Abstract
Neutrophils are highly motile leukocytes that play important roles in the innate immune response to invading pathogens. Neutrophils rapidly migrate to the site of infections and kill pathogens by producing reactive oxygen species (ROS). Neutrophil chemotaxis and ROS production require activation of Rac small GTPase. DOCK2, an atypical guanine nucleotide exchange factor (GEF), is one of the major regulators of Rac in neutrophils. However, because DOCK2 deficiency does not completely abolish fMLF-induced Rac activation, other Rac GEFs may also participate in this process. In this study, we show that DOCK5 acts with DOCK2 in neutrophils to regulate multiple cellular functions. We found that fMLF- and PMA-induced Rac activation were almost completely lost in mouse neutrophils lacking both DOCK2 and DOCK5. Although β2 integrin–mediated adhesion occurred normally even in the absence of DOCK2 and DOCK5, mouse neutrophils lacking DOCK2 and DOCK5 exhibited a severe defect in chemotaxis and ROS production. Similar results were obtained when human neutrophils were treated with CPYPP, a small-molecule inhibitor of these DOCK GEFs. Additionally, we found that DOCK2 and DOCK5 regulate formation of neutrophil extracellular traps (NETs). Because NETs are involved in vascular inflammation and autoimmune responses, DOCK2 and DOCK5 would be a therapeutic target for controlling NET-mediated inflammatory disorders.
Rac is a member of the small GTPases that function as molecular switches by cycling between GDP-bound inactive and GTP-bound active states (1, 2). Once activated, Rac binds to a panel of effector molecules and regulates a plethora of cellular functions, including reorganization of the actin cytoskeleton and production of reactive oxygen species (ROS) (1, 2). The Rac family is composed of three distinct gene products, namely Rac1, Rac2, and Rac3: Rac1 is ubiquitously expressed and Rac3 is enriched in the brain, whereas Rac2 expression is largely restricted to hematopoietic cells (2). Although Rac2 is the predominant isoform in human neutrophils, Rac1 and Rac2 are expressed equally in mouse neutrophils (1). The role of Rac in neutrophil functions has been extensively analyzed with knockout mice lacking Rac1 and/or Rac2, as well as in a human patient with a point mutation in the conserved GTP-binding domain of Rac2 (3–8). These studies clearly indicate that Rac2 is a major Rac isoform that regulates chemotaxis and ROS production in neutrophils. However, the defects in neutrophil chemotaxis and ROS production of Rac2-deficient neutrophils was significantly augmented by additional loss of Rac1 (6, 7), suggesting that Rac1 is also involved in regulation of chemoattractant-induced neutrophil functions in mice.
Neutrophil chemotaxis is initiated when chemoattractants bind to transmembrane receptors that couple to heterotrimeric G proteins. This leads to the dissociation of the G protein into α and βγ subunits, which activates a variety of signaling pathways, including Rac. Because stimulus-induced formation of active Rac is mediated by guanine nucleotide exchange factors (GEFs), significant efforts have been invested to identify the Rac GEFs critical for neutrophil chemotaxis. There are two distinct families of Rac GEFs (9, 10): Dbl homology (DH) domain–containing proteins and DOCK proteins. P-Rex1 is a DH domain–containing GEF that has been purified from neutrophils by its ability to bind to both phospholipids and the Gβγ subunit (11). Although P-Rex1 was initially thought to be a major Rac GEF acting downstream of chemoattractant receptors, neutrophil chemotaxis was only modestly affected by P-Rex1 deficiency (12, 13). Alternatively, we identified DOCK2 as a Rac GEF important for neutrophil chemotaxis (14, 15). In DOCK2-deficient (DOCK2−/−) neutrophils, chemoattractant-induced activation of both Rac1 and Rac2 were severely impaired without affecting Cdc42 activation (14), indicating that DOCK2 is a major Rac GEF acting downstream of chemoattractant receptors in neutrophils. However, because DOCK2 deficiency does not completely abolish Rac activation (14), it is conceivable that other Rac GEFs also participate in this process.
Unlike Dbl-GEFs, the DOCK proteins contain a unique DOCK homology region (DHR)-2 (also know as Docker or CZH2) domain mediating nucleotide exchange on Rac or Cdc42 (10, 16, 17). These GEFs also contain a DHR-1 signature domain that serves to localize the proteins at the membrane, via binding to phospholipids, for GTPase activation (10, 18). This family consists of 11 members subdivided into four subfamilies (DOCK-A, -B, -C, and -D) based on their sequence homology and substrate specificity. For example, DOCK1 and DOCK5, as well as DOCK2, belong to the DOCK-A subfamily and act as Rac-specific GEFs (10, 19). DOCK1 and DOCK5 are widely expressed in various tissues and regulate multiple cellular functions, including myoblast fusion, bone resorption, and migration (20–22); however, their roles in the immune system and immune responses are poorly understood. We found that neutrophils also express DOCK5, but not DOCK1. In this study, we demonstrate that DOCK5 acts with DOCK2 in neutrophils to regulate chemotaxis, ROS production, and formation of neutrophil extracellular traps (NETs) (23).
