Abstract
Objective
To review how disability can develop in older adults with critical illness and to explore ways to reduce long-term disability following critical illness.
Data Sources
Review of the literature describing post-critical illness disability in older adults and expert opinion.
Results
We identified 19 studies evaluating disability outcomes in critically ill patients age 65 years and older. Newly acquired disability in activities of daily living, instrumental activities of daily living and mobility activities was commonplace among older adults who survived a critical illness. Incident dementia and less-severe cognitive impairment was also highly prevalent. Factors related to the acute critical illness, intensive care unit practices such as heavy sedation, physical restraints and immobility as well as aging physiology and coexisting geriatric conditions can combine to result in these poor outcomes.
Conclusion
Older adults who survive critical illness suffer physical and cognitive declines resulting in disability at greater rates than hospitalized, non-critically ill and community dwelling older adults. Interventions derived from widely available geriatric care models in use outside of the ICU, which address modifiable risk factors including immobility and delirium, are associated with improved functional and cognitive outcomes and can be used to complement ICU-focused models such as the ABCDEs.
Introduction
For millions each year, surviving a critical illness represents a life-altering event punctuated by physical and cognitive impairments resulting in new-onset disability (1–8). Patients of all ages are affected (8–10). Older adults (i.e., those 65 years or older), however, bear the lion’s share of this burden as the demographic most likely to become critically ill (11–14). Moreover, because the majority of patients with critical illness are older adults, the aging of the population in coming years, is expected to drive a significant increase in the number of critical illness survivors with physical impairments, cognitive impairments and disabilities (5, 7, 14, 15).
Regardless of age, critical illness survival implies resolution of the underlying illness, yet age may play an important role. In the case of respiratory failure, for example, older adults achieve physiologic recovery from their illness at least as fast as their younger counterparts (16, 17). After adjusting for potential confounders such as severity of illness, however, older adults are more likely to remain intubated and in the ICU (17). These data imply that ongoing and destructive processes—apart from those that resulted in the development of critical illness—may be responsible for poor physical and cognitive outcomes suffered by many older adults.
Critical illness survival also exists on a spectrum ranging from those who are free of disability to those who are severely disabled, a number of whom are “chronically critically ill” or “hospital dependent” (18–21). Why some patients “successfully” recover from critical illness while others do not is unknown. Thus, a better understanding of the contributions to poor long-term physical and cognitive functioning that results in disability is needed to improve the lives of the growing number of older adults who survive a critical illness each year.
The disabling process results from the complex interrelationship between a patient’s pre-illness vulnerability and the acute stress of a critical illness and treatment in an ICU (22). In older adults, the normal aging process, also known as senescence, in combination with systemic pathology from comorbid medical conditions, injuries, environmental and epigenetic factors can reduce physiologic reserves and the ability to “bounce back” from an acute stressor (23–25). Thus, a highly vulnerable patient (e.g., one who is frail or physically or cognitively impaired before their illness) may develop disability following a less severe illness (e.g., urinary tract infection). Alternatively, a patient with low vulnerability will require a greater insult (e.g., septic shock with multiple organ failures) before developing disability.
This manuscript, written by an interdisciplinary team of experts in critical care, geriatrics and gerontology, presents an integrative literature review of the epidemiology of disability in survivors of a critical illness; reviews how critical illness, in the setting of the physiology of aging, can result in disability following a critical illness; and, finally, presents expert opinion on steps that can be taken to make the ICU a more ‘friendly’ place for older adults, with the ultimate goal of reducing the component of post-ICU suffering that is long-term disability.
The development of post-critical illness disability
Optimizing long-term outcomes for survivors of critical illness must begin with a discussion of the disabling process in the setting of critical illness. This understanding will allow researchers and clinicians to communicate using the same terminology, to gain insights into how diseases and treatments may affect outcomes, to define better outcomes of importance to patients and, eventually, to enhance clinical care.
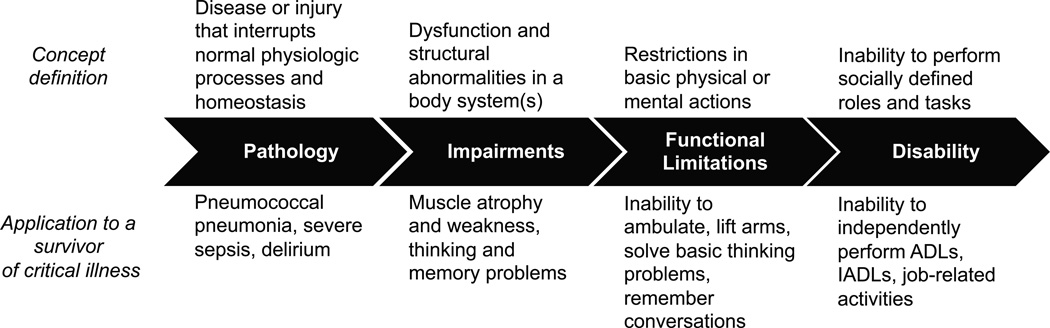
While different conceptual models exist to describe the disabling process, the framework originally proposed by Nagi (26), modified by Verbrugge and Jette (27), provides a robust, informative way to understand how critical illness may lead to disability. According to this model, diseases or injuries (pathology) result in dysfunction of body systems (impairments) leading to the inability to carry out basic physical and cognitive functions (functional limitations) that alter the individual’s capability to meet the demands of his or her environment (disability) (Figure 1). Hence, disability, simply defined, represents the difference between a person’s capabilities and the demands of a particular physical or social environment (27, 28).
Figure 1.
A conceptual model of the disablement process and its application to a survivor of a critical illness. This framework illustrates how diseases (pathology) result in body system dysfunction (impairments) that limits an individual’s ability to perform basic actions (functional limitations) and prevent that individual from performing socially expected activities (disability). When applied to a hypothetical survivor of critical illness, the effects of critical illness alter the functioning of skeletal muscle and the brain to result in the inability to move one’s arms and legs as well as to remember and think clearly, preventing the patient from carrying out activities necessary to live independently such as ADLs (dressing, bathing, walking across a room), IADLs (managing money, cooking a meal) or to remain employed. ARDS, the acute respiratory distress syndrome; ICU, intensive care unit; ADLs, activities of daily living; IADLs, instrumental activities of daily living. Adapted with permission from Verbrugge LM, Jette AM. The disablement process. Social Science & Medicine 1994;38(1):1–14.
To illustrate this process, let us explore a hypothetical case of Mrs. D, a 67 year-old widow who, prior to her illness, lived independently and was employed as an executive secretary (Figure 1). She was mechanically ventilated in the ICU for 7 days due to pneumococcal pneumonia and severe sepsis (pathology). She was, sedated and confined to bed for the first 5 days of her illness and suffered 6 days of delirium while in the ICU (pathology). Following extubation, the ICU physical therapist notes that Mrs. D has significant muscle atrophy and weakness that is attributed to ICU-acquired weakness (impairment). With her delirium now resolved, Mrs. D’s daughter expresses concerns that her mother is having trouble thinking and remembering things (impairment). She notes that Mrs. D was “sharp as a tack” prior to her illness. After being transferred out of the ICU, Mrs. D continues to require assistance to ambulate and to lift her arms (functional limitation). She complains that she cannot complete crossword puzzles that she did easily before her illness and that she cannot recall the details of conversations with her family (functional limitation). As discharge planning progresses, Mrs. D’s daughter is nervous that her mother will be unable to manage her medications and her finances (disability in instrumental activities of daily living). She continues to require assistance to bathe, dress, and transfer from the bed to a chair (disability in basic activities of daily living). As a result of these newly acquired disabilities, Mrs. D is discharged to a skilled nursing facility, where she still resides 1 year later. She is unable to return to work (disability in employment).
