Abstract
Within the body of this review, we provide updates on the mechanisms involved in the renal handling mercury (Hg) and the vicinal dithiol complexing/chelating agents, 2,3-bis(sulfanyl)propane-1-sulfonate (known formerly as 2,3-dimercaptopropane-1-sulfonate, DMPS) and meso-2,3-bis(sulfanyl)succinate (known formerly as meso-2,3-dimercaptosuccinate, DMSA), with a focus on the therapeutic effects of these dithiols following exposure to different chemical forms of Hg. We begin by reviewing briefly some of the chemical properties of Hg, with an emphasis on the high bonding affinity between mercuric ions and reduced sulfur atoms, principally those contained in protein and nonprotein thiols. A discussion is provided on the current body of knowledge pertaining to the handling of various mercuric species within the kidneys, focusing on the primary cellular targets that take up and are affected adversely by these species of Hg, namely, proximal tubular epithelial cells. Subsequently, we provide a brief update on the current knowledge on the handling of DMPS and DMSA in the kidneys. In particular, parallels are drawn between the mechanisms participating in the uptake of various thiol S-conjugates of Hg in proximal tubular cells and mechanisms by which DMPS and DMSA gain entry into these target epithelial cells. Finally, we discuss factors that permit DMPS and DMSA to bind intracellular mercuric ions and mechanisms transporting DMPS and DMSA S-conjugates of Hg out of proximal tubular epithelial cells into the luminal compartment of the nephron, and promoting urinary excretion.
INTRODUCTION
Mercury (Hg) is designated as a transitional metal in row VI of group 12 (IIB) in the periodic table of elements. Elemental mercury (Hg0) has an atomic mass of 200.59 g mol−1 and is unique among metals in that it exists as a liquid at room temperature. It has a high vapor pressure that causes this element to vaporize readily at 100 kPa (equivalent to one standard atmosphere according to the International Union of Pure and Applied Chemistry (IUPAC)). Accordingly, this property facilitates entry of Hg0 to critical cellular targets in humans and other animals subsequent to exposure by inhalation. Once in systemic circulation, Hg0 gains access into a number of critical sensitive target cells due to its nonpolar, lipophilic characteristics. Moreover, Hg0 undergoes oxidation in circulation and/or in the internal compartments of target cells, forming highly reactive mercuric species (Hg2+).1
In the majority of environmental, occupational, and domestic settings, Hg exists as a cation having an oxidation state of either 1+ (mercurous) or 2+ (mercuric). In both aqueous and terrestrial environments, mercuric ions do not exist as free, unbound cations. Rather, they are bonded to a host of inorganic and organic nucleophiles. Thiol-containing biological molecules have an extremely high affinity to bind mercuric ions. Mercuric compounds are by far the primary species of Hg found in environment settings.2,3
In addition to having a 2+ oxidation state, the mercuric ion can exist in states having a valence of 1+ or 2+, depending on whether it is bound covalently to a carbon atom (generally of low-molecular-weight organic chemical groups, such as methyl, ethyl, or phenyl groups). Of the known organic mercuric compounds, the methylmercuric (CH3Hg+) form (generally referred to as simply methylmercury), when it is bound to a monovalent anion such as chloride, is the form most widely disseminated in the environment.2–4 The preponderance of methylmercuric species in environmental settings can be attributed largely to the actions of aquatic and terrestrial prokaryotic organisms capable of methylating inorganic (mercuric) mercury (Hg2+) to CH3Hg+.
HUMAN EXPOSURE TO MERCURY
Since inorganic and organic species of Hg are present or derived from numerous sources in environmental, occupational, and domestic settings, it is nearly impossible for the general population throughout the world to avoid exposure to low levels of some form(s) of Hg on a regular basis. For example, individuals that have had dental amalgams, which are generally composed of at least 50% Hg0, implanted into occlusal surfaces of their teeth as a reconstructive treatment used to treat dental caries, can be exposed to varied amounts Hg0 vapor by inhalation and/or ingestion during the act of mastication or as a result of bruxism.2,3,5–8 It is important to note that Hg0 is still being utilized today by dentists in the United States.
Risk of human exposure to elevated levels of various mercuric species continues to be of considerable concern due to continued and pervasive atmospheric deposition of Hg associated with the industrial use of fossil fuels containing Hg.3,4,9 Exposure to mercuric species at levels that have well-documented toxic effects in humans has been documented in environmental, industrial, medical, educational, governmental, and domestic settings.3,4,9 However, exposure to air, soil, water, and/or ingestion of various animal species contaminated with any of the toxic forms of Hg accounts for the principal means by which Hg gains access into humans and other animals.
There are a number of additional situations in which the general public can be exposed to elevated or toxic levels of Hg. These include breathing in air containing Hg vapor from spills of metallic Hg (such as from broken thermometers) or from areas in proximity to incinerators that burn fossil fuels (especially coal) containing Hg. Ingestion of fish (especially predatory species) contaminated with significant amounts of methylmercury is another way that the general public can be exposed to elevated or toxic levels of Hg, particularly if the fish are consumed frequently.2,3,10,11 Careless use of certain antiseptics, disinfectants, and antifungal agents containing inorganic or organic forms of Hg is an additional means by which humans have been exposed to Hg, although public access to medicinal and domestic chemicals containing mercuric compounds has been reduced greatly due to federal and state regulations and statutes pertaining to the use of such chemicals.
BINDING OF MERCURY IN BIOLOGICAL SYSTEMS
A significant body of evidence indicates that the biological actions and activities of mercurous and mercuric forms of Hg are defined almost exclusively by the complex bonding reactions that occur between the ionic forms of Hg and the sulfur atom(s) of sulfhydryl (-SH) groups (and thiolate anions) present in a host of biomolecules, especially low molecular weight thiol-containing molecules, such as glutathione (GSH), DL-homocysteine (Hcy) and L-cysteine (Cys). Although mercuric ions can bind to any nucleophilic functional group of biological molecules, the affinity constant for a mercuric ion binding to a reduced sulfur atom is on the order of 1015 to 1020.2 In contrast, affinity constants for a Hg atom binding to oxygen- or nitrogen-containing ligands, such as carboxylate or amide groups, respectively, are at least 10 orders of magnitude lower.2 The thermodynamic stability of the bonds formed between inorganic mercuric ions and the reduced sulfur atom of the tripeptide glutathione (GSH) has been studied using 13C NMR.12,13 It was demonstrated that when GSH and Hg2+ are in aqueous solution in a molar ratio of 2:1, each mercuric ion forms thermodynamically stable, linear II coordinate covalent, binds with the sulfur atom of two molecules of GSH, throughout a range of pH from 1 to 14.12,13 Organic mercuric ions, such as the methylmercuric ion (CH3Hg+), form linear I coordinate covalent complexes with thiol-containing molecules.
Despite the thermodynamic stability of the (linear I or II) coordinate covalent bonds formed between the ionic forms of Hg and thiol-containing molecules in aqueous solution, the binding characteristics between a mercuric ion and the reduced sulfur atom of various thiol-containing molecules appear to be more labile in living organisms, especially in mammals.2,12,13 Complex factors such as thiol-competition and electrophilic substitution reactions may be responsible for the labile nature of binding that occurs between mercuric ions and certain thiol-containing molecules in specific cellular and tissue-compartments in mammals. To exemplify this point, the preponderance of mercuric ions present in the plasma of blood have been shown to be bound to proteins possessing one or more reduced cysteinyl residues.14–17 Albumin is one such protein. It is the most abundant protein in plasma and possesses a single –SH group with which mercuric ions can interact and bind. Other nucleophilic domains of albumin (and/or other plasma proteins and nonprotein thiols) may react and bind to mercuric ions with less affinity and with less thermodynamic stability than to SH groups. Interestingly, though, mercuric ions bound to albumin and other plasma proteins appear to be bound to these proteins for relatively short periods. This idea is supported by findings showing that the plasma burden of Hg decreases rapidly after exposure, while there is a concurrent rapid rate of non-endocytotic uptake of mercuric ions in the epithelial cells of the kidneys and liver.2,18
In addition to binding to sulfhydryl groups on proteins, inorganic and organic mercuric ions bind to the sulfur atom of one or more endogenous nonprotein thiols (such as GSH, Hcy, and Cys). Evidence indicates that the thiol S-conjugates formed can serve as transportable substrates of various membrane transporters in the brain, endothelial cells of the blood-brain barrier, and epithelial cells in the small intestine, liver and kidneys.19–22 It also appears that once mercuric ions gain entry into systemic circulation, they undergo one or more complex electrophilic substitution reactions that involve transfer from the reduced sulfur atom(s) of plasma proteins to the reduced sulfur atom of one or more types of low molecular weight, nonprotein, thiols mentioned above.
Due to the nonpolar nature of Hg0, it has the capacity to traverse the plasma membrane of cells by mechanisms that do not require specific membrane transporters.22 It should be emphasized that once Hg0 gains access to systemic circulation, the oxygen-rich environment favors oxidation of Hg0 to Hg2+. Additionally, oxidation of Hg0 likely also occurs within certain target cells, although the amount of oxidation of Hg0 in both intracellular and/or extracellular compartments is not clear at present. However, it does appear that most of the Hg0 that enters into systemic circulation eventually undergoes oxidation, forming one or more mercuric species.23
The tremendously high affinity between oxidized forms of Hg and the reduced sulfur atom of sulfhydryl (and sulfanyl) (SH) groups serves as the therapeutic basis of thiol-containing pharmacological agents, such as penicillamine, N-acetylpenicillamine, dithioerythritol, dithiothreitol, 2,3-bis(sulfanyl)propanol (also known as British Anti-Lewisite or BAL), meso-2,3-bis(sulfanyl)succinate (known formerly as meso-2,3-dimercaptosuccinate; DMSA), and (R,S)-2,3-bis(sulfanyl)propane-1-sulfonate (known formerly as 2,3-dimercaptopropane-1-sulfonate acid; DMPS), forming complexes or chelates with mercuric (and/or mercurous) ions. The formation of mercuric complexes or chelates with these molecules appears to secure Hg2+ or R-Hg+ (where R represents an organic group) in a manner that likely shields the mercuric ion from interacting with biologically relevant nucleophiles. In addition, the polar, negatively charged vicinal dithiols, DMPS and DMSA, have an apparent additional advantage in that, at least in the kidneys, the native molecules gain access into target epithelial cells by sharing, serendipitously, one or more membrane proteins capable of transporting certain thiol S-conjugates of Hg2+ (and the native compound) into the target cells.24–26 After gaining access to the intracellular milieu of renal proximal tubular epithelial cells, the binding affinity between molecules of DMPS or DMSA and mercuric ions is great enough to detach and secure mercuric ions that were bound to a host of intracellular thiols.2 The complexes formed by the binding of DMPS or DMSA to intracellular mercuric ions results in the formation of negatively charged DMPS or DMSA S-conjugates that are water-soluble27,28 and may be transported out of target cells by one or more transporters in the luminal plasma membrane of proximal tubular epithelial cells. The negative charge on the complexes that are transported into the tubular lumen not only promotes their solubility in an aqueous-based luminal fluid but also impedes reabsorption back into peritubular circulation.29
KIDNEY AS A PRIMARY TARGET OF MERCURIC SPECIES
Most chemical species of Hg can induce toxic effects in a number of tissues and organs, although the type and form of toxic effects depend greatly on the chemical species of Hg and the magnitude, duration, and route of exposure. The kidneys in mammals are particularly vulnerable to the toxic effects of Hg, especially mercurous and mercuric forms. Virtually all forms of Hg, including elemental, inorganic, and organic forms, can mediate nephrotoxic effects, depending on the conditions of exposure. In experimental animals and a host of in vitro experimental conditions, mercuric species have been shown to have a great predilection to interact with, and be transported into, renal tubular epithelial cells.19–21,30,31
Systemic distributions of organic forms of Hg are more diffuse than that of inorganic forms. In addition to having toxic effects in the kidneys, organic forms of Hg adversely affect cells in blood, placenta, fetal tissues, and organs and neural tissues, as well as others.2,3,22,32 Differences in cellular mechanisms involved in the transport and metabolism of inorganic and organic forms of Hg (in the various compartments of the body) are likely responsible for the disparity in organ system distribution, pattern of biological effect, and toxic potency of these forms of Hg.
