Significance
We provide one of the few examples of spatiotemporal expression of VEGF-A and VEGF-B, which determine vascular development in zebrafish embryos. Nonoverlapping vascular functions of VEGF-A and VEGF-B are regulated by environmental oxygen tension. We show, for the first time to our knowledge, that downregulation of VEGF-B in zebrafish but not in mouse embryos produces a lethal phenotype owing to vascular defects. These findings indicate that different species use distinct mechanisms by the same factor for vascular development. Our data imply previously unidentified mechanisms for angiogenesis under pathological conditions as compared with healthy counterparts. Thus, differential targeting of the same VEGF-B in pathological and physiological angiogenesis may be potentially achieved by understanding spatiotemporal mechanisms of VEGF-B in relation to VEGF-A.
Keywords: VEGF-B, Neuropilin, zebrafish, vasculature, brain development
Abstract
Physiological functions of vascular endothelial growth factor (VEGF)-B remain an enigma, and deletion of the Vegfb gene in mice lacks an overt phenotype. Here we show that knockdown of Vegfba, but not Vegfbb, in zebrafish embryos by specific morpholinos produced a lethal phenotype owing to vascular and neuronal defects in the brain. Vegfba morpholinos also markedly prevented development of hyaloid vasculatures in the retina, but had little effects on peripheral vascular development. Consistent with phenotypic defects, Vegfba, but not Vegfaa, mRNA was primarily expressed in the brain of developing zebrafish embryos. Interestingly, in situ detection of Neuropilin1 (Nrp1) mRNA showed an overlapping expression pattern with Vegfba, and knockdown of Nrp1 produced a nearly identically lethal phenotype as Vegfba knockdown. Furthermore, zebrafish VEGF-Ba protein directly bound to NRP1. Importantly, gain-of-function by exogenous delivery of mRNAs coding for NRP1-binding ligands VEGF-B or VEGF-A to the zebrafish embryos rescued the lethal phenotype by normalizing vascular development. Similarly, exposure of zebrafish embryos to hypoxia also rescued the Vegfba morpholino-induced vascular defects in the brain by increasing VEGF-A expression. Independent evidence of VEGF-A gain-of-function was provided by using a functionally defective Vhl-mutant zebrafish strain, which again rescued the Vegfba morpholino-induced vascular defects. These findings show that VEGF-B is spatiotemporally required for vascular development in zebrafish embryos and that NRP1, but not VEGFR1, mediates the essential signaling.
Angiogenesis is essential for embryonic development and contributes to the onset and development of many diseases (1). The angiogenic process is tightly regulated by angiogenic factors and inhibitors and involves cooperative and synchronized interactions between vascular endothelial cells and perivascular cells including pericytes and vascular smooth muscle cells. Among all known angiogenic factors, vascular endothelial growth factor (VEGF; also called VEGFA) is probably the best-characterized proangiogenic factor under physiological and pathological conditions (2, 3). There are five structurally and functionally related members in the VEGF family, which includes VEGF-A, -B, -C, and -D and placental growth factor (PlGF) (4). These factors bind primarily to three membrane tyrosine kinase receptors (TKRs), i.e., VEGFR1, VEGFR2, and VEGFR3, to display their biological functions (4). According to their receptor-binding patterns and biological functions, members of the VEGF family are divided into three subgroups: (i) VEGFA as the VEGFR1- and VEGFR2-binding ligand (5); (ii) VEGF-B and PlGF that exclusively bind to VEGFR1 (4, 6–8); and (iii) VEGF-C and VEGF-D as VEGFR3- and VEGFR2-binding ligands (9). Whereas VEGF-A potently stimulates angiogenesis, vascular permeability, and lymphangiogenesis, VEGF-C and VEGF-D primarily induce lymphangiogenesis although they also induce angiogenesis (9). VEGFR2 has been reported as the key receptor that transduces angiogenic and vascular permeability signals, and VEGFR3 is responsible mainly for lymphangiogenesis (10). In addition to TKRs, various heparin-binding isoforms of each member in the VEGF family have been reported to bind to neuropilins (NRPs), which is also crucial for angiogenesis, lymphangiogenesis, axon guidance, cell survival, migration, and invasion (11–14).
VEGF-A is required for embryonic development in mammals, and deletion of only one allele of the Vegfa gene (haploinsufficiency) in mice results in a lethal embryonic phenotype, owing to inappropriate development of the vascular and hematopoietic systems (15, 16). Paradoxically, modest overexpression of VEGF-A in mice also causes embryonic lethality due to cardiovascular deficiency (17). These findings demonstrate that an optimal level of VEGF-A expression is needed for embryonic development. Unlike VEGF-A, deletion of the Vegfb gene in mice does not produce an overt phenotype, except slight cardiovascular impairments (18, 19). Recently, it has been found that VEGF-B–deficient animals exhibit defective lipid uptake in endothelial cells (20, 21). However, these findings could not be reproduced in another study (22). Based on these findings, VEGF-B is probably the least-characterized member in the VEGF-A family, and its physiological functions remain an enigmatic issue in mice (6). The key issue in VEGF-B research is what this factor does under physiological conditions. One of the main differences between developing mouse embryos and zebrafish embryos is the presence of tissue hypoxia during development. In mice and other mammals, embryonic tissues develop under a relatively hypoxic environment, and hypoxia is one of the key mechanisms behind up-regulation of VEGF-A expression (23). The increased VEGFA expression in various tissues would probably compensate the VEGF-B deletion-associated vascular and other defects. However, zebrafish embryos lack this hypoxia-related VEGF-A compensatory mechanism and allow us to study spatiotemporal functions of VEGF-B during embryonic development.
To test this hypothesis, in the present study we have investigated the functions of VEGF-B in developing zebrafish embryos. Surprisingly, knockdown of the Vegfba gene in developing zebrafish embryos produced a lethal phenotype owing to vascular defects in the brain. The functional defects of VEGF-B–deficient zebrafish embryos impeccably correlate with the VEGF-B expression pattern in the developing brain in which VEGF-A expression is modestly low. Importantly, exposure of VEGF-B–defective zebrafish embryos to hypoxia rescues the VEGF-B deficiency-induced vascular defects by a VEGF-A–dependent mechanism. Our findings for the first time to our knowledge demonstrate the indispensable function of VEGF-B in vascular development in zebrafish embryos.
Results
Gross Phenotypes in Vegfba Knockdown Zebrafish.
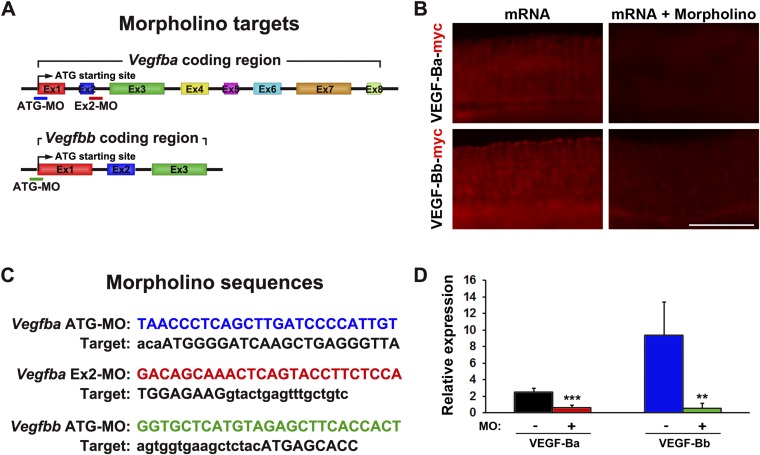
Analysis of the zebrafish genome revealed two Vegfb-related genes, i.e., Vegfba and Vegfbb, and similar duplicate genes also exist in Vegfa (24). Whereas the eight-exon Vegfba gene encodes the full-length VEGF-B protein corresponding to human and mouse VEGF-B, Vegfbb contained only the partial N-terminal coding sequence of VEGF-B (Fig. S1A). According to the available sequences in the zebrafish genome database (transcripts ENSDART00000123364 and ENSDART00000144627 for Vegfba and Vegfbb, respectively), we designed independent morpholinos targeting the ATG starting codon and exon 2 (Fig. S1 A and C). To assess the efficiency of Vegfb knockdown, these morpholinos with or without hybrid Vegfba-myc or Vegfbb-myc mRNA transcripts were injected into the yolk sac of developing zebrafish embryos at the one-cell stage as previously described (25–27). Expectedly, the initiation codon ATG-blocking morpholinos effectively blocked translation of their zebrafish Vegfba and Vegfbb mRNA targets as detected by myc-tag immunostaining of 24-h postfertilization (hpf) embryo trunks (Fig. S1 B and D). This method has been previously described as an effective approach for assessing the knockdown efficacy of morpholinos in zebrafish embryos (24).
Fig. S1.
Morpholino designs and inhibitory efficacies. (A) Schematic representation of the coding sequence of the zebrafish Vegfba and Vegfbb genes. Morpholinos designed in this study were indicated. (B) Whole-mount immunohistochemical staining with an anti-Myc antibody of 24-hpf zebrafish embryos injected with 100 ng Vegfba:myc or Vegfbb:myc mRNAs with or without the corresponding ATG-blocking morpholinos. (Scale bar, 100 μm.) (C) Nucleotide sequences of Vegfb morpholinos and their targets. Lowercase letters in the targets indicate noncoding bases. (D) Quantification of anti-myc reactivity signals in 24-hpf zebrafish embryos injected with 100 ng Vegfba:myc or Vegfbb:myc mRNA with or without the corresponding ATG-blocking morpholinos (average: n = 18). **P < 0.01; ***P < 0.001.
