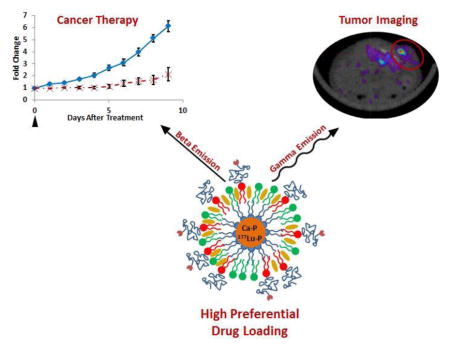
Abstract
We have developed a theranostic nanoparticle delivering the model radionuclide 177Lu based on the versatile lipid-calcium-phosphate (LCP) nanoparticle delivery platform. Characterization of 177Lu-LCP has shown that radionuclide loading can be increased by several orders of magnitude without affecting the encapsulation efficiency or the morphology of 177Lu-LCP, allowing consistency during fabrication and overcoming scale-up barriers typical of nanotherapeutics. The choice of 177Lu as a model radionuclide has allowed in vivo anticancer therapy in addition to radiographic imaging via the dual decay modes of 177Lu. Tumor accumulation of 177Lu-LCP was measured using both SPECT and Cerenkov imaging modalities in live mice, and treatment with just one dose of 177Lu-LCP showed significant in vivo tumor inhibition in two subcutaneous xenograft tumor models. Microenvironment and cytotoxicity studies suggest that 177Lu-LCP inhibits tumor growth by causing apoptotic cell death via double-stranded DNA breaks while causing a remodeling of the tumor microenvironment to a more disordered and less malignant phenotype.
Keywords: Cancer, Theranostic, Nanoparticle, High Specific Drug Loading
Graphical abstract
Introduction
Standard cancer treatment regimens use combinations of surgery, chemotherapy, and radiation therapy to eradicate the disease. The majority of patients receiving radiation treatments undergo external beam radiation therapy, but systemic internal radiotherapy has also achieved substantial clinical success in the last decade [1]. Unlike external beam therapy, which generally bombards the tumor area with photons or electrons emitted from an external source, systemic radiotherapy targets radionuclides to the tumor site via intravenous injection. The radiation source therefore resides inside the body, and the low radiation emission distance only allows the therapy to affect cells positioned close to particle deposition. This is in contrast to external beam therapy in which x-rays or gamma rays pass through the body from an external source. The current gold standard in systemic radiotherapy, called radioimmunotherapy, chelates the radioisotope to a tumor-targeted monoclonal antibody. The radio-immunotherapeutic Zevalin, which is labeled with the radioisotope 90Yttrium, was the first of its kind, approved by the FDA in 2002 for use against B-cell lymphoma [2], but its clinical success has been tempered because of its high cost and antibody-mediated toxicity [3–5].
In this paper we describe a novel method for systemic internal radiotherapy. We have previously developed a targeted and versatile nanoparticle platform termed Lipid Calcium Phosphate (LCP) [6]. LCP is formulated by mixing two reverse microemulsions containing concentrated calcium and phosphate, and can encapsulate phosphorylated small molecules, peptides, and nucleic acids through their co-precipitation into the amorphous calcium-phosphate precipitate [7–9]. In this report we have now extended the application of this LCP platform by developing a theranostic nanoparticle with high specific drug loading for cancer therapy via the encapsulation of the model radioisotope 177Lutetium (177Lu). Trivalent cations such as lutetium can be driven into LCP, as their solubilities with phosphate are many orders of magnitude lower than the solubility of calcium phosphate. Phosphate then prefers to precipitate with these radioisotopes and even a trace amount can be encapsulated with high efficiency.
High encapsulation efficiency of a drug is often attainable only when a low amount of drug is loaded into each particle, requiring large doses of the particle to achieve a therapeutic effect. This can cause particle-mediated toxicity and make clinical scale-up very difficult and expensive. Our method of radioisotope loading does not encounter these issues. Because 177Lu can be driven into LCP due to its low solubility with phosphate, and because only a small mass of 177Lu is needed to provide a therapeutic effect, the drug loading window for a batch of LCP can span several orders of magnitude without affecting the 177Lu encapsulation efficiency or LCP morphology, and without requiring a change in any other input parameters.
177Lu was chosen as a model radionuclide because of its ability to provide simultaneous imaging and therapy: γ-radiation from 177Lu is detectible using Single Photon Emission Computed Tomography (SPECT), while β-decay from 177Lu causes DNA damage to nearby cells in addition to inducing Cerenkov radiation that is detectible using optical imaging techniques. Targeted delivery of these radionuclides therefore allows measurement of tumor accumulation in live mice while simultaneously treating the tumor mass. Unlike chemotherapeutics, radioisotopes like 177Lu decay at a constant rate independent of their environment, providing a low, continuous dose to the tumor consistent with their decay rate. We therefore tested the ability of 177Lu-LCP to achieve a sustained and quantifiable therapeutic effect in vivo after just a single dose.
Materials and Methods
2.0.1: Materials
177LuCl3 was purchased from PerkinElmer (Waltham, MA). 1,2-dioleoyl-sn-glycero-3-phosphate (DOPA), and 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) were purchased from Avanti Polar Lipids (Alabaster, AL). N-(Carbonyl-methoxypolyethyleneglycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine, sodium salt (DSPE-PEG2000) was purchased by NOF America Corporation (White Plains, NY). DSPE-PEG2000-Anisamide (DSPE-PEG-AA) was synthesized in our lab as described previously [10]. Other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
2.0.2: Cell Lines
Three different cell lines were used in the experiments described below: NCI-H460 (H460) human non-small cell lung cancer cells, UMUC3 human bladder cancer cells, and NIH/3T3 (3T3) murine fibroblasts. In some experiments, 3T3 cells that had been stably transfected with green fluorescent protein (GFP) were used.
2.0.3: Experimental Animals
6–8 week old female athymic nude mice were used for all experiments presented in this manuscript. These mice were purchased from the National Cancer Institute (Bethesda, MD) and bred at the Division of Laboratory Animal Medicine at the University of North Carolina-Chapel Hill. All work performed on these animals was approved by the Institutional Animal Care and Use Committee at the University of North Carolina-Chapel Hill.
2.1: 177Lu-LCP Fabrication and Characterization
177Lu-LCP fabrication has been modified [6, 11] from what is described in previous publications. Two reverse microemulsions were prepared in round-bottom flasks. Both microemulsion oil phases contained 73.7:6.7:9.7:10 Cyclohexane:Hexanol:IgepalCO520:TritonX-100 (v:v:v:v). 25mg/ml DOPA was added in chloroform to one oil phase (1:0.023 oil:DOPA v:v). A Phosphate aqueous phase (1:0.0125 oil:aqueous v:v) containing 100mM Na2HPO4 in 0.0125M NaOH was added to the DOPA oil phase with stirring. A Calcium aqueous phase containing 500mM CaCl2 and trace 177LuCl3 in 0.0125M HCl was added to the other oil phase. After five minutes, the oil phase containing phosphate was added to the oil phase containing calcium and stirred for 40 minutes. 100% ethanol was then added to oil (1:1 ethanol:oil v:v) and stirred for 30 minutes. LCP was washed three times via centrifugation at 12,600g, removing the supernatant, replacing the supernatant with 100% ethanol, and vortexing the solution to resuspend the LCP pellet. Lu, Ca, and PO4 that had not precipitated during mixing were discarded in the supernatant of the initial wash (~15% of input 177Lu was removed here), and minimal 177Lu signal was detected in the second and third supernatants. After the final wash, the precipitate was dissolved in chloroform and centrifuged at 10,000g for five minutes. This supernatant then contained the organic-soluble LCP cores, and the pellet contained any precipitate that was not well-coated in DOPA, and therefore not soluble in chloroform (~15% of input 177Lu was removed here). Purifying the LCP cores in these two ways ensured that 177Lu was only present inside the LCP. Outer leaflet lipids (OLLs) dissolved in chloroform were then added to the chloroform-soluble cores (40:40:18:2 DOPC:cholesterol:DSPE-PEG2000:DSPE-PEG2000-AA mol:mol:mol) (0.11:1 total OLL:initial oil phase v:v; all lipids at 20mM concentration). In experiments using DiI-labeled LCP, DiI dissolved in chloroform was added to the cores with the OLLs (1:100 DiI:total OLL mol:mol). Chloroform was evaporated until the particles and lipids coated the vial in a lipid film. Particles were then rehydrated with 80°C water to the injection volume. The solution was vortexed and sonicated liberally, then heated at 80°C for >10 min and passively cooled. The anisamide ligand was used in all formulations to target the sigma receptor overexpressed in UMUC3 and H460 cells [12, 13].