In the kidneys, both inorganic and organic forms of Hg accumulate primarily in the cortex and outer stripe of the outer medulla.18,33–35 Autoradiographic and histochemical data36–43 and tubular microdissection data from mice, rats, and rabbits44,45 indicate that inorganic species of Hg are taken up almost exclusively by the convoluted and straight segments of both cortical and juxtamedullary proximal tubules. Deposits of Hg have also been found in the renal proximal tubules of monkeys exposed to Hg0 originating from dental amalgams.5 Although the segments of the proximal tubule appear to be the predominant sites where mercuric ions are taken up and accumulated (as Hg2+-thiol complexes), there is insufficient data to exclude the possibility that other segments of the nephron and/or collecting duct may also take up, accumulate, and transport inorganic and/ or organic forms of Hg.
Deposits of presumed inorganic Hg have also been identified by various experimental techniques in the epithelial cells lining the entire lengths of renal proximal tubules in rats and mice exposed to organic forms of Hg.34,39–41 Moreover, experimental evidence indicates that a significant fraction of Hg in the kidneys of animals exposed to methylmercury (CH3Hg+) is biotransformed to Hg2+ prior to or after it enters renal tubular epithelial cells.46–48 Additional support for this hypothesis comes from data demonstrating that intracellular conversion of methylmercury to inorganic Hg can occur, albeit by a currently unknown mechanism.49
PROXIMAL TUBULAR UPTAKE AND TRANSPORT OF MERCURY
One of the initial hypotheses regarding the mechanisms of how mercuric species gain access to proximal tubular epithelial cells arose from the notion that filtered complexes of Hg-albumin can gain potential entry into proximal tubular cells via endocytosis. 33,50,51 As mentioned above, albumin is the most abundant protein in plasma that possesses a single free, unbound, SH group (on a terminal cysteinyl residue), which provides a high affinity binding site for a mercuric ion.52 Previous data indicate that the largest percentage of Hg in the plasma is bound to acid-precipitable proteins, such as albumin.14–17,53 Despite the sieving coefficient for albumin being low in renal glomeruli of more highly developed mammals, significant amounts (low gram quantities) of albumin are filtered during each day. Thus, the idea that albumin-Hg complexes are filtered at the renal glomerulus is not an unreasonable one.
In a previous study, where rats were made proteinuric by treatment with the proximal tubular toxicant gentamicin, significant amounts of inorganic Hg were excreted in the urine and were presumed to be conjugated to albumin.50 Assuming, that the permeability of albumin at the glomerular filtration barrier in these rats was unaltered by gentamicin and that the Hg in excreted urine was associated with albumin, the findings tend to support the hypothesis that some fraction of inorganic Hg in the urine may have entered into the luminal compartment of proximal tubules bound to albumin.
In a later study, Zalups and Barfuss33 attempted to implicate a mercuric conjugate of albumin in the luminal uptake of inorganic Hg by simultaneously evaluating the renal disposition of intravenously administered 125I-albumin and 203Hg2+. Their data suggest that mercuric conjugates of albumin are not the primary species of Hg taken up at the luminal membrane of proximal tubular cells. However, endocytosis of a mercuric conjugate of albumin could not be excluded as it may play a minor role in this uptake.
Data from a series of more recent studies provide much more definitive information on the proximal tubular handling of Hg. These data indicate that there are at least two distinct mechanisms involved in the uptake of mercuric ions by proximal tubular epithelial cells. One of the mechanisms involves membrane proteins localized in the luminal plasma membrane, 2,45,54–62 while the other involves transporters in the basolateral plasma membrane.62–70
LUMINAL UPTAKE OF MERCURY BY PROXIMAL TUBULAR CELLS
Role of γ-Glutamyltransferase
In vivo uptake of inorganic Hg (and to a lesser extent organic forms of Hg) at the luminal plasma membrane of tubular epithelial cells in the kidneys has been linked to the activity of γ-glutamyltransferase (γ-GT),2,54,59,71–74 which is presumed to be present exclusively in the luminal (brush border) membrane of the epithelial cells lining both the pars convoluta and pars recta segments of the proximal tubule.75,76 The active site of γ-GT is present in the luminal compartment of the proximal tubule.76 The sole action of this enzyme is to cleave the γ-glutamylcysteine bond in molecules of GSH present in the tubular lumen, thereby forming cysteinylglycine and glutamate. Evidence implicating the activity of the enzyme in the renal tubular uptake of mercuric species comes mainly from in vivo experiments in which renal (and hepatic) γ-glutamyltransferase was inhibited irreversibly by pretreatment with L-(αS,5S)-α-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid (acivicin). Pretreating animals with acivicin decreases the renal uptake and/or accumulation of Hg2+ and causes significant increases in the urinary excretion of Hg2+ in mice71,77 and rats59,78,79 exposed to inorganic Hg or in mice exposed to methylmercury77 or Hg vapor.80 Enhanced urinary excretion of GSH has also been documented in acivicin-pretreated rats injected subsequently with inorganic Hg, providing additional support for the hypothesis that luminal degradation of GSH in proximal tubules is indeed prevented by treatment with acivicin.78
In two isolated perfused tubule studies, Cannon et al. provide direct evidence, linking the activity of γ-GT to the luminal uptake of Hg2+, when delivered as GSH S-conjugates, in proximal tubular segments. These investigators demonstrated that inhibition of the activity of γ-GT (by adding acivicin to the perfusing solution) decreases the luminal uptake (disappearance flux, JD) and cellular accumulation of mercuric ions in the epithelial cells lining intact proximal tubular segments when the mercuric ions were delivered to the luminal compartment as GSH S-conjugates of Hg2+.57,81
Collectively, the aforementioned in vivo and in vitro data indicate that following exposure to inorganic forms of Hg, a significant fraction of inorganic mercuric ions is taken up by proximal tubular epithelial cells by a luminal absorptive mechanism dependent on the actions of γ-GT.
Presence and Formation of Mercuric Conjugates in the Proximal Tubular Lumen
A major implication of the data obtained during in vivo inhibition of γ-GT is that a pool of mercuric ions is present in the luminal compartment of proximal tubules, as GSH S-conjugates, prior to the mercuric ions being taken up. Although it is not known exactly where these mercuric conjugates of GSH are formed or how they gain entry to the luminal compartment of proximal tubules, one must consider the possibility that some of these complexes are formed outside the kidneys (either in systemic circulation or in cells of another organ, such as hepatocytes of the liver) and are then delivered into the lumen of the proximal tubule via glomerular filtration. There are three reasons to suspect that this occurs. First, the formation of mercuric conjugates of GSH in the plasma is possible because the concentration of GSH in plasma (of rats) is approximately 10 μM,82 which provides a pool of GSH to form GSH S-conjugates with mercuric ions in plasma. Second, hepatocytes have been shown to generate significant amounts of GSH that end up in systemic circulation.76 As a result of the significant production of GSH in the liver and the fact that the liver accumulates Hg2+, there is the potential for GSH S-conjugates of Hg2+ being formed in hepatocytes and then being transported out into both biliary and sinusoidal compartments. 83–86 Accordingly, it is possible that some GSH S-conjugates of Hg2+ formed in hepatocytes may enter the systemic circulation along with GSH, where they can then be delivered to the kidneys. Third, GSH and the corresponding mercuric conjugates are small enough to pass readily through the glomerular filtration barrier unimpeded.
One must also consider that a significant pool of GSH S-conjugates of Hg is actually formed in the lumen of the proximal tubule via mechanisms involving thiol-competition, which likely results as a consequence of the secretion of significant amounts of GSH into the proximal tubular lumen. Supporting this notion are data demonstrating that approximately 75% of the GSH synthesized de novo in pars recta segments of proximal tubules is subsequently secreted into the tubular lumen.87 This secretion could potentially provide a high enough concentration of GSH in the luminal compartment of the proximal tubule for thiol-competition to occur.
Another possibility is that GSH S-conjugates of Hg that are formed within proximal tubular cells are secreted into the tubular lumen by export transporters in the luminal plasma membrane. Once in the lumen, the GSH S-conjugates of Hg can then be degraded readily by the tremendous abundance of γ-GT in the luminal plasma membrane. There are in vivo data from mice that tend to support this hypothesis.77 Localization of the multidrug resistance-associated protein, MRP2, in the kidneys also tends to support the possibility of luminal secretion of GSH S-conjugates of both inorganic and organic mercuric ions. MRP2 has been localized in the brush-border membrane of the epithelial cells lining S1, S2, and S3 proximal tubular segments of the rat and in the luminal plasma membrane of human proximal tubular epithelial cells.88,89 This membrane transporter is a member of the ATP-binding cassette (ABC) superfamily of transport proteins and has been shown to be involved in the intracellular to extracellular transport of GSH S-conjugates at the canalicular membrane of hepatocytes.90 On the basis of what is currently known about the cellular location and function of MRP2, it seems reasonable to hypothesize that intracellular GSH S-conjugates of Hg2+ are also transported (in a secretory manner) by this protein in both proximal tubular epithelial cells and hepatocytes.
Cleavage Products of GSH S-conjugates of Mercuric ions as Transportable Molecules
Considering that luminal uptake of mercuric ions by proximal tubular cells is linked to the activity of γ-GT and that GSH S-conjugates of Hg2+ are present in the tubular lumen, the preponderance of the luminal uptake of mercuric ions appears to involve the transport of some product formed by the actions of γ-GT. One such product is a mercuric conjugate of cysteinylglycine (CysGly), which may be transported by one of the small peptide transport systems in the luminal plasma membrane. However, because of the high level of activity of dehydropeptidases (such as cysteinylglycinase) in the luminal membrane, one would predict that if there is transport of this mercuric conjugate along the proximal tubule in vivo, the rate of transport would be very low. On the basis of the high activities of both γ-GT and cysteinylglycinase, it is most likely that the primary species of Hg2+ transported at the luminal membrane is an L-Cys S-conjugate of Hg2+, via one or more amino acid transport systems. In fact, in vitro evidence indicating that sequential enzymatic degradation of GSH to CysGly, and then to L-Cys, is possible while a mercuric ion remains bound to the sulfur atom of the cysteinyl residue (at the site of the –SH group) of GSH.91
Role of Cysteinylglycinase in the Luminal Uptake of Mercuric Species
Cannon et al. have examined the potential for luminal transport of CysGly S-conjugates of Hg2+ in isolated perfused S2 segments of the rabbit proximal tubule.57,81 They demonstrated that inhibition of cysteinylglycinase with the dehydropeptidase-1 inhibitor, Cilastatin, caused significant reductions in the luminal uptake of Hg2+ when it was in the form of a CysGly S-conjugate. These findings support the hypothesis that when Hg2+ is conjugated to CysGly, much of the luminal absorption of Hg is linked to the actions of the dehydropeptidase-1 (i.e., cysteinylglycinase) that cleaves the peptide bond in molecules of CysGly to yield Cys and L-glycine (Gly). Interestingly, however, inhibition of luminal dehydropeptidases did not completely prevent the luminal uptake of Hg when it was in the form of a CysGly S-conjugate of Hg2+. These findings indicate that, at least in isolated perfused proximal tubular segments, some transport of mercuric conjugates of CysGly may actually occur at the luminal membrane, while luminal dehydropeptidases are inhibited. However, before one can make any definitive conclusions about potential transport of mercuric conjugates of CysGly along the three sections of proximal tubule in vivo, one must consider additional factors, such as potential heterogeneity in the handling of GSH and CysGly S-conjugates of Hg2+ along the entire proximal tubule. In fact, there are findings indicating significant axial heterogeneity in the synthesis, secretion, and/or transport of GSH occurring along the length of the rabbit proximal tubule.87,92
Cys S-Conjugates of Mercury as Primary Transportable Substrates
Evidence from both in vivo and in vitro studies provides strong support for the hypothesis that Cys S-conjugates of Hg2+ (in particular, Cys-S-Hg-S-Cys) are one of the principal chemical species of Hg2+ transported into proximal tubular epithelial cells at the luminal membrane. More specifically, in vivo data from rats demonstrate that the renal uptake and subsequent accumulation of Hg2+34,61 as well as the level of renal tubular injury and necrosis induced by Hg2+ are increased in rats when Hg2+ is administered intravenously as Cys-S-Hg-S-Cys.93 In vitro data from renal brush-border membranes demonstrate that mercuric ions gain entry into the intravesicular compartment more readily when they are in the form of Cys S-conjugates than when they are in the form of GSH S-conjugates or even mercuric complexes of chloride, including HgCl2. 94
By far, one of the more convincing bodies of evidence implicating Cys-S-Hg-S-Cys as a transportable substrate at the luminal plasma membrane of proximal tubular cells comes from studies utilizing the isolated perfused tubule technique.57,81 Some of the advantages of this technique are that isolated segments of the rabbit proximal tubule are perfused through the lumen in vitro under physiological and biophysical conditions similar to those found in vivo in intact kidneys. The findings from these isolated perfused tubule studies demonstrate that the rates of luminal uptake (disappearance flux, JD) of mercuric ions in S2 segments of the rabbit proximal tubule are at least 2-fold greater when the segments are perfused through the luminal compartment as Cys S-conjugates than as GSH S-conjugates or CysGly S-conjugates. Additionally, the findings from these studies strongly indicate that Cys-S-Hg-S-Cys is taken up from the luminal compartment of proximal tubular segments by one or more amino acid transporters present in the luminal plasma membrane.57,81 Transport studies performed in the presence and absence of sodium (in both luminal and basolateral compartments) provide additional evidence that the luminal uptake of Cys-S-Hg-S-Cys involves at least two separate amino acid transport systems, one (or more) dependent on the presence of extracellular sodium and the other not dependent on the presence of extracellular sodium.
