Abstract
Rationale
During embryogenesis, the CXC chemokine ligand (CXCL)12 acts on endothelial cells to control cardiac development and angiogenesis. Although biological functions of CXCL12 are exerted in part through activation of the small GTPase Rac, the pathway leading from its receptor CXC chemokine receptor (CXCR)4 to Rac activation remains to be determined.
Objective
DOCK180 (dedicator of cytokinesis), an atypical Rac activator, has been implicated in various cellular functions. Here, we examined the role of DOCK180 in cardiovascular development.
Methods and Results
DOCK180 associates with ELMO (engulfment and cell motility) through the N-terminal region containing a Src homology 3 domain. We found that targeted deletion of the Src homology 3 domain of DOCK180 in mice leads to embryonic lethality with marked reduction of DOCK180 expression at the protein level. These mutant mice, as well as DOCK180-deficient mice, exhibited multiple cardiovascular abnormalities resembling those seen in CXCR4-deficient mice. In DOCK180 knocked down endothelial cells, CXCL12-induced Rac activation was impaired, resulting in a marked reduction of cell motility.
Conclusions
These results suggest that DOCK180 links CXCR4 signaling to Rac activation to control endothelial cell migration during cardiovascular development.
Keywords: cardiovascular development, endothelial cells, CXCL12, Rac, DOCK180
During embryogenesis, progenitor cells migrate to specific locations, where they differentiate into specialized cells that make up different tissues and organs. This process is coordinately regulated by extrinsic guidance cues. The CXC chemokine ligand (CXCL)12 is one of such cues critical for cardiovascular development. Mice lacking CXCL12 or its receptor CXC chemokine receptor (CXCR)4 exhibit ventricular septal defect (VSD) and poor vascularization of the gastrointestinal tract.1,2 In developing heart, CXCR4 is predominantly expressed on endocardial cells in the atrioventricular (AV) canal,3 which are destined to participate in formation of the membranous septa and valves. Similarly, the expression of CXCR4 in developing vessels is limited to arterial endothelial cells.4 Thus, CXCL12 acts on endothelial cells to control cardiac development and angiogenesis during embryogenesis.
On binding to CXCR4, CXCL12 induces dissociation of heterodimeric G proteins into α and βγ subunits, which results in the activation of a variety of signaling pathways including Rac. Like other small GTPases, Rac cycles between GDP-bound inactive and GTP-bound active states, and stimulus-induced formation of the active Rac is mediated by guanine nucleotide exchange factors (GEFs). However, the mechanism controlling Rac activation downstream of CXCR4 is unknown during cardiovascular development.
DOCK180 (dedicator of cytokinesis), a mammalian ortholog of Caenorhabditis elegans CED-5 and Drosophila melanogaster Myoblast City,5,6 controls cell migration and phagocytosis in vitro7,8 and myoblast fusion in vivo.9 DOCK180 does not contain the pleckstrin homology and Dbl homology domains typically found in GEFs for Rho family GTPases.10 However, DOCK180 can bind to phosphatidylinositol 3,4,5-triphosphate through its DHR-1 domain11 and mediate the GTP-GDP exchange for Rac by means of its DHR-2 (also known as Docker) domain.10,12 In this study, we examined the functions of DOCK180 during cardiovascular development.
Methods
Biochemical, histological, and functional analyses were performed by standard methods. An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org.
