotloff KL, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 2.Mondal D, et al. Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis. 2012;54:185–192. doi: 10.1093/cid/cir807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Checkley W, et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. The Lancet Infectious Diseases. 2014 doi: 10.1016/s1473-3099(14)70772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 5.Raja K, et al. Prevalence of cryptosporidiosis in renal transplant recipients presenting with acute diarrhea at a single center in Pakistan. Journal of nephropathology. 2014;3:127–131. doi: 10.12860/jnp.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter PR, Nichols G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin Microbiol Rev. 2002;15:145–154. doi: 10.1128/CMR.15.1.145-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amadi B, et al. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet. 2002;360:1375–1380. doi: 10.1016/S0140-6736(02)11401-2. [DOI] [PubMed] [Google Scholar]
- 8.Amadi B, et al. High dose prolonged treatment with nitazoxanide is not effective for cryptosporidiosis in HIV positive Zambian children: a randomised controlled trial. BMC infectious diseases. 2009;9:195. doi: 10.1186/1471-2334-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Striepen B. Parasitic infections: Time to tackle cryptosporidiosis. Nature. 2013;503:189–191. doi: 10.1038/503189a. [DOI] [PubMed] [Google Scholar]
- 10.Upton SJ, Tilley M, Brillhart DB. Comparative development of Cryptosporidium parvum (Apicomplexa) in 11 continuous host cell lines. FEMS Microbiol Lett. 1994;118:233–236. doi: 10.1111/j.1574-6968.1994.tb06833.x. [DOI] [PubMed] [Google Scholar]
- 11.Gut J, Nelson RG. Cryptosporidium parvum: synchronized excystation in vitro and evaluation of sporozoite infectivity with a new lectin-based assay. J Eukaryot Microbiol. 1999;46:56S–57S. [PubMed] [Google Scholar]
- 12.Hall MP, et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS chemical biology. 2012;7:1848–1857. doi: 10.1021/cb3002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abrahamsen MS, et al. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science. 2004;304:441–445. doi: 10.1126/science.1094786. [DOI] [PubMed] [Google Scholar]
- 14.Theodos CM, Griffiths JK, D’Onfro J, Fairfield A, Tzipori S. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models. Antimicrob Agents Ch. 1998;42:1959–1965. doi: 10.1128/aac.42.8.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mochizuki K. High efficiency transformation of Tetrahymena using a codon-optimized neomycin resistance gene. Gene. 2008;425:79–83. doi: 10.1016/J.Gene.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Gueirosfilho FJ, Beverley SM. On the Introduction of Genetically-Modified Leishmania Outside the Laboratory. Experimental Parasitology. 1994;78:425–428. doi: 10.1006/expr.1994.1048. [DOI] [PubMed] [Google Scholar]
- 17.Fox BA, Ristuccia JG, Gigley JP, Bzik DJ. Efficient gene replacements in Toxoplasma gondii strains deficient for nonhomologous end-joining. Eukaryot Cell. 2009 doi: 10.1128/EC.00357-08. EC.00357-08 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee AH, Symington LS, Fidock DA. DNA repair mechanisms and their biological roles in the malaria parasite Plasmodium falciparum. Microbiol Mol Biol Rev. 2014;78:469–486. doi: 10.1128/MMBR.00059-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooks CF, et al. The Toxoplasma apicoplast phosphate translocator links cytosolic and apicoplast metabolism and is essential for parasite survival. Cell Host Microbe. 2010;7:62–73. doi: 10.1016/j.chom.2009.12.002. S1931-3128(09)00412-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidik SM, Hackett CG, Tran F, Westwood NJ, Lourido S. Efficient Genome Engineering of Toxoplasma gondii Using CRISPR/Cas9. Plos One. 2014;9 doi: 10.1371/journal.pone.0100450. ARTN e100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffiths JK, Theodos C, Paris M, Tzipori S. The gamma interferon gene knockout mouse: a highly sensitive model for evaluation of therapeutic agents against Cryptosporidium parvum. J Clin Microbiol. 1998;36:2503–2508. doi: 10.1128/jcm.36.9.2503-2508.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fayer R, Nerad T, Rall W, Lindsay DS, Blagburn BL. Studies on Cryopreservation of Cryptosporidium-Parvum. Journal of Parasitology. 1991;77:357–361. doi: 10.2307/3283119. [DOI] [PubMed] [Google Scholar]
