Significance
The retina is subject to a variety of insults that lead to degeneration of one or more types of neurons and ultimate visual impairment and blindness. Although the retinas of nonmammalian vertebrates can regenerate new neurons after injury, mammalian retinas largely lack this potential. We have tested whether the expression of the proneural transcription factor Ascl1 may be a key difference between the fish and mouse by targeting this factor to the cells that provide new retinal progenitors in mature retina, the Müller glia. Our results show that at least one of the differences between mammal and fish Müller glia that bears on their difference in regenerative potential is the proneural transcription factor Ascl1.
Keywords: reprogramming, glia, regeneration, neurogenesis, eye
Abstract
Müller glial cells are the source of retinal regeneration in fish and birds; although this process is efficient in fish, it is less so in birds and very limited in mammals. It has been proposed that factors necessary for providing neurogenic competence to Müller glia in fish and birds after retinal injury are not expressed in mammals. One such factor, the proneural transcription factor Ascl1, is necessary for retinal regeneration in fish but is not expressed after retinal damage in mice. We previously reported that forced expression of Ascl1 in vitro reprograms Müller glia to a neurogenic state. We now test whether forced expression of Ascl1 in mouse Müller glia in vivo stimulates their capacity for retinal regeneration. We find that transgenic expression of Ascl1 in adult Müller glia in undamaged retina does not overtly affect their phenotype; however, when the retina is damaged, the Ascl1-expressing glia initiate a response that resembles the early stages of retinal regeneration in zebrafish. The reaction to injury is even more pronounced in Müller glia in young mice, where the Ascl1-expressing Müller glia give rise to amacrine and bipolar cells and photoreceptors. DNaseI-seq analysis of the retina and Müller glia shows progressive reduction in accessibility of progenitor gene cis-regulatory regions consistent with the reduction in their reprogramming. These results show that at least one of the differences between mammal and fish Müller glia that bears on their difference in regenerative potential is the proneural transcription factor Ascl1.
The retina of the teleost fish has a remarkable potential to regenerate new neurons after various types of injury (1, 2). This regenerative potential is much more limited in birds, where neurotoxic damage leads to regeneration of only a subset of inner retinal neurons. In mice and rats, several groups have reported that under certain conditions, these mammalian retinas can also regenerate new neurons after injury or with growth factor stimulation (3, 4); however, the number of new cells is very small, and there is no evidence that regenerated neurons integrate into the retinal circuitry. Although for many years it was known that the source of the regeneration was intrinsic to the retina in teleosts, it is only in the past 15 y that the Müller glial cells were recognized as the primary source of new neurons after retinal damage in fish and birds (5, 6). Attempts to stimulate retinal regeneration in the mammalian retina have therefore focused on the Müller glial cells.
Our understanding of the molecular mechanisms that regulate retinal regeneration from Müller glia in fish has advanced rapidly in the last 10 y. Many of the initial steps in the response to injury that lead to regeneration in fish are also conserved in the mammalian retina (7). In particular, retinal injury in either fish or mammals induces the up-regulation of many mitogenic growth factors (7–9). However, one key difference in the response to injury between fish and mammals is in the expression of proneural transcription factors. In fish and birds, Ascl1 is one of the genes that is rapidly induced in the Müller glia after injury (5, 10). In addition, the expression of the proneural transcription factor Ascl1 is required for retinal regeneration in fish; knocking down Ascl1 in zebrafish prevents the Müller glia from reentering the mitotic cell cycle, and they no longer produce new neurons (10, 11). In mice, Ascl1 is not up-regulated significantly after neurotoxic injury (12) and is one of the differences in transcription factor expression between Müller glia and retinal progenitors (13) (SI Appendix, Fig. S1). We have previously reported that forced expression of Ascl1 in dissociated cultures of mouse Müller glia using a lentiviral approach stimulates mitotic proliferation in the cells, increases expression of many progenitor genes, and induces some of their progeny to develop morphological and electrophysiological characteristics of neurons (14).