In experiments using DiI-labeled Lu-LCP, sucrose gradient ultracentrifugation (50,000g for 4 h) was used to purify DiI-Lu-LCP from excess free DiI and DiI that had incorporated into excess free liposomes. Encapsulation efficiency of 177Lu into 177Lu-LCP was measured by a Model AA2010 “Nucleus” Gamma Scintillation Counter or a Capintec Radioisotope Calibrator CRC-127R and calculated as (signal from 177Lu in LCP cores)/(signal from 177Lu in input aqueous phase). 177Lu-LCP hydrodynamic diameter and zeta potential were measured after sucrose gradient ultracentrifugation using a Malvern Nano ZS dynamic light scattering instrument. Three different batches of LCP were measured. Particle size was corroborated using transmission electron microscopy (TEM).
2.2: Loading Capacity
To simulate an increase in 177Lu loading into 177Lu-LCP, a trace amount of 177Lu was supplemented with additional nonradioactive 175Lu. As the Lu:Ca input ratio was increased from 1:10,000,000 to 1:1, no changes in any inputs were made save for the increase in 175Lu. 177Lu EE was measured using gamma scintillation and 177Lu-LCP morphologies/structural makeups were measured using TEM and energy-dispersive X-ray spectroscopy (EDS) at the Chapel Hill Analytical and Nanofabrication Laboratory at the University of North Carolina at Chapel Hill. TEM images were taken using a TEM JEOL 2010F-FasTEM or a TEM JEOL 100CX II, and EDS measurements were taken using the TEM JEOL 100CX II.
2.3: Pharmacokinetics and Biodistribution
Healthy nude mice were injected with 177Lu-LCP or free 177LuCl3 of known activities. The 177Lu-LCP or 177LuCl3 was injected into the left tail vein and circulating 177Lu-LCP or 177LuCl3 was measured by removing 20–30 μl of blood from the right tail vein at several time points after injection. The signal was read using gamma scintillation and was used to calculate the total signal remaining in the blood. A Microsoft Excel Add-In called PKSolver [14] was used for the pharmacokinetic analysis. In a separate experiment, H460 or UMUC3/3T3 tumor-bearing mice were injected with a therapeutic dose of 177Lu-LCP or free 177LuCl3. Twenty four h after injection, the mice were sacrificed and their organs were dissected and read for 177Lu activity.
2.4: SPECT and Cerenkov Imaging
UMUC3/3T3 tumor-bearing nude mice were i.v. injected with 2.5 mCi of 177Lu-LCP or free 177LuCl3. Twenty four h later, the mice were imaged using a small animal SPECT/CT system (eXplore speCZT, GE Healthcare) at the Small Animal Imaging facility on the UNC campus. SPECT Images were taken with the energy window to be set from 188–229 keV to receive the main gamma photon from Lu-77 peaked at 208 keV. A mouse slit collimator was used to provide whole body imaging with 1.5mm transaxial and 2.5mm axial resolution, and a pinhole collimator was used for partial body imaging with higher resolution (1mm isotropic resolution). Immediately after SPECT/CT imaging, the mice were taken to an in vivo optical imaging system (IVIS-Kinetic, Perkin Elmer) to measure Cerenkov emissions generated by 177Lu.
2.5: In Vivo Tumor Inhibition
Six to eight week old nude mice were subcutaneously inoculated with 100ul PBS containing 5×106 H460 cells or 5×106 UMUC3 cells + 2.5×106 3T3 cells in 25% matrigel (BD matrigel matrix high concentration) on the left flank. After 8–12 days, when tumors had reached a size of 100–150mm3, mice were given a single injection of PBS (Untreated mice), non-radioactive (cold) 175Lu-LCP, free radioactive 177LuCl3, or radioactive 177Lu-LCP. Mice bearing UMUC3/3T3 tumors received a dose of 200 μCi/mouse and mice bearing H460 tumors received a dose of 250 μCi/mouse. Mouse weight was measured every 2 days until untreated mice reached humane endpoints. Tumor volume was measured with digital calipers and calculated as (L x W x D)/2. n = 5–8. Student’s t-test of the final AUC used for statistical analysis.
2.6 Tumor Accumulation
Nude mice bearing s.c. H460 or UMUC3/3T3 tumors were treated with one dose of 250 μCi or 200 μCi 177Lu-LCP, respectively, or appropriate controls. The lipid bilayer of the 177Lu-LCP particles were labeled with the fluorescent small molecule DiI to allow LCP measurement in the tumor. Twenty four h after injection, mice were sacrificed and tumors were dissected and cut in half. One half was frozen in OCT, sectioned, and mounted for DiI Lu-LCP imaging, and the other half was fixed in 4% formalin, embedded in paraffin, and sectioned for CD-31 staining (1:100 dilution rabbit primary Ab, Abcam #ab28364; 1:100 dilution anti-rabbit 647 fluorescent secondary Ab, Cell Signaling #4414) and DAPI counterstain.
2.7 Cytotoxicity Studies
One, 2, and 4 days after treatment with 200 μCi 177Lu-LCP or controls, UMUC3/3T3 tumor-bearing nude mice were sacrificed and their tumors were dissected, fixed, and sectioned in paraffin. Adjacent sections were stained for p-H2AX immunofluorescence (1:100 dilution Rabbit monoclonal primary Ab, Cell Signaling #9718; 1:100 dilution anti-rabbit 647 fluorescent secondary Ab, Cell Signaling #4414) and Terminal deoxynucleotidyl transferase dUTP nick end labeling (DeadEnd™ Fluorometric TUNEL System, Promega) with DAPI counterstains.
2.8 Microenvironment Studies
Nude mice were inoculated with UMUC3/3T3-GFP (fluorescent) tumors using the same cell numbers reported above. When tumors reached ~400 mm3, mice were treated with 200 μCi of fluorescently labeled DiI-177Lu-LCP or controls. After 2 days, mice were sacrificed and their tumors were dissected and prepared for freezing in OCT via a 2-h fixation in 4% formalin followed by overnight incubation in a 30% sucrose solution at 4°C. The frozen tumors were sectioned and imaged with DAPI counterstain to measure GFP and DiI distribution. DiI distribution was quantified using Matlab.
Separate nude mice were inoculated with UMUC3/3T3 (nonfluorescent) tumors. When tumors reached ~300 mm3, mice were treated with 200 μCi of 177Lu-LCP or controls. Four days after treatment, the tumors were dissected, fixed, and sectioned in paraffin. Adjacent sections were trichrome stained and stained for α-SMA immunofluorescence (1:100 dilution Rabbit monoclonal primary Ab, Abcam #ab5694; 1:100 dilution anti-rabbit 647 fluorescent secondary Ab, Cell Signaling #4414) with DAPI counterstain. The stained sections were digitally scanned using the Aperio Scanscope at the University of North Carolina-Chapel Hill’s Translational Pathology Lab. Quantitative analysis was performed on Imagescope and ImageJ software.
2.9: Toxicity Studies
Nude mice and CD-1 mice were treated with 250 μCi of 177Lu-LCP or appropriate controls and were sacrificed 12 days after treatment. Blood and organs were dissected. Serum levels of AST, ALT, BUN, and creatinine were measured, and organs were stained with H&E to measure any changes in tissue morphology. Whole blood was measured for changes in white blood cell count (WBC), hematocrit (HCT), mean cell volume (MCV), red blood cell count (RBC), hemoglobin count (HGB), and platelet count (PLT).
Results
3.1: Lu-LCP Fabrication and Characterization
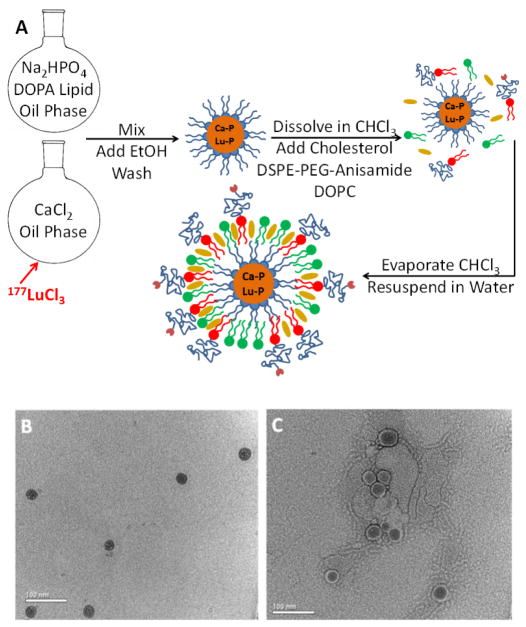
The LCP nanoparticle platform preferentially encapsulates 177Lu in its solid amorphous calcium phosphate core during fabrication (Scheme shown in Figure 1A). Just a trace amount of 177Lu can be efficiently encapsulated because of lutetium’s much lower solubility with phosphate (ksp = 2×10−25) [15] when compared to calcium’s solubility with phosphate (ksp = 1×10−7). The fabrication protocol for 177Lu-LCP was modified [6, 11] from previous publications to maximize 177Lu encapsulation efficiency (EE) at approximately 70%, with the majority of the loss occurring during washing and purification. Particles were measured via dynamic light scattering to have a diameter of 36 ± 9 nm and a polydispersity index of 0.27 ± 0.05, with a zeta potential of −6.7 ± 3.5 mV after separating the particles from excess empty liposomes using sucrose gradient centrifugation (Supplemental Table S1). Particle size was corroborated by transmission electron microscopy (Figure 1B–C). Although cellular entry is not requisite for 177Lu-LCP to achieve therapeutic effect, because anisamide has been proven as an effective targeting ligand against the sigma receptor overexpressed in epithelial cancers such as UMUC3 and H460 [12, 13], and because receptor-mediated endocytosis draws nanoparticles inside the cell and closer to nuclear DNA, DSPE-PEG-Anisamide was exclusively used in all Lu-LCP formulations as described in section 2.1 to provide the greatest statistical chance for treatment efficacy.
