Significance
Ion channels are membrane proteins essential for signal generation and transmission in the nervous system. They are finely regulated, and even small changes in their activity can trigger important physiological or pathological consequences on brain function. A regulatory mechanism of particular importance is phosphorylation. In this study, we assess the impact of phosphorylation on the activity of a specific ion channel, the T-type calcium channel Cav3.2. We show for the first time, to our knowledge, that Cav3.2 is highly phosphorylated in vivo and in a mammalian brain as well as in a human cell line, and we identify many phosphorylation sites critical for the way that the channel opens in response to changes in membrane potential and neuronal excitability.
Keywords: T-type calcium channel, Cav3.2 subunit, patch clamp, mass spectrometry, phosphorylation
Abstract
Phosphorylation is a major mechanism regulating the activity of ion channels that remains poorly understood with respect to T-type calcium channels (Cav3). These channels are low voltage-activated calcium channels that play a key role in cellular excitability and various physiological functions. Their dysfunction has been linked to several neurological disorders, including absence epilepsy and neuropathic pain. Recent studies have revealed that T-type channels are modulated by a variety of serine/threonine protein kinase pathways, which indicates the need for a systematic analysis of T-type channel phosphorylation. Here, we immunopurified Cav3.2 channels from rat brain, and we used high-resolution MS to construct the first, to our knowledge, in vivo phosphorylation map of a voltage-gated calcium channel in a mammalian brain. We identified as many as 34 phosphorylation sites, and we show that the vast majority of these sites are also phosphorylated on the human Cav3.2 expressed in HEK293T cells. In patch-clamp studies, treatment of the channel with alkaline phosphatase as well as analysis of dephosphomimetic mutants revealed that phosphorylation regulates important functional properties of Cav3.2 channels, including voltage-dependent activation and inactivation and kinetics. We also identified that the phosphorylation of a locus situated in the loop I-II S442/S445/T446 is crucial for this regulation. Our data show that Cav3.2 channels are highly phosphorylated in the mammalian brain and establish phosphorylation as an important mechanism involved in the dynamic regulation of Cav3.2 channel gating properties.
Voltage-gated calcium channels (L-, N-, P/Q-, R-, and T-types) mediate calcium entry in many different cell types in response to membrane depolarization and action potentials. Calcium influx through these channels serves as an important second messenger of electrical signaling, initiating a variety of cellular events and physiological functions (1, 2). Among the family of voltage-gated calcium channels, T-type calcium channels (Cav3 family) have unique electrophysiological properties, because they display low voltage-activated calcium currents with rapid activation/inactivation kinetics. In neurons, small changes in the membrane potential near the resting potential can activate T-type channels, favoring further membrane depolarization and repetitive firing of action potentials (3–5). These unique gating properties of T-type channels make them important in many different processes, including neuronal spontaneous firing and pacemaker activities, rebound burst firing, sleep rhythms, sensory processing, and neuronal differentiation, as well as in pathological conditions, such as epilepsy and neuropathic pain (6).
To properly assure this plurality of physiological functions, a tight control of T-type calcium channels is necessary. An important regulatory mechanism is phosphorylation, the fastest and most frequent posttranslational modification for a protein. Ion channels, especially voltage-gated channels, are critically regulated by phosphorylation. Voltage-dependent sodium and potassium channels have been shown to be the target of multiple phosphorylation events, regulating different channel functions and being involved in pathological states, like epilepsy (7–11). Also, several studies have shown the crucial role of the L-type/Cav1 phosphorylation in important physiological functions, like the fight or flight response (12–15).
Regarding T-type channels, phosphorylation remains poorly understood. There are three Cav3 pore-forming proteins (Cav3.1, Cav3.2, and Cav3.3 subunits) all displaying typical properties of T-type channels when expressed in heterologous cell systems (3, 16). Among them, the Cav3.2 channel seems to be particularly sensitive to various types of regulation, including phosphorylation. To date, several serine/threonine kinases, like PKA, PKC, or CamKII, have been shown to regulate Cav3.2 activity (reviewed in refs. 17–20); however, in most cases, this regulation is tissue-dependent, and little is still known about its molecular basis. Cav3.2 channels bear more than 100 multiple intracellular serine and threonine residues that are predicted to be phosphorylated by common prediction algorithms, like NetPhos2.0 (21). However, which of these residues are actually phosphorylated and what functional impact this phosphorylation will have remain to be determined.
In this study, we investigate the phosphorylation pattern of the Cav3.2 isoform of the T-type channels and its role in Cav3.2 channel properties. Using an MS approach, we have established the first, to our knowledge, phosphorylation map of the Cav3.2 channel in a mammalian brain and a human cell line. Then, by using alkaline phosphatase (AP) and dephosphomimetic mutants in patch-clamp experiments, we reveal the importance of phosphorylation in modulating Cav3.2 gating properties. We have also identified a phosphorylation hot spot situated in the loop connecting domains I and II of the channel that plays a crucial role in this regulation. Altogether, this study provides important insights regarding how phosphorylation regulates Cav3.2 channels.
Results
Cav3.2 Is Highly Phosphorylated in Brain and Heterologous Cells.
