Abstract
Introduction
Endothelin 1 (ET1) promotes the growth of osteoblastic breast and prostate cancer metastases. Conversion of big ET1 to mature ET1, catalyzed primarily by endothelin converting enzyme 1 (ECE1), is necessary for ET1’s biological activity. We previously identified the Ece1, locus as a positional candidate gene for a pleiotropic quantitative trait locus affecting femoral size, shape, mineralization, and biomechanical performance.
Methods
We exposed TMOb osteoblasts continuously to 25 ng/ml big ET1. Cells were grown for 6 days in growth medium and then switched to mineralization medium with or without big ET1, for an additional 15 days by which time the TMOb cells form mineralized nodules. We quantified mineralization by alizarin red staining and analyzed levels of miRNAs known to affect osteogenesis. Micro RNA (miR) 126-3p was identified by search as a potential regulator of sclerostin (SOST) translation.
Results
TMOb cells exposed to big ET1 showed greater mineralization than control cells. Big ET1 repressed miRNAs targeting transcripts of osteogenic proteins. Big ET1 increased expression of miRNAs that target transcripts of proteins that inhibit osteogenesis. Big ET1 increased expression of 126-3p 121-fold versus control. To begin to assess the effect of big ET1 on SOST production we analyzed both SOST transcription and protein production with and without the presence of big ET1, demonstrating that transcription and translation were uncoupled.
Conclusion
Our data show that big ET1 signaling promotes mineralization. Moreover, the results suggest that big ET1’s osteogenic effects are potentially mediated through changes in miR expression, a previously unrecognized big ET1 osteogenic mechanism.
Keywords: Osteogenesis, Signaling, Anabolic Activity, Osteoblasts, Mineralization
Introduction
Previously we identified a murine quantitative trait locus (QTL) bmd7 that is responsible for 40% of the variance in bone biomechanical properties in HcB8 x HcB23 F2 intercross mice (1). Phenotypes include differences in bone size, shape and strength, suggesting that the responsible genes alter bone modeling during growth and development. Ece1, a gene within bmd7, encodes endothelin-converting-enzyme-1 (ECE1), a membrane bound protease that converts circulating, inactive, big endothelin-1 (big ET1) to biologically active endothelin-1 (ET1) (1).
ET1 is a short-lived and potent paracrine/autocrine signaling molecule first recognized for its vasoconstrictive effect (2). Multiple studies have shown that big ET1 signaling occurs in many organ system (3, 4). Humans and animals with prostate cancer have increased levels of circulating big and active ET1. In bone, ET1 promotes osteoblastic progression of metastatic prostate and breast cancer. Nelson et. al. proposed that ET1 is responsible for disorganized bone formation characteristic of metastases of both breast and prostate cancers (5, 6). Prostate cancer cells cultured with osteoblasts increase mineralization (7), while inhibition of endothelin receptor A (EDNRA) slows or blocks the growth of osteoblastic bone metastases (8). Animal models of osteoblastic breast cancer metastases show constant ET1 secretion (8). The effect of ET1 on osteogenesis is well known, but it is not established whether osteoblasts are capable of cleaving big ET1 to yield active ET1.
To study the role of ECE1 in big ET1-mediated osteogenesis, we exposed TMOb cells (9) to big ET1, the circulating, biologically inert form of ET1 to demonstrate that ET1 signaling is ECE1 dependent. These experiments show that big ET1 signaling decreases sclerostin (SOST) secretion, a bone specific WNT inhibitor (10, 11) even though Sost mRNA is increased.
Micro RNAs (miRs) down-regulate gene expression posttranscriptionally by binding to the 3′UTRs of mRNAs. Increased levels of a miR decrease protein expression and decreased levels of a miRNA lead to increased levels of protein expression (12). We hypothesized that changes in the expression of micro RNAs, molecules that act post-transcriptionally, are responsible for the effect of big ET1 on osteogenesis.
Materials and Methods
Cell Culture
TMOb cells were cultured in α-MEM (Invitrogen, Carlsbad, CA, USA), 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA), and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C with 5% CO2. Mineralization medium also contained 50 μg/ml ascorbic acid (Sigma-Aldrich, St. Louis, MO), and 10mM -glycerolphosphate (Sigma-Aldrich, St. Louis). The concentration of big ET1 (Sigma Aldrich, St. Louis, MO, USA) was 25 ng/mL.
On day -6, cells harvested from four flasks were pooled in growth medium and transferred to 6-well plates with and without big ET1. The cells were grown for 6 days with one change of medium on day -3. On day 0, mineralization conditions were initiatied and big ET1 supplementation was continued. At 15 days cell were stained with Alizarin Red.