Figure 1.
177Lu-LCP Formulation. A) Schematic depicting general formulation procedure. B) TEM image of final 177Lu-LCP. C) TEM image of final 177Lu-LCP with uranyl acetate negative stain applied. Scale bar represents 100 nm.
3.2: Loading Capacity
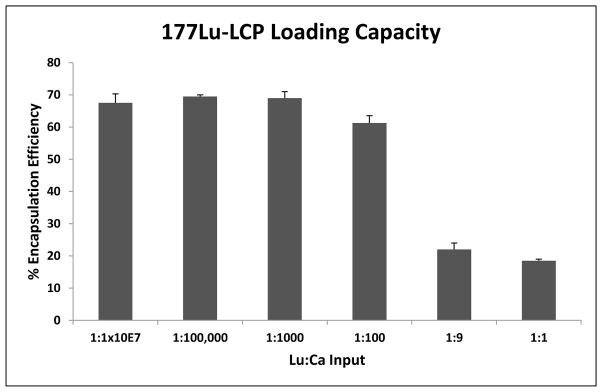
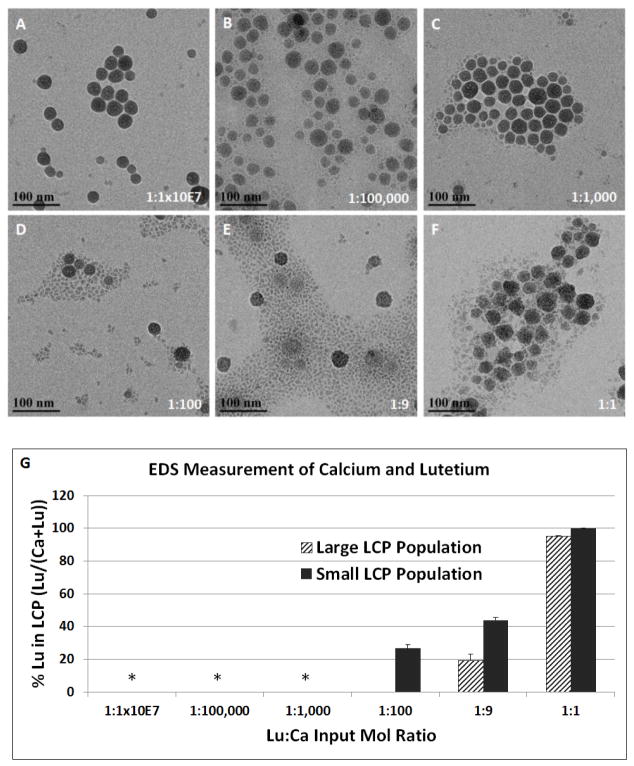
Preferential loading of 177Lu allows 177Lu-LCP to be fabricated with a very high radioisotope concentration, consequently allowing very high drug loading in a particle that is still overwhelmingly comprised of calcium phosphate. Figure 2 and Table 1 show that the drug loading window in equivalent-sized batches of LCP core spans across several orders of magnitude without affecting the 177Lu encapsulation efficiency and not requiring a change in any other input parameters. Additionally, the LCP morphology remains unchanged up to a Lu:Ca input ratio of 1:1000 (Figure 3A–F), above which the Lu starts becoming a structural component of the LCP, as measured by EDS (Figure 3G), which introduces heterogeneity to the formulation. As Lu:Ca is increased to 1:1, Lu forces out nearly all Ca from the LCP to form cores of LuPO4, but the total dose of heavy metal per particle is undesirable due to its inherent toxicity. These data suggest that the maximum Lu:Ca input ratio that maintains desirable encapsulation and morphology is 1:1000. At this input ratio, ~24 mCi of 177Lu—enough to treat over 100 mice—can be encapsulated in a batch size of only 3.2 total mL of oil phase. Considering that it is not uncommon for other types of LCP formulations to be synthesized in batches of 120ml or more for small mouse studies, one can understand how Lu-LCP is able to avoid scale-up complications generally associated with nanoparticles. Moreover, a very small mass of total lutetium—approximately 70 pmol/mouse, or 6×10−4 mg/kg—is used here to achieve therapeutic effect. This low total dose is important in minimizing heavy metal-mediated toxicity. Combined, the high drug loading window and low total mass of lutetium in 177Lu-LCP minimize batch size and toxicity while subsequently allowing the treatment of a large number of animals per batch.
Figure 2.
177Lu-LCP encapsulation efficiencies at increasing levels of lutetium. 177Lu EE decrease begins around 1:100 Lu:Ca. n = 2–3.
Table 1.
177Lu-LCP inputs and output activity based on a specific activity of 20 Ci/mg of lutetium, the minimum standard for PerkinElmer delivery. Ca is always given at a 5x molar excess of PO4. Highest level of stable LCP formulation is 1:1000 Lu:Ca. A trace amount of 177Lu was supplemented with nonradioactive 175Lu to achieve the proper Lu:Ca ratio.
| Lu:Ca Input (mols) | PO4 Input (mols) | Total Lu Input (mols) | Input Activity* (mCi) | 177Lu EE | Output Activity (mCi) |
|---|---|---|---|---|---|
| 1:1×107 | 2.0×10−6 | 1.0×10−12 | 0.0035 | 0.68 | 0.0024 |
| 1:100,000 | ” | 1.0×10−10 | 0.354 | 0.70 | 0.25 |
| 1:1000 | ” | 1.0×10−8 | 35.4 | 0.69 | 24.4 |
| 1:100 | ” | 1.0×10−7 | 354 | 0.61 | 217 |
| 1:9 | ” | 1.1×10−6 | 3933 | 0.22 | 865 |
| 1:1 | ” | 1.0×10−5 | 35400 | 0.19 | 6549 |
Figure 3.
177Lu-LCP morphologies at different Lu:Ca inputs. A–F) TEM images of 177Lu-LCP at varying Lu inputs. G) Percent Lu in output 177Lu-LCP based on Lu:Ca inputs, calculated by energy dispersive X-ray spectroscopy (EDS). *Lu not detected; negligible small LCP population
3.3: Pharmacokinetics and Biodistribution
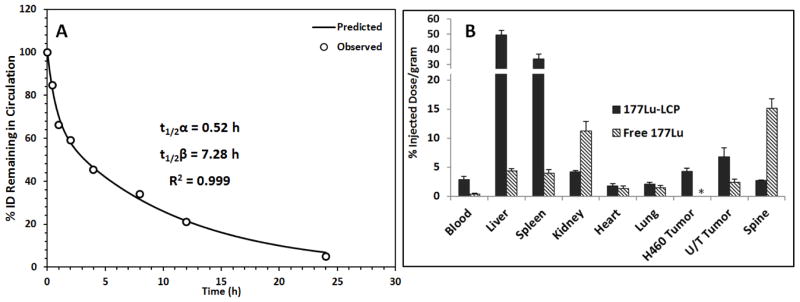
The pharmacokinetics and biodistribution of 177Lu-LCP were determined in tumor-bearing nude mice. A two-compartment model [14] was used to calculate the clearance half-lives, which were 0.52 h for the distribution half-life, t1/2α, and 7.28 h for the elimination half-life, t1/2β, as shown in Figure 4A. Pharmacokinetic analysis was also performed for free 177LuCl3, which showed nearly 80% blood clearance in the first 30 min after injection (Figure S1). It can therefore be concluded that the circulating signal from mice given 177Lu-LCP is indeed from intact particles. The biodistribution of 177Lu-LCP and free 177Lu in tumor-bearing mice is given in Figure 4B. 177Lu-LCP shows significant accumulation in the tumor, as well as accumulation in the nanoparticle clearing organs, the liver and spleen, as it is too large to clear renally. In contrast, free 177Lu is an ion that is plenty small to clear through the kidneys, but also selectively accumulates in the bones, as lanthanide elements are known to do [16]. Free 177Lu also shows some kidney accumulation, which may occur during the quick renal clearance of the element (Figure 4B).
