Significance
Glaucoma is associated with increased pressure in the eye, which can be alleviated by increasing outflow from the eye or reducing inflow of aqueous humor produced in the ciliary epithelium. A special form of Na,K-ATPase (α2β3 isoform), the ion pump that maintains electrochemical gradients of Na+ and K+ across animal cell membranes, powers production of aqueous humor. We describe derivatives of a classical Na,K-ATPase inhibitor, digoxin, with selectivity for the α2β3 isoform over the common α1β1 isoform and show that topical application of α2β3-selective derivatives to rabbit eyes efficiently reduces either pharmacologically raised or basal intraocular pressure. This work confirms the importance of α2β3 in production of aqueous humor and may provide a novel approach for glaucoma drug therapy.
Keywords: Na/K-ATPase, α2β3 isoform, digoxin derivatives, intraocular pressure
Abstract
The ciliary epithelium in the eye consists of pigmented epithelial cells that express the α1β1 isoform of Na,K-ATPase and nonpigmented epithelial cells that express mainly the α2β3 isoform. In principle, a Na,K-ATPase inhibitor with selectivity for α2β3 that penetrates the cornea could effectively reduce intraocular pressure, with minimal systemic or local toxicity. We have recently synthesized perhydro-1,4-oxazepine derivatives of digoxin by NaIO4 oxidation of the third digitoxose and reductive amination with various R-NH2 substituents and identified derivatives with significant selectivity for human α2β1 over α1β1 (up to 7.5-fold). When applied topically, the most α2-selective derivatives effectively prevented or reversed pharmacologically raised intraocular pressure in rabbits. A recent structure of Na,K-ATPase, with bound digoxin, shows the third digitoxose approaching one residue in the β1 subunit, Gln84, suggesting a role for β in digoxin binding. Gln84 in β1 is replaced by Val88 in β3. Assuming that alkyl substituents might interact with β3Val88, we synthesized perhydro-1,4-oxazepine derivatives of digoxin with diverse alkyl substituents. The methylcyclopropyl and cyclobutyl derivatives are strongly selective for α2β3 over α1β1 (22–33-fold respectively), as determined either with purified human isoform proteins or intact bovine nonpigmented epithelium cells. When applied topically on rabbit eyes, these derivatives potently reduce both pharmacologically raised and basal intraocular pressure. The cyclobutyl derivative is more efficient than Latanoprost, the most widely used glaucoma drug. Thus, the conclusion is that α2β3-selective digoxin derivatives effectively penetrate the cornea and inhibit the Na,K-ATPase, hence reducing aqueous humor production. The new digoxin derivatives may have potential for glaucoma drug therapy.
Glaucoma is a disease leading to irreversible blindness. Control of intraocular pressure (IOP) is the mainstay of glaucoma therapy and is achieved by various drugs, such as β-blockers, prostaglandin analogs, α2 adrenergic receptor agonists, cholinergic agonists, and carbonic anhydrase inhibitors given topically or systemically (1). The topical route minimizes systemic side effects and is preferable, provided the drug effectively permeates the cornea. Despite the selection of drugs available, uncontrolled IOP in many patients eventually makes surgical intervention necessary. Thus, fresh approaches to drug treatments are required.
The Na,K-ATPase provides the motive power for aqueous humor production in the ciliary epithelium. The sodium, potassium–adenosine triphosphatase (Na,K-ATPase) is a heterodimer of two subunits, α the catalytic subunit and the β subunit that stabilizes the protein and affects functional properties, together with a regulatory FXYD subunit (FXYD1–7) (2). There are four isoforms of α (α1−α4) and three isoforms of β (β1−β3). The α1β1 complex is the ubiquitous complex. α2 is expressed strongly in skeletal and cardiac muscle, brain astrocytes, and also ciliary epithelium. α3 is the neuronal form and α4 is restricted to testes (3). The ciliary epithelium is a syncitium consisting of an inner layer of pigmented epithelium (PE) facing the stroma and the outer nonpigmented epithelium (NPE) layer facing the aqueous humor. Na,K-ATPase is localized to the basolateral surface of both layers (4). Importantly, it is known that the primary isoform of Na,K-ATPase in PE is α1β1, whereas that in NPE is α2β3 in rodents (5).
Cardiac glycosides (CGs) consist of a steroid core with a five- or six-membered unsaturated lactone ring and a variable number of sugars bound to the C3 position of the steroid. Each of these structural features has specific effects on the binding properties (6). Crystal structures of the Na,K-ATPase are available at moderate resolution in high-affinity complexes with ouabain (7) or bufalin and digoxin (8) and a low-affinity complex with bound K and ouabain (9). The high-affinity binding site is formed by a deep pocket comprised of transmembrane helices αTM1, 2, 4, 5, and 6, with the lactone-steroid moiety pointing inwards and sugars outwards. Binding of K+ occurs via backbone carbonyl oxygens in TM4 that also ligand the lactone moiety in the absence of K+, explaining the well-known antagonistic effect of K+ on CG binding (7–9).
This paper focuses on α2β3-selective digoxin derivatives. The underlying hypothesis is that CGs with selectivity for the principal isoform complex in NPE cells, and sufficiently permeable to enter the eye when applied topically, could effectively inhibit aqueous humor inflow and reduce IOP. As background, digoxin shows moderate selectivity for the α2β1 isoform, and the selectivity is attributable to the tridigitoxose glycan moiety, as inferred from the fact that aglycones such as digoxigenin show no isoform selectivity (10). Following this work, we synthesized a series of perhydro-1,4-oxazepine derivatives of digoxin modified in the third sugar and showed that certain derivatives have enhanced selectivity for α2β1 (up to 7.5-fold) compared with digoxin itself (about fourfold) (11). The two most α2β1-selective derivatives effectively prevented or reversed a pharmacologically induced rise in IOP in rabbits.
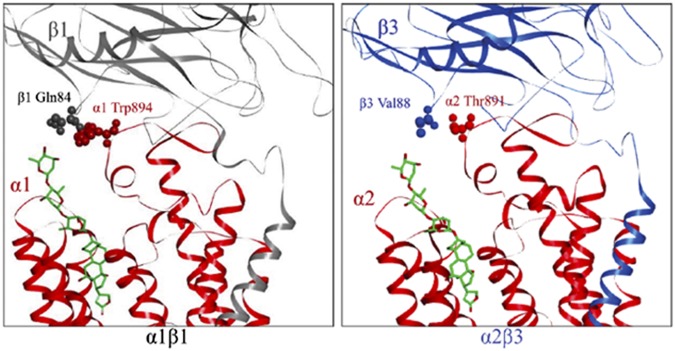
The current work is based on further insight regarding the β subunit, derived from a recent molecular structure of the Na,K-ATPase with bound digoxin (8). The glycan moiety of digoxin points outwards, and most interestingly, the third digitoxose approaches the β1 subunit near a single residue, Gln84 (Fig. 1). Gln84 in β1 is not conserved in β3 but is replaced by Val88 (Fig. 1). We hypothesized that diverse alkyl substitutions in the perhydro-1,4-oxazepine series of digoxin derivatives might interact with the alkyl side chain of Val88 and so raise selectivity for α2β3:α1β1. Several new alkyl derivatives do show strong selectivity for α2β3 over α1β1 and also potently reduce IOP in rabbits when applied topically.