Another set of experiments with isolated perfused S2 segments of rabbit proximal tubules indicate that the luminal uptake of Cys-S-Hg-S-Cys may involve one or more of the same transport systems utilized in the luminal uptake the disulfide amino acid cystine (Cys-S–S-Cys).57 These experiments demonstrated that addition of 3 mML-lysine (Lys) to a perfusate containing 20 μM Hg2+ and 80 μM Cys reduced the net rate of luminal uptake of Hg2+ by approximately 50% in the same tubular segments. Interestingly, Schafer and Watkins had established previously that the presence of 3 mM L-lysine (3 mM) in the perfusate inhibits the luminal uptake of cystine (300 μM) by approximately 50% in isolated perfused S2 segments of the rabbit proximal tubule.95 Their findings provide evidence suggesting that the luminal absorption of cystine occurs through a transporter shared by the basic amino acid L-Lys. Taken together, the findings from these studies suggest that at least some fraction of the luminal uptake of cystine and Cys-S-Hg-S-Cys occurs by a common transport system.
In a more recent study, Zalups and colleagues tested the hypothesis that the heterodimeric amino acid transporter, system b0,+, is capable of individually transporting cystine and Cys-S-Hg-S-Cys.55 The rationale for this hypothesis comes in part from the fact that in the kidneys, system b0,+ is present exclusively in the luminal plasma membrane of proximal tubular cells and that this transport system plays an important role in the sodium-independent luminal absorption of cystine and certain positively charged amino acids (such as L-lysine and L-arginine (Arg)). Using Madin–Darby canine kidney (MDCK) cells (which are derived from distal nephron segments and do not express system b0,+) transfected stably with both rBAT and b0,+AT (the heavy chain and light chain components, respectively, of system b0,+), these investigators demonstrated that MDCK cells expressing a functional form of system b0,+ are capable of transporting not only cystine (and the positively charged amino acids, L-Arg and L-Lys) but also the mercuric complex, Cys-S-Hg-S-Cys.
In a subsequent study utilizing the same transfected MDCK cells, Bridges and Zalups demonstrated that the Hcy S-conjugate of the Hg2+, Hcy-S-Hg-S-Hcy, can also be transported by system b0,+ in a time- and concentration-dependent manner.56 In addition, they demonstrated that the transfected MDCK cells were more susceptible to the toxic effects of Hg2+ than nontransfected cells when the cells were exposed to Hcy-S-Hg-S-Hcy in a concentration-dependent manner. Thus, these two studies provide the most current line of molecular evidence indicating that system b0,+ plays a role in the luminal absorption of both Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy.
Transport systems, other than system b0,+, may also participate in the luminal absorption of thiol S-conjugates of Hg2+ in proximal tubular cells. However, they have not yet been identified.
Potential Role of Molecular Mimicry
On the basis of common features between the structures of cystine and Cys-S-Hg-S-Cys, Zalups and colleagues have hypothesized that some component of the absorptive luminal transport of Cys-S-Hg-S-Cys occurs by a mechanism involving molecular mimicry.20 They postulate that Cys-S-Hg-S-Cys can act as a functional mimic of the amino acid cystine at the site of one or more transporter(s) responsible for the luminal uptake of cystine. Similarly, these investigators postulated that Hcy-S-Hg-S-Hcy may have characteristics similar to the disulfide homocystine (Hcy-S–S-Hcy), which also permits the mercuric conjugate, Hcy-S-Hg-S-Hcy, to be transported into proximal tubular epithelial cells by system b0,+.
Molecular mimicry is not a novel notion. Clarkson has discussed the nature by which ionic forms of Hg and some other metals can form complexes with certain biological molecules that can serve, either in part or in entirety, as molecular mimics of endogenous molecules at critical recognition sites of transporters of these endogenous molecules.23 For example, the complex formed between methylmercury and Cys has been postulated to serve as a mimic of the amino acid L-methionine (Met), as a means to gain entry into the central nervous system via specific amino acid transporters.23,96 This hypothesis is supported, in part, by findings from studies on the extracellular-to-intracellular transport of the S-conjugate methylmercury (Cys-S-CH3Hg) in astrocytes97 and endothelial cells lining the blood–brain barrier.98,99 Clarkson postulates that additional molecular mimics may form when GSH binds to Hg2+ or CH3Hg+. In particular, it was postulated that the complex formed when two molecules of GSH bind to Hg2+, forming the complex GSH-S-Hg-S-GSH, may behave as a functional molecular mimic of glutathione disulfide (GS-SG).23
In recent years, the idea of molecular mimicry has become somewhat controversial. Studies using mass spectrometry, X-ray absorption spectroscopy, and computational chemistry suggested that the structures of Cys-S-CH3Hg and Cys-S-Hg-S-Cys did not possess enough similarity with the amino acids, Met and Cys, respectively, to support a mechanism of molecular mimicry.100 The authors of this study reject the idea that amino acid conjugates of mercuric ions can serve, in their entirety, as mimics of endogenous substrates. Rather, they propose that the ability of mercuric conjugates to be taken up by amino acid transporters may be due to transporter recognition of the amino acid component of the conjugate.
BASOLATERAL UPTAKE OF MERCURY BY PROXIMAL TUBULAR CELLS
Role of Organic Anion Transporters
In addition to luminal mechanisms participating in the uptake of mercuric compounds, certain mercuric species are also taken up at the basolateral membrane of proximal tubular epithelial cells. Experimental findings from rats indicate that approximately 40% of the dose of Hg2+ is present in the total renal mass of rats during the initial hour after intravenous administration of a nontoxic dose of mercuric chloride.64,101–103 Moreover, approximately 40–60% of the renal burden of Hg can be attributed to a basolateral transport mechanism.59,62,64,70,104
Zalups and Minor were the first to demonstrate that renal tubular uptake of administered Hg2+ occurs when glomerular filtration is reduced to negligible levels in one or both kidneys, by using the well established stop-flow technique.62 Pretreatment with mannitol in combination with ureteral ligation causes an approximate 40% decrease in the net renal uptake and accumulation of Hg2+ during the initial hour after administration of a non-nephrotoxic dose of mercuric chloride. In vivo pretreatment with p-aminohippurate (PAH), which is a competitive substrate specific to members of the renal organic anion transporter family,105–112 also significantly reduces the acute renal tubular uptake and accumulation of Hg2+.62,104 In fact, the combination of ureteral ligation and pretreatment with PAH has been shown to cause an approximate 85% reduction in the net renal tubular uptake and accumulation of Hg2+ during the first hour after exposure to mercuric chloride. Overall, these findings indicate that the majority of the basolateral uptake of Hg2+ can be inhibited by PAH, which implicates one or more of the organic anion transporter proteins as a primary mechanism in the basolateral uptake of Hg2+. Data from other recent studies have confirmed that basolateral uptake of inorganic Hg occurs in the kidney and that the primary mechanism involved is linked to the activity of one or more members of the organic anion transport system.59,62,64,101,102,104,113
Organic anion transporters have also been implicated in the basolateral uptake of organic mercuric compounds. It has been demonstrated that renal uptake and/or accumulation58 and toxicity114 of administered methylmercury are reduced significantly in mice pretreated with probenecid, which is another competitive inhibitor of organic anion transporters in renal proximal tubules.110
Two main organic anion transport proteins are present in the basolateral membrane of proximal tubular cells, namely, the organic anion transporter 1 (OAT1) and the other isoform, the organic anion transporter 3 (OAT3).115–119 The predominant organic anion transporter in the basolateral membrane appears to be OAT1, although PAH (which is thought of as the classical competitive inhibitor of organic anion transport in proximal tubular cells) can inhibit the transport-activity of both OAT1 and OAT3.
In a series of recent studies, Zalups and colleagues examined the kinetics and substrate specificity of thiol S-conjugates of Hg2+ and CH3Hg+ in MDCK cells transfected stably to express OAT1 and in oocytes from Xenopus laevis expressing OAT3.65–69,120 Using these cellular models, they demonstrated that Cys and Hcy S-conjugates of Hg2+ and Cys, and Hcy and NAC S-conjugates of CH3Hg+ are all transportable substrates of OAT1. Interestingly, findings from another recent study indicate that the NAC S-conjugate of CH3Hg+ is not transported efficiently in oocytes from Xenopus laevis microinjected with the cDNA for OAT3, indicating that this methylmercuric species is not a high affinity substrate of OAT3.121 Overall, evidence to date indicates that OAT1 has a fairly broad range of substrate-specificity for biologically relevant mercuric species. Moreover, the findings from the aforementioned experiments clearly support a role for OAT1 (and perhaps OAT3) in the basolateral uptake of mercuric species from peritubular blood.
Role of the Basolateral Dicarboxylate Transporter NaC3
In the 1960s, Clarkson and Magos122 demonstrated that when animals are pretreated with the dicarboxylate maleate, reductions occur in the net renal accumulation of administered Hg2+. However, it is not clear whether the changes in the renal disposition of Hg2+ are due to the inhibitory effects of maleate on renal cellular metabolism123 or whether they are due to direct effects at the site of a transporter of one or more S-conjugates. These investigators also found that fumarate, which is an isomer of maleate, did not have the same effects as maleate, indicating isomer-specific effects on the renal disposition of Hg2+.
In the late 1990s, Zalups and Barfuss102 demonstrated that pretreatment with small aliphatic dicarboxylates (made up of 4–6 carbon atoms) such as succinate, glutarate, or adipate (but not malonate, which contains only three carbon atoms), inhibits the renal (basolateral) uptake of intravenously administered Hg2+ (as mercuric chloride) in a dose-dependent manner in normal rats and in rats with ligated ureters. The inhibitory effects of the dicarboxylates on the renal tubular uptake, transport, and accumulation of Hg2+ likely involves the synergistic activity of both the sodium-dependent dicarboxylate transporter NaC3 and the OAT-transporters OAT1 and/or OAT3, which are all present in the basolateral membrane of proximal tubular epithelial cells.
Organic anion transporters in the basolateral membrane function as organic anion/dicarboxylate exchangers.106,109 Numerous data indicate that intracellular generation of α-ketoglutarate contributes to the creation of an intracellular chemical gradient favoring the movement of this dicarboxylate out of the cell. α-Ketoglutarate moves down a concentration gradient out of proximal tubular cells at the basolateral membrane by exchanging with extracellular organic anions (including some dicarboxylates) at the site of OAT1 and OAT3.124 Interestingly, a significant fraction of the α-ketoglutarate (and other dicarboxylates) that exits proximal tubular cells at one or both of the organic anion exchangers is taken back up into the cells at the basolateral membrane via NaC3.125 The activity of this dicarboxylate cotransport system appears to derive electromotive energy from the sodium-gradient generated by the activity of (Na++K+)-stimulated ATPase, which is also present in the basolateral membrane.
Although mechanisms by which succinate, glutarate, and adipate inhibit the renal tubular uptake of inorganic Hg2+ have not been defined fully, it seems likely that an excess of any of these dicarboxylates in the extracellular compartment creates competition for the sodium-dependent entry of α-ketoglutarate at the site of the NaC3. Thus, reduction in the basolateral uptake of α-ketoglutarate likely causes a decrease in the intracellular concentration of this dicarboxylate. As a consequence, there would be transient decreases in the chemical gradient favoring the movement of α-ketoglutarate out of proximal tubular cells in exchange for the uptake of a host of organic anions from the peritubular plasma. Consequently, by changing the activity of NaC3, there would be corresponding changes in the kinetic activity of the organic anion transport system. This would result in a decreased rate of uptake of organic anions and transportable mercuric species by OAT1 and OAT3.