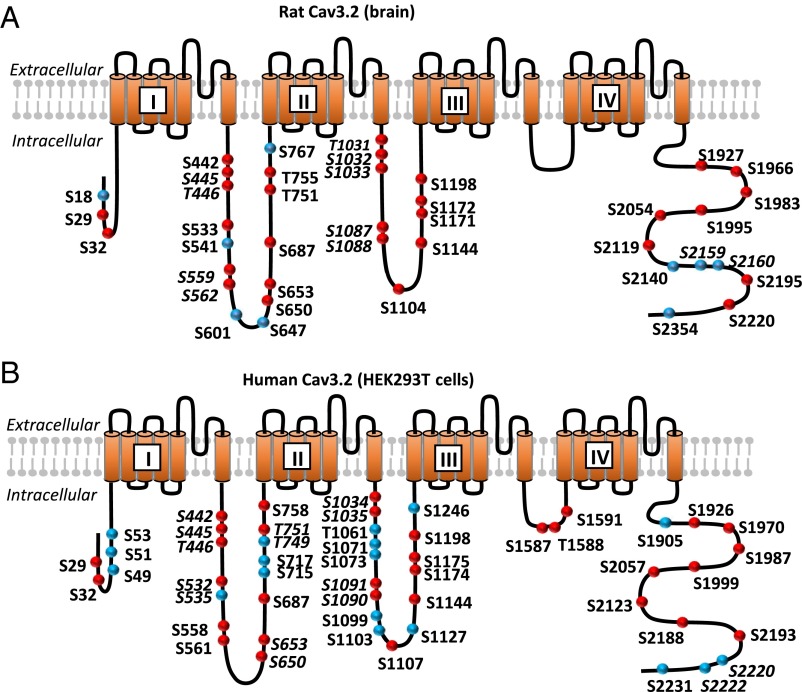
To establish the phosphorylation status of Cav3.2 in brain tissue, we immunopurified Cav3.2 from rat brain lysate, digested the purified protein with proteases, and further analyzed the resulting peptides by high-resolution MS (details are in Materials and Methods); 62% of the protein could be detected by this method. This percentage of protein coverage was even higher (78%) when considering only the major intracellular domains, the principal target of kinases and phosphatases (Fig. S1A).
Based on the MS/MS spectra, in total, 34 different phosphorylation sites were identified (Fig. S2 and Dataset S1 show the representative spectra), 26 of which have not been described elsewhere to our knowledge. Among the others, two of them, S1104 and S1203, have been previously identified as targeted by PKA and CamKII, respectively (22, 23), in adrenocortical carcinoma cells, and six others (S445/T446, S541, S1171, T1172, S2201, and S2360) were described in high-throughput studies using automatic assignation of phosphorylation sites from MS/MS spectra (24, 25). Except the loop connecting the domains III and IV (LIII-IV), which is the smallest, all intracellular loops contained several phosphorylated threonine and serine residues. No tyrosine phosphorylation was detected.
In view of further studying the functional impact of this phosphorylation, we also assessed the phosphorylation status of Cav3.2 expressed in HEK293T cells. This human cell line is a widely used system for studying ion channel function, and it is important to know whether the phosphorylation pattern of the recombinant Cav3.2 protein is similar to the one observed in native tissue (brain). For expression in HEK293T cells, we used the human isoform of Cav3.2, bearing an HA epitope that allows an efficient purification of the protein. The rat and the human Cav3.2 isoforms are highly similar, having a sequence identity of 84%. After expression and immunoprecipitation, we subjected the recombinant Cav3.2 to the same type of analysis as the native protein from rat brain. The overall coverage and the coverage of the major intracellular loops were 71% and 87%, respectively (Fig. S1B). These experiments allowed us to identify of a total of 43 phosphosites (Fig. 1B, Fig. S2, and Dataset S1 show representative MS/MS spectra). Comparing these sites with those identified in rat brain revealed that the vast majority of phosphorylation sites found in the brain were also phosphorylated in HEK293T cells (27 sites from a total of 34). Among the remaining ones, some (S18, S541, S767, and S2354) were not conserved between rat and human Cav3.2 isoforms, and only three were conserved but not detected as phosphorylated in HEK293T cells. More details about phosphosite correspondence between the rat and the human isoforms are given in Table S1. These results suggest that the phosphorylation patterns of Cav3.2 are mostly conserved between the HEK293T cell line and the brain as well as between the human and rat isoforms.
Fig. 1.
Schematic representation of the Cav3.2 MS analysis. (A) Cartoon of the membrane topology of Cav3.2 showing the phosphorylation sites identified in rat brain. (B) Cartoon of the membrane topology of human Cav3.2 showing the phosphorylation sites identified in in HEK293T cells. The red circles indicate the sites identified as phosphorylated in both rat brain and HEK293T cells. Correspondence between the amino acid numbering of the rat and human Cav3.2 isoform is given in Table S1. The unambiguous sites are bold, and the ambiguous sites are bold and italicized. Liquid chromatography–MS/MS sequence coverage of the Cav3.2 protein and the MS/MS spectra of the Cav3.2 peptides can be found in Figs. S1 and S2 and Dataset S1.
Phosphorylation Regulates the Biophysical Properties of Cav3.2 Channels.