Fig. 1.
Model of human Na,K-ATPase α1β1 and α2β3 isoforms. The model depicts the human α1β1 complex and human α2β3 complex on the pig α1β1 structure with bound digoxin (4RET). α1 and α2 subunits, red; β1 subunit, gray; β3 subunit, blue; digoxin, green.
Results
Identification of Digoxin Derivatives with Selectivity for the α2β3 Isoform Complex.
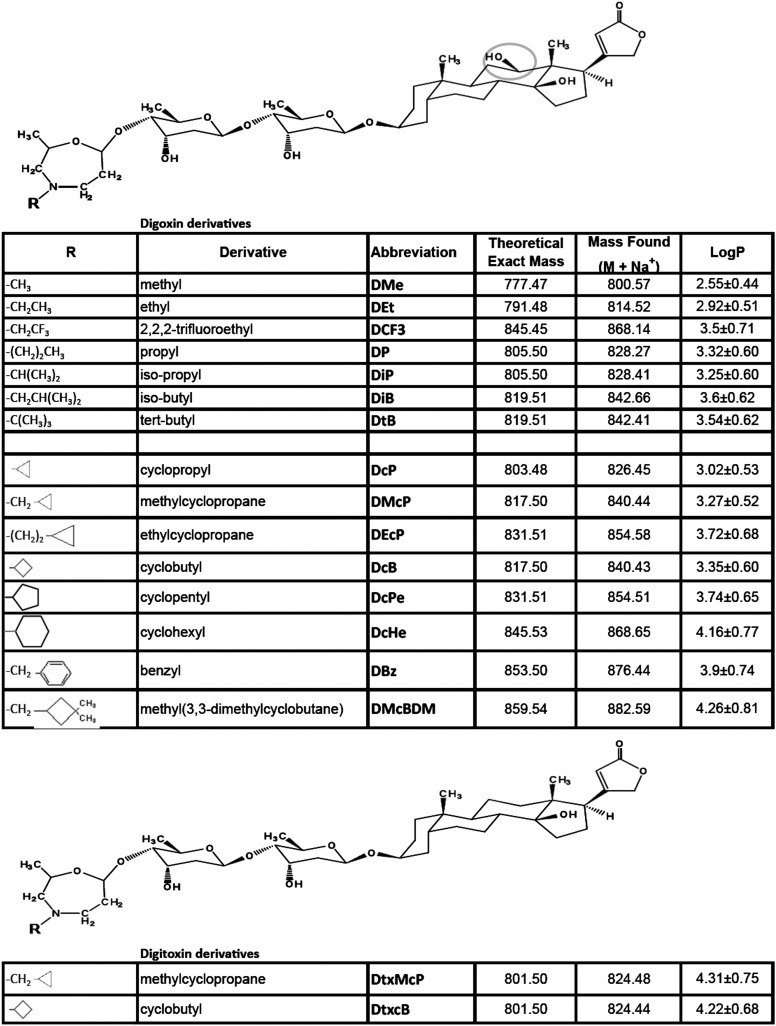
We have synthesized a series of primary, secondary, tertiary, and cyclo alkyl perhydro-1,4-oxazepine derivatives of the third digitoxose of digoxin (and digitoxin) by selective oxidation with NaIO4 and reductive amination. The compounds were purified by HPLC, and masses were determined to verify correctness of the structure and purity. The structure and names of the different amine substituents (R-NH2) and theoretical and experimentally found masses are shown in Fig. S1. 1H-NMR and 13C-NMR spectra, with full assignments, confirm the structures of several derivatives (12). Calculated n-octanol-water partition coefficients (logP) are also shown in Fig. S1. Reference values of LogP for digoxin and digitoxin are 1.26 and 1.85, respectively.
Fig. S1.
Structures, masses, and LogP values of digoxin and digitoxin derivatives. LogP values were calculated using the ALOGPs2.1 programme (www.vcclab.org) and represent the average of seven predictions ± SD. Reference values for Digoxin and Digitoxin are 1.26 and 1.85, respectively.
To screen for isoform selectivity of the digoxin derivatives, we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isoform complexes α1β1FXYD1, α2β1FXYD1 described previously (11, 13, 14), and α2β3FXYD1, which is described briefly in Methods. Although all of the preparations were reconstituted with FXYD1 to stabilize them (14), for simplicity the FXYD1 is omitted in naming of isoform complexes.
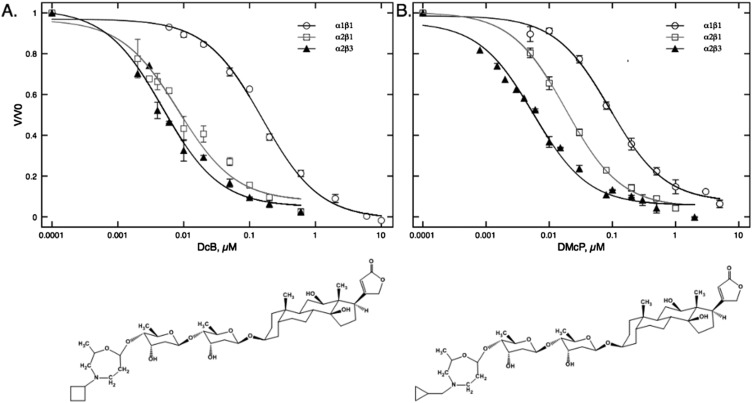
Table 1 shows the Ki values and selectivity ratios (Ki α1β1:α2β1 and α1β1:α2β3) for inhibition of Na,K-ATPase activity of the new compounds compared with digoxin itself and others, such as DMe, described recently (11). (In the current study, we measured Ki rather than intrinsic dissociation constants, KD, because Ki is easier to measure for hydrophobic compounds and is more relevant to physiological effects.) Several new derivatives show significantly improved selectivity for α2β3 compared with α2β1 over α1β1—in particular, DiP, DiB, DcP, DMcP, and DcB. Two derivatives, DMcP and DcB (double asterisk), show strong selectivity for α2β3 of 22- and 33-fold, respectively, and very low Ki values for inhibition of α2β3 (Ki ∼ 4 nM). The full inhibition curves for DMcP and DcB with the structures are shown in Fig. S2. Digoxin itself has moderate selectivity for α2β1 over α1β1 (c. fourfold) (10) and moderately increased selectivity for α2β3 compared with α2β1 (c. sixfold). Bearing in mind the well-known K-CG antagonism (15), in a reaction medium containing 5 mM K ions, the Ki values will be affected by K0.5 K ions for activating Na,K-ATPase activity of α1β1, α2β1, and α2β3, which are 1.24 ± 0.05, 2.7 ± 0.14, and 6.4 ± 0.5 mM, respectively. Thus, moderately increased selectivity of digoxin for α2β3:α2β1 may be attributed to reduced K-CG antagonism. All other derivatives show some increase in Ki α1β1:α2β3 compared with Ki α1β1:α2β1, which must also be partly due to K-CG antagonism. However, the difference for the most α2β3-selective compounds is significantly greater than for digoxin and cannot be explained only by this factor. In particular, there is a distinct structural effect. Maximal α2β3 selectivity is seen for R-substituents with four carbon atoms, such as isobutyl (DiB), methylcyclopropyl (DMcP), and cyclobutyl (DcB) (**), with selectivity for α2β3:α1β1 of 16-, 22-, and 33-fold, respectively. For derivatives with five or a higher number of carbon atoms in the R-substituents (such as DcPe, DcH, and DBz and DMcBDM), selectivity for α2β3 (10–12-fold) and also for R-substituents with three or less carbons was lower (DMe, DEt, DCF3, DP, DiP, DcP). Another striking insight comes from findings with two digitoxin derivatives (DtxMcP and DtxcB). Digitoxin has an identical structure to digoxin, except for the lack of a single 12’OH group in the steroid ring (Fig. S1). The Ki values are very low for α1β1, α2β1, and α2β3 and showed essentially no increase in selectivity for α2β3 (Discussion).