Materials and Methods
Mice
DOCK5- and DOCK2-deficient (DOCK5−/− and DOCK2−/−) mice have been previously described (20, 21, 24). These mice were backcrossed onto a C57BL/6 background for more than eight generations prior to analyses, and age- and sex-matched C57BL/6 mice were used as wild-type (WT) controls. The animals were maintained in specific pathogen-free conditions in the animal facility of Kyushu University. All experiments were done in accordance with the guidelines of the Committee of Ethics of Animal Experiments, Kyushu University.
Neutrophil isolation
Mouse bone marrow (BM) neutrophils were isolated from femurs and tibias of mice and layered onto a discontinuous Percoll (GE Healthcare) gradient. After centrifugation, cells at the 62/81% interface were recovered and washed twice with HBSS (Invitrogen). More than 90% of the recovered cells were Gr-1+CD11b+ mature neutrophils. Human neutrophils were isolated from peripheral blood of healthy donors. Blood samples were mixed with an equal volume of 2% dextran solution by repeated inversion and tubes were set upright for 30 min at room temperature. The straw-colored, leukocyte-rich, erythrocyte-poor upper layer was put onto Histopaque-1077 (Sigma-Aldrich). After centrifugation, pellets were washed with HBSS followed by hemolysis.
Rac activation assays
Mouse BM neutrophils suspended in HBSS were stimulated with fMLF (10 μM) or PMA (100 nM). Aliquots of the cell extracts were kept for total lysate controls, and the remaining extracts were incubated with the GST-fusion Rac-binding domain of PAK1 at 4°C for 60 min. The bound proteins and the total lysate control (5 μg for Rac1 and 2 μg for Rac2) were analyzed by SDS-PAGE, and blots were probed with anti-Rac1 (23A8, Millipore) and anti-Rac2 (3B10-2D9, Sigma-Aldrich) Abs. In some experiments, human peripheral blood neutrophils were treated with CPYPP (100 μM), a small-molecule inhibitor of DOCK-A subfamily members (25–27), for 60 min before stimulation with fMLF (10 μM).
Immunoblot analysis
Mouse BM neutrophils suspended in HBSS were stimulated with fMLF (10 μM) or PMA (100 nM) for the indicated time. Reactions were terminated by adding an equal volume of Laemmli sample buffer (125 mM Tris-HCl, 4% SDS, 20% glycerol, 0.01% bromophenacyl bromide [pH 6.8]) supplemented with 2 mM EGTA, 100 μM DTT, and complete protease inhibitors (Roche), and samples were boiled for 10 min for analyses by immunoblotting. Activation of ERKs, Akt, p38, and MEK was assessed with phosphorylation-specific Abs against Thr202/Tyr204 of p44 and p42 ERKs, Ser473 of Akt, Thr180/Tyr182 of p38, and Ser217/Ser221 of MEK (all from Cell Signaling Technology). The expression of DOCK-A subfamily members, P-Rex1 and Vav, was examined by immunoblotting using anti-DOCK1 (C4C12, Cell Signaling Technology), anti-DOCK2 (Millipore), anti-DOCK5, anti–P-Rex1 (6F12, Millipore), and anti-Vav (C-14, Santa Cruz Biotechnology) Abs. The polyclonal Ab against DOCK5 was produced by immunizing a rabbit with keyhole limpet hemocyanin–coupled synthetic peptide corresponding to the C-terminal sequence (PKARKS-GILSSEPGSQ, residues 1853–1868) of mouse DOCK5.
Chemotaxis assay
Mouse BM neutrophils and human peripheral blood neutrophils with or without CPYPP treatment were allowed to migrate under the fMLF (0–10 μM) or CXCL2 (0–1 μg/ml) gradient in an EZ-TAXIScan chamber (Effector Cell Institute). Phase contrast images of chemotaxing cells were acquired at 30-s intervals during 20 min. Images were imported as stacks to ImageJ (National Institutes of Health, Bethesda, MD) and analyzed with the manual tracking and the chemotaxis and migration tools.
Immunofluorescence microscopy
Mouse BM neutrophils migrating in an EZ-TAXIScan chamber along the fMLF gradient were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100 in PBS. After being suspended in PBS containing 1% BSA, cells were stained with Alexa Fluor 546–conjugated phalloidin and DAPI for 20 min at room temperature and washed twice with the same buffer. Microscopic analysis was performed with a laser scanning confocal microscope (LSM 510 META, Carl Zeiss).
ROS production
Mouse BM neutrophils (5 × 105 for fMLF stimulation, 5 × 104 for PMA stimulation) and human peripheral blood neutrophils (5 × 105 for fMLF stimulation) were suspended in HEPES-buffered saline containing 0.04% BSA and were stimulated at 37°C with fMLF (10 μM) or PMA (324.24 nM). In some experiments, human peripheral blood neutrophils were treated with CPYPP (100 μM) before stimulation. The reaction was terminated by addition of 50 μg/ml superoxide dismutase. ROS production was measured by the enhancer-containing luminol-based detection system (National Diagnostics) with a luminometer (AutoLumat LB 953, Berthold Technologies) or by reduction of ferricytochrome c at 550–540 nm using a Hitachi 557 dual-wavelength spectrophotometer.
