Abstract
Cholinergic stimulation of the cerebral cortex is essential for tasks requiring attention; however, there is still some debate over which cortical regions are required for such tasks. There is extensive cholinergic innervation of both primary and associative cortices, and transient release of acetylcholine (ACh) is detected in deep layers of the relevant primary and/or associative cortex, depending on the nature of the attention task. Here, we investigated the electrophysiological effects of ACh in layer VI, the deepest layer, of the primary somatosensory cortex, the primary motor cortex, and the associative medial prefrontal cortex. Layer VI pyramidal neurons are a major source of top-down modulation of attention, and we found that the strength and homogeneity of their direct cholinergic excitation was region-specific. On average, neurons in the primary cortical regions showed weaker responses to ACh, mediated by a balance of contributions from both nicotinic and muscarinic ACh receptors. Conversely, neurons in the associative medial prefrontal cortex showed significantly stronger excitation by ACh, mediated predominantly by nicotinic receptors. The greatest diversity of responses to ACh was found in the primary somatosensory cortex, with only a subset of neurons showing nicotinic excitation. In a mouse model with attention deficits only under demanding conditions, cholinergic excitation was preserved in primary cortical regions but not in the associative medial prefrontal cortex. These findings demonstrate that the effect of ACh is not uniform throughout the cortex, and suggest that its ability to enhance attention performance may involve different cellular mechanisms across cortical regions.
Keywords: acetylcholine, attention, Chrna5, mouse, muscarinic, nicotinic
Introduction
Attention is a complex cognitive function that focuses the mind on relevant information in the presence of distraction (Crick, 1984). The cholinergic system is considered to be essential for attention (Robbins et al., 1989; Dunnett et al., 1991; Muir et al., 1992; Klinkenberg et al., 2011). In support of this model, cue detection during attention tasks triggers transient increases in acetylcholine (ACh) release in the deep layers of several different cortical regions (Passetti et al., 2000; Fournier et al., 2004; Kozak et al., 2006; Parikh et al., 2007; Parikh & Sarter, 2008). Such release fits with the relatively uniform innervation of cortical regions by cholinergic fibers from the basal forebrain (Mesulam et al., 1983; Mechawar & Descarries, 2001), and raises questions in view of the ongoing debate about which cortical regions are essential for attention (Meyer, 2011). Whereas it is acknowledged that activity in the associative prefrontal cortex is important for particularly challenging attention tasks (Marti et al., 2012), it appears not to be central under conditions of lower demand (Smucny et al., 2013). In fact, it has been suggested that primary cortical regions may carry out certain types of attentional processing on their own (Meyer, 2011). For example, alpha-band activity in the primary somatosensory cortex (SSC) strongly reflects somatosensory attention (Pfurtscheller et al., 1996; Jones et al., 2010), and such oscillations in the primary cortex are powerfully modulated by cholinergic stimulation (Bauer et al., 2012). Consistent with the hypothesis that attention relies on cholinergic modulation of primary as well as associative cortical regions, both primary and associative cortical regions send top-down projections that can control the thalamic and thalamocortical circuitry involved in attention (Guillery, 1995; Sherman, 2005; Zikopoulos & Barbas, 2006).
In particular, layer VI of the cerebral cortex sends and receives projections to thalamic nuclei, forming a corticothalamic feedback loop that is important for top-down control of normal attentional function (Bourassa et al., 1995; Guillery & Sherman, 2002; Gabbott et al., 2005; Parikh et al., 2010; Thomson, 2010). However, layer VI is heterogeneous (Andjelic et al., 2009; Briggs, 2010; Thomson, 2010), and includes corticocortical neurons (Mercer et al., 2005; Kumar & Ohana, 2008; Petrof et al., 2012), which may also be involved in attentional processing. For example, recent work has demonstrated a local circuit within the primary cortex whereby layer VI pyramidal neurons control cortical gain modulation (Olsen et al., 2012).
Layer VI is strongly innervated by cholinergic fibers (Mechawar & Descarries, 2001), and its neurons express ACh receptors of both the nicotinic and muscarinic subtypes (Buckley et al., 1988; Tribollet et al., 2004). However, responses to cholinergic stimulation have only been assessed previously in layer VI of the prelimbic cortex (Tian et al., 2011). The relative consequences of cholinergic stimulation on layer VI pyramidal neurons across primary and associative regions of the cortex are unknown.
Here, we investigated whether ACh exerts similar electrophysiological effects on layer VI neurons in primary and associative cortical regions. Then, we studied the underlying receptor mechanisms involved by using pharmacological manipulations. Heterogeneous responses observed in the SSC were examined further. Finally, we investigated regional variations in cholinergic excitation of layer VI neurons in a population of mice known to have attention deficits under demanding circumstances.
Materials and methods
Animals
Electrophysiological recordings were performed on brain slices from adult male mice (age range, postnatal day 50 to postnatal day 130; mean, 80 ± 6 days; n = 42 mice). To facilitate recording from layer VI neurons in the adult cortex, we employed BAC transgenic mice with extensive labeling of layer VI neurons by enhanced green fluorescent protein (eGFP) expression driven by the synaptotagmin 6 promoter (Syt6–eGFP; MMRRC) on a Swiss Webster background. Labeled neurons have pyramidal somata and apical dendrites that project towards the pial surface. None of the eGFP-positive neurons recorded exhibited electrophysiological characteristics of fast-spiking interneurons. Conversely, fast-spiking interneurons that were visually identified by infrared differential interference contrast microscopy and confirmed electrophysiologically (n = 10 across regions) were eGFP-negative. A subset of experiments were performed in C57BL/6 adult male mice with deletion of the nicotinic receptor α5 subunit (α5−/−) (Salas et al., 2003) and their matched wild-type (WT) controls. Mice were deeply anesthetized with chloral hydrate (400 mg/kg) and killed by decapitation. All animal care and experimental protocols were performed in accordance with the guidelines of the Canadian Council on Animal Care, and were approved by the University of Toronto Animal Care Committee.
Brain slice preparation
Brains were rapidly excised and chilled in 4 °C oxygenated sucrose artificial cerebrospinal fluid (ACSF) (254 mM sucrose, 10 mM D-glucose, 24 mM NaHCO3, 2 mM CaCl2, 2 mM MgSO4, 3 mM KCl, 1.25 mM NaH2PO4; pH 7.4). Coronal slices (thickness, 400 μm; 2.34–0.74 mm from Bregma) were cut on a Dosaka Linear Slicer (SciMedia, Costa Mesa, CA, USA), and allowed to recover in 30°C oxygenated ACSF (128 mM NaCl, 10 mM D-glucose, 26 mM NaHCO3, 2 mM CaCl2, 2 mM MgSO4, 3 mM KCl, 1.25 mM NaH2PO4; pH 7.4) for at least 2 h.
Electrophysiology
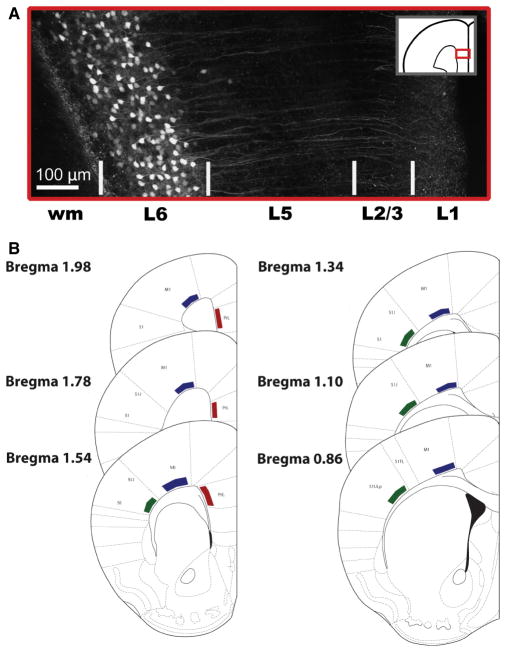
Recovered slices were transferred to a superfusion chamber on the stage of a BX50W1 microscope (Olympus, Richmond Hill, ON, Canada). ACSF was bubbled (95% O2 and 5% CO2 at room temperature) and perfused through the chamber at a rate of 3–4 mL/min. As shown in Fig. 1(A), layer VI was landmarked with fluorescently identified eGFP-positive neurons (X-cite Series 120; Lumen Dynamics, Mississauga, ON, Canada) in Syt6–eGFP mice. Recording electrodes (2–4 MΩ) containing 120 mM potassium gluconate, 5 mM KCl, 2 mM MgCl2, 4 mM K2-ATP, 0.4 mM Na2-GTP, 10 mM Na2-phosphocreatine and 10 mM Hepes buffer (adjusted to pH 7.3 with KOH) were used to patch layer VI pyramidal neurons in acute coronal prefrontal slices. Layer VI neurons in the SSC, primary motor cortex (M1) and associative medial prefrontal cortex (mPFC; prelimbic region) were specifically targeted on the basis of local landmarks, as previously described (Paxinos & Franklin, 2001; Fig. 1B). Currents and membrane potential were recorded in voltage clamp mode (at a holding potential of −75 mV) and in current clamp mode with an EPC10 (HEKA Electroniks, Chester, NS, Canada). All data were acquired at 20 kHz and low-pass filtered at 3 kHz with PCLAMP software (Molecular Devices, Sunnyvale, CA, USA).
