Significance
Signaling pathways regulate the self-renewal and differentiation of embryonic stem cells (ESCs). Suppression of Mek/Erk signaling by pharmacological inhibitors promotes self-renewal and pluripotency maintenance of mouse ESCs, supporting the prevailing view that Erk signaling is dispensable for ESC self-renewal. However, using inducible Erk knockout ESCs, we demonstrate that Erk signaling is critical for ESC self-renewal. ESCs cannot be maintained for more than four passages after Erk depletion, associated with misregulated expression of pluripotency genes, reduced proliferation rate, G1 cell-cycle arrest, increased apoptosis, rapid shortening of telomeres, and impaired genomic stability. We further demonstrate an Erk-independent function of Mek, which may explain the diverse effects of Mek inhibition and Erk knockout on ESC self-renewal.
Keywords: Erk, Mek, embryonic stem cells, self-renewal, genomic stability
Abstract
Inhibition of Mek/Erk signaling by pharmacological Mek inhibitors promotes self-renewal and pluripotency of mouse embryonic stem cells (ESCs). Intriguingly, Erk signaling is essential for human ESC self-renewal. Here we demonstrate that Erk signaling is critical for mouse ESC self-renewal and genomic stability. Erk-depleted ESCs cannot be maintained. Lack of Erk leads to rapid telomere shortening and genomic instability, in association with misregulated expression of pluripotency genes, reduced cell proliferation, G1 cell-cycle arrest, and increased apoptosis. Erk signaling is also required for the activation of differentiation genes but not for the repression of pluripotency genes during ESC differentiation. Furthermore, we find an Erk-independent function of Mek, which may explain the diverse effects of Mek inhibition and Erk knockout on ESC self-renewal. Together, in contrast to the prevailing view, Erk signaling is required for telomere maintenance, genomic stability, and self-renewal of mouse ESCs.
Embryonic stem cells (ESCs) are pluripotent and, hence, promising donor cell sources for regenerative medicine. Transcriptional regulation plays an essential role in pluripotency maintenance of ESCs, and a transcriptional regulation network for pluripotency has been characterized (1, 2). The core component of the pluripotency transcriptional regulation network is a feed-forward self-regulating circuitry formed by transcription factors Oct4, Sox2, and Nanog (3, 4). ESCs are cultured in media supplemented with growth factors. Through signaling pathways, growth factors affect the pluripotency transcriptional regulation network and regulate the self-renewal and differentiation of ESCs. For example, LIF and BMP signaling controls the transcriptional activities of the downstream effectors Stat3 and Smad1 to promote the self-renewal of mouse ESCs (5–7).
The extracellular signal-regulated kinase (Erk)/mitogen-activated protein kinase (MAPK) signal-transduction cascade mediates the effect of growth factors by sequential activation of Ras-like GTPase, Raf kinase, serine/threonine protein kinase Mek, and Erk to regulate cell-cycle progression, proliferation, differentiation, and carcinogenesis (8, 9). Erk signaling also plays a pivotal role in pluripotency maintenance. Inhibition of Mek/Erk signaling constrains the differentiation of mouse ESCs (10). Mouse ESCs can be derived and maintained in medium supplemented with inhibitors of Mek and Gsk3 signaling (2i) (11). Moreover, inhibition of Mek facilitates the conversion of mouse epiblast stem cells (epiSCs) to ESC-like cells (12). Similarly, the Mek inhibitor PD0325901 is used in establishing and maintaining human ground-state pluripotent stem cells (13–16). Conversely, activation of Mek/Erk signaling promotes the differentiation of ESCs. Ectopic expression of an activated H-RAS mutant leads to mouse ESC differentiation toward the trophectodermal lineage. Mek/Erk signaling is the downstream effector of Ras mediating the cell-fate change (17). Attenuating Erk signaling by knocking out the upstream activator Fgf4 or by Mek inhibition impairs the neural differentiation of ESCs (18, 19). Erk signaling may control ESC self-renewal by phosphorylating pluripotency factors. For example, Erk1 and Erk2 phosphorylate Klf4 at Ser123, leading to recruitment of ubiquitin E3 ligase mediated by βTrCP1 and βTrCP2 and to Klf4 ubiquitination and degradation (20). Nanog is also demonstrated to be a substrate of Erk kinases (21, 22). Nanog phosphorylation by Erk reduces Nanog transactivation activity and protein stability (22). Recently, it has been demonstrated that in mouse ESCs, Erk2, in cooperation with PRC2, binds to developmental genes and phosphorylates RNA polymerase II at Ser5 to maintain a poised transcription status (23).
The above data are consistent with the prevailing view that Erk signaling is dispensable for ESC self-renewal but required for the differentiation of ESCs. However, FGF2, an upstream activator of ERK signaling, is a necessary growth factor for conventional human ESCs (24). Knockdown of ERK2 indeed compromises the pluripotency of conventional human ESCs. ERK2 binds to and activates genes involved in pluripotency as well as metabolism, the cell cycle, and translation in conventional human ESCs (25). The conflicting roles of Erk signaling in human and mouse ESCs might be due to species divergence. Human ESCs resemble mouse epiSCs in a primed pluripotency state, whereas mouse ESCs are maintained in a naïve pluripotency state (26, 27). Nevertheless, due to the redundant role of Erk1 and Erk2, many experiments studying Erk signaling rely on Mek inhibitors. Even though these Mek inhibitors are claimed to be of high specificity, we cannot rule out the nonspecific effect of Mek inhibitors. Moreover, Erk1 and Erk2 have long been conceived as the only physiological substrates of Mek (8, 9). However, it remains possible that Mek has other unidentified downstream targets. Indeed, HSF1 has been identified as a novel MEK substrate (28). Therefore, to clarify the function of Erk signaling in ESCs, simultaneous knockout (KO) of Erk1 and Erk2 genes is required. Erk1 and Erk2 have been knocked out individually in mice or ESCs (29–32). However, no Erk1 and Erk2 double KO ESCs have been generated.
In this study, we find that constitutively active Mek1 (caMek1), but not constitutively active Erk1 or Erk2 (caErk1 or caErk2), suppresses the expression of Nanog in ESCs, implying that Mek1 might repress Nanog expression through downstream factors other than Erk1/2. To clarify the function of Erk1/2 in ESCs, endogenous Erk1 and Erk2 genes were disrupted in ESCs harboring an exogenous doxycycline (Dox)-inducible Erk1 gene, resulting in iErk1; Erk KO ESCs. iErk1; Erk KO ESCs grows like wild-type (WT) ESCs when Dox is added to the medium. However, upon Dox withdrawal, Erk1 expression diminishes, leading to rapid telomere shortening, genomic instability, and compromised self-renewal of ESCs such as reduced proliferation rate, altered cell-cycle structure, and apoptosis. Consistent with previous data, ESC differentiation is blocked in the absence of Erk signaling. Moreover, with the inducible Erk KO ESCs, we demonstrate an Erk-independent function of Mek. Altogether, our data show that Erk signaling is indispensable for genomic stability that is critical for self-renewal and differentiation of ESCs, and also suggest that Mek regulates self-renewal and differentiation of mouse ESCs by both Erk-dependent and Erk-independent pathways.
Results
caMek1, but Not caErk1/2, Suppresses the Expression of Nanog.
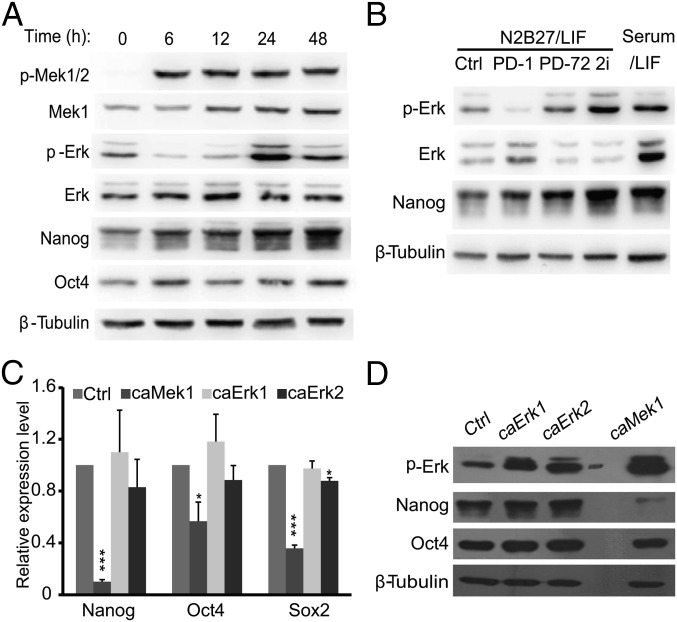
The Mek inhibitor PD0325901 (PD) has been routinely used to inhibit Mek/Erk signaling in 2i medium for mouse ESCs, and Mek inhibition enhances the expression of Nanog in mouse ESCs cultured in a serum/LIF condition (11, 33). We analyzed Erk phosphorylation (p-Erk) at Thr202 and Tyr204 upon Mek inhibition by PD (1 μM), and surprisingly found that the level of p-Erk is only reduced at early time points (6 and 12 h). Nevertheless, p-Erk recovers at late time points (24 and 48 h) to a level similar to or even slightly higher than that of the starting time point (0 h). Interestingly, Nanog expression increases at 24 and 48 h after PD treatment, regardless of the increased level of p-Erk (Fig. 1A). N2B27 medium without serum is used for mouse ESC 2i culture (11). To rule out the possibility of medium effects on Mek inhibition, ESCs cultured in N2B27 medium supplemented with LIF also were treated with PD. The dynamic change of p-Erk is similar to that in a serum/LIF condition. Moreover, both p-Erk and Nanog are higher in ESCs cultured in 2i medium than in ESCs cultured in serum/LIF (Fig. 1B). These data demonstrate that Mek inhibition increases the expression of Nanog and is associated with an elevated p-Erk level, arguing against Mek suppressing Nanog expression through activating Erk signaling.
Fig. 1.
Nanog expression is not negatively correlated with Erk phosphorylation. (A) Short-term PD treatment, but not prolonged PD treatment, reduces the level of p-Erk. V6.5 ESCs were cultured in a serum/LIF condition, and PD0325901 (1 μM) was added to the medium at the indicated time points before harvesting. Cells were harvested and subjected to Western blot analysis. (B) Expression of p-Erk of ESCs in N2B27 medium. V6.5 ESCs were switched from the serum/LIF condition to N2B27/LIF medium for 72 h (Ctrl). PD0325901 was added to the medium at 1 h (PD-1) or 72 h (PD-72) before harvesting cells. V6.5 ESCs cultured in serum/LIF or adapted to the 2i condition (2i) for more than five passages were also included. (C and D) caMeK1, but not caErk1 or caErk2, suppresses Nanog. Plasmids overexpressing caMek1, caErk1, and caErk2 were transfected into V6.5 ESCs. Cells were harvested 48 h after transfection. The expression of pluripotency genes Nanog, Oct4, and Sox2 was determined by quantitative RT-PCR (C) and Western blot (D). *P < 0.05; ***P < 0.001. Error bars are standard deviations (SDs).