Epidemiology of disability following critical illness
The declines in Mrs. D’s physical and cognitive functioning represent a common scenario for the estimated 1.4 million older adults in the United States (and many more worldwide) who survive a critical illness each year (6). We searched PubMed, CINAHL, Web of Science and Google Scholar for studies reporting disability outcomes (i.e., activities of daily living [ADLs], instrumental activities of daily living [IADLs] and mobility activities) and/or cognitive outcomes among patients treated in an ICU who were 65 years or older. We also reviewed the bibliographies of relevant citations to identify additional citations. Overall, 19 studies met these criteria (2, 3, 29–45): 17 studies reported disability outcomes (2, 29–38, 40–42, 44), 2 studies reported cognitive outcomes (3, 45) and 2 studies reported both (39, 43).
Of the studies that reported disability outcomes (Table S1), 13 were single center cohorts and 12 enrolled fewer than 300 patients. Patients were enrolled from mixed (medical and surgical) ICUs in 11 studies, from medical ICUs in 4 studies and from surgical ICUs in 2 studies. The mean age of patients enrolled in the studies ranged from 69 to 89 years. Most (10/14) of the studies that assessed ADL function used the Katz ADL (46), 2 studies used the Barthel Index (47) and 2 other studies used other measures. All four studies that assessed IADLs used the Lawton Index (48). Of the three studies that assessed mobility status (2, 35, 44) each used different scoring measures (35, 49, 50). Most studies assessed outcomes less than 12 months following critical illness.
Disability in ADLs was highly prevalent after a critical illness, and was present in 33% to 58% of patients when follow-up occurred less than three months after the index illness and 12% to 97% for follow-up time points occurring more than six months after the index illness. Among the nine studies that reported baseline (pre-illness) ADL function, new-onset or worsened ADL disability was present in 10% to 63% of patients assessed less than 1 year and in 22% to 37% of patients assessed 1 year or more after their critical illness (29, 31, 34–37, 40–43). New or worsened IADL disability was also common and reported in 22% to 45% of patients evaluated 3 months to almost 2 years following the index illness (34, 38, 43). A single study where a number of patients were disabled in ADLs and IADLs at baseline (43% and 60%, respectively) reported no change in disability at 3-month follow-up (42). Finally, disability in mobility activities was present in 14% to 87% of patients assessed during the first year following their index illness.
Of the four studies that assessed cognitive outcomes, two were single center cohorts. Each study used a different outcome measure and assessed cognition at time points ranging from 3 months to 8 years following the index illness (Table S2) (3, 39, 43, 45). Three out of four studies assessed pre-illness cognitive functioning and reported newly developed (i.e., incident) dementia in 12% to 18% of patients who were assessed between 1 and 8 years after their illness (3, 39, 45). Prevalent dementia (i.e., unknown pre-illness dementia status), was reported in 15% of patients at hospital discharge and in 10% at 1-year follow-up (43). In addition to the incidence of dementia, one study also reported newly acquired mild to moderate cognitive impairment was present in 56% of patients—yielding an overall proportion of cognitive impairment plus dementia of 73% of patients 4 years after critical illness (39).
The findings of this review indicate that disability in ADLs, IADLs and mobility is common among older adults who survive a critical illness. They also highlight the substantial burden of newly acquired cognitive impairment and dementia following critical illness. Nevertheless, several limitations of these studies are worthy of mention. First, few studies have reported data on these important patient-centered outcomes after critical illness. Second, the majority of these studies are small, single-center cohorts that used heterogeneous assessment methods with follow-up time points that varied widely. Third, while some studies reported data on patients’ pre-illness disability, physical and/or cognitive functioning—the majority did not. Thus, the findings may be biased and the true effect of critical illness on these outcomes remains unclear (51). Nevertheless, the rates of post-critical illness disability reported in these studies are substantially higher rates than community dwelling persons (52–54) and older adults who are hospitalized without critical illness (55, 56). Finally, the results of these studies are also biased by the large number of patients who were not included in follow-up assessments due to the competing risk of death among older survivors of critical illness.
Intersections of aging physiology and critical illness
Some older adults carry a low-burden of aging-related disease and disability, remain highly functional and can be thought of as aging “successfully” (57, 58). For the vast majority, however, “normal” aging is characterized by a progressive accumulation of molecular and cellular damage due to illness, injury, environmental and epigenetic factors that lead to physiologic impairments of organ systems and an increased risk of disease, disability and death (23, 24). The rate of this decline of organ systems is controlled by homeostatic maintenance and repair mechanisms (23). Over time, maintenance and repair functions lose complexity, and maladaptive stress responses alter the body’s ability to maintain homeostasis. The degree to which these functions are altered varies from person to person and from organ system to organ system and may explain, in part, variations in the speed of aging (23). Accelerated decline of homeostatic mechanisms, which often characterizes the geriatric condition known as frailty, is present in up to one-third of all older adults and leads to a state of increased vulnerability and disproportionate changes in functional and cognitive status following an acute stressor, such as critical illness (59–64).
While aging-related alterations to homeostatic mechanisms occur in nearly all organ systems, in the context of critical illness, changes to the structure and function of skeletal muscle and/or the brain place older patients at increased risk of developing newly acquired and/or worsening disability. The effects of critical illness on other organ systems such as the aging cardiovascular, pulmonary and renal systems, have been described elsewhere (65–67).
Aging skeletal muscles in critical illness
Roughly half of persons older than 65 years have clinically significant diminished skeletal muscle mass and strength due to age-related changes known as sarcopenia (68). The causes of sarcopenia are multifactorial and include disuse atrophy, changes in endocrine function, inflammation, and nutritional deficiencies (68, 69). Sarcopenia is characterized by a decrease in the size, number and composition of muscle fibers, remodeling of motor units, increased intramuscular lipid concentration, inflammation, oxidative stress and loss of anabolic stimuli (70, 71). The end result of sarcopenia is reduced muscle power and strength, progressive weakness, fatigue, slow gait speeds and difficulty ambulating long distances (68, 70, 72). Sarcopenia is associated with a variety of poor clinical outcomes including increased length of hospital stay, an increased risk of readmission and death (73–76).
During critical illness, inflammation alters the atrophy-hypertrophy-signaling pathways within skeletal muscle, resulting in acute muscle wasting in the first few days of illness, particularly among those with multiple organ failures (77–79). This imbalance between muscle breakdown and recovery represents an additional degenerative insult that cannot be appropriately countered in aging muscle and may, in part, explain the higher incidence of ICU-acquired weakness among older patients (78, 80).
Immobility, even among patients who were ambulatory prior to their illness, is common during hospitalization (81–83). For patients of all ages, bed rest results in losses in muscle mass, strength and aerobic capacity (84); yet, these losses are accelerated by roughly a factor of three among older adults (85). In non-critically ill older patients, even short periods (e.g., 1–2 days) of reduced activity or bed rest can result in disability and nursing home admission (86, 87).
Thus, the skeletal muscles of older adults in the ICU face the concurrent insults of inflammation and bed rest, which are intensified by both the severity and duration of the underlying critical illness. The end result is muscle atrophy, weakness and diminished aerobic capacity, leading to the inability to carryout basic self-care activities (i.e., disability) following critical illness.