Fig. 1.
Layer VI in primary and associative cortical regions is identified in transgenic eGFP-expressing mice. (A) A z-stack projection shows layer VI-specific expression of eGFP in neurons of Syt6–eGFP mice. This laminar specificity is conserved across primary and associative cortical regions. (B) Schematics showing regions of primary and associative cortices targeted for electrophysiological recordings. Bregma coordinates are indicated as described previously (Paxinos & Franklin, 2001). Red: mPFC. Blue: M1. Green: SSC. wm, white matter.
Electrophysiological properties of the layer VI neurons (resting membrane potential, input resistance, and spike amplitude) are shown in Table 1. Input resistance was calculated by measuring the steady-state deflections in membrane potential in response to injections of hyperpolarizing current (50–100 pA, 500 ms). Resting membrane potential was corrected for the liquid junction potential.
Table 1.
Electrophysiological parameters of SSC, M1 and mPFC layer VI neurons
| SSC (n = 73) | M1 (n = 43) | mPFC (n = 65) | P-value (one-way ANOVA) | |
|---|---|---|---|---|
| Resting membrane potential (mV) | −83.5 ± 0.6* | −80.8 ± 0.7 | −81.6 ± 0.7 | 0.01 |
| Spike amplitude (mV) | 90.7 ± 0.8 | 90.3 ± 0.9 | 90.3 ± 1.0 | 0.9 |
| Input resistance (MΩ) | 159.5 ± 7.7 | 179.3 ± 9.7 | 320.2 ± 14.2** | 0.0001 |
Across these regions of the cerebral cortex, there is a small difference in resting membrane potential (F2,178 = 4.4, P = 0.01), and a larger difference in input resistance (F2,178 = 66.2, P < 0.0001). Values are shown as mean ± standard error of the mean. mPFC, medial prefrontal cortex; M1, primary motor cortex; SSC, somatosensory cortex.
P < 0.05 for SSC vs. M1 or mPFC;
P < 0.05 for mPFC vs. SSC or M1.
Pharmacology
Cholinergic responses were probed with the addition of ACh (100 μM or 1 mM, 15 s) in standard ACSF to the bath perfusion after a baseline recording period. ACh applications were timer-controlled. Note that the high level of acetylcholinesterase in cortical slices means that only a fraction of the bath-applied ACh is likely to reach the cholinergic receptors (Bailey et al., 2010). A subset of experiments were performed after blockade of glutamatergic and GABAergic synaptic transmission with a combination of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20 μM), D-(−)-2-amino-5-phos-phonopentanoic acid (APV; 50 μM) and bicuculline (10 μM) added to the bath perfusion. Nicotinic and muscarinic effects were examined individually by applying ACh in the presence of the muscarinic antagonist atropine (200 nM) or the nicotinic antagonist dihydro-β-erythroidine hydrobromide (DHβE; 3 μM). All compounds were obtained from Sigma or Tocris Bioscience, and stored in stock solutions at −20 °C.
Statistical analysis
Current responses and changes in membrane potential resulting from bath-applied ACh were measured with CLAMPFIT software (Molecular Devices) and assessed with one-way ANOVAs and post hoc t-tests. For illustrative purposes, averaged responses to ACh in different groups were calculated by the use of data points extracted from all individual recordings. Comparisons between cholinergic responses and kinetics in SSC neurons were performed with unpaired t-tests. Comparisons between genotype and the regional effects of ACh in α5−/− and WT mice were performed with two-way ANOVAs. Regional and genotype differences in the proportion of neurons depolarized to threshold by ACh were compared by the use of Fisher’s exact tests.
Results
ACh exerts unique effects on layer VI neurons in primary and associative cortical regions
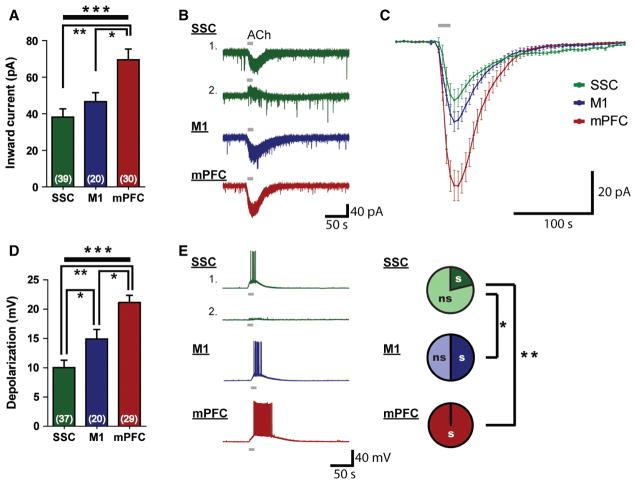
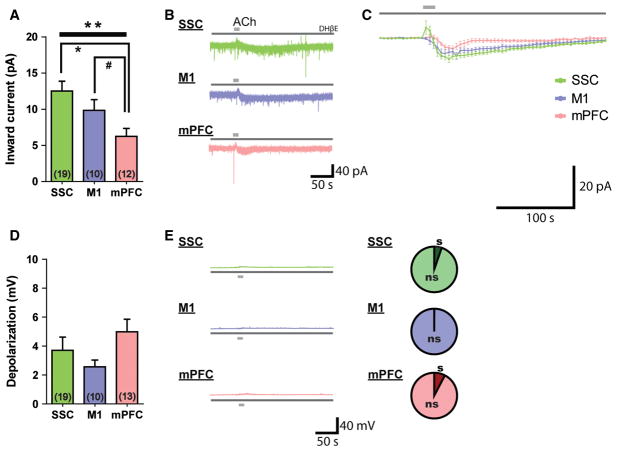
To determine the whole-cell effects of cholinergic stimulation on SSC, M1 and mPFC layer VI pyramidal neurons, we bath-applied ACh (1 mM, 15 s) and quantified the magnitude of the ACh-elicited inward currents in voltage clamp mode, holding neurons at −75 mV in the absence of any pharmacological blockers. There were significant regional differences (F2,86 = 10.5, P < 0.0001, one-way ANOVA; Fig. 2A). In particular, ACh-elicited inward currents were significantly smaller in SSC layer VI neurons (38.2 ± 4.5 pA, n = 39 neurons, t67 = 4.3, P < 0.0001, unpaired two-tailed t-test) and M1 layer VI neurons (46.6 ± 4.9 pA, n = 20 neurons, t48 = 2.8, P = 0.008, unpaired two-tailed t-test) than in mPFC layer VI neurons (69.5 ± 5.9 pA, n = 30 neurons). The responses to cholinergic stimulation were relatively homogenous within the M1 and mPFC, whereas there appeared to be greater diversity in cholinergic responses within the SSC (two examples are shown in Fig. 2B).
Fig. 2.