To clarify the effect of Mek and Erk signaling on Nanog expression, we overexpressed caMek1, caErk1, and caErk2 in mouse ESCs. Surprisingly, only caMek1, but not caErk1 or caErk2, suppresses the expression of pluripotency genes Nanog, Oct4, and Sox2, with the most eminent repression effect on the Nanog gene (Fig. 1C). At the protein level, Nanog expression is also reduced by caMek1 overexpression but not by caErk1 or caErk2 (Fig. 1D). These data imply that Mek1 might repress Nanog expression via an Erk-independent pathway.
Construction of Erk KO ESCs.
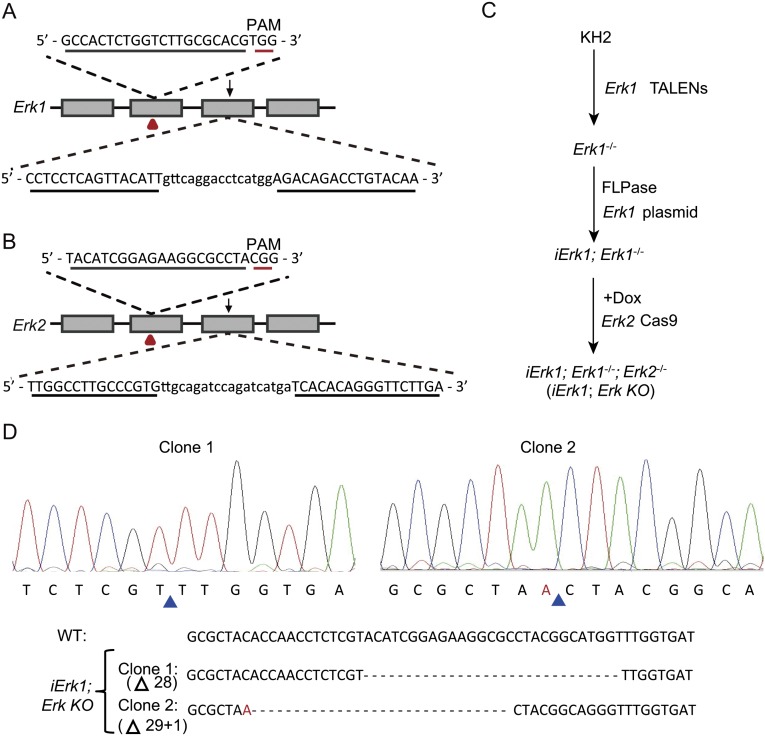
The above data seem to be inconsistent with the prevailing view of Erk signaling in mouse ESCs. It is important to note that previous studies of Erk signaling in mouse ESCs mainly relied on Mek inhibitors (11, 12, 19). However, the possibility of the nonspecific effect of a Mek inhibitor or an additional downstream effector(s) of Mek cannot be ruled out. To elucidate the roles of Erk signaling in ESCs, we applied a genetic approach to delete the Erk1 and Erk2 genes. Erk1−/− or Erk2−/− ESCs were established from WT ESCs (V6.5, KH2, iMek1, and iErk1) by transcription activator-like effector nucleases (TALENs) or clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas) technology (Fig. S1 A and B). The CRISPR/Cas system seems to be more efficient than the TALENs (Table S1). Due to the redundant role of Erk1 and Erk2 (34), it is necessary to generate Erk1 and Erk2 double KO ESCs to study the function of Erk signaling. Thus, we attempted to knock out Erk1 and Erk2 in Erk2−/− and Erk1−/− ESCs, respectively. However, no Erk1−/−; Erk2−/− ESC colonies can be identified, even though the cutting efficiency of TALENs and CRISPR/Cas remains unchanged in Erk2−/− and Erk1−/− ESCs. These data imply that Erk signaling is required for mouse ESCs.
Fig. S1.
Knockout of Erk1 and Erk2 by CRISPR/Cas and TALENs. (A) Schematic illustration of CRISPR/Cas and TALEN design for Erk1. (B) Schematic illustration of CRISPR/Cas and TALEN design for Erk2. CRISPR/Cas and TALEN targeting sites are labeled with red triangles and black arrows, respectively. Recognition sequences for CRISPR/Cas and TALENs are underlined. (C) Experimental outline for the construction of iErk1; Erk KO ESCs. (D) Sequencing results of two clones of iErk1; Erk KO ESCs demonstrate the disruption of the Erk2 alleles. Blue triangles mark the deletion sites. PAM, protospacer adjacent motif.
Table S1.
Knockout of Erk1 and Erk2 in mouse ESCs with TALENs and CRISPR/Cas
| Method | Target gene | Cell line | Screened colonies | Uncut, n (%) | One allele cut, n (%) | Two alleles cut, n (%) |
| CRISPR/Cas | Erk1 | V6.5 | 30 | 21 (70.0) | 3 (10.0) | 6 (20.0) |
| iMek1 | 48 | 23 (47.9) | 7 (14.6) | 18 (37.5) | ||
| Erk2−/− | 24 | 21 (87.5) | 3 (12.5) | 0 (0.0) | ||
| Erk2−/−; Erk1+/− | 39 | — | 39 (100.0) | 0 (0.0) | ||
| Erk2 | KH2 | 39 | 28 (71.8) | 4 (10.3) | 7 (17.9) | |
| iMek1 | 48 | 33 (68.8) | 2 (4.2) | 13 (27.0) | ||
| iErk1; Erk1−/− (+Dox) | 45 | 31 (68.9) | 3 (6.7) | 11 (24.4) | ||
| iErk1; Erk1−/− (−Dox) | 24 | 20 (83.3) | 4 (16.7) | 0 (0.0) | ||
| TALENs | Erk1 | iErk1 | 60 | 50 (83.3) | 8 (13.3) | 2 (3.3) |
| iErk2; Erk2−/− (+Dox) | 71 | 64 (90.2) | 3 (4.2) | 4 (5.6) | ||
| Erk2−/− | 48 | 45 (93.8) | 3 (6.2) | 0 (0.0) | ||
| Erk2−/−; Erk1+/− | 41 | — | 41 (100.0) | 0 (0.0) | ||
| Erk2 | V6.5 | 24 | 20 (83.3) | 0 (0.0) | 4 (16.7) |
n, number of ESC clones; %, percentage of ESC clones.
We then used another strategy to generate Erk KO ESCs (Fig. S1C). First, both alleles of Erk1 were disrupted in KH2 cells by TALENs (Erk1−/− ESCs) (35). An exogenous Erk1 gene driven by a tetracycline-inducible promoter was integrated into the engineered ColA1 locus through FLPe recombinase–mediated recombination, resulting in a Dox-inducible Erk1 transgene (iErk1; Erk1−/− ESCs). Then, iErk1; Erk1−/− cells were transfected with the Cas9 vector targeting Erk2 and cultured in the presence or absence of Dox. A week later, ESC colonies from single cells were picked, and the genotypes of Erk2 in these ESC clones were analyzed. Without Dox, no Erk2−/− ESCs were isolated from iErk1; Erk1−/− ESCs. In contrast, when Erk1 was induced by Dox, both Erk2 alleles were modified in 11 out of 45 picked colonies (Table S1). Sequencing results confirmed that both Erk2 alleles are disrupted in two ESC lines (iErk1; Erk KO ESCs) out of three sequenced clones (Fig. S1D). With the same strategy, iErk2; Erk KO ESCs were also established (Fig. S2A). We selected two iErk1; Erk KO ESC lines and two iErk2; Erk KO ESC lines for subsequent analysis. All data are consistent among these four cell lines. Only one set of data from one iErk1; Erk KO ESC line is shown in the main text, and one set of data from one iErk2; Erk KO ESC line is shown in Supporting Information (Figs. S2–S4).
Fig. S2.
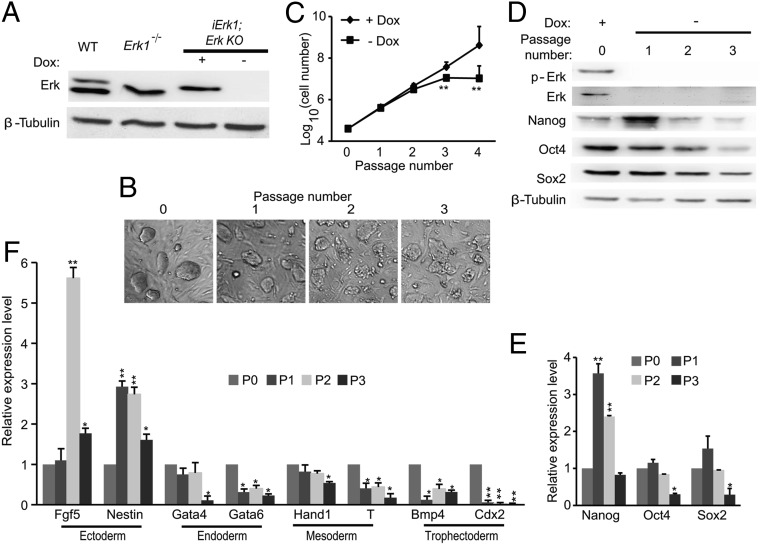
Erk signaling is essential for the self-renewal of ESCs, demonstrated by an independent cell line, iErk2; Erk KO. (A) Experimental outline for the construction of iErk2; Erk KO ESCs. (B) Western blots demonstrate the diminishment of Erk expression and the expression dynamics of pluripotency factors in iErk2; Erk KO ESCs after Dox withdrawal. (C) Colony morphology change upon Erk KO. iErk2; Erk KO ESCs were continuously cultured in the absence of Dox for three passages. Phase-contrast images of ESC colonies at each passage are shown. (D) Reduced proliferation of ESCs after Erk KO. iErk2; Erk KO ESCs were cultured in serum/LIF medium with or without Dox. The cell numbers were counted every passage, and equal amounts of ESCs were plated onto tissue-culture dishes. (E and F) Expression dynamics of pluripotency (E) and differentiation markers (F) after Erk KO. iErk2; Erk KO ESCs were cultured as described in C. *P < 0.05; **P < 0.01. Error bars are SDs.
Fig. S4.
Loss of Erk signaling leads to G1 cell-cycle arrest and apoptosis of ESCs, demonstrated by an independent cell line, iErk2; Erk KO ESCs. Cell-cycle analysis (A) and cell apoptosis analysis (B) of iErk2; Erk KO ESCs at different passages after Dox withdrawal. Experiments were repeated three times. Results from a representative experiment are shown.
Fig. S3.