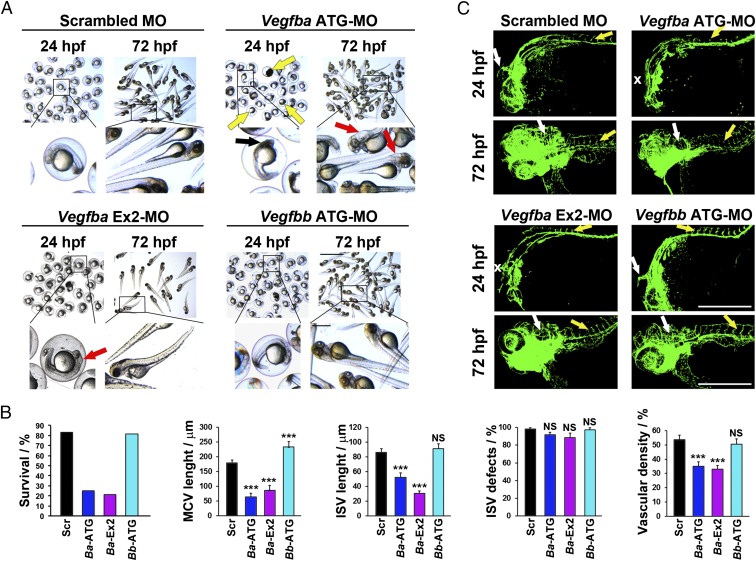
Knowing that these morpholinos effectively inhibited VEGF-B protein synthesis in zebrafish embryos, we next performed functional experiments by injecting these morpholinos into the one-cell-stage zebrafish embryos. Interestingly, knockdown of Vegfba by the Vegfba-ATG-morpholino produced a lethal phenotype, leading to an approximately 80% death rate of zebrafish embryos (Fig. 1 A and B). At 24 hpf, most of the Vegfba-ATG-morpholino–injected embryos exhibited impaired head development, and some surviving embryos showed edema in the head region at 72 hpf (Fig. 1A). A similar defective phenotype was also observed with the Vegfba exon 2-morpholino (Fig. 1A). In contrast, Vegfbb and scrambled morpholinos showed no abnormal phenotypes. These findings demonstrate that Vegfba, but not Vegfbb, is crucially required for embryonic development in zebrafish.
Fig. 1.
Gross and vascular phenotypes of Vegfba and Vegfbb knockdown in zebrafish embryos. (A) Micrographs of zebrafish embryos injected with 0.6 pmol of Scrambled, Vegfba-ATG, Vegfba-Ex2, or Vegfbb-ATG–targeted morpholinos, and embryos were visualized at 24 or 72 hpf. Black arrows indicate embryos with widespread cell death in the brain; yellow arrows indicate dead embryos; red arrows indicate embryos with cerebral edema. (Scale bar, 1 mm; in the amplified picture, 200 μm.) (B) Quantifications of the percentage of surviving embryos, MCV and ISV length at 24 hpf, percentage of defective ISVs at 72 hpf, and the vascular density in the brain of 72-hpf embryos injected with 0.6 pmol of Scrambled, Vegfba-ATG (Ba-ATG), Vegfba-Ex2 (Ba-Ex2), or Vegfbb-ATG (Bb-ATG)-targeted morpholinos (average n = 85 embryos/group). NS, nonsignificant; ***P < 0.001. (C) Confocal micrographs of Tg(fli1a:EGFP) embryos at 24 or 72 hpf injected with 0.6 pmol of Scrambled, Vegfba-ATG, Vegfba-Ex2 or Vegfbb-ATG–targeted morpholinos. White arrows point to the MCV or the brain vasculature affected at 72 hpf. White X symbols mark the positions where MCVs were missing. Yellow arrows point to ISVs. (Scale bars, 500 μm.)
Vascular Phenotypes in Vegfba Knockdown Zebrafish.
VEGF-B in mammals such as humans and mice has been described as a modulator of angiogenesis under various physiological and pathological conditions (28, 29). To study vascular defects, the Fli1a:EGFP transgenic zebrafish strain was used for morpholino injections. Intriguingly, the Vegfba-ATG-morpholino and Vegfba-Ex2-morpholino targeting the Vegfba gene produced nearly identical phenotypes of vascular impairment, which showed reduced vascularization in the head region of developing embryos (Fig. 1 B and C). A substantial number of zebrafish embryos completely lacked visible middle cerebral vessels (MCVs), whereas most others exhibited defective development of MCVs (Fig. 1 B and C). Additionally, development of peripheral vessels including intersegmental vessels was also impaired in most Vegfba morpholino-injected embryos (Fig. 1 B and C). In contrast, vasculatures in Vegfbb and scrambled morpholino-injected embryos developed normally and lacked obvious phenotypes. These findings show that inhibition of VEGF-B production in zebrafish embryos significantly impairs vascular development in the brain.
Defective VEGF-B Expression Induces Apoptosis and Hemorrhages in the Brain.
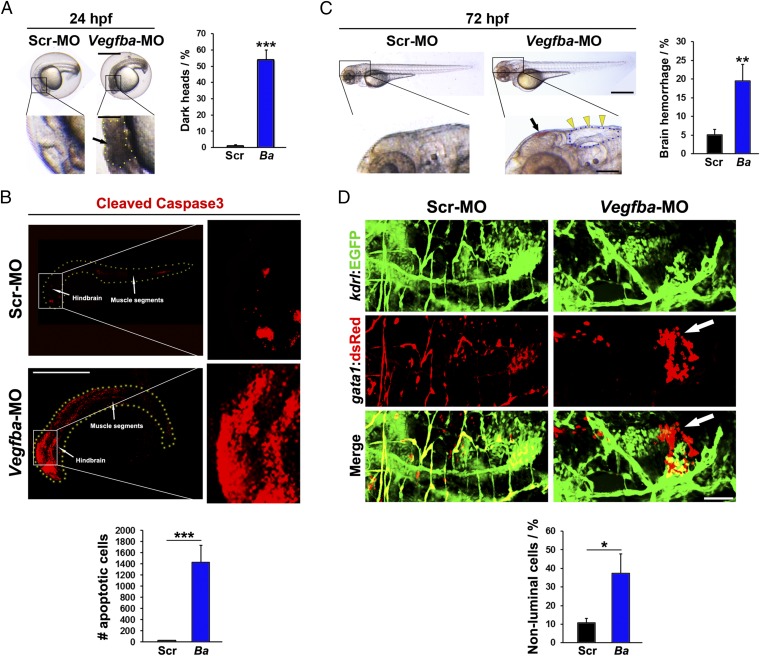
For convenience, we focused the rest of our experimental settings on the Vegfba-ATG-morpholino because the Vegfba-ATG-morpholino and Vegfba-Ex2-morpholino produce nearly identical phenotypes. Gross examination of the Vegfba-ATG-morpholino–treated embryos at 24 hpf showed the presence of a dead area in the brain (Fig. 2A). In fact, more than 50% of the zebrafish embryos exhibited this type of degenerative phenotype in their brains (Fig. 2A). To define cellular apoptosis in zebrafish brains, we stained zebrafish embryos with an antibody against the activated or cleaved form of caspase-3. Consistent with the gross morphological changes, the Vegfba-ATG-morpholino–injected embryos showed tremendously large apoptotic areas in their brains (Fig. 2B). Additionally, the spinal cord proximal to the hindbrain also underwent apoptosis (Fig. 2B). In contrast, injection of scrambled morpholino into zebrafish embryos did not cause overt apoptosis (Fig. 2B).
Fig. 2.
Brain cell death and hemorrhage of VEGF-B–deficient zebrafish embryos. (A) Micrographs of embryos injected with 0.6 pmol Scrambled or Vegfba morpholino; embryos were imaged at 24 hpf. (Scale bar, 200 μm; bar in amplified picture, 50 μm.) Black arrow points to the brain area with dead cells, and dotted line encircles the dead area. Quantification of the percentage of embryos with obvious dead areas in the brain at 24 hpf (average n = 229 embryos/group). ***P < 0.001. (B) Confocal micrographs of embryos injected with 0.6 pmol Scrambled or Vegfba morpholino and stained for cleaved caspase3 activity at 24 hpf. (Scale bar, 500 μm.) Quantification of numbers of cleaved caspase3-positive cells (n = 18–21 embryos/group). ***P < 0.001. (C) Micrographs of embryos injected with 0.6 pmol Scrambled or Vegfba morpholino; embryos were imaged at 72 hpf. (Scale bar, 200 μm; in amplified picture, 50 μm.) Yellow arrowheads point to cerebral edema, and dotted line encircles the edema area. Black arrow points to brain hemorrhage. Quantification of the percentage of embryos with brain hemorrhages at 72 hpf (n = 222–295 embryos/group). **P < 0.01. (D) Confocal micrographs of Tg(Kdrl:egfp;Gata1:DsRed) embryos injected with 0.6 pmol Scrambled or Vegfba morpholino; the injected embryos were imaged at 72 hpf. (Scale bar, 50 μm.) White arrows point to hemorrhagic erythrocytes. Quantification of ratios between extravasated erythrocytes versus the total erythrocyte signals (n = 6 embryos/group). *P < 0.05.
For those few zebrafish embryos that survived beyond the 24 hpf stage, the embryos exhibited a hemorrhagic phenotype (Fig. 2C). To validate the hemorrhagic phenotype, we crossed the Fli1:EGFP strain with a Gata1:DsRed strain that labeled erythrocytes with DsRed (30). Expectedly, large hemorrhagic areas were detected in the head region of the Vegfba-ATG-morpholino–injected embryos, but not in scrambled morpholino-injected embryos (Fig. 2D). Large numbers of Ds-Red–labeled erythrocytes often formed clusters that resided outside of the lumen areas of blood vessels. Taken together, knockdown VEGF-B expression in zebrafish embryos induces massive apoptosis and hemorrhages in the brain of zebrafish embryos. Therefore, VEGF-B is essentially required for embryonic development in zebrafish.