Since a number of small dicarboxylates are themselves organic anions, an excess of any of these dicarboxylates in the extracellular compartment would likely lead to competition with whatever species of Hg that is putatively transported by the organic anion transport system. This could explain the decreased rate of uptake of Hg at the basolateral membrane detected in rats pretreated with small dicarboxylates. There is evidence that both adipate and glutarate but not succinate or malonate can compete directly with α-ketoglutarate at the site of the organic anion transport system.108,109,111,112
ELIMINATION OF MERCURIC SPECIES BY PROXIMAL TUBULAR CELLS
Once mercuric ions gain access to the intracellular compartment of proximal tubular epithelial cells, they tend to be retained in the cells by complex bonding interactions with protein and nonprotein thiols.2 Despite the apparent intracellular retention of mercuric ions, there appears to be some degree of luminal secretion of certain mercuric species,77,126–128 adding to the pool of Hg excreted in the urine.129 This conclusion is derived from recent developments by Bridges and colleagues, who have demonstrated in vivo that low-level, luminal secretion of Hg2+ occurs in proximal tubular cells via the action of the multidrug resistance-associated protein 2 (MRP2).128 The presence of MRP2 in the luminal plasma membrane of proximal tubular epithelial cells has been demonstrated previously.88,89 Bridges and colleagues have demonstrated that without the presence of functional MRP2 protein in the luminal plasma membrane, movement of mercuric species from within proximal tubular cells to the luminal compartment of the nephron is greatly diminished. These data come from in vivo metabolic studies using MRP2-deficient (TR−) and normal (Wistar) rats treated acutely with a non-nephrotoxic dose of HgCl2.
In addition to MRP2, the luminal plasma membrane of proximal tubular cells also contains the multidrug resistance protein 4 (MRP4), another member of the ABC protein family. However, preliminary unpublished findings of Bridges and colleagues tend to indicate that MRP4 does not participate in the luminal secretion of various mercuric species.
HANDLING OF DMPS AND DMSA IN THE KIDNEYS
According to documentation from Heyl Chem,28 DMPS (brand name Dimaval) was first synthesized in 1951. While precise information on when DMSA (brand name Chemet) was first synthesized is less clear, it became available for use in the 1960s. Unlike DMPS, DMSA has been approved for use (mainly for reducing the body-burden of lead (Pb)) by the United States Food and Drug Administration (FDA). Although DMPS has not been approved for use in the U.S., it appears to be widely used internationally. Both molecules are composed of a short, straight-chain, aliphatic carbon skeleton. DMPS has a propane skeleton and has two SH groups on carbons 2 and 3 and a sulfonate (SO−) group on carbon 1. By contrast, DMSA is a dicarboxylic acid with succinate serving as the carbon backbone of the molecule. Carbons 2 and 3 have a single SH group bound to them. Although these two vicinal dithiols are essentially two different types of molecules due to the functional groups on the terminal carbon atoms, they are both polar in nature and are water-soluble, allowing them to be administered easily to patients.27 Studies in human indicate that both compounds are capable of reducing the body burden of mercury.130,131 DMPS is the more water-soluble species of the two, and data from experimental studies in rats tend to indicate that DMPS is slightly more effective at extracting mercuric ions from the kidneys and reducing the body-burden of Hg than DMSA.35,126–128,132–134
In well-oxygenated, aqueous solutions containing electrolytes, the SH groups in both DMPS and DMSA can undergo oxidation forming mixed disulfides. One of the more common disulfides formed involves the oxidation of both SH groups of DMPS or DMSA, with the subsequent formation of disulfide bonds between corresponding sulfur atoms of another molecule of DMPS or DMSA, respectively. In addition, previous experimental evidence indicates that DMPS, and likely DMSA, undergo extensive oxidation and form one or more disulfide forms in vivo.135,136
Diamond and colleagues have shown that DMPS can be secreted from peritubular blood into the luminal compartment of renal tubules of the rat by the organic anion transport systems that transport PAH and other alkanesulfonates.137–139 They also showed that DMPS is effective in reducing the renal burden of Hg2+ when administered in either a reduced or oxidized form, indicating that the organic anion transport systems in mammals are likely capable of transporting reduced and/or oxidized forms of DMPS from the blood into renal tubular epithelial cells. More recently, Wright and colleagues provide molecular data from oocytes expressing human OAT1 that add significant support to the findings of Diamond and colleagues implicating one or more organic anion transporters in the basolateral uptake of reduced and/or oxidized forms of DMPS.24 Diamond and colleagues also demonstrated that oxidized forms of DMPS undergo reduction by a biotransformation reaction in proximal tubular epithelial cells by a mechanism that is currently unknown. Once in the reduced state, the vicinal thiols of DMPS can interact with and compete for mercuric ions bound to intracellular thiols.
It is presently unknown if the organic anion transporter proteins play a role in the basolateral uptake of the dicarboxylate chelator, DMSA, in proximal tubular epithelial cells. There are, however, data showing dicarboxylates composed of five and six carbon atoms (such as glutarate (and its homologue α-ketoglutarate) and adipate) are important substrates (of the organic anion transport system) that can be transported bidirectionally.105,106,109 Moreover, it has been shown that these dicarboxylates can significantly affect the basolateral uptake of mercuric species.102,105,106,109
Addition of one or more polar, negatively charged, functional groups on a short-chain carbon skeleton creates a substrate that has the potential to be transported by one or more of the organic anion transporters (OAT1, 3, and 4) and the dicarboxylate transporter, NaC3. OAT1 and 3 are localized exclusively in the basolateral membrane of proximal tubular epithelial cells, while OAT4 is found only in the apical membrane.124 As a result of the activity of OAT1 and 3, DMPS and DMSA can gain easy access to the intracellular environment of renal proximal tubular epithelial cells.
Even though the pharmaceutical intent in the design of DMPS and DMSA was likely to make the compounds water-soluble (more so than compounds available previously), these negatively charged, vicinal dithiols serve as effective transportable substrates of the organic anion transport system in proximal tubular cells. Fortuitously, this enables these compounds to gain access to the intracellular milieu of the primary target cells that accumulate and are affected adversely by mercuric species.
It should also be mentioned that as a result of the negative potential of the intracellular environment established by the activity of the Na+/K+-ATPase, movement of negatively charged, water-soluble molecules like DMPS and DMSA is impeded in most somatic cells, including other epithelial cells, unless they possess some form of organic anion transporter capable of translocating these negatively charged species from the blood.
COMPLEXATION OF DMPS AND DMSA WITH MERCURIC SPECIES IN VIVO
Currently, there is a paucity of information regarding the precise chemical structure of the complexes formed by DMPS or DMSA binding to inorganic or organic mercuric ions in blood and in proximal tubular epithelial cells. On the basis of results from a number of different methods of analysis, it appears that a variety of potential structures may form. Recent X-ray absorption spectroscopic data and density functional theory (DFT) calculations obtained from simple aqueous solutions containing various ratios of DMPS or DMSA and mercuric nitrate (Hg(NO3)2) tend to suggest that a single mercuric ion is not likely to bond to the vicinal thiols on a single molecule of DMPS or DMSA.140 The authors of these findings postulate that at least two DMPS or DMSA molecules must be involved in the binding of one or two inorganic mercuric ion(s). Interestingly, one of the structures postulated to form between a single inorganic mercuric ion and two molecules of either DMPS or DMSA involves tetrahedral binding, rather than linear II coordinate covalent binding. Although these findings are interesting, there may be an alternate species formed in vivo involving the binding of the vicinal thiols of a single molecule of DMPS or DMSA to a single inorganic mercuric ion. The potential species may involve the binding of the vicinal sulfur atoms of a single molecule of either DMPS or DMSA to a single inorganic mercuric ion, while the single sulfur atom of two molecules of Cys, Hcy, and/or GSH bind to the mercuric ion in a tetrahedral manner. Assuming that Cys and Hcy are present in plasma at concentrations of at least 10 μM82 and that the intracellular concentrations of GSH are in low millimolar concentrations,74,76,141–144 some form of thiol competition may promote intracellular formation of a mixed mercuric conjugate. From a purely chemical standpoint, the authors of the spectroscopic data suggest that DMPS and DMSA are not well optimized for chelation of Hg owing to the observation that neither compound forms a true chelate complex with mercury.140,145 A chelate has been defined as a stable ring complex formed by the binding of a metal ion with two or more functional groups of another molecule.140 Therefore, DMPS and DMSA may be best described as complexing agents. Both of these vicinal thiols have proven to be extremely effective in reducing the renal and plasma burden of Hg, which makes the compounds therapeutically effective despite not being optimized chelators.
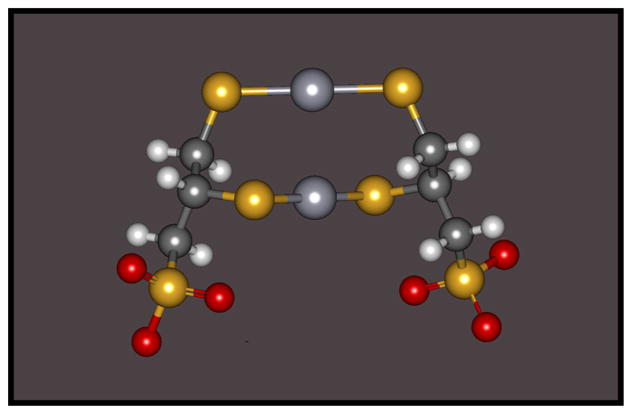
A likely species of a DMPS or DMSA S-conjugate of Hg2+ formed in both blood plasma and the intracellular compartment of proximal tubular epithelial cells that promotes systemic elimination of Hg2+ is a complex formed by the binding of both reduced sulfur atoms of two molecules of either DMSA or DMPS with one or two inorganic mercuric ions. Formation of such a complex in aqueous solution has been demonstrated using X-ray absorption spectroscopic data and density functional theory (DFT) calculations.140 An example of such a complex formed by two molecules of DMPS and two mercuric ions is illustrated in Figure 1.
Figure 1.
A graphical, ball and stick, representation of a mercuric conjugate of 2,3-bis(sulfanyl)propane-1-sulfonate (DMPS) showing linear II coordinate covalent bonding between two molecules of DMPS with two inorganic mercuric ions (Hg2+).
RENAL EXTRACTION OF MERCURIC SPECIES MEDIATED BY DMPS AND DMSA
Numerous sets of data indicate that DMPS and DMSA can efficiently extract mercuric ions from the kidneys of rats, which normally accumulate as much as 50% or more of a single dose of inorganic Hg within the first 24 h after exposure. Two intraperitoneal treatments of DMPS or DMSA, at a dose of 100 mg/kg, have been shown to extract greater than 80% of the renal burden of Hg2+ within 24 h.126,132 Both DMPS and DMSA are slightly less effective in promoting the extraction of mercuric ions from the kidneys of rats exposed to non-nephrotoxic doses of methylmercury.35 Of the approximate 8–10% of the dose present in the kidneys 24 h after exposure to methylmercury, it has been demonstrated that only about 50% of this amount is extracted from the kidneys during the initial 24 h subsequent to treatment with DMPS or DMSA.126,132
Using isolated perfused S2 segments of the rabbit proximal tubule, Zalups and colleagues have provided significant insight for the mechanisms by which DMPS (and likely DMSA) mediate reductions in the renal burden of Hg.146 These investigators demonstrated several important features involved in the renal tubular elimination of Hg2+ mediated by low-molecular-weight, vicinal thiols. One set of their findings from isolated perfused proximal tubular segments indicates that once DMPS S-conjugates of Hg2+ are formed in, or enter into, the extracellular fluid compartment, the conjugates are not transported efficiently into proximal tubular epithelial cells at either the luminal or basolateral plasma membrane. Consequently, this characteristic of the DMPS S-conjugates of Hg2+ allows for both the unimpeded glomerular filtration and the efficient tubular delivery of these conjugates via luminal fluid delivery to the renal pelvis and extra-renal collecting system, thus promoting the urinary excretion of the complexes. These investigators also demonstrated that when proximal tubular (S2) segments were perfused through the lumen with 20 μMGSH-S-Hg-S-GSH complexes for 15 min, rates of disappearance flux (JD) from the lumen averaged between 30 and 50 fmol × min−1 × mm−1 (tubular length). However, when 200 μM DMPS was added to the solution bathing the basolateral surface of the perfused tubules, the JD for Hg2+ changed to approximately −40 to −50 fmol × min−1 × mm−1 within 5 min. This rapid change from a positive to a negative JD indicates that instead of mercuric ions being taken up at the luminal membrane, they were being exported from within proximal tubular cells into the tubular lumen. Substantial reductions in the net tubular content of Hg2+ confirmed the tubular extraction of mercuric ions into the tubular lumen.