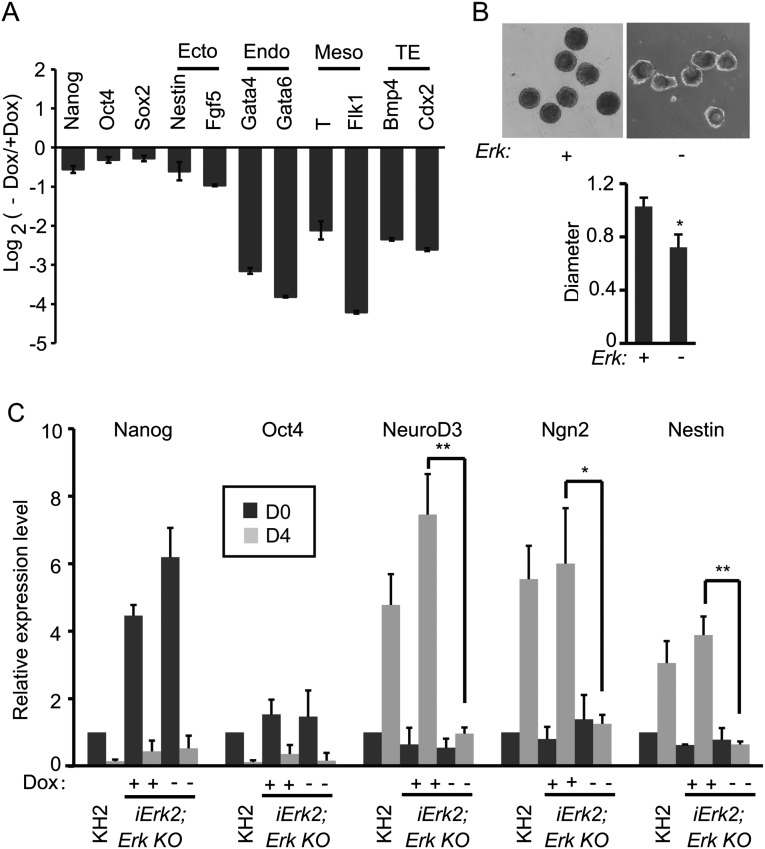
Erk signaling is required for ESC differentiation, demonstrated by an independent cell line, iErk2; Erk KO ESCs. (A and B) iErk2; Erk KO ESCs were cultured with or without Dox for 48 h, and then the cells were used for EB differentiation, again with or without Dox. (A) RNA was isolated from day 4 EBs and analyzed by quantitative RT-PCR. (B) Images of day 4 EBs with or without Dox. The relative diameters of day 4 EBs with or without Dox (n > 10) are plotted. (C) iErk2; Erk KO ESCs were cultured with or without Dox for 48 h (day 0). These cells, as well as KH2 ESCs, were cultured in N2B27 with or without Dox to induce neural differentiation for 4 d. The expression of pluripotency genes and neural genes in the resulting cells was analyzed by quantitative RT-PCR. *P < 0.05; **P < 0.01. Error bars are SDs.
Erk Depletion Compromises ESC Self-Renewal.
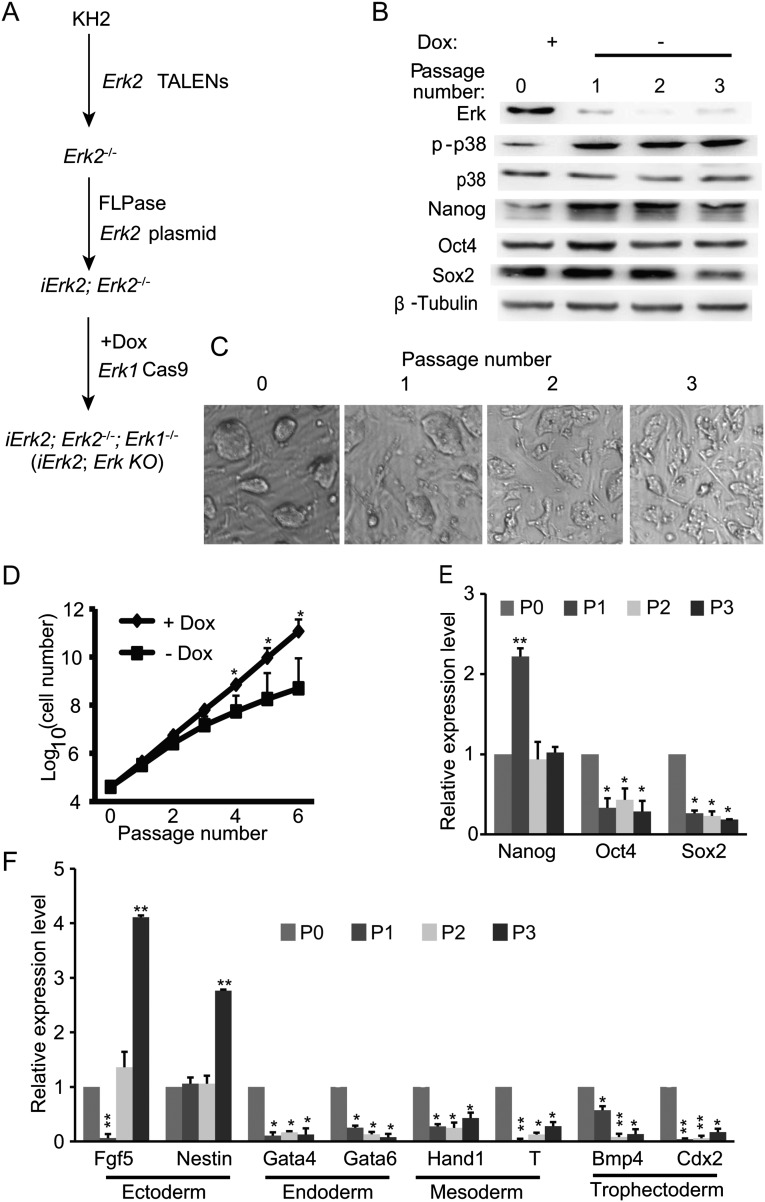
We demonstrated that Erk1 expression diminishes in iErk1; Erk KO ESCs 48 h after Dox withdrawal (Fig. 2A). Thus, we are able to induce Erk depletion by withdrawing Dox, and iErk1; Erk KO ESCs cultured in the absence of Dox for longer than 48 h are considered to be Erk KO ESCs. When iErk1; Erk KO ESCs were continuously cultured in serum/LIF ESC medium without Dox, the colonies became flattened and less compacted (Fig. 2B). After three or four passages in the absence of Dox, cell proliferation rate is significantly reduced and the cell culture crashes (Fig. 2C). The expression of pluripotency genes Nanog, Oct4, and Sox2 gradually decreases after Dox withdrawal, except for elevated expression of Nanog at passage 1 (Fig. 2 D and E). Erk KO leads to the suppression of endodermal, mesodermal, and trophectodermal markers, whereas ectodermal markers, Fgf5 and Nestin, are transiently activated after Erk KO (Fig. 2F). These data suggest that Erk signaling is indispensable for ESC self-renewal.
Fig. 2.
Erk signaling is essential for the self-renewal of ESCs. (A) Western blots demonstrate the diminishment of Erk expression in iErk1; Erk KO ESCs at 48 h after Dox withdrawal. (B) Colony morphology change upon Erk KO. iErk1; Erk KO ESCs were continuously cultured in the absence of Dox for three passages. Phase-contrast images of ESC colonies at each passage are shown. (C) Reduced proliferation of ESCs after Erk KO. iErk1; Erk KO ESCs were cultured in serum/LIF medium with or without Dox. Cell numbers were counted every passage, and equal amounts of ESCs were plated onto tissue-culture dishes. (D–F) Expression dynamics of pluripotency and differentiation markers after Erk KO. Cells were cultured as described in C. The protein (D) and mRNA (E) levels of pluripotency markers Nanog, Oct4, and Sox2 were measured by Western blot and quantitative RT-PCR. (F) Expression of differentiation genes after Erk KO. *P < 0.05; **P < 0.01. Error bars are SDs.
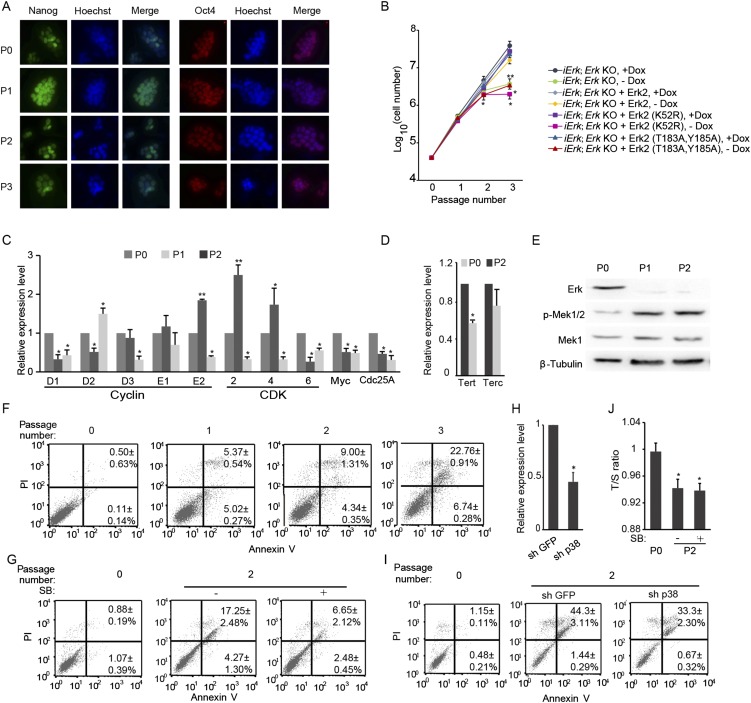
To address whether the kinase activity of Erk is required for ESC self-renewal, WT Erk2 and kinase-dead mutant (K52R) and phosphorylation-site mutant (T183A, Y185A) of Erk were introduced into iErk1; Erk KO ESCs. Only WT Erk2, but not the two Erk2 mutants, is able to rescue the slow proliferation rate induced by Erk depletion (Fig. S5B), suggesting that the kinase activity of Erk indeed is essential for ESC self-renewal.
Fig. S5.
Erk depletion compromises ESC self-renewal. iErk1; Erk KO ESCs were continuously cultured in the absence of Dox for three passages. (A) Immunofluorescence images of Nanog and Oct4. (B) Rescue slow proliferation rate in Erk KO ESCs by WT Erk2 but not by kinase-dead mutant (K52R) or phosphorylation-site mutant (T183A, Y185A). (C) Expression of cell-cycle regulators for G1-to-S transition after Dox withdrawal analyzed by quantitative RT-PCR. (D) The expression of Terc and Tert genes before and two passages after Dox withdrawal analyzed by quantitative RT-PCR. (E) The expression of Erk, Mek1, and p-Mek1 was analyzed by Western blot. (F) Cell apoptosis analysis of iErk1; Erk KO ESCs at different passages after Dox withdrawal. (G) Inhibition of p38 suppresses ESC apoptosis induced by Erk KO. iErk1; Erk KO ESCs were cultured in the absence of Dox for two passages, with or without the p38 inhibitor SB203580. Cell apoptosis analysis was performed with these cells. (H) Quantitative RT-PCR to measure the knockdown efficiency for p38. Two shRNA plasmids targeting p38 were pooled and transfected into iErk1; Erk KO ESCs. Forty-eight hours after transfection, cells were harvested and subjected to quantitative RT-PCR. (I) iErk1; Erk KO ESCs were cultured without Dox for 48 h, and then the control plasmid and p38 shRNA plasmids were transfected into these cells. Forty-eight hours after transfection, cell-cycle analysis was carried out for these cells. iErk1; Erk KO ESCs cultured with Dox were also included as a control. (J) iErk1; Erk KO ESCs were cultured in the absence of Dox for two passages, with or without the p38 inhibitor SB203580. Relative telomere length shown as T/S ratio in these cells was measured by quantitative PCR. *P < 0.05; **P < 0.01. Error bars are SDs.
Erk Signaling Is Essential for ESC Differentiation.