There is regional specificity in the cholinergic excitation of layer VI neurons in primary and associative cortical regions. (A) The average inward current elicited by ACh differs in layer VI of the SSC, M1, and mPFC (F2,86 = 10.5, ***P < 0.0001, one-way ANOVA). SSC neurons (**P < 0.0001, unpaired two-tailed t-test) and M1 neurons (*P = 0.008, unpaired two-tailed t-test) have smaller ACh-elicited currents than mPFC neurons. (B) Representative examples of ACh-elicited currents in the SSC, M1, and mPFC. Two examples are provided to show the greater heterogeneity within the SSC. (C) Averaged plot of all recorded ACh-elicited currents in SSC, M1 and mPFC neurons. (D) Excitation from rest elicited by ACh differs significantly in layer VI of the SSC, M1, and mPFC (F2,83 = 19.2, ***P < 0.0001, one-way ANOVA). Depolarization of layer VI neurons by cholinergic stimulation is smallest in the SSC (vs. M1, *P = 0.02, unpaired two-tailed t-test; vs. mPFC, **P < 0.0001, unpaired two-tailed t-test), larger in the M1 (vs. mPFC, *P = 0.003, unpaired two-tailed t-test), and largest in the mPFC. (E) Representative examples of ACh-elicited depolarization in the SSC, M1, and mPFC. SSC layer VI neurons are less likely to depolarize to threshold in response to cholinergic stimulation than M1 neurons (*P < 0.001, Fisher’s exact test) and mPFC neurons (**P < 0.0001, Fisher’s exact test). s, spiking; ns, non-spiking (in response to ACh).
As a majority of corticothalamic layer VI neurons are typically silent under resting conditions (Sirota et al., 2005; Zhou et al., 2010), we next investigated the ability of cholinergic stimulation to depolarize and elicit action potentials in these neurons in current clamp mode. We found significant regional differences in the degree of ACh-elicited excitation in layer VI neurons (F2,83 = 19.2, P < 0.0001, one-way ANOVA; Fig. 2D). Average depolarization in SSC neurons (10.0 ± 1.3 mV, n = 37 neurons) was significantly smaller than that in M1 neurons (14.9 ± 1.6 mV, n = 20 neurons, t55 = 2.3, P = 0.02, unpaired two-tailed t-test) and mPFC neurons (21.2 ± 1.2 mV, n = 29 neurons, t64 = 6.2, P < 0.0001, unpaired two-tailed t-test). The mPFC neurons showed the strongest excitation by ACh, having significantly larger depolarization than M1 neurons (t47 = 3.1, P = 0.003, unpaired two-tailed t-test). Moreover, the depolarization elicited by ACh within layer VI neurons resulted in action potential firing in some neurons (Fig. 2E). In this regard, the region-specific consequences of cholinergic stimulation also differed between SSC, M1 and mPFC layer VI neurons. SSC neurons were less likely to depolarize to threshold in response to ACh (8/38 neurons, 21%) than M1 neurons (10/20 neurons, 50%, P < 0.001, Fisher’s exact test) or mPFC neurons (30/30 neurons, 100%, P < 0.0001, Fisher’s exact test). mPFC layer VI neurons were unique, in that every neuron depolarized to threshold in response to ACh; this is a significantly larger proportion than found in M1 (P < 0.0001, Fisher’s exact test).
This pattern of strong regional difference in the layer VI cholinergic response was also observed with a lower concentration of ACh (100 μM; F2,35 = 3.8, P < 0.03, one-way ANOVA). ACh elicited significantly smaller average inward currents in SSC neurons (15.3 ± 3.3 pA, n = 11 neurons, t23 = 2.3, P = 0.03, unpaired two-tailed t-test) and M1 neurons (14.2 ± 3.9 pA, n = 13 neurons, t25 = 2.4, P = 0.02, unpaired two-tailed t-test) than in mPFC neurons (26.5 ± 3.4 pA, n = 14 neurons). Significant regional differences were also observed in ACh-elicited depolarization (100 μM; F2,26 = 10.1, P = 0.0006, one-way ANOVA). ACh elicited significantly smaller average depolarization in SSC neurons (6.1 ± 1.5 mV, n = 9 neurons, t18 = 4.8, P = 0.0001, unpaired two-tailed t-test) and M1 neurons (8.8 ± 2.5 mV, n = 9 neurons, t18 = 3.0, P = 0.008, unpaired two-tailed t-test) than in mPFC neurons (17.8 ± 1.8 mV, n = 11 neurons). It is of note that this milder cholinergic stimulus was sufficient to elicit action potentials in the majority of mPFC neurons.
Region-specific effects of ACh on layer VI neurons appear to be postsynaptic in nature
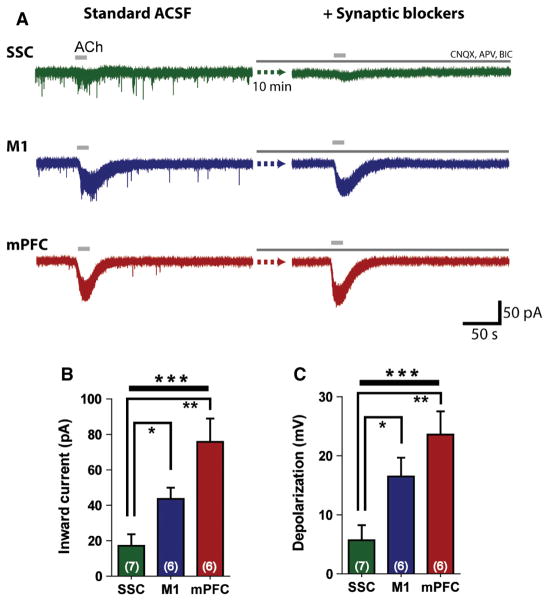
As ACh can affect both presynaptic and postsynaptic aspects of the cortical circuitry (Proulx et al., 2013; Bloem et al., 2014), we examined the effects of ACh in the presence of antagonists of synaptic transmission (20 μM CNQX, 50 μM APV, 10 μM bicuculline; in bath). It is of note that the inward currents elicited by bath application of 1 mM ACh persisted during treatment with glutamatergic and GABAergic antagonists (examples from each region are shown in Fig. 3A). Furthermore, significant regional differences were seen when synaptic transmission was blocked (F2,16 = 10.9, P = 0.001, one-way ANOVA; Fig. 3B). Under these conditions, ACh also elicited smaller responses in SSC neurons (17.2 ± 6.5 pA, n = 7 neurons, t11 = 4.2, P = 0.001, unpaired two-tailed t-test) and M1 neurons (43.6 ± 6.4 pA, n = 6 neurons, t10 = 2.2, P = 0.05, unpaired two-tailed t-test) than in mPFC neurons (75.9 ± 13.2 pA, n = 6 neurons). Likewise, depolarization by ACh in the presence of glutamatergic and GABAergic antagonists was also significantly different across primary and associative cortical regions (F2,16 = 8.2, P = 0.003, one-way ANOVA; Fig. 3C). SSC neurons (5.7 ± 2.6 mV, n = 7 neurons) were significantly less depolarized by ACh than M1 neurons (16.5 ± 3.2 mV, n = 6 neurons, t11 = 2.7, P = 0.02, unpaired two-tailed t-test) and mPFC neurons (23.6 ± 3.9 mV, n = 6 neurons, t11 = 3.9, P = 0.002, unpaired two-tailed t-test). These results suggest that postsynaptic cholinergic receptors are predominantly responsible for the large excitatory responses that we observed in layer VI neurons of primary and associative cortical regions.
Fig. 3.