Results
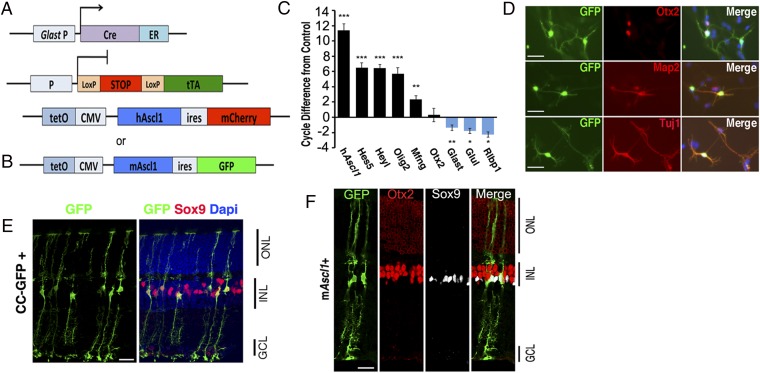
Our previous results led us to hypothesize that Ascl1expression is a critical step in the regeneration process in fish that is absent normally in mouse Müller glia. To test this hypothesis in vivo, we have generated transgenic mouse lines that allow us to direct Ascl1 expression to Müller glia at the time of retinal injury. To determine whether the overexpression of Ascl1 would be sufficient to reprogram Müller glia to retinal progenitors in vivo, we generated transgenic mice that overexpress Ascl1 and a reporter (either nuclear mCherry or cytoplasmic EGFP) under the tetracycline-responsive element (tetO-Ascl1-ires-mCherry; “Ascl1-ires-mCherry” or “Ascl1-ires-EGFP” mice; Fig. 1 A and B). Ascl1-mCherry mice were crossed with Pax6-Cre:Rosa-Flox-stop-rtTA mice to generate Pax6-Cre:Flox-stop-rtTA;Ascl1-mCherry mice (Fig. 1A). We confirmed that Ascl1 was expressed by the transgene using retinal explant cultures from postnatal day 12 (P12) mice. Doxycycline-treated explant cultures showed a large increase in Ascl1 by RT-PCR (Fig. 1C); the Ascl1 targets, Mfng, Heyl, Olig2, and Hes5, showed significant increases, whereas Müller glial genes were reduced (Fig. 1C), similar to what was previously reported for lentiviral expression of Ascl1 in dissociated Müller glial cultures (14). Ascl1 overexpression in dissociated cultures of Müller glial cells using the Glast-CreER (SI Appendix, Mori et al., 2006): Rosa-Flox-stop-tTA;Ascl1-EGFP mice (i.e., Ascl1-EGFP) (Fig. 1B) causes the cells to adopt a neuronal morphology (Fig. 1D) and express markers of neuronal differentiation, Otx2, TuJ1, and Map2 (Fig. 1D). Together these results confirm our previous findings with lentiviral Ascl1 overexpression and show that the level of expression from the transgenic mice is sufficient to initiate the reprogramming process in dissociated and explant cultures of retina.
Fig. 1.
Transgenic Ascl1 overexpression reprograms Müller glia in vitro. (A and B) Diagrams showing the strategy for transgenic Ascl1 overexpression using either AlphaPax6-cre (for retinal-specific expression), rtTAfl-stop, and tetO-Ascl1-IRES-mCherry (hAscl1-mCherry); or Glast-creER (for glial-specific expression), tTAfl-stop, and tetO-Ascl1-IRES-GFP (Ascl1-GFP). (C) Bar plot of RT-PCR–cycle difference from control showing means and SEM for Ascl1, progenitor genes (black), or glial genes (blue) in P12 retinal explants. n = 4; *P < 0.01, **P < 0.005, ***P < 0.0001. (D) Dissociated culture of P12 control and Ascl1-GFP Müller glia, maintained in vitro for 11 d; labeled for antibodies as shown. (Scale bar, 50 μm.) (E) Sections from control mice (CC-GFP = Cag-Cat-eGFP) that received intraperitoneal injections of tamoxifen as adults, colabeled for Sox9 (red). (F) Section through adult mouse retina after 3 wk of Ascl1-GFP expression in the Müller glia, colabeled for Sox9 (white) and Otx2 (red). (Scale bars for E and F, 20 μm.)
To direct the Ascl1 expression to Müller glia in vivo, we used the Glast-CreER: Flox-stop-tTA mice. We tested the specificity of this strategy with a control GFP reporter line (CC-GFP) (SI Appendix). CC-GFP mice over 30 d old were given four to five injections of tamoxifen to activate the cre-recombinase (Fig. 1E). In the CC-GFP reporter mice, the GFP was expressed exclusively in glia in the retina; no retinal neurons were labeled. The vast majority of the labeled cells were Sox9+ Müller glia, with their distinctive morphology (Fig. 1E and Fig. 2), although some astrocytes at the vitreal surface also expressed the reporter.
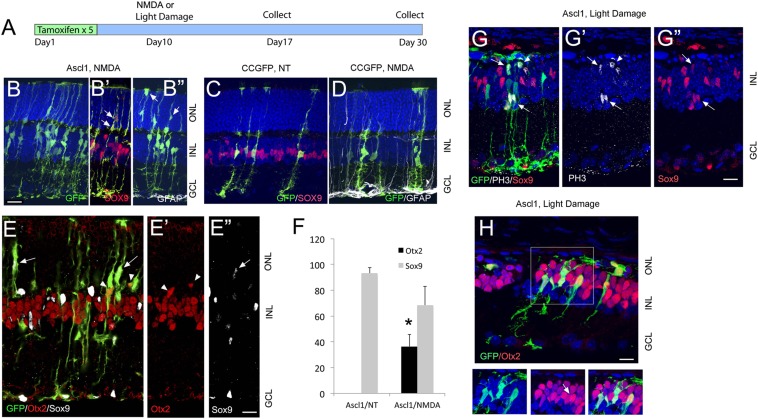
Fig. 2.