The aging brain in critical illness
Aging results in a variable trajectory of declines in cognitive abilities, particularly in working memory, short-term memory and processing speed (88–90). In the aging brain, oxidative stress, epigenetic factors, diminished autophagy, decreased insulin/IGF-1 signaling, impaired stress responses and clearance of toxic proteins in combination, alter hormonal and immunologic feedback mechanisms (89, 91–95). Loss of these feedback mechanisms can result in exuberant inflammatory responses to acute stress, resulting in neurodegeneration, which then drives additional inflammation. Thus, the aging brain can be caught in a vicious cycle that, over time, results in neuronal loss and clinically significant cognitive decline.
The acute stress of critical illness and age-related changes to the brain make critically ill older adults particularly susceptible to developing delirium (96). Delirium results from the complex interaction of a patient’s underlying vulnerability, neurotransmitter imbalances, inflammatory responses, oxidative stress, physiologic stressors and metabolic derangements that result in the large-scale disruption of neural networks, resulting in fluctuating acute confusion, altered consciousness, inattention and disorganized thinking (96–100).
In some cases, delirium may resolve without long-term consequences. Nevertheless, evidence now supports an association between delirium and long-term cognitive sequelae including dementia and accelerated cognitive decline (8, 101–103). In critically ill patients delirium duration is one of the strongest independent predictors of significant cognitive deficits after critical illness (8, 104). Although the precise mechanisms are unclear, it is hypothesized that delirium, triggered by an acute insult, initiates or exacerbates the pathologic age-related structural, immune, neurochemical and neurohormonal brain changes. This results in a cycle of neuroinflammation and neurodegeneration leading to cognitive impairment (95, 105, 106).
Reducing post-critical illness disability
Post-critical illness disability results from the interaction of a patient’s baseline health status and vulnerability to the acute stress of critical illness with the effects of the acute illness itself and treatment practices during and after the ICU admission (55, 107, 108). Thus, because it is not (yet) possible to prevent aging, to reverse vulnerability in the setting of critical illness which is most often an unplanned event, or to completely avoid critical illness-related organ system impairments, the focus of preventing disabilities should lie with the identification of critically ill patients who are at risk for developing/exacerbating disabilities and in addressing specific iatrogenic contributors to post-critical illness disability.
Identifying high-risk older patients
Outside the ICU, several tools exist to identify patients at risk for post-hospital disability(109–112). Although the content of these tools differ slightly, each incorporates the patient’s pre-illness functional and cognitive status, highlighting the important contribution of baseline status to post-hospital outcomes. Despite an association with improved survival and ability to reside in their own home following a hospitalization (113), few hospitalized older adults undergo functional and cognitive status assessments during hospitalization (114). This practice is even less common in the ICU, where few clinicians have training in assessment techniques. Additional barriers to functional and cognitive status assessment in critically ill patients include the inability of many patients to communicate directly due to endotracheal tubes, sedation and/or delirium, as well as time constrains of the busy ICU workflow. These barriers, however, may be overcome using a pragmatic functional and cognitive assessment adapted for the unique needs of critically ill patients (Table 1).
Table 1.
Pragmatic functional and cognitive assessment for older adults with critical illness
| Domain Assessed | |||
|---|---|---|---|
| Activities of Daily Living | Mobility | Cognition | |
|
As soon as possible after ICU admission (use surrogate to obtain information, as needed) |
Katz ADL Index(46)a: Is assistance required to: 1) Bathe or shower 2) Get dressed 3) Get to the restroom 4) Transfer from bed to chair 5) Control bladder or bowels 6) Eat a meal Consider high risk for post-critical illness disability if requires assistance to complete any ADL. |
Sit→Stand→Walk Assess patient’s ability to: 1) Sit up in bed 2) Stand at edge of bed 3) Walk a few feet (using assistive devices, if needed) Consider high risk for post-critical illness disability if unable to get out of bed and stand. |
If patient alert and non-delirious: Mini–Cog, or, If patient comatose or delirious: IQCODE (perform with surrogate) Mini-Cog(158): 1) 3-item recall (e.g., Banana, Sunrise, Chair) 2) Ask patient to draw a clock face showing the time as 11:10 (patient should draw circle, numbers and hands at appropriate time) 3) Ask patient to recall 3 words (1 point for each correct word, 2 points for correct clock) Consider high risk for post-critical illness disability if scores <2 points IQCODE(159)a: 16-question tool comparing current cognitive functioning to 10 years ago. See supplementary materials for IQCODE questionnaire. (Scores range from 1 to 5 where 1 indicates much improved functioning, 5 indicates much worse functioning and 3 indicates no change) Consider high risk for post-critical illness disability if scores 3.44 or greater. |
| Daily while in ICU | Have patient perform ADLs. | Sit→ Stand → Walk | Delirium screening |
| Directly observe or obtain information from patient’s family, bedside nurses, physical or occupational therapists. |
Directly observe or obtain information from patient’s family, bedside nurses, physical or occupational therapists. |
Use CAM-ICU or ICDSC | |
|
Consider high risk for post-critical illness disability if requires assistance to complete any ADL. |
Consider high risk for post-critical illness disability if requires assistance to get out of bed and stand. |
Each day of delirium increases risk for post-critical illness disability |
|
The complete Katz Index of Activities of Daily Living (Katz ADL) instrument and the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) instrument may be found supplementary materials. CAM-ICU, Confusion Assessment Method for the ICU; ICDSC, Intensive Care Delirium Screening Checklist.
Addressing modifiable risk factors for disability
Two of the most common and modifiable risk factors for subsequent functional and cognitive decline are immobility and delirium. Immobility is an “under-recognized epidemic” among hospitalized older adults with deleterious effects on subsequent physical and cognitive function (62, 87, 115, 116). Delirium, present in up to 80% of all mechanically ventilated patients, is among the strongest predictors of subsequent cognitive impairment and also contributes to long-term disability in ADLs (8, 104, 117, 118). Both immobility and delirium exacerbate underlying age-related physiologic changes; thus, efforts to shorten their duration (or prevent their occurrence all together) can have substantial impacts on post-critical illness outcomes (116, 119, 120).
For over half a century, the untoward effects of immobility and delirium have been the focus of clinicians caring for hospitalized older adults outside of the ICU, yet only recently has become the focus of those caring for the critically ill (108, 121–128). Thus, geriatricians have had a significant head start in preventing and managing immobility and delirium through interdisciplinary “geriatric care models” (119, 129–132). Within the last decade, however, ICU-focused interdisciplinary strategies, such as the “ABCDE bundle,” have been described and implemented (133–138). Using a synthesis of the literature and expert opinion, we now will discuss how components of geriatric care models can be used to complement the ABCDE bundle and other “best practices” of ICU care.
Recommended interventions to improve functional and cognitive outcomes
Members of the ICU team should assessment a patient’s functional and cognitive status as soon as possible after admission either through direct patient evaluation or from the patient’s surrogate, to identify patients at high risk for post-critical illness disability. Additionally, because functional and cognitive status can fluctuate during a hospitalization, daily monitoring should be performed to alert clinicians to potential changes.
The ABCDE bundle is advocated by a number of professional societies including the Society for Critical Care Medicine and combines evidence-based strategies to reduce the harms associated with sedation, mechanical ventilation, delirium and immobility in critically ill patients of all ages. While a complete description of each of the components of the ABCDE bundle is beyond the scope of this review, excellent resources that detail the specific components of the ABCDE bundle and the evidence behind them are available at both www.iculiberation.org and www.icudelirium.org. Briefly, the ABCDE bundle includes daily spontaneous Awakening and spontaneous Breathing trial Coordination (“ABC”), Choosing to sedate patients only when necessary and to “lighter” levels (“C”), screening for Delirium (“D”) and the Early mobilization/physical and occupational therapy (“E”). Implementation of the ABCDE bundle is independently associated with a doubling of the odds of a patient being mobilized out of bed and a 45% decrease in the odds of developing delirium (139). Additionally, individual components of the ABCDEs are associated with improved functional status at hospital discharge and decreased mortality following critical illness (116, 140).