Figure 4.
177Lu-LCP Pharmacokinetics and Biodistribution. A) The PK curve of i.v. injected 177Lu-LCP fit to a two-compartment model; n = 5. B) Organ BD at t = 24h after i.v. injection of 177Lu-LCP or free 177LuCl3; n = 3–4. H460 tumor and UMUC3/3T3 (U/T) tumor were subcutaneously inoculated in different mice. *BD of free 177Lu was not tested in H460 tumor-bearing mice.
3.4: SPECT and Cerenkov Imaging
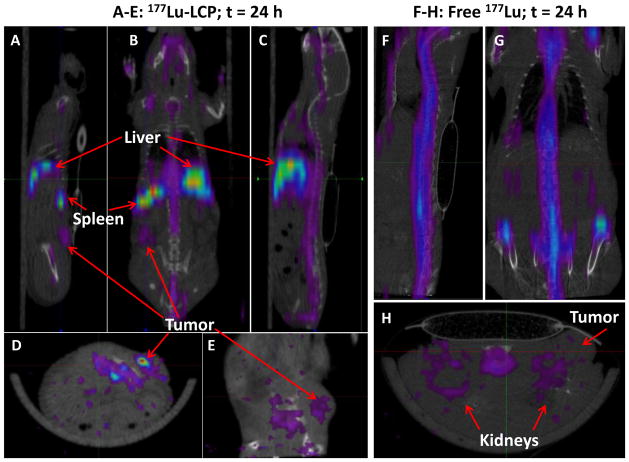
In addition to allowing simple means of detection using gamma scintillation, 177Lu can also be used as a SPECT contrast agent, permitting measurement of 177Lu biodistribution and tumor accumulation in live mice. Figure 5A–E shows the biodistribution of 177Lu-LCP 24 h after injection, including the visible accumulation of 177Lu in the tumor. In contrast, free 177LuCl3 does not accumulate in the tumor and therefore tumor accumulation is not visible using SPECT/CT imaging (Figure 5F–H).
Figure 5.
SPECT/CT images of nude mouse bearing subcutaneous UMUC3/3T3 tumor on left flank at 24 h after injection of 177Lu-LCP (Figures 5A–E) or free 177Lu (Figures 5F–H). Mouse slit collimator was used for whole body imaging: A) left sagittal, B) coronal, C) midsagittal, F) mid-sagittal and G) coronal views. Pinhole collimator was used in D), E), and H) for higher resolution axial and left sagittal views. 177Lu-LCP accumulated in tumor, liver, and spleen, while free 177Lu accumulated in the bone and kidneys, but not in the tumor.
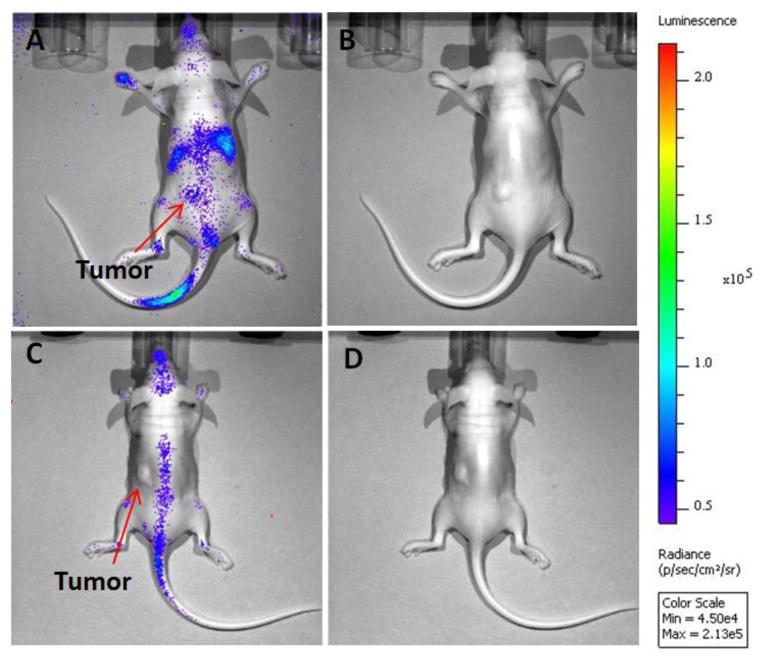
Interestingly, 177Lu also allows imaging via its β-emissions, which are the same decay products (electrons) that induce its therapeutic effect. As the β-particles move through the tissue over their average path length of ~250 μm, they induce photon emissions from the medium with energies in the visible spectrum. This so-called Cerenkov light was measured using an in vivo optical imaging system (Figure 6), also showing tumor uptake of 177Lu-LCP and corroborating the results obtained during SPECT/CT imaging. Cerenkov imaging is a particularly interesting imaging modality because it offers a less expensive and more convenient alternative to SPECT that is not generally used in this field. Although its spatial resolution and depth of penetration is not as high as SPECT or PET, Cerenkov imaging uses less expensive hardware and requires acquisition times of only a few seconds, if the radioisotope and dose are adequate.
Figure 6.
Images of Cerenkov luminescence in nude mice bearing subcutaneous UMUC3/3T3 tumor on left flank. Luminescence image (A) and plain photograph (B) in mouse 24 h after injection of 2.5 mCi 177Lu-LCP. Luminescence image (C) and plain photograph (D) in mouse 24 h after injection of 2.5 mCi free 177LuCl3. Optical images demonstrated 177Lu-LCP accumulation in liver, spleen, and tumor, while free 177LuCl3 mainly accumulated in the spine. Data was acquired by an IVIS optical system.
After imaging was complete, the mouse organs were dissected and their 177Lu signal was read using gamma scintillation. These values are shown in Supplementary Table S2.
3.5: In Vivo Tumor Growth Inhibition
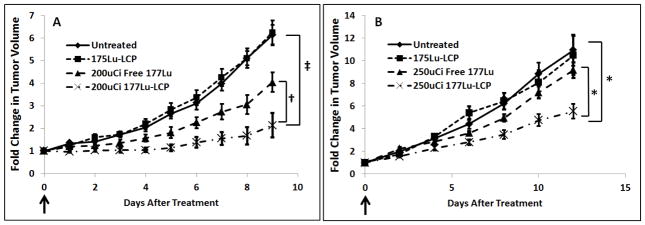
To assess the effectiveness of a single treatment of 177Lu, one dose of 177Lu-LCP was intravenously injected into two different subcutaneous xenograft tumor models. One of the models was an aggressive stroma-rich human bladder cancer model previously developed by our lab. This model uses both UMUC3 bladder cancer cells and NIH-3T3 fibroblasts to generate a tumor that has been shown to be more similar to patient bladder cancer than UMUC3 tumors alone [17]. As shown in Figure 7A, a single 200 μCi dose of 177Lu-LCP showed sustained and significant tumor growth inhibition compared to tumors treated with nonradioactive 175Lu-LCP and tumors receiving only free 177LuCl3. 177Lu-LCP was also tested on the aggressive and radio-resistant [18] H460 human non-small cell lung cancer model; an increased dose of 250 μCi of 177Lu-LCP provided statistically significant tumor growth inhibition as shown in Figure 7B. This boosted dose was still low enough to avoid changes in mouse body weight (Figure S2), and the accumulated dose over the course of the study did not significantly elevate liver/kidney toxicity markers (Table 2).
Figure 7.
In vivo tumor growth inhibition in two subcutaneous xenograft tumor models. Nonradioactive 175Lu was loaded into LCP NPs to act as a vehicle control. One dose per mouse was delivered intravenously on Day 0 (arrows). A) Human UMUC3 bladder cancer supplemented with murine 3T3 fibroblasts. B) Human H460 non-small cell lung cancer. Initial tumor sizes ~100–150 mm3; n = 5–8; †p<0.03, ‡p<5e-5, *p<0.015.
Table 2.
