Abstract
Spatial variation in the composition of communities is the product of many biotic and environmental interactions. A neglected factor in the analysis of community distribution patterns is the multi-scale nature of the data, which has implications for understanding ecological processes and the development of conservation and environmental management practice. Drawing on recently established multivariate spatial analyses, we investigate whether including relationships between spatial structure and abiotic variables enable us to better discern patterns of species and communities across scales. Data comprised 1200 macrozoobenthic samples collected over an array of distances (30 cm to 1 km) in three New Zealand harbours, as well as commonly used abiotic variables, such as sediment characteristics and chlorophyll a concentrations, measured at the same scales. Moran’s eigenvector mapping was used to extract spatial scales at which communities were structured. Benthic communities, representing primarily bivalves, polychaetes and crustaceans, were spatially structured at four spatial scales, i.e. >100 m, 50–100 m, 50–15 m, and < 15 m. A broad selection of abiotic variables contributed to the large-scale variation, whereas a more limited set explained part of the fine-scale community structure. Across all scales, less than 30% of the variation in spatial structure was captured by our analysis. The large number of species (48) making up the 10 highest species scores based on redundancy analyses illustrate the variability of species-scale associations. Our results emphasise that abiotic variables and biodiversity are related at all scales investigated and stress the importance of assessing the relationship between environmental variables and the abundance and distribution of biological assemblages across a range of different scales.
Introduction
Community composition is an integrative response variable encompassing demography, functional traits, and species interactions, influenced by heterogeneity in environmental conditions [1,2]. Broad-scale biodiversity and ecological studies predominantly focus on environmental correlatives of species communities, such as land use or land cover, typically limited to a single spatial scale dictated by available data (but see, e.g. [3]). However, community composition and many factors underpinning ecology are scale-dependent (e.g. [2]). Therefore, the structuring factors underlying heterogeneity of communities should not be tied to a single scale. Moreover, the importance of specific factors likely changes with scale. Neglecting such variation affects assessments of biodiversity and may fail to maximise the extraction of information from available data. Although the role of spatial scale and cross-scale interactions is generally recognised as an important structuring factor in ecosystems [4,5], it is only recently becoming integrated in statistical approaches aimed at downscaling broad-scale data to enable predictive modelling of fine-grained diversity (e.g. [3,6]).
Defining across which scales species, communities and environmental characteristics are related is critical to advance ecological insight into the spatial organisation of communities and ecosystem dynamics [4,7,8]. Such spatial organisation occurs along a continuum from communities displaying certain regular patterns with clear patch boundaries often at a single scale (reviewed by [9]) to others showing multi-scale patterning and fuzzy patch boundaries (e.g. [10,11]). A common simplifying assumption is that biotic processes, such as competition, facilitation or predation [12,13], dominate fine-scale variation, whereas at broader spatial scales abiotic variables, such as hydrodynamics or climatic variables, drive spatial variation [13,14].
Ideally, in addition to embracing scale-dependency, studies addressing spatial variation in community data should apply spatially explicit multivariate models, which encompass multiple predictors and multivariate response variables. Moran’s eigenvector mapping [2,15] is one of the tools available, and employed here. This spatially explicit framework allows us to infer the scale-dependent association between abiotic variables and community distributions without a priori assigning scales, rather letting the data speak for themselves within constraints set by the sampling design. The aim is to understand whether including spatial variation and abiotic variables enables us to differentiate the scale-dependent patterns of communities across scales (from 30 cm to 1 km).
We use a large data set on macrozoobenthic communities, primarily bivalves, crustaceans, and polychaetes, from three estuarine areas of New Zealand explicitly collected for such cross-scale analysis. 1) We explore generality of relationships between abiotic variables and community composition by dissecting variation in species assemblages across a broad set of spatial scales and across multiple sites. In contrast to many species distribution analyses restricted to a single scale and a single species (as criticised by [16,17]), this approach embraces potential changing relevance of abiotic variables with scale. 2) We statistically assess scale-dependent patterns in community-environment relationships. Thereby we discern co-occurrence of species and specific habitat features.
