Significance
Improved therapeutic strategies for transplantation of pancreatic islet cells to selected patients with type-1 diabetes are urgently needed. Growth hormone-releasing hormone (GHRH) agonists to influence growth, function, and engraftment of islet cells were previously reported by us. This study demonstrates greater stimulation by improved GHRH agonists, on proliferation, gene expressions, and the signaling pathways in pancreatic β cells. Agonist MR-409 in vivo reduced the severity of streptozotocin-induced diabetes in nonobese diabetic severe combined immunodeficiency mice. Transplantation of rat islets preconditioned in vitro with MR-409 and its administration in vivo promoted growth, function, and engraftment of exogenous islets, supporting the use of GHRH agonists in type-1 diabetes. The beneficial effects of GHRH agonists on the functions of β cells may also provide approaches to their application in type-2 diabetes.
Keywords: GHRH agonists, diabetes, INS-1, signaling pathways, transplantation
Abstract
Agonists of growth hormone-releasing hormone (GHRH) have been previously reported to promote growth, function, and engraftment of islet cells following transplantation. Here we evaluated recently synthesized GHRH agonists on the proliferation and biological functions of rat pancreatic β-cell line (INS-1) and islets. In vitro treatment of INS-1 cells with GHRH agonists increased cell proliferation, the expression of cellular insulin, insulin-like growth factor-1 (IGF1), and GHRH receptor, and also stimulated insulin secretion in response to glucose challenge. Exposure of INS-1 cells to GHRH agonists, MR-356 and MR-409, induced activation of ERK and AKT pathways. Agonist MR-409 also significantly increased the levels of cellular cAMP and the phosphorylation of cAMP response element binding protein (CREB) in INS-1 cells. Treatment of rat islets with agonist, MR-409 significantly increased cell proliferation, islet size, and the expression of insulin. In vivo daily s.c. administration of 10 μg MR-409 for 3 wk dramatically reduced the severity of streptozotocin (STZ)-induced diabetes in nonobese diabetic severe combined immunodeficiency (NOD/SCID) mice. The maximal therapeutic benefits with respect to the efficiency of engraftment, ability to reach normoglycemia, gain in body weight, response to high glucose challenge, and induction of higher levels of serum insulin and IGF1 were observed when diabetic mice were transplanted with rat islets preconditioned with GHRH agonist, MR-409, and received additional treatment with MR-409 posttransplantation. This study provides an improved approach to the therapeutic use of GHRH agonists in the treatment of diabetes mellitus.
Investigations over the several past decades have explored islet transplantation as an alternative approach to restoration of glucose homeostasis in type-1 diabetes (1–3). The generation of functional insulin-producing cells in vitro also provided a promising source for cell-based therapy (4). Chronic long-term graft survival and islet function are critical issues in transplantation. Numerous studies have reported efforts to improve the survival of islets by preventing the loss of islet cell viability and function during and following transplantation. Strategies to robustly condition pancreatic islets, which include promotion of cell growth and metabolic functions, are particularly important (5–7).
Growth hormone (GH), and various growth factors such as IGF1, glucagon-like peptide (GLP-1) were studied for their ability to stimulate proliferation and survival of pancreatic β cells (8–11). Hypothalamic growth hormone-releasing hormone (GHRH) stimulates production and release of GH from the pituitary gland, exerts some of its effects through the GH/IGF axis, and also directly affects extrapituitary cells expressing GHRH receptors by activating these receptors. GHRH receptor(s) have been detected in various tumor cells, stem cells, and other tissues including pancreatic β cells (12–16). Understanding the action of GHRH on its target cells is important for potential application in the use of synthetic GHRH analogs, with prolonged half-lives, which may provide a promising pharmacologic therapy in various fields. For example, it has been reported that human GHRH[(1–44)NH2] can stimulate insulin secretion from isolated rat islets in vitro (17). Recently, our group demonstrated that synthetic GHRH analog, JI-36, improves β-cell survival, growth, and metabolic function, in vitro. Pretreatment with JI-36 also improves the engraftment and the metabolic function of islets following transplantation to STZ-induced diabetic mice (12). GHRH agonist, JI-38, has been shown to have a cardioprotective effect after myocardial infarction, and to accelerate wound healing (14, 18). In view of these findings of stimulatory effects of GHRH agonists of the JI series, still more potent analogs of GHRH(1–29) have been developed in our laboratory (19). The analogs, designated as “MR series,” display higher endocrine potencies on in vivo GH release and greater binding affinity to GHRH receptor, compared with JI class GHRH analogs. Among these agonists, MR-356 and MR-409 exhibit the highest potency in activating myocardial repair in rats with induced myocardial infarction and efficiently stimulate self-renewal and promote the survival of porcine cardiac stem cells (15). Agonist MR-403 has been shown to augment pancreatic β-cell proliferation and promote the survival of pancreatic islets following transplantation into adrenal gland (13).
To evaluate the efficacy and the utility of the GHRH agonists of MR series on pancreatic β cells in comparison with previously reported JI-36, we tested eight agonists of the MR series with respect to cell proliferation and metabolic function of rat INS-1 cells, a well-established cell line that displays characteristics of pancreatic β cells (20, 21). We demonstrated that at least three signaling pathways are involved in the action of GHRH agonists on INS-1 cells. To further explore the beneficial effect of GHRH agonist in vivo, additional approaches for treating diabetic mice were investigated. We demonstrated the effects of GHRH agonist, MR-409, in the treatment of nonobese diabetic severe combined immunodeficiency (NOD/SCID) mice, and in diabetic mice transplanted with rat islets preconditioned with MR-409. Our findings suggest the merit of further investigation of the agonistic analogs of GHRH in the management of cell replacement therapy in diabetes mellitus.
Results
Stimulatory Effect of GHRH Agonists on Viability and Proliferation of INS-1 Cells.
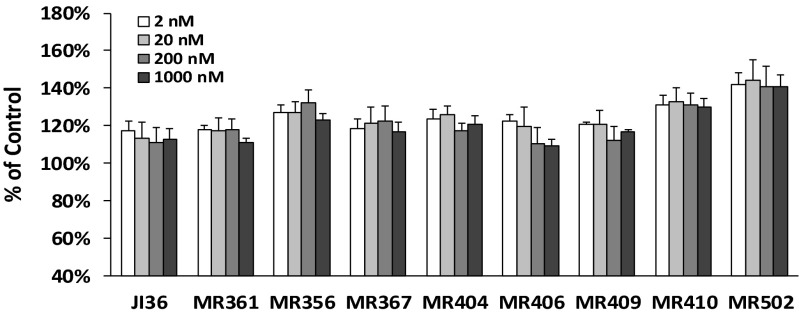
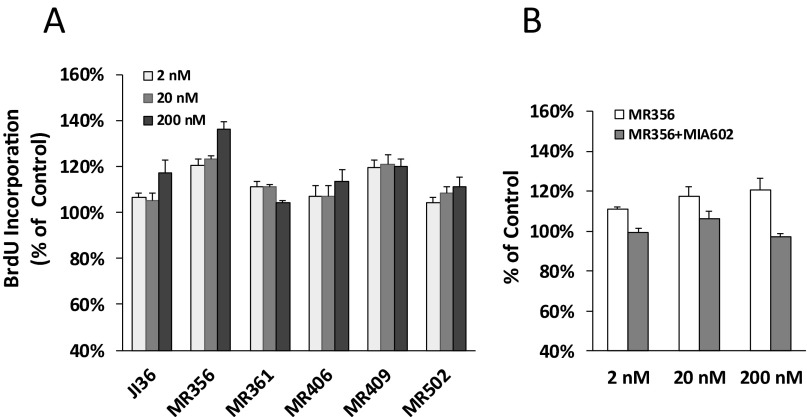
Eight GHRH agonists were tested for their effects on INS-1 cell proliferation. These agonists of the MR series were categorized into three groups based on their chemical structures (19). Analogs MR-356, MR-361, and MR-367 with C-terminal agmatine belonged to group 1; MR-404, -406, -409, and -410 with C-terminal methyl amide to group 2; and MR-502 with C-terminal Gab to group 3. As shown in Fig. 1, treatment of INS-1 cells with these GHRH agonists stimulated cell viability by 10–40% over control at all concentrations tested. MR-502, -356, and -410 displayed significantly greater activities than previously reported GHRH agonist JI-36. In the cell proliferation assay as measured by BrdU incorporation, MR-356 and MR-409 showed greater stimulatory effect than JI-36 (Fig. S1A). Further, cell proliferation stimulated by GHRH agonist, MR-356, could be inhibited by GHRH antagonist, MIA-602 (Fig. S1B), indicating the specificity of the agonist. Similarly, MIA-602 also reversed the cell proliferation induced by MR-361, MR-367, and MR-502.