Older adults, however, face additional risk factors for poor functional and cognitive outcomes not addressed by the ABCDE bundle, including social isolation, enforced dependence in ADLs, restraints, poor nutrition, polypharmacy, and unnecessary medical tests and procedures (119, 129, 132). To address these risk factors, three widely-implemented “geriatric care models” — The Acute Care for Elders (ACE) model, the Hospital Elder Life Program (HELP) and the Nurses Improving Care for Healthsystem Elders (NICHE) — were developed (119, 129, 132). The specific interventions contained in these programs differ; yet, each addresses the risk factors faced more commonly by older adults. In general, each care model reduce falls, prevent functional decline, decrease the proportion of patients who develop delirium, shorten hospital length of stay and increase the likelihood of being discharged to home (130, 141). Whether these same outcomes can be achieved in older adults who are critically ill is an area in need of further research.
Nevertheless, given their association with improved outcomes in less-severely ill older adults, we propose a group of evidence-based interventions that can be used to complement existing ICU best-practices and care bundles to reduce functional and cognitive decline among older adults with critical illness (Figure 2). Because ICUs differ with regard to the specifics by which patient care is delivered, there is no one-size-fits-all approach to implementing these suggested interventions. Yet, because one common thread running thorough modern ICU practice is close, collaborative interdisciplinary patient care, the ICU serves as an ideal environment to adapt and implement components of these geriatric care models. For example, preventing inappropriate medication use requires cooperation between physicians, pharmacists and bedside nurses each of whom contribute to the process of ordering, dispensing and administering medications and communicating these changes to the next level of care. Technologies ranging from simple checklists, to electronic medical records, computerized dashboards and telemedicine have been used to augment therapeutic intervention delivery in severe sepsis and prevent iatrogenic harms such as central line infections and thus could serve as a model for implementing the proposed interventions (142–145).
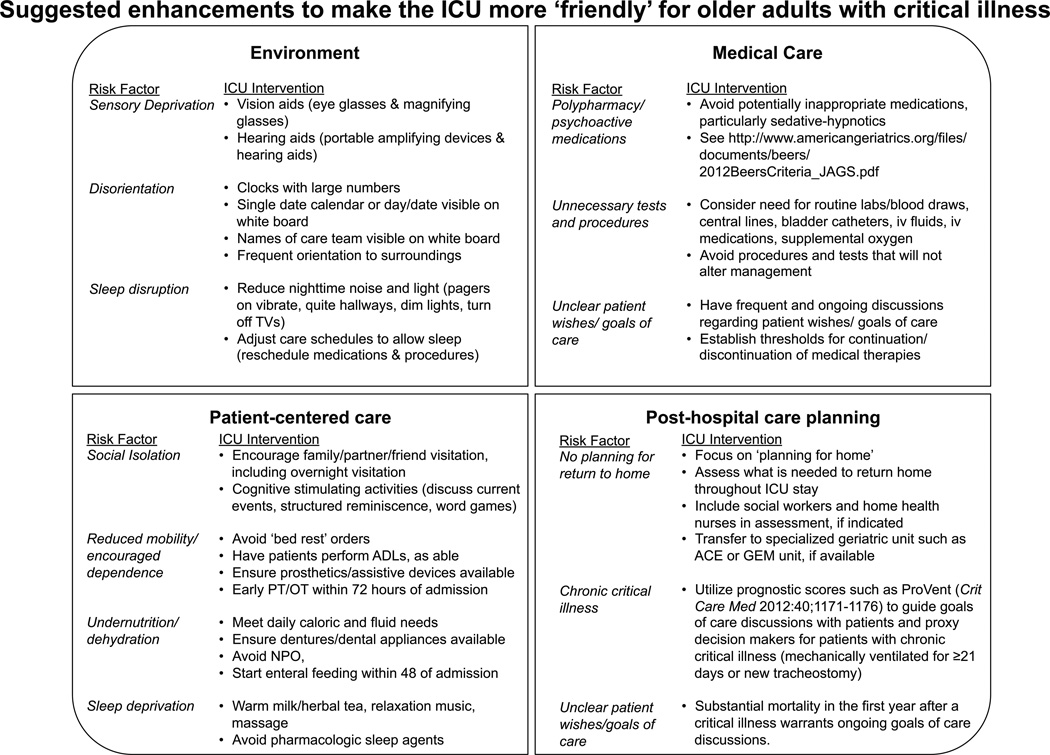
Figure 2.
Interventions adapted for the ICU from geriatric care models may be used to improve care for older adults with critical illness. PT/OT, Physical and Occupational Therapy; ACE, Acute care for elders; GEM, Geriatric evaluation and management
The aforementioned assessment tools and interventions are intended to be a pragmatic approach to caring for critically ill older adults; thus, they are far from comprehensive. To address better the specific age-related issues that affect over half of all ICU patients (1), critical care clinicians are encouraged to further their knowledge of clinical geriatrics and to seek help from experts trained in the care of older adults. Several educational resources are available both in print (e.g., American Geriatrics Society’s Geriatrics at your Fingertips) (146) and online (e.g., the Portal of Geriatrics Online Education [www.pogoe.org] and the Hartford Institute for Geriatric Nursing’s “Try This” series [www.hartfording.org/practice/try_this]). ICU clinicians and educators seeking to develop even greater expertise in the care of older adults may be eligible for “mini-fellowships” in geriatrics sponsored by the Donald W. Reynolds Foundation, which provide intensive courses in geriatrics and geriatrics education as well as follow-up support to enhance these endeavors (147). In the future, the development of collaborative training programs between critical care medicine and geriatrics, two specialties that already share a number of overlapping “Entrustable Professional Activities” (148–150), will enable trainees to face better the important challenges of caring for older adults with critical illness.
Finally, co-management strategies, such as the hip fracture “Orthogeriatric” model (i.e., co-management by both the orthopedic surgeon and a geriatrician) have been used to improve outcomes, including reductions in delirium and length of stay, improve functional status and mortality among older adults could serve as a potential care model for older adults in the ICU (151–154).
Directions for Future Research
Today, older adults who survive a critical illness are suffering with the burdens of disability, physical and/or cognitive impairments that previous generations did not face due to death and effective interventions are needed to aid this growing segment of the population. The central role that hospitalization for a critical illness plays in the development of disability afterwards is becoming clear. Nevertheless, future research is needed to understand better how the trajectory of a patient’s pre-illness functional status as well as factors relating to the patient’s critical illness and ICU treatment result in post-critical illness disabilities. Additionally, deeper knowledge of the unique contributions of post-ICU physical and cognitive dysfunction and mental health impairments to the disabling process should be sought. Interventions, that can be implemented throughout the continuum of critical illness from the earliest days in the ICU to a variety of post-ICU settings (e.g., hospital ward, rehabilitation facilities, nursing facilities and home) to prevent, treat and rehabilitate disabilities in this vulnerable and growing segment of the population should be studied and implemented. Although in need of testing in survivors of critical illness, physical exercise, resistance training and nutritional supplementation which are effective in improving physical functioning among those with aging related muscle loss (e.g., sarcopenia) (155) as well as cognitive rehabilitation that is associated with improve cognitive functioning in patients with acquired brain injuries (e.g., traumatic brain injury and stroke) (156, 157) may serve as readily available platforms by which to reduce disability after critical illness.