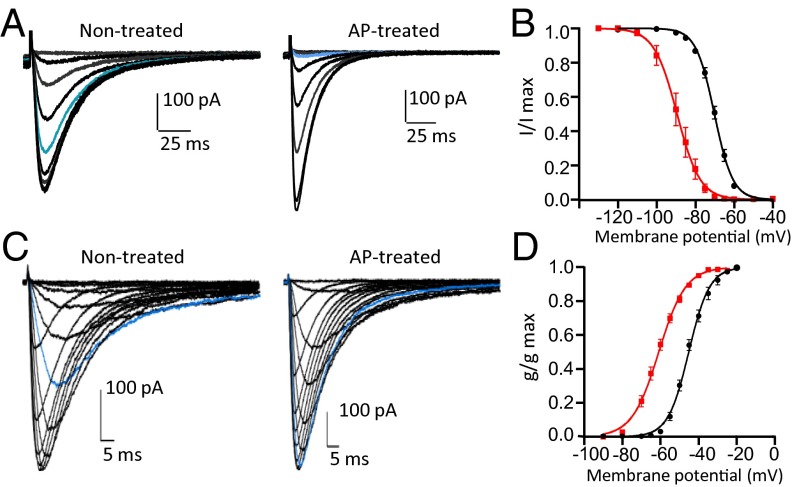
The large number of phosphorylated sites found on Cav3.2 suggests extensive modulation of the channel by phosphorylation. To rapidly evaluate the impact that this phosphorylation could have on channel activity, we used a phosphatase, the AP, in patch-clamp studies. This phosphatase was already successfully used in others studies to show the role that phosphorylation has on Kv channels (26). We, therefore, expressed the human isoform of Cav3.2 in HEK293T cells and measured its activity in a whole-cell patch-clamp configuration. Subsequently, we allowed AP to dialyze into the cell through the patch pipette to induce channel dephosphorylation. The AP dialysis (100 U/mL for 30 min) led to a significant shift toward more negative potentials of the steady-state inactivation and activation curves (Fig. 2). The half-inactivation potential (V1/2) was shifted ∼19 mV from −70.12 ± 0.7 to −89.53 ± 2.2 mV (P < 0.001) (Fig. 2 A and B), and similarly, the half-activation potential was shifted ∼16 mV from −46.02 ± 0.4 to −61.6 ± 1 mV (P < 0.001) (Fig. 2 C and D). Also, the activation and inactivation kinetics became significantly faster on AP treatment. For instance, at −40 mV, the τinact decreased from 28.9 ± 3.3 to 15.5 ± 1.5 ms (P < 0.01) (Fig. S3A), and the τact decreased from 6.5 ± 0.7 to 2.4 ± 0.2 ms (P < 0.001) (Fig. S3B). No significant changes were observed regarding other biophysical properties of the Cav3.2 channel, including recovery from inactivation and deactivation kinetics (Fig. S3 C and D). A set of experiments designed to determine the reversal potential showed no significant effect of the AP treatment on this parameter either (Fig. S3E).
Fig. 2.
AP effect on biophysical properties of Cav3.2 channels. (A) Representative currents elicited by test pulses from different holding potentials (−110, −100, −90, −85, −80, −75, −70, −65, −60, and −50 mV) for 5 s to −30 mV for the cells nontreated or treated with AP. HEK293T cells expressing human Cav3.2 were treated by adding AP into the patch pipette (100 U/mL) and letting it dialyze into the cell for 30 min. For the control cells, the current was recorded after 30 min of dialysis of the internal solution without AP. Note the change in the current amplitude for the trace in blue (holding potential was −75 mV) in the presence of AP as well as the changes in the kinetics. (B) Steady-state inactivation curve fit from traces in A for the control cells (black circles; n = 5) and the AP-treated cells (red squares; n = 11). (C) Representative currents elicited from a holding potential of −100 mV to different test pulse potentials (−90, −80, −70, −65, −60, −55, −50, −45, −40, −35, −30, −25, −20, and −10 mV) for the cells nontreated or treated with AP. Note the change in the amplitude of the current for the trace in blue (test pulse potential was −50 mV) in the presence of AP as well as the changes in the kinetics. (D) Steady-state activation curve fit from traces in C for the control cells (black circles; n = 4) and the AP-treated cells (red squares; n = 12).
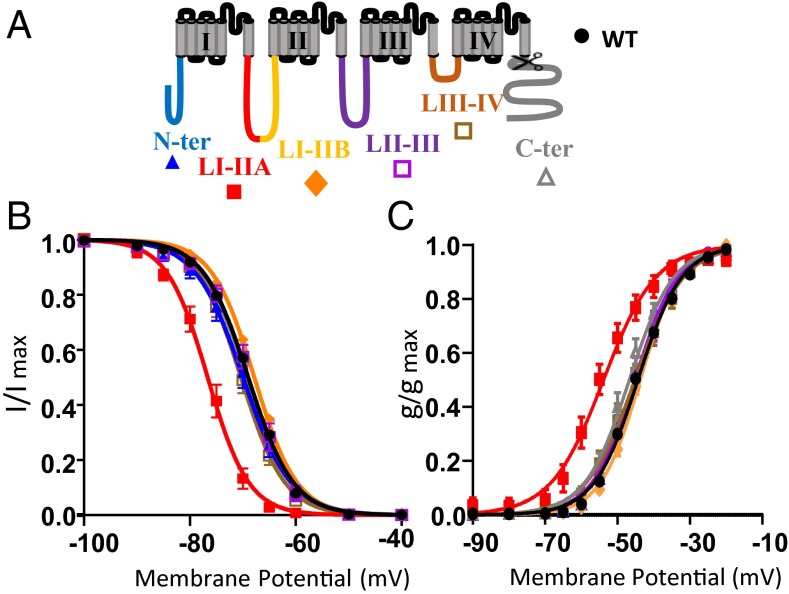
These data suggest that the gating properties of Cav3.2 channels are greatly influenced by the phosphorylation status. To determine whether the AP effect is because of a direct dephosphorylation of the channel, we mutated the serine and threonine residues that we previously identified as phosphorylated to alanine, thus mimicking the dephosphorylation.
In a first series of experiments, six constructs were generated, each of them designed to assess the functionality of the identified phosphoserine and phosphothreonine residues from a particular intracellular region of human Cav3.2 (Table 1 shows details concerning each construct). No significant effect on the Cav3.2 steady-state activation and inactivation properties or kinetics was observed when all of the identified phosphorylated sites of the cytoplasmic N-terminal part of the protein were mutated to alanine (the construct named N-ter Ala is shown in Fig. 3 and Table 1). The same result was obtained when the phosphorylated sites from LII-III and LIII-IV were mutated (constructs LII-III Ala and LIII-IV Ala, respectively, are shown in Fig. 3 and Table 1). To identify a role in gating of the C-terminal part of Cav3.2, we constructed a deletion mutant by introducing a stop codon at Q1886 (C-terΔ). No change in the biophysical properties of the T-type current was observed with the C-terΔ mutant (Fig. 3 and Table 1). To evaluate the role of the large intracellular loop connecting domains I and II (LI-II ; 357 aa), we generated two constructs where all of the identified phosphorylated sites in the proximal part (LI-IIA) or the distal part (LI-IIB), respectively, were mutated to alanines (LI-IIA Ala and LI-IIB Ala are shown in Fig. 3 and Table 1). We did not observe any effect on Cav3.2 current properties with the LI-IIB Ala construct. However, the LI-IIA Ala construct displayed steady-state activation and inactivation curves significantly shifted toward more negative potentials. The extent of the shift was −7 mV for the inactivation curve (V1/2 shifted from −69.02 ± 0.27 to −76.59 ± 0.91 mV; P < 0.001) (Fig. 3B) and −9 mV for the activation curve (V1/2 shifted from −45.07 ± 0.34 to −54.71 ± 1.59 mV; P < 0.001) (Fig. 3C). In addition, the activation and inactivation kinetics were also faster for the LI-IIA Ala mutant (Table 1). No significant change in the current density was seen for any of the mutants.