Differential Vascular Defects by Knocking Down Vegfb and Vegfa.
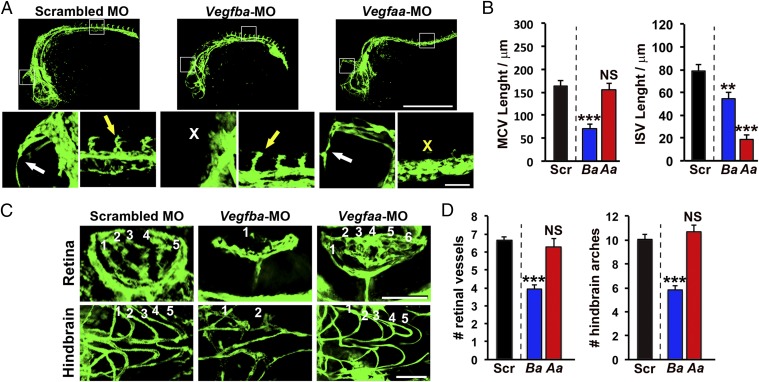
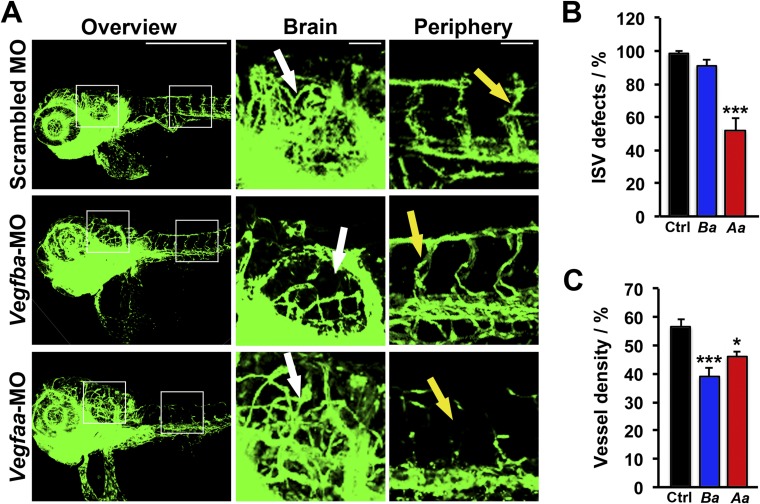
We next compared VEGF-B deficiency-induced defective vascular phenotypes with the VEGF-A deficiency-induced vascular defects. Interestingly, knockdown of Vegfba and Vegfaa by their specific morpholinos produced different patterns of defective vascular development. In Vegfba-deficient zebrafish embryos, the development of MCVs was completely abrogated (Fig. 3 A and B). In contrast, MCV development in Vegfaa-deficient zebrafish embryos was not affected compared with that of zebrafish embryos receiving the scrambled morpholino (Fig. 3 A and B). Conversely, Vegfaa-deficient zebrafish embryos showed severe impairment of intersegmental vessel (ISV) development, whereas Vegfba-deficient zebrafish embryos exhibited only modest defective phenotypes of peripheral vasculatures including ISVs (Fig. 3 A and B and Fig. S2 A and B). Thus, VEGF-B deficiency-triggered vascular defects are generated through a VEGF-A–independent mechanism.
Fig. 3.
Distinct vascular defective phenotypes in VEGF-B and VEGF-A–deficient zebrafish embryos. (A) Confocal micrographs of 24-hpf fli1a:EGFP embryos injected with 0.6 pmol of Scrambled, Vegfba, or Vegfaa morpholinos. White arrows indicate the MCV, and the white X indicates the position where the MCV is missing. Yellow arrows indicate the ISVs, and the yellow X indicates the position where ISVs are missing. (Scale bar, 500 μm; bar in the amplified picture, 50 μm.) (B) Quantifications of MCV or ISV lengths in 24-hpf embryos injected with 0.6 pmol of Scrambled (Ctrl), Vegfba (Ba), or Vegfaa (Aa) morpholinos (n = 8–24 embryos/group). NS, nonsignificant. **P < 0.01; ***P < 0.001. (C) Confocal micrographs of the retina or hindbrain regions in 72-hpf fli1a:EGFP embryos injected with 0.6 pmol of Scrambled, Vegfba, or Vegfaa morpholinos. Sequential numbers indicate the number of retinal vessels or the number of hindbrain arches. (Scale bar, 50 μm.) (D) Quantification of retinal vessel and hindbrain arch numbers in 72-hpf embryos injected with 0.6 pmol of Scrambled (Ctrl), Vegfba (Ba), or Vegfaa (Aa) morpholinos (n = 7–24 embryos/group). NS, nonsignificant; ***P < 0.001.
Fig. S2.
Differential effects of VEGF-Ba and VEGF-Aa on developmental angiogenesis in zebrafish embryos. (A) Confocal micrographs of 72-hpf fli1a:EGFP embryos injected with 0.6 pmol scrambled, Vegfba, and Vegfaa morpholinos. White arrows indicate brain vasculatures. Yellow arrows indicate ISVs. (Scale bar in overview, 500 μm; in brain and periphery, 50 μm.) (B) Quantification of the percentage of embryos with defective ISVs in 72-hpf fli1a:EGFP embryos injected with 0.6 pmol scrambled (Ctrl), Vegfba (Ba), or Vegfaa (Aa) morpholino (n = 7–14 embryos/group). ***P < 0.001. (C) Quantification of brain vessel density of 72-hpf fli1a:EGFP embryos injected with 0.6 pmol scrambled (Ctrl), Vegfba (Ba), and Vegfaa (Aa) morpholinos (n = 8–18 embryos/group). *P < 0.05; ***P < 0.001.
Additionally, knockdown of Vegfba in zebrafish embryos also caused severe impairment of hyaloid vessel development in the retina (Fig. 3 C and D). In fact, hyaloid vessel development in the Vegfba morpholino-injected zebrafish embryos was almost completely prevented. Similarly, vascular development in the hind- and midbrain of the Vegfba morpholino-injected zebrafish embryos also showed severe defective phenotypes with only incomplete development of the vascular networks (Fig. 3 C and D and Fig. S2 A and C). In contrast to Vegfba morpholino-injected zebrafish embryos, knockdown of Vegfaa in zebrafish embryos did not produce overt vascular defects in hyaloid and hind- or midbrain vasculatures (Fig. 3 C and D and Fig. S2 A and C). Taken together, these findings show that down-regulation of VEGF-A and VEGF-B expression in zebrafish embryos produced nonoverlapping vascular defective phenotypes.
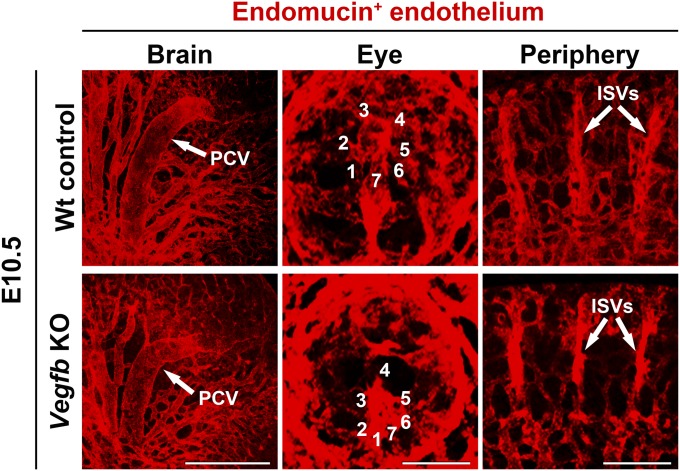
To study the role of VEGF-B in vascular development in mice, the Vegfb gene was genetically deleted and the vasculatures were compared with wild-type stage-matched controls. Analyses of cerebral vessels in the brain, hyaloid vessel development in the eye, and intersegemental vessel development between the somites did not reveal any vascular abnormalities at the embryonic day 10.5 stage of embryonic development (Fig. S3), which corresponded to the stage of zebrafish embryos in our study. Similarly, no vascular abnormalities were detected at other stages of Vegfb-deficient mice compared with wild-type stage-matched controls. These findings show that deletion of the Vegfb gene in mice does not affect vascular development in the brain, eye, and peripheral tissues, which are consistent with previous reports (18, 19, 22). Thus, there are major functional differences of VEGF-B in zebrafish and mice.
Fig. S3.
Vascular development in WT and Vegfb-deficient mice. Confocal micrographs of developing vasculatures in the brain, eye, and somites. PCV, posterior cerebral vessel. Positions of vascular branches from the central hyaloid vessels are numbered and correspond to hyaloid arterial branches in developing zebrafish embryos. (Scale bar in the brain, 500 μm; in the eye, 100 μm; in the periphery, 200 μm.)
Spatiotemporal Expression of Vegfaa and Vegfba in Zebrafish Embryos.