Alizarin Red Staining
Wells were photographed at 1X magnification on a dissecting microscope. SigmaScan (Systat Software International, San Jose, CA, USA) was used to count the number of red pixels per field. Five random fields per well were counted and averaged for each well. Experiments were conducted in sextuplicate. StigmaStat (Systat Software International, San Jose, CA) was used to do statistical analysis. Mineralization was analyzed by Mann-Whitney rank sum test. All data are reported as mean ± SEM.
MiR Analysis
RNA was collected at Day 0 and Day 15. The medium was aspirated and miR was isolated using the miRNeasy mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Levels of miR expression were analyzed with the mouse finder miScript miR PCR Array (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions.
Sost Transcriptional Analysis
Messenger RNA levels of Sost were measured by the ΔΔCt method using GAPDH as a reference. Real-time PCR reactions were performed in a StepOne RT-PCR instrument (Applied Biosystems) using a TaqMan (Mm00599890_M1 Applied Biosystems) assay according to the manufacturer’s instructions. Sost mRNA levels were measured at days 0, 3, 6, 9, 12, and 15.
For analysis of RNA expression of Sost with or without big ET1, each time point was compared in a single experiment and then normalized so that the relative quantity of the control. The experiment was performed in triplicate and expression was compared by t-test at each time point. All data are reported as mean ± SEM.
ELISA
Protein levels of SOST in cell culture media were analyzed using a Quantikine HS kit (R&D Systems), according to the manufacturer’s protocol. For each arm, a time point consisted of media harvested from all wells of a six well plate, measured in duplicate. Results from all six wells were averaged to find the value for a time point. Results were analyzed by two-way repeated measures ANOVA. All values are mean ± SEM
Results
Cells exposed to big ET1 (Figure 1) showed ~3-fold greater mineralization than controls (p<0.001). Table 1 summarizes the day 0 and day 15 levels of miRs that affect osteogenesis (13, 14). The table shows the change in each miR’s level during normal differentiation and mineralization and the effect of exposure of TMOb cells to big ET1 on days 0 and 15 relative to control.
Fig 1.
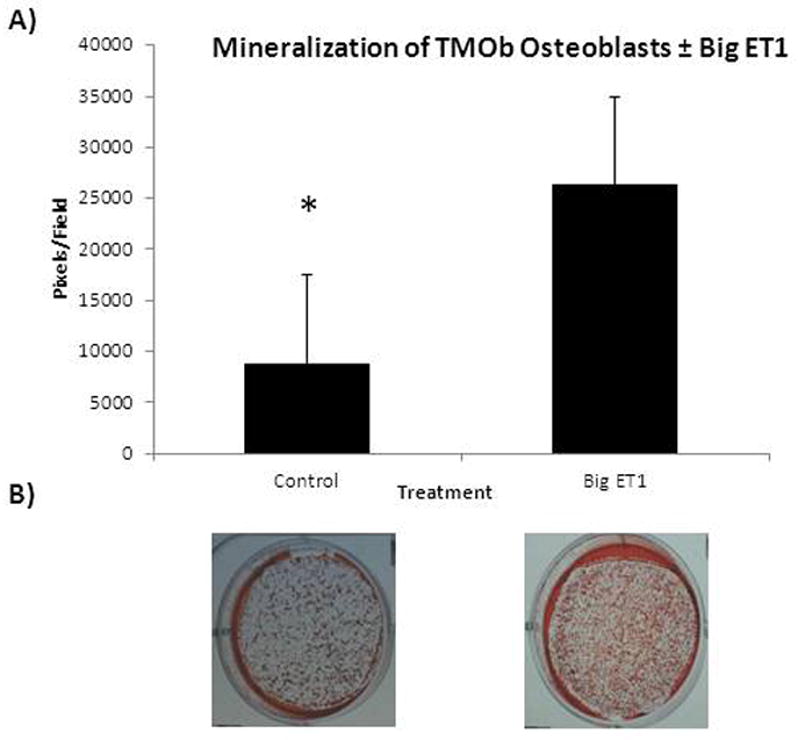
A) Alizarin Red Staining of TMOb cells after 15 days in mineralization media ± big ET1 supplementation. Cells exposed to big ET1 showed greater mineralization (p<0.001). B) Representative images of Alizarin Red staining of TMOb cells after 15 days of mineralization ± big ET1.
Table 1.
