Abstract
Cardiovascular and renal inflammation induced by Aldosterone (Aldo) plays an important role in the pathogenesis of hypertension and renal fibrosis. Toll-like receptor 4 (TLR4) signaling contributes to inflammatory cardiovascular and renal diseases, but its role in Aldo-induced hypertension and renal damage is not clear. In the current study, rats were treated with Aldo-salt combined with TAK-242 (a TLR4 signaling antagonist) for 4 weeks. Hemodynamic, cardiac and renal parameters were assayed at the indicated time. We found that Aldo-salt–treated rats present cardiac and renal hypertrophy and dysfunction. Cardiac and renal expression levels of TLR4 as well as levels of molecular markers attesting inflammation and fibrosis are increased by Aldo infusion, whereas the treatment of TAK-242 reverses these alterations. TAK-242 suppresses cardiac and renal inflammatory cytokines levels (TNF-a, IL-1β and MCP-1). Furthermore, TAK-242 inhibits hypertension, cardiac and renal fibrosis, and also attenuates the Aldo-induced Epithelial-Mesenchymal Transition (EMT). In experimental hyperaldosteronism, upregulation of TLR4 is correlated with cardiac and renal fibrosis and dysfunction, and a TLR4 signaling antagonist, TAK-242, can reverse these alterations. TAK-242 may be a therapeutic option for salt-sensitive hypertension and renal fibrosis.
Introduction
Aldosterone (Aldo) secreted from the adrenal cortex plays an important role in regulating renal sodium transport and electrolytic balance through the activation of mineralocorticoid receptor (MR) in the kidney[1,2].Clinical studies demonstrated that inhibition of MR could decrease the risk of both morbidity and mortality in patients with heart failure, and inhibit albumin excretion in hypertensive and diabetic patients [3,4,5].In addition, MR antagonists also present a renoprotective effect in several experimental models of kidney disease[6,7].
Aldo is implicated in cardiovascular and renal remodeling by inducing inflammation, oxidative stress, fibrosis, and hypertrophy[2,8,9]. Previous studies showed that chronic inflammation has a critical role in the pathogenesis of hypertension[10,11], and renal inflammation is correlated with the development and progression of renal damage [12,13]. These findings suggest that Aldo-induced inflammation could be used as a potential therapeutic target for treating salt-sensitive hypertension and renal fibrosis[14].
The Toll-like receptors (TLRs) are pattern recognition receptors and play a crucial role in regulating inflammatory response[15,16].Emerging studies described that innate immune activation through TLRs is an important driver in the pathogenesis of vascular remodelling and endothelial dysfunction, and renal injury [17,18]. Summers et al. described the role of TLR9 in experimental crescentic glomerulonephritis[19]. They demonstrated that wild-type mice administered a TLR9 ligand develop histological injury with impaired renal function, which is attenuated in TLR9 knockout mice[19]. Activated TLR-3 signaling following ischemia and reperfusion drives strong pro-inflammatory response and results in acute kidney injury. In contrast, this effect was absent inTLR-3-/- mice[20]. Biglycan-induced activation of TLR-2/4 initiates an inflammatory response in kidneys, and results in the release of cytokines and chemokines (such as TNF-α, CXCL1, CCL2 and CCL5)[21]. TLR4 has been described to regulate cardiorenal dysfunction[22,23]. Cardiac TLR4 expression level is increased after angiotensin II infusion, and inhibition of TLR4 signaling markedly suppresses Ang II-induced cardiovascular inflammation, endothelial dysfunction, vascular remodelling and stiffness associated with hypertension [18,24,25,26]. Eissler et al. showed that hypertension is accompanied by upregulated TLR4 expression and activity[27].TLR4 knockout mice develop less-severe left ventricular hypertrophy following aortic banding compared to respective sham controls [28].In the study, we found that cardiac and renal expression of TLR4isincreased by Aldo infusion, which results in an activation of inflammatory response. A TLR4 inhibitor, TAK-242 reverses these alterations. TAK-242 inhibits cardiac and renal inflammation, hypertension, cardiac and renal fibrosis.
Materials and Methods
2.1 Animal models
The study was approved by the Ethics Committee of Nantong University. Adult male Wistar rats (weight, 240± 20 g) were obtained from the Chinese Academy of Sciences (Shanghai, China), and maintained in a pathogen-free facility. The animals were divided into four groups (n = 9/group): (1) Vehicle infusion group treated with vehicle alone, (2) Aldo-salt group treated with an infusion of Aldo-salt (1 mg/kg/day diluted in sunflower oil and administered by subcutaneous injection), (3) Aldo-salt plus TAK-242 group treated with an infusion of Aldo-salt plus TAK-242 at 2 mg/kg/day(MCE, USA).This dose was chosen on the basis of previous studies reporting its anti-inflammatory role[29,30], and TAK242 group treated with TAK242 alone. After 4 weeks of treatment, urine was collected in metabolic cages, and hemodynamic parameters were assayed[31].For example, blood pressure (BP) was measured in conscious but restrained animals, prewarmed to 34°C for 20 minutes. For each group, BP was measured 3 times on 3 separate days, and the mean value of all readings was taken as the average for the rat. Then blood samples, heart and kidney tissues were collected under sedation with sodium pentobarbital anesthesia.