Materials and Methods
Community Data
The abundance and distribution of benthic infauna was sampled in Kaipara (175°56’ S, 37°27’ E), Manukau (174°41’ S, 37°7’ E), and Tauranga (174°17’ S, 36°23’ E) harbours, North Island, New Zealand in the austral summer of 2012 (see, e.g. [18,19] for area descriptions). 400 cores (13 cm diam., 20 cm deep) were sampled on a pre-determined grid (Fig 1) in each harbour during low tide. Transects had a length of 1 km, and the distance between transects was 100 m. This grid was designed to allow sampling at multiple spatial scales and encompass patterns on scales from 30 cm to 1 km (Fig 1), advancing the sampling design previously employed by [20]. Sampling points along transects were spaced at distances of 30 cm, 1 m, 5 m, 10 m, 30 m, 50 m, 100 m, 500 m and 1000 m (Fig 1). This grid covered the intertidal area from the high- to low-water mark to capture tidal variation. Cores were sieved (500 μm mesh) and the residue preserved with 70% isopropyl alcohol. In the laboratory, Rose Bengal (2%) stained species were identified to the lowest practical taxonomic resolution and their abundance assessed (see S1 Appendix). (No specific permissions were required for sampling these locations, as our sampling is a permitted activity. Field studies did not involve endangered or protected species)
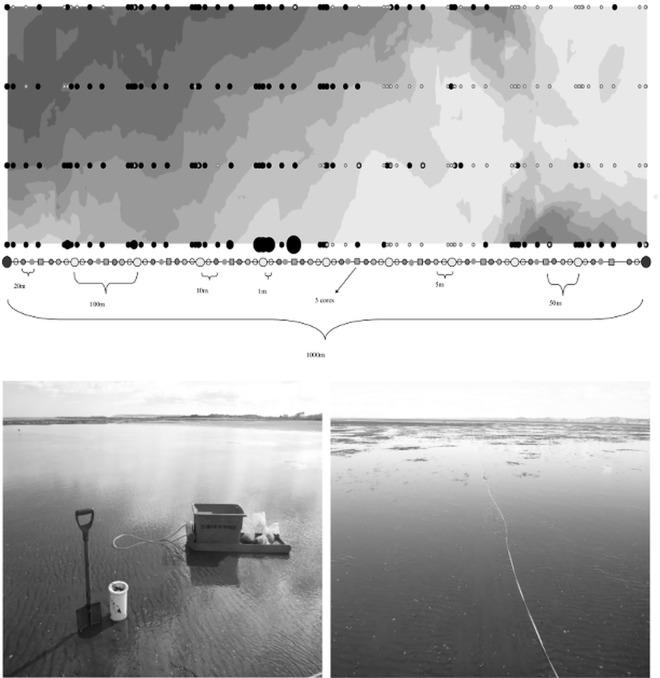
Fig 1. Sampling design matching a number of spatial lags ranging from 0.3 m to 1 km.
Illustrated are the abundances of Macomona liliana (scaled dots, encompassing values between 0 and 40 ind./core) across a sandflat of 300 m by 1000 m at Kaipara Harbour. The background displays an interpolated seascape of median grain size, ranging between 170 and 250 μm (darker grey indicated a larger median grain size, i.e. coarser sands). The middle panel (not to scale) illustrates how sampling points were positioned along a single transect, where the label “3 cores” indicates a sampling distance of 30 cm. The two bottom panels show a sampling area at low tide.
Environmental Variables
Prior to sampling the seafloor surface, at each sampling point (n = 1200) on the grid a photograph of 0.25-m2 of the sediment surface was taken. Coverage (%) of seagrass (Zostera mulleri), bare sand, and shell hash (i.e. broken shell fragments) was estimated based on 75 random points within that photograph using CPCe [21]. To determine sediment median grain size (μm), % sediment fractions (silt < 63 μm, very fine 63–125 μm, fine 125–250 μm, medium 250–500 μm, and coarse > 500 μm), organic content (%), and chlorophyll a concentration (mg/g) at each point, we pooled three surface sediment cores (2 cm diam., 2 cm deep) from each sampling point (n = 1200). These samples were stored in the dark on ice immediately after collecting and freeze-dried upon arrival in the laboratory. Prior to freeze-drying, seagrass and bivalves were removed from the sediment samples. Sediment grain sizes were measured using a Malvern Mastersizer, chlorophyll a concentrations were determined using a fluorometer, and loss on ignition was used to assess organic content (see [22,23] for methodological details). These abiotic variables are commonly associated with coastal benthic diversity (amongst others [10–14, 18–20]).
Statistical Approach
Moran’s eigenvector mapping (MEM, [2,15,24]) was used to evaluate spatial variation in benthic community composition and abiotic variables on multiple scales for each harbour separately. This method is a modification of the Principal Coordinates of Neighbour Matrices approach (PCNM, [25]), using a distance-based (Euclidean) connectivity matrix to define how points are linked across space (see [24,26]).
Employing the MEM-framework involved several steps: 1) Community data were Hellinger-transformed to reduce the importance of the most abundant species [27]. Preliminary analyses indicated that other species transformations, such as relying only on presence-absence information, resulted in poorer model performances; 2) transformed community data were linearly detrended using geographical coordinates to remove a large-scale spatial gradient, and residuals of this model were retained for further analyses (see [24]).
Next, (3) we constructed a spatial weighting matrix (SWM) to define linkages between sampling points, used for the decomposition in orthogonal spatial variables. We trialled connectivity based on Delaunay triangulation, minimum spanning tree, relative neighbourhood, nearest neighbours, Gabriel neighbourhood, and distance thresholds (see [26]), selecting a distance-based SWM (Table 1). This particular matrix optimised performance, as determined by the AICc (Table 2), and reflects a data-driven approach [15,24,26]. Subsequently, (4) this SWM was used in eigen decomposition of community data, providing spatial eigen functions (“MEM-variables”) that can be used as spatial predictors in ordination approaches (see, e.g. [2]). Significant positive MEM-variables, representing positive spatial autocorrelation (p ≤ 0.05, 9999 permutations), were grouped (Fig 2) based on a visual comparison of similarities in their range of spatial autocorrelation. This represents a routine method of clustering as single MEM-variables harbour little significance [28,29]. This grouping in MEM-subsets was constrained by our sampling design, such that “spatial scales” were limited between the smallest (30 cm) and largest (1 km) inter-sample distance.