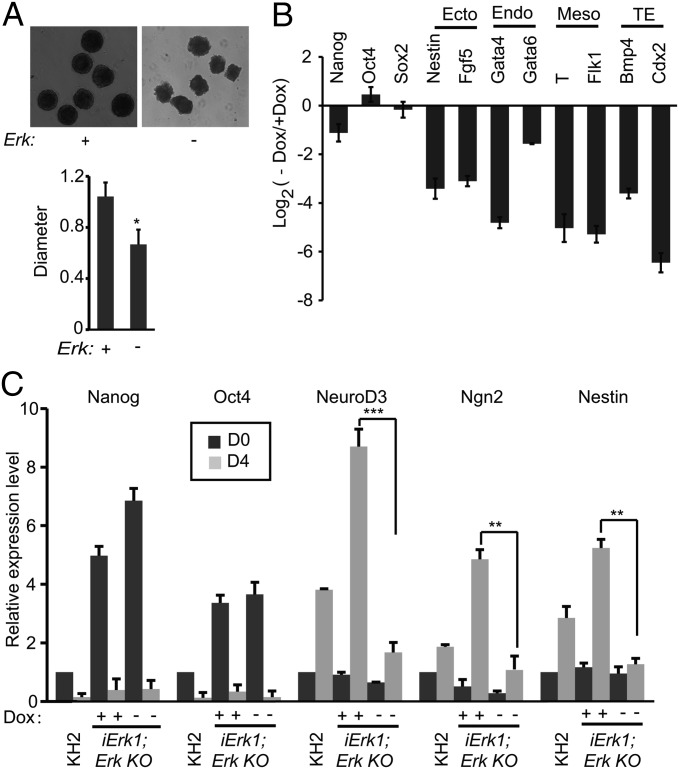
Next, we asked whether Erk signaling is essential for the differentiation of mouse ESCs. iErk1; Erk KO ESCs were cultured in ESC medium with or without Dox for 48 h and allowed to form embryoid bodies (EBs) with the hanging-drop method. EBs formed by Erk KO cells have irregular edges, rather than smooth and round edges, and are smaller than EBs formed by cells with Erk (Fig. 3A). Moreover, without Erk signaling, lineage-specific genes, including ectodermal, endodermal, mesodermal, and trophectodermal genes, are not activated in EBs, whereas the down-regulation of pluripotency markers Nanog, Oct4, and Sox2 is not affected by Erk KO (Fig. 3B). In a neural differentiation system (36), Erk KO ESCs are defective in activating neural markers NeuroD3, Ngn2, and Nestin. Again, pluripotency markers Nanog, Oct4, and Sox2 are repressed after differentiation, regardless of the absence or presence of Erk signaling (Fig. 3C). These data indicate that during ESC differentiation, Erk signaling is essential for the activation of differentiation genes but is not required for the suppression of pluripotency genes.
Fig. 3.
Erk signaling is required for ESC differentiation. (A and B) iErk1; Erk KO ESCs were cultured with or without Dox for 48 h. The cells were then used for EB differentiation, again with or without Dox. (A) Images of day 4 EBs with or without Dox. The relative diameters of day 4 EBs with or without Dox (n > 10) are plotted. (B) RNA was isolated from day 4 EBs and analyzed by quantitative RT-PCR. TE, trophectoderm. (C) iErk1; Erk KO ESCs were cultured with or without Dox for 48 h (D0, day 0). These cells, as well as KH2 ESCs, were cultured in N2B27 with or without Dox to induce neural differentiation for 4 d. The expression of pluripotency genes and neural genes in the resulting cells was analyzed by quantitative RT-PCR. *P < 0.05; **P < 0.01; ***P < 0.001. Error bars are SDs.
Lack of Erk Perturbs the Cell Cycle and Induces Cell Death.
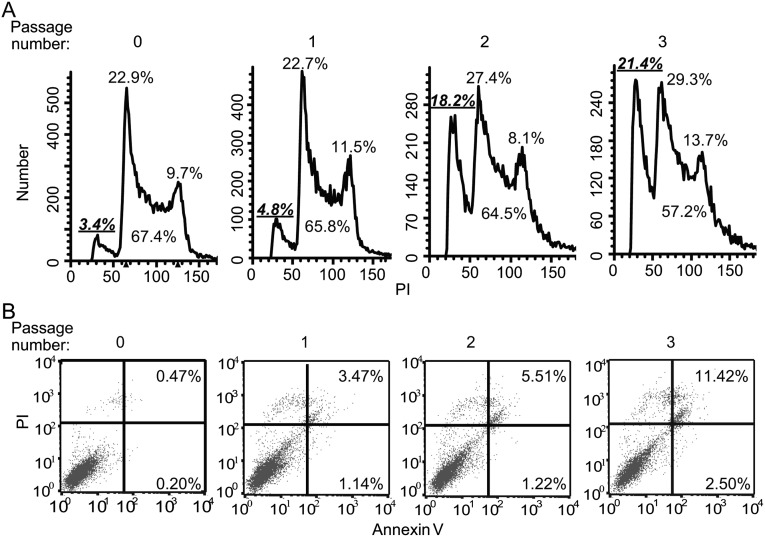
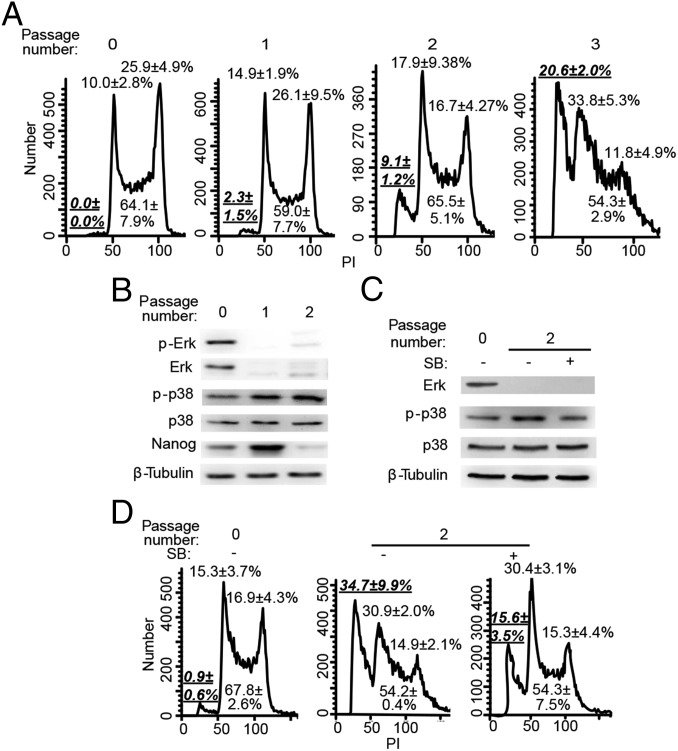
To understand the underlying mechanism for the reduced proliferation rate of Erk KO ESCs, we analyzed the cell-cycle profile of iErk1; Erk KO ESCs after Dox withdrawal by propidium iodide (PI) staining. It is notable that the sub-G1 population (apoptotic cells) and G1 cells increase over time after Dox withdrawal (Fig. 4A), indicating that Erk KO leads to cell arrest at the G1 phase, as well as apoptosis. Consistently, increased cell apoptosis after Erk KO was detected by Annexin V/PI staining (Fig. S5F).
Fig. 4.
Loss of Erk signaling leads to G1 cell-cycle arrest and apoptosis of ESCs. (A) Cell-cycle analysis of iErk1; Erk KO ESCs at different passages after Dox withdrawal. (B) p38 is activated upon Erk KO. The expression of p38 and p-p38 in iErk1; Erk KO ESCs at different passages after Dox withdrawal was determined by Western blot. (C and D) Inhibition of p38 suppresses ESC apoptosis induced by Erk KO. iErk1; Erk KO ESCs were cultured in the absence of Dox for two passages, with or without the p38 inhibitor SB203580. (C) Expression of p38 and p-p38 in Erk KO ESCs treated with or without SB203580 (SB). (D) Cell-cycle analysis was performed with these cells. (A and D) Percentages of sub-G1 cells in total cells (in bold and underlined) and percentages of G1, S, and G2/M cells in nonapoptotic cells are shown.
Three major MAPK pathways, Mek/Erk, JNK, and p38, cross-talk with each other (37). Inhibition of p38 and JNK supports naive pluripotency in human ESCs (13). Moreover, Erk and p38 signaling have been implicated in nitric oxide-induced apoptosis of mouse ESCs (38). We hypothesized that Erk KO might activate p38, consequently triggering apoptosis of ESCs. To test this hypothesis, we first examined the expression dynamics of p38 and phosphorylated p38 (p-p38) at Thr180 and Tyr182 after Erk KO. Indeed, the level of p-p38 increases upon Erk KO, whereas total p38 remains stable (Fig. 4B). We then treated iErk1; Erk KO ESCs with a p38 inhibitor, SB203580, upon Dox withdrawal. The number of apoptotic cells induced by Erk KO is significantly reduced by p38 inhibition, demonstrated by both PI staining and Annexin V/PI staining (Fig. 4 C and D and Fig. S5G). Knockdown of p38 by shRNA also suppresses Erk KO-induced apoptosis (Fig. S5 H and I). Therefore, Erk KO triggers ESC apoptosis through activation of p38. Nevertheless, inhibition of p38 does not affect G1 cell-cycle arrest caused by Erk KO (Fig. 4D).
Erk Depletion Leads to Rapid Telomere Shortening and Genomic Instability.
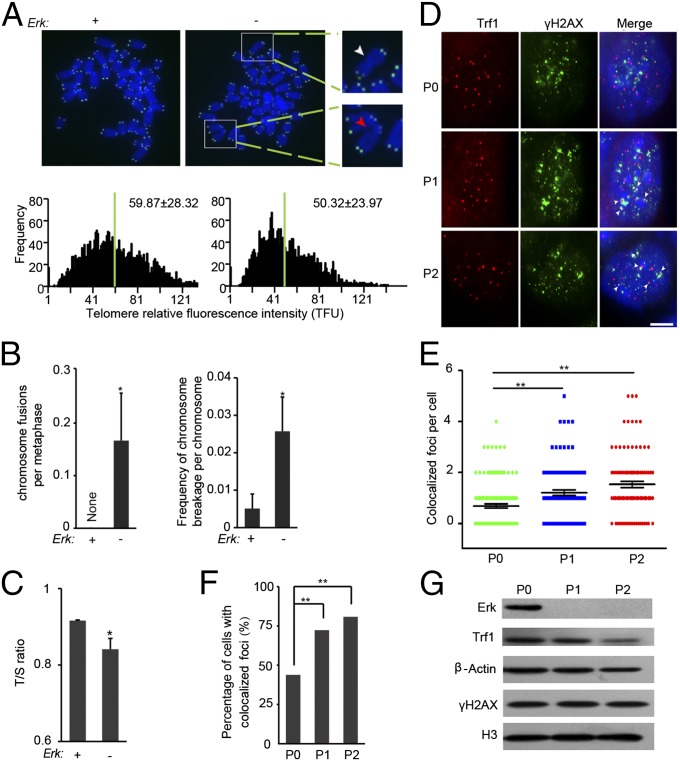
Genomic stability is essential for the self-renewal and potentiality of ESCs (39). Telomere damage and dysfunction can contribute to genomic instability and impair cell proliferation (40, 41). Impaired genomic stability resulting from lack of Erk might also reduce cell proliferation and contribute to increased apoptosis in ESCs. To test this hypothesis, we analyzed chromosome integrity and the length of telomeres in Erk KO ESCs by metaphase spread and telomere quantitative fluorescence in situ hybridization (Q-FISH). Telomere Q-FISH revealed that telomeres of ESCs shorten within only two passages after Erk depletion (Fig. 5A). Rapid telomere shortening in Erk KO ESCs was further validated by quantitative PCR (Fig. 5C). Meanwhile, the genomic stability of ESCs is compromised. Chromosome fusion and breakage events are more frequent in Erk KO ESCs in comparison with those of WT ESCs (Fig. 5 A and B). Telomeres are primarily elongated by telomerase (42). However, the expression levels of key telomerase genes, Tert and Terc, are only reduced by less than twofold in Erk KO ESCs (Fig. S5D). Even in the absence of telomerase activity, around 50 d of culture and 15 passages are required to detect comparable telomere shortening in Terc−/− ESCs and induced pluripotent stem cells (iPSCs), respectively (43, 44). Therefore, telomere shortening induced by Erk KO is unlikely caused by lack of telomerase activity. Instead, rapid telomere shortening might be due to DNA damage. Indeed, we noticed that DNA damage at telomeres, as indicated by γH2AX and Trf1 colocalized foci, gradually increases after Erk KO (Fig. 5 D–F). Reduced expression of Trf1 and an unchanged level of γH2AX (Fig. 5G) imply that Erk signaling is involved in safeguarding telomeric DNA and telomere structure but not overall genomic DNA.