Toxicity of 177Lu in mice, measured 12 days after treatment with 250 μCi 177Lu-LCP or controls to test cumulative toxicity: A) Serum protein values in healthy, but immunocompromised, nude mice; B) Serum protein values in healthy CD-1 mice; C) Whole blood analysis in healthy CD-1 mice, measuring white blood cell count (WBC), hematocrit (HCT), mean cell volume (MCV), red blood cell count (RBC), hemoglobin count (HGB), and platelet count (PLT). 177Lu-LCP treatment does not present significant cumulative renal/hepatotoxicity, but causes a decrease in WBC and PLT.
| A) Nude Mice | BUN (mg/dL) | Creatinine (mg/dL) | AST (U/L) | ALT (U/L) |
|---|---|---|---|---|
| Untreated | 23 ± 0.5 | 0.1 ± 0 | 99 ± 13 | 37 ± 2 |
| 175Lu-LCP | 23 ± 1.0 | 0.1 ± 0 | 102 ± 2 | 34 ± 5 |
| 250 μCi Free 177Lu | 20 ± 1.7 | 0.1 ± 0 | 88 ± 16 | 41 ± 1 |
| 250 μCi 177Lu-LCP | 24 ± 1.9 | 0.1 ± 0 | 73 ± 4 | 40 ± 4 |
| Normal Range | 8–33 | 0.2–0.9 | 54–298 | 17–77 |
| B) CD-1 Mice | BUN (mg/dL) | Creatinine (mg/dL) | AST (U/L) | ALT (U/L) |
|---|---|---|---|---|
| Untreated | 20 ± 0.3 | 0.1 ± 0 | 69 ± 14 | 33 ± 1 |
| 175Lu-LCP | 21 ± 1.3 | 0.2 ± 0.03 | 91 ± 12 | 35 ± 2 |
| 250 μCi Free 177Lu | 15 ± 0.7 | 0.1 ± 0 | 109 ± 15 | 41 ± 3 |
| 250 μCi 177Lu-LCP | 15 ± 0.5 | 0.1 ± 0 | 125 ± 9 | 45 ± 5 |
| Normal Range | 8–33 | 0.2–0.9 | 54–298 | 17–77 |
| C) CD-1 Mice | WBC (103/μl) | HCT (%) | MCV (fl) | RBC (106/μl) | HGB (g/dl) | PLT (103/μl) |
|---|---|---|---|---|---|---|
| Untreated | 4.5 ± 0.2 | 52.6 ± 0.2 | 48.9 ± 0.2 | 10.7 ± 0.1 | 16.3 ± 0.1 | 892 ± 36 |
| 175Lu-LCP | 5.2 ± 0.7 | 50.6 ± 1.1 | 51.0 ± 1.4 | 10.0 ± 0.5 | 15.7 ± 0.3 | 679 ± 54 |
| 250 μCi Free 177Lu | 2.3 ± 0.4 | 47.6 ± 1.7 | 53.8 ± 3.8 | 8.9 ± 0.4 | 14.6 ± 0.3 | 926 ± 34 |
| 250 μCi 177Lu-LCP | 0.3 ± 0.1 | 38.8 ± 2.2 | 50.5 ± 1.6 | 7.7 ± 0.4 | 12.0 ± 0.6 | 217 ± 24 |
| Normal Range | 2.6 – 10.1 | 32.8 – 48.0 | 42.3 – 55.9 | 6.5 – 10.1 | 10.1 – 16.1 | 780 – 1540 |
3.6: Tumor Accumulation
To compare the localization of 177Lu-LCP inside H460 and UMUC3/3T3 tumors, the fluorescent small molecule DiI was incorporated into the outer leaflet of the nanoparticle. DiI has been widely used in liposomal formulations and as a cell membrane marker, and is well known to faithfully remain in the hydrophobic portion of the lipid bilayer [19, 20]. Tumor-bearing mice were sacrificed 24h after injection with DiI-labeled 177Lu-LCP, and tumor sections were imaged for DiI uptake. Figure S3A shows that the bladder cancer tumor has more homogeneous uptake of particles when compared to the H460 lung cancer tumor. This could be because the bladder cancer tumors contain more CD-31-positive area than the H460 tumors (Figure S3B), corresponding to more vasculature. This could also explain the greater tumor accumulation of 177Lu-LCP in the UMUC3/3T3 tumors shown in Figure 4B. The UMUC3/3T3 bladder cancer tumor model was therefore chosen for more detailed mechanistic analysis.
3.7: Mechanisms of Tumor Growth Inhibition: Cytotoxicity Studies
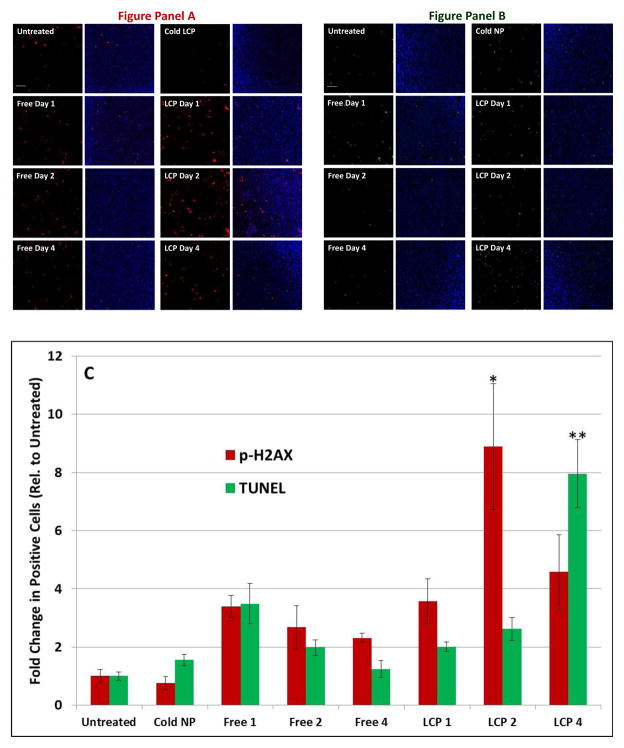
While many chemotherapeutic therapies and treatments using external radiation require multiple doses to generate therapeutic effect, just one dose of 177Lu-LCP provides continuous treatment of the tumor as the 177Lu slowly decays, leading to sustained tumor growth inhibition for several days. We desired to investigate the mechanisms by which this sustained effect occurs, and hypothesized that the cytotoxic β-emissions from 177Lu would persistently induce double-stranded DNA breaks. To study this phenomenon, we looked further into the stroma-rich bladder cancer tumor model. Mice were sacrificed one, two, and four days after treatment with 200 μCi of 177Lu-LCP or appropriate controls. Immunofluorescent staining for p-H2AX, a protein that is phosphorylated in response to DNA double-stranded breaks [21], showed that the maximum induction of the protein occurred two days after treatment and remained elevated even four days after treatment (Figure 8A). This DNA damage led to cell death via apoptotic fragmentation of DNA, which was most induced four days after treatment as measured through the TUNEL assay (Figure 8B). This suggests that the cumulative dose of radiation from 177Lu-LCP causes maximum DNA damage after ~48 h, and this damage translates to apoptotic cell death shortly thereafter. 177Lu-LCP induces significantly higher maximum H2AX phosphorylation and cell death than free 177Lu (p<0.05), and the difference in the pattern of cytotoxic effects between these two treatments may be due to a difference in pharmacokinetics/dynamics, as free 177Lu quickly clears from circulation and may deposit into the tumor much earlier, more transiently, and with a different intratumoral distribution than 177Lu-LCP.
Figure 8.
Cytotoxicity of 177Lu-LCP in vivo: Mice were treated with 200 μCi of 177Lu-LCP or controls and were killed one, two, or four days after administration. Figure panel A shows p-H2AX-positive cells (red) in UMUC3/3T3 tumor sections with or without DAPI (blue). Figure panel B shows TUNEL-positive cells (green) in tumor sections with and without DAPI (blue). Sections in Figure panel B are taken from an adjacent section to those displayed in Figure Panel A. Five representative fields were quantified for TUNEL or p-H2AX-positive cells. Images appearing in Figure panels A and B represent the field with the third-highest quantification. Average values are shown in Figure C: highest p-H2AX upregulation (red) occurred two days after 177Lu-LCP treatment, while highest TUNEL induction (green) occurred 4 days after 177Lu-LCP treatment. *p < 0.007; **p < 0.0005 compared to control. Scale Bar = 100 μm.