Fig. 1.
Effect of GHRH agonists on INS-1 cell viability. INS-1 cells were treated with either MR agonists or JI-36 at concentrations indicated. Total numbers of viable cells were measured at 72 h of treatment using CellTiter 96 aqueous one solution cell proliferation assay and calculated as relative percentage of the control.
Fig. S1.
Effect of GHRH agonists on INS-1 cell viability and proliferation. (A) INS-1 cells were treated by MR agonists and JI-36 at concentrations indicated for 24 h. Total numbers of proliferating cells were measured using the BrdU incorporation assay. Values are shown as percentages of control. (B) INS-1 cells were treated with MR-356 at indicated concentrations in the presence or absence of 2 μM GHRH antagonist, MIA-602. Relative percentages were calculated by comparing the number of viable cells in the treatment to that in the control. Data were averaged from at least three individual tests done in quadruplicate for each test.
Effect of GHRH Agonists on the Expression of Insulin, IGF1, and GHRH Receptor in INS-1 Cells.
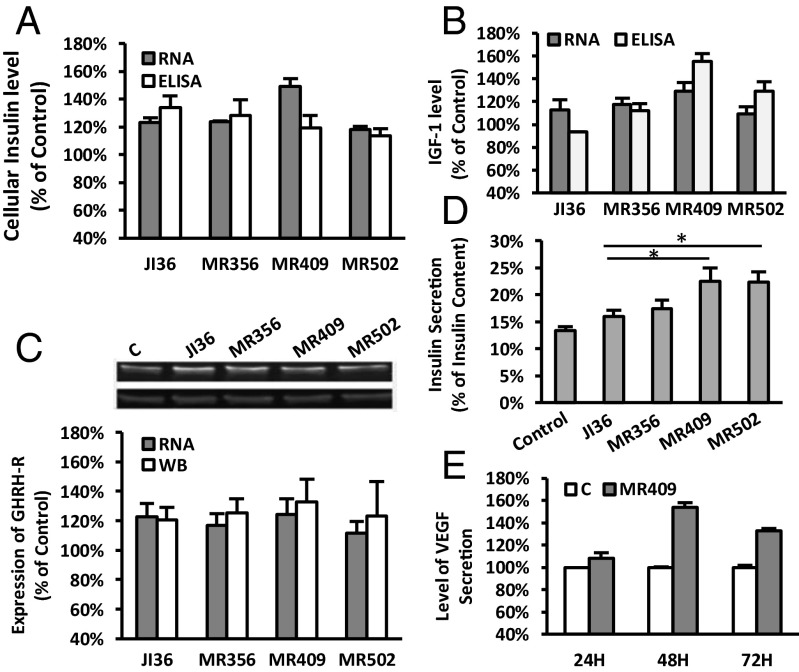
To evaluate the effect of GHRH agonists on the expression of insulin, IGF1 and GHRH receptor in rat INS-1 cells, three agonists, MR-356, MR-409, and MR-502, were selected, one from each structural group, and compared with JI-36. The cells were treated with 500 nM agonists for 48 h. As shown in Fig. 2A, treatment of INS-1 cells with agonists of this class resulted in a 20–50% increase of cellular insulin by mRNA (RT-PCR) or peptide levels (ELISA), relative to control. Of the three agonists MR-409 displayed the greatest capability to stimulate mRNA for insulin (148.9 ± 5.9%). Exposure of INS-1 cells to MR-356, MR-409, or MR-502 also slightly (10–30%) increased the mRNA for IGF1. Secretion of IGF1 in cells treated by MR-409 was also augmented (155.2.9 ± 6.8%, Fig. 2B). Moreover, expressions of GHRH receptor by both mRNA and protein measures was increased 20–30% in cell exposure to MR agonists (Fig. 2C). All three MR agonists showed similar effects on target gene expression as did JI-36 (Fig. 2 A–C). Moreover, MR-409 appeared to have greater potency than the others. Treatment with GHRH agonists also resulted in elevated insulin release upon high glucose challenge (Fig. 2D). Both MR-409 and MR-502 had significantly greater stimulatory effects compared with JI-36 (P < 0.05).
Fig. 2.
Effect of GHRH agonists on expression of insulin, IGF1, GHRH receptor, VEGF, and glucose-stimulated insulin secretion. INS-1 cells were exposed to 500 nM GHRH agonists or respective control medium for 48 h. The expression levels of messenger RNA for (A) insulin, (B) IGF1, and (C) GHRH receptor, are shown as percentages of the control. In addition, the protein levels of (A) intracellular insulin, (B) secreted IGF1, and (C) GHRH receptor, are also shown in parallel. A representative Western blot image for GHRH receptor is shown. (D) Insulin secretion in response to high glucose (17 mM) stimulation; *P < 0.05. (E) Elevated VEGF secretion from MR-409-treated INS-1 cells. All data were calculated from three individual experiments.
Stimulation of Secretion of Vascular Endothelial Growth Factor in INS-1 Cells Treated with GHRH Agonist MR-409.
Fig. 2E demonstrates that agonist MR-409 enhances the secretion of vascular endothelial growth factor (VEGF). Upon exposure to 500 nM MR-409 for 48 and 72 h, the levels of VEGF in the culture media increased 53.6 ± 4.7% and 32.9 ± 1.8%, respectively (P < 0.001).
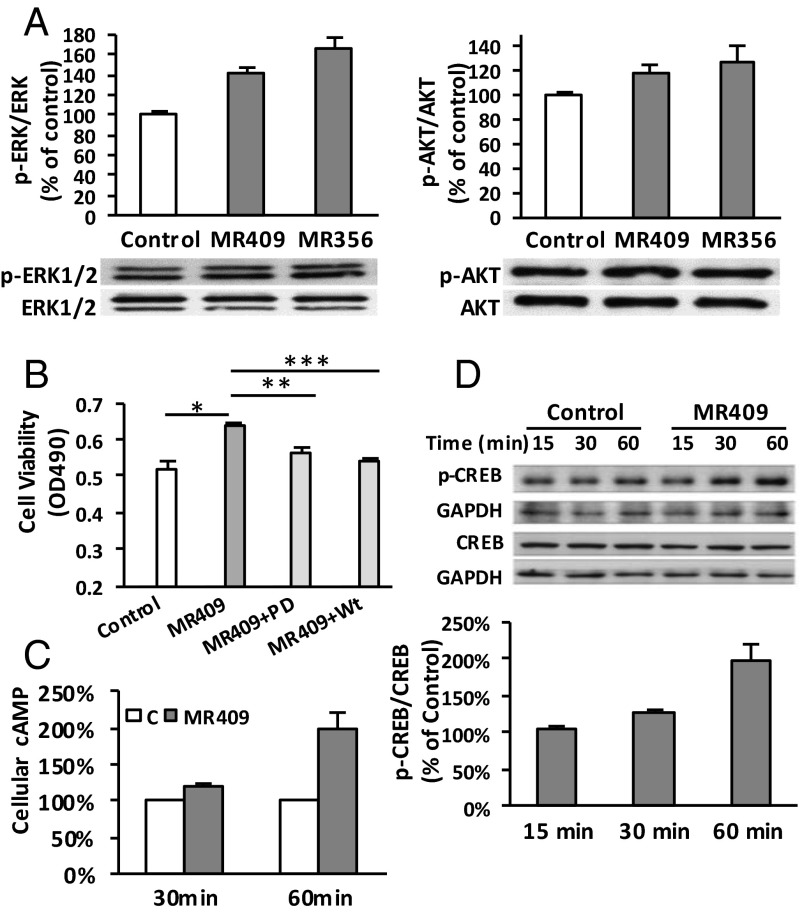
Phosphorylation of ERK, AKT, and cAMP Response Element Binding Protein in INS-1 Cells Treated with GHRH Agonists.