Conclusions
For the 1.4 million older adults in the US (and many more worldwide) who survive a critical illness each year, the subsequent months and years are fraught with significant declines in functional and cognitive status, resulting in long-term disability for as many as 2 out of every 3 patients. We argue that aging physiology, complications of critical illness, and common ICU practices contribute significantly to the development of post-critical illness disability.
Interventions derived from widely available geriatric care models in use outside of the ICU, which address modifiable risk factors including immobility and delirium, are associated with improved functional and cognitive outcomes and can be used to complement ICU-focused models such as the ABCDEs.
Supplementary Material
Acknowledgments
Financial Disclosures:
Dr. Brummel is supported by the National Institute on Aging under award number R03AG045095 and the Vanderbilt Clinical and Translational Scholars Program. Dr. Balas is supported by Alzheimer's Association. Dr. Ferrante is supported by the National Institute on Aging under award number T32AG19134. Dr. Gill is the recipient of an Academic Leadership Award from the National Institute on Aging under award number K07AG043587 and is supported by the National Institute on Aging/Yale Claude D. Pepper Older Americans Independence Center under award number P30AG21342. Dr. Ely is supported by the Veterans Affairs Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC) and by the National Institute on Aging under award number R01AG035117.
Dr. Balas and has received honoraria from ProCe, the France Foundation, Hospira, and Hillrom. Dr. Gill has received honoraria from Novartis. Dr. Ely has received research grants and/or honoraria from Hospira, Orion, and Abbott.
Footnotes
Conflicts of Interest:
The other authors report no other conflicts of interest.
Copyright form disclosures: Dr. Brummel received grant support and support for article research from the National Institutes of Health (NIH). Dr. Balas consulted for ProCe, the France Foundation, Hospira, Hillrom, Centers for Disease Control, and Cynosure Health. Her institution received grant support from the Alzheimer’s Association and from the Robert Wood Johnson Foundation Interdisciplinary Nursing Quality Research Initiative. Dr. Ferrante received support for article research from the NIH. Her institution received grant support from the NIA/NIH. Thomas M. Gill C/F (received grant support from the NIH; received support for article research from the NIH. Dr. Ely received grant support from the NIH and VA; consulted for Hospira, Abbott, Orion, and Masimo; and received support for article research from the NIH. Dr. Morandi disclosed that he does not have any potential conflicts of interest.
References
- 1.Angus DC, Shorr AF, White A, et al. Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006;34(4):1016–1024. doi: 10.1097/01.CCM.0000206105.05626.15. [DOI] [PubMed] [Google Scholar]
- 2.Barnato AE, Albert SM, Angus DC, et al. Disability among elderly survivors of mechanical ventilation. Am J Respir Crit Care Med. 2011;183(8):1037–1042. doi: 10.1164/rccm.201002-0301OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303(8):763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus DC, Kelley MA, Schmitz RJ, et al. Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA. 2000;284(21):2762–2770. doi: 10.1001/jama.284.21.2762. [DOI] [PubMed] [Google Scholar]
- 6.Wunsch H, Guerra C, Barnato AE, et al. Three-Year Outcomes for Medicare Beneficiaries Who Survive Intensive Care. JAMA. 2010;303(9):849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 7.Iwashyna TJ, Cooke CR, Wunsch H, et al. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070–1077. doi: 10.1111/j.1532-5415.2012.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins RO, Weaver LK, Collingridge D, et al. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171(4):340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 11.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 12.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29(7 Suppl):S109–S116. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 13.Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000–2007) Chest. 2011;140(5):1223–1231. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 14.Carson SS, Cox CE, Holmes GM, et al. The changing epidemiology of mechanical ventilation: a population-based study. J Intensive Care Med. 2006;21(3):173–182. doi: 10.1177/0885066605282784. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Census Bureau: Population Projections [online] 2010 [cited Available from: http://www.census.gov/population/projections/
- 16.Ely EW, Evans GW, Haponik EF. Mechanical ventilation in a cohort of elderly patients admitted to an intensive care unit. Ann Intern Med. 1999;131(2):96–104. doi: 10.7326/0003-4819-131-2-199907200-00004. [DOI] [PubMed] [Google Scholar]
- 17.Ely EW, Wheeler AP, Thompson BT, et al. Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Ann Intern Med. 2002;136(1):25–36. [PubMed] [Google Scholar]
- 18.Nelson JE, Cox CE, Hope AA, et al. Chronic critical illness. Am J Respir Crit Care Med. 2010;182(4):446–454. doi: 10.1164/rccm.201002-0210CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson JE, Tandon N, Mercado AF, et al. Brain dysfunction: another burden for the chronically critically ill. Arch Intern Med. 2006;166(18):1993–1999. doi: 10.1001/archinte.166.18.1993. [DOI] [PubMed] [Google Scholar]
- 20.Kahn JM, Benson NM, Appleby D, et al. Long-term acute care hospital utilization after critical illness. JAMA. 2010;303(22):2253–2259. doi: 10.1001/jama.2010.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reuben DB, Tinetti ME. The hospital-dependent patient. N Engl J Med. 2014;370(8):694–697. doi: 10.1056/NEJMp1315568. [DOI] [PubMed] [Google Scholar]
- 22.Gill TM. Disentangling the disabling process: insights from the precipitating events project. Gerontologist. 2014;54(4):533–549. doi: 10.1093/geront/gnu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120(4):437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Otin C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinlan N, Marcantonio ER, Inouye SK, et al. Vulnerability: the crossroads of frailty and delirium. J Am Geriatr Soc. 2011;59(Suppl 2):S262–S268. doi: 10.1111/j.1532-5415.2011.03674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Committee on a National Agenda for the Prevention of Disabilities IoM. Disability in America: Toward a National Agenda for Prevention. Washington, D.C.: National Adademy Press; 1991. [Google Scholar]
- 27.Verbrugge LM, Jette AM. The disablement process. Social science & medicine. 1994;38(1):1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 28.Jette AM. Toward a common language for function, disability, and health. Physical therapy. 2006;86(5):726–734. [PubMed] [Google Scholar]
- 29.Parno JR, Teres D, Lemeshow S, et al. Two-year outcome of adult intensive care patients. Med Care. 1984;22(2):167–176. doi: 10.1097/00005650-198402000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Mahul P, Perrot D, Tempelhoff G, et al. Short- and long-term prognosis, functional outcome following ICU for elderly. Intensive Care Med. 1991;17(1):7–10. doi: 10.1007/BF01708401. [DOI] [PubMed] [Google Scholar]
- 31.Kass JE, Castriotta RJ, Malakoff F. Intensive-Care Unit Outcome in the Very Elderly. Critical Care Medicine. 1992;20(12):1666–1671. doi: 10.1097/00003246-199212000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Chelluri L, Pinsky MR, Donahoe MP, et al. Long-term outcome of critically ill elderly patients requiring intensive care. JAMA. 1993;269(24):3119–3123. [PubMed] [Google Scholar]
- 33.Rockwood K, Noseworthy TW, Gibney RT, et al. One-year outcome of elderly and young patients admitted to intensive care units. Crit Care Med. 1993;21(5):687–691. doi: 10.1097/00003246-199305000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Broslawski GE, Elkins M, Algus M. Functional abilities of elderly survivors of intensive care. The Journal of the American Osteopathic Association. 1995;95(12):712–717. [PubMed] [Google Scholar]