Results and Discussion
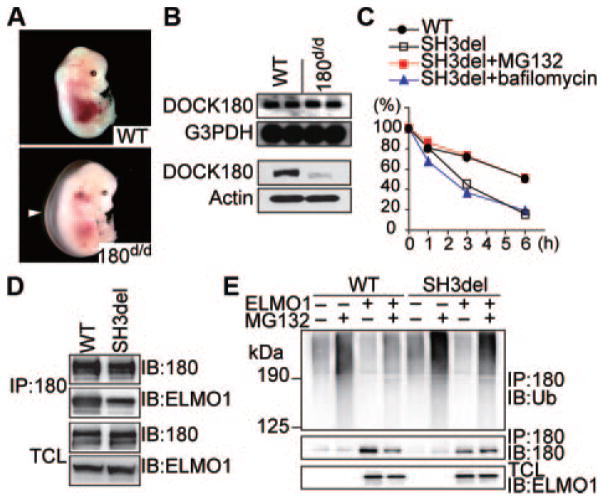
DOCK180 associates with ELMO (engulfment and cell motility) through the N-terminal region containing a Src homology (SH)3 domain and a putative α-helical region.7,13 To investigate the physiological role of the SH3 domain of DOCK180, we specifically deleted a portion of this domain in mice by gene targeting (Online Figure I). Although mating between heterozygous mice yielded homozygous mutants nearly at Mendelian ratio throughout gestation, no viable homozygous offspring was obtained (Online Table I). Close examination of umbilical blood flow indicated that some homozygous mutants were dead at embryonic day (E)17.5 and E18.5. As in DOCK180-deficient (DOCK180−/−) embryos,9 a defect in myogenesis was also observed in homozygous mutants lacking DOCK180 SH3 domain (DOCK180d/d) (Online Figure II). In addition, both DOCK180d/d and DOCK180−/− embryos exhibited severe edema in the large area of the back at E14.5 (Figure 1A; Online Figure III). Whereas Northern blot analysis of whole embryos revealed that DOCK180 gene was normally transcribed in DOCK180d/d mice, DOCK180 protein level was reduced to <10% of the wild-type (WT) level (Figure 1B).
Figure 1. Targeted deletion of the SH3 domain of DOCK180 in mice causes edema with marked reduction of DOCK180 protein expression level.
A, Gross morphology of a DOCK180d/d embryo at E14.5. Arrowhead indicates edema. B, Northern (top) and Western (bottom) blot analyses of DOCK180 expression in whole embryos. C, Pulse-chase experiments showing proteasome-dependent degradation of SH3del mutant. D, Association of ELMO1 with WT DOCK180 or SH3del mutant in HEK293T cells. IB indicates immunoblotting; IP, immunoprecipitation; TCL, total cell lysates. E, Differential effect of ELMO1 on ubiquitination of WT DOCK180 or SH3del mutant. In all assays, pCGN-HA-ubiquitin (Ub) was cotransfected in HEK293T cells.
To understand the mechanism of protein decrease in the mutant animals, we examined protein degradation using CHO cells stably expressing green fluorescent protein (GFP)-tagged WT DOCK180 or its mutant bearing the same deletion as in DOCK180d/d mice (designated SH3del). Pulse-chase experiments revealed that the SH3del mutant was more rapidly degraded than WT DOCK180 (Figure 1C). The proteasome inhibitor MG132 blocked this degradation, but bafilomycin had no effect, indicating that deletion of the SH3 domain leads to proteasome-dependent degradation of DOCK180 (Figure 1C). When the SH3del mutant was transiently expressed with ELMO1 in HEK293T cells, its binding to ELMO1 was modestly diminished (Figure 1D), in agreement with a nonessential role of this domain for ELMO-binding.13 However, whereas ubiquitination of WT DOCK180 was suppressed by coexpression of ELMO1 in the presence of MG132, such inhibitory effect was less profound for the SH3del mutant (Figure 1E), suggesting that the interaction of DOCK180 SH3 domain with ELMO is required to inhibit ubiquitination and degradation of DOCK180.
Because DOCK180d/d mice exhibited severe edema, we hypothesized that they might have defects in cardiovascular development. Indeed, cardiac abnormalities such as sub-membranous VSD and double outlet right ventricle (DORV) were detected in all DOCK180d/d embryos examined at E14.5 (Figure 2A). Similar results were obtained when DOCK180−/− embryos were analyzed (Online Figure III). In both DOCK180d/d and DOCK180−/− embryos, mitral valve leaflets were also thickened and in some of them fused, which accounted for the blood retention in left atrium (Figure 2A; Online Figure III). Side-by-side analyses revealed that cardiac abnormalities of DOCK180d/d embryos resembled those seen in CXCR4-deficient (CXCR4−/−) mice (Figure 2A). When DOCK180 SH3 domain was specifically deleted in endothelial cells using Tie2-Cre transgenic mice, these mice also exhibited VSD and DORV (Online Figure IV), thus confirming the endothelial origin of the cardiac defects in DOCK180d/d embryos.