To evaluate the effect of GHRH agonists, MR-356 and MR-409, on major signaling pathways related to cell proliferation and survival, the phosphorylation of ERK and AKT in agonist-treated INS-1 cells was analyzed. As shown in Fig. 3A, ERK phosphorylation (p-ERK/ERK) increased by 65.0 ± 14.0% and 41.6 ± 6.6%, respectively, after cells were exposed to 1 μM of either MR-356 or MR-409 for 30 min. This treatment also led to 26.5 ± 14.4% and 18.8 ± 5.6% increases, respectively, in AKT phosphorylation (p-AKT/AKT). Both PD98059 (25 μM) and wortmannin (0.5 μM) significantly inhibited cell proliferation induced by MR-409 (59.2 ± 1.8% and 79.0 ± 1.7%, respectively, Fig. 3B), indicating the involvement of signaling transduction through both ERK and AKT pathways. Furthermore, exposure of cells to 1 μM MR-409 for 30 and 60 min increased intracellular levels of cAMP by 21.63 ± 1.5% (P < 0.05), and 99.1 ± 14.9% (P < 0.05), respectively (Fig. 3C). Simultaneously, phosphorylation of downstream cAMP response element binding protein (CREB) at Ser-133 (p-CREB/CREB) was also increased 29.0 ± 3.0% (P < 0.05) and 95.9 ± 14.9% (P < 0.001), respectively (Fig. 3D).
Fig. 3.
Stimulatory effect of GHRH agonists on ERK, AKT, and cAMP/CREB pathways. (A) Stimulation of p-ERK/ERK and p-AKT/AKT after a 30-min treatment with MR-356 and MR-409. (B) Inhibition of MR-409-induced cell proliferation by PD98059 (PD, 25 µM) or Wortmannin (Wt, 0.5 µM). Data shown are a representative experiment done in quintuplicate in each treatment; *P < 0.05, **P < 0.01, ***P < 0.001. (C) Stimulation of cellular cAMP in MR-409-treated INS-1 cells. (D) Elevated pCREB/CREB in MR-409-treated INS-1 cells. Data were averaged from individual experiments. A representative Western blot image is shown.
Stimulation of the Proliferation of Rat Islets with GHRH Agonists.
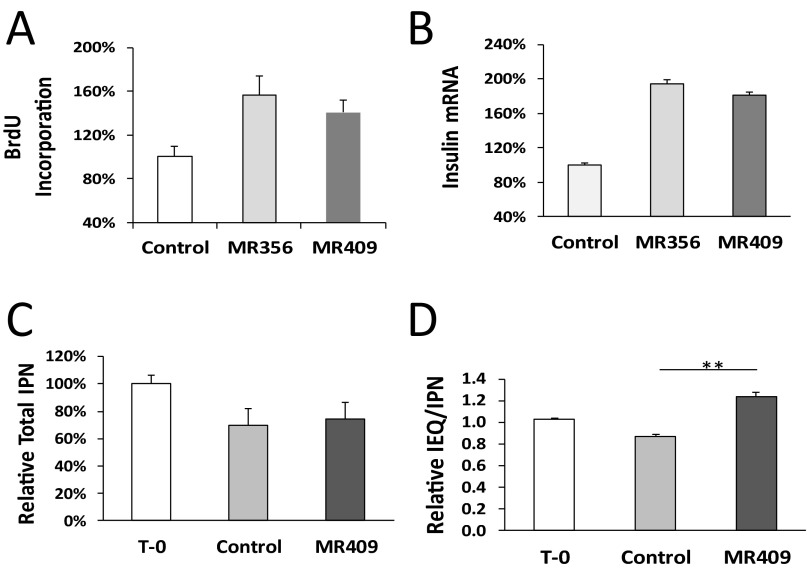
We also tested the effect of GHRH agonists on the proliferation of freshly prepared rat islets. As shown in Fig. S2A, GHRH agonists, MR-356 and MR-409, significantly increased proliferation of cells in the rat islets preparations (157.1 ± 17.1% and 140.6 ± 11.7%, respectively). RT-PCR analysis indicated that the treatment significantly enhanced the expression of cellular insulin levels (194.0 ± 4.5% and 181.7 ± 2.7% for MR-356 and MR-409, respectively, Fig. S2B). For preconditioning of islets before transplantation, isolated rat islets were cultured with 1 μM agonist MR-409 for 2 d. There was a reduction of total numbers of islet particles (IPN); however, no relevant differences were otherwise seen between control and agonist-treated islets, (69.9% ± 5.5% vs. 74.4 ± 5.3%, respectively, Fig. S2C). Exposure of isolated islets to MR-409 greatly enhanced the growth of islets; the ratio of islet equivalents/islet particle number (IEQ/IPN) increased 42.5 ± 1.1% (P < 0.01, Fig. S2D).
Fig. S2.
Effect of GHRH agonists on the proliferation of rat islets. (A) Proliferation of rat islet cells after 24 h of treatment with MR-356 or MR-409. (B) Relative expression levels of insulin in rat islets treated with 1 μM MR-356 or MR-409 for 48 h. (C and D) Comparison of total IPN and IEQ/IPN in rat islets treated with either 1 μM MR409 or vehicle for 48 h; T-0, islets before culture. **P < 0.01.
Beneficial Effects of GHRH Agonist MR-409 in Vivo on Nontransplanted, Otherwise Untreated NOD/SCID Mice.
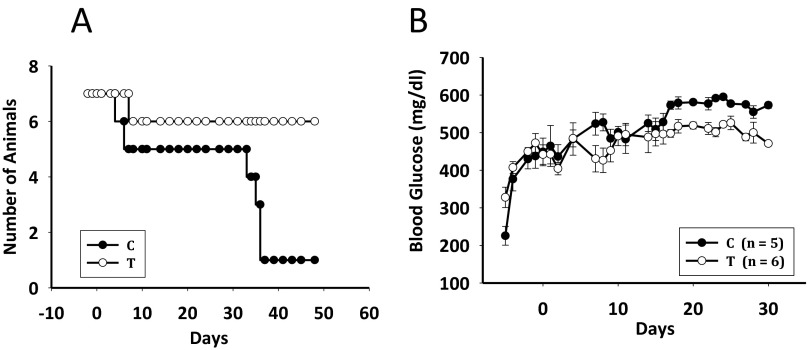
Agonist MR-409 was evaluated for the in vivo treatment of streptozotocin (STZ)-induced NOD/SCID mice. The s.c. administration of MR-409 at 10 μg/day for 3 wk dramatically reduced the severity of their diabetic status. The treated group (T) had a higher survival rate and less severe diabetic status compared with the control group (C). There were seven animals in each group; however two animals from the C and one from the T group died at around 1 wk of treatment. In the 4th wk, animals in group C started to deteriorate and by the end of week 5, only one survived (Fig. S3A), whereas all six animals in the group T were alive through the end of the experiment (45 d). The improved survival rate in the T group was correlated with less severe hyperglycemia. Determination of blood glucose showed a slight reduction in group T during the first week of treatment, a minor increase by the end of the 2nd, and thereafter remained unchanged (Fig. S3B). Mice remained hyperglycemic with an average glucose of 506.0 ± 9.1 and 509.5 ± 7.42 mg/dL (n = 6) in the 3rd and 4th wk, respectively. In the control group, average blood glucose levels increased gradually; the levels of 554.8 ± 10.0 and 578.6 ± 3.63 mg/dL (n = 5) in the 3rd and 4th wk, respectively, were significantly higher than those of the treated group (P < 0.001). Blood samples collected at the end of 3-wk treatment showed no obvious difference between groups C and T for serum insulin (C, 0.342 ± 0.020 ng/mL; T, 0.348 ± 0.066 ng/mL), serum IGF1 (C, 641.7 ± 16.1 ng/mL; T, 652.0 ± 13.0 ng/mL), or serum GH (C, 3.493 ± 2.083 ng/mL; T, 4.119 ± 0.825 ng/mL).
Fig. S3.
Effect of GHRH agonist MR-409 on survival and blood glucose levels of NOD/SCID mice. (A) Cumulative survival of the diabetic animals in control (C, ●) and MR-409-treated group (T, ○). (B) Blood glucose (average ± SEM) of the diabetic mice treated by either control (C, ●) or MR-409 (T, ○). Animals in group T received 10 μg of MR-409 per day s.c. from day 0 to day 21.
Beneficial Effects of GHRH Agonist MR-409 on NOD/SCID Mice Transplanted with Rat Islets.