Region-dependent effects of ACh are preserved in the presence of antagonists of synaptic transmission. (A) Example traces from layer VI neurons in primary and associative cortical regions of inward currents elicited by ACh. Traces on the left show responses to ACh in standard ACSF. Traces on the right show responses to ACh in the same neuron in the presence of glutamatergic and GABAergic receptor antagonists [20 μM CNQX; 50 μM APV; 10 μM bicuculline (BIC)] after a 10-min wash-in period. (B) Prominent regional differences in the inward currents elicited by ACh are seen in the presence of synaptic blockers (F2,16 = 10.9, ***P = 0.001, one-way ANOVA). Currents are smallest in SSC neurons (vs. M1, *P = 0.01, unpaired two-tailed t-test; vs. mPFC, **P = 0.001, unpaired two-tailed t-test), larger in M1 neurons (vs. mPFC, P = 0.05, unpaired two-tailed t-test), and largest in mPFC neurons. (C) Depolarization by ACh in the presence of synaptic blockers is significantly different across the SSC, M1, and mPFC (F2,16 = 8.2, ***P = 0.003, one-way ANOVA). Depolarization in SSC neurons is significantly smaller than in M1 neurons (*P = 0.02, unpaired two-tailed t-test) and mPFC neurons (**P = 0.002, unpaired two-tailed t-test).
The balance of nicotinic and muscarinic receptor contributions depends on cortical region
To determine the respective contributions of nicotinic and muscarinic ACh receptors to the regional dependence of the ACh responses in layer VI, we tested the effects of ACh in the continued presence of either the non-specific muscarinic antagonist atropine (200 nM) or the selective nicotinic antagonist DHβE (3 μM). In a subset of experiments, a combination of these antagonists blocked all responses to ACh in layer VI pyramidal neurons of all three cortical regions examined (data not shown).
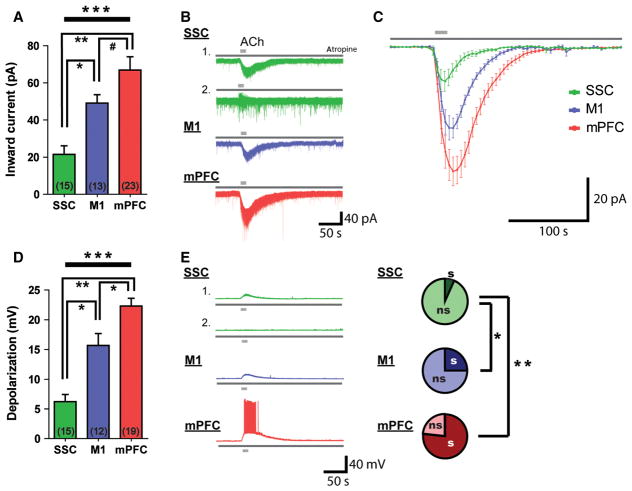
Nicotinic receptor-mediated inward currents elicited by ACh in the presence of atropine differed significantly across the cortical regions (F2,48 = 13.3, P < 0.0001, one-way ANOVA; Fig. 4A). Nicotinic stimulation of layer VI neurons resulted in smaller inward currents, on average, in SSC neurons (21.5 ± 4.6 pA, n = 15 neurons) than in M1 neurons (49.1 ± 4.5 pA, n = 13 neurons, t26 = 4.2, P = 0.0002, unpaired two-tailed t-test) and mPFC neurons (66.9 ± 7.2 pA, n = 23 neurons, t36 = 4.7, P < 0.0001, unpaired two-tailed t-test). Again, there was greater heterogeneity in the responses in the SSC than in the other two regions (two examples are shown in Fig. 4B).
Fig. 4.
There is regional specificity in the nicotinic receptor-mediated responses to ACh in layer VI neurons of primary and associative cortical regions. (A) The average inward current elicited by nicotinic stimulation differs among the SSC, M1, and mPFC (F2,48 = 13.3, ***P < 0.0001, one-way ANOVA). Nicotinic receptor-evoked inward currents are smallest in the SSC (vs. M1, *P = 0.0002, unpaired two-tailed t-test; vs. mPFC, **P < 0.0001, unpaired two-tailed t-test), and larger in the M1 (vs. mPFC, #P = 0.09, unpaired two-tailed t-test) and mPFC. (B) Representative examples of nicotinic currents in the SSC, M1, and mPFC. (C) Averaged plot of all recorded nicotinic responses in SSC, M1 and mPFC neurons. (D) Depolarization by nicotinic stimulation differs significantly between layer VI neurons of primary and associative cortal regions (F2,42 = 32.9, ***P < 0.0001, one-way ANOVA). This depolarization is smallest in SSC neurons (vs. M1, *P = 0.0003, unpaired two-tailed t-test; vs. mPFC, **P = 0.0001, unpaired two-tailed t-test), larger in M1 neurons (vs. mPFC, *P = 0.007, unpaired two-tailed t-test), and largest in mPFC neurons. (E) Representative examples of nicotinic receptor-mediated depolarization in the SSC, M1, and mPFC. SSC layer VI neurons are less likely to depolarize to threshold in response to nicotinic stimulation than M1 neurons (*P = 0.008, Fisher’s exact test) and mPFC neurons (**P < 0.0001, Fisher’s exact test). s, spiking; ns, non-spiking.
Depolarization by ACh in the presence of atropine was significantly different across regions (F2,42 = 32.9, P < 0.0001, one-way ANOVA; Fig. 4D). Significantly smaller average depolarization was observed in SSC neurons (6.2 ± 1.2 mV, n = 15 neurons) than in M1 neurons (15.7 ± 2.0 mV, n = 12 neurons, t25 = 4.2, P = 0.0003, unpaired two-tailed t-test) and mPFC neurons (22.3 ± 1.3 mV, n = 19 neurons, t32 = 8.8, P = 0.0001, unpaired two-tailed t-test). Consistently, mPFC layer VI neurons were also more likely to depolarize to threshold (15/19 neurons, 78%) than M1 neurons (3/12 neurons, 25%, P = 0.008, Fisher’s exact test) and SSC neurons (1/15 neurons, 7%, P < 0.0001, Fisher’s exact test).
Next, we investigated muscarinic stimulation of layer VI neurons by ACh in the presence of the nicotinic receptor antagonist DHβE. The response to muscarinic stimulation was region-specific (F2,38 = 5.6, P = 0.007, one-way ANOVA; Fig. 5A). The largest average currents were observed in SSC layer VI neurons (12.5 ± 1.3 mV, n = 12 neurons), and they were significantly larger than those in mPFC neurons (6.3 ± 1.1 mV, n = 19 neurons, t29 = 3.3, P = 0.003, unpaired two-tailed t-test); M1 neurons showed intermediate responses (9.8 ± 1.5 mV, n = 10 neurons). Unlike the ACh and nicotinic responses, muscarinic responses were relatively homogeneous within the SSC (Fig. 5B).
Fig. 5.
There is some regional specificity, albeit less pronounced, in muscarinic receptor-mediated responses to ACh in layer VI of primary and associative cortical regions. (A) Inward currents elicited by muscarinic stimulation differ among the SSC, M1, and mPFC (F2,38 = 5.6, **P = 0.007, one-way ANOVA). Muscarinic receptor-mediated currents are largest in SSC and M1 neurons and smallest in mPFC neurons (vs. SSC, *P = 0.003, unpaired two-tailed t-test; vs. M1, #P = 0.06, unpaired two-tailed t-test). (B) Representative sample traces of muscarinic receptor-mediated currents in the SSC, M1, and mPFC. (C) Averaged plot of all recorded muscarinic currents in SSC, M1 and mPFC neurons. (D) Depolarization from rest by muscarinic stimulation does not differ significantly between SSC, M1 and mPFC layer VI neurons (F2,39 = 1.6, P = 0.2, one-way ANOVA). (E) Examples of representative responses to muscarinic stimulation. There are no differences in the proportions of neurons reaching threshold in response to muscarinic receptor-mediated excitation. s, spiking; ns, non-spiking.
Despite our observation that muscarinic receptor-mediated inward currents were significantly different across the three regions, depolarization by ACh in the presence of DHβE did not differ across regions (F2,39 = 1.6, P = 0.2, one-way ANOVA; Fig. 5D). Therefore, there are no regional differences in the ability of ACh to depolarize neurons to threshold by muscarinic stimulation (SSC, 1/19 neurons, 5%; M1, 0/10 neurons, 0%; mPFC, 1/13 neurons, 8%; Fig. 5E). These paradoxical findings may be explained by the significantly higher average input resistance of mPFC layer VI neurons than M1 and SSC neurons, as shown in Table 1.