2.2 Quantitative real-time PCR (qPCR)
Total RNA was extracted from rat heart or kidney using Trizol reagent (Invitrogen, CA, USA). The reverse transcription (RT) was carried out using Oligo dT primer (Takara, Osaka, Japan). qPCR was performed using a standard protocol from the SYBR Green PCR kit (Toyobo, Osaka, Japan) on Applied Biosystems 7300 real-time PCR system. β-actin was used as internal control. The qPCR primers are as follows: TLR4 forward, CTCTGGCATCATCTTCATTG, TLR4 reverse,
GTTGCTTCTTGTTCTTCCTCT; TNF-a forward,
CTTCTGTCTACTGAACTTCGGG, TNF-a reverse,
GTTGTCTTTGAGATCCATGCC; MCP-1 forward,
GCTGACCCCAATAAGGAATG, MCP-1 reverse,
CTTGAGGTGGTTGTGGAAAAGA; IL-1beta forward,
ATGATGACGACCTGCTAGTGTGT, IL-1beta reverse,
TGGCTTATGTTCTGTCCATTGAG; TGF-beta forward,
CAACGCAATCTATGACAAAACC, TGF-beta reverse,
ACAAGAGCAGTGAGCACTGAAG; Col1 forward,
TCCTTCTGGTCCTCGTGGTCTCC, Col1 reverse,
TTCCCCATCATCTCCGTTCTTGC.
2.3 Western blot and antibodies
Western blot analysis to assess rat TLR4, E-cadherin, fibronectin, a-SMA and β-actin protein expression was performed as previously described [32]. The anti-TLR4/E-cadherin/fibronectin/a-SMA primary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). β-actin primary antibodies were purchased from Sigma (MO, USA).
2.4 Enzyme-linked immunosorbent assay (ELISA) for TNF-a, MCP-1 and IL-1β in heart and kidney
The hearts and kidneys were removed and homogenized at the indicated time. Homogenates were sonicated for 30 s and then centrifuged at 2500 g and 4°C. The supernatants were used for measurement of TNF-a, MCP-1 and IL-1β. ELISAs were performed using a TNF-a kit (R&D Systems, Minneapolis, MN), MCP-1 kit (R&D Systems) and IL-1β kit (R&D Systems) according to the manufacturers’ protocols.
2.5 Histological analysis
Kidney tissues or left ventricles were quickly fixed with buffered 4% paraformaldehyde, embedded in paraffin and cut into 4-μm-thick sections. Periodic acid–Schiffand Masson’s trichrome staining were performed using serial sections. Tubulointerstitial fibrosis areas were semiquantified using image J software and expressed as a percentage of the total area. Perivascular fibrosis was assessed by calculating the percentage of Trichrome-stained collagen deposits surrounding the vessel to the total perivascular area using the software’s color cube function.
2.6 Statistical analysis
All data are expressed as mean ± SEM, computed from the average measurements obtained from each group of animals. Results were analysed using unpaired Student’s t-test or the Mann–Whitney U test. Analyses were conducted using GraphPad Prism (4.0) (software, Inc. San Diego, CA). Differences were deemed statistically significant at p< 0.05.
Results
3.1 Cardiac and renal expression of TLR4 is increased in Aldo-salt-treated rats
Aldo-salt-treated rats present a significant increase in systolic blood pressure (SBP) and diastolic BP (DBP)(Table 1). Meanwhile, Aldo-salt treatment results in an increase in ratio of heart weight to body weight and a decrease in heart rate(Table 1). Both cardiac dysfunction and hypertrophy are reversed by TAK-242, a TLR4 signaling antagonist(Table 1). In addition, urine volume, serum creatinine, creatinine clearance and kidney weight/body weight ratio of each group at the end of the 4-weekexperiment are present in Table 2. Aldo-salt-treated rats induce renal hypertrophy (increased ratio of kidney weight to body weight),increase glomerular filtration rate (assessed by the creatinine clearance), and results in a significant increase in serum creatinine compared with the other groups. Both renal dysfunction and hypertrophy are prevented by TAK-242 treatment.
Table 1. Physiological and hematological parameters in Aldo-salt–treated rats.
| Control | Aldo-salt | Aldo-salt +TAK-242 | |
|---|---|---|---|
| SBP, mm Hg | 131 ± 0.9 | 150 ± 0.7* | 139 ± 1.1 # |
| DBP, mm Hg | 94 ± 0.8 | 112 ± 0.6* | 103 ± 0.9 # |
| HR, beats/min | 338 ± 5.8 | 271 ± 6.5* | 309 ± 6.7 # |
| HW/BW, mg/g | 2.62 ± 0.02 | 2.85 ± 0.01* | 2.69 ± 0.02 # |
Aldo = aldosterone; SBP = systolic blood pressure; DBP = diastolic blood pressure; HW = heart weight; BW = body weight.