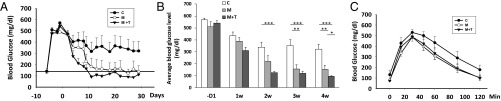
Following islet transplantation influence of GHRH agonist, MR-409, on engraftment and other effects of rat islets was further evaluated in STZ-induced NOD/SCID mice. Animals were divided into three groups (Materials and Methods). Animals transplanted with rat islets treated with vehicle were designated as group C. In general, animals transplanted with islets preconditioned with MR-409 (group M, preconditioned only; and group M + T, preconditioned plus also treated in vivo with 10 μg MR-409 per day for 3 wk) performed better and showed a significantly improved engraftment rate (animals became normoglycemic). At day 28, the engraftment rates of either group M + T (7/7, 100%), or group M (4/5, 80.0%) were much higher than those of control (3/7, 42.9%). Average blood glucose levels were monitored for 4 wk and are shown in Fig. 4A. Animals in group M + T became normoglycemic within 9 d following transplantation. The treatment for animals in group M + T was most effective in the control of blood glucose (Fig. 4B). From week 2 to week 4 blood glucose levels in group M + T remained significantly (P < 0.001) lower than those of control. A significant relief of hyperglycemia was also observed between group M versus group C during the 3rd and 4th wk (P < 0.01). These results suggest that maximally improved outcomes result from the use of MR-409 preconditioned islets and then continuing administration of MR-409 posttransplantation. In the 4th wk, blood glucose levels in group M + T dropped to 96.21 ± 4.9 mg/dL, which was lower than that in group M (154.6 ± 32.9 mg/dL, P < 0.05) and also even lower than that of nondiabetic mice (147.3 ± 7.6 mg/dL, n = 25, P < 0.05).
Fig. 4.
Effect of GHRH agonist MR-409 in the transplanted NOD/SCID mice. (A) Blood glucose follow-up (average ± SEM) in the diabetic mice after transplantation. Control (C, ●); M (○); and M + T (▼). Transplantation was conducted at day 0, animals in the group M + T were injected s.c. with 10 μg/day of MR-409 from day 2 to day 24. The horizontal line represents the average blood glucose level in nondiabetic mice (147.3 ± 7.6 mg/dL, n = 25). (B) Average weekly blood glucose, the data represent the average glucose of three measurements per week (mean ± SEM). Open box, control; light gray box, group M, and gray box, group M + T. −D1, 1 d before transplantation. (C) The IPGTT performed at 2 wk after transplantation.
The body weight in each group increased steadily following transplantation. The average body weights (mean ± SEM) in the 4th wk for groups C, M, and M + T were 24.56 ± 0.62 g, 27.32 ± 0.36 g, and 27.05 ± 0.25 g, respectively. Body weights in groups M and M + T were significantly (P < 0.01) higher than those of control. One month following transplantation, the islet-bearing left kidneys were surgically removed from the animals. The animals became hyperglycemia following nephrectomy. The survival rates, at day 7 after nephrectomy in groups M (60%, 3/5) and M + T (57.1%, 4/7) were much higher than those in group C (14.3% 1/7).
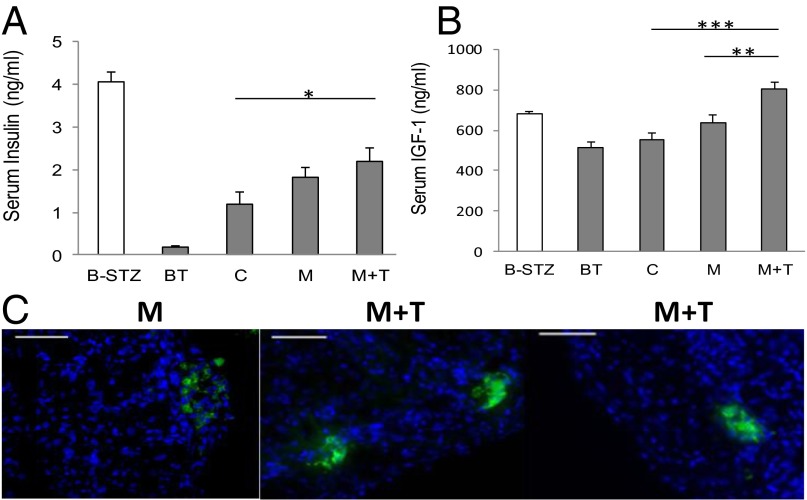
In the i.p. glucose tolerance test (IPGTT), performed on day 15 following transplantation, animals in the control group showed an inferior response to glucose challenge compared with those in groups M and M + T (Fig. 4C). Serum insulin levels were measured after 3 wk of treatment (Fig. 5A). Compared with the insulin levels before transplantation (BT, 0.190 ± 0.035 ng/mL) the serum insulin levels in all three groups increased. The average insulin levels in group M + T were much higher (2.196 ± 0.328 ng/mL) than those in the control group (1.196 ± 0.272 ng/mL, P < 0.05) and were also slightly higher than those in group M (1.822 ± 0.219 ng/mL). Meanwhile, serum IGF1 levels in group M + T (801.9 ± 33.9 ng/mL) were higher than those in group M (639 ± 38.6 ng/mL), control (555.5 ± 29.1 ng/mL) and normal nondiabetic mice (680.6 ± 9.4 ng/mL, M + T vs. C, P < 0.001) (Fig. 5B).
Fig. 5.
Serum levels of insulin and IGF1 in transplanted NOD/SCID mice. The serum levels of (A) insulin and (B) IGF1 were analyzed after 3 wk of treatment. B-STZ, before STZ induction, n = 12); BT, diabetic mice before transplantation (n = 8); C, control (n = 7), M (n = 5), M + T (n = 7); *P < 0.05, **P < 0.01, ***P < 0.001. (C) Immunohistochemistry analysis of insulin expression. Representative immunostained tissue sections of islet-bearing kidneys retrieved from the animals in M and M + T groups. (Scale bar, 100 μm.)
Expression of insulin in tissue sections of islet-bearing kidneys was detected by immunohistochemistry analysis. The strong insulin signals in the kidney retrieved 1 mo after transplantation revealed the stable engraftment of rat islets. Interestingly, in the M + T group examination of the retrieved kidney revealed that the insulin positive cells had retained their islet-like clustering, whereas in the M group, the insulin positive cells tended to disperse under the kidney capsules (Fig. 5C).
Discussion
In view of the stimulatory effects of previous GHRH agonists on pancreatic islets, cardiomyocytes, and wound healing, an improved class of GHRH agonists (designated MR) was designed and synthesized in our laboratory. The MR series agonists display increased endocrine and cardiac activities compared with the previous JI analogs (19). In this study, we tested GHRH agonists of the MR series for their effects on the proliferation of pancreatic β cells and islets. In the in vitro assay these agonists increased cell proliferation in rat β-cell line INS-1 cells by 10–40%. Similar effects were also observed when these agonists were tested on mouse β-cell line MIN-6 (21). GHRH agonists increased the expression of insulin, IGF1, and GHRH receptor in INS-1 cells. The potency of MR agonists in the stimulation of cell proliferation and expression of target genes in INS-1 cells was in general somewhat greater than those of the previously reported GHRH agonist JI-36. Moreover, agonist MR-409 displayed a significantly greater stimulatory effect on insulin release in response to high-glucose challenge, compared with JI-36 (Fig. 2D, P < 0.05). Importantly, in tests with isolated rat islets, GHRH agonists, MR-356 and MR-409, exhibited greater potency in inducing proliferation of rat islets (Fig. S2A) and also caused a greater increase in levels of mRNA for insulin (Fig. S2B).