Table 1.
Ki values for inhibition of Na,K-ATPase activity of individual isoforms by digoxin derivatives and isoform selectivity ratios
| CG | R = C atoms | Ki, nM ± SEM | Selectivity, Ki | ||||
| α1β1 | α2β1 | α2β3 | α1β1/α2β1 | α1β1/α2β3 | |||
| Digoxin | 0 | 268 ± 13.8 | 58.7 ± 5.4 | 42.8 ± 3.0 | 4.5 | 6.2 | |
| DMe | 1 | 103 ± 5.6 | 15.3 ± 1.2 | 10.8 ± 0.6 | 6.7 | 9.5 | |
| DEt | 2 | 137.9 ± 12.6 | 23.2 ± 0.9 | 14.4 ± 1.27 | 5.9 | 9.5 | |
| DCF3 | 2 | 119 ± 15.0 | 28.6 ± 0.9 | 12.4 ± 1.5 | 4.1 | 9.6 | |
| DP | 3 | 87.7 ± 7.9 | 18.3 ± 1.68 | 9.8 ± 1.1 | 4.8 | 8.8 | |
| DiP | 3 | 149 ± 20.7 | 28.9 ± 1.7 | 10.3 ± 1.8 | 5.1 | 14.4 | |
| DcP | 3 | 109 ± 6.2 | 14.6 ± 11.6 | 8.1 ± 1.36 | 7.5 | 13.4 | |
| DtB | 4 | 135 ± 12.1 | 21.6 ± 5.6 | 16.3 ± 0.28 | 6.2 | 8.2 | |
| DiB | 4 | 92 ± 8.9 | 20.6 ± 1.4 | 5.8 ± 0.6 | 4.4 | 16** | |
| DMcP | 4 | 95.8 ± 13.7 | 18.3 ± 1.6 | 4.3 ± 0.6 | 5.2 | 22.2** | |
| DcB | 4 | 135 ± 11 | 8 ± 1.25 | 4 ± 0.15 | 16.9 | 33.6** | |
| DcPe | 5 | 138 ± 21 | 33.4 ± 7.5 | 27.6 ± 9.5 | 4.1 | 5.0 | |
| DcH | 6 | 70.4 ± 4.1 | 15.2 ± 3.7 | 11.7 ± 4.5 | 4.6 | 10.1 | |
| DBz | 6 | 57.9 ± 15.5 | 10.1 ± 2.2 | 5.6 ± 1.6 | 5.7 | 10.3 | |
| DMcBDM | 7 | 31.6 ± 0.5 | 8.6 ± 1.4 | 3.9 ± 0.7 | 3.7 | 8.2 | |
| Digitoxin | 0 | 89 ± 15.8 | 29.5 ± 2.7 | 28.8 ± 5.9 | 3.0 | 3.1 | |
| Dtx.McP | 4 | 25 ± 4 | 4.2 ± 0.4 | 3.7 ± 0.5 | 5.9 | 6.7 | |
| Dtx.cB | 4 | 30.7 ± 7.2 | 5.4 ± 0.5 | 4.3 ± 0.6 | 5.6 | 7.1 | |
The following amines were used: DBz, benzylamine; DcB, cyclobutylamine; DCF3, trifluorethylamine; DcH, cyclohexylamine; DcP, cyclopropylamine; DcPe, cyclopentylamine; DEt, ethylamine; DiB, isobutylamine; DiP, isopropylamine; DMcBDM, 3,3-dimethylcycobutylmethanamine; DMcP, cyclopropylmethanamine; DMe, methylamine; DP, propylamine; DtB, tert-butylamine; Dtx.cB, cyclobutylamine; Dtx.McP, cyclopropylmethanamine.
Fig. S2.
Inhibition curves for α1β1, α2β1, and α2β3 and structures of (A) DcB and (B) DMcP.
Inhibition of Na,K-ATPase Activity in Permeabilized Bovine NPE Cells.
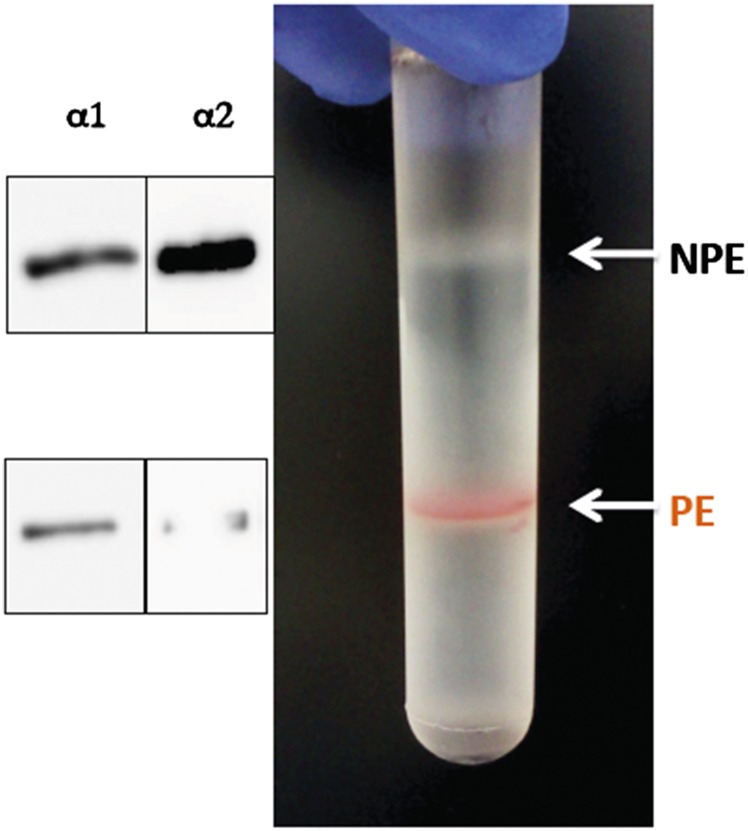
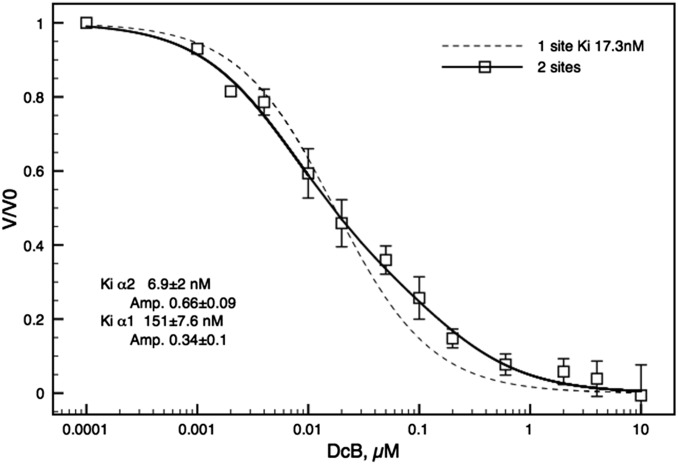
One can ask legitimately whether the selectivity features for the digoxin derivatives observed with purified detergent-soluble recombinant human isoforms apply also to the Na,K pumps in native ciliary epithelium. As a test of this question we have looked at inhibition of Na,K-ATPase in NPE cells isolated from the bovine ciliary body. Fig. S3 shows separation of NPE and PE cells on a density gradient, and Western blots probed with isoform-selective antibodies, calibrated using purified human isoforms, show that NPE cells contain about 70% α2 to 30% α1, whereas PE cells have about 90% α1 to 5–10% α2. After unmasking with alamethicin, Na,K-ATPase activity was found to be 0.195 ± 0.027 and 0.035 ± 0.008 μmoles·mg protein·min, with ouabain-sensitive fractions of total ATPase activity of about 65% and 35%, in NPE and PE cells, respectively. Average Ki values ± SEM from three to four separate experiments for inhibition of NPE Na,K-ATPase activity by digoxin, DMe, DMcP, and DcB, fitted to a single site inhibition model, were 91.7 ± 10.2, 15. 6 ± 1.3, 7.9 ± 2.2, and 17.3 ± 2.5 nM, respectively. However, because NPE cells contain about 70% α2 to 30% α1, in principle the inhibition of Na,K-ATPase activity should reflect the properties of the isoform mixture. Fig. 2 shows the detailed inhibition curve for DcB. The curve is clearly fitted better by a two-site model than a one site-model using the average Ki value of 17.3 nM. The best fit parameters—66% α2:34% α1 and Ki 6.9 ± 2 nM:Ki 151 ± 7.6 nM (Kiα1/α2, c. 22-fold)—in the inset are quite close to the proportions of α2:α1 estimated in the Western blots (Fig. S3) and the selectivity ratio Kiα1β1/α2β3 ∼ 33 in Table 1. Thus, selectivity properties of DcB observed with purified human isoforms are relevant also to intact NPE cells.