Values are presented as mean ± SEM.
*p<0.05 vs. control
# p<0.05 vs. Aldo-salt group.
Table 2. Physiological and renal parameters in Aldo-salt–treated rats.
| Control | Aldo-salt | Aldo-salt +TAK-242 | |
|---|---|---|---|
| Creatinine clearance,ml/min | 1.15± 0.22 | 1.32± 0.46 | 1.20± 0.48 |
| Urine volume (mL/day) | 8.2± 2.6 | 38.7± 6.8* | 25.4± 5.7 # |
| Serum creatinine (mg/dL) | 0.76± 0.21 | 1.32± 0.25* | 0.98± 0.31 # |
| KW/BW, mg/g | 2.70± 0.01 | 3.72± 0.01* | 3.15± 0.02 # |
Aldo = aldosterone; KW = kidney weight; BW = body weight.
Values are presented as mean ± SEM.
*p<0.05 vs. control
# p<0.05 vs. Aldo-salt group.
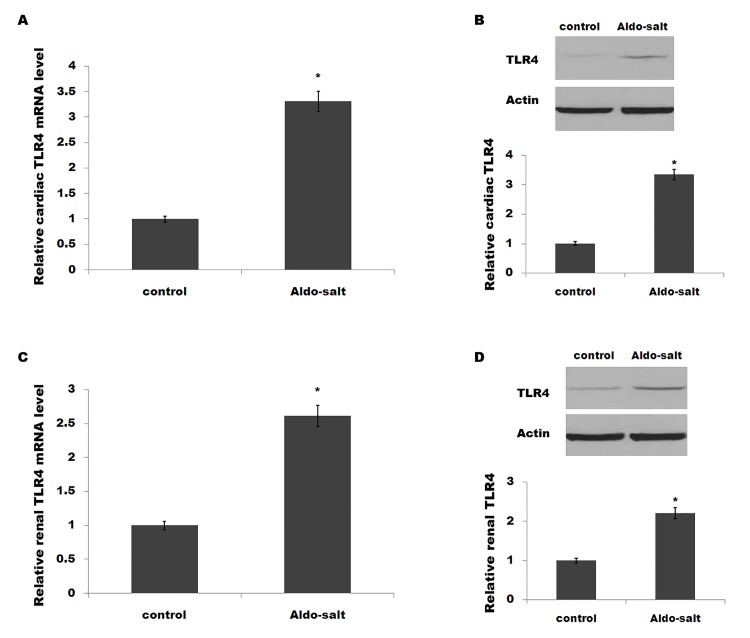
We then assessed whether the expression level of TLR4 is aberrant after Aldo-salt treatment. As shown in Fig 1A and 1B, Aldo-salt-treated rats show an increased cardiac TLR4 expression at both the mRNA and protein levels. At the same time, renal TLR4 expression levels are upregulated after Aldo-salt treatment (Fig 1C and 1D).
Fig 1. Cardiac and renal expression of TLR4 is increased in Aldo-salt-treated rats.
(A and B) Rats were infused with Aldo-salt at 1 mg/kg/day for 4weeks, and cardiac mRNA levels (A) and protein levels (B) of TLR4 were assayed using qPCR and western blot, respectively. Rats were infused with Aldo-salt at 1 mg/kg/day for 4weeks, and renal mRNA levels (C) and protein levels (D) of TLR4 were assayed using qPCR and western blot, respectively. *p< 0.05.