Analysis of miRNAs involved in osteogenesis in TMOb osteoblasts during differentiation and mineralization, in the absence or presence of big ET1.
| Micro RNA | Target | Δ in miRNA expression during mineralization of TMOb cells without big ET1 | Function during Mineralization | Δ miRNA expression in TMOb cells plus big ET1 relative to Δ in expression in control. (Day 0) | Δ miRNA expression in TMOb cells plus ET1 relative to Δ in expression in control (Day 15) | Big ET1 Effect |
|---|---|---|---|---|---|---|
| 29a(11,12) | Collagens | ↓20X | ↑Collagen | ↑2X | NC | ↓Early Collagen |
| 29b(11,12) | DAC4, DUSPP2 TGF3 | ↓20X | ↑ Inhibitors | ↑3.5X | ↑25X | ↓Inhibitors |
| 20a(11,12) | BAMB1, CRIM, PPAR | ↓100X | ↓BMP | NC | ↑ 4.3 | ↑BMP |
| 19a(11,12) | TNF | ↓10000X | ↑TNF | NC | ↑13X | ↓TNF |
| 126-3p (15) | SOST | ↓2.6X | ↓WNT | ↑18X | ↑121X | ↑WNT |
| 27a(11,12) | RUNX2 | ↓1000X | ↑RUNX2 | ↓2X | ↓12 X | ↑RUNX2 |
| 101a (15) | RUNX2 | ↓2.5X | ↑RUNX2 | NC | ↓20X | ↑RUNX2 |
| 34c(11,12) | RUNX2 | Down 100X | ↑RUNX2 | ↓10 X | NC | ↑RUNX2 |
| 23a(11,12) | RUNX2 | NC | NA | ↓2X | NC | ↑RUNX2 |
| 335-5p (15) | IGF1 | ↑18X | ↓IGF1 | NC | ↓10X | ↑IGF1 |
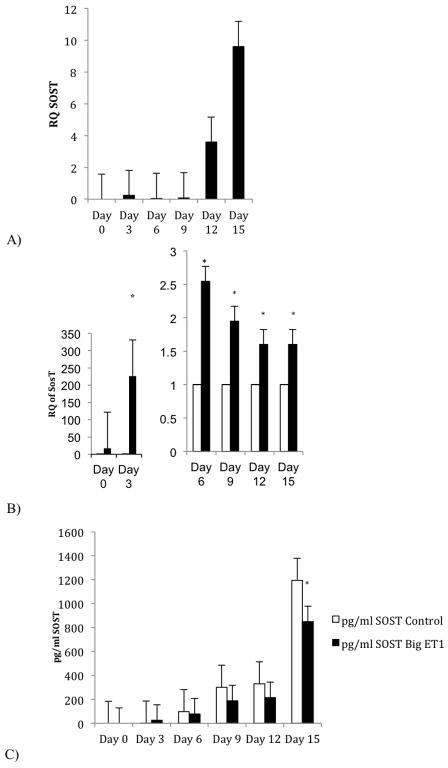
Relative to control, big ET1 increases expression of miRs that down-regulate osteogenesis inhibitors. Big ET1 decreases expression of miRs that down-regulate proteins involved in the anabolic activity of mineralization. We observed a 121-fold increase in miR 126-3p relative to control in big ET1 treated cells (Table 1). Transcriptional data (figure 2A) show the normal expression of Sost during differentiation and mineralization. Figure 2B and 2C show that big ET1 exposure elicits discordant effects on Sost mRNA (p<0.05 on days 3, 6, 9, 12 and 15) and SOST protein production (p<0.001).
Fig 2.
Transcriptional expression and protein production of sclerostin (SOST) in TMOb cells during mineralization with and without big ET1. Panel A) Expression of Sost mRNA in TMOb cell during control mineralization. Sost has low expression until late in differentiation and mineralization of TMOb cells. Panel B) Expression of Sost mRNA with and without big ET1during TMOb differentiation and mineralization with SOST control expression normalized to one. Individual differences appear at days 3, 6, 9, 12 and 15 (p<0.05) with increased expression of Sost mRNA in the big ET1 treated arm. Panel C) SOST protein production during TMOb cell differentiation and mineralization with and without big ET1. Two-way repeated measures ANOVA finds significant differences between the control and big ET1 arm over time (p<0.001), between treatment (p<0.001), and time vs treatment (p<0.001). While the general production of SOST protein shown in panel C follows the transcription pattern of panel A, with increasing SOST over time, the protein expression in the big ET1 arm does not follow the transcriptional results shown in panel B which would suggest and increase in SOST production. This result shows that in the presence of big ET1 production of SOST protein is uncoupled from transcription suggesting a post-transcriptional regulation.
Discussion
Breast and prostate cancer metastases cause formation of disorganized bone and both types of cancer are known to secrete ET1 constitutively (5, 6). Endogenous big ET1 signaling in bone has not been studied in detail and its mechanism of action has not been fully defined. Relative to control, we have shown that exogenous big ET1 causes significant changes in expression levels of miRNAs known to affect osteogenesis, always in favor of increased anabolic behavior, suggesting that big ET1 increases mineralization by regulating the miRNA environment of osteoblasts. As big ET1 is inert prior to cleavage by ECE1, our data demonstrate that TMOb cells are able to cleave big ET1 to active ET1.