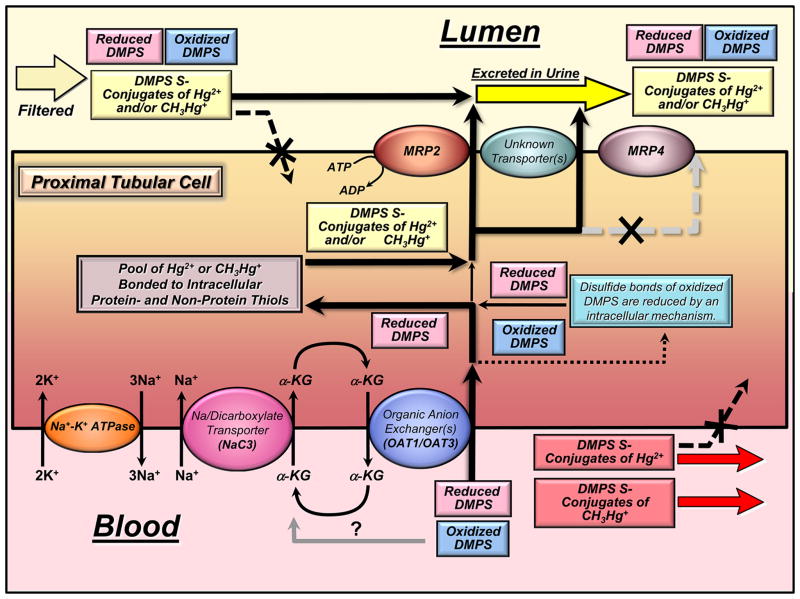
More recent studies from our laboratory demonstrate that at least some extraction of inorganic and organic mercuric ions from within proximal tubular segments into the luminal extracellular compartment within the kidneys appears to be mediated by the MRP2. As mentioned above, this membrane protein is a member of the ATP-binding cassette transporter family, which in the kidneys is located exclusively in the luminal membrane of proximal tubular epithelial cells.89 This transporter has been shown to mediate the transport of a broad range of substrates into the lumen of proximal tubules.147–149 Moreover, recent sets of evidence indicate that MRP2 likely plays a significant role in DMPS- and DMSA-mediated extraction of mercuric ions from proximal tubular cells and contributes to the subsequent urinary excretion of Hg.35,126,127 Figure 2 provides a graphical representation summarizing the known and putative mechanisms participating in the handling of DMPS and DMPS S-conjugates of Hg2+ and CH3Hg+ by proximal tubular cells. Figure 2 shows that in humans and other mammals exposed to inorganic mercuric or methylmercuric forms of mercury and then treated subsequently with DMPS, both DMPS (in reduced and oxidized forms) and DMPS S-conjugates of Hg2+ or CH3Hg+ can be present in the plasma of systemic blood shortly after treatment. During each passage of circulating blood through the kidneys, about 20–25% of the DMPS and the DMPS S-conjugates of Hg2+ or CH3Hg+ in the plasma are filtered efficiently through the glomerular filtration barrier into the luminal compartment of proximal tubules. The remaining fraction of these compounds in plasma (approximately 75–80%) is delivered first to the basolateral compartment of proximal tubules via peritubular capillary blood flow. In the luminal compartment of the proximal tubule and the remainder of the nephron, oxidized DMPS, reduced DMPS, or DMPS S-conjugates of Hg2+ or CH3Hg+ are not absorbed efficiently because of the polar negative charge associated with the sulfonate group of DMPS, thus promoting the urinary excretion of DMPS and mercuric complexes of DMPS. However, at the basolateral plasma membrane of proximal tubular cells, both oxidized and reduced forms of DMPS (not bonded to mercuric ions) are taken up efficiently into the proximal tubular epithelial cells by one or both of the organic anion transporters present in the basolateral membrane, namely, the organic transporter 1 (OAT1) and (OAT3).
Figure 2.
Graphical summary of the mechanisms by which the vicinal, dithiol, metal-complexing agent 2,3-bis(sulfanyl)propane-1-sulfonate (DMPS) reduces the renal proximal tubular content of inorganic mercuric (Hg2+) and methylmercuric (CH3Hg+) ions.
It should be mentioned that DMPS is taken up from the extracellular basolateral compartment by OAT1 and/or OAT3 by an outwardly driven intracellular to extracellular gradient for α-ketoglutarate, which is generated by intracellular metabolism and by transport back into the proximal tubular cells. One of the key transporters linked to the activity of OAT1 and OAT3 and the extracellular to intracellular transport of α-ketoglutarate is the Na+-dicarboxylate cotransporter, NaC3, which is also located in the basolateral membrane. The electromotive force utilized by this membrane transporter is derived from the actions of the sodium (Na+)–potassium (K+) ATPase in the basolateral membrane, which pumps Na+ out of the proximal tubular cells in an electrogenic manner, generating an extracellular to intracellular gradient of 140 mM to 10 mM, which favors the movement of Na+ back into the cells.
Interestingly, evidence from isolated perfused segments of the proximal tubule indicates that OAT1 and/or OAT3 are not involved in the basolateral uptake of DMPS S-conjugates of Hg2+. In fact, it appears that DMPS S-conjugates of Hg2+ are not taken up efficiently, or at all, from peritubular blood into proximal tubular epithelial cells.
Experimental evidence does indicate that the therapeutic actions of DMPS for extracting mercuric species from within proximal tubular cells involve the following steps: (1) as mentioned above, DMPS (in a reduced and/or oxidized state) is taken up avidly at the basolateral membrane by OAT1 and/or OAT3. (2) Oxidized forms of DMPS taken up at the basolateral membrane are reduced by an intracellular mechanism not yet known. (3) Reduced forms of DMPS within the proximal tubular cells interact with, compete for, and then remove mercuric ions bound to a host of potential intracellular thiol-containing molecules, including large intracellular proteins, metallothioneins (MT), glutathione (GSH), cysteine (Cys), and others. (4) After one or two mercuric ions bind to DMPS and a sufficient intracellular concentration of DMPS S-conjugates of Hg2+ or CH3Hg+ has formed to generate a sufficient gradient favoring the outward movement of these complexes, the conjugates are transported out of the cells into the tubular lumen by the multidrug resistance-associated protein 2 (MRP2) and possibly by another currently unknown transporter. Preliminary unpublished data indicate that MRP4 does not likely participate in the export of mercuric conjugates of DMPS. (5) Finally, the DMPS S-conjugates of Hg2+ or CH3Hg+ conjugates are excreted into the urine because they do not appear to be transportable substrates that can be taken up by any segment of the nephron or collecting duct beyond the proximal tubule.
CONCLUSIONS
Although a great deal of new information regarding mechanisms by which specific epithelial cells, particularly the proximal tubular epithelial cells in the kidneys handle various species of mercury, a great deal of new information is needed to gain a more complete understanding of how mercuric species are handled in both epithelial and nonepithelial target cells. Moreover, design of new more effective, nontoxic, chelating or complexing agents is needed to gain access to and extract mercuric ions from within target cells adversely affected by the various forms of mercury. Perhaps one of the more challenging tasks at hand is to design chemical compounds that can not only extract mercuric species from the kidney but also find a means to gain access through the blood–brain barrier to bind mercuric ions in neurons of the CNS. The compounds not only need to gain access to the target neurons but also need to be designed to utilize one or more neuronal membrane transporters to extract mercuric ions in a manner that can permit the formed mercuric complexes to gain access to systemic circulation, where they may be excreted by the kidneys and/or liver.
Acknowledgments
Funding
Some of the contents of this review were supported, in part, by the following grants awarded by the National Institute of Environmental Health (NIEHS): ES05980 (to R.K.Z.), ES019991 (to C.C.B.), and ES015511 (to C.C.B.).
ABBREVIATIONS
- ABC
ATP-binding cassette
- DMSA
meso-2,3-bis(sulfanyl)-succinate
- DMPS
2,3-bis(sulfanyl)propane-1-sulfonate
- DFT
density functional theory
- CH3Hg+
methylmercury or methylmercuric ion
- Hg
mercury
- Hg0
elemental mercury or metallic mercury
- HgCl2
mercuric chloride
- CysGly
cysteinylglycine
- FDA
United States Food and Drug Administration
- JD
disappearance flux
- DL-Hcy
homocysteine
- γ-GT
γ-glutamyltransferase
- GSH
glutathione
- Hg+
mercurous ion
- Hg2+
mercuric ion or Hg
- Hg(NO3)2
mercuric nitrate
- IUPAC
International Union of Pure and Applied Chemistry
- NAC
N-acetylcysteine
- MRP2
multidrug resistance-associated protein 2
- MRP4
multidrug resistance-associated protein 4
- NaC3
sodium-dependent dicarboxylate transporter 3
- OAT1
organic anion transporter 1
- OAT3
organic anion transporter 3
- OAT4
organic anion transporter 4
- SH
sulfhydryl or thiol group
- SO−
sulfonate
- PAH
p-aminohippurate
- TR−
MRP2-deficient
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Zalups RK. Evidence for basolateral uptake of cadmium in the kidneys of rats. Toxicol Appl Pharmacol. 2000;164:15–23. doi: 10.1006/taap.1999.8854. [DOI] [PubMed] [Google Scholar]
- 2.Zalups RK. Molecular interactions with mercury in the kidney. Pharmacol Rev. 2000;52:113–143. [PubMed] [Google Scholar]
- 3.ATSDR. Toxicological Profile for Mercury. United States Public Health Service/Agency for Toxic Substance Registry; Atlanta, GA: 1999. pp. 1–617. [Google Scholar]
- 4.Clarkson TW. Mercury: major issues in environmental health. Environ Health Perspect. 1993;100:31–38. doi: 10.1289/ehp.9310031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danscher G, Horsted-Bindslev P, Rungby J. Traces of mercury in organs from primates with amalgam fillings. Exp Mol Pathol. 1990;52:291–299. doi: 10.1016/0014-4800(90)90070-t. [DOI] [PubMed] [Google Scholar]
- 6.George GN, Singh SP, Hoover J, Pickering IJ. The chemical forms of mercury in aged and fresh dental amalgam surfaces. Chem Res Toxicol. 2009;22:1761–1764. doi: 10.1021/tx900309c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahn LJ, Kloiber R, Leininger RW, Vimy MJ, Lorscheider FL. Whole-body imaging of the distribution of mercury released from dental fillings into monkey tissues. FASEB J. 1990;4:3256–3260. doi: 10.1096/fasebj.4.14.2227216. [DOI] [PubMed] [Google Scholar]
- 8.Hahn LJ, Kloiber R, Vimy MJ, Takahashi Y, Lorscheider FL. Dental “silver” tooth fillings: a source of mercury exposure revealed by whole-body image scan and tissue analysis. FASEB J. 1989;3:2641–2646. doi: 10.1096/fasebj.3.14.2636872. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald WF, Clarkson TW. Mercury and monomethylmercury: present and future concerns. Environ Health Perspect. 1991;96:159–166. doi: 10.1289/ehp.9196159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarkson TW. The biological properties and distribution of mercury. Biochem J. 1972;130:61P–63P. doi: 10.1042/bj1300061pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris HH, Pickering IJ, George GN. The chemical form of mercury in fish. Science. 2003;301:1203. doi: 10.1126/science.1085941. [DOI] [PubMed] [Google Scholar]
- 12.Fuhr BJ, Rabenstein DL. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. IX The binding of cadmium, zinc, lead, and mercury by glutathione. J Am Chem Soc. 1973;95:6944–6950. doi: 10.1021/ja00802a013. [DOI] [PubMed] [Google Scholar]
- 13.Rabenstein DL, editor. Metal Complexes of Glutathione and Their Biological Significance. Vol. 3. Wiley Press; New York: 1989. [Google Scholar]