Figure 2. DOCK180d/d embryos exhibit cardiac abnormalities similar to those of CXCR4−/− mice.
A, Histology of cardiac tissues of E14.5 embryos. *Endocardial cushion. Arrowhead or arrow indicates VSD or DORV, respectively. B, Cellularity of endocardial cushion of E12.5 embryos. Data are expressed as ratios to the values of WT littermates. **P<0.01. C and D, AV explant assay. C, AV explants were stained with phalloidin (green) and Hoechst 33342 (blue). Scale bar, 50 μm. Arrowheads indicate cells invading the collagen gel, and the corresponding section is shown in the xy image. D, the number of invading cells was compared between WT and DOCK180d/d embryos. Data are expressed as ratios to the values of WT littermates. **P<0.01. E, In vitro EMT assay. Data are expressed as percentage of α-SMA–positive mesenchymal cells. TGF indicates transforming growth factor.
Cardiac endothelial cells in the AV canal undergo transforming growth factor-β–dependent epithelial–mesenchymal transformation (EMT) and migrate into an extracellular matrix referred to as the cardiac jelly.14 This process is important to form endocardial cushion from which AV valves and membranous septa originate. At E8.5, before EMT initiates, DOCK180d/d and WT littermates were indistinguishable concerning the heart development. However, the endocardial cushion of DOCK180d/d embryos showed a significantly decreased cellularity at E12.5 (Figure 2B). In heart explant assays, cardiac endothelial cells from both WT and DOCK180d/d embryos were widely spread on the gel, showing spindle-like mesenchymal morphology (Figure 2C). However, although cells from the WT explants effectively invaded the gel, the number of invading cells significantly decreased in the case of DOCK180d/d explants (Figure 2D). It is clear that DOCK180 is not required for EMT itself, because endothelial cells from WT and DOCK180d/d embryos comparably transformed into mesenchymal cells expressing α-smooth muscle actin (α-SMA) (Figure 2E).
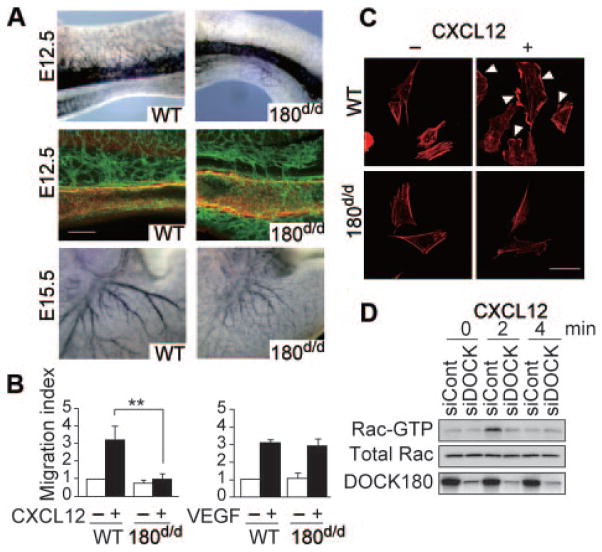
Because CXCR4 is required for vascularization of the gastrointestinal tract,2,4 we examined whether DOCK180 also functions in vascular endothelial cells. During embryogenesis, the small intestine begins to develop as a simple tube called a midgut loop. When the midgut loop of WT embryos was analyzed at E12.5, many branches were found to arise from the α-SMA–positive superior mesenteric artery (Figure 3A). However, as in CXCR4−/− embryos, the interconnecting vessels between superior mesenteric artery and the capillary plexus were severely reduced in DOCK180d/d midgut loop (Figure 3A). Similarly, at E15.5, large vessels poorly developed in the stomach of DOCK180d/d embryos (Figure 3A).