GHRH has been shown to induce proliferation of pituitary somatotroph cells; the pathway involving cAMP/protein kinase A is important in cells targeted by GHRH (22, 23). It was also reported that GHRH(1–29)NH2 and its agonists can activate mitogen-activated protein kinase (MAPK) in pituitary cells, porcine stem cells (15), and in cell lines overexpressing the GHRH receptor (24, 25), whereas GHRH antagonists inhibit cAMP production and inactivate ERK and AKT kinases in cancer cells (26, 27). However, the mechanism of GHRH and its agonists on pancreatic β cells has not yet been elucidated. In this study we demonstrated that GHRH agonists, MR-356 and MR-409, increase phosphorylation of both ERK and AKT in INS-1 cells and, in the presence of specific inhibitors of these pathways, cell proliferation was significantly inhibited (Fig. 3B). We also demonstrated that agonist MR-409 dramatically elevates the levels of cellular cAMP and phosphorylated CREB of INS-1 cells. The results suggested that at least three signaling pathways, MAPK/ERK, PI3K/AKT, and cAMP/PKA are involved in the action of GHRH agonists upon their binding to GHRH receptors on INS-1 cells. GH and various growth factors stimulate proliferation and survival of β cells; these well-characterized growth factors include IGF1 and glucagon-like peptide (GLP-1) (8, 11, 28–30). It has been reported that the binding of GH to GH-receptor triggers downstream signal transduction through the JAK2-STAT5a/b pathway, which leads to increase β-cell proliferation and survival (30, 31). The effect of IGF1/IGF1 receptor (IGF1-R) and elements of the IGF1 signal transduction pathway have been well studied in pancreatic β cells (9, 32). Insulin receptor substrate 2 (IRS-2)-mediated signaling is crucial in this action (33). IGF1 activates phosphorylation of AKT and ERK in β cells, which leads to increased cell survival and up-regulates the expression of genes including the insulin gene (34–36). Studies showed that GLP-1 improves β-cell function and increases proliferation by induction of the cAMP pathway; activation of cAMP promotes pancreatic β-cell survival by stimulating IRS-2 (29, 37, 38). Our work shows that in INS-1 cells, GHRH agonists elevate cell proliferation; activate three signaling pathways; increase expression of GHRH receptor, cellular insulin, and IGF1; and enhance insulin release in response to glucose challenge; GHRH antagonists completely inhibited cell proliferation induced by the agonists. All these findings suggest that the interaction of GHRH receptor and its ligands plays an important role in the proliferation and metabolic function of pancreatic β cells. The GHRH–GHRH receptor complex may enable signal transduction independently or in cooperation with other pathways, probably the IGF1 signaling pathway, in the regulation of development and function of pancreatic β cells. The detailed downstream elements (effectors) involved in the action of GHRH agonists need to be further elucidated. Studies using gene knockout technology and transgenic animal models may shed light on the mechanisms of cross-talk between the GHRH and other signal transduction pathways.
Agonists MR-356 and MR-409 are equally effective in tests in vitro. MR-409 was selected for investigation in vivo because MR-356 belongs to the agmatine group, which is more difficult to synthesize on a large scale. The animal model used in the study represents type-1 diabetes; in vivo treatment of diabetic animals with GHRH agonist, MR-409, clearly reduced the severity of the disease, prolonging life span and reducing hyperglycemia. Actually, the hyperglycemia in the untreated mice was generally undervalued because often the glucose levels in untreated animals were above the maximum of the glucose meter’s top of scale (600 mg/dL); in such cases, 600 mg/dL was taken as the blood glucose level. The blood glucose level in the treated mice fell somewhat in the 1st wk of treatment, increased slightly by the 2nd wk, and was stable in the 3rd and 4th wk. The situation was worse in the untreated animals as the blood glucose increased gradually through 4 wk. The high survival rate of treated diabetic mice, 45 d after onset of diabetes, correlated well with lesser severity of the disease even though the levels of serum insulin in the treated animals remained unchanged. These results imply a possible beneficial effect of treatment with GHRH agonists in the earlier stage of diabetes. In this study, no obvious side effects were observed after administration of 10 μg/day of MR-409 s.c. for 3 wk. GHRH has shown antiinflammatory effects in rats injected with endotoxin; GHRH agonist, JI-34, was shown to protect against bacterial toxin-induced pulmonary barrier dysfunction in mice (39, 40), suggesting GHRH may act as an immune modulator. The effects of GHRH agonists in immune-competent diabetic animal models need to be further tested. GHRH stimulates the release of GH from the pituitary; however the function of the GH/IGF1 axis in the causes and development of diabetes is complex (41–43). Sustained expression of GH prevents the development of autoimmune diabetes in NOD mice by modification of immune response (44). In our current study we did not observe elevated serum GH or IGF1 levels in diabetic mice after administration of MR-409 for 3 wk. It is possible that administration of GHRH agonists promotes the proliferation of the remaining β cells in pancreas after administration of STZ, or affects the peripheral levels of glucagon and GLP-1, which in turn could influence glucose homeostasis (10, 45). The underlining mechanism of reduced severity of diabetes in these immune-deficient mice is yet to be fully explained. It might be important to investigate whether GHRH agonists play a role in regeneration of endogenous islets, in preventing progressive loss of β-cell mass and function in type-2 diabetes, and in reduction of hyperlipidemia in diabetic animal models.
Transplantation of rat islets when preconditioned with GHRH agonist JI-36 leads to a better survival of engrafted rat islets, which was previously reported by our group, which demonstrates the importance of preconditioning of the islets. Islets preconditioned with GHRH agonist JI-36 displayed an increase in size as determined by measurement of relative IEQ/IPN after 24–72 h in culture (12, 13). In this study we found that treatment of rat islets with agonist MR-409 for 2 d increases islet volume and expression of insulin at RNA levels (Fig. S2). Further, we showed that superior beneficial effects with respect to regaining normoglycemia (engraft rate), elevated serum blood insulin, gain of body weights, and longer lifespan following nephrectomy of the islets-bearing kidney can result from transplantation of islets preconditioned with GHRH agonist MR-409 and then followed by administration of MR-409 posttransplantation. In the group M + T, engraftment succeeded in all animals (n = 7); the animals became normoglycemic in 8–9 d compared with 2 wk in the M group (Fig. 4A). Interestingly, the average blood glucose levels in group M + T were lower than those in normal animals or in group M through 20 d. Serum insulin levels in all three groups (C, M, and M + T) were elevated following transplantation compared with those of diabetic animals before transplantation (Fig. 5A). Immunostaining indicating that the rat islets preconditioned with GHRH agonist were stably engrafted and were functioning 1 mo after transplantation. Although serum insulin levels in “cured animals” (based on blood glucose level) in groups M and M + T were still below the average of the healthy animals (4.06 ± 0.22 ng/mL, n = 12). The limited quantity (∼160 IEQ) of transplanted rat islets was able to produce enough insulin to restore glucose homeostasis in diabetic mice. The efficacy of islet transplantation can be substantially improved by preconditioning of islets with GHRH agonist; this leads to a reduction of the islet mass necessary for metabolic control.
Numerous studies have reported efforts to improve the survival of islets by preventing loss of viability and function of islet cells during and following the transplant period. Preconditioning islets with protein kinase Ce (PKCe) activator improves islet graft function (46). Cotransplanting pituitary cells or bone marrow cells with islets also significantly improves the performance of islet grafts (47, 48). It has been reported that administration of GH increases proliferation of β cells in transplanted islets (49). Nerve growth factor (NGF) and VEGFs play a critical role in development of β cells and are also associated with the survival of islets in vivo following transplantation (50–52). GHRH(1–29)NH2 was reported to stimulate the expression of VEGF in vitro (53), and, correspondingly, GHRH antagonists strongly inhibited the expression of VEGF in tumor (54). Our work shows that exposure of INS-1 cells to GHRH agonist, MR-409, greatly enhanced the secretion of VEGF in INS-1 cells (Fig. 2E); this may explain the role of GHRH agonists in the improvement of islet engraftment following transplantation.
In conclusion, our study provides evidence for the beneficial action of synthetic GHRH agonists on pancreatic β cells in vitro and in vivo. The use of GHRH agonists offers a physiologic approach to cytoprotection of viability of islets. Long-acting GHRH agonists may fulfill the requirements of effective drugs for improving engraftment of islets following transplantation. Consequently, synthetic GHRH agonists appear to be a useful addition to the armamentarium for the therapy of diabetes mellitus. Our finding suggests the merit of further investigation of possible use of GHRH agonists for the development of new approaches to management of diabetes mellitus.
Materials and Methods
Animal Model and Treatment of Diabetic Animals.
Diabetes was induced in male NOD/SCID mice (Charles River) by single i.p. injection of STZ (Sigma, 180 mg/kg) following guidelines established by the institutional animal care and use committee of the University of Miami. Serum glucose was monitored using an AimStrip Plus glucose meter (Germaine Laboratories). In cases when blood glucose level was above the maximal reading of the meter, 600 mg/dL was taken for calculation. Mice were considered diabetic when nonfasting blood glucose rose above 400 mg/dL for at least 2 consecutive days. To determine the effect of GHRH agonist, MR-409, on nontransplanted diabetic animals, animals (n = 7) in the treatment group, designated group T, were injected s.c. with 10 μg/day MR-409 [in 10% (vol/vol) propylene glycol], whereas animals (n = 7) in the control group, designated group C, received vehicle only. The nonfasting blood glucose levels and body weight were recorded three times per week. Blood samples were collected after 3 wk of treatment; serum insulin and IGF1 were measured.