Table 1. Optimal connectivity network.
| Kaipara | Tauranga | Manukau | |
|---|---|---|---|
| Optimal distance (m) | 104 | 199 | 131 |
| AICc | -355 | -540 | -571 |
| Nvar | 40 | 26 | 4 |
| α | 2 | 3 | 5 |
| Radj | 28 | 19 | 41 |
| Positive MEM-variables (n) | 39 | 23 | 40 |
AICc (corrected Akaike Information Criterion), Nvar (number of MEM variables), α (parameter of concave spatial weighting function dictating how similarity decays with distance; see [26]), and Radj. (% adjusted explained variance) summarise the optimal connectivity. See Materials and Methods for details.
Table 2. Mean environmental characteristics (standard deviation) and number of macrozoobenthic species encountered during macrobenthic sampling.
Highest mean environmental characteristics, as identified by single variable ANOVA’s, in bold.
| Kaipara | Tauranga | Manukau | |
|---|---|---|---|
| Sampling 2012 | 18 & 19 April | 23 & 24 April | 4 & 5 May |
| Species (n) | 114 | 81 | 109 |
| Individuals (n) | 21846 | 25394 | 26573 |
| Median grain size (μm) | 213 (14.7) | 197 (23.4) | 166 (35.1) |
| Silt (%) | 1 (2.3) | 5 (3.1) | 14 (10.5) |
| Very fine sediments (%) | 6 (2.9) | 17 (4.8) | 17 (5.9) |
| Fine sediments (%) | 61 (4.6) | 44 (5.7) | 48 (10.8) |
| Medium sediments (%) | 32 (6.5) | 28 (5.4) | 18 (7.2) |
| Coarse sediments (%) | 0.4 (0.5) | 6 (3.4) | 3 (4.7) |
| Organic content (%) | 0.8 (0.2) | 2 (0.6) | 2 (1.1) |
| Chlorophyll a (mg/g) | 5 (3.1) | 11 (4.2) | 23 (7.4) |
| Bare sand cover (%) | 84 (28.1) | 73 (18.9) | 79 (23.5) |
| Shell hash cover (%) | 2 (3.3) | 3 (4.2) | 16 (17.6) |
| Sea grass cover (%) | 13 (27.2) | 23 (18.3) | 5 (18.4) |
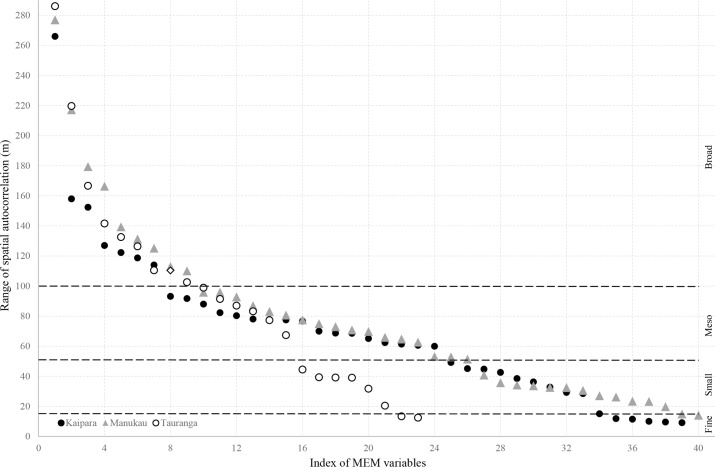
Fig 2. Range of spatial autocorrelation of each significant positive MEM variable.
Broad: MEM variables with a range > 100 m; Meso: MEM variables with a range < 100 m and > 50 m; Small: MEM variables with a range < 50 m and > 15 m; Fine: MEM variables with a range < 15 m. Delineation into 4 distinct spatial scales is based on visual appraisal (see Materials and Methods).
Then, (5) the spatial variables of each MEM-subset were used in a redundancy analysis (RDA) with environmental variables in order to identify the abiotic variables linked to that scale. We included quadratic functions of abiotic variables, in case of continuous variables, to enhance fitting more complex relationships (e.g. [25,30]). Few missing abiotic variables (n = 2 in Tauranga and Manukau, n = 19 in Kaipara) values were estimated using interpolations based on inverse distance weighting (e.g. [31,32]). Forward selection with a significance level of 0.05 and 9999 random permutations of explanatory variables was then used to obtain the model with the most parsimonious set of abiotic variables ([33], see Fig 3). Finally, (6) we identified characteristic species, defined as the 10 benthic species with the highest positive or negative scores on the first two environmental ordination axes in the RDA of species abundances and environmental variables for each MEM-subset (e.g. [34]).