3.2 TAK-242 suppresses cardiorenal inflammation and fibrosis caused by Aldo-salt in vivo
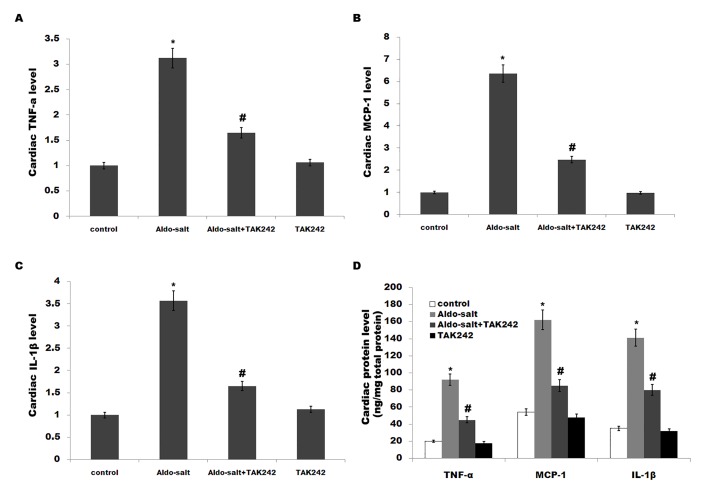
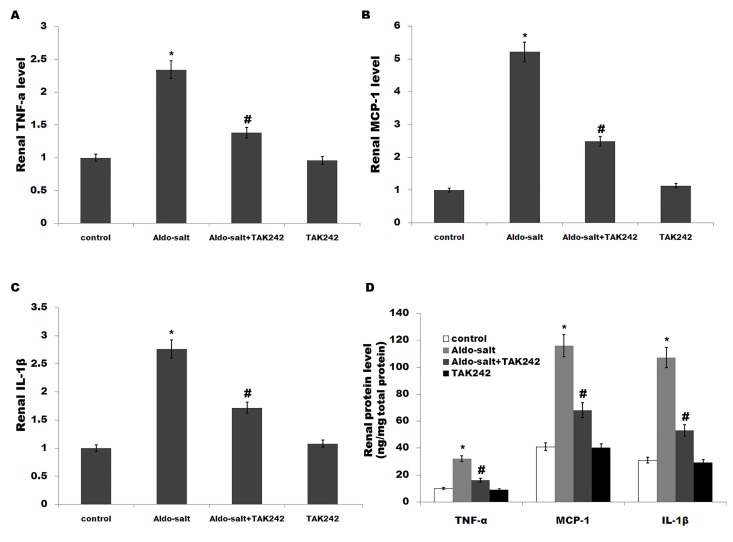
Activation of TLR4 leads to downstream release of inflammatory modulators including TNF-α, IL-1β and MCP-1[33].Moreover, previous studies demonstrated that inflammation plays a crucial role in the pathogenesis of hypertension, and the development and progression of renalfibrosis[13,34]. We thus speculated whether TLR4 mediates Aldo-salt-induced cardiorenal inflammation. We assayed the expression of genes for various proinflammatory cytokines by qPCR and ELISA. Fig 2A–2D showed that the cardiac mRNA and protein levels of TNF-a, MCP-1 and IL-1β are markedly increased by Aldo-salt infusion, whereasTAK-242 treatment inhibits cardiac inflammatory cytokines levels (Fig 2A–2D). Similarly, the renal inflammatory cytokines levels (TNF-a, MCP-1 and IL-1β) are enhanced after Aldo-salt treatment, whereas the expression of these genes is inhibited by TAK-242 (Fig 3A–3D).
Fig 2. TAK-242 suppresses cardiac inflammation and fibrosis caused by Aldo-salt in vivo.
(A-C) Cardiac mRNA levels of TNF-α, MCP-1 and IL-1β in rats treated with Aldo-salt or with Aldo-salt plus TAK-242. *p< 0.05 vs control, #p< 0.05 vs Aldo-salt. (D) Cardiac protein levels of TNF-α, MCP-1 and IL-1β in rats treated with Aldo-salt or with Aldo-salt plus TAK-242 were assayed using ELISA. *p< 0.05 vs control, #p< 0.05 vs Aldo-salt.
Fig 3. TAK-242 suppresses renal inflammation and fibrosis caused by Aldo-salt in vivo.
(A-C) Renal mRNA levels of TNF-α, MCP-1 and IL-1β in rats treated with Aldo-salt or with Aldo-salt plus TAK-242. *p< 0.05 vs control, #p< 0.05 vs Aldo-salt. (D) Renal protein levels of TNF-α, MCP-1 and IL-1β in rats treated with Aldo-salt or with Aldo-salt plus TAK-242 were assayed using ELISA. *p< 0.05 vs control, #p< 0.05 vs Aldo-salt.
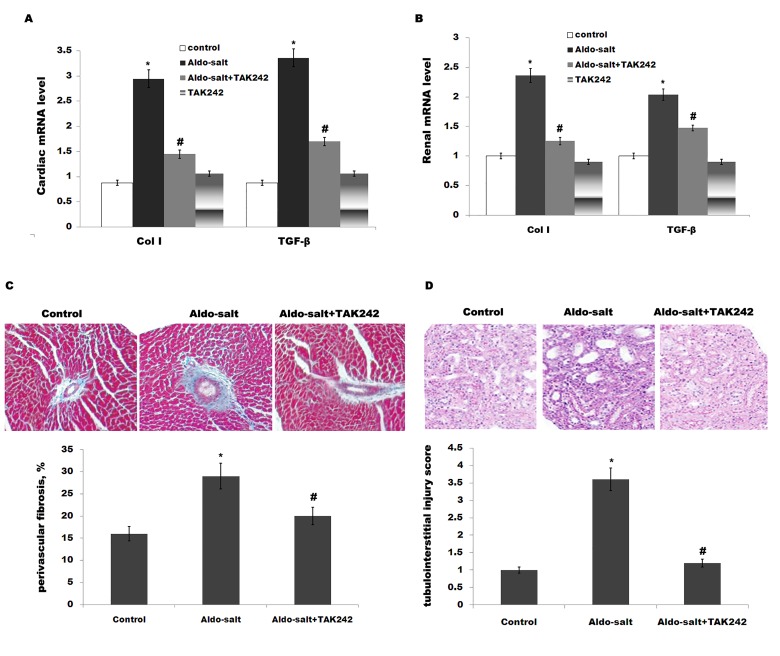
To evaluate cardiorenal fibrosis, we assayed the expression of collagen type I (Col I) and transforming growth factor-β(TGF-β), which are extracellular matrix protein and profibrotic marker, respectively. Although the Aldo-salt-treated group shows an increase of Col I and TGF-β in rat heart, the expression Col I and TGF-β is inhibited byTAK-242 treatment (Fig 4A). As in the case of heart, Aldo-salt treatment upregulates the expression of renal Col I and TGF-β, and TAK-242suppresses this increase (Fig 4B). Perivascular fibrosis in the left ventricle was assessed by deposition of collagen around the vasculature. Fig 4C presents representative images of collagen deposition and quantitation of fibrosis.TAK242 treatment markedly suppresses Aldo-induced perivascular fibrosis. Meanwhile, periodic acid Schiff-stained sections revealed thatTAK242 inhibits Aldo-induced tubulointerstitial damage (Fig 4D). These results suggest that TAK-242 treatment inhibits cardiorenal inflammation and fibrosis induced by Aldo-salt.
