Significance
Autumnal phenological shifts (leaf senescence and dormancy) because of climate change bring substantial impacts on community and ecosystem processes (e.g. altered C and N cycling and phenological mismatches) and the fall foliage ecotourism industry. However, the understanding of the environmental control of autumn phenology has changed little over the past 60 y. We found that cold, frost, wet, and high heat-stress lead to earlier dormancy dates across temperate deciduous forest communities, whereas moderate heat- and drought-stress delayed dormancy. Divergent future responses of fall dormancy timing were predicted: later for northern regions and earlier for southern areas. Our findings improve understanding of autumn phenology mechanisms and suggests complex interactions among environmental conditions affecting autumn phenology now and in the future.
Keywords: Land-surface phenology, dormancy date, frost, New England
Abstract
Changes in spring and autumn phenology of temperate plants in recent decades have become iconic bio-indicators of rapid climate change. These changes have substantial ecological and economic impacts. However, autumn phenology remains surprisingly little studied. Although the effects of unfavorable environmental conditions (e.g., frost, heat, wetness, and drought) on autumn phenology have been observed for over 60 y, how these factors interact to influence autumn phenological events remain poorly understood. Using remotely sensed phenology data from 2001 to 2012, this study identified and quantified significant effects of a suite of environmental factors on the timing of fall dormancy of deciduous forest communities in New England, United States. Cold, frost, and wet conditions, and high heat-stress tended to induce earlier dormancy of deciduous forests, whereas moderate heat- and drought-stress delayed dormancy. Deciduous forests in two eco-regions showed contrasting, nonlinear responses to variation in these explanatory factors. Based on future climate projection over two periods (2041–2050 and 2090–2099), later dormancy dates were predicted in northern areas. However, in coastal areas earlier dormancy dates were predicted. Our models suggest that besides warming in climate change, changes in frost and moisture conditions as well as extreme weather events (e.g., drought- and heat-stress, and flooding), should also be considered in future predictions of autumn phenology in temperate deciduous forests. This study improves our understanding of how multiple environmental variables interact to affect autumn phenology in temperate deciduous forest ecosystems, and points the way to building more mechanistic and predictive models.
Plant phenological shifts in recent decades are iconic bio-indicators of climate change (1–4). These phenological changes in turn have cascading ecological effects on species demography, biotic interactions, and ecosystem functions (5–8). Whereas mechanisms of spring phenology (i.e., bud burst, leafing out, and flowering) are well studied (9–13), fall phenology (i.e., leaf senescence and dormancy, indicated by visual signals from leaf coloration and leaf drop) remains little studied (14–16). Changes in timing of autumn phenology play a significant role in growing season length prediction, C and N cycling, and biotic interactions (8, 17–19). Furthermore, delayed leaf coloration and more muted autumn foliage in response to climate change will likely significantly affect the multibillion dollar fall foliage ecotourism industry (20–22). Although delayed leaf coloration and leaf drop in deciduous forests have been observed across the northern hemisphere in recent decades (14, 23, 24), the full range of environmental triggers and how they influence fall phenological changes now or in the future remain poorly understood.
Autumn phenology of deciduous woody plant species in temperate regions is the timing of the developmental stages of leaf senescence and dormancy. Plant physiologists demark leaf senescence beginning with onset of leaf coloration, and dormancy with leaf drop and the development of dormant apical meristems (25, 26). As detected by remotely sensed satellite images, autumn phenology dates describe the timing of loss of leaf greenness. Leaf senescence dates correspond to when greenness starts to decrease (i.e., onset of leaf coloration) and dormancy dates occur when greenness reaches a minimum value (brown leaves with leaf drop) (27) (SI Appendix, Figs. S1 and S2). Currently, most studies consider short day length and low temperature as the primary or only external triggers of autumn phenology (28, 29). However, over the past 60 y (25, 30, 31), researchers studying the physiology of leaf senescence and dormancy have enumerated a range of other environmental conditions that may influence autumn phenology, including frost, moisture conditions, and extreme weather events (e.g., drought- and heat-stress, and flooding). Although the effects of a subset of these factors on plant leaf coloration and leaf drop were reported by a handful of physiological experiments (32, 33), few studies have quantified the response of fall phenology to a full suite of potential explanatory factors. Ongoing climate changes are likely to introduce higher frequency and intensity of climatic stress factors (34), so it is important to include these in developing more predictive, mechanistic models of fall phenology.
To study landscape-scale forest phenology, we used satellite remotely sensed autumn dormancy dates of deciduous forests in New England, United States, from the Moderate Resolution Imaging Spectroradiometer (MODIS) data product (27) (SI Appendix, Fig. S1). Greenness of forest canopy reaches the minimum values at the dormancy date (27), a proxy for plant fall dormancy (SI Appendix, Figs. S1 and S2). We examined dormancy dates of deciduous forest communities in two eco-regions (NH, Northeastern Highlands; NCZ, Northeastern Coastal Zone) from 2001 to 2012 (Figs. 1 and 2). Multiple environmental factors affecting fall forest dormancy were identified representing spatially and temporally varying chill and frost-stress, heat-stress, drought-stress, precipitation, and flooding events (Table 1), as well as latitude (a proxy for photoperiod) and elevation. We built models independently using eight different statistical regression methods [including multiple linear regression, penalized regression methods, Bayesian model averaging (BMA) (35), and Bayesian spike and slab regression (36)] to select significant environmental drivers of dormancy dates of deciduous forest communities, and to assess spatiotemporal responses of dormancy dates to those drivers. By model selection criteria and root mean square error (RMSE), the best models were selected to predict future dormancy dates of deciduous forest communities in two 10-y periods (2040–2050 and 2090–2099) with two greenhouse gas concentration scenarios [representative concentration pathway (RCP) 4.5 and RCP 8.5] under future climate change projections (37).