Transgenic Ascl1 overexpression reprograms Müller glia after injury. (A) Diagram showing the experimental design. (B) Retinal section of adult Ascl1- GFP mouse after tamoxifen injections, followed by a single intraocular injection of NMDA, and collected 1 wk later. Anti-GFAP (white) shows Müller glial reactivity after the NMDA (B′, B′′, and D). Müller glia in the Ascl1-GFP retina migrate to the ONL (arrows). (Scale bar, 20 μm.) Red shows anti-Sox9. (C and D) Retinal sections of CC-GFP mice that received no treatment (NT, C) or NMDA (D). The Müller glia retain Sox9 (red) expression in controls and do not migrate into the ONL. (E) Ascl1-GFP–expressing Müller glia express Otx2 (red, arrowheads) and down-regulate Sox9 (white, arrows). (Scale bar, 20 μm.) (F) Graph showing the percentages of Sox9+ and Otx2+ Müller glia in CC-GFP and Ascl1-GFP retinas and in Ascl1-GFP+ retinas following NMDA or light damage. The increase in Otx2 expression after damage in the Ascl1-GFP retinas was significant by a Student’s t test at P = 0.027 (n = 3 for each group). (G) Ascl1-GFP+ light-damaged retina showing Sox9+ (red) Müller glia expressing the mitotic cell-cycle marker, Phospho-Histone3 (white) (arrows). (H) Section through the retinas of light-damaged, Otx2+ progeny of Ascl1-GFP+ Müller glia migrate to the ONL. (Boxed region, Right) Ascl1-GFP+ Müller glia express Otx2. (Scale bar, 20 μm.)
We next tested whether expression of Ascl1 in mature Müller glia is sufficient to reprogram them to a neurogenic state in vivo using the Glast-CreER: Flox-stop-tTA;Ascl1-GFP mice. In these retinas, all of the GFP+ Müller glial cells were also immunoreactive for Ascl1, and RT-PCR analysis showed substantial Ascl1 expression and an up-regulation in known target genes (SI Appendix, Fig. S3). We assessed the morphology of the Ascl1-GFP+ Müller glia in mice that survived for 1–4 wk after the tamoxifen treatment. Although Ascl1 expression was successfully induced in adult Müller glia, there were only relatively minor changes detected in the Müller glia even 1 mo after the tamoxifen injections. The Müller glia continued to express Sox9 and did not express the neural marker, Otx2 (Fig. 1F), and there was no evidence of mitotic proliferation. These results indicate that transgenic overexpression of Ascl1 in mature mouse Müller glia is not sufficient to reprogram these cells to a neurogenic state as it does in vitro.
Although the Müller glia appeared unaltered by Ascl1 expression in the Ascl1-GFP mice, we hypothesized that their expression of Ascl1 might be sufficient to promote regeneration of new neurons after injury. To test this hypothesis, we used two types of injury: NMDA and excessive light (Fig. 2A). Ascl1 expression (day 1) was induced with five daily injections of tamoxifen followed by an intraocular injection of NMDA to induce death of amacrine and ganglion cells (SI Appendix, Fig. S4). We assessed the degree of neurotoxic damage by immunolabeling for GFAP to examine glial reactivity and Brn3 or HuC/D to verify retinal ganglion cell and amacrine cell loss, respectively.