Fig 4. Histological findings.
Cardiac (A) or renal (B) mRNA levels of Col I and TGF-β in rats treated with Aldo-salt or with Aldo-salt plus TAK-242. *p< 0.05 vs control, #p< 0.05 vs Aldo-salt. (C)Representative images and quantitation of perivascular fibrosis in left ventricles of rats treated with Aldo-salt or with Aldo-salt plus TAK-242. *p< 0.05 vs control, #p< 0.05 vs Aldo-salt. (D) Histological staining with periodic acid Schiff (PAS), also showing tubulointerstitial damage of rats treated with Aldo-salt or with Aldo-salt plus TAK-242. *p< 0.05 vs control, #p< 0.05 vs Aldo-salt. n = 9, 200× magnification.
3.3 TAK-242 suppresses Aldo-salt-induced EMT
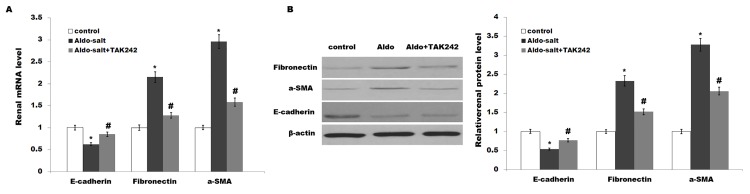
Epithelial-mesenchymal transition (EMT) is gradually being accepted as a mechanism by which injured renal tubular cells transform into mesenchymal cells, which might contribute to the development and progression of fibrosis[35,36]. Sun et al. demonstrated that uremic toxins induce renal fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated EMT [37].Therefore, we further investigated the role of TAK-242 in renal tubular epithelial EMT. As shown in Fig 5A and 5B, Aldo-salt treatment results in a significant decrease in E-cadherin (an epithelial marker) as well as an increase in fibronectin and a-SMA (mesenchymal markers) mRNA and protein expressions in proximal tubules of rat kidneys compared with controls. These changes are reversed by TAK-242 treatment (Fig 5A and 5B).
Fig 5. TAK-242 suppresses Aldo-salt-induced EMT.
Renal mRNA (A) and protein (B) expression of E-cadherin (an epithelial marker), and Fibronectin and a-SMA (mesenchymal markers) were assayed in rats treated with Aldo-salt or with Aldo-salt plus TAK-242. TAK-242 reversed the Aldo-salt–induced renal epithelial-mesenchymal transition. *p< 0.05 vs control, #p< 0.05 vs Aldo-salt.
Discussion
The activation of the renin-angiotensin-aldosterone system (RAAS), such as aniotensin II (Ang II) or aldosterone (Aldo), plays an important role in the pathogenesis of hypertension and chronic kidney damage via the production of inflammation [25]. Previous studies show that TLR4-mediated inflammatory response promotes Angiotensin II-induced cardiac hypertrophy and dysfunction[25].Several studies also indicate that inhibition of TLR4 improve cardiac function and attenuate myocardial fibrosis[24,26].However the role of TLR4 in aldosterone-induced cardiac damage is not clear. On the basis of referring to their researches, we initiated the study to investigate the role of TLR4 in aldosterone-induced cardiac injury. Furthermore, we investigated the role of TLR4 in aldosterone-induced renal injury. In experimental hyperaldosteronism, we demonstrated that upregulation of TLR4iscorrelated with cardiac and renal fibrosis and dysfunction. Especially, a TLR4 signaling antagonist, TAK-242, can reverse these alterations. Therefore, TAK-242 may be a therapeutic option for salt-sensitive hypertension and renal fibrosis.
Cardiovascular and renal fibrosis is found to be linked to inflammation in Aldo-salt-treated models[38].Previous studies demonstrated that the inhibition of inflammatory cytokines ameliorates cardiac and/or renal injury in several experimental models[39].Their data support the conclusion that MCP-1and TNF-a secreted by activated macrophages in the kidneys cause tissue damage[14].Here we also found that Aldo treatment induces the production of pro-inflammatory cytokines (such as TNF-a, IL-1β and MCP-1), whereas TAK-242 markedly reverses these alterations in kidney and heart.