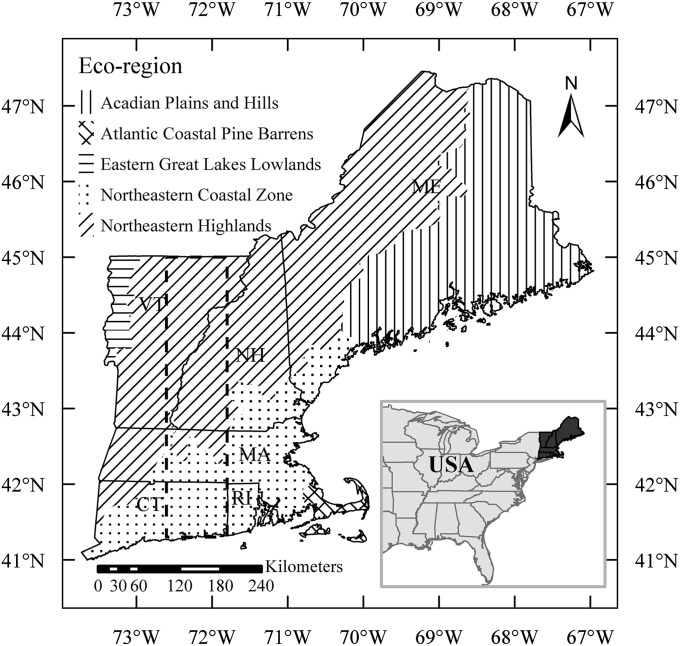
Fig. 1.
Study area (dashed rectangular box) in New England, United States, covering the Connecticut River Valley from 41.3 to 45°N, 71.8 to 72.6°W, and parts of the states of Connecticut (CT), Rhode Island (RI), Massachusetts (MA), New Hampshire (NH), and Vermont (VT). Study area covers two eco-regions, NH and NCZ.
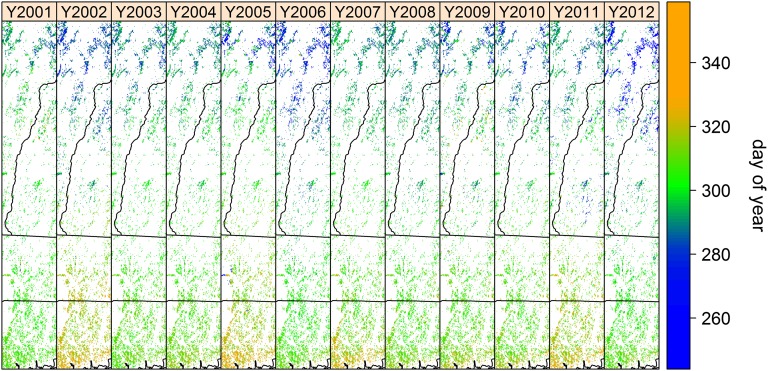
Fig. 2.
Dormancy dates (day of year) for deciduous forests across study area from 2001 to 2012. Small values (blue pixels) indicate early dormancy dates and large values (orange pixels) indicate late dormancy dates. Black lines are state boundaries (Connecticut at the bottom, Massachusetts next, then Vermont upper left, and New Hampshire upper right). White areas indicate nondeciduous forest area; see also study area shown in Fig. 1.
Table 1.
Candidates of explanatory variables for dormancy dates
| Name | Description |
| Cold degree day (CDD) | ∑(Tb − Ti) |
| Hot days (HD) | No. of days with Tmax ≥ 32 or 35 °C |
| Frost days (FD) | No. of days with Tmin ≤ 0 °C |
| Growing season drought (GDR) | No. of events when ≥ 7 consecutive days without precipitation |
| Rainy days (RD) | No. of days with precipitation ≥ 2 mm |
| Heavy rainy days (ECA) | No. of days with precipitation ≥ 20 mm |
Tb: base temperature; Ti: daily mean temperature; Tmax: daily maximum temperature; Tmin: daily minimum temperature.
Results
Statistical regressions between fall dormancy dates and the suite of predictor variables showed that a number of environmental/weather conditions significantly affect dormancy dates. The different variable selection methods converged on very similar, best-fit models with only slightly different values for predictor coefficients (SI Appendix, Tables S1 and S2). The posterior median model (38) from the BMA procedure was selected as the best model explaining deciduous forest dormancy dates in the NH eco-region, and the multiple linear regression model as the best model in the NCZ eco-region. Predictors included in the two best models (Table 2) show that dormancy dates of deciduous forests in New England were significantly affected by latitude (a proxy for photoperiod), elevation, plus temperature conditions [cold degree days (CCD), frosts, and heat-stress], and precipitation [rain days (RD), drought-stress, and extreme flooding events] summarized from daily data over the course of the growing season. Deciduous forests at higher latitude and elevation showed earlier fall dormancy dates. Generally, cold, wet, or extremely hot conditions tended to induce earlier dormancy, whereas moderately hot and dry conditions delayed fall dormancy. Quadratic terms in the models indicated significant nonlinear relationships between dormancy dates and frost, drought, and rainfall conditions.
Table 2.
Coefficients of variables in the best models of two eco-regions (mean value and SD)
| Variables | Northeastern Highlands | Northeastern Coastal Zone |
| Latitude | −4.255 (0.097) | −3.955 (0.151) |
| Elevation | −0.007 (0.0004) | −0.017 (0.001) |
| CDD20(Aug.1–Nov.15) | −0.022 (0.001) | −0.029 (0.001) |
| FD(Sep.1–Nov.15) | — | −0.269 (0.018) |
| FD(Sep.1–Nov.15)2 | 0.004 (0.0003) | — |
| FD(Apr.1-–un.30) | −0.059 (0.009) | — |
| HD32(Jul.1–Aug.31) | 1.111 (0.076) | 1.611 (0.033) |
| HD35(Jul.1–Aug.31) | −0.821 (0.161) | −1.706 (0.051) |
| GDR(May.1–Jun.30) | — | −0.079 (0.012) |
| GDR(Jul.1–Aug.31) | 0.088 (0.022) | 0.050 (0.013) |
| GDR(Sep.1–Nov.15) | 0.755 (0.028) | 0.111 (0.009) |
| GDR(Sep.1–Nov.15)2 | −0.029 (0.002) | — |
| RD(May.1–Jun.30) | −0.192 (0.012) | — |
| RD(Jul.1–Aug.31) | 0.578 (0.068) | −0.099 (0.012) |
| RD(Jul.1–Aug.31)2 | −0.012 (0.002) | — |
| RD(Sep.1–Nov.15) | −0.067 (0.012) | 0.743 (0.085) |
| RD(Sep.1–Nov.15)2 | — | −0.019 (0.002) |
| ECA(May.1–Jun.30) | −0.206 (0.025) | −0.243 (0.022) |
| ECA(Jul.1–Aug.31) | 0.124 (0.022) | 0.372 (0.023) |
| ECA(Sep.1–Nov.15) | 0.286 (0.021) | −0.795 (0.064) |
| ECA(Sep.1–Nov.15)2 | — | 0.083 (0.006) |
| FD(Sep.1–Nov.15) × Elevation | — | 0.002 (0.0008) |
| HD32(Jul.1–Aug.31) × RD(Jul.1–Aug.31) | −0.058 (0.006) | −0.093 (0.002) |
All coefficients are significantly different from zero. Tb of CDD is 20 °C, and HD has two thresholds, 32 °C and 35 °C. The time period calculated for the variables are shown in subscript in brackets for different seasons (spring: May–June; summer: July–August; fall: September1–November 15). Positive coefficients promote later fall dormancy; negative coefficients promote earlier fall dormancy. Dash indicates variables not included in the best models.