Table 1.
Electrophysiological properties of WT and mutant Cav3.2 channels
| Constructions | Mutations | Activation V1/2 (mV) and K (mV) | Inactivation V1/2 (mV) and K (mV) | Kinetics at −45 mV τact (ms) and τinact (ms) |
| WT | — | −45.07 ± 0.34 (65) | −69.02 ± 0.27 (68) | 9.476 ± 0.33 (62) |
| 5.26 ± 0.07 (65) | 4.06 ± 0.06 (68) | 29 ± 0.7 (62) | ||
| N-ter Ala | S29A, S32A, S49A, S51A, S53A | −45.97 ± 1.0 (8) | −69.97 ± 1.02 (8) | 9.036 ± 1.07 (7) |
| 4.963 ± 0.15 (8) | 4.199 ± 0.2 (8) | 26.14 ± 2.75 (8) | ||
| LI-IIA Ala | S442A, S4445A, T446A, S532A, S535A, S558A, S561A | −54.71 ± 1.59(7)* | −76.59 ± 0.914(7)* | 4.347 ± 0.43 (5)* |
| 5.354 ± 0.41(7) | 3.609 ± 0.07(7) | 17.96 ± 1.67(5)* | ||
| LI-IIB Ala | S650A, S653A, S687A, S715A, S717A, S749A, T751A, S758A | −44.24 ± 0.6 (6) | −67.84 ± 0.25 (7) | 12.14 ± 0.42 (5) |
| 5.029 ± 0.16 (6) | 4.021 ± 0.09 (7) | 32.75 ± 3.5 (5) | ||
| LII-III Ala | T1034A, S1035A, T1061A, S1071A, S1073A, S1091A, S1090A, S1099A, S1103A, S1107A, S1127A, S1144A, S1174A, S1175A, S1198A, S1246A | −45.6 ± 1.52 (5) | −68.97 ± 0.87 (5) | 8.235 ± 0.94 (4) |
| 5.264 ± 0.16 (5) | 4.102 ± 0.05 (5) | 25.83 ± 3.6 (4) | ||
| LIII-IV Ala | S1587A, T1588A, S1591A | −46.03 ± 1.62 (8) | −70.35 ± 0.78 (10) | 8.203 ± 0.64 (11) |
| 5.604 ± 0.18 (8) | 3.873 ± 0.08 (10) | 29.76 ± 2.1 (11) | ||
| C-terΔ | Q1886Stop | −47.79 ± 1.4 (7) | −69.09 ± 0.97 (8) | 8.558 ± 1.08 (5) |
| 4.961 ± 0.34 (7) | 4.087 ± 0.1 (8) | 26.02 ± 2.16 (5) | ||
| LI-II A1 | S442A, S4445A, T446A | −54.05 ± 0.7 (11)* | −76.17 ± 0.7 (11)* | 4.561 ± 0.24 (11)* |
| 5.297 ± 0.1 (11) | 3.509 ± 0.11 (11)† | 23.49 ± 1.06 (11)‡ | ||
| LI-II A2 | S532A, S535A | −45.69 ± 1.03 (5) | −69.09 ± 0.4 (5) | 7.927 ± 0.48 (5) |
| 5.326 ± 0.35 (5) | 4.189 ± 0.18 (5) | 27.34 ± 2.17 (5) | ||
| LI-II A3 | S558A, S561A | −44.04 ± 1.69 (6) | −66.4 ± 0.866 (6) | 11.28 ± 1.20 (7) |
| 5.356 ± 0.36 (6) | 4.045 ± 0.25 (6) | 27.2 ± 2.8 (7) | ||
| WT AP | — | −61.6 ± 1.04 (12)* | −89.53 ± 2.2 (11)* | 2.919 ± 0.26 (11)* |
| 6.124 ± 0.14 (12) | 4.224 ± 0.04 (11) | 15.82 ± 1.5 (11)* | ||
| LI-II A1 AP | S442A, S4445A, T446A | −64.58 ± 1.08 (8)* | −89.3 ± 1.3 (9)* | 2.584 ± 0.211 (8)* |
| 5.572 ± 0.32 (8) | 3.763 ± 0.06 (9) | 21.33 ± 2.3 (8)† |
V1/2 represents the half-activation and half-inactivation potentials, respectively; K is the slope factor; and τact and τinact are the activation and inactivation kinetics, respectively. The kinetics values were obtained by fitting the current traces obtained using the I-V curve protocol with a double-exponential function. The number of cells is indicated in parentheses.
P < 0.001 compared with the WT channels.
P < 0.01 compared with the WT channels.
P < 0.05 compared with the WT channels.
Fig. 3.
Electrophysiological analysis of the phosphorylation mutants. (A) Cartoon of the membrane topology of Cav3.2 showing the region mutated for the different constructions (Table 1 shows details regarding the mutated residues for each construction). (B and C) Steady-state inactivation curves and steady-state activation curves, respectively, for the WT (black circles), N-ter Ala (blue triangles), LI-II A Ala (red squares), LI-II B Ala (orange diamonds), LII-III Ala (purple squares), LIII-IV Ala (brown squares), and C-terΔ (gray triangles).