The family of Toll-like receptors (TLRs)is pattern recognition receptors that recognize specific pathogen associated molecular patterns (PAMPs), which are found in pathogens but not in mammalian cells[40].TLRs are known to be highly expressed in innate immune cells in response to pathogens and environmental stressors[41]. Recently, TLRs have been found to express in renal tubular epithelial cells[17]. Emerging evidence suggests that endogenous ligands could activate TLRs, resulting in the antigen-independent inflammation that accompanies acute kidney injury (AKI), solid organ transplant rejection, and immune-mediated glomerulonephritis[42]. lipopolysaccharide (LPS) activates TLR4, and this activation increases the inflammation that characterizes LPS-induced AKI[43]. Devaraj et al. demonstrated that patients with T1DM (Type 1 diabetes mellitus)show an increased expression and activity of the TLR-2 and -4 on monocytes, which contributes to the accentuated proinflammatory state and complications of T1DM[44].TLR2 knockout reduces the pro-inflammatory state of diabetes and incipient diabetic nephrophthy[45]. They further showed that knockout of TLR4attenuatesrenal inflammation, fibrosis and podocytopathy[46]. In addition, TLR4 is also upregulated by Ang II infusion and TLR4 overexpression contributes to the inflammation, endothelial dysfunction, vascular remodelling and stiffness associated with hypertension [18].
In the study we investigated the role of TLR4 in Aldo-induced cardiac and renal injury. Aldo-salt treatment results in a significant increase in SBP, DBP and ratio of heart weight to body weight, whereas cardiac dysfunction and hypertrophy are reversed by TAK-242. Meanwhile, both renal dysfunction and hypertrophy are also prevented by TAK-242 in Aldo-salt-treated rats. Furthermore, TAK-242 inhibits cardiorenal inflammation and fibrosis caused by Aldo-salt in vivo. Different to previous study, we did not find that TAK-242 significantly suppresses TLR4 expression [47]. It is possible that TAK242 inhibits TLR4 signaling pathway, but not TLR4 itself. Therefore, it is very important to investigate the underlying relationship among Aldo, TAK-242 and TLR4 signaling pathway. Several studies reported that TAK-242 have a potential role in treating inflammation-related diseases. Garate et al. showed that TAK-242 decreases neuroinflammation in rat brain frontal cortex after stress, and could be considered as a potential therapeutic adjuvant strategy to constrain the inflammatory process in stress-related neuropsychiatric diseases[47].Other studies also demonstrated a similar anti-inflammatory/pro-survival profile of TAK-242 in mouse endotoxic shock models[48]. Conclusion: Our results suggest a key role for TLR4 signaling in cardiorenal remodeling and dysfunction induced by Aldo, and TLR4 antagonist might be used as a potential therapeutic target for treating salt-sensitive hypertension and renal fibrosis.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Brown NJ (2013) Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol 9: 459–469. 10.1038/nrneph.2013.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Irita J, Okura T, Jotoku M, Nagao T, Enomoto D, et al. (2011) Osteopontin deficiency protects against aldosterone-induced inflammation, oxidative stress, and interstitial fibrosis in the kidney. Am J Physiol Renal Physiol 301: F833–844. 10.1152/ajprenal.00557.2010 [DOI] [PubMed] [Google Scholar]
- 3. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, et al. (1999) The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 4. Williams GH, Burgess E, Kolloch RE, Ruilope LM, Niegowska J, et al. (2004) Efficacy of eplerenone versus enalapril as monotherapy in systemic hypertension. Am J Cardiol 93: 990–996. [DOI] [PubMed] [Google Scholar]
- 5. White WB, Duprez D, St Hillaire R, Krause S, Roniker B, et al. (2003) Effects of the selective aldosterone blocker eplerenone versus the calcium antagonist amlodipine in systolic hypertension. Hypertension 41: 1021–1026. [DOI] [PubMed] [Google Scholar]
- 6. de las Heras N, Ruiz-Ortega M, Miana M, Ruperez M, Sanz-Rosa D, et al. (2007) Interactions between aldosterone and connective tissue growth factor in vascular and renal damage in spontaneously hypertensive rats. J Hypertens 25: 629–638. [DOI] [PubMed] [Google Scholar]
- 7. Brown NJ, Nakamura S, Ma L, Nakamura I, Donnert E, et al. (2000) Aldosterone modulates plasminogen activator inhibitor-1 and glomerulosclerosis in vivo. Kidney Int 58: 1219–1227. [DOI] [PubMed] [Google Scholar]