Coefficients in the best models indicate sensitivities of fall dormancy dates to changes of environmental conditions. Dormancy dates for the deciduous forest communities in the two eco-regions showed different sensitivities to environmental variation (Table 2). We found that deciduous forests in the NH were more sensitive to changes of latitude (i.e., photoperiod), drought, and summer rainfall than the NCZ forests, whereas deciduous forests in the NCZ were more sensitive to change of elevation, chill-stress, autumn rainfall, and heavy rain events (Table 2 and SI Appendix, Fig. S3). The interaction between summer rain and heat in two models suggested that to some extent summer rain reduced the effect of heat-stress on forest dormancy dates, especially for NCZ forests.
The variables that were unique to each of the two models indicate that dormancy dates for deciduous forests in two eco-regions had different responses to frost, seasonal drought-stress, and seasonal rainfall conditions (Table 2). Dormancy dates of NH deciduous forests were influenced by frost in both spring (earlier) and autumn (nonlinear), whereas dormancy dates in the NCZ were affected significantly only by autumn frost (earlier). Moreover, the response of dormancy dates in NCZ forests to frost was also related to elevation; the significant interaction between frost days (FD) and elevation in the model indicated that the sensitivity of deciduous forests at higher elevation to FD was smaller than the forests at lower elevation. In terms of drought effects, dormancy dates in NCZ forests were affected by spring, summer, and autumn drought-stress (respectively earlier, later, and later dormancy dates), but dormancy dates in the NH were more sensitive to summer and autumn drought (later dormancy dates and nonlinear effects). Although droughts in autumn lead to later dormancy dates in the two eco-regions, the quadratic term (growing season drought, GDR) GDR(Sep.1–Nov.15)2 indicates a delaying effect on dormancy dates in NH forests, but that as drought continues to increase, dormancy comes earlier.
Precipitation affected fall dormancy dates of deciduous forests in the NH across the growing season from spring to autumn. For NCZ forests, dormancy dates were affected by rainfall in summer (earlier) and autumn (later and with a nonlinear response). More summer rainfall lead to later dormancy dates in the NH but earlier dormancy dates in the NCZ; but the quadratic effect of summer rainfall [RD(Jul.1–Aug.31)2] in the NH indicates that initially as rainfall increases, fall dormancy is later but as the rainfall further increases, dormancy progressively comes earlier. More autumn rainfall lead to earlier dormancy dates in the NH but later dormancy dates in the NCZ, but given the quadratic coefficient associated with autumn rainfall in the NCZ [RD(Sep.1–Nov.15)2], initially as rainfall increases, dormancy is later, and as this continues to increase, dormancy comes earlier. Because there are more heavy rainfall days in autumn in the NCZ, dormancy is earlier, but with a significant positive quadratic term (heavy rainy days, ECA) [ECA(Sep.1–Nov.15)2] there is nonlinearity to this trend.
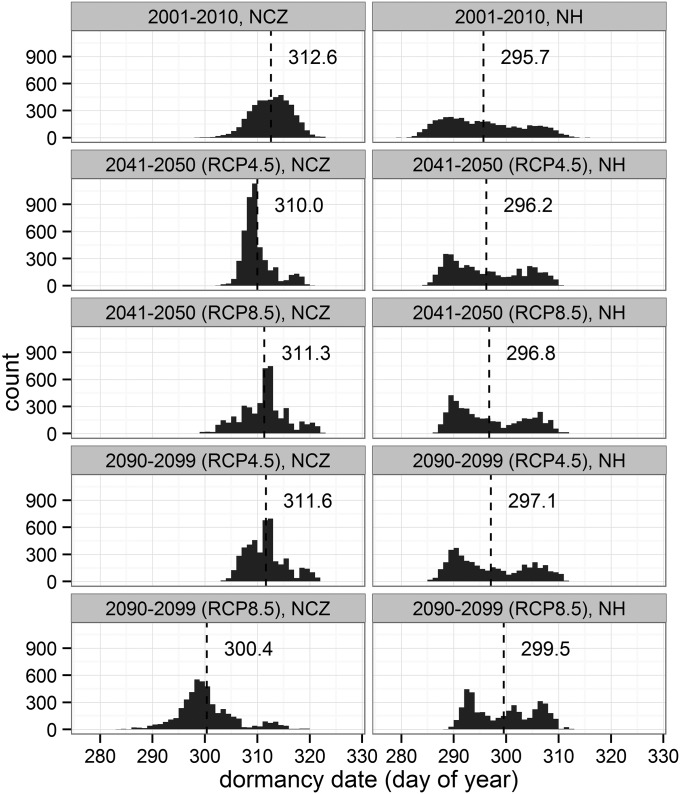
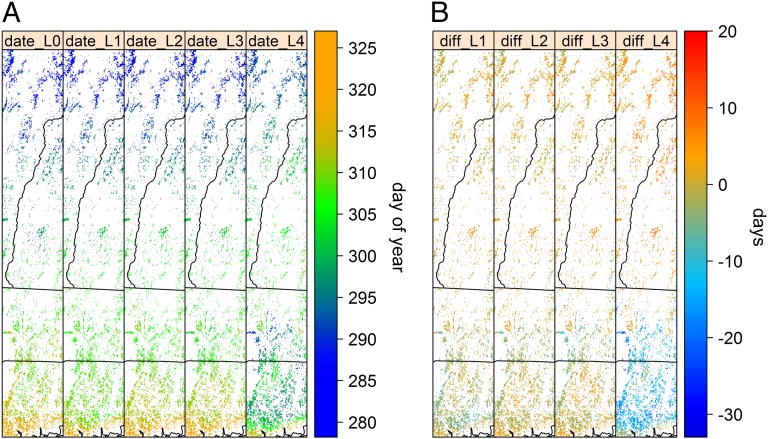
Predicted fall dormancy dates in the two eco-regions showed different responses to projected future climate change. Although later dormancy dates were predicted in the NH, earlier dormancy dates were predicted for the NCZ (Fig. 3). Dormancy dates in the NH were predicted to be 0.5 ± 3 d (RCP 4.5) and 1.1 ± 3 d (RCP 8.5) later on average (±SD) for 2041–2050 compared with recent dates (2001–2010). In contrast, dormancy dates for NCZ forests were predicted to be 2.6 ± 3 d (RCP 4.5) and 1.3 ± 3 d (RCP 8.5) earlier on average across the region compared with current (2001–2010) conditions (first to third rows in Fig. 3). For the two scenarios in 2090–2099, dormancy dates in the NH were projected to be further delayed: 1.4 ± 3 d (RCP 4.5) and 3.8 ± 3 d (RCP 8.5). However, dormancy dates in the NCZ in 2090–2099 were 1.0 ± 3 d earlier (RCP 4.5) and were strongly advanced (12.2 ± 5 d earlier) under the RCP 8.5 scenario (fourth and fifth rows in Fig. 3). Across the landscape, dormancy dates of deciduous forest in northern region occurred earlier than in southern region over 2001–2012. Similar spatial variation of dormancy dates was predicted under the RCP 4.5 scenario for the two 10-y periods and under the RCP 8.5 scenario for the 2041–2050 period (Fig. 4A). However, the spatial pattern in the late century RCP 8.5 scenario is projected to be quite different (Fig. 4A, fifth column): the latest dormancy dates are projected for midlatitude forests and earlier dormancy is projected for southern latitudes, mirroring northern latitudes. Note that for the NCZ, dormancy dates are projected to be as much as 20+ days earlier than at present, whereas for the NH eco-region dormancy dates are projected to be as much as 10+ days later (Fig. 4B).