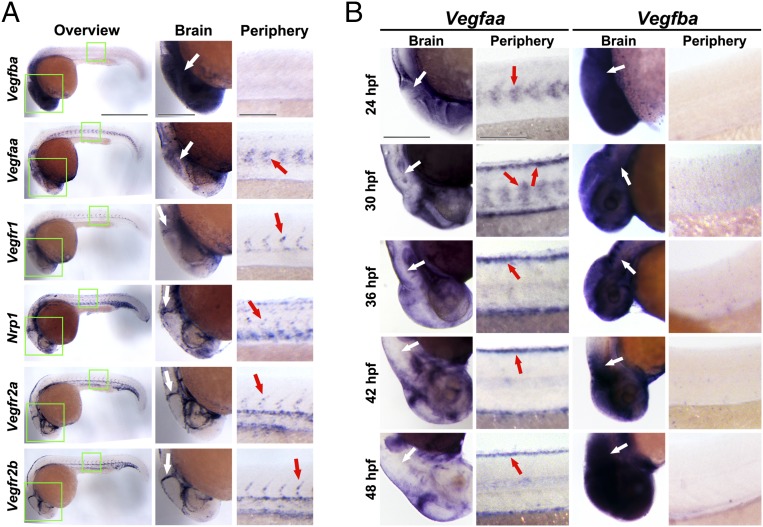
To study the mechanisms underlying VEGF-A– and VEGF-B–mediated differential functions in regulation of vascular development, we performed in situ hybridization to localize Vegfaa and Vegfba mRNA expression patterns. At 24 hpf, Vegfba mRNA was prominently expressed in the brain of developing embryos and nearly undetectable levels were found in the trunk region (Fig. 4 A and B). In contrast, Vegfaa mRNA levels were modestly expressed in the brain at this stage of embryonic development. However, Vegfaa mRNA was expressed to a relatively high level in somites (Fig. 4 A and B). These findings were consistent with the functional phenotype arising from morpholino-mediated knockdown of Vegfaa during this stage, where deficient embryos completely lacked ISVs (Fig. 3). Surprisingly, Vegfaa mRNA in somites showed only a transient expression pattern at 24 hpf and became progressively decreased during embryonic development (Fig. 4B). At 36 hpf and onward, Vegfaa mRNA was virtually undetectable in somites (Fig. 4B). The time-course–related expression patterns of Vegfaa mRNA in developing zebrafish embryos were consistent with ISV development because ISVs reached the maximal length at ∼30 hpf (24). Similar to the peripheral expression pattern, Vegfaa mRNA levels in the brain were also progressively decreased during embryonic development (Fig. 4B).
Fig. 4.
mRNA expression levels of Vegfa, Vegfb, and Vegfrs in developing zebrafish embryos. (A) In situ hybridization micrographs of Vegfba, Vegfaa, Vegfr1, Nrp1, Vegfr2a, or Vegfr2b expression in 24-hpf zebrafish embryos. In situ positive signals are in dark blue. White arrows indicate MCVs (Vegfr1, Nrp1, Vegfr2a, and Vegfr2b images) or the MCV area (Vegfba and Vegfaa images). Red arrows indicate somites (Vegfaa) or ISVs. (Scale bar in overview: 500 μm; in brain: 200 μm; in periphery: 100 μm.) (B) In situ hybridization micrographs of Vegfba or Vegfaa expression in the brain or peripheral trunk regions of zebrafish embryos at 24, 30, 36, 42, or 48 hpf. In situ positive signals are in dark blue. White arrows indicate the MCV area. Red arrows indicate the peripheral expression-pattern of Vegfaa mRNA. (Scale bars in the brain, 200 μm; in periphery, 100 μm.)
We next studied mRNA expression levels of VEGF-B and VEGF-A receptors in developing embryos. Vegfr1 expression levels in the brain and in the peripheral trunk of 24-hpf–developing embryos were modest (Fig. 4A). In contrast to Vegfr1, Neuropilin1 (Nrp1) mRNA was expressed to high levels in the brain and peripheral vasculatures (Fig. 4A). The expression pattern of Nrp1 mRNA supports its vascular functions, as blood vessels appeared to be the primary structures that showed positive signals. Notably, the distribution pattern of Nrp1 mRNA in the brain matches the defective phenotype of Vegfba knockdown phenotype because high expression levels of Vegfba were found in the region where MCVs develop between 24 and 36 hpf (Fig. 4 A and B). Interestingly, Vegfr2a and Vegfr2b were also expressed in the brain and peripheral vessels (Fig. 4A) although these receptors do not bind to VEGF-B. Taken together, these localization studies provide potential structural bases for the VEGF-B knockdown-related vascular defects.
Knockdown of NRP1 Reproduces VEGF-B Deficiency-Related Vascular Phenotypes.
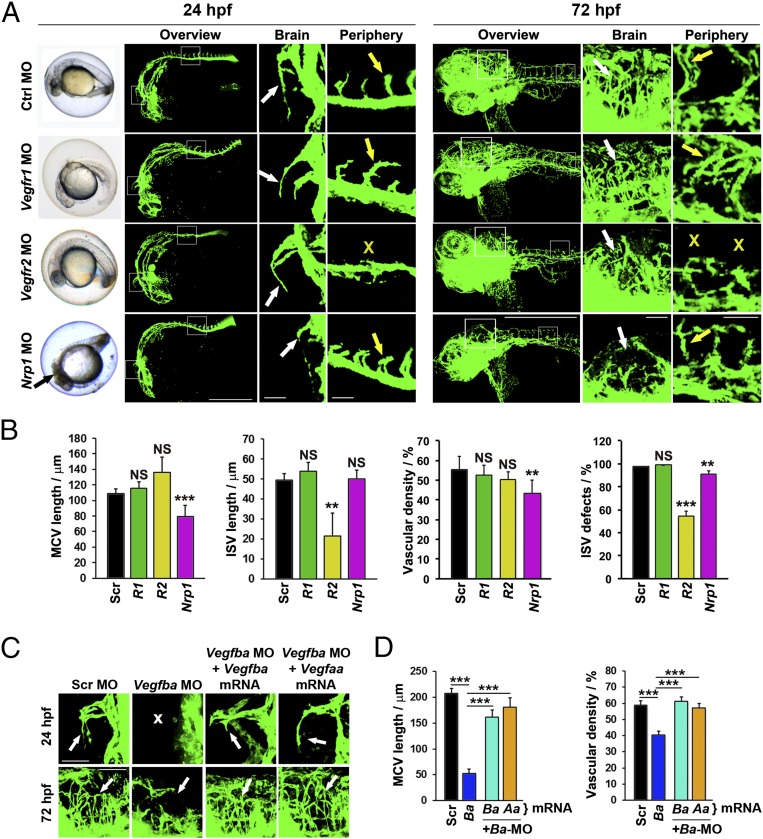
To functionally study VEGFRs and NRPs in mediating VEGF-B–induced vascular functions, specific morpholinos against Vegfr1, Vegfr2, and Nrp1 were administered to zebrafish embryos. Despite being an important VEGF-B–binding receptor, knockdown of Vegfr1 in zebrafish embryos by a specific morpholino did not produce vascular defects in the brain or in peripheral regions (Fig. 5 A and B), indicating that VEGFR1 is not primarily responsible for VEGF-B deficiency-related phenotypes. Unlike VEGFR1, knockdown of Vegfr2 produced a vascular defective phenotype that was similar to Vegfaa knockdown-related phenotypes showing severe impairments of ISV development (Fig. 5 A and B). However, knockdown of Vegfr2 did not produce overt vascular defects in the brain, and MCV development was not affected (Fig. 5A). Perhaps the VEGFR2 knockdown-related vascular phenotypes are expectedly different from VEGF-B deficiency because this receptor does not bind VEGF-B. Interestingly, knockdown of Nrp1 virtually reproduced the vascular phenotype of Vegfba knockdown-related vascular defects (Fig. 5 A and B), showing severe impairment of brain vasculature development. Development of MCVs was significantly impaired in Nrp1-knockdown zebrafish embryos. Despite these vascular defects in the brain, development of ISVs was not altered in Nrp1-knockdown zebrafish embryos. The brain vascular defects of Nrp1 knockdown in principle imitate the vascular defects seen in VEGF-B–knockdown zebrafish embryos. These findings suggest that NRP1 is likely the receptor that mediates VEGF-B–related vascular functions in the brain.
Fig. 5.
VEGFR deficiency-associated vascular phenotypes and functional rescue by Vegfba or Vegfaa mRNAs. (A) Bright-field and confocal micrographs of 24- and 72-hpf fli1a:EGFP zebrafish embryos injected with 0.6 pmol of Scrambled, Vegfr1, Vegfr2, or Nrp1 morpholinos. Black arrow indicates zebrafish brain with dead cells. White arrows indicate 24-hpf MCVs and the 72-hpf brain vasculatures. Yellow arrows indicate ISVs. Yellow X symbols indicate absence of ISVs. (Scale bars in overviews, 500 μm; in brain and periphery, 50 μm.) (B) Quantifications of 24-hpf MCV length, ISV length, 72-hpf brain vascular density, and percentage of defective ISVs after treatment with 0.6 pmol of Scrambled (Ctrl), Vegfr1 (R1), Vegfr2 (R2), or Nrp1 morpholinos (average of n = 14 embryos/group). NS, nonsignificant. **P < 0.01, ***P < 0.001. (C) Confocal images of 24- and 72-hpf fli1a:EGFP embryos injected with 0.6 pmol of Scrambled or Vegfba morpholinos alone or in combinations with 100 ng Vegfba or Vegfaa mRNAs. White arrows indicate 24- MCVs and 72-hpf brain vasculatures. White X indicates absence of the MCV. (Scale bars, 50 μm.) (D) Quantification of 24-hpf MCV length and 72-hpf brain vascular density of fli1a:EGFP embryos injected with 0.6 pmol of Scrambled (Ctrl) or Vegfba (Ba) morpholinos alone or in combinations with 100 ng Vegfba or Vegfaa (Aa) mRNAs (average of n = 16 embryos/group). ***P < 0.001.