Fig. 5.
Rapid telomere shortening occurs in Erk KO ESCs. iErk1; Erk KO ESCs with Dox or two passages after Dox withdrawal were subjected to telomere Q-FISH analysis (A) and telomere/single copy gene 36B4 (T/S) ratio analysis (C). (A, Upper) Telomere Q-FISH images of chromosome spreads. Blue, DAPI-stained chromosomes; green dots, telomeres. Blown-up images are examples of chromosome fusion (marked with a white arrowhead) and chromosome breakage (indicated by a red arrowhead). (A, Lower) Histograms showing the distribution of relative telomere length displayed as TFU by Q-FISH. Green bars mark the average telomere length. Mean ± SD of telomere length is shown in each panel. Heavy black bars on the y axis show the frequency of telomere signal-free ends. Fifteen chromosome spreads were quantified for each group. (B) Quantification of chromosome fusion and chromosome breakage frequencies from the Q-FISH data in A. Mean ± SE are plotted (n ≥ 15). (C) Relative telomere length shown as T/S ratio measured by quantitative PCR. (D–G) iErk1; Erk KO ESCs cultured with Dox, 48 and 96 h after Dox withdrawal, were subjected to immunofluorescence and Western blot analyses. (D) Immunofluorescence images of TRF1 (red) and γH2AX (green). Colocalized foci are indicated by arrowheads. (Scale bar, 5 μm.) Approximately 100 nuclei from images captured in D were quantified for colocalized TRF1 and γH2AX foci per cell (E) and percentage of cells with TRF1 and γH2AX colocalized foci (F). Mean ± SE are shown in E. (G) Protein levels of TRF1 and γH2AX as measured by Western blot. *P < 0.05; **P < 0.01.
We have shown that activation of p38 induces apoptosis of Erk KO ESCs. Telomere shortening and genome instability might trigger apoptosis. We then asked whether p38 activation is upstream of telomere shortening in order to cause apoptosis. p38 inhibition cannot prevent rapid telomere shortening in Erk KO ESCs (Fig. S5J), suggesting that the impaired genome stability and rapid telomere shortening are not caused by p38 activation.
Erk-Dependent and -Independent Effect of Mek Inhibition in ESCs.
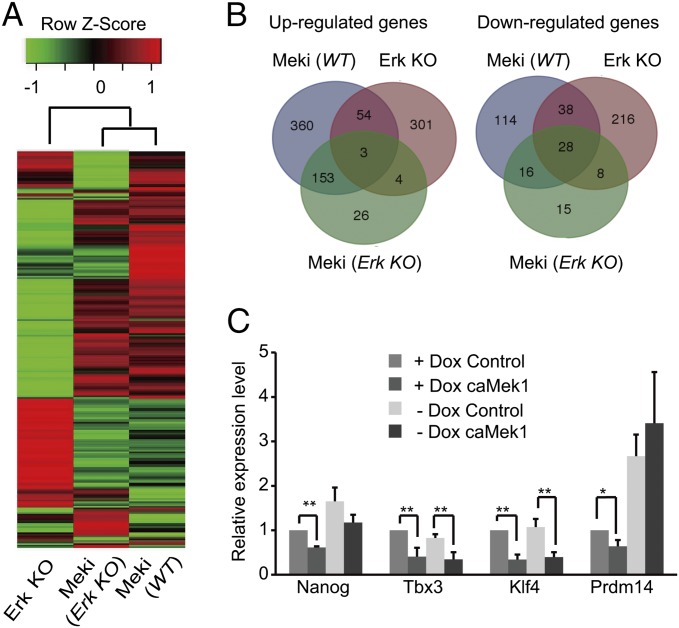
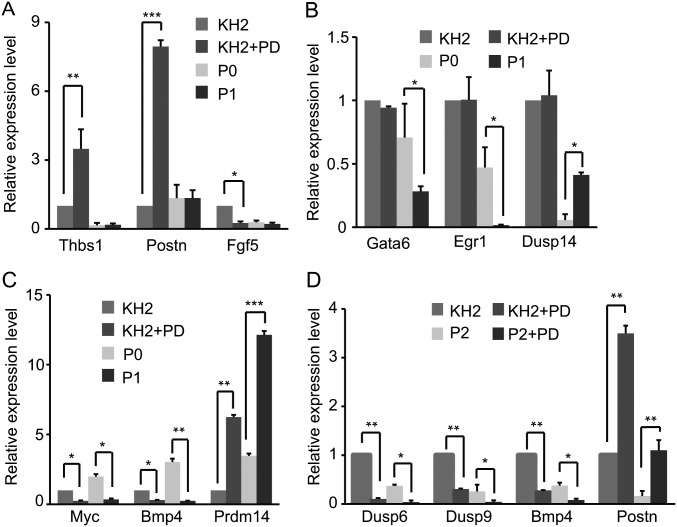
To gain insights into the role of Erk signaling in Mek inhibition of ESCs, six samples, WT KH2 ESCs treated with or without PD for 48 h (KH2+PD and KH2, respectively), iErk1; Erk KO ESCs cultured with Dox (P0) and 48 and 96 h after Dox withdrawal (P1 and P2, respectively), and iErk1; Erk KO ESCs cultured without Dox for 96 h and treated with PD in the last 48 h (P2+PD), were subjected to RNA-sequencing (RNA-seq) analysis. By comparing KH2+PD with KH2 [denoted Meki (WT)], 570 up-regulated genes and 196 down-regulated genes were identified; comparison between P1 and P0 (named Erk KO) identified 362 up-regulated genes and 290 down-regulated genes; and 186 up-regulated genes and 67 down-regulated genes were identified by comparing P2+PD with P2 [called Meki (Erk KO)] (Dataset S1). Consistent with the role of Erk signaling in ESC differentiation, the differentially expressed genes in the three comparisons are enriched for differentiation- and development-related genes (Dataset S2). Clustering analysis revealed that Meki (WT) is more closely related to Meki (Erk KO) than to Erk KO (Fig. 6A). Venn diagrams also show that only a small proportion of genes, 57 up-regulated genes and 66 down-regulated genes, are shared by Meki (WT) and Erk KO (Fig. 6B). The RNA-seq results were confirmed by quantitative RT-PCR for selected genes, including Meki-regulated genes (Thbs1, Postn, and Fgf5), Erk KO-regulated genes (Gata6, Egr1, and Dusp14), and both Meki- and Erk KO-regulated genes (Myc, Bmp4, and Prdm14) (Fig. S6 A–C). These data suggest that Mek inhibition and Erk KO have quite distinct effects on the transcriptional profile of ESCs.
Fig. 6.
Erk-dependent and -independent functions of Mek. (A and B) RNA-seq analysis of six samples: WT KH2 ESCs treated with or without PD for 48 h (KH2+PD and KH2, respectively), iErk1; Erk KO ESCs cultured in the presence of Dox (P0) and 48 and 96 h after Dox withdrawal (P1 and P2, respectively), and iErk1; Erk KO ESCs cultured without Dox for 96 h and treated with PD in the last 48 h (P2+PD). Meki (WT), Meki (Erk KO), and Erk KO are paired comparisons of KH2+PD and KH2, P2+PD and P2, and P1 and P0, respectively. Differentially expressed genes were identified in these three comparisons with the criteria of more than twofold change and false discovery rate (FDR) <0.001 (Dataset S1). (A) Clustering analysis of differentially regulated genes. (B) Venn diagrams of up- and down-regulated genes from three conditions. (C) caMek1 represses the expression of Klf4, Tbx3, and Nanog in the absence of Erk signaling. iErk1; Erk KO ESCs with Dox or 48 h after Dox withdrawal were transfected with empty vector or caMek1 overexpression vector. Forty-eight hours after transfection, cells were harvested for quantitative RT-PCR analysis. *P < 0.05; **P < 0.01. Error bars are SDs.
Fig. S6.
Validation of RNA-seq results for selected genes. (A) Genes affected in Meki (WT) but not in Erk KO. (B) Genes affected in Erk KO but not in Meki (WT). (C) Genes affected in both Meki (WT) and Erk KO. (D) Genes affected in both Meki (WT) and Meki (Erk KO). *P < 0.05; **P < 0.01; ***P < 0.001. Error bars are SDs.
When comparing Meki (WT) with Meki (Erk KO), fewer genes are affected by PD treatment in Erk KO ESCs than in WT ESCs, consistent with the current view that PD inhibits Mek and subsequently Erk signaling to regulate gene expression. Nevertheless, a significant fraction of genes, 156 up-regulated genes and 44 down-regulated genes, are regulated by PD treatment in the same way, regardless of the presence or absence of Erk signaling (Fig. 6B). The RNA-seq results of selected genes, including Dusp6, Dusp9, Bmp4, and Postn, were validated by quantitative RT-PCR (Fig. S6D). These data demonstrate that PD regulates a group of genes through an Erk-independent mechanism, whereas Erk signaling mediates the PD effect for the majority of genes.
The Erk-independent effect of PD might be due to the off-target effect of PD or the Erk-independent function of Mek. To demonstrate that Mek might have a biological function independent of Erk signaling, caMek1 was overexpressed in iErk1; Erk KO ESCs with or without Dox, and the expression of several known Mek downstream genes was analyzed by quantitative RT-PCR. caMek1 fails to suppress Prdm14 in Erk KO ESCs. Thus, Mek1 regulates Prm14 through Erk signaling. In contrast, Klf4 and Tbx3 are suppressed by caMek1 in both WT and Erk KO cells. caMek1 also down-regulates the expression of Nanog in Erk KO ESCs, even though the difference is not statistically significant (Fig. 6C). These data indicate that Mek may regulate gene expression independent of Erk signaling.