When Ascl1 is expressed in Müller glia before NMDA-induced amacrine and ganglion cell death, the Müller glia show distinct morphological changes that differ substantially from the CC-GFP–expressing Müller glia shown in Fig. 2 C and D. In NMDA-damaged retinas, some of the Ascl1-GFP+ Müller glia migrate to the ONL (outer nuclear layer) (Fig. 2 B′, B′′, and E, arrows), and ∼50% of the Ascl1-GFP+ Müller glia down-regulate Sox9 and instead express the photoreceptor/bipolar cell transcription factor Otx2 (Fig. 2 E and F). NMDA damage in control mice did not result in these changes. These early changes in the Müller glia after injury resemble the dedifferentiation and acquisition of neurogenic markers observed in fish after retinal injury; however, even after 3 wk, the cells did not acquire morphology or markers of further neuronal differentiation. Similar results were obtained using excessive light to induce photoreceptor cell death in CC-GFP+ and in Ascl1-GFP–expressing Müller glia (Fig. 2 G and H). Light damage caused the migration of a subset of Ascl1-GFP+ Müller glia to the outer nuclear layer (Fig. 2 G and H and SI Appendix, Fig. S5), and some of the migrating Ascl1-GFP+ Müller glia expressed the mitotic markers, PH3 and Ki67 (SI Appendix, Fig. S5); however, less than 5% of the Ascl1-GFP+ cells were labeled for a proliferation marker at the 3-wk survival period. Many of the Müller glia that migrated to the ONL were immunoreactive for Otx2, a transcription factor normally expressed in photoreceptor and bipolar cells (Fig. 2H), and the cells lost expression of the glial marker, Sox9 (SI Appendix, Fig. S5). Similar to NMDA damage, light damage did not result in further differentiation of mature neuronal properties: the Ascl1-GFP+ cells did not acquire a photoreceptor morphology, even after 3 wk. Thus, although Ascl1 can promote the dedifferentiation of Müller glia after injury (loss of Sox9) and initiate a neurogenic response (proliferation, Otx2 expression), more mature markers of neural/photoreceptor differentiation are not expressed by the Ascl1-GFP–expressing cells in adult mice.
The above results show that Ascl1 is not sufficient to stimulate regeneration of differentiated neurons from Müller glia in the mature retina. We next asked whether expression of Ascl1 in the Müller glia of young mice can stimulate their ability to regenerate neurons after injury (Fig. 3A). By postnatal day 7 in the mouse, all of the Müller glia have been generated and have differentiated by postnatal day 10. In mice injected with tamoxifen at postnatal day 12 (in the CC-GFP+ mice) and analyzed at P21, as in the adult, all of the cells labeled in the Glast-creER retina are Müller glia (Fig. 3B). There are no photoreceptors, bipolar cells, or amacrine cells expressing the reporter. When we induce Ascl1 expression in Müller glia by injections of tamoxifen at P12 or P14, the Müller glial morphology is similar to the control CC-GFP–expressing cells (Fig. 3B), indicating that the expression of Ascl1 alone does not stimulate neurogenesis in Müller glia.
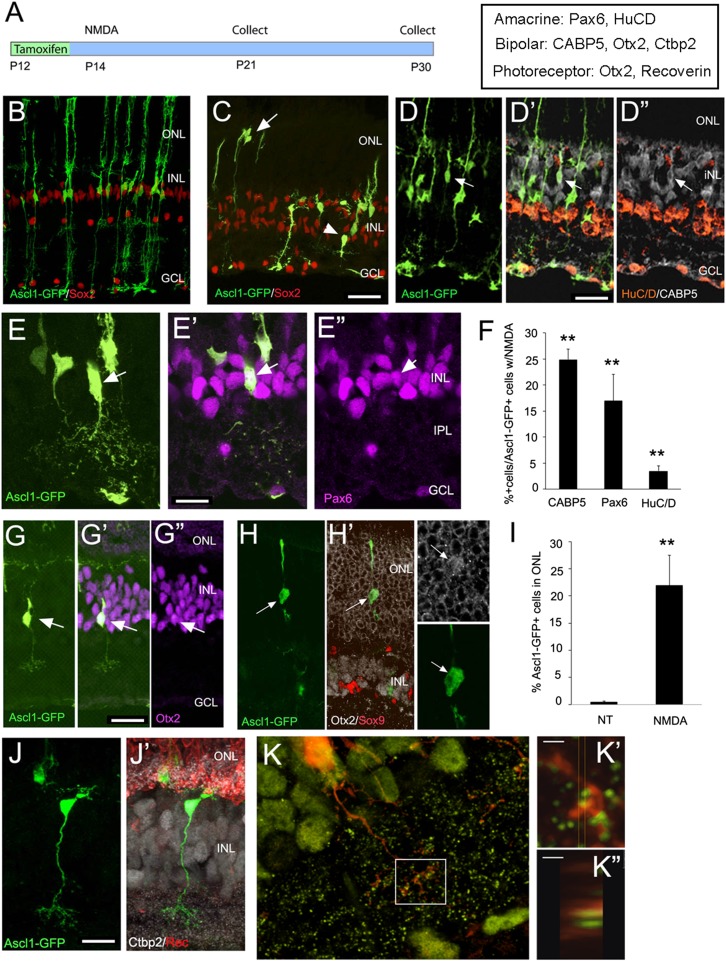
Fig. 3.