Figure 3. DOCK180 acts downstream of CXCR4 to control endothelial cell migration.
A, Vasculature of midgut and stomach in WT and DOCK180d/d embryos. Top, Midgut of E12.5 embryos stained with anti–PECAM-1 antibody. Middle, Midgut of E12.5 embryos stained with anti–PECAM-1 (green) and anti–α-SMA (red) antibodies. Scale bar, 100 μm. Bottom, Stomach of E15.5 embryos stained with anti–PECAM-1 antibody. B, Chemotactic response to CXCL12 or VEGF. Data are expressed as migration indexes after normalization of WT value without stimulation to an arbitrary value of 1. **P<0.01. C, CXCL12-induced membrane ruffle formation (arrowhead). Images of cells stained with phalloidin are shown. Scale bar, 10 μm. D, CXCL12-induced Rac activation in human aorta endothelial cells. After treatment with DOCK180-specific or control siRNA, human aorta endothelial cells were stimulated with CXCL12 (100 ng/mL).
To examine whether DOCK180 functions downstream of CXCR4, we purified arterial endothelial cells from WT and DOCK180d/d embryos. In chemotaxis assays, WT endothelial cells efficiently migrated to the lower chamber in response to either CXCL12 or vascular endothelial growth factor (VEGF). However, migratory response to CXCL12, but not to VEGF, was almost completely lost for DOCK180d/d endothelial cells (Figure 3B). Consistent with this finding, CXCL12-induced peripheral membrane ruffle formation was scarcely detected in DOCK180d/d endothelial cells (Figure 3C). To obtain biochemical evidence that DOCK180 controls Rac activation downstream of CXCR4, we used human aorta endothelial cells. In these cells, knockdown of DOCK180 expression by RNA interference abrogated CXCL12-induced Rac activation (Figure 3D).
Here we have shown that DOCK180 is required for cardiovascular development. Importantly, DOCK180 knockdown does not affect the expression or localization of CXCL12 and CXCR4 in vivo (Online Figure V). In addition, DOCK180 and CXCR4 are coexpressed in cardiac and vascular endothelial cell lineage (Online Figure VI). Our results thus indicate that DOCK180 links CXCR4 signaling to Rac activation to control endothelial cell migration during cardiovascular development.
Supplementary Material
Novelty and Significance.
What Is Known?
The chemokine CXCL12 and its receptor CXCR4 are required for cardiac development and angiogenesis.
On activation, Rac provides the force necessary to extend membrane protrusion in the direction of migration.
DOCK180, an atypical Rac activator, controls cell migration and phagocytosis in vitro and myoblast fusion in vivo.
What New Information Does This Article Contribute?
Mice with defective DOCK180 expression exhibit multiple cardiovascular abnormalities similar to those seen in CXCR4-deficient mice.
DOCK180 regulates CXCL12-induced endothelial cell migration.
DOCK180 is required for CXCR4-mediated Rac activation in endothelial cells.
Cardiovascular malformations are the most common congenital anomalies. Therefore, elucidation of the signaling pathways involved in heart and vessel pattering is important in clinical medicine. Although previous studies have shown that the chemokine CXCL12 and its receptor CXCR4 play important roles in cardiovascular development, the downstream signaling events are poorly understood. In this study, we show that DOCK180, an atypical Rac activator, controls cardiac development and angiogenesis in vivo by acting downstream of CXCR4 in endothelial cells. Our results provide the first evidence showing that DOCK180 is a key signaling molecule that regulates cardiovascular development.
Acknowledgments
Sources of Funding
This work was supported by grants for the CREST Program from the Japan Science and Technology Agency; the Targeted Proteins Research Program from the Ministry of Education, Culture, Sports, Science and Technology of Japan; Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science; and the Canadian Institute of Health Research.