Discussion
To clarify the role of Erk signaling in mouse ESCs, we constructed inducible Erk KO ESCs and demonstrated that Erk signaling is indispensable for both self-renewal and differentiation of ESCs. Erk negatively regulates the expression of pluripotency genes, especially for Nanog, in mouse ESCs. At 48 h after Dox withdrawal, Nanog expression is increased in iErk1; Erk KO ESCs (Fig. 2 D and E), likely due to the suppression of the heterogeneous expression of Nanog in ESCs (Fig. S5A). Consistently, enhanced expression of pluripotency genes, including Nanog, Tbx3, Prdm14, and Klf4, has been observed in Erk2 KO ESCs and Erk2 KO/Erk1 knockdown ESCs (23, 30). In addition to transcriptional regulation, Erk may regulate the protein stability of pluripotency factors, such as Nanog and Klf4, by phosphorylation modification (20–22). The down-regulation of pluripotency genes after prolonged culture of Erk KO ESCs might be an indirect effect of loss of Erk signaling. For example, loss of Erk signaling activates Mek (Fig. S5E), which in turn may function through an Erk-independent mechanism to suppress pluripotency genes. However, Erk signaling is not required for suppressing pluripotency genes during ESC differentiation. In EB and neural differentiation experiments, pluripotency genes Oct4, Sox2, and Nanog are repressed in Erk KO cells (Fig. 3 B and C). In contrast, the activation of differentiation genes requires Erk signaling. Erk KO leads to down-regulation of differentiation genes in ESCs (Fig. 2F), and failure to activate differentiation genes, when ESCs differentiate into EBs or neural cells (Fig. 3 B and C). It is consistent with the role of Erk in phosphorylating RNA polymerase II and maintaining poised transcription status of differentiation genes (23). It has been reported that Erk2 is dispensable for multilineage commitment (30). In that study, only Erk2 is knocked out, and the redundant function of Erk1 ensures normal differentiation of ESCs.
The pluripotency status of ESCs is associated with rapid progression through the cell cycle and a short G1 phase (45). Even though Erk signaling promotes the G1-to-S transition by activating cyclin D1 expression in somatic cells (46), Erk phosphorylation has been shown to be dispensable for the progression from G1 to S phase in ESCs (47). However, we observed that loss of Erk signaling induces G1 cell-cycle arrest and cell death of ESCs (Fig. 4A). ESCs express low levels of D-type cyclins and undetectable cyclin D-Cdk4–associated kinase activity (48). Rather, increased cyclin E and extended cyclin E-Cdk2 activity lead to hyperphosphorylation of Rb and facilitate the G1-to-S phase transition (49, 50). When the expression of cell-cycle regulators for the G1-to-S transition was examined, we found that cyclins D1, D3, E2, Cdk2, Cdk4, Cdk6, Cdc25A, and c-Myc are down-regulated at passage 2 after Erk KO (Fig. S5C). Given the importance of cyclin E-Cdk2 activity in cell-cycle progression of ESCs, the reduced expression of Cdk2 and cyclin E2 might contribute to G1 cell-cycle arrest in Erk KO ESCs. In addition, down-regulation of c-Myc, which activates the transcription of cyclin E and Cdc25A (51), might be another reason for Erk KO-induced G1 arrest.
Erk KO triggers apoptosis of ESCs through the activation of p38. It has been shown that dual-specificity phosphatases (Dusps) mediate the cross-talk among three major MAPK cascades, Erk, JNK, and p38 (37). RNA-seq detected down-regulation of multiple Dusp genes, including Dusp4, Dusp5, Dusp6, and Dusp9, upon Erk KO, which might lead to the activation of p38 and apoptosis subsequently. In addition, genes regulating cell death, which are differentially expressed upon Erk KO but not by PD treatment, were also identified by RNA-seq. These genes, including up-regulated positive regulators for cell death Fgd4, Smpd2, and Tiam2 and down-regulated negative regulators for cell death Cntfr, Nrbp2, and Nrg1, might contribute to the apoptosis in Erk KO ESCs. Moreover, Erk KO-induced rapid shortening of telomeres might initiate apoptosis as well. A component of the shelterin complex, Trf1, deletion of which leads to apoptosis of iPSCs (52), is down-regulated upon Erk KO (Fig. 5G). This might be another reason for Erk KO-induced apoptosis.
Notably, telomere damage and rapid shortening occur in Erk KO ESCs. Within two passages, the average length of telomeres is reduced by about 9 kb (Fig. 5A). This is unlikely due to lack of telomerase activity. The rate of telomere loss has been measured as 50–100 bp per cell division in somatic cells, in which telomerase activity is low or absent (53). Around six or seven cell doublings occur during two passages of mouse ESC culture. If lacking telomerase is the only reason for telomere shortening, telomeres should become shorter by less than 1 kb. Moreover, much slower telomere shortening was observed in telomerase-deficient mouse ESCs and iPSCs than in Erk KO ESCs (43, 44). In fact, increased DNA damage was detected at telomeres in Erk KO ESCs, whereas the overall DNA damage signal remains stable (Fig. 5 D–G). Therefore, Erk signaling might be involved in maintaining telomere structure and preventing DNA damage. Erk KO is associated with down-regulation of Trf1 (Fig. 5G). It has been shown that deletion of Trf1 leads to telomere fusion and increased telomere fragility but not telomere shortening (54, 55). Thus, reduced expression of Trf1 might account for the increased chromosome fusion and breakage in Erk KO ESCs. However, rapid telomere shortening in Erk KO ESCs could be caused by other mechanisms. Targeted telomere insertion (TTI) might contribute to telomere shortening (56). Whether TTI is activated in Erk KO ESCs needs to be validated experimentally. Additional causes for genetic and epigenetic instability following the depletion of Erk cannot be excluded, and remain to be explored.
Our results do not support the prevailing view that Erk signaling is dispensable for ESC self-renewal. Almost all previous data are from experiments using pharmacological inhibitors of Mek. Thus, the discrepancy might be due to the difference between pharmacological inhibition of Mek and genetic KO of Erk. Obviously, the off-target effect of Mek inhibitors might account for the difference between Mek inhibition and Erk KO. Nevertheless, the off-target effect of Mek inhibitors is unlikely to be the reason, because multiple Mek inhibitors, including U0126, PD98059, PD184352, and PD0325901, have been shown to have similar effects in promoting pluripotency maintenance (10, 11, 57). Instead, Mek inhibition and Erk KO have distinct effects on Erk signaling. Upon Erk KO, Erk signaling is completely blocked. However, during Mek inhibition, Erk signaling is only attenuated, because the Mek-Erk cascade has properties of a negative-feedback amplifier, stabilizing outputs in response to drug-induced perturbations (58, 59). Furthermore, we found that Mek inhibition only reduces p-Erk levels at an early time point, and the level of p-Erk comes back to a level similar to or even higher than that before Mek inhibition (Fig. 1 A and B). Activation of Mek and down-regulation of Erk phosphatase might contribute to the elevated p-Erk level after prolonged Mek inhibition. The level of phosphorylated Mek1/2 at Ser217/221 is enhanced after Mek inhibition (Fig. 1A). Our RNA-seq and quantitative RT-RCR results demonstrate the down-regulation of several dual-specificity phosphatase genes, including Dusp6 and Dusp9, upon Mek inhibition (Fig. S6D). The latter has been shown to be a key downstream target gene of BMP4 signaling to modulate Erk activity in mouse ESCs (60). Moreover, Mek might function through another immediate downstream target(s), in addition to Erk1/2. Even though Erk1/2 have been conceived as the only substrates for Mek1/2 (8, 9), a recent study identified HSF1 as a novel MEK substrate (28). Our data also provide evidence that Mek might have a function(s) independent of Erk1/2. First, Mek inhibition regulates a large group of genes (643 genes), which are not affected by Erk KO (Fig. 6B). Second, with or without Erk signaling, a significant portion of genes (200 genes) responds to PD treatment in the same way (Fig. 6B and Fig. S6D). This group of genes is likely regulated by Mek through an Erk-independent mechanism. Third, in Erk KO cells, overexpression of caMek1 represses the expression of pluripotency genes Nanog, Klf4, and Tbx3, further confirming the Erk-independent function of Mek (Fig. 6C). More investigation is required to identify a novel Mek substrate(s) involved in pluripotency maintenance.
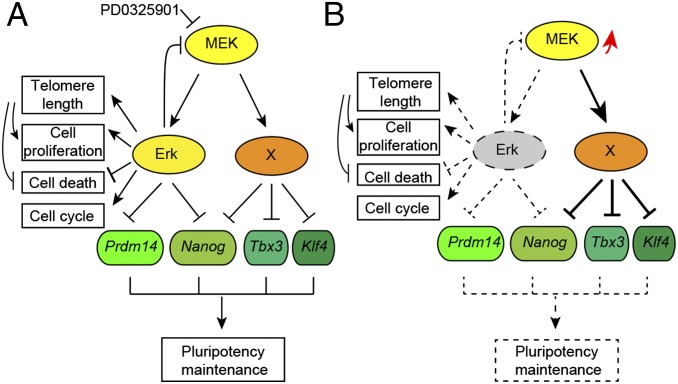
In summary, our work has demonstrated that Erk signaling is indispensable not only for ESC differentiation but also for the self-renewal of mouse ESCs. Erk signaling has dual roles in pluripotency regulation. On one hand, it represses the expression of pluripotency genes such as Prdm14 and Nanog. On the other hand, Erk is required for cell proliferation, cell-cycle progression, and telomere-length maintenance, as well as suppression of apoptosis in ESCs (Fig. 7A). Thus, Erk KO leads to reduced proliferation, G1 arrest, rapid telomere shortening, and apoptosis of ESCs. In addition, upon Erk KO, Mek is activated, and may subsequently exert its Erk-independent function to suppress the expression of pluripotency genes, including Tbx3 and Klf4, perturbing the pluripotency maintenance network (Fig. 7B). In contrast to the effect of Erk KO, Mek inhibition by PD0325901 facilitates the maintenance of pluripotency, because both Erk-dependent and -independent functions of Mek are suppressed. Moreover, during PD0325901 treatment, residual Erk activity remains to facilitate the self-renewal of ESCs. Our data suggest an Erk-independent function of Mek. Characterization of this Erk-independent function of Mek is of great significance not only for understanding the role of Mek in pluripotency maintenance but also for cancer therapy targeting MEK.
Fig. 7.
Working model for Mek/Erk signaling in mouse ESCs. (A) Mek might suppress the expression of pluripotency genes through Erk-dependent and -independent pathways. In addition to its role in repressing Prdm14 and Nanog, Erk signaling is required for cell proliferation, cell-cycle progression, and telomere-length maintenance as well as suppression of apoptosis. (B) Upon Erk KO, Mek and its Erk-independent pathway are activated (marked by a red arrow) to perturb the pluripotency regulation network.
Materials and Methods
Quantitative RT-PCR.
Total RNA was extracted from cells using an RNeasy Mini Kit (Qiagen). cDNA synthesis was performed using a Transcriptor First Strand cDNA Synthesis Kit (Roche) with random primers according to the manufacturer’s instructions. PCR was performed with FastStart Universal SYBR Green Master (Roche) in a Bio-Rad iQ5 System. PCR cycling conditions were 95 °C for 2 min and 40 cycles of 95 °C for 15 s, 58 °C for 15 s, and 72 °C for 30 s, and then a melting curve of the amplified DNA was acquired. Quantification of target genes was normalized with β-actin. Primer information is listed in Table S2.
Table S2.