3.8: Mechanisms of Tumor Growth Inhibition: Microenvironment Studies
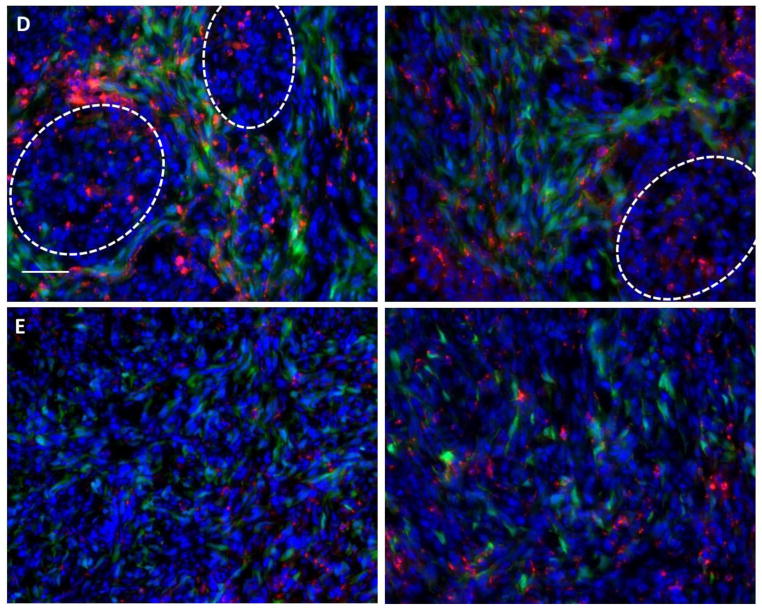
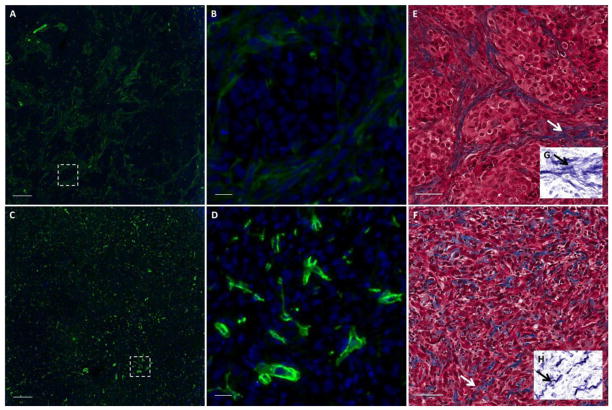
Changes in the tumor microenvironment were observed as well: Figure 9 shows that a diffuse and disordered fibroblast structure was present two days after treatment with 177Lu-LCP, in contrast to the organized fibroblast structure present in untreated and control-treated tumors of the same size (see higher magnification images in Figure 9 D–E). These images clearly show that the fibroblasts in untreated tumors can organize into a defined morphology to form tumor nests, while those fibroblasts in tumors that have been treated with 177Lu-LCP are dispersed and without this mature organization. Four days after treatment, the disorganized fibroblast structure persisted (Figure 10 A–D), along with changes in the collagen structure in the tumor, in which the long swaths of collagen in the untreated tumor had been replaced with shorter and more tortuous collagen fibers (Figure 10 E–H). It is known that the organized fibroblast structure and more ordered, linearized, and bundled collagen fibers present in the untreated tumor are associated with a more mature and malignant phenotype, while a disordered fibroblast structure with short, tortuous collagen fibers like those of the treated tumor suggest a tumor microenvironment less structurally and functionally capable of growth and progression [22–26].
Figure 9.
UMUC3/3T3-GFP (green) tumor sections, with tumor nests outlined in white. Row A) Untreated tumors. Row B) t = 2 days after treatment with cold DiI-175Lu-LCP (red). Row C) t = 2 days after treatment with hot DiI-177Lu-LCP (red). Row D) High magnification images t = 2 days after treatment with cold DiI-175Lu-LCP (red). Row E) High magnification images t = 2 days after treatment with hot DiI-177Lu-LCP (red); disorganized fibroblast structure does not allow tumor nest formation. Each row shows representative photographs from each group, consisting of several tumors and tumor sections. Scale Bar = 300 μm for rows A–C and 50 μm for rows D–E.
Figure 10.
Microenvironment structure in UMUC3/3T3 tumor sections t = 4 days after treatment with 177Lu-LCP: A–D) Stain for fibroblast marker α-SMA (green) in UMUC3/3T3 tumor sections. Figures A and C show α-SMA expression in an untreated tumor and in a tumor at t = 4 days after treatment with 177Lu-LCP; scale bars = 200 μm. White dashed boxes are zoomed in in Figures B and D; scale bars = 20 μm. E–H) Trichrome stain in tumors that are untreated (E) or t = 4 days after treatment (F). Inserts G–H on bottom right have been processed to show a more isolated view of the collagen stain. Arrows show the location of the region in the insert. Scale bar = 50 μm.
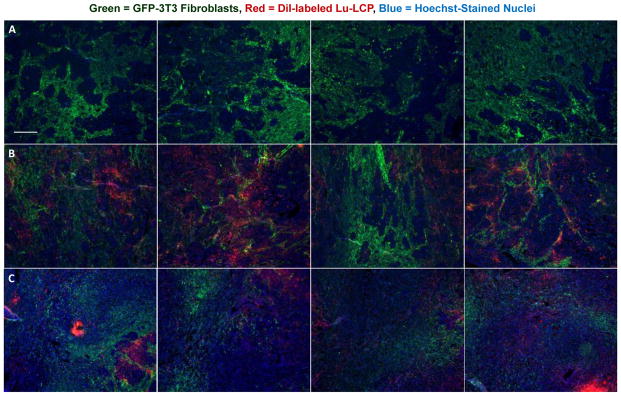
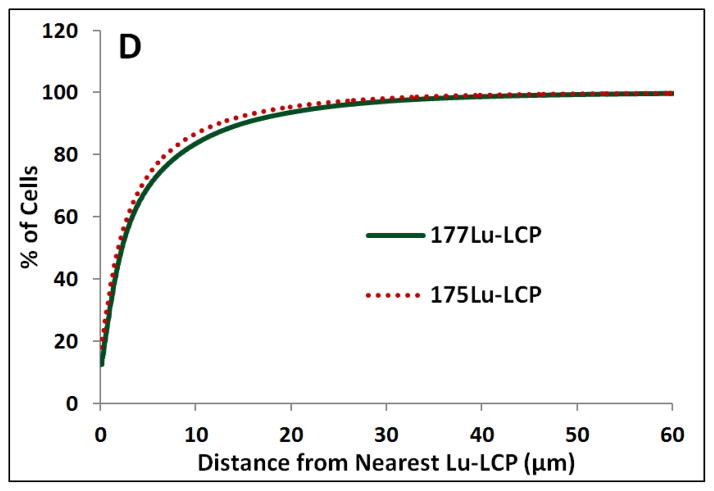
We also desired to measure the intratumoral localization of Lu-LCP. Unlike traditional chemotherapeutics that must enter the target cell in order to impart their therapeutic effect, internal radiotherapy can damage cells at a distance several cell lengths from the source. β-particles from 177Lu, for example, have a maximum energy of ~500 keV, allowing an average path length in tissue of 200–300 μm [27] and a maximum path length of ~4x that value [28]. 177Lu-LCP may then be able to provide therapeutic effect to cells that did not take up the particles, which is especially important given the intratumoral heterogeneity of nanoparticle delivery in general. We supposed that a majority of cells in a tumor would be “in range” for β-particle damage after 177Lu-LCP distribution, but desired to measure the intratumoral localization of 177Lu-LCP in order to calculate this important spatial relationship between cells and particles. The lipid membrane of the LCPs used in the experiment described in Figures 10 and 12 were labeled with fluorescent DiI, and excess DiI was purified away using sucrose gradient centrifugation in order to measure the dispersion of the particles throughout the tumor. Tumor sections from several areas in different tumors were then analyzed to quantify distances between cells and nanoparticles. Figure 11 shows that nearly 100% of cells in the field are within just 50 μm of the nearest Lu-LCP, well within the average path length of a β particle, even though the only nanoparticles considered are those present in that 2-D plane—Lu-LCPs residing in the z-direction would only increase the particles in range for a given cell. This substantiates the claim that although not all tumor cells have taken up particles, all are susceptible to the delivered therapeutic payload.
Figure 12.
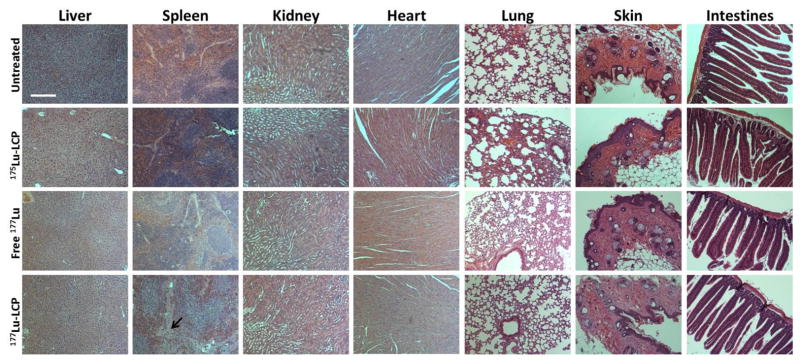
Healthy nude mouse organs fixed, sectioned, and stained with hematoxylin and eosin 12 days after treatment with 250 μCi 177Lu-LCP or controls to test cumulative toxicity. Some acellular regions (arrow) are visible in spleens treated with 177Lu-LCP. Scale bar = 150 μm.
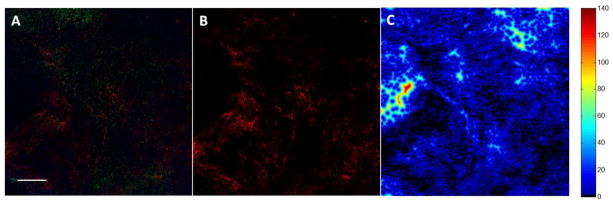
Figure 11.