(A) Diagram showing the experimental design for Ascl1 transgenic expression in Müller glia in young mice. (B) Retinal section through P21 Ascl1- GFP mouse that received tamoxifen at P12. All labeled cells are Sox9+ (red) and have Müller glial morphology. (C–E, G, H, J, and K) Retinal sections through P21 Ascl1-GFP mouse that received tamoxifen at P12 and a single intraocular injection of NMDA at P14. (C) The Ascl1-GFP+ cells now take on a variety of different morphologies and migrate to the ONL (arrows) and inner part of the INL (arrowhead). (D–D′′) Example of a CABP5+ bipolar cell (arrow). (E–E′′) Example of Pax6+ amacrine cell. (F) Percentage of Ascl1-GFP+ cells that express the marker shown. Significance compared with undamaged Ascl1-GFP+ retinas, where no cells with these labels were observed (**P < 0.001, n = 5) (G) Example of Otx2+ bipolar cell derived from Müller glia. (H) Example of Otx2+ (white) rod photoreceptor-like cell, negative for Sox9 (red). (Inset) The Otx2 labeling in the GFP+ cell has acquired the rod-like morphology. (I) Graph of the percent of Ascl1-GFP+ cells in the ONL in undamaged Ascl1-GFP+ retinas and after NMDA damage. **P = 0.0017. (n = 5) (J and J′) Example of bipolar cell derived from Ascl1-GFP+ Müller glia, showing CtBP2 labeling (white) and recoverin (red) labeled photoreceptors and termini in the OPL. (K, K′, K′′) Overlap between CtBP2 (green) and GFP (red) in a Müller glial-derived bipolar cell. (Scale bars, 1 μm.)
We next asked whether at this age the Müller glia would show a more complete regenerative response. NMDA injections are neurotoxic for amacrine cells and ganglion cells in 2-wk-old mice, much as in adults (SI Appendix, Fig. S4). We induced Ascl1 expression at P12 with tamoxifen and then at P14 made intraocular injections of NMDA and analyzed the retinas at P21. We found that in the NMDA-damaged retinas of the young mice, the response is quite different from that observed in the adults. In NMDA-treated retinas in young mice, many of the Ascl1-GFP+ cells in the INL (inner nuclear layer) acquire morphological features of bipolar cells and amacrine cells, whereas those that migrate to the ONL resemble rod photoreceptors (Fig. 3 C–E, G, H, and J). Some of the cells that resemble amacrine cells also express the amacrine markers, HuC/D and Pax6 (Fig. 3 E and F and SI Appendix, Fig. S6), whereas many of the cells that develop morphological features of bipolar cells also express bipolar markers Otx2 and Cabp5 (Fig. 3 D, F, and G). Approximately 20% of the Müller glial-derived cells in the ONL had a rod-like morphology and expression of Otx2 in a rod-like nuclear pattern (Fig. 3 H and I), and some of the cells express the photoreceptor-marker, recoverin (SI Appendix, Fig. S7). In addition, the arbors of putative bipolar cells in the inner plexiform layer show overlap with the ribbon synaptic marker, CtBP2, suggesting that the cells form connections with the existing retinal circuitry (Fig. 3 J and K and SI Appendix, Fig. S8). These neuronal cells are only present after NMDA treatment and are generated in response to the retinal damage. Cell-cycle markers Ki67 and EdU show that some of the Ascl1-expressing cells reenter the mitotic cycle at P12 (SI Appendix, Fig. S9). Thus, directed overexpression of Ascl1 to Müller glia in young mice enables them to respond to retinal injury and regenerate retinal neurons, including amacrine cells. Although NMDA likely only damages amacrine and ganglion cells, we consistently find bipolar cells and photoreceptors generated in response to the neurotoxin. In fish, selective damage of a particular cell type typically induces regeneration of other cell types as well (1). Thus, Ascl1-expressing mouse Müller glia appear to undergo a similar response. The regenerative response to NMDA damage observed in Ascl1-expressing Müller glia does not occur after the second postnatal week. NMDA damage at postnatal day 16 or day 18 results in an adult-like response, with an increase in Otx2 and a decrease in Sox9 in Müller glia, and morphological changes similar to those in the adult, but with no clear examples of bipolar, amacrine, or photoreceptor differentiation (SI Appendix, Fig. S10 B and D). One difference that might account for this change in the response is that Sox9 is reduced in nearly all of the Müller glia that express Otx2 at P14, but most Müller glia retain Sox9 expression after P16, even though they express Otx2 (SI Appendix, Fig. S10C).