Primers used for quantitative RT-PCR
| Gene | Forward, 5′-3′ | Reverse, 5′-3′ |
| Oct4 | ATCAGCTTGGGCTAGAGAAGGATG | AAAGGTGTCCCTGTAGCCTCATAC |
| Sox2 | GCGGAGTGGAAACTTTTGTCC | CGGGAAGCGTGTACTTATCCTT |
| Nanog | TACAAGGGTCTGCTACTGAGATGC | TTGGGACTGGTAGAAGAATCAGGG |
| Cdx2 | CAGTCCCTAGGAAGCCAAGTGAAA | AAGTGAAACTCCTTCTCCAGCTCC |
| Bmp4 | ACAGCGGTCCAGGAAGAAGAAT | TGCACAATGGCATGGTTGGT |
| Gata4 | GCTATGCATCTCCTGTCACTCAGA | CCAAGTCCGAGCAGGAATTTGAAG |
| Gata6 | CTTCTCCTTCTACACAAGCGACCA | ATACTTGAGGTCACTGTTCTCGGG |
| Nestin | CTGGATCTGGAAGTCAACAGAGGT | ATCCTCAGTTTCCACTCCTGTAGC |
| Hand1 | AAGGATGCACAAGCAGGTGAC | TTTAATCCTCTTCTCGCCGGG |
| T | CATCGGAACAGCTCTCCAACCTAT | TACCATTGCTCACAGACCAGAGAC |
| Fgf5 | GAAACTCGGATACAGCATCCCTCT | GGATCGCTACAGAGAATCCCACTT |
| Tbx3 | TTCACAACTCTCGGTGGATGGT | CGCTTGGGAAGGCCAAAGTAAA |
| Prdm14 | GCATCCTGGTTCCCACAGAG | CTGCAGAACACGCCAAAGTG |
| Egr1 | AGCGCCTTCAATCCTCAAG | TTTGGCTGGGATAACTCGTC |
| Klf4 | AGCCACCCACACTTGTGACTAT | AGTGGTAAGGTTTCTCGCCTGT |
| Myc | GCTGTTTGAAGGCTGGATTTC | GATGAAATAGGGCTGTACGGAG |
| Cyclin D1 | GCCCTCCGTATCTTACTTCAAG | GCGGTCCAGGTAGTTCATG |
| Cyclin D2 | GTGTTCCTATTTCAAGTGCGTG | AGCCAAGAAACGGTCCAG |
| Cyclin D3 | GCGTGCAAAAGGAGATCAAG | GATCCAGGTAGTTCATAGCCAG |
| Cyclin E1 | GCGAGGATGAGAGCAGTTC | AAGTCCTGTGCCAAGTAGAAC |
| Cyclin E2 | CTCCGCAAGAAACCCAGATAA | GCTTCTTGGTGATCTCCTCTTT |
| CDK2 | AAGATGGACGGAGCTTGTTATC | GGCACTGGTTTAGTTACATCCT |
| CDK4 | ACAAGTAATGGGACCGTCAAG | GGGTGTTGCGTATGTAGACTG |
| CDK6 | GTTCAGACGTGGATCAACTAGG | TGTCACAAACTTCTCGATGGG |
| Cdc25A | AAGAACCCTATTGTGCCTACTG | GTACTCATTGCCGAGCCTATC |
| NeuroD3 | CCTTCTTTGTGACTGGCTCA | CCCTTTTCCAAACCACACTG |
| Ngn2 | CATGCACAACCTAAACGCC | CAGATGTAATTGTGGGCGAAG |
| Dusp6 | ACTGGAATGAGAACACTGGTG | GTCTAGATTGGTCTCGCAGTG |
| Dusp9 | CCCAACCTTCCTAACCTCTTTG | GAGCACCCCACAGTTCTG |
| Dusp14 | CTCTGGCTGACATTCCTCATG | GTACGCAATACAGAGGGTGG |
| Postn | CAAGGCAGTCTTCAGCCTATTA | CACCGTTTCGCCTTCTTTAATC |
| Thbs1 | CTATGACAAGGACGGGATTGG | ATACTGGGCTGGGTTGTAATG |
| p38 | GTGATTGGTCTGTTGGATGTG | TGAGAAACTGAACGTGGTCG |
| Tect | ACTGGTGGAGATCATCTTTCTGGG | ACCTGAGGAGTCTGACATATTGGC |
| Terc | CATTAGCTGTGGGTTCTGGTCT | TCCTGCGCTGACGTTTGTTT |
| mTel | CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT | GCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT |
| 36B4 | ACTGGTCTAGGACCCGAGAAG | AATGGTGCCTCTGGAGATT |
| β-Actin | CAGAAGGAGATTACTGCTCTGGCT | CAGAAGGAGATTACTGCTCTGGCT |
Telomere Q-FISH.
Telomere length and function (telomere integrity and chromosome stability) were measured by telomere Q-FISH, as described previously (61). Telomeres were denatured at 80 °C and hybridized with telomere PNA probe (0.5 μg/mL) (Panagene). Chromosomes were stained with 0.5 μg/mL DAPI. Fluorescence from chromosomes and telomeres was digitally imaged on a Zeiss microscope with FITC/DAPI filters, using AxioCam and AxioVision software 4.6. Telomere length shown as telomere fluorescence unit (TFU) was integrated using the TFL-TELO program (a gift kindly provided by P. Lansdorp, Terry Fox Laboratory, Vancouver, Canada).
RNA Sequencing.
Construction of an RNA-sequencing library, sequencing with Illumina HiSeq 2000, and bioinformatic analysis were performed by BGI Tech. The RNA-seq dataset (accession no. GSE70304) has been deposited in the Gene Expression Omnibus database.
Statistical Analysis.
Data were analyzed by two-tailed Student’s t test, except for Fig. 5F, in which χ2 test was performed to calculate the P value. Statistically significant P values are indicated in the figures as follows: *P < 0.05; **P < 0.01; ***P < 0.001. Averages and SDs of at least three independent experiments are shown in the figures when applicable.
Detailed information is provided in SI Materials and Methods.
SI Materials and Methods
Plasmid Construction.
caMek1 (S218D, S222D), caErk1 (R84S), and caErk2 (R65S, D319N) genes (62, 63) were cloned into the pCAGIPuro expression plasmid (a gift from Hitoshi Niwa, RIKEN Center for Developmental Biology, Kobe, Japan). For CRISRP/Cas plasmids, a pair of oligos (5′-CACCGCCACTCTGGTCTTGCGCACG-3′, 5′-AAACCGTGCGCAAGACCAGAGTGGC-3′) for Erk2 and a pair of oligos (5′-CACCGTACATCGGAGAAGGCGCCTA-3′, 5′-AAACTAGGCGCCTTCTCCGATGTAC-3′) for Erk1 were annealed and ligated to the linearized pX330 vector. TALENs targeting Erk1 (left: 5′-CCTCCTCAGTTACATT-3′; right: 5′-TGTACAGGTCTGTC-3′) and Erk2 (left: 5′-TTGGCCTTGCCCGTGT-3′; right: 5′-CAAGAACCCTGTGTGA-3′) were designed using online software (tale-nt.cac.cornell.edu). The construction of DNA fragments encoding TAL effector repeat arrays and the insertion of the TEL effector DNA into pCS2 FokI expression vectors were performed as described elsewhere (64). p38 gene knockdown was performed with the pSuper.puro System (OligoEngine) following the manufacturer’s instructions. The targeting sequences for p38 were 5′-AGCCCAGCAACCTAGCTGT-3′ and 5′-TCAGATAATACCACTGGTTAA-3′.
Cell Culture and Cell Lines.
Unless specified, mouse ESCs were cultured in serum/LIF medium consisting of 85% (vol/vol) DMEM (Invitrogen), 15% (vol/vol) FBS (HyClone), 2 mM l-glutamine, 5,000 U/mL penicillin and streptomycin, 0.1 mM nonessential amino acids (Invitrogen), 0.1 mM 2-mercaptoethanol (Sigma), and 1,000 U/mL LIF (Chemicon). 2i medium for mouse ESCs: 50% (vol/vol) DMEM/F12, 50% (vol/vol) Neurobasal, 1 × N2, 1 × B27 (Invitrogen), 2 mM l-glutamine, 5,000 U/mL penicillin and streptomycin, 0.1 mM nonessential amino acids, 0.1 mM 2-mercaptoethanol, 1,000 U/mL LIF, 25 μg/mL BSA, 1 μM PD0325901 (Sigma), and 3 μM CHIR99021 (Stemgent). iErk1; Erk KO ESCs were cultured in serum/LIF medium with 2 μg/mL Dox. To form EBs, ESCs were cultured in 30-μL hanging drops (100 cells per drop, ESC medium without LIF), and EBs were collected on day 4. To induce neural differentiation, undifferentiated ESCs were plated on a gelatin-coated culture dish in N2B27 medium as described elsewhere (36). Inducible Erk1 and Erk2 ESCs were constructed from the parent cell line, KH2, as described previously (35). To knock out Erk1 or Erk2 by CRISPR/Cas or TALENs, plasmids expressing Cas9/single-guide RNA (sgRNA) or TALENs were transfected into ESCs using Lipofectamine 3000 (Invitrogen). Two days after transfection, ESCs were plated down at low density to allow the formation of colonies from single cells. Individual colonies were 5–7 d later picked up and subjected to further culture and analysis.
Western Blotting.
Cells were lysed in lysis buffer (Beyotime), and protein concentration was measured using a BCA protein assay kit (Beyotime) to ensure an equal amount of loading. The samples were resolved by SDS/PAGE, followed by transferring onto a PVDF membrane (Millipore). Membranes were probed with anti-Sox2 (GeneTex), –β-tubulin (Huada), -Oct4, -Erk1/2 (Santa Cruz Biotechnology), -Nanog (Bethyl Laboratories), –p-Erk, -Mek1, –p-Mek, -p38, –p-p38 (Cell Signaling Technology), -γH2AX (Millipore), and -Trf1 (Alpha Diagnostic) antibodies. Bound primary antibodies were recognized by HRP-linked secondary antibodies (GE Healthcare). Immunoreactivity was detected by ECL Plus (Beyotime). Digital images were taken with an automatic chemiluminescence imaging analysis system (Tanon).
Immunofluorescence.
Cells were fixed in 4% (wt/vol) paraformaldehyde for 20 min and then permeabilized with 0.2% Triton X-100 for 30 min. After blocking with 5% (vol/vol) goat serum for 2 h, cells were incubated with anti-Nanog (Bethyl Laboratories), -Oct4 (Santa Cruz Biotechnology), -Trf1 (Alpha Diagnostic), and -γH2AX (Millipore) antibodies overnight at 4 °C. Then, cells were washed and incubated with Alexa Fluor 488 anti-mouse and Alexa Fluor 594 anti-rabbit secondary antibodies (Molecular Probes). Hoechst 33342 (Sigma) was used for nucleus staining. Images were captured with a fluorescence microscope.
Cell-Cycle Analysis.
Single-cell suspensions were prepared by trypsinization and washed once in PBS. Cells were fixed in ice-cold 70% (vol/vol) ethanol and stored at 4 °C overnight. Following RNaseA treatment, total DNA was stained with propidium iodide (Beyotime). Cells were analyzed with a FACSCalibur flow cytometer (BD Biosciences) and the data were processed using FlowJo.
Cell Apoptosis Analysis.
Cells were collected by trypsinization and washed with PBS. To detect cell death, an Annexin V apoptosis detection kit (Beyotime) was used. Briefly, 105 cells were mixed and incubated for 15 min at room temperature in the dark with Annexin V FITC and propidium iodide. Stained cells were analyzed with a FACSCalibur flow cytometer (BD Biosciences).