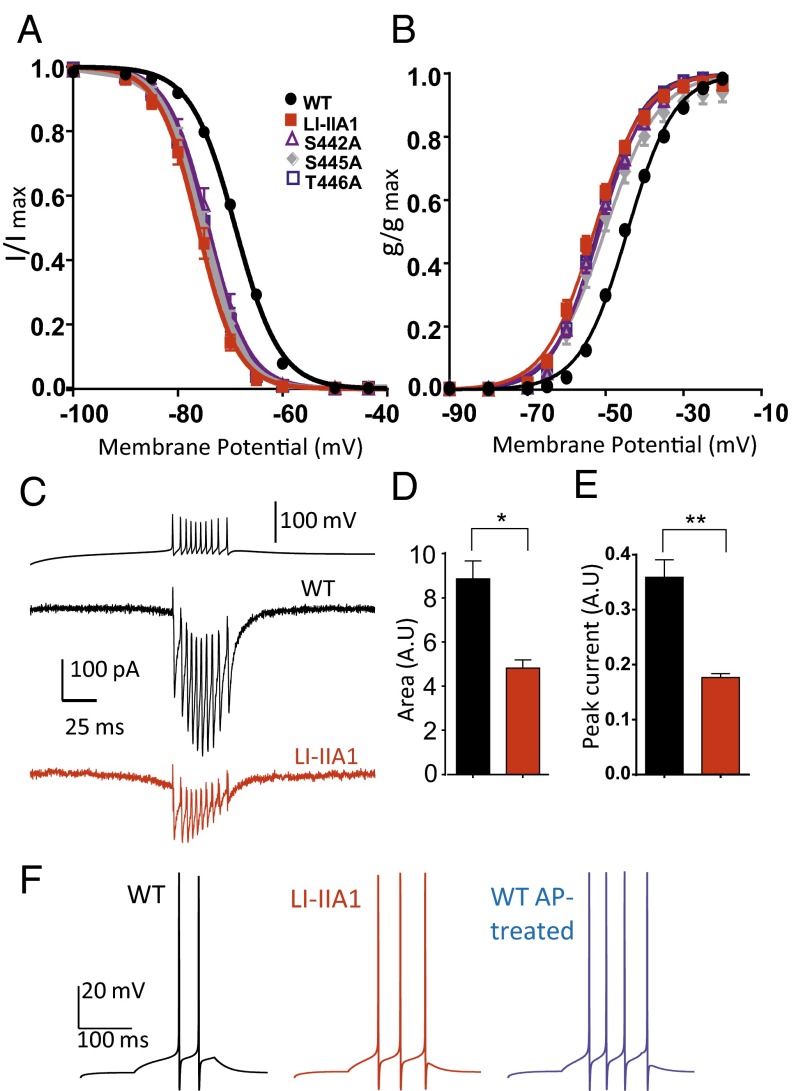
Because the LI-IIA Ala construct has seven mutated serine and threonine residues, we sought to determine which of them was responsible for the gating effect. Therefore, three additional constructs were generated: LI-IIA1 (carrying the mutations S442A, S445A, and T446A), LI-IIA2 (S532A and S535A), and LI-IIA3 (S558A and S561A). Whereas LI-IIA2 and LI-IIA3 had current properties similar to the WT, the LI-IIA1 mutant exhibited a shift in both the steady-state half-activation and half-inactivation potentials and faster activation and inactivation kinetics similar to the LI-IIA Ala construct (Table 1), indicating that the phosphosites from this region are important for the voltage-dependent gating of the channel. Furthermore, treatment of the LI-IIA1 mutant with AP resulted in a hyperpolarizing shift in voltage-dependent activation and inactivation to the same end points as the AP-treated WT Cav3.2 channel (Table 1). The shift observed for the LI-IIA1 mutant in the presence of AP was significantly reduced compared with the one obtained for the WT. For the mutant channel, the steady-state activation and inactivation curves were shifted by 10 and 13 mV, respectively, compared with the 16- and 19-mV shifts measured for the WT. These experiments further support that the Cav3.2 gating properties are modulated by the phosphorylation of the S442/S445/T446 cluster.
The LI-IIA1 construct is a triple mutant carrying the following mutations: S442A, S445A, and T446A. Individual mutation of these sites led to three single alanine mutants each exhibiting a shift in the steady-state activation and inactivation properties as well as accelerated kinetics, similar to those observed for LI-IIA Ala or the triple mutant LI-IIA1 (Fig. 4 A and B). Importantly, these specific voltage-dependent biophysical properties of the LI-IIA1 mutant could also be observed in another cell line, the neuroblastoma NG108-15 cell line, which constitutively expresses Cav3.2. In this neuronal environment, expression of LI-IIA1 also yielded a hyperpolarizing shift of the steady-state activation and inactivation curves as well as faster activation and inactivation kinetics (Fig. S4). In addition, we have performed action potential voltage-clamp experiments. Because the role of T-type channels in promoting burst activity in thalamic neurons is well documented (27), these experiments were done using a burst firing activity typical of the thalamic reticular neurons as a voltage-clamp command (28). Using this protocol on cells displaying similar T-type current density, we observed that, at a physiological membrane potential (−76 mV), the LI-IIA1 mutant generated significantly smaller calcium current compared with the WT channel (Fig. 4C). This result was further confirmed by the quantification of both the peak current density (Fig. 4D) and the inward current area (Fig. 4E), which accounts for total calcium entry during burst activity protocol. To evaluate the impact of Cav3.2/T-type channel phosphorylation on neuronal excitability, we have used a NEURON model of thalamic reticular neuron (28). Introduction of our experimental values for the WT, LI-IIA1 mutant, and AP-treated WT channels revealed marked differences in firing patterns (Fig. 4F). We found that both mutant and AP-treated WT channels led to increased firing. The number of spikes was increased for the LI-IIA1 mutant compared with the WT channel, an increase that was even stronger for the AP-treated WT channel. Also, the latency for the first spike was shorter for the dephosphorylated channels (50.7 ms for AP-treated WT and 55.7 ms for LI-IIA1 mutant compared with 82.9 ms for the WT), and the average interspike interval was decreased (36.1 ms for AP-treated WT and 36.8 ms for LI-IIA1 mutant compared with 45.1 ms for the WT).