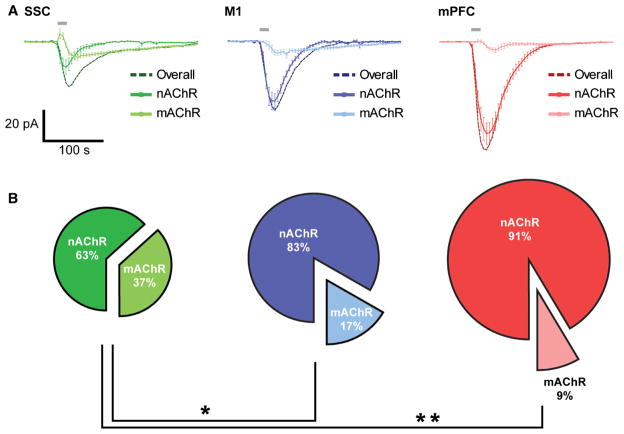
On the basis of our pharmacological dissection of the cholinergic response in layer VI neurons across primary and associative cortical regions, there appear to be significant differences in the respective contributions of nicotinic and muscarinic receptors to the overall cholinergic response between regions. These differences are illustrated in Fig. 6, and are calculated on the basis of the mean magnitudes of ACh-elicited inward currents in the presence of either atropine or DHβE. On average, there appears to be a shift from a combination of moderate nicotinic and muscarinic responses in SSC layer VI neurons (nicotinic ACh receptors, 63%; muscarinic ACh receptors, 37%) to larger and more dominant nicotinic responses in M1 neurons (nicotinic ACh receptors, 83%; muscarinic ACh receptors, 17%; P = 0.002, Fisher’s exact test), and the strongest and most dominant nicotinic responses in mPFC neurons (nicotinic ACh receptors, 91%; muscarinic ACh receptors, 9%; P < 0.0001, Fisher’s exact test).
Fig. 6.
Cortical region determines the overall cholinergic excitation of layer VI neurons and the degree of nicotinic and muscarinic contributions to the average response. (A) Superimposed average plots of all nicotinic [nicotinic ACh receptor (nAChR)], muscarinic [muscarinic ACh receptor (mAChR)] and overall responses to ACh in SSC, M1 and mPFC layer VI neurons. (B) Pie charts represent the relative contributions of nicotinic receptor-mediated and muscarinic receptor-mediated responses to ACh in SSC, M1 and mPFC neurons. Layer VI neurons are more dependent on nicotinic stimulation in the M1 (*P = 0.002, Fisher’s exact test) and mPFC (**P < 0.0001, Fisher’s exact test) than in the SSC.
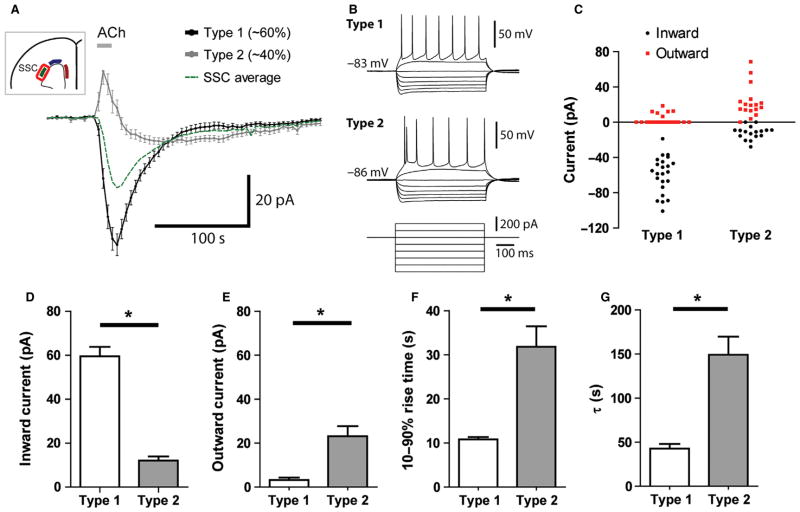
Characterization of two distinct populations of cholinergic responses among SSC neurons
We observed greater heterogeneity in the SSC than in the M1 and mPFC for responses to cholinergic (Fig. 2B and E) and nicotinic (Fig. 4B and E) stimulation, but not for responses to muscarinic stimulation (Fig. 5B and E). On the basis of these results, we hypothesized that SSC layer VI neurons could be subdivided into two subtypes according to their responses to ACh. As summarized in Fig. 7, ‘type 1’ neurons showed prominent inward currents (57%, 22 of 39 SSC neurons) and ‘type 2’ neurons showed more subtle and complex responses (43%, 17 of 39 SSC neurons). The averaged ACh-elicited currents of type 1 and type 2 neurons are shown in Fig. 7(A) and compared with the overall average response in the SSC. Interestingly, some evidence indicates that these subgroups may reflect distinct subclasses of layer VI neurons, as characterized by their responses to depolarizing current injections. Previous work has suggested that layer VI corticothalamic neurons have regular-spiking firing patterns, whereas corticocortical neurons have initial-doublet firing patterns (Mercer et al., 2005; Kumar & Ohana, 2008; Petrof et al., 2012). Here, we found a highly significant difference in the prevalence of these different firing patterns in type 1 and type 2 neurons (Fisher’s exact test, P < 0.0001), with the majority of regular-firing cells (22 of 27 neurons) yielding a type 1 response to ACh, and all initial-doublet neurons yielding a type 2 response (12 of 12 neurons).
Fig. 7.
Further characterization of the two distinct cholinergic responses observed in SSC layer VI neurons. (A) Averaged plots of ACh-elicited currents in these ‘type 1’ and ‘type 2’ SSC neurons. (B) These different responses to ACh may arise from two distinct subclasses of layer VI neurons, as illustrated by their typical spiking patterns in response to injection of depolarizing current. (C) Scatter plot showing uni-directional and bi-directional responses to ACh (outward current shown in red; inward current shown in black) in both populations of SSC neurons. Neurons with either no inward or no outward currents are plotted as zeroes. (D and E) Average inward and outward currents in type 1 and type 2 neurons. Type 1 neurons have significantly larger inward currents (*P < 0.0001, unpaired two-tailed t-test), but smaller outward currents on average (*P < 0.0001, unpaired two-tailed t-test). (F and G) Type 2 neurons have slower rise time (*P < 0.0001, unpaired two-tailed t-test) and longer response duration (*P < 0.0001, unpaired two-tailed t-test) following cholinergic stimulation.
Acetylcholine-elicited inward currents were significantly larger in type 1 neurons (type 1, 58.4 ± 4.4 pA, n = 22 neurons; type 2, 11.9 ± 1.7 pA, n = 17 neurons; t37 = 8.9, P < 0.0001, unpaired two-tailed t-test; Fig. 7C and D). These inward currents also had different kinetics in terms of speed of onset (type 1, 10.8 ± 0.6 s, n = 23 neurons; type 2, 31.8 ± 4.6 s, n = 13 neurons; t34 = 6.0, P < 0.0001, unpaired two-tailed t-test; Fig. 7F) and duration of response (type 1, 42.7 ± 5.3 s, n = 23 neurons; type 2, 149.2 ± 20.4 s, n = 13 neurons; t34 = 6.4, P < 0.0001, unpaired two-tailed t-test; Fig. 7G). In the presence of the muscarinic antagonist atropine, fast inward currents were found in 63% (10/16) of SSC neurons, whereas 37% (6/16) showed a minimal or no response. It is of note that the type 2 SSC neurons consistently showed a transient outward current at the beginning of the ACh response, but this was only infrequently observed in type 1 SSC neurons (Fig. 7C). Such outward currents were also prominent among the muscarinic responses in all regions observed in the presence of the nicotinic antagonist DHβE. On the basis of the heterogeneity in the ACh and nicotinic responses and the homogeneity in the muscarinic responses in the SSC, we speculate that type 1 responses are composed of a mixture of nicotinic and muscarinic components, whereas type 2 responses are primarily muscarinic in nature. This heterogeneity of responses to ACh in the SSC contrasts with the relative homogeneity of the responses in the M1 and mPFC.