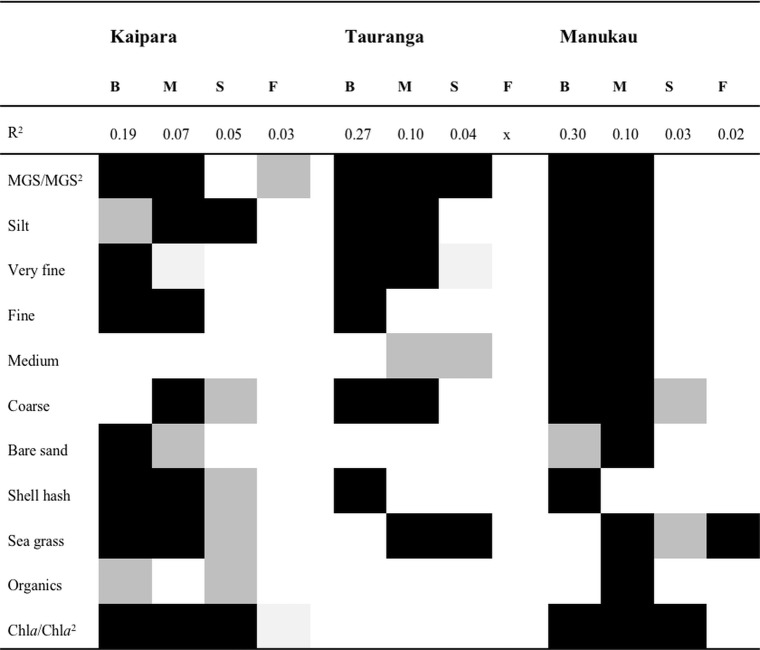
Fig 3. Environmental variables linked to community distributions at each distinct scale.
Blocks from left to right represent b(road) scale (> 100 m), m(eso) scale (< 100 m and > 50 m), s(mall) scale (< 50 m and > 15 m) and f(ine) scale (< 15 m) spatial subsets. Fine scale spatial subset for Tauranga not shown, since none of the environmental variables linked to this scale. Light grey = p ≤ 0.05; dark grey = p ≤ 0.01; black = p ≤ 0.001.
To determine how much of the captured community variation was related to measured abiotic variables or spatial structure we performed variance partitioning (see, e.g. [24,26]) in each harbour for each spatial submodel.
All analyses were done in R [35] using the packages spacemakeR, ncf, packfor, spdep, and vegan. Detailed explanations of the MEM-framework and variation partitioning, including R-scripts and formal matrix notations, can be found in [2,24], and [26].
Results
Benthic Communities
Across the three sites, we identified 146 taxa, comprising 73813 individuals (Table 2). Species accumulation curves indicated these were representative of the complete benthic communities present (Greenfield, Kraan, and Thrush, unpublished data). The spionid polychaetes Prionospio aucklandia (n = 9142, 813 sampling points) and Aonides trifida (n = 8097, 674 sampling points) were the most abundant, followed by the bivalves Austrovenus stutchburyi (n = 6520, 786 sampling points), Macomona liliana (n = 5347, 1135 sampling points) and Nucula hartvigiana (n = 4638, 524 sampling points). Concentrating on individual sites, in Kaipara A. trifida (n = 3915, 229 sampling points) was the most abundant species, whereas M. liliana (n = 1952, 379 sampling points) was the most widespread species. A. stutchburyi (n = 4235, 281 sampling points) was the most abundant species in Manukau. M. liliana (n = 1936, 384 sampling points) again was the most widespread species. In Tauranga, the most abundant and most widely distributed species was P. aucklandia (n = 7214, 385 sampling points).
The three locations differed significantly in this set of environmental characteristics (single variable ANOVA’s, df = 2, residuals = 1195, all p-values ≤ 0.05). Manukau Harbour was the muddiest site (identified by the highest % silt) and contained the highest concentration of chlorophyll a (Table 2). The coarsest sediment was encountered in Tauranga Harbour, which also contained the highest coverage of sea grass and shell hash. Kaipara Harbour had the highest coverage of bare sand (Table 2). All three sites had a median grain size classified as fine sands.
Spatial Scales
The best spatial weighting matrix was based on a different optimal distance for all three harbours, i.e. Kaipara 104 m, Tauranga 199 m, and Manukau 131 m. This optimal distance indicates the spatial distance across which sampling points share similar communities. Across the three estuaries, broad-scale subsets captured 19% to 30% of the variation in benthic community composition (Fig 3). Meso-scale subsets explained 7% to 10% of the variation, whereas small-scale and fine-scale subsets captured between 2% and 5% of the variation (Fig 3). For Tauranga none of the available explanatory variables explained the fine-scale variation in community composition (Fig 3).
At spatial scales larger than 50 m most of the selected abiotic explanatory variables contributed significantly to explaining community composition, particularly at Kaipara and Manukau sites (Fig 3). In Tauranga only the smaller grain-size fractions related to community patterns. Small- and fine-scale subsets in all sites were mostly associated with coverage of seagrass and chlorophyll a content. There was no consistent pattern of relationships between abiotic variables and community patterns across spatial scales (Fig 3). A common feature was the decrease of explanatory power at finer scales.