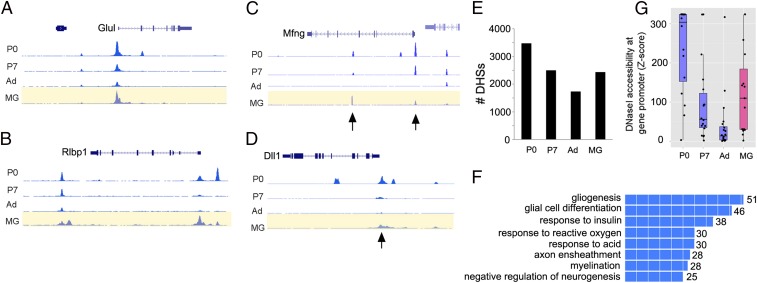
The difference in the response of young and adult Müller glia may reflect epigenetic changes in chromatin accessibility that occur as the cells mature, and evidence from zebrafish indicates epigenetic changes affect regeneration (15). To assess chromatin accessibility at potential Ascl1 progenitor targets, we carried out DNaseI-seq in Müller glial cultures and compared the DHSs (DNase Hypersensitive Sites) with those of developing (P0, P7) and mature retina. At P0, the retina has a mix of progenitors and differentiated neurons, whereas at P7, neurogenesis is complete. Genome-wide analysis of Müller glial DHSs shows that ∼34% of the known promoters have a DHS in Müller glia, and the other DHSs are distributed primarily over intronic and intergenic regions, with a smaller percentage in exons, similar to those present in total retina (16). Fig. 4 shows examples of signal near genes known to be highly expressed in Müller glia (Fig. 4 A and B), and progenitors (Fig. 4 C and D). Surprisingly, although the progenitor genes Mfng and Dll1 are not expressed in Müller glia (SI Appendix, Fig. S1), the chromatin showed some accessibility in the P12 cells and the P7 retina. To identify potential Ascl1 targets, we overlapped the DHSs from P0 with ChIP-seq data for Ascl1 from neural progenitors (17). We then asked whether these same DHSs were accessible in the P12 Müller glia, the P7 retina, and the adult retina. The majority of the P0 DHSs with an Ascl1 ChIP-seq peak were also present in both P7 retina and P12 Müller glial cells and shared between these ages (Fig. 4E and SI Appendix, Fig. S11). These DHSs were analyzed with GREAT (Genomic Regions Enrichment of Annotations Tool) (18), and the GO (Gene Ontology) Biological Process terms were highly enriched for CNS development (Fig. 4F). Many of these DHSs are not present in the adult retina, however, and those that remain are not enriched for neural development GO terms when analyzed by GREAT (SI Appendix, Fig. S11). We further assessed DNase accessibility at the promoters of a set of 15 progenitor genes (SI Appendix, Table S3) and found that these showed greater accessibility in the P12 glia than in adult retina (Fig. 4G). These data suggest that the cis-regulatory regions of retinal progenitor genes are accessible in young Müller glia, but not in the adult, and may explain why Ascl1 is able to reprogram the young Müller glia effectively.
Fig. 4.
DNaseI-seq analysis of Müller glia shows accessible chromatin at progenitor gene loci. (A and B) Examples of Müller glial-expressed genes, glutamate synthetase (Glul), and cellular retinaldehyde-binding protein (Rlbp1) at three stages of retinal development and in P12 Müller glia. (C and D) Examples of progenitor genes, Mfng and Dll1, in P12 Müller glia. Arrows point to peaks present in P0 retina that remain accessible at P7 and in P12 Müller glia. (E) P0 DHSs that overlap with Ascl1-ChIP-seq in neural progenitors that are also present at P7, adult retina and P12 Müller glia. (F) The P0-DHS/Ascl1-ChIP-seq peaks DHSs shared with Müller glia are enriched for GO Biological processes associated with neural developmental genes. (G) The degree of DNase1 hypersensitivity at the promoters of 15 progenitor genes in P0, P7, or adult retina, compared with Müller glia, using z scores (e−x). Box plots with means and data points shown.
Discussion
Taken together, our results demonstrate that transgenic expression of Ascl1 in mouse Müller glia, either in vitro or in vivo, provides them with the potential to respond to retinal injury in a manner reminiscent of the regenerative response observed in nonmammalian vertebrates. In the absence of retinal damage, in vivo expression of Ascl1 in Müller glia of adult mice does not overtly disrupt the cells; however, when the retinas are injured with either a neurotoxin or excessive light, many of the cells migrate from their normal layer, some reenter the mitotic cell cycle, and ∼50% express early neural determination markers, like Otx2, and reduce their expression of glial genes, e.g., Sox9. The response to Ascl1 expression in Müller glia in young mice is even more effective at driving regeneration; when 2-wk-old mouse retinas are damaged, the transgenic expression of Ascl1 gives the cells the potential to regenerate new neurons. The progeny of the Müller glia express markers of bipolar cells, amacrine cells, and photoreceptors, and they acquire the appropriate laminar position and morphology consistent with the markers. The cells appear to integrate with the existing retinal cells, making extensive arbors in the inner plexiform layer and expressing puncta of the ribbon synaptic marker, CtBP2.