Fig. 3.
Histogram of 10-y averaged dormancy dates in three 10-y periods (current: 2001–2010, and projected future: 2041–2050, 2090–2099) with two climate change projection scenarios (RCP 4.5 and RCP 8.5) across the two eco-regions (NCZ and NH). Dashed lines and numbers indicate mean values of predicted dormancy dates.
Fig. 4.
(A) Maps of 10-y averaged dormancy dates for deciduous forests across the region over different 10-y periods with two climate change projection scenarios. L0: 2001–2010; L1: 2041–2050, RCP 4.5; L2: 2090–2099, RCP 4.5; L3: 2041–2050, RCP 8.5; L4: 2090–2099, RCP 8.5. (B) Difference of dormancy dates between current period and predicted periods in study area. Positive values (warm color) indicate delayed dormancy dates in the future compared with the current period, and negative values (cold color) indicate earlier dormancy dates in the future. In both plots, black lines are state boundaries and white areas indicate nondeciduous forest areas; see also study area shown in Fig. 1.
Discussion
Autumn phenology determining timing of the end of the growing season (i.e., dormancy), reflects plant strategic responses to all environmental stressors during the entire growing season in maximizing survival (39). Leaf senescence and dormancy result from the integration of developmental and environmental signals (40). Developmental signals including hormones and molecular regulations of growth cessation, bud set, and leaf senescence leading to dormancy have been identified in studies (30, 40, 41). However, the controlling environmental factors and how they interact to affect timing of these events is still unclear. We found that timing of fall dormancy of deciduous forest communities was affected not only by temperature, but also by a number of other environmental conditions over the growing season, including frost events, heat-stress, rainfall patterns, and drought-stress. Moreover, our models suggest nonlinear responses of dormancy to these factors, implying a complex environmental regulation of plant growth and development. Our findings support the results from previous studies: regression models with monthly precipitation had smaller RMSE than CDD-photoperiod models for autumn phenology of selected tree species (28), and summer severe drought followed by rains in autumn caused delayed leaf senescence of selected deciduous tree species in Europe in 2013 (42).
At least two possible interpretations relating plant phenological responses in the autumn to environmental stresses may be relevant in interpreting our results. One is viewed as the traditional explanation for autumnal dormancy in plants, that arresting growth and entering dormancy early avoids unfavorable, or damaging growing season conditions and facilitates the diversion of more resources for use in subsequent years, thus maximizing longer-term fitness (25, 30, 43). This finding explains negative correlations between dormancy dates and autumnal CDD, frost, and high heat-stress days (Table 2). An alternative possibility is that some environmental stressors may actually delay leaf senescence; a very limited number of studies that have examined the effects of a range of environmental stressors on fall phenology support this interpretation. We found that moderate summer heat-stress, summer and fall drought-stress, and summer heavy rain events, all lead to later autumn dormancy, counter to the traditional expectations. Studies on economically important plants (e.g., apples, soybeans, birch) have shown that under moderate heat- or water-stress in summer and fall, associated changes occur in gene-regulated levels of plant hormones and stress-shock proteins (44–46). The regulatory and physiological changes not only induce greater drought tolerance in plant leaves (47), but also prevent drought-induced cell death in leaves (that would otherwise lead to leaf senescence), and alter photosynthate source-sink relationships among plant organs (roots, stems, and leaves). The net result is delayed autumn senescence and dormancy. This finding suggests a long-term advantage in resource storage and reallocation for plant growth in the spring. However, this phenomenon has been little studied (45). Few studies have mentioned either the effects of heat- and drought-stress on fall phenology (45, 48) or the potential role of the underlying molecular and physiological mechanisms (44, 46). We have an ongoing project of ground-based phenological observations (SI Appendix, Fig. S2A) suggesting delayed effects from autumn drought on leaf coloration and leaf drop based on preliminary results. Clearly there is much to be done to fully elucidate the role that environmental factors, other than simply cold and frost, have on determining autumnal phenological events, as well as their underlying gene-regulatory and physiological bases.