We have demonstrated that treatment with big ET1 changes the production of SOST protein in a manner consistent with miR regulation. Big ET1 supplementation increases Sost transcription while simultaneously inhibiting SOST protein production. Inspection of the Microcosm miRNA database (15) revealed that miR 126-3p targets the 3′UTR of the Sost gene. It has been demonstrated that changes in Sost expression and protein production significantly impact bone mass by regulation of canonical WNT signaling (10, 11).
Furthermore, big ET1 regulates multiple miRs previously reported to impact osteoblast differentiation and mineralization. In each case, big ET1 induces changes in miR expression that favor increased osteogenesis. Future experiments include transfection of selected miRNAs and inhibitors to evaluate their effects on mineralization and protein expression, as well as the effects of blocking big ET1 signaling ex vivo in bone cores and in vivo in animal models.
Conclusions
Big ET1 is an important signaling molecule in osteogenesis and changes the miRNA environment of TMOb osteoblasts in favor of increased anabolic metabolism.
Footnotes
Declaration of Interests
All authors have no conflicts of interest.
References
- 1.Saless N, Litscher SJ, Lopez Franco GE, Houlihan MJ, Sudhakaran S, Raheem KA, et al. Quantitative trait loci for biomechanical performance and femoral geometry in an intercross of recombinant congenic mice: restriction of the Bmd7 candidate interval. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2009;23(7):2142–54. doi: 10.1096/fj.08-118679. [DOI] [PubMed] [Google Scholar]
- 2.Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. Journal of hypertension Supplement: official journal of the International Society of Hypertension. 1988;6(4):S188–91. doi: 10.1097/00004872-198812040-00056. [DOI] [PubMed] [Google Scholar]
- 3.Bagnato A, Loizidou M, Pflug BR, Curwen J, Growcott J. Role of the endothelin axis and its antagonists in the treatment of cancer. British journal of pharmacology. 2011;163(2):220–33. doi: 10.1111/j.1476-5381.2011.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annual review of pharmacology and toxicology. 2001;41:851–76. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- 5.Nelson JB, Hedican SP, George DJ, Reddi AH, Piantadosi S, Eisenberger MA, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nature medicine. 1995;1(9):944–9. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- 6.Nelson JB, Nguyen SH, Wu-Wong JR, Opgenorth TJ, Dixon DB, Chung LW, et al. New bone formation in an osteoblastic tumor model is increased by endothelin-1 overexpression and decreased by endothelin A receptor blockade. Urology. 1999;53(5):1063–9. doi: 10.1016/s0090-4295(98)00658-x. [DOI] [PubMed] [Google Scholar]
- 7.Chiao JW, Moonga BS, Yang YM, Kancherla R, Mittelman A, Wu-Wong JR, et al. Endothelin-1 from prostate cancer cells is enhanced by bone contact which blocks osteoclastic bone resorption. British journal of cancer. 2000;83(3):360–5. doi: 10.1054/bjoc.2000.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin JJ, Mohammad KS, Kakonen SM, Harris S, Wu-Wong JR, Wessale JL, et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(19):10954–9. doi: 10.1073/pnas.1830978100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao ZS, Crenshaw M, Guo R, Nesbitt T, Drezner MK, Quarles LD. Intrinsic mineralization defect in Hyp mouse osteoblasts. The American journal of physiology. 1998;275(4 Pt 1):E700–8. doi: 10.1152/ajpendo.1998.275.4.E700. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. The Journal of biological chemistry. 2005;280(20):19883–7. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 11.Ott SM. Sclerostin and Wnt signaling--the pathway to bone strength. The Journal of clinical endocrinology and metabolism. 2005;90(12):6741–3. doi: 10.1210/jc.2005-2370. [DOI] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Lian JB, Stein GS, van Wijnen AJ, Stein JL, Hassan MQ, Gaur T, et al. MicroRNA control of bone formation and homeostasis. Nature reviews Endocrinology. 2012;8(4):212–27. doi: 10.1038/nrendo.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taipaleenmaki H, Bjerre Hokland L, Chen L, Kauppinen S, Kassem M. Mechanisms in endocrinology: micro-RNAs: targets for enhancing osteoblast differentiation and bone formation. European journal of endocrinology/European Federation of Endocrine Societies. 2012;166(3):359–71. doi: 10.1530/EJE-11-0646. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic acids research. 2006;34(Database issue):D140–4. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]