VEGF-Aa Rescues VEGF-B Deficiency-Related Vascular Phenotypes.
We next performed gain-of-function experiments to rescue VEGF-B deficiency-related vascular defects. In the Vegfba morpholino-injected zebrafish embryos, we injected excessive amounts of zebrafish Vegfba or Vegfaa mRNAs. Expectedly, injection of the excessive amount of Vegfba mRNA in Vegfba morpholino-treated embryos largely rescued the defective development of MCVs and vasculatures in the brain (Fig. 5 C and D). Interestingly, injection of Vegfaa mRNA also significantly rescued Vegfba morpholino-induced brain vascular defects (Fig. 5 C and D). These findings show that VEGF-A can completely rescue VEGF-B deficiency-related vascular defects. Given the fact that VEGF-A binds to NRP1, rescuing VEGF-B deficiency-related vascular defects by VEGF-A is probably not surprising.
Zebrafish VEGF-B Binds to Zebrafish NRP1.
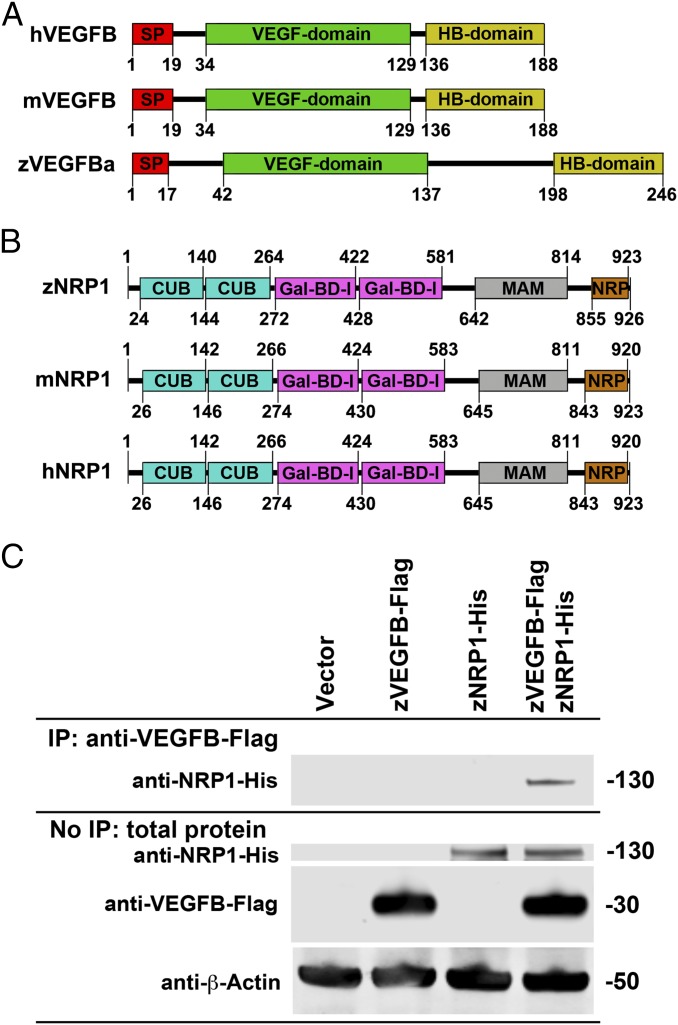
To provide conclusive evidence of an interaction between zebrafish VEGF-B (zVEGF-B) and zebrafish NRP1 (zNRP1), we performed coimmunoprecipitation experiments using Flag-tagged zVEGF-B and His-tagged NRP1 recombinant proteins. First, we analyzed the protein sequence structures of zVEGF-B, mouse VEGF-B (mVEGF-B), and human VEGF-B (hVEGF-B) and compared the structural similarities among these species. Similar to hVEGF-B and mVEGF-B, the primary zVEGF-B amino acid sequence also contains a heparin-binding domain enriched in positively charged amino acids (Fig. 6A). In this heparin-binding domain, an extremely high cluster of positively charged amino acids, including lysine and arginine residues of mVEGF-B or hVEGF-B, have been claimed to be the motif that binds to NRP1. Thus, the primary amino acid sequence of zVEGF-B exhibits NRP1-binding potential. Likewise, zebrafish NRP1 (zNRP1) also shares high sequence similarities with mouse NRP1 (mNRP1) and human NRP1 (hNRP1) (Fig. 6B). Consistent with structural predictions, immunoprecipitation of zVEGF-B-Flag by a specific anti-Flag antibody also coprecipitated zNRP1 (Fig. 6C). These findings provide compelling evidence that zVEGF-B physically binds to zNRP1 and thus provide a convincing structural basis for the observed overlapping phenotypes in Vegfb- and Nrp1-deficient zebrafish embryos.
Fig. 6.
Binding of zebrafish VEGF-B to zebrafish NRP1. (A) Primary structures of VEGF-B proteins in human (hVEGF-B), mouse (mVEGF-B), and zebrafish (zVEGF-Ba) based on Ensembl classifications of protein domains. Numbers indicate the amino acid residues in their corresponding proteins. SP, signal peptide; HB, heparin-binding. (B) Primary structures of NRP1 proteins in human (hNRP1), mouse (mNRP1), and zebrafish (zNRP1) based on Ensembl classifications of protein domains. Numbers indicate the amino acid residues in their corresponding proteins. CUB, complement C1r/C1s, Uegf and Bmp domain; Gal-BD-l, Gal-binding domain-like; MAM, mephrin, A-5 protein, mu domain; NRP, neuropilin C-terminal domain. (C) Immunoblots of SDS/PAGE-separated proteins derived from cells transfected with empty (vector), zVegfb:flag (zVEGF-B-Flag), zNrp1:his (zNRP1-His), or both zVegfb:flag- and zNrp1:his-containing vectors. (Top) Protein samples that were first immunoprecipitated with an anti-Flag antibody, and the immunoprecipitated complexes were SDS/PAGE-separated and blotted with the anti–NRP1-his antibody. The numbers on the right indicate molecular weights.
Exposure of Zebrafish Embryos to Hypoxia Rescues Vegfb Morpholino-Induced Vascular Defects.
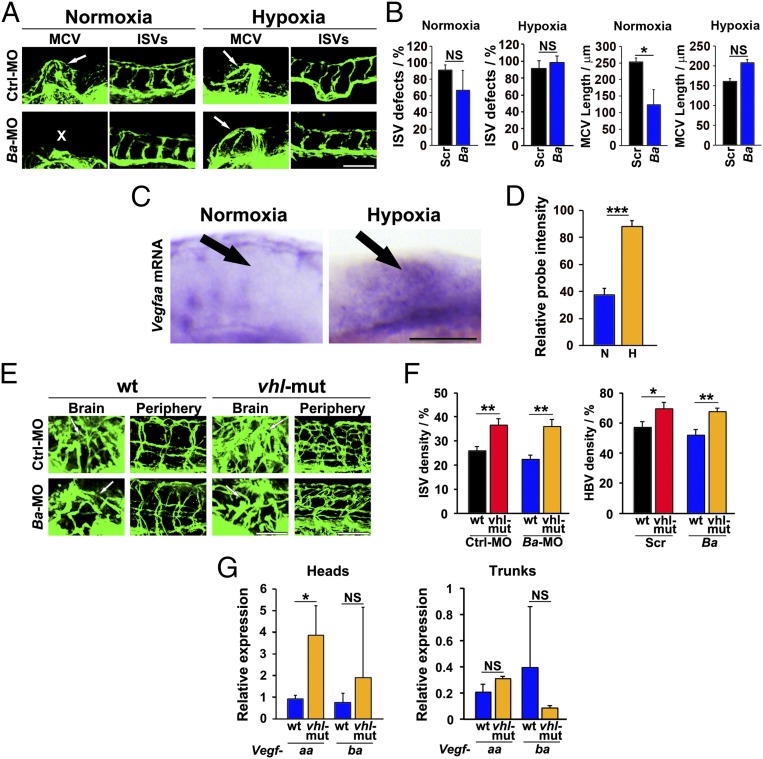
We next addressed an important issue of the spatiotemporal relation between VEGF-B and VEGF-A in vascular development in different regions of zebrafish embryos. It is known that tissue hypoxia is one of the primary driving forces for VEGF-A expression (23, 31). Thus, we hypothesized that exposure of zebrafish embryos to a hypoxic environment would increase VEGF-A expression and that hypoxia-induced VEGF-A expression would potentially display a redundant function to that of VEGF-B in Vegfb morpholino-treated zebrafish embryos. Expectedly, exposure of the Vegfb morpholino-treated zebrafish embryos to hypoxic water completely rescued the vascular defects of the MCVs (Fig. 7 A and B). Unlike MCVs, hypoxia did not significantly affect the development of peripheral ISVs in Vegfb morpholino-treated zebrafish embryos compared with scrambled morpholino-treated zebrafish embryos (Fig. 7 A and B). These findings show that hypoxia rescues the Vegfb-induced brain vascular defects in developing zebrafish embryos.
Fig. 7.