The results with Ascl1 expression in Müller glia are similar to those obtained by reprogramming brain glial cells, where proneural transcription factors like Ascl1 (in combination with MyT1 and Brn2), Neurog2, or Neurod1 have the potential to reprogram astrocytes to neurons (17, 19, 20). Several dozen papers report neuronal reprogramming in culture, but more recently, similar results have shown this can occur in vivo, although in most cases at a very low efficiency (21–27). Similar to the retina, these reports have found that early neural markers, like Dcx and β-Tubulin type III, are expressed in a higher percentage of the infected glia than more mature markers like NeuN. It is also interesting that neural injury potentiates the reprogramming response from astrocytes (25, 26), possibly through the release of growth factors (8), as these have been shown to replace damage in zebrafish and chicken retinal regeneration (7, 28). The age dependence in the response of the Müller glia to expression of a proneural transcription factor is similar to that observed after expression of Atoh1 in the support cells of the cochlea; Atoh1 causes the cells to transdifferentiate into sensory hair cells in young postnatal mice, but not in adult animals (29–31). Our results suggest that the restriction in regenerative ability as the animals mature may be due to epigenetic changes (32); progenitor gene loci are largely accessible in P12 Müller glia, but these become less accessible in adult retina.
Overall, our results show that at least one of the differences between mammal and fish Müller glia that bears on their difference in regenerative potential is likely the proneural transcription factor Ascl1. The response to damage in Ascl1-expressing Müller glia has some features in common with the response of these cells in the fish in that they down-regulate Rlbp1 and other glial genes, while increasing their expression of progenitor genes (33–35). However, in the zebrafish, the initial mitotic division of the Müller glia produces progenitor cells that continues to divide to generate new neurons (2, 34), whereas in Ascl1-expressing mouse Müller glia, the amount of mitotic division is much lower, even with Ascl1 expression. Thus, additional factors will be required to produce a full regenerative response in adult mice more like that in fish. Nevertheless, the targeted expression of this single proneural transcription factor to Müller glia produces a coordinated response to injury reminiscent of that in zebrafish and provides a way forward for the stimulation of retinal regeneration in the treatment of eye diseases.
Materials and Methods
Detailed methods are provided in SI Appendix. Most techniques are based on previously published protocols. The TetO-Ascl1-IRES-EGFP transgene was constructed from mouse Ascl1 under the control of tetO promoter, and the internal ribosome entry site (IRES) and enhanced green fluorescent protein (EGFP) cDNA are further placed downstream of Ascl1. Mice carrying the transgene were generated by the pronuclear injection using standard techniques. The TetO-hAscl1-IRES-mCherry mice were similarly generated. Mice were injected with tamoxifen in corn oil intraperitoneally to activate overexpression of Ascl1 and GFP to label recombinant cells.
Supplementary Material
Acknowledgments
The authors thank Drs. Rachel Wong, David Raible, Leah Vandenbosch, and Olivia Bermingham-McDonogh for critical comments on the manuscript. We thank members of Dr. Rachel Wong’s laboratory for help with the imaging and image processing, particularly Clare Gamlin. We thank members of the T.A.R. and Bermingham-McDonogh laboratories, for valuable discussion and technical advice. The authors also thank Ed Levine for the Rlbp1-creER mouse line and the laboratory of Dr. John Stamatoyannopoulos for assistance with the DNase-seq. This work was supported by a grant from the International Retinal Research Foundation and F32 EY023498-01A1 (to Y.U.), 1R01EY021482 (to T.A.R.), and 1RO1EY021374 (to Kang Zhang), an NSF Fellowship (to M.S.W.) (DGE-0718124), and the Vision Core Grant P30EY01730 for use of the imaging facilities.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the Sequence Read Archive (SRA) database, www.ncbi.nlm.nih.gov/sra (accession nos. GSM1014188, GSM1014198, and GSM1014175).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1510595112/-/DCSupplemental.