The mouse attention deficit model has disrupted associative and preserved primary responses
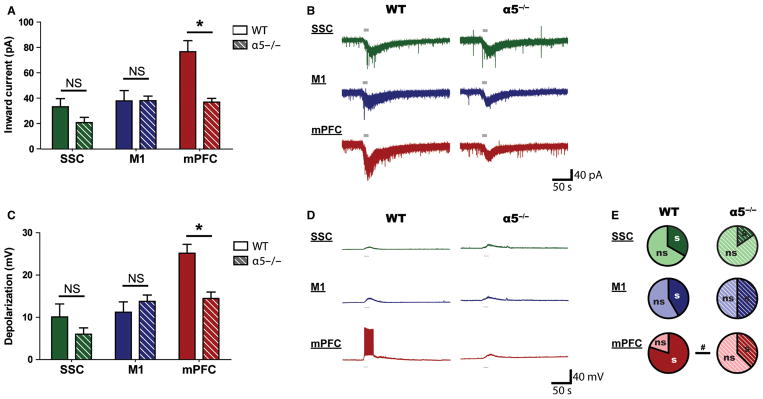
Associative cortical regions such as the mPFC are thought to be necessary for attention tasks only under conditions of high demand (Marti et al., 2012). Here, we examined ACh responses in layer VI pyramidal neurons across primary and associative regions in a mouse model that shows deficient attention only under demanding conditions (Bailey et al., 2010). This mouse is deleted for the Chrna5 gene, which encodes the α5 nicotinic ACh receptor subunit expressed in layer VI across the cerebral cortex (Wada et al., 1990; Salas et al., 2003; Winzer-Serhan & Leslie, 2005). Recorded ACh responses from α5−/− mice and their WT controls showed a significant interaction between genotype and cortical region (F2,63 = 3.9, P = 0.03, two-way ANOVA; Fig. 8A and B). This interaction arises not from changes to ACh responses within the primary cortex, but from a significant reduction in the responses within the associative mPFC (t = 3.9, P < 0.001, Bonferroni post hoc test).
Fig. 8.
The regional specificity of cholinergic responses is attenuated in the absence of the nicotinic receptor α5 subunit. (A) Inward currents elicited by ACh in primary and associative cortical regions of α5−/− mice and WT controls. There is a significant interaction between genotype and cortical region on cholinergic responses (F2,63 = 3.9, P = 0.03, two-way ANOVA). ACh-elicited inward currents in mPFC layer VI neurons are significantly smaller in α5−/− mice than in WT controls (*P < 0.001, Bonferroni post hoc tests), but a genotype difference is not observed in primary cortical regions. (B) Representative examples of cholinergic responses across primary and associative cortical regions in WT and α5−/− mice. (C) Depolarization by ACh in SSC, M1 and mPFC layer VI neurons of α5−/− mice and WT controls. A significant interaction exists between genotype and cortical region on ACh-elicited depolarization (F2,57 = 7.8, P = 0.01, two-way ANOVA). Depolarization by ACh in mPFC layer VI neurons, but not in those of primary cortical regions, is significantly smaller in α5−/− mice than in WT controls (*P < 0.01, Bonferroni post hoc tests). (D) Representative recordings of depolarization by cholinergic stimulation in the SSC, M1, and mPFC. (E) Proportions of neurons depolarized to threshold across primary and associative cortical regions in WT and α5−/− mice. mPFC layer VI neurons in α5−/− mice tend to be less likely to depolarize to threshold than those of WT controls (#P = 0.07, Fisher’s exact test). s, spiking; ns, non-spiking; NS, not significant.
A similar interaction was found between genotype and cortical region on ACh-elicited depolarization (F2,57 = 7.8, P = 0.01, two-way ANOVA; Fig. 8C and D). Again, this interaction arises from a significant decrease in ACh-elicited depolarization only within the mPFC (t = 3.5, P < 0.01, Bonferroni post hoc test). Likewise, mPFC layer VI neurons in α5−/− mice appeared to be less likely to depolarize to threshold than those in WT controls (WT, 12/15 neurons, 80%; α5−/−, 3/8 neurons; P = 0.07, Fisher’s exact test). This decrease in cholinergic excitation was not observed in layer VI M1 or SSC neurons. These data show that a manipulation that only interferes with the performance of attention tasks under conditions of high demand (Bailey et al., 2010) has selective effects on ACh-elicited responses in the associative cortex but not on those in primary cortical regions.
Discussion
There are prominent regional differences in the strength and nature of cholinergic responses in layer VI pyramidal neurons. These responses are primarily mediated by postsynaptic mechanisms, and are not a consequence of ACh-elicited changes in synaptic transmission. Pharmacological dissection of the cholinergic responses reveals that the balance between nicotinic and muscarinic ACh receptor-mediated responses is region-specific. SSC layer VI neurons showed the weakest average response to cholinergic stimulation. Their responses were not homogeneous: slightly more than half of the neurons responded to both nicotinic and muscarinic receptors; however, a large minority showed only muscarinic responses. In contrast, M1 neurons responded more strongly and homogeneously to ACh, with both nicotinic and muscarinic receptors contributing to cholinergic excitation. Finally, mPFC layer VI neurons were the most powerfully excited by cholinergic stimulation, an effect driven predominantly by large nicotinic responses that showed region-specific dependence on the nicotinic receptor α5 subunit. The physiological importance of these responses likely corresponds to their role in modulating normal attention, depending on the modality and demand of the attention task.
Layer VI neurons, attention, and the importance of cholinergic neurotransmission
We chose to target our recordings to layer VI neurons because they constitute a major source of top-down feedback to the thalamus, innervating both primary and higher-order thalamic nuclei (Bourassa et al., 1995; Guillery & Sherman, 2002; Gabbott et al., 2005). Coupled with thalamocortical inputs, they form a loop between the cortex and thalamus that is considered to be essential for normal attentional function (Parikh et al., 2010; Thomson, 2010). The strong cholinergic stimulation that we observed is consistent with the presence of both nicotinic and muscarinic receptors within neurons of deep cortical layers (Buckley et al., 1988; Tribollet et al., 2004). These receptors are likely involved in mediating in vivo responses to local release of ACh upon cue detection during attention tasks, as observed in both primary (Fournier et al., 2004; Parikh & Sarter, 2008) and associative (Parikh et al., 2007; Rasmusson et al., 2007) cortical regions. Within the SSC, ACh release has been extensively linked to the shifting of attentional focus in response to visual–sensory stimulation (Fournier et al., 2004; Haegens et al., 2011; Bauer et al., 2012). In particular, top-down modulated alpha-band activity in the SSC reflects an anticipatory state that predicts the direction of attentional focus, as well as attentional performance (Jones et al., 2010; Haegens et al., 2011). In the M1, evoked tonic release of ACh through periods of attentional demand has been linked to performance in attention tasks (Parikh & Sarter, 2008), and functionally correlated with motor planning or the execution of necessary movements that contribute to normal performance in these tasks (Sirota et al., 2005; Conner et al., 2010). On the other hand, ACh release within the mPFC is observed just before cue detection, and, in fact, predicts the success of such detection (Parikh et al., 2007; Howe et al., 2013).
Within primary regions, cholinergic activity triggered by stimuli is affected by, but not dependent on, the presence of distractors (Kelly et al., 2006; Sauseng et al., 2009). However, cholinergic activity within the mPFC is not necessarily present across all attention tasks. Some evidence suggests that it may not be central under conditions of lower attentional demand (Smucny et al., 2013), and that it may be employed only in particularly challenging conditions (Marti et al., 2012). Supporting this theory is the observation that the influence of cholinergic neuromodulation is highest when attention is taxed by distractors or prolonged trials (Kozak et al., 2006; Sarter et al., 2006; St Peters et al., 2011).