Fig. 4.
Effect of mutation of the phosphorylation loci S442, S445, and T446. (A and B) Steady-state inactivation curves and steady-state activation curves, respectively, for the WT (black circles; n = 62–68), LI-IIA1 (red squares; n = 11), S442A (purple triangles; n = 8), S445A (gray diamonds; n = 7), and T446A (blue squares; n = 8). (C) Representative calcium current trace for the WT (black trace) and the LI-IIA1 mutant (red trace) recorded during a voltage-clamp firing pattern of a thalamic reticular neuron generated by the NEURON model. Please note that these representative recordings were obtained in cells exhibiting similar current density when recorded at −30 mV from a holding potential (HP) of −110 mV. (D) Calcium current area quantification from traces in C. (E) Peak calcium current quantification from traces in C. The WT is represented in black (n = 3), and the LI-IIA1 mutant is represented in red (n = 3). (F) Reticular thalamic neuron firing simulation using values obtained with WT (black trace), LI-IIA1 mutant (red trace), and AP-treated WT (blue trace) channels. The current clamp responses were elicited by a 0.037-nA current injection for 150 ms in a dendrite of the detailed cell model from a holding potential of −70 mV.
Discussion
Phosphorylation is a major mechanism regulating the activity of ion channels that remains poorly understood for T-type calcium channels (17–20). Taking advantage of the MS approach, we provide here the first, to our knowledge, phosphorylation map of the Cav3.2 T-type calcium channel purified from both native tissue (rat brain) and recombinant system (human HEK293T cells). Importantly, we show that phosphorylation highly modulates channel gating properties, and we report that phosphorylation of a locus within the intracellular loop connecting domains I and II is directly involved in this gating control.
Our MS experiments reveal that a large number of serine and threonine residues of the Cav3.2 protein is phosphorylated: 34 for the native channel in the rat brain and 43 for the recombinant human Cav3.2 channel in the HEK293T cells. At least 27 phosphosites identified in vivo were also found to be phosphorylated in the heterologous expression system (Table S1), suggesting that the phosphorylation pattern of the Cav3.2 protein is broadly conserved among species (rat and human) and cellular systems (recombinant channel in HEK293T and native channel in the brain). Our work has validated 8 sites identified elsewhere (Table S1) and identified 26 never described in vivo phosphosites. Among these discovered sites, some (S18, S647, S687, and S2193 in the rat isoform) were not even predicted to be phosphorylated by widely used computer algorithms, like NetPhos2.0. This underscores the limitations of algorithm-based predictions and shows the importance of undertaking MS-based proteomic studies to characterize the phosphorylation status of a protein.
The striking extent of Cav3.2 phosphorylation raises questions as to its role in Cav3.2 regulation. The remarkable hyperpolarizing shift in the activation and inactivation curves of the channel (16 and 19 mV, respectively) obtained by treatment with a phosphatase (AP) indicates that the phosphorylation level of the cell will greatly influence the biophysical properties of the channel. Mutating all of the identified phosphosites of human Cav3.2 shows that dephosphorylation of S442, S445, and /or T446 is, at least partially, responsible for the shift observed in the presence of AP. Future studies are needed to determine if the additional shift observed with AP (7 mV for the activation and 12 mV for the inactivation curve) is because of an indirect action through channel partners or the direct dephosphorylation of other Cav3.2 phosphosites that could not be identified in this study. Indeed, some phosphorylation sites, particularly those with low stoichiometry, might have been missed by our MS approach. Either way, our data show that the phosphorylation level of the cell can drastically influence the voltage-dependent gating of Cav3.2 channel.
Our results also show that the phosphorylation locus S442/S445/T446 has a particular importance for the voltage-dependent gating of Cav3.2. These three amino acids were detected by MS on the ARHLS442NDS445T446LASFSEPGSCYEELLK peptide (spectra are shown in Dataset S1) for the human isoform expressed in HEK293T cells and the YLS442NDS445T446LASFSEPGSCYEELLK peptide (Fig. S2) for the rat brain isoform. The latter was detected in the monophosphorylated as well as the diphosphorylated forms. For the monophosphorylated peptide, the MS/MS data showed a hybrid spectrum with b and y ions supporting both the phosphorylation of S442 and S445 or T446 (Fig. S2). Analysis of the MS/MS spectrum of the human peptide allowed us to determine that one of the residues S442, S445, or T446 is phosphorylated, but data were not sufficient to pinpoint exactly which one of the three is actually phosphorylated. From a functional point of view, individual mutations of S442, S445, and T446 to alanine led to single mutants exhibiting the same biophysical properties as the triple-alanine mutant, suggesting that mutation/dephosphorylation of either one of these residues is sufficient to trigger functional changes in Cav3.2 activity.