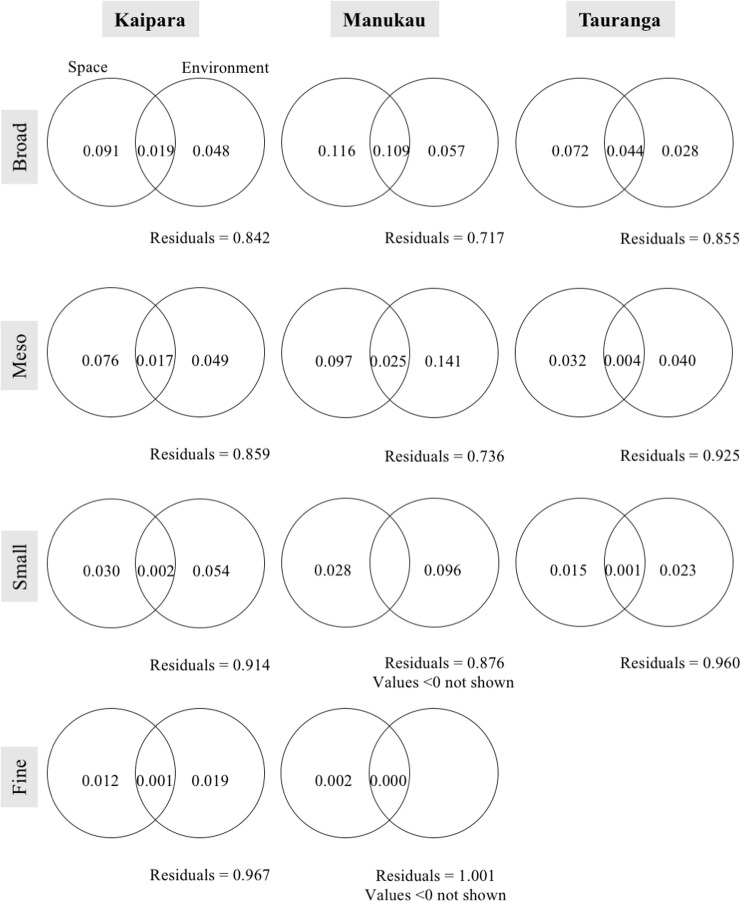
Partitioning of the variance showed that for the broad-scale sub-models little variation of the spatial components could be captured by the abiotic variables (Fig 4). This lack of variation explained by abiotic variables was generally more pronounced for the other spatial sub-models. Also, part of the variation was captured by spatially structured abiotic factors (overlapping parts of the circles in Fig 4). All fractions were significant (p < 0.05) with the exception of the Manukau fine-scale partitioning.
Fig 4. Partitioning the variation in an environmental component and a spatial component.
No results are shown for the fine-scale MEM-subset for Tauranga, since none of the abiotic variables were linked to that scale. Numbers indicate values for adjusted R2.
Characteristic Species
48 different species (Table 3), encompassing bivalves (n = 6), polychaetes (n = 28), crustaceans (n = 10) and gastropods (n = 4), represented the most characteristic species (see Materials and Methods for their definition). Most of these, such as the bivalve M. liliana or the crustacean Paracalliope novizealandiae, were associated with more than one spatial scale. A minority of these characteristic species, such as the polychaete Travisia olens, were captured best by a single scale model (Table 3), suggesting they are specialist in their resource requirements. Interestingly, most crustaceans and gastropods were characteristic species only at single sites. In contrast, bivalves were characteristic at more than one site. Polychaetes were either characteristic in all three sites or limited to just a single site (Table 3).
Table 3. Characteristic macrozoobenthic species associated with broad, meso, small and fine scale MEM models.
| Kaipara | Tauranga | Manukau | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Broad | AusMod | AusStu | Euchon | AntAur | AonTri | AusStu | AntAur | AonTri | Aricid |
| HetFil | MacLil | MacSte | Cerato | HetFil | Lumbri | AusStu | BocSyr | BumCir | |
| NucHar | OrbPap | OwePet | Lysian | MacSte | NucHar | CosCon | Dexami | HetFil | |
| PapAus | ParNou | Phoron | PerVal | Phoxoc | ScoCyl | MacLil | MacSte | Nemert | |
| PriAuc | PseFat | SolSil | ScoLel | ZeaSub | NotSca | NucHar | OwePet | ||
| TraOle | TroDen | WaiBre | PriAuc | ||||||
| Meso | AonTri | AusMod | AusStu | AntAur | Cerato | HetFil | AntAur | AonTri | Aricid |
| Euchon | Hesion | HetFil | LasPar | Lumbri | Lysian | AusStu | CosCon | HetFil | |
| MacLil | MagDak | Nemert | MacLil | NucHar | PerVal | MacLil | MacSte | MagDak | |
| NucHar | OwePet | Phoron | Phoxoc | PriAuc | ScoBen | Nemert | NotSca | NucHar | |
| PriAuc | SolSil | ScoCyl | Scolel | ZeaLut | ParNou | PriAuc | SolSil | ||
| ZeaSub | TroDen | ||||||||
| Small | AonTri | AusMod | BumCir | AntAur | AonTri | Cerato | AntAur | AonTri | AusStu |
| Cerato | Euchon | LasPar | ColLem | DilSub | LasPar | BocSyr | CosCon | HetFil | |
| MacLil | MacSte | NicAes | Lumbri | Lysian | MacLil | MacLil | MagDak | NicAes | |
| OrbPap | PapAus | ParNou | PerVal | Phoxoc | PriAuc | NotSca | NucHar | ParLyr | |
| PriAuc | PseThi | ScoBen | ScoCyl | Scolel | ZeaSub | PriAuc | |||
| SolSil | WaiBre | ||||||||
| Fine | AonTri | AusMod | BocSyr | AntAur | AusStu | BocSyr | |||
| BumCir | ColLem | Euchon | ComGla | HalWhi | HetFil | ||||
| MacLil | MacSte | MagDak | MacLil | MacSte | MagDak | ||||
| Nemert | NucHar | OwePet | Nemert | NicAes | NucHar | ||||
| PapAus | ParNou | Phoron | OwePet | ParLyr | PlaAus | ||||
| PseFat | TroDen | PriAuc | PseFat |