- 35.Ip SP, Leung YF, Ip CY, et al. Outcomes of critically ill elderly patients: Is high-dependency care for geriatric patients worthwhile? Crit Care Med. 1999;27:2351–2357. doi: 10.1097/00003246-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Montuclard L, Garrouste-Orgeas M, Timsit JF, et al. Outcome, functional autonomy, and quality of life of elderly patients with a long-term intensive care unit stay. Crit Care Med. 2000;28(10):3389–3395. doi: 10.1097/00003246-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Udekwu P, Gurkin B, Oller D, et al. Quality of life and functional level in elderly patients surviving surgical intensive care. J Am Coll Surg. 2001;193(3):245–249. doi: 10.1016/s1072-7515(01)00994-2. [DOI] [PubMed] [Google Scholar]
- 38.Boumendil A, Maury E, Reinhard I, et al. Prognosis of patients aged 80 years and over admitted in medical intensive care unit. Intensive Care Med. 2004;30(4):647–654. doi: 10.1007/s00134-003-2150-z. [DOI] [PubMed] [Google Scholar]
- 39.de Rooij SE, Govers AC, Korevaar JC, et al. Cognitive, functional, and quality-of-life outcomes of patients aged 80 and older who survived at least 1 year after planned or unplanned surgery or medical intensive care treatment. J Am Geriatr Soc. 2008;56(5):816–822. doi: 10.1111/j.1532-5415.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 40.Balas MC, Happ MB, Yang W, et al. Outcomes Associated With Delirium in Older Patients in Surgical ICUs. Chest. 2009;135(1):18–25. doi: 10.1378/chest.08-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Somme D, Andrieux N, Guerot E, et al. Loss of autonomy among elderly patients after a stay in a medical intensive care unit (ICU): a randomized study of the benefit of transfer to a geriatric ward. Arch Gerontol Geriatr. 2010;50(3):e36–e40. doi: 10.1016/j.archger.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Daubin C, Chevalier S, Seguin A, et al. Predictors of mortality and short-term physical and cognitive dependence in critically ill persons 75 years and older: a prospective cohort study. Health Qual Life Outcomes. 2011;9:35. doi: 10.1186/1477-7525-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sacanella E, Perez-Castejon JM, Nicolas JM, et al. Functional status and quality of life 12 months after discharge from a medical ICU in healthy elderly patients: a prospective observational study. Crit Care. 2011;15(2):R105. doi: 10.1186/cc10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavoni V, Gianesello L, Paparella L, et al. Outcome and quality of life of elderly critically ill patients: an Italian prospective observational study. Arch Gerontol Geriatr. 2012;54(2):e193–e198. doi: 10.1016/j.archger.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Guerra C, Linde-Zwirble WT, Wunsch H. Risk factors for dementia after critical illness in elderly medicare beneficiaries. Crit Care. 2012;16(6):R233. doi: 10.1186/cc11901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychological function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 47.Mahoney FI, Barthel DW. Functional Evaluation: The Barthel Index. Maryland state medical journal. 1965;14:61–65. [PubMed] [Google Scholar]
- 48.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 49.Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21(4):556–559. doi: 10.1093/geronj/21.4.556. [DOI] [PubMed] [Google Scholar]
- 50.The EuroQol G. EuroQol--a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 51.Iwashyna TJ, Netzer G, Langa KM, et al. Spurious inferences about long-term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185(8):835–841. doi: 10.1164/rccm.201109-1660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gill TM, Hardy SE, Williams CS. Underestimation of disability in community-living older persons. J Am Geriatr Soc. 2002;50(9):1492–1497. doi: 10.1046/j.1532-5415.2002.50403.x. [DOI] [PubMed] [Google Scholar]
- 53.Wiener JM, Hanley RJ, Clark R, et al. Measuring the activities of daily living: comparisons across national surveys. J Gerontol. 1990;45(6):S229–S237. doi: 10.1093/geronj/45.6.s229. [DOI] [PubMed] [Google Scholar]
- 54.Federman AD, Penrod JD, Livote E, et al. Development of and recovery from difficulty with activities of daily living: an analysis of national data. Journal of aging and health. 2010;22(8):1081–1098. doi: 10.1177/0898264310375986. [DOI] [PubMed] [Google Scholar]
- 55.Boyd CM, Xue QL, Guralnik JM, et al. Hospitalization and development of dependence in activities of daily living in a cohort of disabled older women: the Women's Health and Aging Study I. J Gerontol A Biol Sci Med Sci. 2005;60(7):888–893. doi: 10.1093/gerona/60.7.888. [DOI] [PubMed] [Google Scholar]
- 56.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 57.Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997;37(4):433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- 58.McLaughlin SJ, Connell CM, Heeringa SG, et al. Successful aging in the United States: prevalence estimates from a national sample of older adults. J Gerontol B Psychol Sci Soc Sci. 2010;65(2):216–226. doi: 10.1093/geronb/gbp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57(3):B115–B125. doi: 10.1093/gerona/57.3.b115. [DOI] [PubMed] [Google Scholar]
- 61.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 62.Gill TM, Allore HG, Gahbauer EA, et al. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304(17):1919–1928. doi: 10.1001/jama.2010.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bagshaw SM, Stelfox HT, McDermid RC, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2014;186(2):E95–E102. doi: 10.1503/cmaj.130639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marik PE. Management of the critically ill geriatric patient. Crit Care Med. 2006;34:S176–S182. doi: 10.1097/01.CCM.0000232624.14883.9A. [DOI] [PubMed] [Google Scholar]
- 66.Pisani MA. Considerations in caring for the critically ill older patient. J Intensive Care Med. 2009;24(2):83–95. doi: 10.1177/0885066608329942. [DOI] [PubMed] [Google Scholar]
- 67.Balas M, Casey CM, Happ MB. Assessment and management of older adults with complex illness in the critical care unit. Hartford Institute for Geriatric Nursing; 2008. http://hartfordign.org/uploads/File/gnec_state_of_science_papers/gnec_critical_care.pdf. [Google Scholar]
- 68.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. Journal of the American Medical Directors Association. 2011;12(4):249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frontera WR, Hughes VA, Fielding RA, et al. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol (1985) 2000;88(4):1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 71.Frontera WR, Zayas AR, Rodriguez N. Aging of human muscle: understanding sarcopenia at the single muscle cell level. Physical medicine and rehabilitation clinics of North America. 2012;23(1):201–207. xiii. doi: 10.1016/j.pmr.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985) 2003;95(5):1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 73.Bunout D, de la Maza MP, Barrera G, et al. Association between sarcopenia and mortality in healthy older people. Australasian journal on ageing. 2011;30(2):89–92. doi: 10.1111/j.1741-6612.2010.00448.x. [DOI] [PubMed] [Google Scholar]
- 74.Landi F, Liperoti R, Fusco D, et al. Sarcopenia and mortality among older nursing home residents. Journal of the American Medical Directors Association. 2012;13(2):121–126. doi: 10.1016/j.jamda.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 75.Arango-Lopera VE, Arroyo P, Gutierrez-Robledo LM, et al. Mortality as an adverse outcome of sarcopenia. The journal of nutrition, health & aging. 2013;17(3):259–262. doi: 10.1007/s12603-012-0434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gariballa S, Alessa A. Sarcopenia: prevalence and prognostic significance in hospitalized patients. Clin Nutr. 2013;32(5):772–776. doi: 10.1016/j.clnu.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 77.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 78.Batt J, dos Santos CC, Cameron JI, et al. Intensive care unit-acquired weakness: clinical phenotypes and molecular mechanisms. Am J Respir Crit Care Med. 2013;187(3):238–246. doi: 10.1164/rccm.201205-0954SO. [DOI] [PubMed] [Google Scholar]