Fig. S3.
Density gradient separation of bovine NPE and PE cells and Western blots of α1 and α2 isoforms.
Fig. 2.
Inhibition of Na,K-ATPase activity of bovine NPE cells by DcB. Inhibition of Na,K-ATPase activity in NPE cells by DcB was fitted to the two sites model [VCG/V0 = Aα2 * Ki2/([CG] + Ki2) + Aα1 * Ki1/([CG] + Ki1), where A and Ki represent the amplitudes and Ki values for α2 and α1, respectively]. The dashed line shows the theoretical fit for the one site model [VCG/V0 = Ki/([CG] + Ki), with a Ki of 17.3 nM]. Each point represents the average value from four independent experiments ± SEM.
Reduction of IOP in Rabbits.
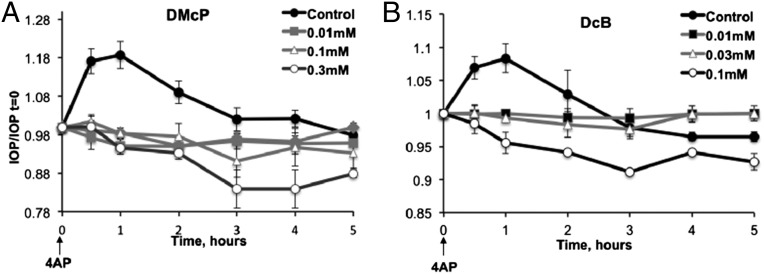
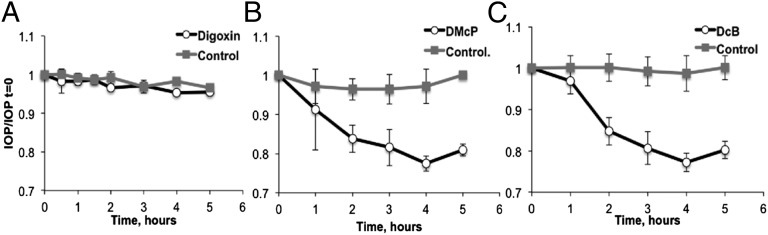
The experiments in this section examined the effects of topically applied α2β3-selective digoxin derivatives, DMcP and DcB, on IOP in rabbits. Due to the much lower Ki for inhibition of α2β3, compared with digoxin, and their hydrophobic character (logP: DMcP, 3.27 and DcB, 3.35 versus digoxin, 1.26) (Fig. S1), these derivatives should both permeate the cornea very well and efficiently inhibit the α2β3 in the NPE cells, thus reducing inflow of aqueous humor and IOP. The first set of experiments examined the effects of these compounds when applied just before 4-aminopyridine (4AP), used to transiently raise IOP (11). Fig. 3 A and B shows that 10 μM or higher concentrations of either DMcP or DcB prevented the 4AP-induced rise in IOP. DMcP and DcB are both considerably more effective than the optimal compound in our previous study, DMe (11), and also DiB, described here. More interestingly, higher concentrations (0.1–0.3 mM) of either DMcP or DcB reduced IOP to levels significantly below the starting value. This observation implies that DMcP and DcB could reduce basal IOP, even in the absence of 4AP, as tested in the experiment of Fig. 4. Using groups of three rabbits, one drop of 1 mM digoxin, DMcP, and DcB was applied to one eye, whereas the other eye received PBS and served as the control. Over the test period of 4–5 h, digoxin did not affect basal IOP, confirming our previous observations (11). By contrast, both DMcP and DcB significantly reduced the basal IOP by 20–25% (∼4 mmHg for rabbits with a basal IOP of 17 mmHg). DcB (2 mM) did not reduce the IOP more than 1 mM.
Fig. 3.
Effects of DMcP (A) and DcB (B) on 4AP-induced ocular hypertension. One drop of DMcP or DcB was added to both eyes 30 min before addition of 4AP. During this preincubation period, there was little or no change in IOP. IOP was measured at the indicated times after addition of 4AP. The values of IOP/IOP t = 0 represent the average for three rabbits (i.e., six eyes) ± SEM.
Fig. 4.
Effects of Digoxin (A), DMcP (B), and DcB (C) on basal IOP. One drop of 1 mM of digoxin, DMcP, or DcB was added to the RE and one drop of PBS to the LE as the control, at time 0. IOP was measured at the indicated times. The values of IOP/IOP t = 0 represent the average for three rabbits ± SEM.