- 14.Cember H, Gallagher P, Faulkner A. Distribution of mercury among blood fractions and serum proteins. Am Ind Hyg Assoc J. 1968;29:233–237. doi: 10.1080/00028896809342994. [DOI] [PubMed] [Google Scholar]
- 15.Friedman HL. Relationship between chemical structure and biological activity in mercurial compounds. Ann NY Acad Sci. 1957;65:461–470. doi: 10.1111/j.1749-6632.1956.tb36651.x. [DOI] [PubMed] [Google Scholar]
- 16.Lau S, Sarkar B. Inorganic mercury(II)-binding components in normal human blood serum. J Toxicol Environ Health. 1979;5:907–916. doi: 10.1080/15287397909529800. [DOI] [PubMed] [Google Scholar]
- 17.Mussini E. Bonds of mercurial diuretics to blood proteins. Boll Soc Ital Biol Sper. 1958;34:1588–1590. [PubMed] [Google Scholar]
- 18.Zalups RK. Early aspects of the intrarenal distribution of mercury after the intravenous administration of mercuric chloride. Toxicology. 1993;79:215–228. doi: 10.1016/0300-483x(93)90213-c. [DOI] [PubMed] [Google Scholar]
- 19.Aschner M, Aschner JL. Mercury neurotoxicity: mechanisms of blood-brain barrier transport. Neurosci Biobehav Rev. 1990;14:169–176. doi: 10.1016/s0149-7634(05)80217-9. [DOI] [PubMed] [Google Scholar]
- 20.Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol. 2005;204:274–308. doi: 10.1016/j.taap.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bridges CC, Zalups RK. Transport of inorganic mercury and methylmercury in target tissues and organs. J Toxicol Environ Health, Part B. 2010;13:385–410. doi: 10.1080/10937401003673750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 23.Clarkson TW. Molecular and ionic mimicry of toxic metals. Annu Rev Pharmacol Toxicol. 1993;33:545–571. doi: 10.1146/annurev.pa.33.040193.002553. [DOI] [PubMed] [Google Scholar]
- 24.Islinger F, Gekle M, Wright SH. Interaction of 2,3-dimercapto-1-propane sulfonate with the human organic anion transporter hOAT1. J Pharmacol Exp Ther. 2001;299:741–747. [PubMed] [Google Scholar]
- 25.Rodiger M, Zhang X, Ugele B, Gersdorff N, Wright SH, Burckhardt G, Bahn A. Organic anion transporter 3 (OAT3) and renal transport of the metal chelator 2,3-dimercapto-1-propanesulfonic acid (DMPS) Can J Physiol Pharmacol. 2010;88:141–146. doi: 10.1139/Y09-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lungkaphin A, Chatsudthipong V, Evans KK, Groves CE, Wright SH, Dantzler WH. Interaction of the metal chelator DMPS with OAT1 and OAT3 in intact isolated rabbit renal proximal tubules. Am J Physiol Renal Physiol. 2004;286:F68–76. doi: 10.1152/ajprenal.00075.2003. [DOI] [PubMed] [Google Scholar]
- 27.Aposhian HV. DMSA and DMPS: water soluble antidotes for heavy metal poisoning. Annu Rev Pharmacol Toxicol. 1983;23:193–215. doi: 10.1146/annurev.pa.23.040183.001205. [DOI] [PubMed] [Google Scholar]
- 28.Ruprecht J. Dimaval. Heyl Chem-Pharm Fabrik G; Berlin: 2008. [Google Scholar]
- 29.Chang IJ, Fischbach BV, Sile S, Golper TA. Extracorporeal Treatment of Poisoning. In: Brenner BM, editor. The Kidney. Saunders; Philadelphia, PA: 2004. pp. 2733–2757. [Google Scholar]
- 30.Clarkson TW, Vyas JB, Ballatori N. Mechanisms of mercury disposition in the body. Am J Ind Med. 2007;50:757–764. doi: 10.1002/ajim.20476. [DOI] [PubMed] [Google Scholar]
- 31.Zalups RK, Bridges CC. Molecular and Cellular Biology of Mercury in the Kidneys. In: Zalups RK, Koropatnick J, editors. Cellular and Molecular Biology of Metals. Taylor and Francis; Boca Raton, FL: 2010. pp. 35–77. [Google Scholar]
- 32.Farina M, Rocha JB, Aschner M. Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sci. 2011;89:555–563. doi: 10.1016/j.lfs.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zalups RK, Barfuss DW. Intrarenal distribution of inorganic mercury and albumin after coadministration. J Toxicol Environ Health. 1993;40:77–103. doi: 10.1080/15287399309531777. [DOI] [PubMed] [Google Scholar]
- 34.Zalups RK, Barfuss DW. Renal disposition of mercury in rats after intravenous injection of inorganic mercury and cysteine. J Toxicol Environ Health. 1995;44:401–413. doi: 10.1080/15287399509531969. [DOI] [PubMed] [Google Scholar]
- 35.Zalups RK, Bridges CC. MRP2 involvement in renal proximal tubular elimination of methylmercury mediated by DMPS or DMSA. Toxicol Appl Pharmacol. 2009;235:10–17. doi: 10.1016/j.taap.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Hultman P, Enestrom S. Localization of mercury in the kidney during experimental acute tubular necrosis studied by the cytochemical Silver Amplification method. Br J Exp Pathol. 1986;67:493–503. [PMC free article] [PubMed] [Google Scholar]
- 37.Hultman P, Enestrom S. Dose-response studies in murine mercury-induced autoimmunity and immune-complex disease. Toxicol Appl Pharmacol. 1992;113:199–208. doi: 10.1016/0041-008x(92)90115-9. [DOI] [PubMed] [Google Scholar]
- 38.Hultman P, Enestrom S, von Schenck H. Renal handling of inorganic mercury in mice. The early excretion phase following a single intravenous injection of mercuric chloride studied by the Silver Amplification method. Virchows Arch, B. 1985;49:209–224. [PubMed] [Google Scholar]
- 39.Magos L, Brown AW, Sparrow S, Bailey E, Snowden RT, Skipp WR. The comparative toxicology of ethyl- and methylmercury. Arch Toxicol. 1985;57:260–267. doi: 10.1007/BF00324789. [DOI] [PubMed] [Google Scholar]
- 40.Rodier PM, Kates B. Histological localization of methylmercury in mouse brain and kidney by emulsion autoradiography of 203Hg. Toxicol Appl Pharmacol. 1988;92:224–234. doi: 10.1016/0041-008x(88)90382-1. [DOI] [PubMed] [Google Scholar]
- 41.Rodier PM, Kates B, Simons R. Mercury localization in mouse kidney over time: autoradiography versus silver staining. Toxicol Appl Pharmacol. 1988;92:235–245. doi: 10.1016/0041-008x(88)90383-3. [DOI] [PubMed] [Google Scholar]
- 42.Taugner R, Winkel Kz, Iravani J. On the localization of mercuric chloride concentration in the rat kidney. Virchows Arch Pathol Anat Physiol Klin Med. 1966;340:369–383. [PubMed] [Google Scholar]
- 43.Zalups RK. Autometallographic localization of inorganic mercury in the kidneys of rats: effect of unilateral nephrectomy and compensatory renal growth. Exp Mol Pathol. 1991;54:10–21. doi: 10.1016/0014-4800(91)90039-z. [DOI] [PubMed] [Google Scholar]
- 44.Zalups RK. Method for studying the in vivo accumulation of inorganic mercury in segments of the nephron in the kidneys of rats treated with mercuric chloride. J Pharmacol Methods. 1991;26:89–104. doi: 10.1016/0160-5402(91)90058-d. [DOI] [PubMed] [Google Scholar]
- 45.Zalups RK, Barfuss DW. Accumulation of inorganic mercury along the renal proximal tubule of the rabbit. Toxicol Appl Pharmacol. 1990;106:245–253. doi: 10.1016/0041-008x(90)90244-o. [DOI] [PubMed] [Google Scholar]
- 46.Gage JC. Distribution and excretion of methyl and phenyl mercury salts. Br J Ind Med. 1964;21:197–202. doi: 10.1136/oem.21.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norseth T, Clarkson TW. Studies on the biotransformation of 203Hg-labeled methyl mercury chloride in rats. Arch Environ Health. 1970;21:717–727. doi: 10.1080/00039896.1970.10667325. [DOI] [PubMed] [Google Scholar]
- 48.Norseth T, Clarkson TW. Biotransformation of methylmercury salts in the rat studied by specific determination of inorganic mercury. Biochem Pharmacol. 1970;19:2775–2783. doi: 10.1016/0006-2952(70)90104-8. [DOI] [PubMed] [Google Scholar]
- 49.Dunn JD, Clarkson TW. Does mercury exhalation signal demethylation of methylmercury? Health Phys. 1980;38:411–414. [PubMed] [Google Scholar]
- 50.Madsen KM. Mercury accumulation in kidney lysosomes or proteinuric rats. Kidney Int. 1980;18:445–453. doi: 10.1038/ki.1980.157. [DOI] [PubMed] [Google Scholar]
- 51.Madsen KM, Hansen JC. Subcellular distribution of mercury in the rat kidney cortex after exposure to mercuric chloride. Toxicol Appl Pharmacol. 1980;54:443–453. doi: 10.1016/0041-008x(80)90171-4. [DOI] [PubMed] [Google Scholar]
- 52.Brown DL, Shockley P. Serum albumin: Structure and Characterization of Its Ligand Binding Sites. In: Jost PV, Griffith P, editors. Lipid-Protein Interactions. John Wiley & Sons; New York: 1982. pp. 25–68. [Google Scholar]
- 53.Mussini E. Distribution of mercurial diuretics in the organism. Boll Soc Ital Biol Sper. 1958;34:1487–1490. [PubMed] [Google Scholar]
- 54.Baggett JM, Berndt WO. The effect of depletion of nonprotein sulfhydryls by diethyl maleate plus buthionine sulfoximine on renal uptake of mercury in the rat. Toxicol Appl Pharmacol. 1986;83:556– 562. doi: 10.1016/0041-008x(86)90238-3. [DOI] [PubMed] [Google Scholar]
- 55.Bridges CC, Bauch C, Verrey F, Zalups RK. Mercuric conjugates of cysteine are transported by the amino acid transporter system b(0,+): implications of molecular mimicry. J Am Soc Nephrol. 2004;15:663–673. doi: 10.1097/01.ASN.0000113553.62380.F5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bridges CC, Zalups RK. Homocysteine, system b0,+ and the renal epithelial transport and toxicity of inorganic mercury. Am J Pathol. 2004;165:1385–1394. doi: 10.1016/S0002-9440(10)63396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cannon VT, Zalups RK, Barfuss DW. Amino acid transporters involved in luminal transport of mercuric conjugates of cysteine in rabbit proximal tubule. J Pharmacol Exp Ther. 2001;298:780–789. [PubMed] [Google Scholar]
- 58.Tanaka T, Naganuma A, Imura N. Routes for renal transport of methylmercury in mice. Eur J Pharmacol. 1992;228:9–14. doi: 10.1016/0926-6917(92)90005-w. [DOI] [PubMed] [Google Scholar]
- 59.Zalups RK. Organic anion transport and action of gamma-glutamyl transpeptidase in kidney linked mechanistically to renal tubular uptake of inorganic mercury. Toxicol Appl Pharmacol. 1995;132:289–298. doi: 10.1006/taap.1995.1110. [DOI] [PubMed] [Google Scholar]
- 60.Zalups RK, Barfuss DW. Transport and toxicity of methylmercury along the proximal tubule of the rabbit. Toxicol Appl Pharmacol. 1993;121:176–185. doi: 10.1006/taap.1993.1143. [DOI] [PubMed] [Google Scholar]
- 61.Zalups RK, Barfuss DW. Participation of mercuric conjugates of cysteine, homocysteine, and N-acetylcysteine in mechanisms involved in the renal tubular uptake of inorganic mercury. J Am Soc Nephrol. 1998;9:551–561. doi: 10.1681/ASN.V94551. [DOI] [PubMed] [Google Scholar]
- 62.Zalups RK, Minor KH. Luminal and basolateral mechanisms involved in the renal tubular uptake of inorganic mercury. J Toxicol Environ Health. 1995;46:73–100. doi: 10.1080/15287399509532019. [DOI] [PubMed] [Google Scholar]