- 8. Rafiq K, Hitomi H, Nakano D, Nishiyama A (2011) Pathophysiological roles of aldosterone and mineralocorticoid receptor in the kidney. J Pharmacol Sci 115: 1–7. [DOI] [PubMed] [Google Scholar]
- 9. Zhang YD, Dai HY, Xie HL, Zhou QL, Liu ZH (2013) The role of renal damage on cardiac remodeling in patients with diabetic nephropathy. Clin Nephrol 80: 249–255. 10.5414/CN107875 [DOI] [PubMed] [Google Scholar]
- 10. Savoia C, Schiffrin EL (2006) Inflammation in hypertension. Curr Opin Nephrol Hypertens 15: 152–158. [DOI] [PubMed] [Google Scholar]
- 11. Li JJ, Chen JL (2005) Inflammation may be a bridge connecting hypertension and atherosclerosis. Med Hypotheses 64: 925–929. [DOI] [PubMed] [Google Scholar]
- 12. Lee SB, Kalluri R (2010) Mechanistic connection between inflammation and fibrosis. Kidney Int Suppl: S22–26. 10.1038/ki.2010.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meng XM, Nikolic-Paterson DJ, Lan HY (2014) Inflammatory processes in renal fibrosis. Nat Rev Nephrol 10: 493–503. 10.1038/nrneph.2014.114 [DOI] [PubMed] [Google Scholar]
- 14. Doi T, Doi S, Nakashima A, Ueno T, Yokoyama Y, et al. (2014) Mizoribine ameliorates renal injury and hypertension along with the attenuation of renal caspase-1 expression in aldosterone-salt-treated rats. PLoS One 9: e93513 10.1371/journal.pone.0093513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frangogiannis NG (2014) The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 11: 255–265. 10.1038/nrcardio.2014.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang SC, Yiu WH, Lin M, Lai KN (2015) Diabetic nephropathy and proximal tubular damage. J Ren Nutr 25: 230–233. 10.1053/j.jrn.2014.10.020 [DOI] [PubMed] [Google Scholar]
- 17. Stribos EG, van Werkhoven MB, Poppelaars F, van Goor H, Olinga P, et al. (2015) Renal expression of Toll-like receptor 2 and 4: dynamics in human allograft injury and comparison to rodents. Mol Immunol 64: 82–89. 10.1016/j.molimm.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 18. Hernanz R, Martinez-Revelles S, Palacios R, Martin A, Cachofeiro V, et al. (2015) Toll-like receptor 4 contributes to vascular remodelling and endothelial dysfunction in angiotensin II-induced hypertension. Br J Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Summers SA, Steinmetz OM, Ooi JD, Gan PY, O'Sullivan KM, et al. (2010) Toll-like receptor 9 enhances nephritogenic immunity and glomerular leukocyte recruitment, exacerbating experimental crescentic glomerulonephritis. Am J Pathol 177: 2234–2244. 10.2353/ajpath.2010.100153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paulus P, Rupprecht K, Baer P, Obermuller N, Penzkofer D, et al. (2014) The early activation of toll-like receptor (TLR)-3 initiates kidney injury after ischemia and reperfusion. PLoS One 9: e94366 10.1371/journal.pone.0094366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moreth K, Frey H, Hubo M, Zeng-Brouwers J, Nastase MV, et al. (2014) Biglycan-triggered TLR-2- and TLR-4-signaling exacerbates the pathophysiology of ischemic acute kidney injury. Matrix Biol 35: 143–151. 10.1016/j.matbio.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jenke A, Wilk S, Poller W, Eriksson U, Valaperti A, et al. (2013) Adiponectin protects against Toll-like receptor 4-mediated cardiac inflammation and injury. Cardiovasc Res 99: 422–431. 10.1093/cvr/cvt118 [DOI] [PubMed] [Google Scholar]
- 23. Shah N, Mohamed FE, Jover-Cobos M, Macnaughtan J, Davies N, et al. (2013) Increased renal expression and urinary excretion of TLR4 in acute kidney injury associated with cirrhosis. Liver Int 33: 398–409. 10.1111/liv.12047 [DOI] [PubMed] [Google Scholar]
- 24. Echem C, Bomfim GF, Ceravolo GS, Oliveira MA, Santos-Eichler RA, et al. (2015) Anti-toll like receptor 4 (TLR4) therapy diminishes cardiac remodeling regardless of changes in blood pressure in spontaneously hypertensive rats (SHR). Int J Cardiol 187: 243–245. 10.1016/j.ijcard.2015.03.190 [DOI] [PubMed] [Google Scholar]
- 25. Matsuda S, Umemoto S, Yoshimura K, Itoh S, Murata T, et al. (2015) Angiotensin Activates MCP-1 and Induces Cardiac Hypertrophy and Dysfunction via Toll-like Receptor 4. J Atheroscler Thromb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dange RB, Agarwal D, Masson GS, Vila J, Wilson B, et al. (2014) Central blockade of TLR4 improves cardiac function and attenuates myocardial inflammation in angiotensin II-induced hypertension. Cardiovasc Res 103: 17–27. 10.1093/cvr/cvu067 [DOI] [PubMed] [Google Scholar]
- 27. Eissler R, Schmaderer C, Rusai K, Kuhne L, Sollinger D, et al. (2011) Hypertension augments cardiac Toll-like receptor 4 expression and activity. Hypertens Res 34: 551–558. 10.1038/hr.2010.270 [DOI] [PubMed] [Google Scholar]