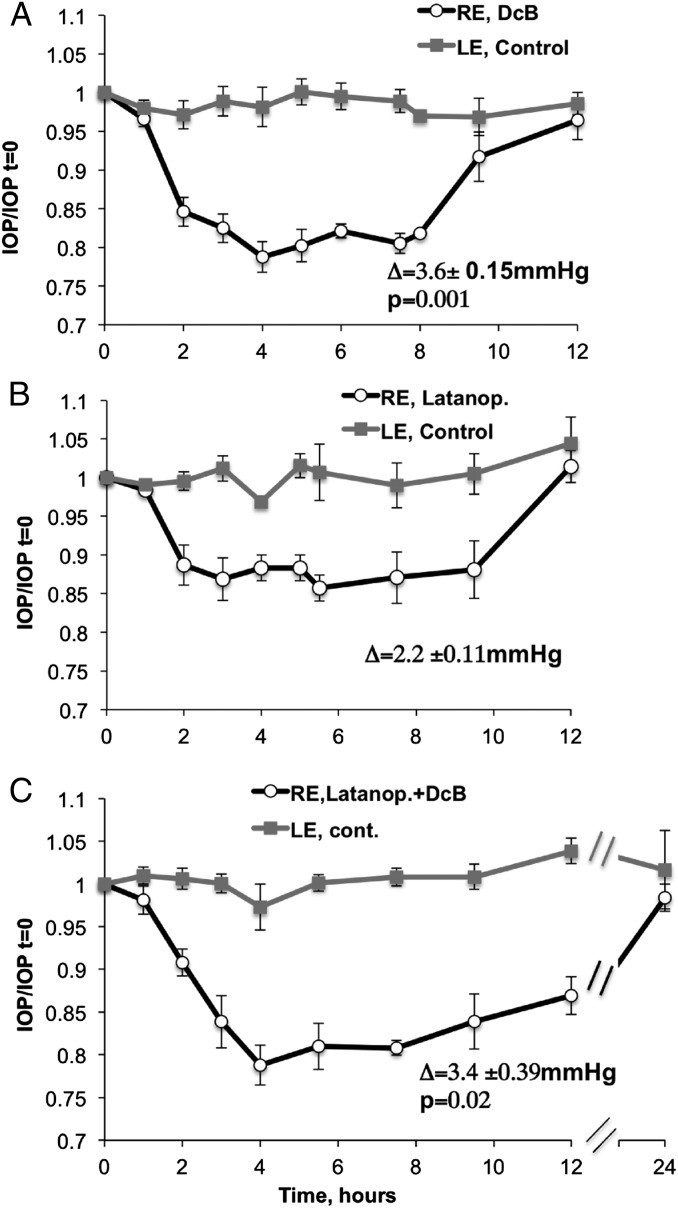
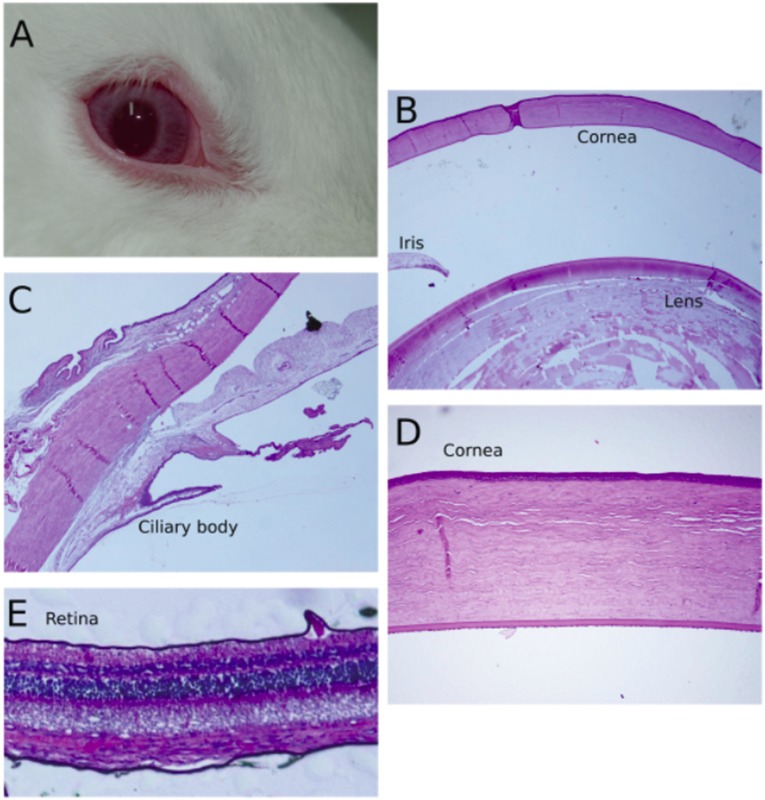
We have compared the effectiveness and duration of the effects on basal IOP of topical DcB with the antiglaucoma drug Latanoprost, applied alone or in combination (Fig. 5 A–C). The steady-state IOP was lower by 3.5 ± 0.15, 2.6 ± 0.11, and 3.44 ± 0.39 mmHg for DcB, Latanoprost, and DcB plus Latanoprost, respectively, whereas the control was unchanged. Reduced IOP was maintained by DcB for about 8 h and returned to the control value by 12 h (Fig. 5A). This is considerably longer than the 4–5 h observed for the optimal compound, DMe, in our previous study, using the 4AP protocol (11). A similar time course was seen with Latanoprost (Fig. 5B). By contrast, with combined DcB plus Latanoprost, the low IOP was maintained for significantly longer, returning to the control value only after 24 h (Fig. 5C). Corneal thickness was measured after DcB (four rabbits) or Latanoprost (two rabbits) was applied once a day for 6 d. As seen in Table S1, there was no detectable effect of either drug on corneal thickness. Furthermore, histologic examination did not reveal retinal damage or significant damage to tissues of the anterior chamber, including the lens, iris, and ciliary body, and no significant external redness or ocular irritation was observed (Fig. S4, DcB-treated eye).
Fig. 5.
Effect of DcB and Latanoprost, separately and combined, on basal IOP. RE: (A) DcB, 1 mM; (B) Latanoprost, 0.005%; (C) 1 mM DcB + Latanoprost 0.005%. LE: PBS, control. Within each figure, the inserted symbol Δ indicates the absolute difference in IOP (in mmHg) between time IOP t = 0 and the average of IOP between 3 and 8 h ± SEM. The P values refer to the difference between DcB versus Latanoprost (A) or DcB/Latanoprost versus Latanoprost (C). Points represent averages IOP/IOP t = 0 from five rabbits ± SEM.
Table S1.
Corneal thickness after application of DcB or Latanoprost
| Pachymetry, μm | |||
| Rabbit | Treatment to RE | RE | LE |
| 1 | DcB | 455 | 456 |
| 2 | DcB | 480 | 456 |
| 3 | DcB | 400 | 396 |
| 4 | DcB | 382 | 374 |
| 5 | Latanoprost | 467 | 471 |
| 6 | Latanoprost | 489 | 498 |
Fig. S4.
Exterior view and histological sections of rabbit eye after DcB treatment. (A) whole eye; (B) cornea, iris, lens; (C) ciliary body; (D) cornea; and (E) retina.
Discussion
The β Subunit and Binding of Digoxin Derivatives with α2β3 Selectivity.
The data in Table 1 show clearly that, compared with digoxin itself, the isobutyl, methylcyclopropyl, and cyclobutyl derivatives—DiB, DMcP, and DcB—have rather strong selectivity for α2β3:α1β1, reaching 16-, 22-, and >33-fold, respectively. As judged by the preference for R-substituents with 4C atoms, especially DMcP and DcB, and the fact that digitoxin derivatives DtxMcP and DtxcB do not show increased selectivity for α2β3, the findings provide a clear indication for a selective structural interaction of the modified sugar of digoxin with the β3 subunit.