The distinct regional responses that we report here show that layer VI neurons across the cortex do not respond uniformly to ACh. The heightened influence of muscarinic receptor-mediated responses to ACh in the primary regions suggests they may have a greater role in the sensorimotor components of attention, as seen in pharmacological studies involving behaving rodents and primates (Robbins et al., 1998; Davidson & Marrocco, 2000). In particular, we found two distinct populations of neurons within layer VI of the SSC on the basis of their cholinergic response, which may reflect differences in this response among neurons within the SSC with different projection targets (Mercer et al., 2005; Kumar & Ohana, 2008). The known heterogeneity of neurons within layer VI (Andjelic et al., 2009; Briggs, 2010; Thomson, 2010) raises questions about their potentially distinct contributions, in terms of timing and direction of response, to the performance of somatosensory attention tasks. In addition to the postsynaptic effects of ACh reported here, cholinergic stimulation can elicit changes at presynaptic sites in the cerebral cortex (Bloem et al., 2014). Although ACh can elicit strong postsynaptic responses in the presence of antagonists of synaptic transmission, ACh release in vivo likely plays an important role in the fine-tuning of activity within the local circuitry that can affect attention. More experiments, including the characterization of the two neuronal subtypes in the SSC, are necessary for a better understanding of the general circuitry within this region and how their unique responses to ACh can modulate attentional behavior.
The ACh response in the associative cortex and the nicotinic receptor α5 subunit
The larger overall responses to cholinergic stimulation and the dominant nature of the nicotinic receptor-mediated component sets layer VI neurons of the associative mPFC apart from the primary cortical regions. Our results suggest that the nicotinic receptor α5 subunit is required to maintain these large cholinergic responses within layer VI of the mPFC in adulthood, but does not play a role in the ACh responses in the primary cortical regions examined. These results are broadly consistent with studies suggesting that the α5 subunit shows not only laminar specificity in its expression (Birtsch et al., 1997; Salas et al., 2003; Winzer-Serhan & Leslie, 2005), but also some degree of regional specificity within the cortex (Wada et al., 1990). Although incorporation of the α5 subunit into α4β2* receptors can modulate nicotinic responses directly by increasing the channel Ca2+ permeability (Kuryatov et al., 1997), our experiments do not conclusively show a direct effect of this subunit. The constitutive deletion of the α5 subunit in our mice does not preclude the potential for developmental effects of α5 subunits. Indeed, the nicotinic response is strongest during the juvenile period in the rodent mPFC, peaking during the second and third weeks of postnatal life (Kassam et al., 2008; Bailey et al., 2012, 2014). Therefore, cholinergic signaling mediated by α5-containing receptors, either directly within mPFC layer VI neurons or within other regions during development, may have long-lasting consequences for the strength of cholinergic responses in adulthood.
Conclusions
These findings provide concrete functional data that demonstrate how layer VI neurons respond to cholinergic stimulation across several regions that are important in attentional performance. There is clear regional specificity in the direct effect of ACh on layer VI neurons of primary and associative cortical regions. Such neurons in M1 and the SSC have smaller responses to ACh, with a substantial muscarinic component, whereas the SSC is unusual for having two populations of layer VI neurons that show distinct responses to ACh. In contrast, mPFC layer VI neurons show dramatically greater cholinergic excitation, driven predominantly by nicotinic receptors. This region-specific pattern appears to be dependent on the presence of the nicotinic α5 subunit, and is lost in a mouse model of attention deficits under demanding conditions.
Acknowledgments
This work was supported by grants to E. K. Lambe from the Canadian Institutes of Health Research (MOP 89825), and the Canada Research Chairs Program. M. K. Tian is supported by a Banting and Best Canada Graduate Scholarship. C. D. C. Bailey is funded by an NSERC Discovery Grant. We thank Dr Mariella De Biasi of Baylor College of Medicine for the original gift of the nicotinic ACh receptor α5 subunit knockout mice.
Abbreviations
- ACh
acetylcholine
- ACSF
artificial cerebrospinal fluid
- APV
D-(−)-2-amino-5-phosphonopentanoic acid
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- DHβE
dihydro-β-erythroidine hydrobromide
- eGFP
enhanced green fluorescent protein
- M1
primary motor cortex
- mPFC
medial prefrontal cortex
- SSC
primary somatosensory cortex
- Syt6
synaptotagmin 6
- WT
wild-type
References
- Andjelic S, Gallopin T, Cauli B, Hill EL, Roux L, Badr S, Hu E, Tamas G, Lambolez B. Glutamatergic nonpyramidal neurons from neocortical layer VI and their comparison with pyramidal and spiny stellate neurons. J Neurophysiol. 2009;101:641–654. doi: 10.1152/jn.91094.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CD, De Biasi M, Fletcher PJ, Lambe EK. The nicotinic acetylcholine receptor alpha5 subunit plays a key role in attention circuitry and accuracy. J Neurosci. 2010;30:9241–9252. doi: 10.1523/JNEUROSCI.2258-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CD, Alves NC, Nashmi R, de Biasi M, Lambe EK. Nicotinic α5 subunits drive developmental changes in the activation and morphology of prefrontal cortex layer VI neurons. Biol Psychiat. 2012;71:120–128. doi: 10.1016/j.biopsych.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CD, Tian MK, Kang L, O’Reilly R, Lambe EK. Chrna5 genotype determines the long-lasting effects of developmental in vivo nicotine exposure on prefrontal attention circuitry. Neuropharmacology. 2014;77:145–155. doi: 10.1016/j.neuropharm.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Kennett S, Driver J. Attentional selection of location and modality in vision and touch modulates low-frequency activity in associated sensory cortices. J Neurophysiol. 2012;107:2342–2351. doi: 10.1152/jn.00973.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birtsch C, Wevers A, Traber J, Maelicke A, Bloch W, Schroder H. Expression of alpha 4-1 and alpha 5 nicotinic cholinoceptor mRNA in the aging rat cerebral cortex. Neurobiol Aging. 1997;18:335–342. doi: 10.1016/s0197-4580(97)80316-8. [DOI] [PubMed] [Google Scholar]
- Bloem B, Poorthuis RB, Mansvelder HD. Cholinergic modulation of the medial prefrontal cortex: the role of nicotinic receptors in attention and regulation of neuronal activity. Front Neural Circuits. 2014;8:2–16. doi: 10.3389/fncir.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa J, Pinault D, Deschênes M. Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: a single-fibre study using biocytin as an anterograde tracer. Eur J Neurosci. 1995;7:19–30. doi: 10.1111/j.1460-9568.1995.tb01016.x. [DOI] [PubMed] [Google Scholar]
- Briggs F. Organizing principles of cortical layer 6. Front Neural Circuits. 2010;4:3. doi: 10.3389/neuro.04.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley NJ, Bonner TI, Brann MR. Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci. 1988;8:4646–4652. doi: 10.1523/JNEUROSCI.08-12-04646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Kulczycki M, Tuszynski MH. Unique contributions of distinct cholinergic projections to motor cortical plasticity and learning. Cereb Cortex. 2010;20:2739–2748. doi: 10.1093/cercor/bhq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci USA. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M, Marrocco R. Local infusion of scopolamine into intraparietal cortex slows covert orienting in rhesus monkeys. J Neurophysiol. 2000;83:1536–1549. doi: 10.1152/jn.2000.83.3.1536. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Everitt BJ, Robbins TW. The basal forebrain–cortical cholinergic system: interpreting the functional consequences of excito-toxic lesions. Trends Neurosci. 1991;14:494–501. doi: 10.1016/0166-2236(91)90061-x. [DOI] [PubMed] [Google Scholar]