Fine scale subset for Tauranga not shown, since none of the environmental variables linked to this scale. Bivalves: AusStu = Austrovenus stutchburyi, LasPar = Lasaea parangaensis, MacLil = Macomona liliana, NucHar = Nucula hartvigiana, PapAus = Paphies australis, SolSil = Soletellina siliqua. Crustaceans: AusMod = Austrominius modestus, ColLem = Colurostylis lemurum, Dexami = Dexaminidea, HalWhi = Halicarcinus whitei, ParNou = Paracalliope nouzealandia, Phoxoc = Phoxocephalidea, WaiBre = Waitangi brevirostris. Polychaetes: AonTri = Aonides trifida, Aricid = Aricidea, BocSyr = Boccardia syrtis, BumCir =“Bumpy cirrisyllid”, Cerato = Ceratonereis sp., Euchon = Euchone sp., CosCon = Cossura consimilis, Hesion = Hesionidea, HetFil = Heteromastus filiformis, Lumbri = Lumbrineridea, Lysian = Lysianassidae, MacSte = Macroclymenella stewartensis, MagDak = Magelona dakini, Nemert = Nemertean, NicAes = Nicon aestuariensis, OrbPap = Orbinia papillosa, OwePet = Owenia petersonae, ParLyr = Paradonereis lyra, PerVal = Perinereis vallata, Phoron = Phoronis sp., PlaAus = Platynereis australis, PriAuc = Prionospio aucklandia, PseFat = Pseudopolydora “fat”, PseThi = Pseudopolydora “thin”, ScoBen = Scolecolepides benhami, ScoCyl = Scoloplos cylindrifer, Scolel = Scolelepis sp., TraOle = Travisia olens, TroDen = Trochodata dendyi. Gastropods: ComGla = Cominella glandiformis, DilSub = Diloma subrostrata, NotSca = Notoacmea scapha, ZeaLut = Zeacumantus lutulentus, ZeaSub = Zeacumantus subcarinatus. Cnidaria: AntAur = Anthopleura aureoradiata.
Discussion
Addressing the role of scale-dependency in the spatial structuring of communities in relationship to abiotic variables to infer processes has been recognised as critical in ecology (e.g. [36]). This helps to focus studies on the scales that are most relevant to community composition, as well as identifying abiotic variables most associated with these scales. In return this provides information on the mechanisms that likely underpin community diversity and distributions. The inferences drawn from such analysis can help support conservation or environmental management measures due to improved ecological understanding. Realising such research goals requires sampling communities with purposefully cross-scale sampling designs and using models capable of capturing such information. Yet, to our knowledge, our work offers a scarce example of studies to gather response and explanatory data at corresponding scales across such a large range of spatial scales in coastal ecosystems (but see, amongst others, [32, 37, 38, 39]).
Moran’s eigenvector mapping, as applied here to intertidal communities living in sandy sediments, allowed us to define distinct spatial scale ranges by explicitly including spatial autocorrelation in the analysis of multivariate biodiversity data [15,40]. While we found strong support for the links between abiotic variables and communities on scales from 1 km to just 15 m, at spatial scales finer than 15 m other factors seem to be associated with spatial structuring of communities (see below). The variation captured by the various spatial subsets is similar to previous studies (e.g. [24,30]), demonstrating less spatial variation in community composition captured from broader to finer scales. Ecological studies incorporate many sources of variation; therefore the explained spatial variation is likely to remain limited, especially in dynamic systems [24].
The fine-scale subsets explained limited variability, particularly in the Tauranga site. Explanatory abiotic variables linked to fine-scale variation were not measured or the patterns are due to biotic variables (see [24,30]). Other studies (e.g. [29]) discussed the possibility of their sampling design being inadequate to detect fine-scale variability due to lack of replication at fine spatial scales. However, our spatial sampling grid was explicitly designed to capture such patterns, foregoing arguments of a lack of power at finer scales. Alternatively, community-environment relationships might not be apparent at such fine scales simply due to the fact that macrozoobenthic communities respond to abiotic variables at scales larger than 15 m. Partitioning of variation indicated that our set of abiotic variables had a significant, yet relatively minor (up to 14%), contribution to small-scale community structure. This suggests that biotic variables are a likely explanation for small-scale community structure. Prior to the development of multivariate spatial modelling approaches, such quantitative insight into the organisation of community structure would have been difficult to obtain.