Effects of GHRH agonist, MR-409, on diabetic animals transplanted with rat islets were evaluated in three groups. For preconditioning of islets, freshly isolated rat islets were cultured in islet medium containing either 1 μM GHRH agonist, MR-409, or vehicle (0.05% DMSO) for 48 h. Islets sample (∼160 IEQ) was then transplanted beneath the left kidney capsule (12). Animals transplanted with rat islet-treated vehicle were designated group C. Animals transplanted with MR409-treated islets were further divided into two groups, which were designated group M and group M + T, respectively. Two days after transplantation, mice in group M + T received s.c. MR-409 (10 μg/day) for 3 wk, whereas mice in both group C and group M received vehicle solution [10% (vol/vol) propylene glycol] only. The nonfasting blood glucose levels and body weights were recorded three times per week. At day 14, mice were subjected to an IPGTT test. Blood samples were collected after 3 wk of treatment, and serum insulin and IGF1 were measured. At day 30, the islet-bearing left kidney was removed. Animals were observed for 1 wk following this nephrectomy.
All other materials and methods are provided in SI Materials and Methods.
SI Materials and Methods
Cell Culture.
Rat insulinoma cell line INS-1 was cultured in RPMI medium 1640 supplemented with 2 mM l-glutamine, 10% (vol/vol) FBS (heat inactivated FBS, Gibco/Invitrogen), 20 mM Hepes (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 1 mM sodium pyruvate, 50 μM β-mercaptoethanol, 100 units/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a 5% (vol/vol) CO2 atmosphere. For all experiments, cells at passages 21–30 were used.
GHRH Agonists.
Synthesis, purification, and chemical structures of our GHRH agonists have been previously reported (19). GHRH agonists JI-36 and MR series MR-356, -361, -367, -404, -406, -409, -410, and -502 were used in this study. Stock solutions of the 2-mM agonists were made in dimethyl sulfoxide (DMSO). Before use, stock solutions were diluted to working concentrations in cell culture medium. For in vivo tests, stock solution was diluted in 10% (vol/vol) propylene glycol.
Cell Proliferation Assay.
INS-1 cells were treated with GHRH agonists at selected concentrations in quadruplicate or quintuplicate; medium was replaced once after 48-h treatment. Total numbers of the living cells were chemiluminescently measured at 72 h using CellTiter 96 aqueous one solution cell proliferation assay kits (Promega) or at 24 h using BrdU cell proliferation kits (Millipore). Relative cell viability or proliferation relative to control (medium containing 0.05% DMSO) is presented. In experiments including inhibitors for MAPK/ERK and PI3K/AKT pathways, cells were treated with 1 µM GHRH agonist, MR-409, for 48 h in the presence or absence of 25 µM PD98059 or 0.5 µM wortmannin, (Sigma-Aldrich), respectively.
RNA Isolation and Quantitative Real-Time PCR.
Total RNA was isolated using RNeasy Mini kits (Qiagen). A total of 1 μg of DNase-I treated RNA was reverse transcribed to cDNA using iScript cDNA Synthesis kit (Bio-Rad). For quantitative real-time PCR (qRT-PCR), 1–5 ng cDNA and 0.5 μM of each primer in 20 μL of 1× iQ SYBR Green Supermix (Bio-Rad) was amplified using CFX96 Touch Real-Time PCR Detection System (Bio-Rad). Thermal cycling conditions included an initial denaturation at 95 °C for 3 min, followed by 40 cycles of 95 °C, 30 s and 62 °C, 1 min. The absence of nonspecific PCR products was confirmed by melting the PCR products at 0.5 °C per minute from 60 °C to 95 °C. All reactions were done in quadruplicate, and gene expression analysis was conducted using CFX manager software (Bio-Rad). The PCR primers used for each target were: rat insulin (Fw: GCCCAGGCTTTTGTCAAACA, Rev: TCCACCCAGCTCCAGTTGTG); rat IGF1 (Fw: ACAGGCTATGGCTCCAGCAT, Rev: CTCCAGCCTCCTCAGATCACA); rat GHRH receptor (Fw: CCTTCCAGGGTTTTGTTGTTG, Rev: GGTGAGCACCTTCACTCTCGAT); and rat GAPDH (Fw: CTCCCATTCTTCCACCTTTGAT, Rev: CCCTGTTGCTGTAGCCATATTC).
Western Blots.
In Western blot studies for GHRH receptors, INS-1 cells were treated with GHRH agonists (500 nM) for 48 h. Cells were lysed in lysis buffer containing 1% protease inhibitors mixture (Sigma-Aldrich). Equal amounts of protein (60 μg) were subjected to electrophoresis in 10% (vol/vol) SDS/PAGE and subsequently transferred to PVDF membrane (Bio-Rad). After blocking in 5% (wt/vol) nonfat milk, membranes were incubated 2 h with anti-GHRH receptors (Abcam ab76263) or tubulin (CalBiochem no. CP06), respectively; followed by a 1-h incubation with fluorescence-tagged secondary antibodies (LI-COR no. 926–32211, no. 926–68020). Images of the bands were captured using an Odyssey Imaging system and analyzed by Odyssey software v 3.0 (LI-COR Biotechnology). To calculate the expression levels of GHRH receptors, the intensity of the band for GHRH receptor for each treatment was first normalized against the respective tubulin reference, and then compared with the control, with normalized band intensity designated as 100%. In Western blots for AKT and ERK pathways, INS-1 cells were starved in serum-free RPMI medium 1640 for 24 h, 1 μM GHRH agonist was then added to the cell culture medium. At 30 min, cell lysate was collected and used for Western blots. Antibodies for p-ERK, ERK, p-AKT, and AKT were purchased from Cell Signaling Technology (nos. 9101, 9102, 9271, and 9272), HRP-conjugated secondary antibody from Santa Cruz (SC-2004).
Measurement of Insulin Secretion by Static Challenge with Glucose.
INS-1 cells were seeded in 24-well plates, treated with GHRH agonists (500 nM) for 48 h, and then incubated with Krebs-Ringer bicarbonate buffer (KRBB) (7), containing 3.3 mM of glucose at 37 °C for 1 h. The cells were then further treated with KRBB containing either 3.3 mM or 17 mM glucose for an additional 1 h at 37 °C. Medium was collected and the cells were washed and lysed with 0.05 N hydrochloric acid (HCl). Insulin in the medium and cell pellet was measured by ELISA and normalized with protein concentration. Percentage of insulin secretion was calculated as insulin in the medium compared with total insulin content (which consists of insulin in the medium plus cells). The experiments were repeated at least three times in triplicate for each treatment.
Measurement of cAMP.
INS-1 cells were first starved in serum-free RPMI medium 1640 for 24 h. For treatment, cells were kept in serum-free medium containing 100 μM of 3-isobutyl-1-methylxanthine (IBM-X, Sigma) and 1 μM of GHRH agonist MR-409 for 15–60 min. Cellular cAMP levels were measured by cAMP EIA kit (Cayman) following manufacturer’s instructions. Meanwhile, cell lysate was prepared and used to analyze the expression of CREB and its phosphorylated isoform by Western blots. The primary antibodies against CREB and p-CREB (Ser-133) were purchased from Cell Signaling Technology (9197 and 9198); anti-GAPDH from Santa Cruz (sc47724).
Measurement of Insulin and IGF1.
INS-1 cells were treated with 500 nM GHRH agonists for 48 h, then lysed in 0.05 N HCl. Cellular levels of insulin were determined using rat insulin ELISA kits (Millipore) and further normalized to protein concentrations. IGF1 in the cell culture medium was measured using mouse/rat IGF1 ELISA kit (R&D Systems). For serum insulin and IGF1 measurements, blood samples were collected after 3 wk of s.c. administration of MR-409 and used for ELISA analysis.
Measurement of VEGF.
INS-1 cells were treated with 500 nM GHRH agonist MR-409; cell culture medium was collected at 24, 48, and 72 h following treatment. VEGF was measured by rat VEGF ELISA kit (R&D Systems).
Isolation of Rat Pancreatic Islets.