Hypoxia rescues VEGF-B deficiency-induced vascular defects in zebrafish embryos. (A) Confocal micrographs of 36-hpf stage Fli1a:EGFP zebrafish embryos injected with 0.6 pmol Scrambled (Ctrl) and Vegfba (Ba) morpholinos. The injected embryos were subjected to an E3 medium at normal oxygen tension (normoxia) or to a hypoxic environment where the oxygen tension was reduced to 1.5% (hypoxia). White arrows indicate the MCVs. White X indicates the absence of the MCV. (Scale bar, 100 μm.) (B) Quantification of ISV defects and MCV lengths at the 36-hpf stage of Fli1a:EGFP zebrafish embryos injected with 0.6 pmol Scrambled (Ctrl) or Vegfba (Ba) morpholinos. The injected embryos were subjected to an E3 medium under normoxia or hypoxia conditions (n = 11–21 embryos/group). NS, nonsignificant. *P < 0.05. (C) Detection of Vegfaa mRNA by in situ hybridization of 36-hpf stage embryos exposed to an E3 medium under normoxia or hypoxia. Black arrows indicate the MCV area. (Scale bar, 100 μm.) (D) Quantification of in situ hybridization positive signals under normoxia (N) and hypoxia (H) conditions (n = 35 embryos/group). ***P < 0.001. (E) Confocal micrographs of Fli1a:EGFP-wt (wt) or Fli1a:EGFP-vhl-mutant (vhl-mut) 120-hpf zebrafish embryos injected with 0.6 pmol scrambled (Ctrl) or Vegfba (Ba) morpholinos. White arrows indicate the brain vasculatures. (Scale bar in , 50 μm; bar in periphery, 100 μm.) (F) Quantification of ISV and brain vascular (HBV) density in Fli1a:EGFP-wt (wt) or Fli1a:EGFP-vhl-mutant (vhl-mut) 120-hpf zebrafish embryos injected with 0.6 pmol scrambled (Ctrl) or Vegfba (Ba) morpholinos (n = 9–17 embryos/group). *P < 0.05; **P < 0.01. (G) qPCR analysis of the relative expression of Vegfaa or Vegfba mRNA levels in heads and trunks of 120-hpf Fli1a:EGFP-wt (wt) or Fli1a:EGFP-vhl-mutant (vhl-mut) zebrafish embryos (60–150 embryos/group). NS, nonsignificant. *P < 0.05.
Consistent with phenotypic rescues, in situ hybridization showed that Vegfaa mRNA expression levels in the brain were markedly increased in hypoxia-exposed zebrafish embryos (Fig. 7 C and D). It should be emphasized that the level of Vegfaa mRNA in zebrafish embryos under a normoxic condition was barely detectable in the hind- and midbrain between 24 and 48 hpf (Fig. 4). These findings also support the fact that hypoxia potently induces VEGF-A expression in zebrafish as previously described (25–27, 32).
Genetic Inactivation of von Hippel-Lindau in Zebrafish Embryos Rescues Vegfb Morpholino-Induced Vascular Defects.
In addition to hypoxia, we performed Vegfb morpholino treatment in von Hippel-Lindau (Vhl)-inactivated zebrafish embryos in which the VHL-directed HIF-1α degradation pathway is impaired (33). Interestingly, Vegfb morpholino-induced vascular defects of MCVs and brain vessels were completely rescued, and no obvious vascular defects could be found in Vhl-inactivated zebrafish embryos (Fig. 7 E and F). Again, development of peripheral ISVs was not significantly affected by Vegfb knockdown, albeit a slight increase of ISV density in Vhl-inactivated zebrafish embryos could be observed following 96 hpf. These findings are consistent with other findings that VHL targets HIF-1α to a degradation pathway, leading to reduced VEGF expression.
We next quantified Vegfaa mRNA expression levels in wild type and Vhl-inactivated zebrafish embryos. Interestingly, brain Vegfaa mRNA levels of Vhl-inactivated zebrafish embryos were significantly higher than those in wild-type embryos (Fig. 7G). In contrast, Vegfba expression levels were not affected in Vhl-inactivated zebrafish embryos (Fig. 7G). These data provide further supportive evidence that VEGF-A could compensate the VEGF-B deficiency-induced vascular defects in developing zebrafish embryos.
Discussion
Formation of a functional vascular network in developing embryos requires an intimate coordination between various signaling pathways that modulate vessel growth, remodeling, and maturation. VEGF-A plays a nonredundant function in the initial formation of vascular progenitor cells and subsequent establishment of the entire vascular network during embryonic development in mice (15, 16). Deletion of only one allele of the Vegfa gene in mice leads to early embryonic lethality owing to lack of vascular and hematopoietic systems, a haploinsufficiency phenotype that is rarely seen in genetic deletion animal models. These findings indicate that optimal expression levels of VEGF-A are essential to ensure accurate development of the vascular system. Unlike mice, zebrafish embryonic development occurs in an in vitro environment, which is independent from maternal supply of nutrients and oxygen. Our present study provides, to our knowledge, one of the first examples to demonstrate that the hypoxia-associated environmental changes in embryonic tissues control their development.
The environment surrounding embryos may play a pivotal role in determination of signaling pathways for vascular development. In rodents and humans, embryos develop in a relative hypoxic environment. Hypoxia is one of the most potent driving forces for up-regulation of VEGF expression (23, 31). As a result, infant prematurity owing to hyperoxia can cause severe vascular impairments, leading to defective tissue and organ development (34). By contrast, zebrafish embryos are exposed to the same oxygenated environment as the adult zebrafish. Oxygen from the surrounding water is present in sufficient amounts to keep all tissues normoxic during embryonic development. The hypoxia–VEGF-A expression axis is unlikely to be as pronounced as seen in mice. Therefore, other factors, especially nonhypoxia-responsive angiogenic factors, may play a crucial role in ensuring vascular development. Our localization data show that VEGF-A is only modestly expressed in the brain of zebrafish embryos at the early stage of development. The expression levels of VEGF-A were further decreased during embryonic development. Consistent with modest expression levels, knockdown of VEGF-A did not result in an overt vascular defect in the brain, but rather in severe defects of peripheral vasculatures. Collectively, these findings show that VEGF-A is not the primary factor responsible for development of vasculatures in the brain of zebrafish embryos.
One of our surprising findings is that VEGF-B is expressed at extremely high levels in the brain of developing embryos. VEGF-B, as a VEGFR1-binding ligand, lacks obvious biological functions in mouse-based experimental models (18, 19, 22). Genetic deletion of the Vegfb gene in mice does not result in any obvious vascular phenotype, and Vegfb-null mice develop normally. Despite this fact, it is plausible that functional defects of the VEGF-B–mediated vascular functions are redundantly replaced by VEGF-A. This is probably particularly true in the brain as both factors are expressed at high levels in the mouse brain. In zebrafish embryos, however, VEGF-A is not prominently expressed in the brain, probably owing to the lack of hypoxia-induced activation at the Vegfa promoter; functional knockdown of Vegfb in the absence of VEGF-A would produce a defective phenotype of vascular development. If so, exposure of zebrafish embryos to a hypoxic environment would rescue Vegfb knockdown-related vascular phenotypes in the brain due to induction of VEGF-A expression. In this study, we provide compelling evidence that exposure of zebrafish embryos to hypoxia induces brain VEGF-A expression that rescues Vegfb knockdown-related vascular defects, particularly the MCV and brain microvascular developmental defects. These findings provide previously unidentified mechanistic insights on spatiotemporal relations between VEGF-A and VEGF-B in controlling embryonic vessel development. Tissue hypoxia is the key determinant to functionally switch these two factors as redundant angiogenic signals. In zebrafish, and perhaps in other species that use in vitro fertilization as a mechanism of embryonic development, such a hypoxia-operated VEGF-A expression mechanism is lacking, and interference with VEGF-B expression would lead to severe impairments of vascular development in regions where VEGF-B and VEGF-A are not redundantly expressed. Therefore, our data provide a previously unidentified mechanistic explanation of functional differences of the same protein such as VEGF-B between mammals and zebrafish.
Despite VEGFR1 being the key receptor of VEGF-B, knockdown of Vegfr1 does not produce overt vascular defects, indicating that VEGFR1 is not primarily involved in VEGF-B–mediated vascular functions. This finding is in general agreement with other findings in mammals, supporting the fact that VEGFR1 is a potential decoy or negative receptor for angiogenesis (4). What, then, would be the receptor signaling that mediates VEGF-B–induced angiogenesis? We show that NRP1 is expressed at a high level in the brain and that its expression pattern is comparable to VEGF-B. Notably, knockdown of Nrp1 in zebrafish embryos produced a nearly identical vascular phenotype as seen in Vegfb knockdown zebrafish embryos. We provide further evidence that zebrafish VEGF-B physically binds to NRP1. NRP1 acts as a guidance factor for nerve and vascular development, and members of the VEGF family including VEGF-A and VEGF-B, are important ligands for NRP-1. Unlike VEGFRs, the signaling events mediated by VEGF-B-NRP1 in zebrafish remain uncharacterized. However, it is known that the VEGF-B-NRP1–signaling pathway plays a pivotal role in other physiophathological situations in rodents (35). It is highly plausible that VEGF-B-NRP1 signaling also transduces active signals in neuronal cells as seen in vascular endothelial cells. In the light of the important functions of VEGF-NRP1–signaling mouse models (36–38), we reasonably speculate that the neurotrophic effect of VEGF-B-NRP1 on neuronal cells would be also impaired in response to morpholino-mediated inhibition. Thus, it is possible that both vascular defects and neuronal impairment contribute to impaired brain development and lethality. These speculations warrant future experimental validation using genetic models to ablate NRP1 in specific cell types.
Taken together, our present data provide previously unidentified mechanistic insights on VEGF-B–mediated cerebral functions in developing zebrafish embryos through modulation of vascular development. These findings provide one of the few examples in which inactivation of a growth factor results in dissimilar vascular phenotypes in different species. The mechanism underlying phenotypic differences between zebrafish and mice involves the lack of hypoxia-associated compensatory and redundant mechanisms in zebrafish embryos. Although our present findings are confined to zebrafish embryos, the VEGF-B–mediated vascular functions in relation to tissue hypoxia may potentially be extended to pathological conditions in humans. For example, VEGF-B may significantly contribute to development of retinopathy of prematurity in human infants and in solid cancers where hypoxia in the tumor microenvironment undergoes constant changes. Another extended clinical implication of our findings is that VEGF-B might play a role in brain tumor development and invasion as seen in other systems (39–41). These interesting clinically related issues coupled to VEGF-B warrant future investigation.