It is becoming increasingly clear that purely environmental based species distribution models are too simple to capture complex community responses to habitat change (e.g. [16,41]). In addition, experimental field studies indicate a profound role for complex interactions across scales governing community dynamics [19,42,43]. The MEM-framework offers a quantitative approach towards understanding scale-dependent community interactions associated with abiotic variables (also see, e.g. [44]). This is essential if the role of biodiversity in affecting response to changing environmental conditions (e.g. climate change) is to be fully realised. Indeed, the merit of our study is providing an ecological case study on the impact of including spatial structure and abiotic variables to better differentiate biogeographical patterns of species communities across scales. Yet, an open, non trivial, question remains how such MEM-derived spatial templates can be incorporated in predictive species distribution models to accommodate cross-scale variation in biodiversity-environment links.
In conclusion, our study emphasises the lack of one right scale to study spatial variation in species communities. As highlighted by the MEM-framework, different species and different habitat features are linked to various spatial scales. This emphasises the importance of assessing the relationship between environmental variables and the abundance and distribution of biological assemblages across a range of different scales.
Supporting Information
(PDF)
Acknowledgments
Collecting and processing the data would not have been feasible without Katie Cartner, Kelly Carter, Rebecca Gladstone-Gallagher, Sarah Hailes, Silvia de Juan Mohan, Andrés Ospina-Álvarez, Henrike Andresen and Stéphane Arnaud. Comments by two reviewers greatly improved clarity of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
CK was funded by a Marie-Curie International Outgoing Fellowship (298380). CK, CFD, BLG, and SFT were funded by the Marsden fund of the Royal Society of New Zealand (NIW1102). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Schneider DC (2001) The rise of the concept of scale in ecology. BioScience 51: 545–553. [Google Scholar]
- 2. Dray S, Pélissier R, Couteron P, Fortin MJ, Legendre P, Peres-Neto PR, et al. (2012) Community ecology in the age of multivariate multiscale spatial analysis. Ecol Monogr 82: 257–275. [Google Scholar]
- 3. Belmaker J, Jetz W (2011) Cross-scale variation in species richness-environment associations. Global Ecol Biogeogr 20: 464–474. [Google Scholar]
- 4. Wiens JA (1989) Spatial scaling in ecology. Funct Ecol 3: 385–397. [Google Scholar]
- 5. Levin SA (1992) The problem of pattern and scale in ecology. Ecology 73: 1943–1967. [Google Scholar]
- 6. Keil P, Wilson AM, Jetz W (2014) Uncertainty, priors, autocorrelation and disparate data in downscaling of species distributions. Div Distr 20: 797–812. [Google Scholar]
- 7. Jombart T, Dray S, Dufour A-B (2009) Finding essential scales of spatial variation in ecological data: a multivariate approach. Ecography 32: 161–168. [Google Scholar]
- 8. McGill BJ (2010) Matters of scale. Science 328: 575–576. 10.1126/science.1188528 [DOI] [PubMed] [Google Scholar]
- 9. Rietkerk M, van de Koppel J (2008) Regular pattern formation in real ecosystems. Trends Ecol Evol 23: 169–175. 10.1016/j.tree.2007.10.013 [DOI] [PubMed] [Google Scholar]
- 10. Thrush SF, Hewitt JE, Pridmore RD (1989) Patterns in the spatial arrangements of polychaetes and bivalves on intertidal sandflats. Mar Biol 102: 529–535. [Google Scholar]
- 11. Kraan C, van der Meer J, Dekinga A, Piersma T (2009) Patchiness of macrobenthic invertebrates in homogenized intertidal habitats: hidden spatial structure at a landscape scale. Mar Ecol Progr Ser 383: 211–224. [Google Scholar]
- 12. Thrush SF (1991) Spatial patterns in soft-bottom communities. Trends Ecol Evol 6: 75–79. 10.1016/0169-5347(91)90178-Z [DOI] [PubMed] [Google Scholar]
- 13. Legendre P, Thrush SF, Dayton PK, Grant J, Hewitt JE, Hines AH (1997) Spatial structure of bivalves in a sandflat: scale and generating processes. J Exp Mar Biol Ecol 216: 99–128. [Google Scholar]
- 14. Gray JS, Elliott M (2009) Ecology of marine sediments Oxford University Press, UK. [Google Scholar]
- 15. Dray S, Legendre P, Peres-Neto PR (2006) Spatial modelling: a comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol Mod 196: 483–493. [Google Scholar]
- 16. Araújo MB, Luoto M (2007) The importance of biotic interactions for modelling species distributions under climate change. Global Ecol Biogeogr 16: 743–753. [Google Scholar]
- 17. Dormann CF (2007) Promising the future? Global change projections of species distributions. Basic Appl Ecol 8: 387–397. [Google Scholar]