Pancreatic islets were isolated from male Wistar rats. Briefly, animals were anesthetized by 3% (vol/vol) isoflurane, followed by injection of digestion solution (PBS containing 1 mg/mL collagenase V and 100 μg/mL DNase I, Sigma-Aldrich) through the pancreatic common bile duct (55) to expand pancreas tissue. Islets were purified using a discontinuous islet gradient (CellGro) under centrifugation at 1,600 × g for 20 min at 4 °C. Purified islets were maintained in islet medium [RPMI medium 1640 containing 10% (vol/vol) FBS, 10 mM Hepes, 5.5 mM glucose, 100 units/mL penicillin, and 100 μg/mL streptomycin]. To evaluate the effect of GHRH analogs on islet cell proliferation, isolated rat islets were treated by 1 µM GHRH analogs for 24 h. Total numbers of proliferating cells were then assayed using a BrdU cell proliferation assay kit (Millipore). The numbers of islets (IPN) of each preparation were counted and sized under an inverted microscope with quadruplicate samples of ∼300 islets stained with dithizone (Sigma-Aldrich). To calculate islet equivalents (IEQ), the islets with diameters >50 μm were divided into classes of 50-μm increments and, each diameter class was converted into mean volume of 150-μm diameter by a relative conversion factor (12). Relative islet volume (ratio of IEQ/IPN) was compared between islets treated for 48 h with either vehicle or 1 µM MR-409. In addition, total RNA was isolated and subjected to analysis for insulin expression by quantitative RT-PCR.
IPGTT.
For IPGTT, mice were fasted overnight and injected with glucose solution at 3 g/kg. Blood glucose levels were measured at 0, 15, 30, 45, 60, 90, and 120 min after injection.
Immunohistochemistry Analysis.
Frozen cryosections (14 μm thick) were prepared from the retrieved islet-bearing kidney. Sections were fixed in 4% (wt/vol) paraformaldehyde, permeabilized in PBS containing 0.2% Triton-X, and blocked with PBS containing 0.2% Triton-X and 5% (vol/vol) goat serum. Immunostaining was performed using rabbit anti-insulin antibody (Cell Signaling no. 4590), Alexa-488 goat anti-rabbit (Jackson ImmunoResearch Laboratories), and counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma). Images were acquired using a Nikon Eclipse Ti fluorescence microscope.
Statistical Analysis.
Program Graph-Pad Prism version 5.0 was used for statistical analysis. One-way ANOVA, followed by Bonferroni multiple comparison test and unpaired Student’s t test were performed. Data are expressed as mean ± SEM. P < 0.05 values were considered to be significant.
Acknowledgments
This work was supported by the Medical Research Service of Veterans Affairs Department (A.V.S.); Department of Medicine, Division of Hematology/Oncology, University of Miami, Millar School of Medicine (A.V.S.); the South Florida Veterans Affairs Foundation for Research and Education (A.V.S.); and the L. Austin Weeks Endowment for Urologic Research (N.L.B.). P.P. was supported by a stipend program of the Department of Medicine, Dresden, and by the Helmholtz Alliance Imaging and Curing Environmental Metabolic Diseases through the Initiative and Networking Fund of the Helmholtz Association.
Footnotes
Conflict of interest statement: R.C., A.V.S., and N.L.B. are co-inventors on the patent on GHRH agonists, assigned to the University of Miami and the Veterans Affairs. N.L.B. is a member of the board of directors of Biscayne Pharmaceuticals, Inc.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1518540112/-/DCSupplemental.
References
- 1.Lehmann R, Spinas GA, Moritz W, Weber M. Has time come for new goals in human islet transplantation? Am J Transplant. 2008;8(6):1096–1100. doi: 10.1111/j.1600-6143.2008.02214.x. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig B, et al. Islet transplantation at the Dresden diabetes center: Five years’ experience. Horm Metab Res. 2015;47(1):4–8. doi: 10.1055/s-0034-1385876. [DOI] [PubMed] [Google Scholar]
- 3.Bruni A, Gala-Lopez B, Pepper AR, Abualhassan NS, Shapiro AJ. Islet cell transplantation for the treatment of type 1 diabetes: Recent advances and future challenges. Diabetes Metab Syndr Obes. 2014;7:211–223. doi: 10.2147/DMSO.S50789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagliuca FW, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159(2):428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro AM, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 6.Lakey JR, Burridge PW, Shapiro AM. Technical aspects of islet preparation and transplantation. Transpl Int. 2003;16(9):613–632. doi: 10.1007/s00147-003-0651-x. [DOI] [PubMed] [Google Scholar]
- 7.Fotino N, Fotino C, Pileggi A. Re-engineering islet cell transplantation. Pharmacol Res. 2015;98:76–85. doi: 10.1016/j.phrs.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhodes CJ, White MF. Molecular insights into insulin action and secretion. Eur J Clin Invest. 2002;32(Suppl 3):3–13. doi: 10.1046/j.1365-2362.32.s3.2.x. [DOI] [PubMed] [Google Scholar]
- 9.Ma F, et al. Signaling cross talk between growth hormone (GH) and insulin-like growth factor-I (IGF-I) in pancreatic islet β-cells. Mol Endocrinol. 2011;25(12):2119–2133. doi: 10.1210/me.2011-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, et al. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem. 2003;278(1):471–478. doi: 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen JH, et al. Regulation of β-cell mass by hormones and growth factors. Diabetes. 2001;50(Suppl 1):S25–S29. doi: 10.2337/diabetes.50.2007.s25. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig B, et al. Agonist of growth hormone-releasing hormone as a potential effector for survival and proliferation of pancreatic islets. Proc Natl Acad Sci USA. 2010;107(28):12623–12628. doi: 10.1073/pnas.1005098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schubert U, et al. Transplantation of pancreatic islets to adrenal gland is promoted by agonists of growth-hormone-releasing hormone. Proc Natl Acad Sci USA. 2013;110(6):2288–2293. doi: 10.1073/pnas.1221505110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanashiro-Takeuchi RM, et al. Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction. Proc Natl Acad Sci USA. 2010;107(6):2604–2609. doi: 10.1073/pnas.0914138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Florea V, et al. Agonists of growth hormone-releasing hormone stimulate self-renewal of cardiac stem cells and promote their survival. Proc Natl Acad Sci USA. 2014;111(48):17260–17265. doi: 10.1073/pnas.1420375111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busto R, et al. The expression of growth hormone-releasing hormone (GHRH) and splice variants of its receptor in human gastroenteropancreatic carcinomas. Proc Natl Acad Sci USA. 2002;99(18):11866–11871. doi: 10.1073/pnas.182433099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green IC, Southern C, Ray K. Mechanism of action of growth-hormone-releasing hormone in stimulating insulin secretion in vitro from isolated rat islets and dispersed islet cells. Horm Res. 1990;33(5):199–204. doi: 10.1159/000181509. [DOI] [PubMed] [Google Scholar]
- 18.Dioufa N, et al. Acceleration of wound healing by growth hormone-releasing hormone and its agonists. Proc Natl Acad Sci USA. 2010;107(43):18611–18615. doi: 10.1073/pnas.1013942107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai R, et al. Synthesis of new potent agonistic analogs of growth hormone-releasing hormone (GHRH) and evaluation of their endocrine and cardiac activities. Peptides. 2014;52:104–112. doi: 10.1016/j.peptides.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asfari M, et al. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130(1):167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- 21.Skelin M, Rupnik M, Cencic A. Pancreatic beta cell lines and their applications in diabetes mellitus research. ALTEX. 2010;27(2):105–113. doi: 10.14573/altex.2010.2.105. [DOI] [PubMed] [Google Scholar]
- 22.Cunha SR, Mayo KE. Ghrelin and growth hormone (GH) secretagogues potentiate GH-releasing hormone (GHRH)-induced cyclic adenosine 3′,5′-monophosphate production in cells expressing transfected GHRH and GH secretagogue receptors. Endocrinology. 2002;143(12):4570–4582. doi: 10.1210/en.2002-220670. [DOI] [PubMed] [Google Scholar]