T/S Ratio.
For telomere-length measurement, DNA samples were extracted from ESCs using a DNeasy Blood & Tissue Kit (Qiagen). Quantitative real-time PCR assay was used to measure the average telomere length as previously described (61).
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grants 31271547, 31470081, and 31271587), Natural Science Foundation of Tianjin, China (14JCYBJC23600), Program for New Century Excellent Talents (NCET-13-0293), Program for Changjiang Scholars and Innovative Research Team in University (IRT13023), China Ministry of Science and Technology National Key Basic Research Program (2012CB911202), and 111 Project (Grant B08011).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The RNA-seq dataset reported in this paper has been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE70304).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1516319112/-/DCSupplemental.
References
- 1.Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132(4):567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young RA. Control of the embryonic stem cell state. Cell. 2011;144(6):940–954. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyer LA, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122(6):947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loh YH, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38(4):431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 5.Raz R, Lee CK, Cannizzaro LA, d’Eustachio P, Levy DE. Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci USA. 1999;96(6):2846–2851. doi: 10.1073/pnas.96.6.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12(13):2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115(3):281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 8.Shaul YD, Seger R. The MEK/ERK cascade: From signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773(8):1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Pearson G, et al. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr Rev. 2001;22(2):153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 10.Burdon T, Stracey C, Chambers I, Nichols J, Smith A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev Biol. 1999;210(1):30–43. doi: 10.1006/dbio.1999.9265. [DOI] [PubMed] [Google Scholar]
- 11.Ying QL, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453(7194):519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou H, et al. Conversion of mouse epiblast stem cells to an earlier pluripotency state by small molecules. J Biol Chem. 2010;285(39):29676–29680. doi: 10.1074/jbc.C110.150599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gafni O, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504(7479):282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- 14.Chan YS, et al. Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell. 2013;13(6):663–675. doi: 10.1016/j.stem.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Takashima Y, et al. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell. 2014;158(6):1254–1269. doi: 10.1016/j.cell.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theunissen TW, et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell. 2014;15(4):471–487. doi: 10.1016/j.stem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu CW, et al. Ras-MAPK signaling promotes trophectoderm formation from embryonic stem cells and mouse embryos. Nat Genet. 2008;40(7):921–926. doi: 10.1038/ng.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunath T, et al. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134(16):2895–2902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- 19.Stavridis MP, Lunn JS, Collins BJ, Storey KG. A discrete period of FGF-induced Erk1/2 signalling is required for vertebrate neural specification. Development. 2007;134(16):2889–2894. doi: 10.1242/dev.02858. [DOI] [PubMed] [Google Scholar]
- 20.Kim MO, et al. ERK1 and ERK2 regulate embryonic stem cell self-renewal through phosphorylation of Klf4. Nat Struct Mol Biol. 2012;19(3):283–290. doi: 10.1038/nsmb.2217. [DOI] [PubMed] [Google Scholar]
- 21.Brumbaugh J, et al. NANOG is multiply phosphorylated and directly modified by ERK2 and CDK1 in vitro. Stem Cell Rep. 2014;2(1):18–25. doi: 10.1016/j.stemcr.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SH, et al. ERK1 phosphorylates Nanog to regulate protein stability and stem cell self-renewal. Stem Cell Res (Amst) 2014;13(1):1–11. doi: 10.1016/j.scr.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Tee WW, Shen SS, Oksuz O, Narendra V, Reinberg D. Erk1/2 activity promotes chromatin features and RNAPII phosphorylation at developmental promoters in mouse ESCs. Cell. 2014;156(4):678–690. doi: 10.1016/j.cell.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 25.Göke J, Chan YS, Yan J, Vingron M, Ng HH. Genome-wide kinase-chromatin interactions reveal the regulatory network of ERK signaling in human embryonic stem cells. Mol Cell. 2013;50(6):844–855. doi: 10.1016/j.molcel.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 26.Brons IG, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448(7150):191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 27.Tesar PJ, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448(7150):196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 28.Tang Z, et al. MEK guards proteome stability and inhibits tumor-suppressive amyloidogenesis via HSF1. Cell. 2015;160(4):729–744. doi: 10.1016/j.cell.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saba-El-Leil MK, et al. An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO Rep. 2003;4(10):964–968. doi: 10.1038/sj.embor.embor939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamilton WB, Kaji K, Kunath T. ERK2 suppresses self-renewal capacity of embryonic stem cells, but is not required for multi-lineage commitment. PLoS One. 2013;8(4):e60907. doi: 10.1371/journal.pone.0060907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao Y, et al. Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proc Natl Acad Sci USA. 2003;100(22):12759–12764. doi: 10.1073/pnas.2134254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pagès G, et al. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999;286(5443):1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 33.Silva J, et al. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138(4):722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frémin C, Saba-El-Leil MK, Lévesque K, Ang SL, Meloche S. Functional redundancy of ERK1 and ERK2 MAP kinases during development. Cell Reports. 2015;12(6):913–921. doi: 10.1016/j.celrep.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Beard C, Hochedlinger K, Plath K, Wutz A, Jaenisch R. Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells. Genesis. 2006;44(1):23–28. doi: 10.1002/gene.20180. [DOI] [PubMed] [Google Scholar]
- 36.Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21(2):183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 37.Fey D, Croucher DR, Kolch W, Kholodenko BN. Crosstalk and signaling switches in mitogen-activated protein kinase cascades. Front Physiol. 2012;3:355. doi: 10.3389/fphys.2012.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JH, et al. p38 MAP kinase and ERK play an important role in nitric oxide-induced apoptosis of the mouse embryonic stem cells. Toxicol In Vitro. 2013;27(1):492–498. doi: 10.1016/j.tiv.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Weissbein U, Benvenisty N, Ben-David U. Quality control: Genome maintenance in pluripotent stem cells. J Cell Biol. 2014;204(2):153–163. doi: 10.1083/jcb.201310135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldser DM, Hackett JA, Greider CW. Telomere dysfunction and the initiation of genome instability. Nat Rev Cancer. 2003;3(8):623–627. doi: 10.1038/nrc1142. [DOI] [PubMed] [Google Scholar]
- 41.McEachern MJ, Krauskopf A, Blackburn EH. Telomeres and their control. Annu Rev Genet. 2000;34:331–358. doi: 10.1146/annurev.genet.34.1.331. [DOI] [PubMed] [Google Scholar]
- 42.Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51(6):887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 43.Wang F, et al. Molecular insights into the heterogeneity of telomere reprogramming in induced pluripotent stem cells. Cell Res. 2012;22(4):757–768. doi: 10.1038/cr.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niida H, et al. Severe growth defect in mouse cells lacking the telomerase RNA component. Nat Genet. 1998;19(2):203–206. doi: 10.1038/580. [DOI] [PubMed] [Google Scholar]
- 45.Savatier P, Lapillonne H, Jirmanova L, Vitelli L, Samarut J. Analysis of the cell cycle in mouse embryonic stem cells. Methods Mol Biol. 2002;185:27–33. doi: 10.1385/1-59259-241-4:27. [DOI] [PubMed] [Google Scholar]
- 46.Lavoie JN, L’Allemain G, Brunet A, Müller R, Pouysségur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271(34):20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 47.Jirmanova L, Afanassieff M, Gobert-Gosse S, Markossian S, Savatier P. Differential contributions of ERK and PI3-kinase to the regulation of cyclin D1 expression and to the control of the G1/S transition in mouse embryonic stem cells. Oncogene. 2002;21(36):5515–5528. doi: 10.1038/sj.onc.1205728. [DOI] [PubMed] [Google Scholar]
- 48.Savatier P, Lapillonne H, van Grunsven LA, Rudkin BB, Samarut J. Withdrawal of differentiation inhibitory activity/leukemia inhibitory factor up-regulates D-type cyclins and cyclin-dependent kinase inhibitors in mouse embryonic stem cells. Oncogene. 1996;12(2):309–322. [PubMed] [Google Scholar]
- 49.Savatier P, Huang S, Szekely L, Wiman KG, Samarut J. Contrasting patterns of retinoblastoma protein expression in mouse embryonic stem cells and embryonic fibroblasts. Oncogene. 1994;9(3):809–818. [PubMed] [Google Scholar]
- 50.Stead E, et al. Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene. 2002;21(54):8320–8333. doi: 10.1038/sj.onc.1206015. [DOI] [PubMed] [Google Scholar]
- 51.Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002;12(9):432–438. doi: 10.1016/s0962-8924(02)02352-8. [DOI] [PubMed] [Google Scholar]
- 52.Schneider RP, et al. TRF1 is a stem cell marker and is essential for the generation of induced pluripotent stem cells. Nat Commun. 2013;4:1946. doi: 10.1038/ncomms2946. [DOI] [PubMed] [Google Scholar]
- 53.Allsopp RC, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 1992;89(21):10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iwano T, Tachibana M, Reth M, Shinkai Y. Importance of TRF1 for functional telomere structure. J Biol Chem. 2004;279(2):1442–1448. doi: 10.1074/jbc.M309138200. [DOI] [PubMed] [Google Scholar]
- 55.Martínez P, et al. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev. 2009;23(17):2060–2075. doi: 10.1101/gad.543509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marzec P, et al. Nuclear-receptor-mediated telomere insertion leads to genome instability in ALT cancers. Cell. 2015;160(5):913–927. doi: 10.1016/j.cell.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 57.Niwa H, Ogawa K, Shimosato D, Adachi K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature. 2009;460(7251):118–122. doi: 10.1038/nature08113. [DOI] [PubMed] [Google Scholar]
- 58.Fritsche-Guenther R, et al. Strong negative feedback from Erk to Raf confers robustness to MAPK signalling. Mol Syst Biol. 2011;7:489. doi: 10.1038/msb.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sturm OE, et al. The mammalian MAPK/ERK pathway exhibits properties of a negative feedback amplifier. Sci Signal. 2010;3(153):ra90. doi: 10.1126/scisignal.2001212. [DOI] [PubMed] [Google Scholar]
- 60.Li Z, et al. BMP4 signaling acts via dual-specificity phosphatase 9 to control ERK activity in mouse embryonic stem cells. Cell Stem Cell. 2012;10(2):171–182. doi: 10.1016/j.stem.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 61.Huang J, et al. Association of telomere length with authentic pluripotency of ES/iPS cells. Cell Res. 2011;21(5):779–792. doi: 10.1038/cr.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levin-Salomon V, Kogan K, Ahn NG, Livnah O, Engelberg D. Isolation of intrinsically active (MEK-independent) variants of the ERK family of mitogen-activated protein (MAP) kinases. J Biol Chem. 2008;283(50):34500–34510. doi: 10.1074/jbc.M806443200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schramek H, Feifel E, Healy E, Pollack V. Constitutively active mutant of the mitogen-activated protein kinase kinase MEK1 induces epithelial dedifferentiation and growth inhibition in Madin-Darby canine kidney-C7 cells. J Biol Chem. 1997;272(17):11426–11433. doi: 10.1074/jbc.272.17.11426. [DOI] [PubMed] [Google Scholar]
- 64.Huang P, et al. Heritable gene targeting in zebrafish using customized TALENs. Nat Biotechnol. 2011;29(8):699–700. doi: 10.1038/nbt.1939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.