- 63.Torres AM, Dnyanmote AV, Bush KT, Wu W, Nigam SK. Deletion of multispecific organic anion transporter Oat1/Slc22a6 protects against mercury-induced kidney injury. J Biol Chem. 2011;286:26391–26395. doi: 10.1074/jbc.M111.249292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zalups RK. Basolateral uptake of inorganic mercury in the kidney. Toxicol Appl Pharmacol. 1998;151:192–199. doi: 10.1006/taap.1998.8416. [DOI] [PubMed] [Google Scholar]
- 65.Zalups RK, Ahmad S. Homocysteine and the renal epithelial transport and toxicity of inorganic mercury: role of basolateral transporter organic anion transporter 1. J Am Soc Nephrol. 2004;15:2023–2031. doi: 10.1097/01.ASN.0000135115.63412.A9. [DOI] [PubMed] [Google Scholar]
- 66.Zalups RK, Ahmad S. Handling of cysteine S-conjugates of methylmercury in MDCK cells expressing human OAT1. Kidney Int. 2005;68:1684–1699. doi: 10.1111/j.1523-1755.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- 67.Zalups RK, Ahmad S. Handling of the homocysteine S-conjugate of methylmercury by renal epithelial cells: role of organic anion transporter 1 and amino acid transporters. J Pharmacol Exp Ther. 2005;315:896–904. doi: 10.1124/jpet.105.090530. [DOI] [PubMed] [Google Scholar]
- 68.Zalups RK, Ahmad S. Transport of N-acetylcysteine s-conjugates of methylmercury in Madin-Darby canine kidney cells stably transfected with human isoform of organic anion transporter 1. J Pharmacol Exp Ther. 2005;314:1158–1168. doi: 10.1124/jpet.105.086645. [DOI] [PubMed] [Google Scholar]
- 69.Zalups RK, Aslamkhan AG, Ahmad S. Human organic anion transporter 1 mediates cellular uptake of cysteine-S conjugates of inorganic mercury. Kidney Int. 2004;66:251–261. doi: 10.1111/j.1523-1755.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- 70.Zalups RK, Barfuss DW. Renal organic anion transport system: a mechanism for the basolateral uptake of mercurythiol conjugates along the pars recta of the proximal tubule. Toxicol Appl Pharmacol. 2002;182:234–243. doi: 10.1006/taap.2002.9448. [DOI] [PubMed] [Google Scholar]
- 71.Tanaka T, Naganuma A, Imura N. Role of gamma-glutamyltranspeptidase in renal uptake and toxicity of inorganic mercury in mice. Toxicology. 1990;60:187–198. doi: 10.1016/0300-483x(90)90142-4. [DOI] [PubMed] [Google Scholar]
- 72.Tanaka T, Naganuma A, Miura N, Imura N. Role of testosterone in gamma-glutamyltranspeptidase-dependent renal methylmercury uptake in mice. Toxicol Appl Pharmacol. 1992;112:58–63. doi: 10.1016/0041-008x(92)90279-2. [DOI] [PubMed] [Google Scholar]
- 73.Zalups RK, Lash LH. Advances in understanding the renal transport and toxicity of mercury. J Toxicol Environ Health. 1994;42:1–44. doi: 10.1080/15287399409531861. [DOI] [PubMed] [Google Scholar]
- 74.Zalups RK, Lash LH. Depletion of glutathione in the kidney and the renal disposition of administered inorganic mercury. Drug Metab Dispos. 1997;25:516–523. [PubMed] [Google Scholar]
- 75.Meister A. Transport and metabolism of glutathione and gamma-glutamyl amino acids. Biochem Soc Trans. 1983;11:793–794. doi: 10.1042/bst0110793. [DOI] [PubMed] [Google Scholar]
- 76.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 77.Tanaka-Kagawa T, Naganuma A, Imura N. Tubular secretion and reabsorption of mercury compounds in mouse kidney. J Pharmacol Exp Ther. 1993;264:776–782. [PubMed] [Google Scholar]
- 78.Berndt WO, Baggett JM, Blacker A, Houser M. Renal glutathione and mercury uptake by kidney. Fundam Appl Toxicol. 1985;5:832–839. doi: 10.1016/0272-0590(85)90166-6. [DOI] [PubMed] [Google Scholar]
- 79.de Ceaurriz J, Payan JP, Morel G, Brondeau MT. Role of extracellular glutathione and gamma-glutamyltranspeptidase in the disposition and kidney toxicity of inorganic mercury in rats. J Appl Toxicol. 1994;14:201–206. doi: 10.1002/jat.2550140310. [DOI] [PubMed] [Google Scholar]
- 80.Kim CY, Watanabe C, Satoh H. Effects of buthionine sulfoximine (BSO) on mercury distribution after Hg(o) exposure. Toxicology. 1995;98:67–72. doi: 10.1016/0300-483x(94)02960-3. [DOI] [PubMed] [Google Scholar]
- 81.Cannon VT, Barfuss DW, Zalups RK. Molecular homology and the luminal transport of Hg2+ in the renal proximal tubule. J Am Soc Nephrol. 2000;11:394–402. doi: 10.1681/ASN.V113394. [DOI] [PubMed] [Google Scholar]
- 82.Lash LH, Jones DP. Distribution of oxidized and reduced forms of glutathione and cysteine in rat plasma. Arch Biochem Biophys. 1985;240:583–592. doi: 10.1016/0003-9861(85)90065-7. [DOI] [PubMed] [Google Scholar]
- 83.Ballatori N, Clarkson TW. Dependence of biliary secretion of inorganic mercury on the biliary transport of glutathione. Biochem Pharmacol. 1984;33:1093–1098. doi: 10.1016/0006-2952(84)90519-7. [DOI] [PubMed] [Google Scholar]
- 84.Ballatori N, Clarkson TW. Biliary secretion of glutathione and of glutathione-metal complexes. Fundam Appl Toxicol. 1985;5:816–831. doi: 10.1016/0272-0590(85)90165-4. [DOI] [PubMed] [Google Scholar]
- 85.Ballatori N, Gatmaitan Z, Truong AT. Impaired biliary excretion and whole body elimination of methylmercury in rats with congenital defect in biliary glutathione excretion. Hepatology. 1995;22:1469–1473. [PubMed] [Google Scholar]
- 86.Ballatori N. Molecular Mechanisms of Hepatic Metal Transport. In: Zalups RK, Koropatnick J, editors. Molecular Biology and Toxicology of Metals. Taylor and Francis; London: 2000. pp. 346–381. [Google Scholar]
- 87.Parks LD, Zalups RK, Barfuss DW. Heterogeneity of glutathione synthesis and secretion in the proximal tubule of the rabbit. Am J Physiol. 1998;274:F924–931. doi: 10.1152/ajprenal.1998.274.5.F924. [DOI] [PubMed] [Google Scholar]
- 88.Schaub TP, Kartenbeck J, Konig J, Spring H, Dorsam J, Staehler G, Storkel S, Thon WF, Keppler D. Expression of the MRP2 gene-encoded conjugate export pump in human kidney proximal tubules and in renal cell carcinoma. J Am Soc Nephrol. 1999;10:1159–1169. doi: 10.1681/ASN.V1061159. [DOI] [PubMed] [Google Scholar]
- 89.Schaub TP, Kartenbeck J, Konig J, Vogel O, Witzgall R, Kriz W, Keppler D. Expression of the conjugate export pump encoded by the mrp2 gene in the apical membrane of kidney proximal tubules. J Am Soc Nephrol. 1997;8:1213–1221. doi: 10.1681/ASN.V881213. [DOI] [PubMed] [Google Scholar]
- 90.Keppler D, Leier I, Jedlitschky G, Konig J. ATP-dependent transport of glutathione S-conjugates by the multidrug resistance protein MRP1 and its apical isoform MRP2. Chem-Biol Interact. 1998;111–112:153–161. doi: 10.1016/s0009-2797(97)00158-0. [DOI] [PubMed] [Google Scholar]
- 91.Naganuma A, Oda-Urano N, Tanaka T, Imura N. Possible role of hepatic glutathione in transport of methylmercury into mouse kidney. Biochem Pharmacol. 1988;37:291–296. doi: 10.1016/0006-2952(88)90731-9. [DOI] [PubMed] [Google Scholar]
- 92.Parks LD, Zalups RK, Barfuss DW. Luminal and basolateral membrane transport of glutathione in isolated perfused S(1), S(2), and S(3) segments of the rabbit proximal tubule. J Am Soc Nephrol. 2000;11:1008–1015. doi: 10.1681/ASN.V1161008. [DOI] [PubMed] [Google Scholar]
- 93.Zalups RK, Barfuss DW. Nephrotoxicity of inorganic mercury co-administrated with L-cysteine. Toxicology. 1996;109:15–29. doi: 10.1016/0300-483x(95)03297-s. [DOI] [PubMed] [Google Scholar]
- 94.Zalups RK, Lash LH. Binding of mercury in renal brush-border and basolateral membrane-vesicles. Biochem Pharmacol. 1997;53:1889–1900. doi: 10.1016/s0006-2952(97)00138-x. [DOI] [PubMed] [Google Scholar]
- 95.Schafer JA, Watkins ML. Transport of L-cystine in isolated perfused proximal straight tubules. Pflugers Arch. 1984;401:143–151. doi: 10.1007/BF00583874. [DOI] [PubMed] [Google Scholar]
- 96.Ballatori N. Transport of toxic metals by molecular mimicry. Environ Health Perspect. 2002;110(Suppl 5):689–694. doi: 10.1289/ehp.02110s5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aschner M, Eberle NB, Goderie S, Kimelberg HK. Methylmercury uptake in rat primary astrocyte cultures: the role of the neutral amino acid transport system. Brain Res. 1990;521:221–228. doi: 10.1016/0006-8993(90)91546-s. [DOI] [PubMed] [Google Scholar]
- 98.Aschner M, Clarkson TW. Methyl mercury uptake across bovine brain capillary endothelial cells in vitro: the role of amino acids. Pharmacol Toxicol. 1989;64:293–297. doi: 10.1111/j.1600-0773.1989.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 99.Kerper LE, Ballatori N, Clarkson TW. Methylmercury transport across the blood-brain barrier by an amino acid carrier. Am J Physiol. 1992;262:R761–765. doi: 10.1152/ajpregu.1992.262.5.R761. [DOI] [PubMed] [Google Scholar]
- 100.Hoffmeyer RE, Singh SP, Doonan CJ, Ross AR, Hughes RJ, Pickering IJ, George GN. Molecular mimicry in mercury toxicology. Chem Res Toxicol. 2006;19:753–759. doi: 10.1021/tx0503449. [DOI] [PubMed] [Google Scholar]
- 101.Zalups RK. Basolateral uptake of mercuric conjugates of N-acetylcysteine and cysteine in the kidney involves the organic anion transport system. J Toxicol Environ Health, Part A. 1998;55:13–29. doi: 10.1080/009841098158593. [DOI] [PubMed] [Google Scholar]
- 102.Zalups RK, Barfuss DW. Small aliphatic dicarboxylic acids inhibit renal uptake of administered mercury. Toxicol Appl Pharmacol. 1998;148:183–193. doi: 10.1006/taap.1997.8320. [DOI] [PubMed] [Google Scholar]
- 103.Zalups RK, Diamond GL. Intrarenal distribution of mercury in the rat: effect of administered dose of mercuric chloride. Bull Environ Contam Toxicol. 1987;38:67–72. doi: 10.1007/BF01606560. [DOI] [PubMed] [Google Scholar]
- 104.Zalups RK, Barfuss DW. Pretreatment with p-aminohippurate inhibits the renal uptake and accumulation of injected inorganic mercury in the rat. Toxicology. 1995;103:23–35. doi: 10.1016/0300-483x(95)03099-2. [DOI] [PubMed] [Google Scholar]
- 105.Dantzler WH, Evans KK. Effect of alpha-KG in lumen on PAH transport by isolated perfused rabbit renal proximal tubules. Am J Physiol. 1996;271:F521–526. doi: 10.1152/ajprenal.1996.271.3.F521. [DOI] [PubMed] [Google Scholar]
- 106.Dantzler WH, Evans KK, Wright SH. Kinetics of interactions of para-aminohippurate, probenecid, cysteine conjugates and N-acetyl cysteine conjugates with basolateral organic anion transporter in isolated rabbit proximal renal tubules. J Pharmacol Exp Ther. 1995;272:663–672. [PubMed] [Google Scholar]
- 107.Ferrier B, Martin M, Roch-Ramel F. Effects of p-aminohippurate and pyrazinoate on the renal excretion of salicylate in the rat: a micropuncture study. J Pharmacol Exp Ther. 1983;224:451–458. [PubMed] [Google Scholar]