- 23.Rekasi Z, et al. Antiproliferative actions of growth hormone-releasing hormone antagonists on MiaPaCa-2 human pancreatic cancer cells involve cAMP independent pathways. Peptides. 2001;22(6):879–886. doi: 10.1016/s0196-9781(01)00413-2. [DOI] [PubMed] [Google Scholar]
- 24.Miller TL, Godfrey PA, Dealmeida VI, Mayo KE. The rat growth hormone-releasing hormone receptor gene: Structure, regulation, and generation of receptor isoforms with different signaling properties. Endocrinology. 1999;140(9):4152–4165. doi: 10.1210/endo.140.9.6977. [DOI] [PubMed] [Google Scholar]
- 25.Pombo CM, Zalvide J, Gaylinn BD, Diéguez C. Growth hormone-releasing hormone stimulates mitogen-activated protein kinase. Endocrinology. 2000;141(6):2113–2119. doi: 10.1210/endo.141.6.7513. [DOI] [PubMed] [Google Scholar]
- 26.Rick FG, et al. Antagonists of growth hormone-releasing hormone inhibit growth of androgen-independent prostate cancer through inactivation of ERK and Akt kinases. Proc Natl Acad Sci USA. 2012;109(5):1655–1660. doi: 10.1073/pnas.1120588109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Csernus V, Schally AV, Groot K. Antagonistic analogs of growth hormone releasing hormone (GHRH) inhibit cyclic AMP production of human cancer cell lines in vitro. Peptides. 1999;20(7):843–850. doi: 10.1016/s0196-9781(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 28.Lingohr MK, Buettner R, Rhodes CJ. Pancreatic β-cell growth and survival: A role in obesity-linked type 2 diabetes? Trends Mol Med. 2002;8(8):375–384. doi: 10.1016/s1471-4914(02)02377-8. [DOI] [PubMed] [Google Scholar]
- 29.Hui H, Nourparvar A, Zhao X, Perfetti R. Glucagon-like peptide-1 inhibits apoptosis of insulin-secreting cells via a cyclic 5′-adenosine monophosphate-dependent protein kinase A- and a phosphatidylinositol 3-kinase-dependent pathway. Endocrinology. 2003;144(4):1444–1455. doi: 10.1210/en.2002-220897. [DOI] [PubMed] [Google Scholar]
- 30.Friedrichsen BN, Galsgaard ED, Nielsen JH, Møldrup A. Growth hormone- and prolactin-induced proliferation of insulinoma cells, INS-1, depends on activation of STAT5 (signal transducer and activator of transcription 5) Mol Endocrinol. 2001;15(1):136–148. doi: 10.1210/mend.15.1.0576. [DOI] [PubMed] [Google Scholar]
- 31.Cousin SP, et al. Stimulation of pancreatic beta-cell proliferation by growth hormone is glucose-dependent: signal transduction via janus kinase 2 (JAK2)/signal transducer and activator of transcription 5 (STAT5) with no crosstalk to insulin receptor substrate-mediated mitogenic signalling. Biochem J. 1999;344(Pt 3):649–658. [PMC free article] [PubMed] [Google Scholar]
- 32.Gahete MD, et al. Insulin and IGF-I inhibit GH synthesis and release in vitro and in vivo by separate mechanisms. Endocrinology. 2013;154(7):2410–2420. doi: 10.1210/en.2013-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Withers DJ, et al. Irs-2 coordinates Igf-1 receptor-mediated β-cell development and peripheral insulin signalling. Nat Genet. 1999;23(1):32–40. doi: 10.1038/12631. [DOI] [PubMed] [Google Scholar]
- 34.Tuttle RL, et al. Regulation of pancreatic β-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat Med. 2001;7(10):1133–1137. doi: 10.1038/nm1001-1133. [DOI] [PubMed] [Google Scholar]
- 35.Dickson LM, et al. Differential activation of protein kinase B and p70(S6)K by glucose and insulin-like growth factor 1 in pancreatic β-cells (INS-1) J Biol Chem. 2001;276(24):21110–21120. doi: 10.1074/jbc.M101257200. [DOI] [PubMed] [Google Scholar]
- 36.Lingohr MK, et al. Activation of IRS-2-mediated signal transduction by IGF-1, but not TGF-alpha or EGF, augments pancreatic β-cell proliferation. Diabetes. 2002;51(4):966–976. doi: 10.2337/diabetes.51.4.966. [DOI] [PubMed] [Google Scholar]
- 37.Hussain MA, et al. Increased pancreatic β-cell proliferation mediated by CREB binding protein gene activation. Mol Cell Biol. 2006;26(20):7747–7759. doi: 10.1128/MCB.02353-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jhala US, et al. cAMP promotes pancreatic β-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003;17(13):1575–1580. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talhouk RS, Saadé NE, Mouneimne G, Masaad CA, Safieh-Garabedian B. Growth hormone releasing hormone reverses endotoxin-induced localized inflammatory hyperalgesia without reducing the upregulated cytokines, nerve growth factor and gelatinase activity. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(4):625–631. doi: 10.1016/j.pnpbp.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 40.Czikora I, et al. Protective effect of Growth Hormone-Releasing Hormone agonist in bacterial toxin-induced pulmonary barrier dysfunction. Front Physiol. 2014;5:259. doi: 10.3389/fphys.2014.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holly JM, Amiel SA, Sandhu RR, Rees LH, Wass JA. The role of growth hormone in diabetes mellitus. J Endocrinol. 1988;118(3):353–364. doi: 10.1677/joe.0.1180353. [DOI] [PubMed] [Google Scholar]
- 42.Diz Chaves Y, Spuch Calvar C, Pérez Tilve D, Mallo Ferrer F. GH responses to GHRH and GHRP-6 in Streptozotocin (STZ)-diabetic rats. Life Sci. 2003;73(26):3375–3385. doi: 10.1016/j.lfs.2003.06.026. [DOI] [PubMed] [Google Scholar]
- 43.Chen NY, et al. Effects of streptozotocin treatment in growth hormone (GH) and GH antagonist transgenic mice. Endocrinology. 1995;136(2):660–667. doi: 10.1210/endo.136.2.7835300. [DOI] [PubMed] [Google Scholar]
- 44.Villares R, et al. Growth hormone prevents the development of autoimmune diabetes. Proc Natl Acad Sci USA. 2013;110(48):E4619–E4627. doi: 10.1073/pnas.1314985110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conarello SL, et al. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia. 2007;50(1):142–150. doi: 10.1007/s00125-006-0481-3. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton D, et al. A preconditioning regimen with a PKCε activator improves islet graft function in a mouse transplant model. Cell Transplant. 2014;23(7):913–919. doi: 10.3727/096368913X665567. [DOI] [PubMed] [Google Scholar]
- 47.Davalli AM, et al. Pituitary cotransplantation significantly improves the performance, insulin content, and vascularization of renal subcapsular islet grafts. Diabetes. 1999;48(1):59–65. doi: 10.2337/diabetes.48.1.59. [DOI] [PubMed] [Google Scholar]
- 48.Sakata N, Chan NK, Chrisler J, Obenaus A, Hathout E. Bone marrow cell cotransplantation with islets improves their vascularization and function. Transplantation. 2010;89(6):686–693. doi: 10.1097/TP.0b013e3181cb3e8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Höglund E, Mattsson G, Tyrberg B, Andersson A, Carlsson C. Growth hormone increases beta-cell proliferation in transplanted human and fetal rat islets. JOP. 2009;10(3):242–248. [PubMed] [Google Scholar]
- 50.Brissova M, et al. Pancreatic islet production of vascular endothelial growth factor--a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55(11):2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 51.Lee BW, et al. Effect of hypoxia-inducible VEGF gene expression on revascularization and graft function in mouse islet transplantation. Transpl Int. 2011;24(3):307–314. doi: 10.1111/j.1432-2277.2010.01194.x. [DOI] [PubMed] [Google Scholar]
- 52.Zhang N, et al. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes. 2004;53(4):963–970. doi: 10.2337/diabetes.53.4.963. [DOI] [PubMed] [Google Scholar]
- 53.Stepień T, et al. Stimulatory effect of growth hormone-releasing hormone (GHRH(1-29)NH2) on the proliferation, VEGF and chromogranin A secretion by human neuroendocrine tumor cell line NCI-H727 in vitro. Neuropeptides. 2009;43(5):397–400. doi: 10.1016/j.npep.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Letsch M, Schally AV, Busto R, Bajo AM, Varga JL. Growth hormone-releasing hormone (GHRH) antagonists inhibit the proliferation of androgen-dependent and -independent prostate cancers. Proc Natl Acad Sci USA. 2003;100(3):1250–1255. doi: 10.1073/pnas.0337496100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol Proced Online. 2009;11:3–31. doi: 10.1007/s12575-009-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]