S442, S445, and T446 are located within the loop connecting domains I and II (I-II loop) in a region that was previously described as a “gating brake” (29–31). Indeed, Vitko et al. (29) showed that deletion of the first 62 aa of the I-II loop (429–491) allows the Cav3.2 channel to open at more negative membrane potential by shifting the steady-state activation and inactivation curves, a phenotype recapitulated in our LI-IIA1 mutant. It is tempting to postulate that phosphorylation/dephosphorylation of the residues S442, S445, and/or T446 would affect the conformation of this region, leading to a change in channel gating. Our data are, therefore, consistent with the critical role of this region in the gating of the Cav3.2 channel and provide a possible physiological regulation of the gating brake. It is interesting to notice that the vast majority of the described Cav3.2 absence epilepsy-linked mutations are also on the I-II loop (32). One of these mutations, C456S, which induces a 5-mV shift in the activation curve and an increase in surface expression of the protein (29, 33), has been shown to increase the spontaneous firing rate of hippocampal neurons and thus, an increase in seizure susceptibility (34). This underscores the importance of Cav3.2 gating properties in neuronal firing, a property that could be dynamically regulated by loop I-II phosphorylation/dephosphorylation as documented in this study.
Our modeling experiments predict that neuronal firing patterns depend on T-type channel phosphorylation. The LI-IIA1 mutant and the AP-treated WT channels could trigger an increased firing in a thalamic reticular neuron model, suggesting that, in their native environment, these neurons would be more excitable under dephosphorylated conditions. It remains, however, possible that the complex interplay between the gating properties of the Cav3.2 channel would give a different output in another neuronal environment. It is interesting to notice that the values reported in the literature for the half-activation and half-inactivation potentials (V1/2) of the T-type current markedly vary between neurons. For instance, the half-activation potential ranges from −45 to −60 mV (35), values that are consistent with our findings (−45 mV for WT and −61 mV for AP-treated WT). Although different contributions of T-type channel isoforms or methodological differences could explain this wide range in half-activation potentials, it could also be caused by different phosphorylation states. Our MS approach is a global one, not taking into account the cell type specificity. Future experiments should be designed to assess the phosphorylation pattern of the channel protein in a more specific set of neurons, allowing better correlation with the gating properties of the channel.
Except for S442, S445, and T446, alanine mutations of all of the other identified phosphosites resulted in no apparent effect on Cav3.2 biophysical properties. Most of our alanine mutations were done concomitantly for all of the phosphosites found in a particular loop, and it is, indeed, possible that some phosphosites individually shift gating into different directions, thereby resulting in the absence of a detectable biophysical effect. A functional role of some of the other Cav3.2 phosphosites may be related to other modes of channel regulation not investigated in this study or depend on a particular channel environment. For instance, Hu et al. (22) showed that phosphorylation of S1107 (S1104 in the rat isoform) could induce an inhibition of the T-type current only in the presence of G protein βγ-subunits (Gβ2γ2), which are absent in HEK293T cells. It is, therefore, possible that protein partners are needed to reveal the functional role of a particular phosphorylation site of Cav3.2 channel. It could also be possible that a functional effect may be observed when two or several residues from distinct intracellular domains are concomitantly phosphorylated/dephosphorylated. The shift observed with AP treatment may have more complex underpinnings that are comprised of multiple components going in both directions. Finally, it should also be noticed that the phosphorylation maps provided in this study correspond to the basal state of the channel in brain tissue and HEK293T cells. It may, therefore, be possible that other serine and threonine residues of the channel could be phosphorylated in response to physiological or pathological stimuli and signaling pathways that are not constitutively active in brain or HEK293T cells.
Taken together, our results show that the Cav3.2 T-type calcium channel is highly phosphorylated both in vivo (brain) and in vitro (HEK293T cells) and that phosphorylation greatly contributes to the biophysical properties of the channel, likely leading to important physiological consequences. Our findings pave the way to a better understanding of the dynamic regulation of Cav3.2 activity by phosphorylation in physiology and pathology.
Materials and Methods
HEK-293T cells were transfected with human Cav3.2-pcDNA3.1 constructs using standard protocols. Mutagenesis was performed using the QuikChange II XL Kit (Agilent). For MS experiments, Cav3.2 channel immunoprecipitation was performed from 6-wk-old rat brain and transfected HEK-293T cells. T-type calcium currents were recorded from HEK-293T cells using the whole-cell patch-clamp technique. AP experiments were conducted by adding 100 U/mL enzyme (Roche) into the patch pipette solution with cells dialyzed for 30 min. Details on constructs, immunoprecipitation experiments, MS, electrophysiology, and NEURON modeling are provided in SI Materials and Methods. Data are presented as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Dr. Maithé Corbani for technical help and Dr. François Rassendren, Dr. Nathalie Guérineau, and Dr. Chris Jopling for helpful comments and critical reading of the manuscript. This work was supported by CNRS, INSERM, the Université de Montpellier, Agence Nationale pour la Recherche Grants ANR-10-BLAN-1601 and ANR-09-MNPS-035, and LabEx Ion Channel Science and Therapeutics.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1511740112/-/DCSupplemental.