- 108.Pritchard JB. Coupled transport of p-aminohippurate by rat kidney basolateral membrane vesicles. Am J Physiol. 1988;255:F597–604. doi: 10.1152/ajprenal.1988.255.4.F597. [DOI] [PubMed] [Google Scholar]
- 109.Pritchard JB, Miller DS. Mechanisms mediating renal secretion of organic anions and cations. Physiol Rev. 1993;73:765–796. doi: 10.1152/physrev.1993.73.4.765. [DOI] [PubMed] [Google Scholar]
- 110.Shimomura A, Chonko AM, Grantham JJ. Basis for heterogeneity of para-aminohippurate secretion in rabbit proximal tubules. Am J Physiol. 1981;240:F430–436. doi: 10.1152/ajprenal.1981.240.5.F430. [DOI] [PubMed] [Google Scholar]
- 111.Ullrich KJ, Rumrich G, Fritzsch G, Kloss S. Contraluminal para-aminohippurate (PAH) transport in the proximal tubule of the rat kidney. I Kinetics, influence of cations, anions, and capillary preperfusion. Pflugers Arch. 1987;409:229–235. doi: 10.1007/BF00583470. [DOI] [PubMed] [Google Scholar]
- 112.Ullrich KJ, Rumrich G, Fritzsch G, Kloss S. Contraluminal para-aminohippurate (PAH) transport in the proximal tubule of the rat kidney. II Specificity: aliphatic dicarboxylic acids. Pflugers Arch. 1987;408:38–45. doi: 10.1007/BF00581838. [DOI] [PubMed] [Google Scholar]
- 113.Zalups RK. Enhanced renal outer medullary uptake of mercury associated with uninephrectomy: implication of a luminal mechanism. J Toxicol Environ Health. 1997;50:173–194. doi: 10.1080/009841097160564. [DOI] [PubMed] [Google Scholar]
- 114.Ban M, de Ceaurriz J. Probenecid-induced protection against acute hexachloro-1,3-butadiene and methyl mercury toxicity to the mouse kidney. Toxicol Lett. 1988;40:71–76. doi: 10.1016/0378-4274(88)90184-1. [DOI] [PubMed] [Google Scholar]
- 115.Bhatnagar V, Xu G, Hamilton BA, Truong DM, Eraly SA, Wu W, Nigam SK. Analyses of 5′ regulatory region polymorphisms in human SLC22A6 (OAT1) and SLC22A8 (OAT3) J Hum Genet. 2006;51:575–580. doi: 10.1007/s10038-006-0398-1. [DOI] [PubMed] [Google Scholar]
- 116.Nagle MA, Truong DM, Dnyanmote AV, Ahn SY, Eraly SA, Wu W, Nigam SK. Analysis of three-dimensional systems for developing and mature kidneys clarifies the role of OAT1 and OAT3 in antiviral handling. J Biol Chem. 2011;286:243–251. doi: 10.1074/jbc.M110.139949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J Biol Chem. 2002;277:26934–26943. doi: 10.1074/jbc.M203803200. [DOI] [PubMed] [Google Scholar]
- 118.Vallon V, Rieg T, Ahn SY, Wu W, Eraly SA, Nigam SK. Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. Am J Physiol Renal Physiol. 2008;294:F867–873. doi: 10.1152/ajprenal.00528.2007. [DOI] [PubMed] [Google Scholar]
- 119.Xu G, Bhatnagar V, Wen G, Hamilton BA, Eraly SA, Nigam SK. Analyses of coding region polymorphisms in apical and basolateral human organic anion transporter (OAT) genes [OAT1 (NKT), OAT2, OAT3, OAT4, URAT (RST)] Kidney Int. 2005;68:1491–1499. doi: 10.1111/j.1523-1755.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 120.Aslamkhan AG, Han YH, Yang XP, Zalups RK, Pritchard JB. Human renal organic anion transporter 1-dependent uptake and toxicity of mercuric-thiol conjugates in Madin-Darby canine kidney cells. Mol Pharmacol. 2003;63:590–596. doi: 10.1124/mol.63.3.590. [DOI] [PubMed] [Google Scholar]
- 121.Koh AS, Simmons-Willis TA, Pritchard JB, Grassl SM, Ballatori N. Identification of a mechanism by which the methylmercury antidotes N-acetylcysteine and dimercaptopropanesulfonate enhance urinary metal excretion: transport by the renal organic anion transporter-1. Mol Pharmacol. 2002;62:921–926. doi: 10.1124/mol.62.4.921. [DOI] [PubMed] [Google Scholar]
- 122.Clarkson TW, Magos L. The effect of sodium maleate on the renal deposition and excretion of mercury. Br J Pharmacol Chemother. 1967;31:560–567. doi: 10.1111/j.1476-5381.1967.tb00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rogulski J, Angielski S. Effect of maleic acid on the kidney. IV Synthesis of amino acids in the kidney of maleate-treated rats. Acta Biochim Pol. 1963;10:133–139. [PubMed] [Google Scholar]
- 124.Wright SH, Dantzler WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev. 2004;84:987–1049. doi: 10.1152/physrev.00040.2003. [DOI] [PubMed] [Google Scholar]
- 125.Pritchard JB, Miller DS. Comparative insights into the mechanisms of renal organic anion and cation secretion. Am J Physiol. 1991;261:R1329–1340. doi: 10.1152/ajpregu.1991.261.6.R1329. [DOI] [PubMed] [Google Scholar]
- 126.Bridges CC, Joshee L, Zalups RK. MRP2 and the DMPS- and DMSA-mediated elimination of mercury in TR(−) and control rats exposed to thiol S-conjugates of inorganic mercury. Toxicol Sci. 2008;105:211–220. doi: 10.1093/toxsci/kfn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bridges CC, Joshee L, Zalups RK. Multidrug resistance proteins and the renal elimination of inorganic mercury mediated by 2,3-dimercaptopropane-1-sulfonic acid and meso-2,3-dimercaptosuccinic acid. J Pharmacol Exp Ther. 2008;324:383–390. doi: 10.1124/jpet.107.130708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bridges CC, Joshee L, Zalups RK. MRP2 and the handling of mercuric ions in rats exposed acutely to inorganic and organic species of mercury. Toxicol Appl Pharmacol. 2011;251:50–58. doi: 10.1016/j.taap.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zalups RK, Koropatnick J. Temporal changes in metallothionein gene transcription in rat kidney and liver: relationship to content of mercury and metallothionein protein. J Pharmacol Exp Ther. 2000;295:74–82. [PubMed] [Google Scholar]
- 130.Aposhian HV, Maiorino RM, Gonzalez-Ramirez D, Zuniga-Charles M, Xu Z, Hurlbut KM, Junco-Munoz P, Dart RC, Aposhian MM. Mobilization of heavy metals by newer, therapeutically useful chelating agents. Toxicology. 1995;97:23–38. doi: 10.1016/0300-483x(95)02965-b. [DOI] [PubMed] [Google Scholar]
- 131.Aposhian HV, Maiorino RM, Rivera M, Bruce DC, Dart RC, Hurlbut KM, Levine DJ, Zheng W, Fernando Q, Carter D, et al. Human studies with the chelating agents, DMPS and DMS. J Toxicol Clin Toxicol. 1992;30:505–528. doi: 10.3109/15563659209017938. [DOI] [PubMed] [Google Scholar]
- 132.Zalups RK. Influence of 2,3-dimercaptopropane-1-sulfonate (DMPS) and meso-2,3-dimercaptosuccinic acid (DMSA) on the renal disposition of mercury in normal and uninephrectomized rats exposed to inorganic mercury. J Pharmacol Exp Ther. 1993;267:791–800. [PubMed] [Google Scholar]
- 133.Zalups RK, Bridges CC. Seventy-five percent nephrectomy and the disposition of inorganic mercury in 2,3-dimercaptopropanesulfonic acid-treated rats lacking functional multidrug-resistance protein 2. J Pharmacol Exp Ther. 2010;332:866–875. doi: 10.1124/jpet.109.163774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zalups RK, Gelein RM, Cernichiari E. DMPS as a rescue agent for the nephropathy induced by mercuric chloride. J Pharmacol Exp Ther. 1991;256:1–10. [PubMed] [Google Scholar]
- 135.Gabard B, Walser R. Note on the metabolism of the mercury chelating agent sodium 2,3-dimercaptopropane-1-sulfonate. J Toxicol Environ Health. 1979;5:759–764. doi: 10.1080/15287397909529785. [DOI] [PubMed] [Google Scholar]
- 136.Maiorino RM, Weber GL, Aposhian HV. Fluorometric determination of 2,3-dimercaptopropane-1-sulfonic acid and other dithiols by precolumn derivatization with bromobimane and column liquid chromatography. J Chromatogr. 1986;374:297–310. doi: 10.1016/s0378-4347(00)83285-5. [DOI] [PubMed] [Google Scholar]
- 137.Diamond GL, Klotzbach JM, Stewart JR. Complexing activity of 2,3-dimercapto-1-propanesulfonate and its disulfide auto-oxidation product in rat kidney. J Pharmacol Exp Ther. 1988;246:270–274. [PubMed] [Google Scholar]
- 138.Klotzbach JM, Diamond GL. Complexing activity and excretion of 2,3-dimercapto-1-propane sulfonate in rat kidney. Am J Physiol. 1988;254:F871–878. doi: 10.1152/ajprenal.1988.254.6.F871. [DOI] [PubMed] [Google Scholar]
- 139.Stewart JR, Diamond GL. In vivo renal tubular secretion and metabolism of the disulfide of 2,3-dimercaptopropane-1-sulfonate. Drug Metab Dispos. 1988;16:189–195. [PubMed] [Google Scholar]
- 140.George GN, Prince RC, Gailer J, Buttigieg GA, Denton MB, Harris HH, Pickering IJ. Mercury binding to the chelation therapy agents DMSA and DMPS and the rational design of custom chelators for mercury. Chem Res Toxicol. 2004;17:999–1006. doi: 10.1021/tx049904e. [DOI] [PubMed] [Google Scholar]
- 141.Lash LH, Zalups RK. Activities of enzymes involved in renal cellular glutathione metabolism after uninephrectomy in the rat. Arch Biochem Biophys. 1994;309:129–138. doi: 10.1006/abbi.1994.1095. [DOI] [PubMed] [Google Scholar]
- 142.Zalups RK, Barfuss DW, Lash LH. Relationships between alterations in glutathione metabolism and the disposition of inorganic mercury in rats: effects of biliary ligation and chemically induced modulation of glutathione status. Chem-Biol Interact. 1999;123:171–195. doi: 10.1016/s0009-2797(99)00135-0. [DOI] [PubMed] [Google Scholar]
- 143.Zalups RK, Barfuss DW, Lash LH. Disposition of inorganic mercury following biliary obstruction and chemically induced glutathione depletion: dispositional changes one hour after the intravenous administration of mercuric chloride. Toxicol Appl Pharmacol. 1999;154:135–144. doi: 10.1006/taap.1998.8562. [DOI] [PubMed] [Google Scholar]
- 144.Zalups RK, Lash LH. Effects of uninephrectomy and mercuric chloride on renal glutathione homeostasis. J Pharmacol Exp Ther. 1990;254:962–970. [PubMed] [Google Scholar]
- 145.Fu J, Hoffmeyer RE, Pushie MJ, Singh SP, Pickering IJ, George GN. Towards a custom chelator for mercury: evaluation of coordination environments by molecular modeling. J Biol Inorg Chem. 2011;16:15–24. doi: 10.1007/s00775-010-0695-1. [DOI] [PubMed] [Google Scholar]
- 146.Zalups RK, Parks LD, Cannon VT, Barfuss DW. Mechanisms of action of 2,3-dimercaptopropane-1-sulfonate and the transport, disposition, and toxicity of inorganic mercury in isolated perfused segments of rabbit proximal tubules. Mol Pharmacol. 1998;54:353–363. doi: 10.1124/mol.54.2.353. [DOI] [PubMed] [Google Scholar]
- 147.Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 148.Zhou SF, Wang LL, Di YM, Xue CC, Duan W, Li CG, Li Y. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem. 2008;15:1981–2039. doi: 10.2174/092986708785132870. [DOI] [PubMed] [Google Scholar]
- 149.Aremu DA, Madejczyk MS, Ballatori N. N-acetylcysteine as a potential antidote and biomonitoring agent of methylmercury exposure. Environ Health Perspect. 2008;116:26–31. doi: 10.1289/ehp.10383. [DOI] [PMC free article] [PubMed] [Google Scholar]