References
- 1.Goldman D. Müller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014;15(7):431–442. doi: 10.1038/nrn3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenkowski JR, Raymond PA. Müller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retin Eye Res. 2014;40:94–123. doi: 10.1016/j.preteyeres.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karl MO, Reh TA. Regenerative medicine for retinal diseases: Activating endogenous repair mechanisms. Trends Mol Med. 2010;16(4):193–202. doi: 10.1016/j.molmed.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karl MO, Reh TA. Studying the generation of regenerated retinal neuron from Müller glia in the mouse eye. Methods Mol Biol. 2012;884:213–227. doi: 10.1007/978-1-61779-848-1_15. [DOI] [PubMed] [Google Scholar]
- 5.Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4(3):247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- 6.Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Müller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26(23):6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan J, Ramachandran R, Goldman D. HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration. Dev Cell. 2012;22(2):334–347. doi: 10.1016/j.devcel.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan J, Zhao XF, Vojtek A, Goldman D. Retinal injury, growth factors, and cytokines converge on β-catenin and pStat3 signaling to stimulate retina regeneration. Cell Reports. 2014;9(1):285–297. doi: 10.1016/j.celrep.2014.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kassen SC, et al. CNTF induces photoreceptor neuroprotection and Müller glial cell proliferation through two different signaling pathways in the adult zebrafish retina. Exp Eye Res. 2009;88(6):1051–1064. doi: 10.1016/j.exer.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Fausett BV, Gumerson JD, Goldman D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J Neurosci. 2008;28(5):1109–1117. doi: 10.1523/JNEUROSCI.4853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Müller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol. 2010;12(11):1101–1107. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karl MO, et al. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci USA. 2008;105(49):19508–19513. doi: 10.1073/pnas.0807453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson BR, et al. Genome-wide analysis of Müller glial differentiation reveals a requirement for Notch signaling in postmitotic cells to maintain the glial fate. PLoS One. 2011;6(8):e22817. doi: 10.1371/journal.pone.0022817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollak J, et al. ASCL1 reprograms mouse Müller glia into neurogenic retinal progenitors. Development. 2013;140(12):2619–2631. doi: 10.1242/dev.091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell C, Grant AR, Cornblath E, Goldman D. Analysis of DNA methylation reveals a partial reprogramming of the Müller glia genome during retina regeneration. Proc Natl Acad Sci USA. 2013;110(49):19814–19819. doi: 10.1073/pnas.1312009110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilken MS, et al. DNase I hypersensitivity analysis of the mouse brain and retina identifies region-specific regulatory elements. Epigenetics Chromatin. 2015 doi: 10.1186/1756-8935-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wapinski OL, et al. Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell. 2013;155(3):621–635. doi: 10.1016/j.cell.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLean CY, et al. GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol. 2010;28(5):495–501. doi: 10.1038/nbt.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vierbuchen T, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robel S, Berninger B, Götz M. The stem cell potential of glia: Lessons from reactive gliosis. Nat Rev Neurosci. 2011;12(2):88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- 21.Heinrich C, Spagnoli FM, Berninger B. In vivo reprogramming for tissue repair. Nat Cell Biol. 2015;17(3):204–211. doi: 10.1038/ncb3108. [DOI] [PubMed] [Google Scholar]
- 22.Heinrich C, Götz M, Berninger B. Reprogramming of postnatal astroglia of the mouse neocortex into functional, synapse-forming neurons. Methods Mol Biol. 2012;814:485–498. doi: 10.1007/978-1-61779-452-0_32. [DOI] [PubMed] [Google Scholar]
- 23.Niu W, et al. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013;15(10):1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu W, et al. SOX2 reprograms resident astrocytes into neural progenitors in the adult brain. Stem Cell Rep. 2015;4(5):780–794. doi: 10.1016/j.stemcr.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grande A, et al. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat Commun. 2013;4:2373. doi: 10.1038/ncomms3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Z, et al. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014;14(2):188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torper O, et al. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci USA. 2013;110(17):7038–7043. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer AJ, Reh TA. Exogenous growth factors stimulate the regeneration of ganglion cells in the chicken retina. Dev Biol. 2002;251(2):367–379. doi: 10.1006/dbio.2002.0813. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, Fang J, Dearman J, Zhang L, Zuo J. In vivo generation of immature inner hair cells in neonatal mouse cochleae by ectopic Atoh1 expression. PLoS One. 2014;9(2):e89377. doi: 10.1371/journal.pone.0089377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z, et al. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J Neurosci. 2012;32(19):6600–6610. doi: 10.1523/JNEUROSCI.0818-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly MC, Chang Q, Pan A, Lin X, Chen P. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J Neurosci. 2012;32(19):6699–6710. doi: 10.1523/JNEUROSCI.5420-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raposo AA, et al. Ascl1 coordinately regulates gene expression and the chromatin landscape during neurogenesis. Cell Reports. 2015;10:1544–1556. doi: 10.1016/j.celrep.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin Z, Barthel LK, Raymond PA. Genetic evidence for shared mechanisms of epimorphic regeneration in zebrafish. Proc Natl Acad Sci USA. 2009;106(23):9310–9315. doi: 10.1073/pnas.0811186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thummel R, et al. Pax6a and Pax6b are required at different points in neuronal progenitor cell proliferation during zebrafish photoreceptor regeneration. Exp Eye Res. 2010;90(5):572–582. doi: 10.1016/j.exer.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thummel R, et al. Characterization of Müller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp Eye Res. 2008;87(5):433–444. doi: 10.1016/j.exer.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.