Quantification of DiI distribution at t = 2 days: A) UMUC3/3T3-GFP tumor given DiI-177Lu-LCP; scale bar = 500 μm; B) Isolated DiI signal from panel A; C) Heat map showing each cell’s distance from its nearest Lu-LCP, where black and blue areas show areas of close proximity between cells and particles; D) Quantification of relative cell distance from Lu-LCP; n = 1.75×106 cells in 8 sections; n = 4 sections each for 177Lu-LCP and 175Lu-LCP.
3.9: Toxicity Studies
To gauge the cumulative toxicity of 177Lu-LCP in mice, we treated healthy immunocompromised nude mice and healthy immunocompetent CD-1 mice with a single dose of 250 μCi of 177Lu-LCP, the highest dose used in this study. Mice were sacrificed 12 days after treatment to assess the cumulative effect of the radiation. At the conclusion of the 12-day study, mouse organs were dissected and sectioned, serum markers for kidney and liver toxicity were measured (ALT, AST, BUN, and creatinine), and whole blood from the immunocompetent CD-1 mice was analyzed for changes in white blood cell count (WBC), hematocrit (HCT), mean cell volume (MCV), red blood cell count (RBC), hemoglobin count (HGB), and platelet count (PLT). These results were compared against appropriate controls and against the normal range for these values. Morphologies of kidney, liver, heart, lung, skin, and small intestine sections after H&E staining showed no difference from normal tissue in nude mice (Figure 12) or in CD-1 mice (Figure S4). Serum markers for renal and hepatotoxicity also remained in the normal range in both nude and CD-1 mice (Table 2A–B) after treatment. The data do show some well-tolerated side effects as a result of this treatment, including differences in spleen morphology as well as a decrease in white blood cell and platelet counts outside the normal range. Hematological side effects are common during cancer therapy and are easily handled using immune boosters, etc., which were not used in this study. None of these side effects translated to a decrease in mouse body weight (Figure S2).
Discussion
In this paper, we have described a novel method for systemic internal radiotherapy by extending the anticancer application of our LCP platform to include a theranostic nanoparticle with high specific drug loading. The preferential uptake of 177Lu into LCP allows a huge amount of 177Lu to be encapsulated in a very small batch of particles without changing the LCP’s morphology or encapsulation efficiency. The difficulty of nanoparticle scale-up that is pervasive throughout this field is therefore eliminated, as a therapeutic dose for a mouse or a human can be made on the same benchtop.
The theranostic advantage of radioisotope loading into LCP should also not be understated. 177Lu’s γ emissions permitted live in vivo imaging using SPECT to measure tumor accumulation of 177Lu-LCP, paving the way for future delivery to orthotopic tumors that will represent a more clinically relevant “diagnostic” procedure. The β decay from 177Lu also causes significant tumor inhibition via DNA double-stranded breaks while simultaneously inducing Cerenkov radiation for imaging. Because therapeutic β-emissions are also the imaging contrast agent, Cerenkov imaging is a wonderful example of a theranostic application. Compared to SPECT, Cerenkov imaging correctly estimated the general biodistribution of 177Lu-LCP at a fraction of the time and cost. This opens up opportunity for several applications such as dynamically looking at early stages of biodistribution and clearance by taking many images in quick succession. Because the high loading of Lu into LCP is broadly applicable to several other polycationic radiometals, such as the β-emitter and effective Cerenkov agent 90Y, it is worthwhile to discuss Cerenkov imaging as an interesting and useful theranostic application.
As a follow-up to our tumor inhibition data, we were interested in assessing any significant changes in the tumor microenvironment as a result of this treatment, and we evaluated these changes by measuring the fibroblast and collagen structure in the tumor. The changes in the tumor microenvironment may be explained by looking deeper into how β-radiation causes the double-stranded DNA breaks that are shown to be elevated in the treated tumors. Generally, β-radiation is classified as having low linear energy transfer (LET); that is, the β-particles deposit a small fraction of their energy per unit length as they travel along their path [28]. In most cases, the DNA damage does not occur via direct interaction between a DNA strand and the β-particle itself. In the case of low LET emitters like 177Lu, it is known that most DNA damage is caused by an increase in reactive oxygen species (ROS) generated as the emitted β-particles interact with and radicalize water molecules along their path. The induced ROS then damage DNA and cause single and double-stranded breaks in an “indirect radiation effect” [28].
Oxidative stress is known to cause many changes in the cellular and extracellular environment, especially in rapidly remodeling tissues like cancer, where actin and collagen are just some of the proteins susceptible to oxidative damage [29–33]. Actin is an important structural component of cells and is also a structural player in the adherens junctions between 3T3 fibroblasts [34]. The cysteine residues on actin are susceptible to oxidative damage, and this damage can prevent polymerization, cause depolymerization, and affect the organization of actin in cells, potentially restricting the organization of fibroblasts in the tumor. Collagen stability is also affected by oxidative damage, as scissions in the collagen α-bands can decrease the degradation temperature of the collagen to below body temperature and prevent the formation of collagen fibrils [30, 31]. These results suggest that cumulative ROS damage may cause the observed changes in the overall in vivo collagen and fibroblast structure shown in Figures 10 and 11, prohibiting the dynamic formation of bundled collagen and organized fibroblast structures that have been implicated in the formation of resistant tumor nests found in more advanced and aggressive tumors.
Tumor growth inhibition via 177Lu-LCP may then occur by taking advantage of both the uncontrolled growth and the excessive microenvironment remodeling observed in cancer. The low, continuous dose of radiation damages tumor cell DNA that can have fewer active repair mechanisms and divide much more rapidly than healthy cells while inhibiting the progression of the microenvironment toward malignancy. This allows sustained cancer treatment at a dose that preserves the health of the subject.
Part of the beauty of this approach to radioisotope loading is its broad application for many trivalent radiometals, such as other therapeutic radionuclides like 90Y or 192Ir, which are great candidates for future work in this subject. Combination therapy using a radiotherapeutic and a radiosensitizing chemotherapeutic residing in the same LCP particle should also be possible, given that many small molecule drugs have previously been encapsulated in LCP.
Future work should also investigate whether systemic administration of a radiotherapeutic LCP is the most effective dosing method. Macro and micro-dosimetric calculations should be used to determine absorbed doses in the mouse and how the distribution of nanoparticles necessitates different calculations than doses from an external beam. In one potential local delivery scenario, highly loaded 177Lu-LCP, 192Ir-LCP, or 90Y-LCP could be directly injected into prostate tumors to create a sort of nano-brachytherapy, in which modified particles would disseminate locally and stably in the prostate but remain out of the systemic circulation.
Conclusions
Radionuclide loading into 177Lu-LCP can be increased by several orders of magnitude without affecting the 177Lu encapsulation efficiency or LCP morphology, generating a nanoparticle with high radioisotope concentration that overcomes scale-up barriers typical of nanotherapeutics and minimizes heavy metal-based toxicities. The choice of 177Lu as a model radionuclide has allowed in vivo anticancer therapy via DNA damage and microenvironment remodeling, in addition to permitting live animal SPECT/CT and Cerenkov imaging. As studies in LCP-mediated radionuclide therapy continue, other nuclides may be considered, perhaps in combination with chemotherapeutics, for several different therapeutic approaches.
Supplementary Material
Figure S1: 177Lu-LCP and Free 177 Lu-Cl3 Pharmacokinetics. Data represented as mean ± SEM. n = 5 for 177Lu-LCP and n = 3 for free 177LuCl3.
Figure S2: Relative change in body weight in H460 tumor-bearing mice used in tumor growth inhibition experiment shown in Figure 7B. Mice did not significantly lose or gain weight during the experiment.
Figure S3: A) 24 h after injection with DiI-177Lu-LCP, UMUC3/3T3 tumors show more homogeneous tumor accumulation of DiI-labelled NP (red). B) Untreated UMUC3/3T3 tumors have more vasculature than H460 tumors, shown as more CD-31 positive area (orange).
Figure S4: Healthy CD-1 mouse organs fixed, sectioned, and stained with hematoxylin and eosin 12 days after treatment with 250 μCi 177Lu-LCP or controls to test cumulative toxicity. Scale bar = 150 μm.
Table S1: 177Lu-LCP Characterization. Diameter, zeta potential, and PDI measurements taken in triplicate after sucrose gradient purification.
Table S2: Biodistribution of 177Lu in mice shown in Figures 5–7. Mice were sacrificed after SPECT and Cerenkov measurements at t=24 h and organs were harvested. Left columns show organ accumulation in mouse given free 177LuCl3. Right columns show organ accumulation in mouse given 177Lu-LCP. Organ biodistributions measured using gamma scintillation.
Acknowledgments
ABS was supported by the National Science Foundation’s Graduate Research Fellowship Program. We thank the Small Animal Imaging facility, especially staff Kevin Guley and Joseph Merrill, at the UNC Biomedical Imaging Research Center for providing the SPECT/CT and Cerenkov imaging service. The imaging core is supported in part by NCI Grant #P30-CA016086-35-37. The work was supported by NIH grants CA149363, CA151652 and CA149387.