References
- 1.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57(4):411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 2.Simms BA, Zamponi GW. Neuronal voltage-gated calcium channels: Structure, function, and dysfunction. Neuron. 2014;82(1):24–45. doi: 10.1016/j.neuron.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Chemin J, et al. Specific contribution of human T-type calcium channel isotypes (alpha(1G), alpha(1H) and alpha(1I)) to neuronal excitability. J Physiol. 2002;540(Pt 1):3–14. doi: 10.1113/jphysiol.2001.013269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83(1):117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Reyes E. Molecular characterization of T-type calcium channels. Cell Calcium. 2006;40(2):89–96. doi: 10.1016/j.ceca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Cheong E, Shin HS. T-type Ca2+ channels in normal and abnormal brain functions. Physiol Rev. 2013;93(3):961–992. doi: 10.1152/physrev.00010.2012. [DOI] [PubMed] [Google Scholar]
- 7.Berendt FJ, Park KS, Trimmer JS. Multisite phosphorylation of voltage-gated sodium channel alpha subunits from rat brain. J Proteome Res. 2010;9(4):1976–1984. doi: 10.1021/pr901171q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baek JH, Rubinstein M, Scheuer T, Trimmer JS. Reciprocal changes in phosphorylation and methylation of mammalian brain sodium channels in response to seizures. J Biol Chem. 2014;289(22):15363–15373. doi: 10.1074/jbc.M114.562785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park KS, Mohapatra DP, Misonou H, Trimmer JS. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science. 2006;313(5789):976–979. doi: 10.1126/science.1124254. [DOI] [PubMed] [Google Scholar]
- 10.Yang JW, Vacher H, Park KS, Clark E, Trimmer JS. Trafficking-dependent phosphorylation of Kv1.2 regulates voltage-gated potassium channel cell surface expression. Proc Natl Acad Sci USA. 2007;104(50):20055–20060. doi: 10.1073/pnas.0708574104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerda O, Trimmer JS. Analysis and functional implications of phosphorylation of neuronal voltage-gated potassium channels. Neurosci Lett. 2010;486(2):60–67. doi: 10.1016/j.neulet.2010.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hulme JT, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac CaV1.2 channels during beta1-adrenergic regulation. Proc Natl Acad Sci USA. 2006;103(44):16574–16579. doi: 10.1073/pnas.0607294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci Signal. 2010;3(141):ra70. doi: 10.1126/scisignal.2001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emrick MA, Sadilek M, Konoki K, Catterall WA. Beta-adrenergic-regulated phosphorylation of the skeletal muscle Ca(V)1.1 channel in the fight-or-flight response. Proc Natl Acad Sci USA. 2010;107(43):18712–18717. doi: 10.1073/pnas.1012384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuller MD, Fu Y, Scheuer T, Catterall WA. Differential regulation of CaV1.2 channels by cAMP-dependent protein kinase bound to A-kinase anchoring proteins 15 and 79/150. J Gen Physiol. 2014;143(3):315–324. doi: 10.1085/jgp.201311075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cain SM, Snutch TP. Contributions of T-type calcium channel isoforms to neuronal firing. Channels (Austin) 2010;4(6):475–482. doi: 10.4161/chan.4.6.14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Jiang X, Snutch TP, Tao J. Modulation of low-voltage-activated T-type Ca²⁺ channels. Biochim Biophys Acta. 2013;1828(7):1550–1559. doi: 10.1016/j.bbamem.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 18.Iftinca MC, Zamponi GW. Regulation of neuronal T-type calcium channels. Trends Pharmacol Sci. 2009;30(1):32–40. doi: 10.1016/j.tips.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Huc S, et al. Regulation of T-type calcium channels: Signalling pathways and functional implications. Biochim Biophys Acta. 2009;1793(6):947–952. doi: 10.1016/j.bbamcr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Chemin J, Traboulsie A, Lory P. Molecular pathways underlying the modulation of T-type calcium channels by neurotransmitters and hormones. Cell Calcium. 2006;40(2):121–134. doi: 10.1016/j.ceca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294(5):1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 22.Hu C, Depuy SD, Yao J, McIntire WE, Barrett PQ. Protein kinase A activity controls the regulation of T-type CaV3.2 channels by Gbetagamma dimers. J Biol Chem. 2009;284(12):7465–7473. doi: 10.1074/jbc.M808049200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao J, et al. Molecular basis for the modulation of native T-type Ca2+ channels in vivo by Ca2+/calmodulin-dependent protein kinase II. J Clin Invest. 2006;116(9):2403–2412. doi: 10.1172/JCI27918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trinidad JC, et al. Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Mol Cell Proteomics. 2012;11(8):215–229. doi: 10.1074/mcp.O112.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huttlin EL, et al. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143(7):1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murakoshi H, Shi G, Scannevin RH, Trimmer JS. Phosphorylation of the Kv2.1 K+ channel alters voltage-dependent activation. Mol Pharmacol. 1997;52(5):821–828. doi: 10.1124/mol.52.5.821. [DOI] [PubMed] [Google Scholar]
- 27.Cain SM, Snutch TP. T-type calcium channels in burst-firing, network synchrony, and epilepsy. Biochim Biophys Acta. 2013;1828(7):1572–1578. doi: 10.1016/j.bbamem.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 28.Destexhe A, Contreras D, Steriade M, Sejnowski TJ, Huguenard JR. In vivo, in vitro, and computational analysis of dendritic calcium currents in thalamic reticular neurons. J Neurosci. 1996;16(1):169–185. doi: 10.1523/JNEUROSCI.16-01-00169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vitko I, et al. The I-II loop controls plasma membrane expression and gating of Ca(v)3.2 T-type Ca2+ channels: A paradigm for childhood absence epilepsy mutations. J Neurosci. 2007;27(2):322–330. doi: 10.1523/JNEUROSCI.1817-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arias-Olguín II, et al. Characterization of the gating brake in the I-II loop of Ca(v)3.2 T-type Ca(2+) channels. J Biol Chem. 2008;283(13):8136–8144. doi: 10.1074/jbc.M708761200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez-Reyes E. Characterization of the gating brake in the I-II loop of CaV3 T-type calcium channels. Channels (Austin) 2010;4(6):453–458. doi: 10.4161/chan.4.6.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, et al. Association between genetic variation of CACNA1H and childhood absence epilepsy. Ann Neurol. 2003;54(2):239–243. doi: 10.1002/ana.10607. [DOI] [PubMed] [Google Scholar]
- 33.Vitko I, et al. Functional characterization and neuronal modeling of the effects of childhood absence epilepsy variants of CACNA1H, a T-type calcium channel. J Neurosci. 2005;25(19):4844–4855. doi: 10.1523/JNEUROSCI.0847-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckle VS, et al. Mechanisms by which a CACNA1H mutation in epilepsy patients increases seizure susceptibility. J Physiol. 2014;592(Pt 4):795–809. doi: 10.1113/jphysiol.2013.264176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.