Non-standard Abbreviations and Acronyms
- AV
atrioventricular
- CXCL
CXC chemokine ligand
- CXCR
CXC chemokine receptor
- CXCR4−/−
CXCR4-deficient
- DOCK
dedicator of cytokinesis
- DOCK180d/d
mice lacking DOCK180 Src homology 3 domain
- DOCK180−/−
DOCK180-deficient
- DORV
double outlet right ventricle
- E
embryonic day
- ELMO
engulfment and cell motility
- EMT
epithelial–mesenchymal transformation
- GEF
guanine nucleotide exchange factor
- GFP
green fluorescent protein
- SH3
Src homology 3
- SMA
smooth muscle actin
- VEGF
vascular endothelial growth factor
- VSD
ventricular septal defect
- WT
wild type
Footnotes
Disclosures
None.
References
- 1.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 2.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 3.Sierro F, Biben C, Martínez-Muñoz L, Mellado M, Ransohoff RM, Li M, Woehl B, Leung H, Groom J, Batten M, Harvey RP, Martínez-A C, Mackay CR, Mackay F. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A. 2007;104:14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ara T, Tokoyoda K, Okamoto R, Koni PA, Nagasawa T. The role of CXCL12 in the organ-specific process of artery formation. Blood. 2005;105:3155–3161. doi: 10.1182/blood-2004-07-2563. [DOI] [PubMed] [Google Scholar]
- 5.Wu YC, Horvitz HR. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature. 1998;392:501–504. doi: 10.1038/33163. [DOI] [PubMed] [Google Scholar]
- 6.Kiyokawa E, Hashimoto Y, Kobayashi S, Sugimura H, Kurata T, Matsuda M. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 1998;12:3331–3336. doi: 10.1101/gad.12.21.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gumienny TL, Brugnera E, Tosello-Trampont AC, Kinchen JM, Haney LB, Nishiwaki K, Walk SF, Nemergut ME, Macara IG, Francis R, Schedl T, Qin Y, Van Aelst L, Hengartner MO, Ravichandran KS. CED-12/ELMO, a novel member of the CRKII/DOCK180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107:27–41. doi: 10.1016/s0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- 8.Grimsley CM, Kinchen JM, Tosello-Trampont AC, Brugnera E, Haney LB, Lu M, Chen Q, Klingele D, Hengartner MO, Ravichandran KS. Dock180 and ELMO1 proteins cooperate to promote evolutionarily conserved Rac-dependent cell migration. J Biol Chem. 2004;279:6087–6097. doi: 10.1074/jbc.M307087200. [DOI] [PubMed] [Google Scholar]
- 9.Laurin M, Fradet N, Blangy A, Hall A, Vuori K, Côté JF. The atypical Rac activator Dock180 (Dock1) regulates myoblast fusion in vivo. Proc Natl Acad Sci U S A. 2008;105:15446–15451. doi: 10.1073/pnas.0805546105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Côté JF, Vuori K. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J Cell Sci. 2002;115:4901–4913. doi: 10.1242/jcs.00219. [DOI] [PubMed] [Google Scholar]
- 11.Côté JF, Motoyama AB, Bush JA, Vuori K. A novel and evolutionarily conserved PtdIns(3,4,5)P3-binding domain is necessary for DOCK180 signalling. Nat Cell Biol. 2005;7:797–807. doi: 10.1038/ncb1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, Tosello-Trampont AC, Macara IG, Madhani H, Fink GR, Ravichandran KS. Unconventional Rac-GEF activity is mediated through the Dock180 ELMO complex. Nat Cell Biol. 2002;4:574–582. doi: 10.1038/ncb824. [DOI] [PubMed] [Google Scholar]
- 13.Komander D, Patel M, Laurin M, Fradet N, Pelletier A, Barford D, Côté JF. An α-helical extension of the ELMO1 plekstrin homology domain mediates direct interaction to DOCK180 and is critical in Rac signaling. Mol Biol Cell. 2008;19:4837–4851. doi: 10.1091/mbc.E08-04-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong EJ, Bischoff J. Heart valve development. Endothelial cell signaling and differentiation. Cir Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.