The hypothesis that hydrophobic perhydro-1,4-oxazepine derivatives of digoxin could interact better with Val88 of β3 than with Gln94 of β3 has provided the rationale for preparing derivatives that do show strong selectivity for the α2β3 isoform. Nevertheless, the exact nature of the β-selective interaction with DMcP and DcB is yet to be unequivocally demonstrated. The cyclobutyl or methyl cyclopropane substituents could interact directly with the Val88 of β3 or fit better into the space between the α2 and β3 subunits or raise selectivity by other interactions. Because the cyclobutyl or methyl cyclopropane derivatives of digitoxin (lacking the 12OH) do not show the increased α2β3 selectivity, quite small structural modifications of the steroid core must affect the disposition of the sugar chain. There is some independent evidence for a difference in binding of digoxin and digitoxin. The recent structure of renal Na,K-ATPase with bound digoxin shows that the 12OH of digoxin is close enough to Asn122 of TM2 of the α subunit to interact via an H bond (8). Digitoxin is known to bind to renal Na,K-ATPase with a higher affinity than digoxin (10, 16) (and see also Table 1), and because the 12OH–α interaction is absent, other interactions must tip the balance toward greater binding affinity for digitoxin. One could speculate that a tilt or rotation of the bound digitoxin steroid would suffice to twist the third digitoxose away from the β subunit.
Overall, an important conclusion is that both α and β subunits play a role in digoxin binding, and both α and β subunits are relevant in the design of isoform-selective digoxin derivatives. The term “design” must, of course, be qualified as semirational in that the syntheses use clues from the structures to introduce various substituents into derivatives, which are screened with the purified isoform complexes. In any case, the findings strongly support a role of the β subunit in digoxin binding suggested by the structure itself (8) and also older data on photoaffinity labeling (17).
Effects of Digoxin Derivatives on IOP.
One basic conclusion from this work is that α2β3 plays a central role in the production of aqueous humor, consistent with the prominent expression of α2β3 in NPE cells (5). In principal, drugs may reduce IOP either by inhibiting inflow or stimulating outflow of aqueous humor (18). Although stimulation of outflow by the digoxin derivatives is not excluded (see, for example, ref. 19), especially as expression of α2 in the trabecular meshwork is not known, the current findings are more consistent with inhibition of aqueous humor inflow by DcB and DMcP. One indication is the high affinity for inhibition of Na,K-ATPase by DcB in the NPE cells (Fig. 2). Derivatives with the lowest Ki for inhibition of α2β3 in NPE cells and highest permeability via the cornea are predicted to inhibit active Na and K fluxes and aqueous humor inflow most potently. IOP measurements do not indicate which individual property predominates, but the low Ki and high logP values of DMcP or DcB (3.27 and 3.55, respectively; Fig. S1) suggest that both factors are important. The finding that DMcP and DcB reduce basal IOP as well as prevent acutely raised IOP (Fig. 4) provides a compelling kinetic argument for inhibition of aqueous humor inflow. The transepithelial NaCl flux involves a combination of Na/H plus Cl/HCO3 exchange or 2Cl/Na/K cotransport into the PE cells, passage of the NaCl into the NPE cells via tight junctions, and extrusion of NaCl as the aqueous humor via a Cl channel and the Na,K pump of the NPE baso-lateral membrane (20). In basal conditions, net transepithelial NaCl inflow is limited by the Cl flux via the Cl channel (20). Because Na extrusion via the Na,K pump is not limiting in basal conditions, partial inhibition of the Na,K pump cannot greatly affect fluid transport. When fluid transport is stimulated by 4AP (21), active Na extrusion may become rate-limiting for net salt flux, and so inhibition of active Na and K fluxes can normalize IOP. To reduce IOP in basal conditions, inhibition of active Na transport must be severe enough to make it rate-limiting for transepithelial NaCl flux. In practice, this is achieved only with the most permeable and potent inhibitors, DMcP and DcB, at high concentrations, whereas digoxin itself and other derivatives such as DMe have no effect and, presumably, are not potent or permeable enough (logP 1.25 and 2.55, respectively; Fig. S1). In theory, full inhibition of the Na,K pump could completely block production of aqueous humor. However, maximal IOP reduction (25–30% at 2 mM DcB) may be limited by factors such as dissociation and washout of the inhibitor or counterpressures within the eye that prevent its collapse. In any case, reduction of basal IOP by DMcP and DcB is explained simply by the accepted physiological model on the assumption that they inhibit inflow. A word of caution concerning an exclusive role of α2β3 is appropriate. There are reports of heterogeneity of the NPE cell layer in human and bovine eyes with diversity and gradient of α isoform expression and abundant β2 expression (4, 22), although β3 was not looked for and questions concerning the specificity of the antibodies used to detect β2 have been raised (5). We have shown separately that DcB also displays enhanced selectivity for the human α2β2 complex, with the order Ki α2β3 < α2β2 < α2β1 < α1β1. Thus, the effects of the digoxin derivatives on IOP are unlikely to be significantly altered depending on whether NPE cells express only α2β3 as in rodents or α2β2 as reported for humans and cows (Fig. 2, which shows a high selectivity of DcB for α2:α1, was done with bovine NPE cells).
Regarding potential application of the digoxin derivatives as topical ophthalmological drugs, improved efficacy, duration of effects, or reduced side effects compared with currently available glaucoma drugs are necessary requirements (18). It is significant that DcB produced a 25–30% larger reduction in basal IOP than Latanoprost, the current first-line drug. Furthermore, the DcB/Latanoprost combination reduced IOP for considerably longer than DcB or Latanoprost alone. In principle, the ability of DMcP and DcB to reduce the basal IOP could be relevant not only to optical hypertension and primary open angle glaucoma but also to normotensive glaucoma for which reduction of IOP below the basal level is mandatory (1). Regarding local toxicity, α2β3 (or α2β2)-selective digoxin derivatives could have an additional benefit of producing only minimal side effects on corneal endothelium (23) or lens epithelium (24) because these tissues express mainly the ubiquitous α1 isoform. On the other hand, other ocular tissues such as retina express various combination of α1−3 and β1−3 isoforms (25), making it essential to examine possible local toxic effects of the digoxin derivatives. Corneal swelling was not observed (Table S1), histological examination did not reveal damage to ocular tissues, and local redness and irritation were not observed (Fig. S4). Concerning possible systemic toxicity, such as cardiotoxicity observed in clinical use of digoxin, α2-selective digoxin derivatives are likely to be intrinsically noncardiotoxic (26). Furthermore, the circulating concentration is likely to be very low. Using conventional pharmacokinetic assumptions (27), one can make a worst-case calculation of the concentration in the region of 10 pM, which is negligible compared with the Kis for inhibition of either α1 or α2 (Table 1). [Assume that 10% of the 30 μL drop of DcB enters the eye and is diluted by the aqueous humor (i.e., 3 μL of a 1 mM solution into 250 μL to about 12 μM). Then assume that over 1–2 h the aqueous humor is washed into the general circulation and the DcB is distributed in the same apparent volume as oral digoxin (c. 300 L for a 75 kg man) (27). The calculated concentration is c. 10 pM.]