- 18. Thrush SF, Hewitt JE, Norkko A, Nicholls PE, Funnell GA, Ellis GI (2003) Habitat change in estuaries: predicting broad-scale responses of intertidal macrofauna to sediment mud content. Mar Ecol Progr Ser 263: 101–112. [Google Scholar]
- 19. Thrush SF, Hewitt JE, Lohrer AM (2012) Interaction networks in coastal soft-sediments highlight the potential for change in ecological resilience. Ecol Appl 22: 1213–1223. [DOI] [PubMed] [Google Scholar]
- 20. Hewitt JE, Legendre P, McArdle BH, Thrush SF, Bellehumeur C, Lawrie SM (1997) Identifying relationships between adult and juvenile bivalves at different spatial scales. J Exp Mar Biol Ecol 216: 77–98. [Google Scholar]
- 21. Kohler KE, Gill SM (2006) Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comp Geosci 32: 1259–1269. [Google Scholar]
- 22. Jones HFE, Pilditch CA, Bruesewitz DA, Lohrer AM (2011) Sedimentary environment influences the effect of an infaunal suspension feeding bivalve on estuarine ecosystem function. PLoS One 6: e27065 10.1371/journal.pone.0027065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Needham HR, Pilditch CA, Lohrer AM, Thrush SF (2011) Context-specific bioturbation mediates changes to ecosystem functioning. Ecosystems 14: 1096–1109. [Google Scholar]
- 24. Legendre P, Legendre L (2013) Numerical ecology Third edition Elsevier, the Netherlands. [Google Scholar]
- 25. Borcard D, Legendre P (2002) All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol Mod 153: 51–68. [Google Scholar]
- 26. Borcard D, Gillet F, Legendre P (2011) Numerical ecology with R Springer, USA. [Google Scholar]
- 27. Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129: 271–280. [DOI] [PubMed] [Google Scholar]
- 28. Bellier E, Monestiez P, Durbec J-P, Candau J-N (2007) Identifying spatial relationships at multiple scales: principal coordinates of neighbour matrices (PCNM) and geostatistical approaches. Ecography 30: 385–399. [Google Scholar]
- 29. Benedetti-Cecchi L, Iken K, Konar B, Cruz-Motta J, Knowlton A, Pohle G, et al. (2010) Spatial relationships between polychaete assemblages and environmental variables over broad geographical scales. PLoS One 5: e12946 10.1371/journal.pone.0012946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Borcard D, Legendre P, Avois-Jacquet C, Tuomisto H (2004) Dissecting the spatial structure of ecological data at multiple scales. Ecology 85: 1826–1832. [Google Scholar]
- 31. Cressie NAC (1993) Spatial statistics for spatial data Wiley-Interscience, USA. [Google Scholar]
- 32. Kraan C, Aarts G, van der Meer J, Piersma T (2010) The role of environmental variables in structuring landscape-scale species distributions in seafloor habitats. Ecology 91: 1583–1590. [DOI] [PubMed] [Google Scholar]
- 33. Blanchet FG, Legendre P, Borcard D (2008) Forward selection of explanatory variables. Ecology 89: 2623–2632. [DOI] [PubMed] [Google Scholar]
- 34. Jones MM, Szyska B, Kessler M (2011) Microhabitat partitioning promotes plant diversity in a tropical montane forest. Global Ecol Biogeogr 20: 558–569. [Google Scholar]
- 35.R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing. Available: www.r-project.org.
- 36. Legendre P (1993) Spatial autocorrelation: trouble or new paradigm. Ecology 74: 1659–1673. [Google Scholar]
- 37. Underwood AJ, Chapman MG (1998) A method for analysing spatial scales of variation in composition of assemblages. Oecologia 117:570–578. [DOI] [PubMed] [Google Scholar]
- 38. Murphy RJ, Tolhurst TJ, Chapman MG, Underwood AJ (2008) Spatial variation of chlorophyll on estuarine mudflats determined by field-based remote sensing. Mar Ecol Progr Ser 365:45–55. [Google Scholar]
- 39. Chapman MG, Underwood AJ (2008) Scales of variation of densities of gastropods over multiple spatial scales: comparison of common and rare species. Mari Ecol Progr Ser 354: 147–160. [Google Scholar]
- 40. Peres-Neto PR, Legendre P (2010) Estimating and controlling for spatial structure in the study of ecological communities. Global Ecol Biogeogr 19:174–184. [Google Scholar]
- 41. Wisz MS, Pottier J, Kissling WD, Pellissier L, Lenoir J, Damgaard CF, et al. (2012) The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biol Rev 88: 15–30. 10.1111/j.1469-185X.2012.00235.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thrush SF, Hewitt JE, Dayton PK, Coco G, Lohrer AM, Norkko A (2009) Forecasting the limits of resilience: integrating empirical research with theory. Proc Royal Soc B 276: 3209–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lohrer AM, Thrush SF, Hewitt JE, Kraan C (2015) Ecological self-organisation and the up-scaling of ecosystem functions in a heterogeneous world. Sci Rep 5: 10439 10.1038/srep10349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sharma S, Legendre P, De Cáceres M, Boisclair D (2011) The role of environmental and spatial processes in structuring native and non-native fish communities across thousands of lakes. Ecography 34: 762–771. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.