- 79.Puthucheary Z, Harridge S, Hart N. Skeletal muscle dysfunction in critical care: wasting, weakness, and rehabilitation strategies. Crit Care Med. 2010;38(10 Suppl):S676–S682. doi: 10.1097/CCM.0b013e3181f2458d. [DOI] [PubMed] [Google Scholar]
- 80.Muscaritoli M, Lucia S, Molfino A. Sarcopenia in critically ill patients: the new pandemia. Minerva Anestesiol. 2013;79(7):771–777. [PubMed] [Google Scholar]
- 81.Brown CJ, Redden DT, Flood KL, et al. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660–1665. doi: 10.1111/j.1532-5415.2009.02393.x. [DOI] [PubMed] [Google Scholar]
- 82.Zisberg A, Shadmi E, Sinoff G, et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59(2):266–273. doi: 10.1111/j.1532-5415.2010.03276.x. [DOI] [PubMed] [Google Scholar]
- 83.Pedersen MM, Bodilsen AC, Petersen J, et al. Twenty-four-hour mobility during acute hospitalization in older medical patients. J Gerontol A Biol Sci Med Sci. 2013;68(3):331–337. doi: 10.1093/gerona/gls165. [DOI] [PubMed] [Google Scholar]
- 84.Puthucheary Z, Montgomery H, Moxham J, et al. Structure to function: muscle failure in critically ill patients. The Journal of physiology. 2010;588(Pt 23):4641–4648. doi: 10.1113/jphysiol.2010.197632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kortebein P, Ferrando A, Lombeida J, et al. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297(16):1772–1774. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 86.Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52(8):1263–1270. doi: 10.1111/j.1532-5415.2004.52354.x. [DOI] [PubMed] [Google Scholar]
- 87.Gill TM, Allore HG, Holford TR, et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292(17):2115–2124. doi: 10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
- 88.Mattson MP. Cellular and Neurochemical Aspects of the Aging Human Brain. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, editors. Hazzard's geriatric medicine and gerontology. 6th ed. New York: McGraw-Hill Medical; 2009. pp. 739–750. [Google Scholar]
- 89.Yankner BA, Lu T, Loerch P. The aging brain. Annual review of pathology. 2008;3:41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- 90.Hayden KM, Reed BR, Manly JJ, et al. Cognitive decline in the elderly: an analysis of population heterogeneity. Age Ageing. 2011;40(6):684–689. doi: 10.1093/ageing/afr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Whalley LJ, Deary IJ, Appleton CL, et al. Cognitive reserve and the neurobiology of cognitive aging. Ageing research reviews. 2004;3(4):369–382. doi: 10.1016/j.arr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 92.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464(7288):529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miller DB, O'Callaghan JP. Aging, stress and the hippocampus. Ageing research reviews. 2005;4(2):123–140. doi: 10.1016/j.arr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 94.Luo XG, Ding JQ, Chen SD. Microglia in the aging brain: relevance to neurodegeneration. Molecular neurodegeneration. 2010;5:12. doi: 10.1186/1750-1326-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain BehavImmun. 2004;18(5):407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 96.Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190–1222. doi: 10.1016/j.jagp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 97.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852–857. [PubMed] [Google Scholar]
- 99.Sanders RD. Hypothesis for the pathophysiology of delirium: role of baseline brain network connectivity and changes in inhibitory tone. Med Hypotheses. 2011;77(1):140–143. doi: 10.1016/j.mehy.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 100.Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789–856. ix. doi: 10.1016/j.ccc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 101.Gross AL, Jones RN, Habtemariam DA, et al. Delirium and Long-term Cognitive Trajectory Among Persons With Dementia. Arch Intern Med. 2012;172(17):1324–1331. doi: 10.1001/archinternmed.2012.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davis DH, Muniz Terrera G, Keage H, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain. 2012;135(Pt 9):2809–2816. doi: 10.1093/brain/aws190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72(18):1570–1575. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Critical Care Medicine. 2010;38(7):1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Gool WA, van de BD, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375(9716):773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 106.Khan BA, Zawahiri M, Campbell NL, et al. Biomarkers for delirium--a review. J Am Geriatr Soc. 2011;59(Suppl 2):S256–S261. doi: 10.1111/j.1532-5415.2011.03702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: "She was probably able to ambulate, but I'm not sure". JAMA. 2011;306(16):1782–1793. doi: 10.1001/jama.2011.1556. [DOI] [PubMed] [Google Scholar]
- 108.Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219–223. doi: 10.7326/0003-4819-118-3-199302010-00011. [DOI] [PubMed] [Google Scholar]
- 109.Braes T, Flamaing J, Sterckx W, et al. Predicting the risk of functional decline in older patients admitted to the hospital: a comparison of three screening instruments. Age Ageing. 2009;38(5):600–603. doi: 10.1093/ageing/afp097. [DOI] [PubMed] [Google Scholar]
- 110.McCusker J, Kakuma R, Abrahamowicz M. Predictors of functional decline in hospitalized elderly patients: a systematic review. J Gerontol A Biol Sci Med Sci. 2002;57(9):M569–M577. doi: 10.1093/gerona/57.9.m569. [DOI] [PubMed] [Google Scholar]
- 111.Mehta KM, Pierluissi E, Boscardin WJ, et al. A clinical index to stratify hospitalized older adults according to risk for new-onset disability. J Am Geriatr Soc. 2011;59(7):1206–1216. doi: 10.1111/j.1532-5415.2011.03409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Inouye SK, Wagner DR, Acampora D, et al. A predictive index for functional decline in hospitalized elderly medical patients. J Gen Intern Med. 1993;8(12):645–652. doi: 10.1007/BF02598279. [DOI] [PubMed] [Google Scholar]
- 113.Ellis G, Whitehead MA, O'Neill D, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2011;(7):CD006211. doi: 10.1002/14651858.CD006211.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bogardus ST, Jr, Towle V, Williams CS, et al. What does the medical record reveal about functional status? A comparison of medical record and interview data. J Gen Intern Med. 2001;16(11):728–736. doi: 10.1111/j.1525-1497.2001.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brown CJ, Roth DL, Allman RM, et al. Trajectories of life-space mobility after hospitalization. Ann Intern Med. 2009;150(6):372–378. doi: 10.7326/0003-4819-150-6-200903170-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abelha FJ, Luis C, Veiga D, et al. Outcome and quality of life in patients with postoperative delirium during an ICU stay following major surgery. Crit Care. 2013;17(5):R257. doi: 10.1186/cc13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brummel NE, Jackson JC, Pandharipande PP, et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation*. Crit Care Med. 2014;42(2):369–377. doi: 10.1097/CCM.0b013e3182a645bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 120.Morris PE, Griffin L, Berry M, et al. Receiving early mobility during an intensive care unit admission is a predictor of improved outcomes in acute respiratory failure. AmJMed Sci. 2011;341(5):373–377. doi: 10.1097/MAJ.0b013e31820ab4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Asher RA. The dangers of going to bed. Br Med J. 1947;2(4536):967. doi: 10.1136/bmj.2.4536.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Engel GL, Romano J. Delirium, a syndrome of cerebral insufficiency. J Chronic Dis. 1959;9(3):260–277. doi: 10.1016/0021-9681(59)90165-1. [DOI] [PubMed] [Google Scholar]