We found higher sensitivities to CDD and heat stress for the southern region (NCZ) than the northern region (NH) of the study area. These results are similar to previous studies (23, 24) reporting higher phenological sensitivities of deciduous trees to temperature change at lower latitudes. However, we also found the sensitivity of fall dormancy dates to drought was higher in northern areas than in southern areas. These differences are likely a result of differences in forest tree species compositions in two eco-regions. Maples and birches dominate (45% biomass on average) the north (NH), whereas oaks dominate (35% biomass on average) the south (NCZ) (SI Appendix, Table S3). With higher drought tolerance, oaks may show lower sensitivity to drought stress than maples or birches (49, 50).
The studies that typically only considered projected warming effects (28, 29) all showed a consistent, progressive delay in the onset of fall leaf senescence and dormancy under projected climate change, but our study projected a slightly later dormancy in northern areas and earlier dormancy in coastal areas, especially under the RCP 8.5 scenario. Comparing climatic variables between the current and future periods, we found that lower values for CDD, fewer frosts, and more moderate heat-stress days in summer, were the main drivers for delaying dormancy dates in two eco-regions (SI Appendix, Figs. S4 and S5). However, there are also antagonistic factors operating at the same time that lead to earlier fall dormancy: that is, in the future higher heat-stress, slightly less drought, and significant interaction effects between moderate heat-stress and summer rainfall. The heat-stress effects were stronger than the lower chill effects in NCZ eco-region, leading to earlier dormancy dates (SI Appendix, Fig. S5). Although warmer autumn may extend forest growing season, earlier leaf dormancy can be forced under higher heat-stress during the summer from climate change. This finding suggests that multiple impacts from projected climate with more identified stresses, in addition to warming, will in concert affect autumn phenology of deciduous forest trees in the future. Recent studies pointing out the positive correlation between spring and fall phenology (51, 52), further support our finding that autumn phenology responds to weather spanning the full growing season. Moreover, these responses will be spatially and temporally complex: different phenological responses will likely occur in different regions, given the spatial variation in climate variables across the landscape.
From the variable selection methods, the very slight difference in predictor coefficients, model selection criteria, and RMSE (SI Appendix, Tables S1 and S2) suggested that multicollinearity does not significantly affect model fitting or predictions. RMSE in model validation (2011–2012) suggested predictive uncertainties were about 14.2 (NH) and 6.7 (NCZ) days (SI Appendix, Tables S1 and S2), which include data uncertainty in the MODIS and climate data because of data quality and model uncertainties; this is within the limits of the temporal resolution of MODIS phenology data summarized at 8-d intervals.
We encourage further investigations on physiological responses of autumn phenology to multiple environmental stresses, including interactions among stresses and nonlinear effects, and collecting long-term datasets across more species, communities, and ecosystems, including field observations and physiological experiments, to better inform future predictions and narrow model uncertainties. Species-specific phenological responses also need to be integrated into forest community phenology models in the future to better predict individual species- and community- or landscape-level responses (53). Indeed the bimodality in dormancy responses for the NH (Fig. 3) may reflect this issue.
Materials and Methods
Study Area.
A rectangular area (72.6°W to 71.8°W, 41.3°N to 45°N) was selected in New England, United States as the study area (Fig. 1). This area covers two ecological regions: the Northeastern Highlands (NH) and Northeastern Coastal Zone (NCZ) (archive.epa.gov/wed/ecoregions/web/html/na_eco.html). These two eco-regions are geographically and ecologically different representing a large variation of landscape, species composition, and environmental conditions in deciduous forest communities in New England (SI Appendix, Fig. S6). The NH is a mountainous area with elevations up to 1,000 m higher than the NCZ, which comprises coastal plains with hills rising to about 400 m, and overall the NH has a cooler and wetter climate than the NCZ. For forest tree species composition, deciduous forests in the NH are dominated by maples and birches, whereas the NCZ deciduous forest is dominated by oaks (SI Appendix, Table S3).
Data and Processing.
The MODIS Land Cover Dynamics (MCD12Q2) product (the NASA Land Processes Distributed Active Archive Center, US Geological Survey/Earth Resources Observation and Science Center) provides estimates of the timing of vegetation phenology at regional to global scale based on the remotely sensed vegetation index summarized at 8-d temporal resolution. The MODIS data product derives four phenological transition dates (green-up, maturity, senescence, and dormancy) from 2001 to 2012 with a spatial resolution of 500 m (28) (SI Appendix, Fig. S1). This study focuses on dormancy dates in fall. By using 30-m resolution land cover data from National Oceanic and Atmospheric Association’s Coastal Services Center C-CAP 2001 dataset (coast.noaa.gov/dataregistry/search/collection/info/ccapregional), we extracted MODIS pixels corresponding to deciduous forest in the study area. The percentage of deciduous forest cover in each MODIS grid cell was calculated by combining land cover and phenology data. MODIS pixels with at least 75% deciduous forests were retained for analysis. We removed outliers from the analyses that showed dormancy occurring before Julian day 244 (September 1 or August 31) or after Julian day 360 (December 26 or 25). These outliers are less than 1% of the MODIS phenology data in the deciduous forest region, and are likely because of subpixel patches of agricultural fields being plowed, forest patches being defoliated or harvested, or the occurrence of grassy areas that remain green well into the winter. The final phenology data set included about 9,500 grid cells for each year for two eco-regions. Digital elevation data (srtm.csi.cgiar.org/) with a spatial resolution of 90 m were aggregated to generate elevation data for the 500-m MODIS grid cells.
We used gridded daily weather data from 2001 to 2012 obtained from PRISM climate group to develop the explanatory weather variables. The data included daily mean, maximum, and minimum temperature and daily precipitation with a spatial resolution of 4 km (54), which can be used to summarize a broad range of different weather indices (55). Statistically downscaled climate projection data for one global climate model (GCM, GFDL-ESM2G) with two future scenarios (RCP 4.5 and RCP 8.5) were obtained from Multivariate Adaptive Constructed Analogs group for model predictions (37). We used daily maximum and minimum temperature and daily precipitation over two 10-y periods (2041–2050 and 2090–2099) with a spatial resolution of 4 km, which is comparable to the PRISM data.