Conclusion.
Digoxin derivatives, such as DcB, might become interesting candidates for development as drugs for treatment of glaucoma, either as monotherapy or combined with other drugs. Of course, realization of the pharmacological potential will depend on tests of potency and toxicity in additional animal models, with natural or induced glaucoma (18), toward possible human trials.
Materials
Digoxin (D6003), 4AP (A78403), and Alamethicin (A5361) were obtained from Sigma, Latanoprost (XalatanTM, Pfizer). The methanol HPLC grade was from Baker. All of the organic solvents, reagents, and amines were of the highest purity analytical grade.
Methods
Molecular Modeling.
The human homology models were prepared with Discovery Studio 4.0 Homology Model module (BioVia; www.3ds.com/products-services/biovia/) using the recently published structure of the Na,K-ATPase in complex with digoxin [α1β1 Protein Data Bank ID code 4RET] as the template. The α1, β1, α2, and β3 sequences were aligned to the template structure using the Align Sequence to Templates module, with manual adjustments when needed, and the Built Homology Model module was built based on this structural alignment. The models were verified using the Verify Protein (MODELER) module. The Discrete Optimized Protein Energy (DOPE) Score and Normalized DOPE Score DOPE method were calculated for each structure. In addition, probability density function Total Energy and probability density function Physical Energy were taken into consideration.
Synthesis of Perhydro-1,4-Oxazepine Derivatives of Digoxin and Digitoxin.
Synthesis of derivatives of digoxin and digitoxin, by oxidation with NaIO4 and reductive amination with different amines, R-NH2, HPLC purification, and structural characterization by mass spectroscopy and in several cases 1H-NMR and 13C-NMR, was done as described before (11). The n-octanol-water partition coefficients and LogP values were calculated using the ALOGPs2.1 program (www.vcclab.org) and represent the average of seven trials (± SD).
Expression, Purification, and Activity of Human Na,K-ATPase Isoforms.
Pichia pastoris transformation, yeast growth, membrane preparation, and purification of recombinant human α1β1, α2β1, and Na,K-ATPase were done as described (10, 11). The procedure for α2β3 is similar. Purified isoform complexes (0.3–0.5 mg/mL) were eluted from the BD-Talon beads in a solution containing 200 mM Imidazole, 100 mM NaCl, 20 mM Tricine∙HCl pH 7.4, 0.1 mg/mL C12E8, 0.07 mg/mL 1-Stearoyl-2-Oleoyl-sn-Glycero-3-[Phospho-L-Serine], 0.01 mg/mL cholesterol, and 25% (vol/vol) glycerol, by gravity column. The isoforms were reconstituted together with purified FXYD1 on the BD-Talon beads before elution, as described (13, 14). Proteins were stored at –80 °C. Protein concentration was determined with BCA (B9643 Sigma). Na,K-ATPase activity of αβFXYD1 complexes was measured over 1 h at 37 °C (10) followed by addition of PiColorLock gold malachite green (Innova Biosciences) (10). Na,K-ATPase activities for α1β1, α2β1, and α2β3 were 21.5 ± 5.3, 18.7 ± 1.8, and 10.7 ± 1.9 µmoles∙min∙mg, respectively. Inhibition was estimated in 3–5 experiments, and average Ki ± SEM was calculated.
Isolation of NPE Cells, Isoform Content, and Na,K-ATPase Activity.
Isolation of ciliary epithelium PE and NPE cells was as in ref. 28, with minor modifications. We layered 2 mL cell suspension onto layers of 30% and 15% Metrizamide (in Ca and Mg-free Hepes-Ringer with 2 mM EDTA) and centrifuged it at 218,000 × g (SW 40Ti) for 2 h. The white layer (NPE cells) was collected at the 30% boundary and the red layer (PE cells) between the 15–30% layers (Fig. S3). The NPE and PE cells were diluted in Ca and Mg-free Hepes–Ringer solution, centrifuged at 160,000 × g (60 Ti) (SW 60) for 30 min, the pellets resuspended in 25 mM Histidine and 25% glycerol, and frozen at –80 °C.
Immunoblots.
We separated 40 μg of NPE or PE cells on 10% polyacrylamide SDS-Laemmli gel. Immunoblots were blotted with selective antibodies, anti-α1 subunit (Upstate Biotechnology 05–369) and anti-α2 subunit (Upstate Biotechnology 07–674).
Na,K-ATPase activity.
The cells were incubated with 0.8 mg/mL Alamecithin for 30 min at 20–22 °C, before transfer to the reaction medium containing 130 mM NaCl, 5 mM KCl, 3 mM MgCl2, 25 mM Histidine, pH 7.4, 1 mM EGTA, 1 mM Azide, and 0.5 mM ATP. Cells were incubated for 45 min at 37 °C, with or without inhibitors as indicated, or 0.5 mM ouabain.
IOP Measurements.
New Zealand white rabbits (3–3.5 kg) about 1 y old, of either sex, were housed in pairs in a cage in animal room conditions on a 12-h light/dark cycle (7 AM to 7 PM). Experiments were started at 8:00 AM. IOP measurements were made with a Pneumatonometer (Model 30, Reichert Technologies) either after raising IOP with 4AP (one drop of 40mg/mL) (11) or on basal IOP after addition of one drop of 1 mM digoxin derivatives to the right eye (RE) and one drop of PBS to the left eye (LE) as control. The Weizmann Institutional Animal Care and Use Committee (IACUC) approved IACUC application no. 04270911-2.
For comparison of DcB with Latanoprost, three groups of five rabbits were used. Rabbits treated with Latanoprost received the medication daily for 5 d. On the day of the experiment, rabbits were treated at 5-min intervals with one drop of 1 mM DcB, one drop of 0.005% Latanoprost (XalatanTM, Pfizer), or one drop each of DcB and Latanoprost (RE), or normal saline (LE, Control). IOP was measured every hour for 12 h (DcB and Latanoprost alone) and after 24 h (DcB with Latanoprost). Basal IOPs in both eyes, without any medication, were measured 5 d before and on the day of the experiment. All eyes were examined routinely by ophthalmic examinations and were free of any abnormalities. Corneal thickness (μm) was measured using an ultrasonic pachometer (Sonogage). For histologic examination, after 6 d of treatment with DcB or no treatment, animals were killed and eyes were removed, fixed in 10% neutral buffered formalin, trimmed at 4 µm, and stained with hematoxylin and eosin.
Drug Preparation.
Stock solutions of CGs were dissolved in ethanol and freshly diluted in PBS for each experiment, such that the final ethanol concentration did not exceed 1%. For rabbit experiments, CGs were dissolved in DMSO and diluted in PBS to indicated concentrations (final DMSO 1–2%).
Acknowledgments
We thank Dr. Bella Finarov and Dr. Ori Brenner of the Weizmann Institute Veterinary Service for help with the rabbit experiments and histological preparations. We thank also Prof. Zvi Farfel for helpful discussions. This work was supported by grants from the Israel Science Foundation (789/12), Israel Ministry of Trade and Industry Kamin-Yeda programme (Project 47146), Yeda CEO fund, and Mauerberger Foundation, South Africa.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.J.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514569112/-/DCSupplemental.