- 123.Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263(8):1097–1101. [PubMed] [Google Scholar]
- 124.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 125.Bergeron N, Dubois MJ, Dumont M, et al. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27(5):859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 126.Bailey P, Thomsen GE, Spuhler VJ, et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35(1):139–145. doi: 10.1097/01.CCM.0000251130.69568.87. [DOI] [PubMed] [Google Scholar]
- 127.Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 128.Inouye SK, Wagner DR, Acampora D, et al. A controlled trial of a nursing-centered intervention in hospitalized elderly medical patients: the Yale Geriatric Care Program. J Am Geriatr Soc. 1993;41(12):1353–1360. doi: 10.1111/j.1532-5415.1993.tb06487.x. [DOI] [PubMed] [Google Scholar]
- 129.Fulmer T, Mezey M, Bottrell M, et al. Nurses Improving Care for Healthsystem Elders (NICHE): using outcomes and benchmarks for evidenced-based practice. Geriatr Nurs. 2002;23(3):121–127. doi: 10.1067/mgn.2002.125423. [DOI] [PubMed] [Google Scholar]
- 130.Baztan JJ, Suarez-Garcia FM, Lopez-Arrieta J, et al. Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: meta-analysis. BMJ. 2009;338:b50. doi: 10.1136/bmj.b50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bachmann S, Finger C, Huss A, et al. Inpatient rehabilitation specifically designed for geriatric patients: systematic review and meta-analysis of randomised controlled trials. BMJ. 2010;340:c1718. doi: 10.1136/bmj.c1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Landefeld CS, Palmer RM, Kresevic DM, et al. A Randomized Trial of Care in a Hospital Medical Unit Especially Designed to Improve the Functional Outcomes of Acutely Ill Older Patients. New England Journal of Medicine. 1995;332(20):1338–1344. doi: 10.1056/NEJM199505183322006. [DOI] [PubMed] [Google Scholar]
- 133.Vasilevskis EE, Pandharipande PP, Girard TD, et al. A screening, prevention, and restoration model for saving the injured brain in intensive care unit survivors. Crit Care Med. 2010;38(10):S683–S691. doi: 10.1097/CCM.0b013e3181f245d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vasilevskis EE, Ely EW, Speroff T, et al. Reducing Iatrogenic Risks. ICU -Acquired Delirium and Weakness- Crossing the Quality Chasm. Chest. 2010;138(5):1224–1233. doi: 10.1378/chest.10-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Balas MC, Vasilevskis EE, Burke WJ, et al. Critical Care Nurses' Role in Implementing the "ABCDE Bundle" Into Practice. Crit Care Nurse. 2012;32(2):35–47. doi: 10.4037/ccn2012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Morandi A, Brummel NE, Ely EW. Sedation, delirium and mechanical ventilation: the 'ABCDE' approach. Curr Opin Crit Care. 2011;17(1):43–49. doi: 10.1097/MCC.0b013e3283427243. [DOI] [PubMed] [Google Scholar]
- 137.Balas M, Buckingham R, Braley T, et al. Extending the ABCDE bundle to the post-intensive care unit setting. J Gerontol Nurs. 2013;39(8):39–51. doi: 10.3928/00989134-20130530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Balas MC, Burke WJ, Gannon D, et al. Implementing the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle into everyday care: opportunities, challenges, and lessons learned for implementing the ICU Pain, Agitation, and Delirium Guidelines. Crit Care Med. 2013;41(9 Suppl 1):S116–S127. doi: 10.1097/CCM.0b013e3182a17064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Balas MC, Vasilevskis EE, Olsen KM, et al. Effectiveness and Safety of the Awakening and Breathing Coordination, Delirium Monitoring/Management, and Early Exercise/Mobility Bundle. Crit Care Med. 2014 doi: 10.1097/CCM.0000000000000129. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 141.Fox MT, Persaud M, Maimets I, et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta-analysis. J Am Geriatr Soc. 2012;60(12):2237–2245. doi: 10.1111/jgs.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pronovost PJ. Enhancing physicians' use of clinical guidelines. JAMA. 2013;310(23):2501–2502. doi: 10.1001/jama.2013.281334. [DOI] [PubMed] [Google Scholar]
- 143.Kahn JM, Gunn SR, Lorenz HL, et al. Impact of nurse-led remote screening and prompting for evidence-based practices in the ICU*. Crit Care Med. 2014;42(4):896–904. doi: 10.1097/CCM.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014–2020. doi: 10.1097/01.ccm.0000142399.70913.2f. [DOI] [PubMed] [Google Scholar]
- 145.Waitman LR, Phillips IE, McCoy AB, et al. Adopting real-time surveillance dashboards as a component of an enterprisewide medication safety strategy. Joint Commission journal on quality and patient safety / Joint Commission Resources. 2011;37(7):326–332. doi: 10.1016/s1553-7250(11)37041-9. [DOI] [PubMed] [Google Scholar]
- 146.American Geriatrics Society. Geriatrics at your fingertips. Belle Mead, N.J.: Excerpta Medica; 2013. [Google Scholar]
- 147.Heflin MT, Bragg EJ, Fernandez H, The Donald W, et al. Reynolds Consortium for Faculty Development to Advance Geriatrics Education (FD~AGE): a model for dissemination of subspecialty educational expertise. Academic medicine : journal of the Association of American Medical Colleges. 2012;87(5):618–626. doi: 10.1097/ACM.0b013e31824d5251. [DOI] [PubMed] [Google Scholar]
- 148.Fessler HE, Addrizzo-Harris D, Beck JM, et al. Entrustable professional activities and curricular milestones for fellowship training in pulmonary and critical care medicine: executive summary from the Multi-Society Working Group. Crit Care Med. 2014;42(10):2290–2291. doi: 10.1097/CCM.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 149.Fessler HE, Addrizzo-Harris D, Beck JM, et al. Entrustable professional activities and curricular milestones for fellowship training in pulmonary and critical care medicine: report of a multisociety working group. Chest. 2014;146(3):813–834. doi: 10.1378/chest.14-0710. [DOI] [PubMed] [Google Scholar]
- 150.Leipzig RM, Sauvigne K, Granville LJ, et al. What is a geriatrician? American Geriatrics Society and Association of Directors of Geriatric Academic Programs end-of-training entrustable professional activities for geriatric medicine. J Am Geriatr Soc. 2014;62(5):924–929. doi: 10.1111/jgs.12825. [DOI] [PubMed] [Google Scholar]
- 151.Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 152.Gonzalez-Montalvo JI, Alarcon T, Mauleon JL, et al. The orthogeriatric unit for acute patients: a new model of care that improves efficiency in the management of patients with hip fracture. Hip international : the journal of clinical and experimental research on hip pathology and therapy. 2010;20(2):229–235. doi: 10.1177/112070001002000214. [DOI] [PubMed] [Google Scholar]
- 153.Adunsky A, Lusky A, Arad M, et al. A comparative study of rehabilitation outcomes of elderly hip fracture patients: the advantage of a comprehensive orthogeriatric approach. J Gerontol A Biol Sci Med Sci. 2003;58(6):542–547. doi: 10.1093/gerona/58.6.m542. [DOI] [PubMed] [Google Scholar]
- 154.Boddaert J, Cohen-Bittan J, Khiami F, et al. Postoperative admission to a dedicated geriatric unit decreases mortality in elderly patients with hip fracture. PloS one. 2014;9(1):e83795. doi: 10.1371/journal.pone.0083795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age Ageing. 2014;43(6):748–759. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cicerone KD, Langenbahn DM, Braden C, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil. 2011;92(4):519–530. doi: 10.1016/j.apmr.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 157.Cicerone KD, Dahlberg C, Malec JF, et al. Evidence-based cognitive rehabilitation: Updated review of the literature from 1998 through 2002. Archives of Physical Medicine and Rehabilitation. 2005;86(8):1681–1692. doi: 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 158.Borson S, Scanlan J, Brush M, et al. The mini-cog: a cognitive 'vital signs' measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021–1027. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 159.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24(1):145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.