To find the relationships between environmental factors and dormancy of deciduous forests, we first built a list of weather variables of potential environmental conditions that may affect fall phenology including cold, frost, heat, rainfall, drought, and flood events (Table 1 and SI Appendix, Fig. S6). Accumulating CDDs (28, 56) and decreasing day length that occur in fall have long been considered as the primary triggers of leaf senescence and dormancy. Because day length does not have year-to-year variation, we did not investigate the effect of day length effect; rather this effect is taken into account in the latitudinal variation (57). The other variables in Table 1 represent environmental/weather stressors potentially affecting tree performance (55, 58, 59). Plant responses to stresses may differ depending on when stresses occur in different seasons, and the specific species involved (56, 60–62). The physiological requirements of trees may also differ in different phenophases (62, 63). We calculated three sets of weather variables, growing season drought, rainy days, and heavy rainy days, for three periods (May 1 to June 30, July 1 to August 31, and September 1 to November 15). For CDD, we examined the effects of three different base temperatures (10°, 15°, and 20 °C) and starting dates (July 1, August 1, and September 1) to determine which period of CDD with what base temperature may best explain dormancy timing variation across the deciduous forest landscape. The end date of CDD was set as November 15, the 90th percentile of dormancy dates in the whole study region. We also used different threshold temperatures (32° and 35 °C) for hot days and we found hot days only occurred in July and August in the study area. There was no frost between June 30 and September 1 in study area, so we only calculated FD for two periods (April 1 to May 31 and September 1 to November 15), representing spring and fall growing season frosts.
Statistical Modeling.
Datasets from two eco-regions were analyzed separately because dormancy dates in two eco-regions fall in two different normal distributions. Data from 2001 to 2010 were used as model training data, and data from 2011 to 2012 were used in model validation. From initial exploratory data analyses, we selected one CDD variable with the highest correlation coefficient with dormancy dates plus other variables with a limited number of quadratic and interaction terms between predictors for each eco-region in subsequent analyses. Large number of explanatory variables from a large-scale dataset with multicollinearity among variables (e.g., correlations between temperature and latitude, latitude, and elevation) make variable selection and interpretation quite challenging (64). Thus, in addition to multiple linear regression, we used several complementary statistical methods to select important predictors explaining variation in dormancy dates. Variable selection methods include penalized regression methods, BMA (35), and Bayesian spike and slab regression (36). The penalized regression methods used were: ridge regression (65), Bayesian Least Absolute Shrinkage and Selection Operator (Bayesian LASSO) (66), the Elastic Net (67), and Pairwise Absolute Clustering and Sparsity method (PACS) (68). Penalized regression methods apply penalties to estimate coefficients with shrinkage effects of driving coefficients to be zero, which can simultaneously select important variables and estimate coefficients in the model. PACS and Elastic Net can especially select groups of correlated variables to deal with multicollinearity (67, 68). BMA provides a coherent mechanism to take account of model uncertainty by determining the coefficient of each variable using the weighted average of the parameter’s posterior estimate in each model on the entire model space (35). To choose a model for future prediction, the model consisting of those variables that have overall posterior inclusion probability equal to or greater than 0.5 was considered as the optimal predictive model (38); this is termed the “posterior median model” and is easily found in BMA procedures. Bayesian spike and slab regression used a mixture of “slab distribution” (e.g., normal distribution) and “spike distribution” (e.g., a probability mass at zero) as a prior distribution to segregate the variable coefficients to be exactly zero in the induced posterior (36). Data were analyzed using software R (69) (see SI Appendix for R codes).
The Akaike Information Criterion (AIC) (70), Bayesian Information Criterion (BIC) (71), and RMSE were used for model selection. Models from all eight methods were used to predict dormancy dates for 2011–2012 as model validation. AIC, BIC, and RMSE were calculated for model estimation (2001–2010) and validation (2011–2012) (SI Appendix, Tables S1 and S2). Best models were selected by smallest AIC, BIC, and RMSE indicating best model fitting and prediction. Based on future climate projection data, dormancy dates of deciduous forests in two eco-regions were predicted by the best models for two 10-y periods, 2041–2050 and 2090–2099, with two scenarios (RCP 4.5 and RCP 8.5). We calculated 10-y average dormancy dates of period, 2001–2010, as a base line, and then compared these to 10-y averaged dormancy dates in future periods.
Supplementary Material
Acknowledgments
We thank R. Primack, J. Allen, M. Aiello-Lammens, C. Merow, E. Adams, and two anonymous reviewers for helpful comments. This study was supported in part by National Science Foundation Grant DEB 0842465 (to J.A.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509991112/-/DCSupplemental.
References
- 1.Peñuelas J, Filella I. Phenology. Responses to a warming world. Science. 2001;294(5543):793–795. doi: 10.1126/science.1066860. [DOI] [PubMed] [Google Scholar]
- 2.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421(6918):37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 3.Miller-Rushing AJ, Primack RB. Global warming and flowering times in Thoreau’s Concord: A community perspective. Ecology. 2008;89(2):332–341. doi: 10.1890/07-0068.1. [DOI] [PubMed] [Google Scholar]