- 24.Liu JY, Bolstad DB, Bolstad ESD, Wright DL, Anderson AC. Towards New Antifolates Targeting Eukaryotic Opportunistic Infections. Eukaryotic Cell. 2009;8:483–486. doi: 10.1128/Ec.00298-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Striepen B, et al. Gene transfer in the evolution of parasite nucleotide biosynthesis. Proc Natl Acad Sci U S A. 2004;101:3154–3159. doi: 10.1073/pnas.0304686101. 0304686101 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. P Natl Acad Sci USA. 2008;105:2415–2420. doi: 10.1073/Pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Striepen B. In: Antimicrobial Drug resistance. Mayers DL, Lerner SA, Ouellette M, Sobel JD, editors. Vol. 1. Springer; 2009. pp. 605–621. [Google Scholar]
- 28.Sheoran A, Wiffin A, Widmer G, Singh P, Tzipori S. Infection with Cryptosporidium hominis provides incomplete protection of the host against Cryptosporidium parvum. J Infect Dis. 2012;205:1019–1023. doi: 10.1093/infdis/jir874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald V, Deer R, Uni S, Iseki M, Bancroft GJ. Immune responses to Cryptosporidium muris and Cryptosporidium parvum in adult immunocompetent or immunocompromised (nude and SCID) mice. Infect Immun. 1992;60:3325–3331. doi: 10.1128/iai.60.8.3325-3331.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang L, Lee PC, White J, Rathod PK. Potent and selective activity of a combination of thymidine and 1843U89, a folate-based thymidylate synthase inhibitor, against Plasmodium falciparum. Antimicrob Agents Ch. 2000;44:1047–1050. doi: 10.1128/Aac.44.4.1047-1050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harb OS, Roos DS. The Eukaryotic Pathogen Databases: A Functional Genomic Resource Integrating Data from Human and Veterinary Parasites. Parasite Genomics Protocols, Second Edition. 2015;1201:1–18. doi: 10.1007/978-1-4939-1438-8_. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Dooren GG, Tomova C, Agrawal S, Humbel BM, Striepen B. Toxoplasma gondii Tic20 is essential for apicoplast protein import. Proc Natl Acad Sci U S A. 2008;105:13574–13579. doi: 10.1073/pnas.0803862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gubbels MJ, Li C, Striepen B. High-Throughput Growth Assay for Toxoplasma gondii Using Yellow Fluorescent Protein. Antimicrob Agents Chemother. 2003;47:309–316. doi: 10.1128/AAC.47.1.309-316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saeij JP, Boyle JP, Grigg ME, Arrizabalaga G, Boothroyd JC. Bioluminescence imaging of Toxoplasma gondii infection in living mice reveals dramatic differences between strains. Infect Immun. 2005;73:695–702. doi: 10.1128/IAI.73.2.695-702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakrabarti K, et al. Structural RNAs of known and unknown function identified in malaria parasites by comparative genomics and RNA analysis. RNA. 2007;13:1923–1939. doi: 10.1261/rna.751807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Striepen B, Soldati D. In: Toxoplasma gondii: The Model Apicomplexan - Perspective and Methods. Weiss LM, Kim K, editors. Elsevier; 2007. pp. 391–415. [Google Scholar]
- 38.Sharling L, et al. A screening pipeline for antiparasitic agents targeting cryptosporidium inosine monophosphate dehydrogenase. PLoS neglected tropical diseases. 2010;4:e794. doi: 10.1371/journal.pntd.0000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bessoff K, Sateriale A, Lee KK, Huston CD. Drug repurposing screen reveals FDA-approved inhibitors of human HMG-CoA reductase and isoprenoid synthesis that block Cryptosporidium parvum growth. Antimicrob Agents Chemother. 2013;57:1804–1814. doi: 10.1128/AAC.02460-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mary C, et al. Multicentric Evaluation of a New Real-Time PCR Assay for Quantification of Cryptosporidium spp. and Identification of Cryptosporidium parvum and Cryptosporidium hominis. Journal of Clinical Microbiology. 2013;51:2556–2563. doi: 10.1128/Jcm.03458-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Upton SJ. In: Cryptosporidium and Cryptosporidiosis. Fayer R, editor. CRC Press; 1997. pp. 181–207. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.