Abbreviations
- AA
Anisamide
- DOPA
1,2-dioleoyl-sn-glycero-3-phosphate
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- DSPE-PEG2000
N-(Carbonyl-methoxypolyethyleneglycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine, sodium salt
- EDS
Energy-Dispersive X-ray Spectroscopy
- EE
Encapsulation Efficiency
- Ksp
Solubility Product Constant
- LCP
Lipid-Calcium-Phosphate
- Lu
Lutetium
- SPECT
Single Photon Emission Computed Tomography
- TEM
Transmission Electron Microscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldsmith SJ. Radioimmunotherapy of lymphoma: Bexxar and Zevalin. Semin Nucl Med. 2010;40(2):122–35. doi: 10.1053/j.semnuclmed.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Grillo-Lopez AJ. Zevalin: the first radioimmunotherapy approved for the treatment of lymphoma. Expert Rev Anticancer Ther. 2002;2(5):485–93. doi: 10.1586/14737140.2.5.485. [DOI] [PubMed] [Google Scholar]
- 3.van der Kolk LE, et al. Complement activation plays a key role in the side-effects of rituximab treatment. Br J Haematol. 2001;115(4):807–11. doi: 10.1046/j.1365-2141.2001.03166.x. [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni HS, Kasi PM. Rituximab and cytokine release syndrome. Case Rep Oncol. 2012;5(1):134–41. doi: 10.1159/000337577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rezvani AR, Maloney DG. Rituximab resistance. Best Pract Res Clin Haematol. 2011;24(2):203–16. doi: 10.1016/j.beha.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Yang Y, Huang L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. J Control Release. 2012;158(1):108–14. doi: 10.1016/j.jconrel.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, et al. Codelivery of VEGF siRNA and gemcitabine monophosphate in a single nanoparticle formulation for effective treatment of NSCLC. Mol Ther. 2013;21(8):1559–69. doi: 10.1038/mt.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, et al. Combinational delivery of c-myc siRNA and nucleoside analogs in a single, synthetic nanocarrier for targeted cancer therapy. Biomaterials. 2013;34(33):8459–68. doi: 10.1016/j.biomaterials.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu Y, et al. A highly efficient synthetic vector: nonhydrodynamic delivery of DNA to hepatocyte nuclei in vivo. ACS Nano. 2013;7(6):5376–84. doi: 10.1021/nn4012384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banerjee R, et al. Anisamide-targeted stealth liposomes: a potent carrier for targeting doxorubicin to human prostate cancer cells. Int J Cancer. 2004;112(4):693–700. doi: 10.1002/ijc.20452. [DOI] [PubMed] [Google Scholar]
- 11.Tseng YC, et al. Lipid-calcium phosphate nanoparticles for delivery to the lymphatic system and SPECT/CT imaging of lymph node metastases. Biomaterials. 2014;35(16):4688–98. doi: 10.1016/j.biomaterials.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miao L, et al. Nanoparticles with Precise Ratiometric Co-Loading and Co-Delivery of Gemcitabine Monophosphate and Cisplatin for Treatment of Bladder Cancer. Adv Funct Mater. 2014;24(42):6601–6611. doi: 10.1002/adfm.201401076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Kim WY, Huang L. Systemic delivery of gemcitabine triphosphate via LCP nanoparticles for NSCLC and pancreatic cancer therapy. Biomaterials. 2013;34(13):3447–58. doi: 10.1016/j.biomaterials.2013.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010;99(3):306–14. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Byrne XLaRH. Rare earth and yttrium phosphate solubilities in aqueous solution. Geochimica et Cosmochimica Acta. 1997;61(8):1625–1633. [Google Scholar]
- 16.Zaichick S, et al. Accumulation of rare earth elements in human bone within the lifespan. Metallomics. 2011;3(2):186–94. doi: 10.1039/c0mt00069h. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, et al. Synergistic anti-tumor effects of combined gemcitabine and cisplatin nanoparticles in a stroma-rich bladder carcinoma model. J Control Release. 2014;182:90–6. doi: 10.1016/j.jconrel.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres-Roca JF, et al. Prediction of radiation sensitivity using a gene expression classifier. Cancer Res. 2005;65(16):7169–76. doi: 10.1158/0008-5472.CAN-05-0656. [DOI] [PubMed] [Google Scholar]
- 19.Honig MG, Hume RI. Dil and diO: versatile fluorescent dyes for neuronal labelling and pathway tracing. Trends Neurosci. 1989;12(9):333–5. 340–1. [PubMed] [Google Scholar]
- 20.Guo S, et al. Lipid-coated Cisplatin nanoparticles induce neighboring effect and exhibit enhanced anticancer efficacy. ACS Nano. 2013;7(11):9896–904. doi: 10.1021/nn403606m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogakou EP, et al. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273(10):5858–68. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 22.Ingber DE. Can cancer be reversed by engineering the tumor microenvironment? Semin Cancer Biol. 2008;18(5):356–64. doi: 10.1016/j.semcancer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Provenzano PP, et al. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4(1):38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4(2):165–78. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baglole CJ, et al. More than structural cells, fibroblasts create and orchestrate the tumor microenvironment. Immunol Invest. 2006;35(3–4):297–325. doi: 10.1080/08820130600754960. [DOI] [PubMed] [Google Scholar]
- 26.Clark AK, et al. A bioengineered microenvironment to quantitatively measure the tumorigenic properties of cancer-associated fibroblasts in human prostate cancer. Biomaterials. 2013;34(20):4777–85. doi: 10.1016/j.biomaterials.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Sofou S. Radionuclide carriers for targeting of cancer. Int J Nanomedicine. 2008;3(2):181–99. doi: 10.2147/ijn.s2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bednarczyk EM. Ann Pharmacother. 3. 2012. Radiopharmaceuticals in Nuclear Pharmacy and Nuclear Medicine. [Google Scholar]
- 29.Terman JR, Kashina A. Post-translational modification and regulation of actin. Curr Opin Cell Biol. 2013;25(1):30–8. doi: 10.1016/j.ceb.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metreveli NO, et al. UV-vis and FT-IR spectra of ultraviolet irradiated collagen in the presence of antioxidant ascorbic acid. Ecotoxicol Environ Saf. 2010;73(3):448–55. doi: 10.1016/j.ecoenv.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Miles CA, et al. Identification of an intermediate state in the helix-coil degradation of collagen by ultraviolet light. J Biol Chem. 2000;275(42):33014–20. doi: 10.1074/jbc.M002346200. [DOI] [PubMed] [Google Scholar]
- 32.Fujimori E. Ultraviolet light-induced change in collagen macromolecules. Biopolymers. 1965;3(2):115–9. doi: 10.1002/bip.360030202. [DOI] [PubMed] [Google Scholar]
- 33.Sionkowska A, Kaminska A. Thermal helix-coil transition in UV irradiated collagen from rat tail tendon. Int J Biol Macromol. 1999;24(4):337–40. doi: 10.1016/s0141-8130(99)00047-1. [DOI] [PubMed] [Google Scholar]
- 34.Gluck U, et al. Regulation of adherens junction protein expression in growth-activated 3T3 cells and in regenerating liver. Exp Cell Res. 1992;202(2):477–86. doi: 10.1016/0014-4827(92)90102-e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: 177Lu-LCP and Free 177 Lu-Cl3 Pharmacokinetics. Data represented as mean ± SEM. n = 5 for 177Lu-LCP and n = 3 for free 177LuCl3.
Figure S2: Relative change in body weight in H460 tumor-bearing mice used in tumor growth inhibition experiment shown in Figure 7B. Mice did not significantly lose or gain weight during the experiment.
Figure S3: A) 24 h after injection with DiI-177Lu-LCP, UMUC3/3T3 tumors show more homogeneous tumor accumulation of DiI-labelled NP (red). B) Untreated UMUC3/3T3 tumors have more vasculature than H460 tumors, shown as more CD-31 positive area (orange).
Figure S4: Healthy CD-1 mouse organs fixed, sectioned, and stained with hematoxylin and eosin 12 days after treatment with 250 μCi 177Lu-LCP or controls to test cumulative toxicity. Scale bar = 150 μm.
Table S1: 177Lu-LCP Characterization. Diameter, zeta potential, and PDI measurements taken in triplicate after sucrose gradient purification.
Table S2: Biodistribution of 177Lu in mice shown in Figures 5–7. Mice were sacrificed after SPECT and Cerenkov measurements at t=24 h and organs were harvested. Left columns show organ accumulation in mouse given free 177LuCl3. Right columns show organ accumulation in mouse given 177Lu-LCP. Organ biodistributions measured using gamma scintillation.