- 4.Ibáñez I, et al. Forecasting phenology under global warming. Philos Trans R Soc Lond B Biol Sci. 2010;365(1555):3247–3260. doi: 10.1098/rstb.2010.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cleland EE, Chuine I, Menzel A, Mooney HA, Schwartz MD. Shifting plant phenology in response to global change. Trends Ecol Evol. 2007;22(7):357–365. doi: 10.1016/j.tree.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Cook BI, Wolkovich EM, Parmesan C. Divergent responses to spring and winter warming drive community level flowering trends. Proc Natl Acad Sci USA. 2012;109(23):9000–9005. doi: 10.1073/pnas.1118364109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ovaskainen O, et al. Community-level phenological response to climate change. Proc Natl Acad Sci USA. 2013;110(33):13434–13439. doi: 10.1073/pnas.1305533110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estiarte M, Peñuelas J. Alteration of the phenology of leaf senescence and fall in winter deciduous species by climate change: Effects on nutrient proficiency. Glob Change Biol. 2014;21(3):1005–1017. doi: 10.1111/gcb.12804. [DOI] [PubMed] [Google Scholar]
- 9.Chuine I. A unified model for budburst of trees. J Theor Biol. 2000;207(3):337–347. doi: 10.1006/jtbi.2000.2178. [DOI] [PubMed] [Google Scholar]
- 10.Körner C, Basler D. Plant science. Phenology under global warming. Science. 2010;327(5972):1461–1462. doi: 10.1126/science.1186473. [DOI] [PubMed] [Google Scholar]
- 11.Polgar CA, Primack RB. Leaf-out phenology of temperate woody plants: From trees to ecosystems. New Phytol. 2011;191(4):926–941. doi: 10.1111/j.1469-8137.2011.03803.x. [DOI] [PubMed] [Google Scholar]
- 12.Clark JS, Melillo J, Mohan J, Salk C. The seasonal timing of warming that controls onset of the growing season. Glob Change Biol. 2014;20(4):1136–1145. doi: 10.1111/gcb.12420. [DOI] [PubMed] [Google Scholar]
- 13.Allen JM, et al. Modeling daily flowering probabilities: Expected impact of climate change on Japanese cherry phenology. Glob Change Biol. 2014;20(4):1251–1263. doi: 10.1111/gcb.12364. [DOI] [PubMed] [Google Scholar]
- 14.Estrella N, Menzel A. Responses of leaf colouring in four deciduous tree species to climate and weather in Germany. Clim Res. 2006;32(3):253–267. [Google Scholar]
- 15.Vitasse Y, Porté AJ, Kremer A, Michalet R, Delzon S. Responses of canopy duration to temperature changes in four temperate tree species: Relative contributions of spring and autumn leaf phenology. Oecologia. 2009;161(1):187–198. doi: 10.1007/s00442-009-1363-4. [DOI] [PubMed] [Google Scholar]
- 16.Gallinat AS, Primack RB, Wagner DL. Autumn, the neglected season in climate change research. Trends Ecol Evol. 2015;30(3):169–176. doi: 10.1016/j.tree.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Weih M. Genetic and environmental variation in spring and autumn phenology of biomass willows (Salix spp.): Effects on shoot growth and nitrogen economy. Tree Physiol. 2009;29(12):1479–1490. doi: 10.1093/treephys/tpp081. [DOI] [PubMed] [Google Scholar]
- 18.Fridley JD. Extended leaf phenology and the autumn niche in deciduous forest invasions. Nature. 2012;485(7398):359–362. doi: 10.1038/nature11056. [DOI] [PubMed] [Google Scholar]
- 19.Pépino M, Proulx R, Magnan P. Fall synchrony between leaf color change and brook trout spawning in the Laurentides Wildlife Reserve (Québec, Canada) as potential environmental integrators. Ecol Indic. 2013;30:16–20. [Google Scholar]
- 20.Spencer DM, Holecek DF. A profile of the fall foliage tourism market. J Vacation Marketing. 2007;13(4):339–358. [Google Scholar]
- 21.Rustad L, et al. 2011. Changing Climate, Changing Forests: The Impacts of Climate Change on Forests of the Northeastern United States and Easter Canada. USDA Forest Service Northern Research Station General Technical Report NRS-99. (US Department of Agriculture, Forest Service, Newtown Square, PA) 48 pp.
- 22.Ge Q, Dai J, Liu J, Zhong S, Liu H. The effect of climate change on the fall foliage vacation in China. Tour Manage. 2013;38:80–84. [Google Scholar]
- 23.Doi H, Takahashi M. Latitudinal patterns in the phenological responses of leaf colouring and leaf fall to climate change in Japan. Glob Ecol Biogeogr. 2008;17(4):556–561. [Google Scholar]
- 24.Dragoni D, Rahman AF. Trends in fall phenology across the deciduous forests of the Eastern USA. Agric For Meteorol. 2012;157:96–105. [Google Scholar]
- 25.Paul LK, Rinne PL, van der Schoot C. Shoot meristems of deciduous woody perennials: Self-organization and morphogenetic transitions. Curr Opin Plant Biol. 2014;17:86–95. doi: 10.1016/j.pbi.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Hänninen H, Tanino K. Tree seasonality in a warming climate. Trends Plant Sci. 2011;16(8):412–416. doi: 10.1016/j.tplants.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, et al. Monitoring vegetation phenology using MODIS. Remote Sens Environ. 2003;84(3):471–475. [Google Scholar]
- 28.Archetti M, Richardson AD, O’Keefe J, Delpierre N. Predicting climate change impacts on the amount and duration of autumn colors in a New England forest. PLoS One. 2013;8(3):e57373. doi: 10.1371/journal.pone.0057373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong S-J, Medvigy D. Macroscale prediction of autumn leaf coloration throughout the continental United States. Glob Ecol Biogeogr. 2014;23(11):1245–1254. [Google Scholar]
- 30.Lim PO, Kim HJ, Nam HG. Leaf senescence. Annu Rev Plant Biol. 2007;58:115–136. doi: 10.1146/annurev.arplant.57.032905.105316. [DOI] [PubMed] [Google Scholar]
- 31.Samish RM. Dormancy in woody plants. Annu Rev Plant Physiol. 1954;5(1):183–204. [Google Scholar]
- 32.Rosenthal SI, Camm EL. Photosynthetic decline and pigment loss during autumn foliar senescence in western larch (Larix occidentalis) Tree Physiol. 1997;17(12):767–775. doi: 10.1093/treephys/17.12.767. [DOI] [PubMed] [Google Scholar]
- 33.Fracheboud Y, et al. The control of autumn senescence in European aspen. Plant Physiol. 2009;149(4):1982–1991. doi: 10.1104/pp.108.133249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia RA, Cabeza M, Rahbek C, Araújo MB. Multiple dimensions of climate change and their implications for biodiversity. Science. 2014;344(6183):1247579. doi: 10.1126/science.1247579. [DOI] [PubMed] [Google Scholar]
- 35.Hoeting JA, Madigan D, Raftery AE, Volinsky CT. Bayesian Model Averaging: A tutorial. Stat Sci. 1999;14(4):382–417. [Google Scholar]
- 36.Ishwaran H, Rao JS. Spike and slab variable selection: Frequentist and Bayesian strategies. Ann Stat. 2005;33(2):730–773. [Google Scholar]