Materials and Methods
Zebrafish Strains and Husbandry.
Zebrafish embryos and larvae of the Tg(fli1a:EGFP)y1, Tg(fli1a:EGFP)y1;vhlhu2081/hu2081, and Tg(fli1a:EGFP)y1;(gata1a:dsRed)sd2 strains (all from ZIRC) were produced by natural mating at the Department of Medical Biochemistry and Biophysics, Karolinska Institute (Stockholm) or at the Linköping University (Linköping, Sweden), according to our standard methods as previously described (24). Fertilized embryos were maintained in E3 medium at 28.5 °C and carefully staged before analyses according to previously established guidelines (24, 42). All experiments were performed according to experimental guidelines approved by the North Stockholm and Linköping Experimental Animal Ethical Committees, Sweden.
Identification of Zebrafish Vegfb Gene.
Zebrafish Vegfb exists in two isoforms, Vegfba and Vegfbb, located in chromosomes 14 and 21, respectively. Zebrafish Vegfba is not currently mapped, but a blast against the human VEGF-B protein sequence revealed that the entry PREDICTED: VEGFA-like isoform ×1 (Danio rerio) was the most similar non-VEGFA protein in zebrafish. We found that this predicted protein mapped to a gene (LOC101885552 or BX511266.1) of high homology to the gene structure of human Vegfb. This same Vegfba gene was also previously studied by others (43) and contains in its ORF the gene CO353295 (DR_ATE_FL13_ D11 adult testis full-length Danio rerio cDNA, mRNA), which is annotated as vegfba by ZFIN. The zebrafish Vegfbb gene was previously mapped (accession no. ENSDART:00000144627). The Ensembl Genome Browser was used to predict the gene and protein structures in different species.
In Vitro Generation of mRNAs Coding for Vegfaa and Vegfba.
Capped mRNAs were produced by the mMessage/Machine Kit (Ambion), according to the manufacturer’s instructions. A plasmid clone used as a template for generating full-length Vegfaa mRNA was purchased from Source BioScience (Table S1). The clone was sequenced for verification. The sequence coding for zebrafish Vegfba was cloned from the full-length cDNA into a pCS2+ vector. The primers were the following: forward primer—5′-cacacaGAATTCATGGGGATCAAGCTGAGGG-3′; and reverse primer—5′-cacacaGAATTCATGAGCACCCTTTGCAAGC-3′. EcoR1 and Xho1 restriction sites were designed on the flanking regions. A myc-tagged version of zebrafish Vegfba mRNA was used to determine knockdown efficiency as previously described (24). The full-length zebrafish Vegfba-coding sequence was subcloned into a pCS2-Myc vector using the following primers: forward primer—5′-cacacaGAATTCATGGGGATCAAGCTGAGGG-3′; and reverse—5′-cacacaCTCGAGTTCACTTTGGGTTTCACATC-3′. Capped mRNAs were dissolved in nuclease-free water and injected at a concentration of 100 pg/nL.
Table S1.
Designation of various gene clones
| Gene | Refseq | IMAGE clone |
| Nrp1 | NM_205674 | 3717155 |
| Vegfr1 | NM_001014829 | 7046937 |
| Vegfr2a | NM_131472 | 7913248 |
| Vegfr2b | NM_001024653 | 6911239 |
| Vegfaa | AF016244 | 9037521 |
Morpholino and mRNA Treatment.
Each zebrafish embryo at the one-cell stage was injected in the yolk with 1 nL morpholino at a concentration of 0.6 mM, 1 nL of mRNA at a concentration of 100 ng/nL, or a combination of morpholino and mRNA. All nucleotides were dissolved in nuclease-free water. Morpholinos were purchased from Gene Tools. Morpholinos included the following: Vegfaa MO (5′-GTATCAAATAAACAACCAAGTTCAT-3′); Vegfab MO (5′-GGAGCACGCGAACAGCAAAGTTCAT-3′); Vegfba-ATG MO (5′-TAACCCTCAGCTTGATCCCCATTGT-3′); Vegfba-e2i2 MO (5′-GACAGCAAACTCAGTACCTTCTCCA-3′); Vegfbb MO (5′-GGTGCTCATGTAGAGCTTCACCACT-3′); Vegfr1 MO (5′-ATATCGAACATTCTCTTGGTCTTGC-3′); Vegfr2a MO (5′-CCGAATGATACTCCGTATGTCAC-3′); Vegfr2b MO (5′-CAGTTATGCTCTATTAGATGCCTGT-3′); Nrp1 (Nrp1a MO + Nrp1b MO) (5′-GAGGATCAACACTAATCCACAATGC-3′ + 5′-ACACAGAGCAAACACCAGTACATCC-3′); and standard scrambled control MO (5′-CCTCTTACCTCAGTTACAATTTATA-3′). Following injection, embryos were placed in a fresh E3 medium and incubated for various periods as indicated in figures.
Quantitative Real-Time PCR.
Heads and trunks of zebrafish were lyzed in 1 mL QIAzol solution (Qiagen), followed by homogenization for 30 s using a VDI 12 Homogenizer. The GeneJET RNA purification kit (Fermentas) was used for RNA purification according to the manufacturer’s instruction. Total RNA of 100 ng was reversely transcribed using a RevertAid H minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Inc.) according to the manufacturer’s protocol. For first-strand cDNA synthesis, a mixture of random hexamer nucleotides was used. Reverse transcription was performed at 42 °C for 60 min, followed by enzyme inactivation at 70 °C for 5 min. Samples were directly subjected to quantitative PCR (qPCR) using a StepOne Plus Real Time PCR system (Applied Biosystems) or stored at −20 °C until further use. A 20-μL reaction contained 10 μL SYBR Green reagent (Applied Biosystems), 150 nm forward and reverse primers, and 1 μL cDNA template. qPCR was performed in triplicates, and the protocol was executed for 40 cycles. Each cycle contained denaturation at 95 °C for 15 s, annealing at 60 °C for 1 min, and extension at 72 °C for 1 min. The primer pairs specific for different genes were listed in Table S2. Threshold cycle (Ct) values were obtained for all cDNA samples. GAPDH was used as an internal control. All calculated Ct values for the gene of interest were normalized to the Ct value of GAPDH. The relative prevalence of transcripts was calculated using the ΔΔCt method.
Table S2.
Primer sequences for cloning zebrafish genes
| Gene | Forward primer | Reverse primer |
| zVegfaa | 5′-tgctcctgcaaattcacacaa-3′ | 5′-atcttggcttttcacatctgcaa-3′ |
| zVegfba | 5′-aatcacctctttctgccgtcg-3′ | 5′-gctgcacaagttcatgcttc-3′ |
| zGapdh | 5′-taacggattcggtcgcattg-3′ | 5′–taacggattcggtcgcattg-3′ |
Histology.
Cellular apoptosis was detected by staining for activated (cleaved) caspase3 reactivity using a rabbit anti-cleaved caspase3 antibody (1:200, Cell Signaling, #9661, lot 42) according to previously described protocols (44). Briefly, zebrafish embryos were fixed at the 24-hpf stage in cold 4% (mass/mass) paraformaldehyde overnight at 4 °C, permeabilized, blocked with 3% (mass/mass) milk powder in 0.3% Triton-X 100 in PBS, and stained with the primary antibody for 24 h at 4 °C. After rigorous washing, embryos were reblocked with blocking buffer, incubated for 2 h with a secondary goat anti-rabbit-cy3 antibody, followed by washing, and were mounted in VectaShield (Vector Laboratories).
Zebrafish in situ hybridization was performed using a previous published protocol (24). For generation of in situ probes, clones for the respective genes were purchased from Source BioScience. (Table S1). These clones were used directly for generation of digoxigenin-tagged antisense RNAs as previously described (24) using the mMessage/Machine Kit (Ambion) according to the manufacture’s instruction. Stained zebrafish embryos were analyzed using a Leica DFC300FX camera equipped with a Leica MZ16 microscope.
Microscopy and Imaging Analysis.
Zebrafish embryos were whole-mounted in VectaShield (Vector Laboratories) and examined by confocal microscopy (Nikon D-Eclipse C1, Nikon) as previously described (32, 44). Imaging analyses were repeated on different sample sets three times in each experimental group. Captured images were subsequently analyzed in either Photoshop CS4 (Adobe) or ImageJ (NIH).
Coimmunoprecipitation Assay.