References
- 1.Tsai JC, Forbes M. 2nd Ed Professional Communications, Inc.; West Islip, NY: 2004. Medical Management of Glaucoma. [Google Scholar]
- 2.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 3.Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: Heterogeneity in structure, diversity in function. Am J Physiol. 1998;275(5 Pt 2):F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh S, Hernando N, Martín-Alonso JM, Martin-Vasallo P, Coca-Prados M. Expression of multiple Na+,K(+)-ATPase genes reveals a gradient of isoforms along the nonpigmented ciliary epithelium: Functional implications in aqueous humor secretion. J Cell Physiol. 1991;149(2):184–194. doi: 10.1002/jcp.1041490203. [DOI] [PubMed] [Google Scholar]
- 5.Wetzel RK, Sweadner KJ. Immunocytochemical localization of NaK-ATPase isoforms in the rat and mouse ocular ciliary epithelium. Invest Ophthalmol Vis Sci. 2001;42(3):763–769. [PubMed] [Google Scholar]
- 6.Forbush B. Cardiotonic steroid binding to Na,K-ATPase. Current Topics in Membranes and Transport. 1983;19:167–201. [Google Scholar]
- 7.Laursen M, Yatime L, Nissen P, Fedosova NU. Crystal structure of the high-affinity Na+K+-ATPase-ouabain complex with Mg2+ bound in the cation binding site. Proc Natl Acad Sci USA. 2013;110(27):10958–10963. doi: 10.1073/pnas.1222308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laursen M, Gregersen JL, Yatime L, Nissen P, Fedosova NU. Structures and characterization of digoxin- and bufalin-bound Na+,K+-ATPase compared with the ouabain-bound complex. Proc Natl Acad Sci USA. 2015;112(6):1755–1760. doi: 10.1073/pnas.1422997112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa H, Shinoda T, Cornelius F, Toyoshima C. Crystal structure of the sodium-potassium pump (Na+,K+-ATPase) with bound potassium and ouabain. Proc Natl Acad Sci USA. 2009;106(33):13742–13747. doi: 10.1073/pnas.0907054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz A, et al. Selectivity of digitalis glycosides for isoforms of human Na,K-ATPase. J Biol Chem. 2010;285(25):19582–19592. doi: 10.1074/jbc.M110.119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz A, et al. Digoxin derivatives with enhanced selectivity for the α2 isoform of Na,K-ATPase: Effects on intraocular pressure in rabbits. J Biol Chem. 2014;289(30):21153–21162. doi: 10.1074/jbc.M114.557629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tal DM, Karlish SJD, Gottlieb H. An NMR study of new cardiac glycoside derivatives. Magn Reson Chem. 2015 doi: 10.1002/mrc.4380. in press. [DOI] [PubMed] [Google Scholar]
- 13.Kapri-Pardes E, et al. Stabilization of the α2 isoform of Na,K-ATPase by mutations in a phospholipid binding pocket. J Biol Chem. 2011;286(50):42888–42899. doi: 10.1074/jbc.M111.293852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra NK, et al. FXYD proteins stabilize Na,K-ATPase: Amplification of specific phosphatidylserine-protein interactions. J Biol Chem. 2011;286(11):9699–9712. doi: 10.1074/jbc.M110.184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wasserstrom JA, Aistrup GL. Digitalis: New actions for an old drug. Am J Physiol Heart Circ Physiol. 2005;289(5):H1781–H1793. doi: 10.1152/ajpheart.00707.2004. [DOI] [PubMed] [Google Scholar]
- 16.Paula S, Tabet MR, Ball WJ., Jr Interactions between cardiac glycosides and sodium/potassium-ATPase: Three-dimensional structure-activity relationship models for ligand binding to the E2-Pi form of the enzyme versus activity inhibition. Biochemistry. 2005;44(2):498–510. doi: 10.1021/bi048680w. [DOI] [PubMed] [Google Scholar]
- 17.Hall C, Ruoho A. Ouabain-binding-site photoaffinity probes that label both subunits of Na+,K+-ATPase. Proc Natl Acad Sci USA. 1980;77(8):4529–4533. doi: 10.1073/pnas.77.8.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toris CB. Pharmacotherapies for glaucoma. Curr Mol Med. 2010;10(9):824–840. doi: 10.2174/156652410793937778. [DOI] [PubMed] [Google Scholar]
- 19.Dismuke WM, Mbadugha CC, Faison D, Ellis DZ. Ouabain-induced changes in aqueous humour outflow facility and trabecular meshwork cytoskeleton. Br J Ophthalmol. 2009;93(1):104–109. doi: 10.1136/bjo.2008.142133. [DOI] [PubMed] [Google Scholar]
- 20.Do CW, Civan MM. Basis of chloride transport in ciliary epithelium. J Membr Biol. 2004;200(1):1–13. doi: 10.1007/s00232-004-0688-5. [DOI] [PubMed] [Google Scholar]
- 21.Socci RR, Chu E, Bayorh MA, Chu TC. 4-aminopyridine transiently increases intraocular pressure in rabbits. Pharmacology. 2003;69(2):108–114. doi: 10.1159/000072364. [DOI] [PubMed] [Google Scholar]
- 22.Coca-Prados M, Fernández-Cabezudo MJ, Sánchez-Torres J, Crabb JW, Ghosh S. Cell-specific expression of the human Na+,K(+)-ATPase beta 2 subunit isoform in the nonpigmented ciliary epithelium. Invest Ophthalmol Vis Sci. 1995;36(13):2717–2728. [PubMed] [Google Scholar]
- 23.Huang B, Blanco G, Mercer RW, Fleming T, Pepose JS. Human corneal endothelial cell expression of Na+,K+-adenosine triphosphatase isoforms. Arch Ophthalmol. 2003;121(6):840–845. doi: 10.1001/archopht.121.6.840. [DOI] [PubMed] [Google Scholar]
- 24.Tamiya S, Dean WL, Paterson CA, Delamere NA. Regional distribution of Na,K-ATPase activity in porcine lens epithelium. Invest Ophthalmol Vis Sci. 2003;44(10):4395–4399. doi: 10.1167/iovs.03-0287. [DOI] [PubMed] [Google Scholar]
- 25.Wetzel RK, Arystarkhova E, Sweadner KJ. Cellular and subcellular specification of Na,K-ATPase alpha and beta isoforms in the postnatal development of mouse retina. J Neurosci. 1999;19(22):9878–9889. doi: 10.1523/JNEUROSCI.19-22-09878.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Despa S, Lingrel JB, Bers DM. Na(+)/K(+)-ATPase α2-isoform preferentially modulates Ca2(+) transients and sarcoplasmic reticulum Ca2(+) release in cardiac myocytes. Cardiovasc Res. 2012;95(4):480–486. doi: 10.1093/cvr/cvs213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thummel KE, Shen DD, Isoherran N. McGraw Hill; NY: 2011. Goodman Gilman’s the Pharmacological Basis of Therapeutics. 12 Ed, pp 1921. [Google Scholar]
- 28.Edelman JL, Sachs G, Adorante JS. Ion transport asymmetry and functional coupling in bovine pigmented and nonpigmented ciliary epithelial cells. Am J Physiol. 1994;266(5 Pt 1):C1210–C1221. doi: 10.1152/ajpcell.1994.266.5.C1210. [DOI] [PubMed] [Google Scholar]