- 37.Abatzoglou JT, Brown TJ. A comparison of statistical downscaling methods suited for wildfire applications. Int J Climatol. 2012;32(5):772–780. [Google Scholar]
- 38.Barbieri MM, Berger JO. Optimal predictive model selection. Ann Stat. 2004;32(3):870–897. [Google Scholar]
- 39.Günthardt-Goerg MS, Vollenweider P. Linking stress with macroscopic and microscopic leaf response in trees: New diagnostic perspectives. Environ Pollut. 2007;147(3):467–488. doi: 10.1016/j.envpol.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 40.Cooke JE, Eriksson ME, Junttila O. The dynamic nature of bud dormancy in trees: Environmental control and molecular mechanisms. Plant Cell Environ. 2012;35(10):1707–1728. doi: 10.1111/j.1365-3040.2012.02552.x. [DOI] [PubMed] [Google Scholar]
- 41.Jibran R, A Hunter D, P Dijkwel P. Hormonal regulation of leaf senescence through integration of developmental and stress signals. Plant Mol Biol. 2013;82(6):547–561. doi: 10.1007/s11103-013-0043-2. [DOI] [PubMed] [Google Scholar]
- 42.Leuzinger S, Zotz G, Asshoff R, Körner C. Responses of deciduous forest trees to severe drought in Central Europe. Tree Physiol. 2005;25(6):641–650. doi: 10.1093/treephys/25.6.641. [DOI] [PubMed] [Google Scholar]
- 43.Larcher W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups. 4th Ed Springer; New York: 2003. [Google Scholar]
- 44.Carvalho HH, et al. The molecular chaperone binding protein BiP prevents leaf dehydration-induced cellular homeostasis disruption. PLoS One. 2014;9(1):e86661. doi: 10.1371/journal.pone.0086661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naschitz S, Naor A, Wolf S, Goldschmidt EE. The effects of temperature and drought on autumnal senescence and leaf shed in apple under warm, east Mediterranean climate. Trees (Berl) 2014;28(3):879–890. [Google Scholar]
- 46.Xu Y, Huang B. Heat-induced leaf senescence and hormonal changes for thermal bentgrass and turf-type bentgrass species differing in heat tolerance. J Am Soc Hortic Sci. 2007;132(2):185–192. [Google Scholar]
- 47.Rivero RM, et al. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc Natl Acad Sci USA. 2007;104(49):19631–19636. doi: 10.1073/pnas.0709453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pääkkönen E, Vahala J, Holopainen T, Kärenlampi L. Physiological and ultrastructural responses of birch clones exposed to ozone and drought stress. Chemosphere. 1998;36(4):679–684. [Google Scholar]
- 49.Hinckley TM, Dougherty PM, Lassoie JP, Roberts JE, Teskey RO. A severe drought: Impact on tree growth, phenology, net photosynthetic rate and water relations. Am Midl Nat. 1979;102(2):307–316. [Google Scholar]
- 50.Caspersen JP, Kobe RK. Interspecific variation in sapling mortality in relation to growth and soil moisture. Oikos. 2001;92(1):160–168. [Google Scholar]
- 51.Fu YSH, et al. Variation in leaf flushing date influences autumnal senescence and next year’s flushing date in two temperate tree species. Proc Natl Acad Sci USA. 2014;111(20):7355–7360. doi: 10.1073/pnas.1321727111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keenan TF, Richardson AD. The timing of autumn senescence is affected by the timing of spring phenology: Implications for predictive models. Glob Chang Biol. 2015;21(7):2634–2641. doi: 10.1111/gcb.12890. [DOI] [PubMed] [Google Scholar]
- 53.Diez JM, et al. Forecasting phenology: From species variability to community patterns. Ecol Lett. 2012;15(6):545–553. doi: 10.1111/j.1461-0248.2012.01765.x. [DOI] [PubMed] [Google Scholar]
- 54.PRISM Climate Group 2004. Prism Climate Data, Available at prism.oregonstate.edu. Accessed June 25, 2014.
- 55.Wilson AM, Silander JA., Jr Estimating uncertainty in daily weather interpolations: A Bayesian framework for developing climate surfaces. Int J Climatol. 2014;20(8):1251–1263. [Google Scholar]
- 56.Richardson AD, Bailey AS, Denney EG, Martin CW, O’Keefe J. Phenology of a northern hardwood forest canopy. Glob Change Biol. 2006;12(7):1174–1188. [Google Scholar]
- 57.Forsythe WC, Rykiel EJ, Stahl RS, Wu HI, Schoolfield RM. A model comparison for day length as a function of latitude and day of year. Ecol Modell. 1995;80(1):87–95. [Google Scholar]
- 58.Duque AS, et al. Abiotic stress responses in plants: Unraveling the complexity of genes and networks to survive. In: Vahdati K, Leslie C, editors. Abiotic Stress—Plant Responses and Applications in Agriculture. InTech; Rijeka, Croatia: 2013. pp. 49–55. [Google Scholar]
- 59.Niinemets Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For Ecol Manage. 2010;260(10):1623–1639. [Google Scholar]
- 60.Bréda N, et al. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann Sci. 2006;63(6):625–644. [Google Scholar]
- 61.Primack RB, et al. Spatial and interspecific variability in phenological responses to warming temperatures. Biol Conserv. 2009;142(11):2569–2577. [Google Scholar]
- 62.Wilczek AM, et al. Genetic and physiological bases for phenological responses to current and predicted climates. Philos Trans R Soc Lond B Biol Sci. 2010;365(1555):3129–3147. doi: 10.1098/rstb.2010.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hwang T, et al. Divergent phenological response to hydroclimate variability in forested mountain watersheds. Glob Change Biol. 2014;20(8):2580–2595. doi: 10.1111/gcb.12556. [DOI] [PubMed] [Google Scholar]
- 64.Dormann CF, et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36(1):27–46. [Google Scholar]
- 65.Hoerl AE, Kennard RW. Ridge regression: Biased estimation for nonorthogonal problems. Technometrics. 1970;12(1):55–67. [Google Scholar]
- 66.Park T, Casella G. The Bayesian LASSO. J Am Stat Assoc. 2008;103(482):681–686. [Google Scholar]
- 67.Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc, B. 2005;67(2):301–320. [Google Scholar]
- 68.Sharma DB, Bondell HD, Zhang HH. Consistent group identification and variable selection in regression with correlated predictors. J Comput Graph Stat. 2013;22(2):319–340. doi: 10.1080/15533174.2012.707849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. [Google Scholar]
- 70.Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Caski S, editors. Proceedings of the Second International Symposium on Information Theory. Akademiai Kaido; Budapest, Hungary: 1973. pp. 267–281. [Google Scholar]
- 71.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6(2):461–464. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.