The full-length cDNA coding for zebrafish vegfba was cloned from a zebrafish cDNA library into the multicloning sites of a pFLAG-CMVTM-5a (Sigma-Aldrich) vector using the forward primer 5′-GGGAATTCGCCACCATGGGGATCAAGCTGAGGGTTATTC-3′ and the reverse primer 5′-CCGCGGCCGCTTCACTTTGGGTTTCAC-3′. A His-tag was engineered to follow the carboxyl terminus of the full-length zebrafish Nrp1a cDNA using a PCR protocol. PCR amplification of the full-length zebrafish Nrp1a from a zebrafish cDNA library was accomplished using the forward primer 5′-GGGAATTCGCCACCATGCATTGTGGATTAGTGTTG-3′ and the reverse primer 5′-CCGCGGCCGCTCAGTGGTGATGGTGATGATGCGCTTCCGAGTACGAG-3′. The amplified PCR fragment was cloned into a pMXs-IG vector. The correct sequences coding for zebrafish Vegfba and Nrp1 were verified by DNA sequencing. 293T cells were transfected with the pFLAG-CMVTM-5a:zVegfba and/or pMXs-IG:Nrp1a-His using the Polyetylenimine Max (Polysciences Inc.). At 48 h after transfection, cells were lyzed with a CelLytic MT Cell Lysis Reagent (Sigma-Aldrich). zVEGF-B protein was immunoprecipitated from the cell lysates (400 μg of protein) by incubation with an anti-Flag M2 antibody (1 μg; Sigma-Aldrich) for 1 h, followed by subsequent incubation with protein A/G-agarose (20 μL; sc-2003) for 2 h at 4 °C. Immune complexes were washed four times with a lysis buffer, eluted in an SDS sample buffer, separated by a 10% SDS/PAGE gel, and immunoblotted with an anti-His antibody (GenScript, A00174).
Exposure of Zebrafish Embryos to Hypoxia.
Zebrafish hypoxia was achieved by incubating the embryos in a custom-made hypoxia chamber as previously described (25, 32). Briefly, an oxygen tension of 7.5% air saturation (aproximately 1.5% oxygen) in the aquarium water was achieved through bubbling N2 gas in the water. An automated regulatory unit (Loligo) coupled to an oxygen electrode inserted into the aquarium controlled the perfusion of N2 gas. Zebrafish embryos at the 32-cell stage were exposed to this hypoxic condition and incubated for 72 h. Normoxia exposure was used as a parallel control.
Quantification of Vascularization.
Vasculatures in developing zebrafish embryos were analyzed at 24, 36, 72, and 120 hpf. At 24 hpf, the length of MCVs and ISVs in each zebrafish embryo was measured. In hypoxia experiments, ISV and peripheral neovascularization in embryos at 36 and 72 hpf were quantitatively measured. At 72 hpf, the density of brain vasculatures in the region between the MCV and the posterior cerebral vessel were measured. The same measurements were used to quantify vascularization at 120 hpf (VHL-mutant fish), with additional measurement of peripheral ISV density in a set area spanning from the dorsal aorta to the dorsal longitudinal anastomotic vessel and over three ISV branches dorsal to the yolk extension.
Statistical Analysis.
Statistical analyses of results were performed using the standard two-tailed Student t test, and *P < 0.05, **P < 0.01, and ***P < 0.001 were deemed statistically significant. Data were presented as means ± SEM.
Acknowledgments
Y.C.’s laboratory is supported by research grants from the Swedish Research Council; the Swedish Cancer Foundation; the Karolinska Institute Foundation; the Karolinska Institute Distinguished Professor Award; the Torsten Soderbergs Foundation; the European Research Council advanced grant ANGIOFAT (project no. 250021); a NOVO Nordisk Foundation advanced grant; and the Alice Wallenberg Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1510245112/-/DCSupplemental.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 3.Senger DR, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 4.Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2(59):re1. doi: 10.1126/scisignal.259re1. [DOI] [PubMed] [Google Scholar]
- 5.Shibuya M. Vascular endothelial growth factor and its receptor system: Physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153(1):13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X. VEGF-B: A thing of beauty. Cell Res. 2010;20(7):741–744. doi: 10.1038/cr.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci USA. 1991;88(20):9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olofsson B, et al. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci USA. 1998;95(20):11709–11714. doi: 10.1073/pnas.95.20.11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev. 2002;82(3):673–700. doi: 10.1152/physrev.00005.2002. [DOI] [PubMed] [Google Scholar]
- 10.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21(2):154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Pan Q, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11(1):53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 12.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90(4):739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 13.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92(6):735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 14.Caunt M, et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell. 2008;13(4):331–342. doi: 10.1016/j.ccr.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 15.Carmeliet P, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380(6573):435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara N, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380(6573):439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 17.Miquerol L, Langille BL, Nagy A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development. 2000;127(18):3941–3946. doi: 10.1242/dev.127.18.3941. [DOI] [PubMed] [Google Scholar]
- 18.Aase K, et al. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation. 2001;104(3):358–364. doi: 10.1161/01.cir.104.3.358. [DOI] [PubMed] [Google Scholar]
- 19.Bellomo D, et al. Mice lacking the vascular endothelial growth factor-B gene (Vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ Res. 2000;86(2):E29–E35. doi: 10.1161/01.res.86.2.e29. [DOI] [PubMed] [Google Scholar]
- 20.Hagberg CE, et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature. 2012;490(7420):426–430. doi: 10.1038/nature11464. [DOI] [PubMed] [Google Scholar]
- 21.Hagberg CE, et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464(7290):917–921. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- 22.Dijkstra MH, et al. Lack of cardiac and high-fat diet induced metabolic phenotypes in two independent strains of Vegf-b knockout mice. Sci Rep. 2014;4:6238. doi: 10.1038/srep06238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 24.Jensen LD, et al. Opposing effects of circadian clock genes bmal1 and period2 in regulation of VEGF-dependent angiogenesis in developing zebrafish. Cell Reports. 2012;2(2):231–241. doi: 10.1016/j.celrep.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Rouhi P, et al. Hypoxia-induced metastasis model in embryonic zebrafish. Nat Protoc. 2010;5(12):1911–1918. doi: 10.1038/nprot.2010.150. [DOI] [PubMed] [Google Scholar]
- 26.Cao Z, et al. Hypoxia-induced retinopathy model in adult zebrafish. Nat Protoc. 2010;5(12):1903–1910. doi: 10.1038/nprot.2010.149. [DOI] [PubMed] [Google Scholar]
- 27.Cao R, Jensen LD, Söll I, Hauptmann G, Cao Y. Hypoxia-induced retinal angiogenesis in zebrafish as a model to study retinopathy. PLoS One. 2008;3(7):e2748. doi: 10.1371/journal.pone.0002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, et al. VEGF-B: A survival, or an angiogenic factor? Cell Adhes Migr. 2009;3(4):322–327. doi: 10.4161/cam.3.4.9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nash AD, Baca M, Wright C, Scotney PD. The biology of vascular endothelial growth factor-B (VEGF-B) Pulm Pharmacol Ther. 2006;19(1):61–69. doi: 10.1016/j.pupt.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Zhu H, et al. Regulation of the lmo2 promoter during hematopoietic and vascular development in zebrafish. Dev Biol. 2005;281(2):256–269. doi: 10.1016/j.ydbio.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 31.Makino Y, et al. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature. 2001;414(6863):550–554. doi: 10.1038/35107085. [DOI] [PubMed] [Google Scholar]
- 32.Lee SL, et al. Hypoxia-induced pathological angiogenesis mediates tumor cell dissemination, invasion, and metastasis in a zebrafish tumor model. Proc Natl Acad Sci USA. 2009;106(46):19485–19490. doi: 10.1073/pnas.0909228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Rooijen E, et al. Zebrafish mutants in the von Hippel-Lindau tumor suppressor display a hypoxic response and recapitulate key aspects of Chuvash polycythemia. Blood. 2009;113(25):6449–6460. doi: 10.1182/blood-2008-07-167890. [DOI] [PubMed] [Google Scholar]
- 34.Bates ML, Farrell ET, Eldridge MW. Abnormal ventilatory responses in adults born prematurely. N Engl J Med. 2014;370(6):584–585. doi: 10.1056/NEJMc1311092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lähteenvuo JE, et al. Vascular endothelial growth factor-B induces myocardium-specific angiogenesis and arteriogenesis via vascular endothelial growth factor receptor-1- and neuropilin receptor-1-dependent mechanisms. Circulation. 2009;119(6):845–856. doi: 10.1161/CIRCULATIONAHA.108.816454. [DOI] [PubMed] [Google Scholar]
- 36.Erskine L, et al. VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron. 2011;70(5):951–965. doi: 10.1016/j.neuron.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarz Q, et al. Vascular endothelial growth factor controls neuronal migration and cooperates with Sema3A to pattern distinct compartments of the facial nerve. Genes Dev. 2004;18(22):2822–2834. doi: 10.1101/gad.322904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: Direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci USA. 2000;97(18):10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munson J, et al. Identifying new small molecule anti-invasive compounds for glioma treatment. Cell Cycle. 2013;12(14):2200–2209. doi: 10.4161/cc.25334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munson JM, et al. Anti-invasive adjuvant therapy with imipramine blue enhances chemotherapeutic efficacy against glioma. Sci Transl Med. 2012;4(127):127ra36. doi: 10.1126/scitranslmed.3003016. [DOI] [PubMed] [Google Scholar]
- 41.Owonikoko TK, et al. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol. 2014;11(4):203–222. doi: 10.1038/nrclinonc.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maves L, Kimmel CB. Dynamic and sequential patterning of the zebrafish posterior hindbrain by retinoic acid. Dev Biol. 2005;285(2):593–605. doi: 10.1016/j.ydbio.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 43.van Rooijen E, et al. von Hippel-Lindau tumor suppressor mutants faithfully model pathological hypoxia-driven angiogenesis and vascular retinopathies in zebrafish. Dis Model Mech. 2010;3(5-6):343–353. doi: 10.1242/dmm.004036. [DOI] [PubMed] [Google Scholar]
- 44.Dahl Ejby Jensen L, et al. Nitric oxide permits hypoxia-induced lymphatic perfusion by controlling arterial-lymphatic conduits in zebrafish and glass catfish. Proc Natl Acad Sci USA. 2009;106(43):18408–18413. doi: 10.1073/pnas.0907608106. [DOI] [PMC free article] [PubMed] [Google Scholar]