- 28. Ha T, Li Y, Hua F, Ma J, Gao X, et al. (2005) Reduced cardiac hypertrophy in toll-like receptor 4-deficient mice following pressure overload. Cardiovasc Res 68: 224–234. [DOI] [PubMed] [Google Scholar]
- 29. Sha T, Iizawa Y, Ii M (2011) Combination of imipenem and TAK-242, a Toll-like receptor 4 signal transduction inhibitor, improves survival in a murine model of polymicrobial sepsis. Shock 35: 205–209. 10.1097/SHK.0b013e3181f48942 [DOI] [PubMed] [Google Scholar]
- 30. Yao L, Kan EM, Lu J, Hao A, Dheen ST, et al. (2013) Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia. J Neuroinflammation 10: 23 10.1186/1742-2094-10-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clemitson JR, Pratt JR, Frantz S, Sacks S, Samani NJ (2002) Kidney specificity of rat chromosome 1 blood pressure quantitative trait locus region. Hypertension 40: 292–297. [DOI] [PubMed] [Google Scholar]
- 32. Kapinas K, Kessler C, Ricks T, Gronowicz G, Delany AM (2010) miR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop. J Biol Chem 285: 25221–25231. 10.1074/jbc.M110.116137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beg AA (2002) Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol 23: 509–512. [DOI] [PubMed] [Google Scholar]
- 34. Parissis JT, Korovesis S, Giazitzoglou E, Kalivas P, Katritsis D (2002) Plasma profiles of peripheral monocyte-related inflammatory markers in patients with arterial hypertension. Correlations with plasma endothelin-1. Int J Cardiol 83: 13–21. [DOI] [PubMed] [Google Scholar]
- 35. Jia L, Ma X, Gui B, Ge H, Wang L, et al. (2015) Sorafenib ameliorates renal fibrosis through inhibition of TGF-beta-induced epithelial-mesenchymal transition. PLoS One 10: e0117757 10.1371/journal.pone.0117757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Masola V, Gambaro G, Tibaldi E, Brunati AM, Gastaldello A, et al. (2012) Heparanase and syndecan-1 interplay orchestrates fibroblast growth factor-2-induced epithelial-mesenchymal transition in renal tubular cells. J Biol Chem 287: 1478–1488. 10.1074/jbc.M111.279836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun CY, Chang SC, Wu MS (2012) Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS One 7: e34026 10.1371/journal.pone.0034026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Briet M, Schiffrin EL (2010) Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol 6: 261–273. 10.1038/nrneph.2010.30 [DOI] [PubMed] [Google Scholar]
- 39. Elmarakby AA, Quigley JE, Olearczyk JJ, Sridhar A, Cook AK, et al. (2007) Chemokine receptor 2b inhibition provides renal protection in angiotensin II—salt hypertension. Hypertension 50: 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takeda K, Akira S (2004) TLR signaling pathways. Semin Immunol 16: 3–9. [DOI] [PubMed] [Google Scholar]
- 41. Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124: 783–801. [DOI] [PubMed] [Google Scholar]
- 42. Shirali AC, Goldstein DR (2008) Tracking the toll of kidney disease. J Am Soc Nephrol 19: 1444–1450. 10.1681/ASN.2008010123 [DOI] [PubMed] [Google Scholar]
- 43. Cohen J (2002) The immunopathogenesis of sepsis. Nature 420: 885–891. [DOI] [PubMed] [Google Scholar]
- 44. Devaraj S, Jialal I, Yun JM, Bremer A (2011) Demonstration of increased toll-like receptor 2 and toll-like receptor 4 expression in monocytes of type 1 diabetes mellitus patients with microvascular complications. Metabolism 60: 256–259. 10.1016/j.metabol.2010.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Devaraj S, Tobias P, Kasinath BS, Ramsamooj R, Afify A, et al. (2011) Knockout of toll-like receptor-2 attenuates both the proinflammatory state of diabetes and incipient diabetic nephropathy. Arterioscler Thromb Vasc Biol 31: 1796–1804. 10.1161/ATVBAHA.111.228924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jialal I, Major AM, Devaraj S (2014) Global Toll-like receptor 4 knockout results in decreased renal inflammation, fibrosis and podocytopathy. J Diabetes Complications 28: 755–761. 10.1016/j.jdiacomp.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 47. Garate I, Garcia-Bueno B, Madrigal JL, Caso JR, Alou L, et al. (2014) Toll-like 4 receptor inhibitor TAK-242 decreases neuroinflammation in rat brain frontal cortex after stress. J Neuroinflammation 11: 8 10.1186/1742-2094-11-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sha T, Sunamoto M, Kitazaki T, Sato J, Ii M, et al. (2007) Therapeutic effects of TAK-242, a novel selective Toll-like receptor 4 signal transduction inhibitor, in mouse endotoxin shock model. Eur J Pharmacol 571: 231–239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.