- Fournier GN, Semba K, Rasmusson DD. Modality- and region-specific acetylcholine release in the rat neocortex. Neuroscience. 2004;126:257–262. doi: 10.1016/j.neuroscience.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Guillery RW. Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. J Anat. 1995;187(Pt 3):583–592. [PMC free article] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Haegens S, Handel BF, Jensen O. Top-down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. J Neurosci. 2011;31:5197–5204. doi: 10.1523/JNEUROSCI.5199-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe W, Berry A, Francois J, Gilmour G, Carp J, Tricklebank M, Lustig C, Sarter M. Prefrontal cholinergic mechanisms instigating shifts from monitoring for cues to cue-guided performance: converging electrochemical and fMRI evidence from rats and humans. J Neurosci. 2013;33:8742–8752. doi: 10.1523/JNEUROSCI.5809-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Buckingham S, Sattelle D. Proteins interacting with nicotinic acetylcholine receptors: expanding functional and therapeutic horizons. Trends Pharmacol Sci. 2010;31:455–462. doi: 10.1016/j.tips.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Kassam SM, Herman PM, Goodfellow NM, Alves NC, Lambe EK. Developmental excitation of corticothalamic neurons by nicotinic acetylcholine receptors. J Neurosci. 2008;28:8756–8764. doi: 10.1523/JNEUROSCI.2645-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol. 2006;95:3844–3851. doi: 10.1152/jn.01234.2005. [DOI] [PubMed] [Google Scholar]
- Klinkenberg I, Sambeth A, Blokland A. Acetylcholine and attention. Behav Brain Res. 2011;221:430–442. doi: 10.1016/j.bbr.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Kozak R, Bruno JP, Sarter M. Augmented prefrontal acetylcholine release during challenged attentional performance. Cereb Cortex. 2006;16:9–17. doi: 10.1093/cercor/bhi079. [DOI] [PubMed] [Google Scholar]
- Kumar P, Ohana O. Inter- and intralaminar subcircuits of excitatory and inhibitory neurons in layer 6a of the rat barrel cortex. J Neurophysiol. 2008;100:1909–1922. doi: 10.1152/jn.90684.2008. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Gerzanich V, Nelson M, Olale F, Lindstrom J. Mutation causing autosomal dominant nocturnal frontal lobe epilepsy alters Ca2+ permeability, conductance, and gating of human alpha4beta2 nicotinic acetylcholine receptors. J Neurosci. 1997;17:9035–9047. doi: 10.1523/JNEUROSCI.17-23-09035.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti S, Sigman M, Dehaene S. A shared cortical bottleneck underlying attentional blink and psychological refractory period. NeuroImage. 2012;59:2883–2898. doi: 10.1016/j.neuroimage.2011.09.063. [DOI] [PubMed] [Google Scholar]
- Mechawar N, Descarries L. The cholinergic innervation develops early and rapidly in the rat cerebral cortex: a quantitative immunocytochemical study. Neuroscience. 2001;108:555–567. doi: 10.1016/s0306-4522(01)00389-x. [DOI] [PubMed] [Google Scholar]
- Mercer A, West DC, Morris OT, Kirchhecker S, Kerkhoff JE, Thomson AM. Excitatory connections made by presynaptic cortico-cortical pyramidal cells in layer 6 of the neocortex. Cereb Cortex. 2005;15:1485–1496. doi: 10.1093/cercor/bhi027. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Meyer K. Primary sensory cortices, top-down projections and conscious experience. Prog Neurobiol. 2011;94:408–417. doi: 10.1016/j.pneurobio.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Muir JL, Dunnett SB, Robbins TW, Everitt BJ. Attentional functions of the forebrain cholinergic systems: effects of intraventricular hemicholinium, physostigmine, basal forebrain lesions and intracortical grafts on a multiple-choice serial reaction time task. Exp Brain Res. 1992;89:611–622. doi: 10.1007/BF00229886. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Bortone DS, Adesnik H, Scanziani M. Gain control by layer six in cortical circuits of vision. Nature. 2012;483:47–52. doi: 10.1038/nature10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Sarter M. Cholinergic mediation of attention. Ann NY Acad Sci. 2008;1129:225–235. doi: 10.1196/annals.1417.021. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Ji J, Decker MW, Sarter M. Prefrontal beta2 subunit-containing and alpha7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling. J Neurosci. 2010;30:3518–3530. doi: 10.1523/JNEUROSCI.5712-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passetti F, Dalley JW, O’Connell MT, Everitt BJ, Robbins TW. Increased acetylcholine release in the rat medial prefrontal cortex during performance of a visual attentional task. Eur J Neurosci. 2000;12:3051–3058. doi: 10.1046/j.1460-9568.2000.00183.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic; San Diego, CA: 2001. [Google Scholar]
- Petrof I, Viaene AN, Sherman SM. Two populations of corticothalamic and interareal corticocortical cells in the subgranular layers of the mouse primary sensory cortices. J Comp Neurol. 2012;520:1678–1686. doi: 10.1002/cne.23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Neuper C. Event-related synchronization (ERS) in the alpha band – an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol. 1996;24:39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- Proulx E, Piva M, Tian MK, Bailey CDC, Lambe EK. Nicotinic acetylcholine receptors in attention circuitry: the role of layer VI neurons of prefrontal cortex. Cell Mol Life Sci. 2013;71:1225–1244. doi: 10.1007/s00018-013-1481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson D, Smith S, Semba K. Inactivation of prefrontal cortex abolishes cortical acetylcholine release evoked by sensory or sensory pathway stimulation in the rat. Neuroscience. 2007;149:232–241. doi: 10.1016/j.neuroscience.2007.06.057. [DOI] [PubMed] [Google Scholar]
- Robbins T, Everitt B, Marston H, Wilkinson J, Jones G, Page K. Comparative effects of ibotenic acid- and quisqualic acid-induced lesions of the substantia innominata on attentional function in the rat: further implications for the role of the cholinergic neurons of the nucleus basalis in cognitive processes. Behav Brain Res. 1989;35:221–240. doi: 10.1016/s0166-4328(89)80143-3. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Granon S, Muir JL, Durantou F, Harrison A, Everitt BJ. Neural systems underlying arousal and attention.Implications for drug abuse. Ann NY Acad Sci. 1998;846:222–237. [PubMed] [Google Scholar]
- Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, de Biasi M. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol Pharmacol. 2003;63:1059–1066. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring W, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, Glennon M, Gerloff C, Birbaumer N, Hummel FC. Brain oscillatory substrates of visual short-term memory capacity. Curr Biol. 2009;19:1846–1852. doi: 10.1016/j.cub.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Thalamic relays and cortical functioning. Prog Brain Res. 2005;149:107–126. doi: 10.1016/S0079-6123(05)49009-3. [DOI] [PubMed] [Google Scholar]
- Sirota MG, Swadlow HA, Beloozerova IN. Three channels of corticothalamic communication during locomotion. J Neurosci. 2005;25:5915–5925. doi: 10.1523/JNEUROSCI.0489-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Rojas DC, Eichman LC, Tregellas JR. Neuronal effects of auditory distraction on visual attention. Brain Cognition. 2013;81:263–270. doi: 10.1016/j.bandc.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Peters M, Cherian AK, Bradshaw M, Sarter M. Sustained attention in mice: expanding the translational utility of the SAT by incorporating the Michigan Controlled Access Response Port (MICARP) Behav Brain Res. 2011;225:574–583. doi: 10.1016/j.bbr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM. Neocortical layer 6, a review. Front Neuroanat. 2010;4:1–14. doi: 10.3389/fnana.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Bailey C, de Biasi M, Picciotto M, Lambe E. Plasticity of prefrontal attention circuitry: upregulated muscarinic excitability in response to decreased nicotinic signaling following deletion of alpha5 or beta2 subunits. J Neurosci. 2011;31:16458–16463. doi: 10.1523/JNEUROSCI.3600-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E, Bertrand D, Marguerat A, Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain. Neuroscience. 2004;124:405–420. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Wada E, McKinnon D, Heinemann S, Patrick J, Swanson LW. The distribution of mRNA encoded by a new member of the neuronal nicotinic acetylcholine receptor gene family (alpha 5) in the rat central nervous system. Brain Res. 1990;526:45–53. doi: 10.1016/0006-8993(90)90248-a. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Leslie FM. Expression of alpha5 nicotinic acetylcholine receptor subunit mRNA during hippocampal and cortical development. J Comp Neurol. 2005;481:19–30. doi: 10.1002/cne.20357. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liu B, Wu G, Kim Y, Xiao Z, Tao H, Zhang L. Preceding inhibition silences layer 6 neurons in auditory cortex. Neuron. 2010;65:706–717. doi: 10.1016/j.neuron.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci. 2006;26:7348–7361. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]