NET formation
Mouse BM neutrophils (5 × 105/ml) suspended in serum-free RPMI 1640 medium were seeded on poly-D-lysine–coated glass-bottom dishes (Mat-Tek). Cells were stimulated with PMA (20 nM) for 18 h at 37°C, and samples were stained with Sytox Green (for live cells) and Sytox Orange (for NETs; both from Invitrogen). Quantification of NETs was performed as described (28). Briefly, NETs were incubated in the presence of 500 mU/ml microccocal nuclease for 15 min. Nuclease activity was stopped with 5 mM EDTA, and insoluble debris was removed by centrifugation. Total DNA was extracted from neutrophils with DNazol (Invitrogen) supplemented with 1% polyacryl carrier (Molecular Research Center) and dissolved in TE buffer. DNA was quantified with a PicoGreen dsDNA kit (Invitrogen). Percentage of NET DNA was calculated by dividing the amount of NET DNA by the total amount of DNA.
Adhesion assay
Mouse BM neutrophils suspended in HBSS containing 20 mM HEPES (pH 7.4) and 0.1% BSA were stimulated with fMLF (10 μM) or PMA (162.12 nM). Cells were then allowed to adhere for 30 min at 37°C in 96-well plates coated with ICAM-1 and C3bi. Wells were washed twice with HBSS containing 20 mM HEPES (pH 7.4) and 0.1% BSA, and adherent neutrophils were quantified using a CytoTox 96 nonradioactive cytotoxicity assay system (Promega).
Statistical analysis
Statistical analysis was performed using analysis of Kruskal–Wallis H test or variance (ANOVA) followed by a two-tailed multiple t test with a Bonferroni correction.
Results
DOCK2 and DOCK5 act additively to regulate chemoattractant-induced Rac activation in mouse BM neutrophils
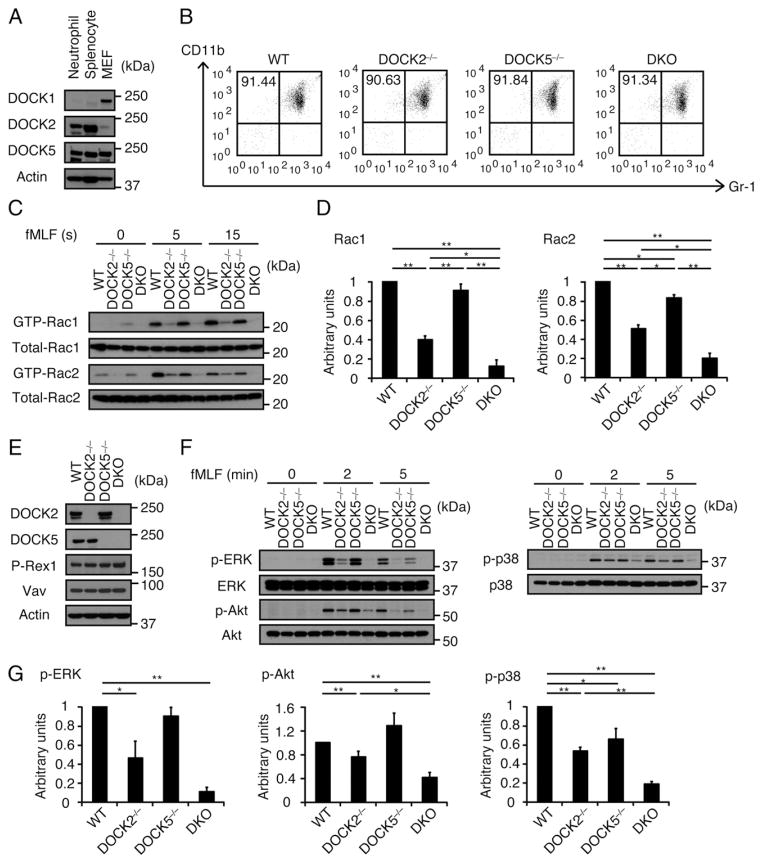
Western blot analysis revealed that mouse neutrophils express DOCK2 and DOCK5, but not DOCK1 (Fig. 1A). This finding led us to examine the extent of functional overlap between these Rac GEFs in neutrophil functions. When the expression of CD11b and Gr-1 on freshly isolated BM neutrophils was compared among WT, DOCK2−/−, DOCK5−/−, and double knockout (DKO) mice lacking both DOCK2 and DOCK5, no difference was found, indicating that deficiency of DOCK2 and/or DOCK5 does not affect neutrophil maturation (Fig. 1B). In WT BM neutrophils stimulated with the bacterial peptide fMLF, activated Rac1 and Rac2 were readily detected at 5 and 15 s (Fig. 1C). This Rac activation was substantially reduced in DOCK2−/− neutrophils, whereas DOCK5 deficiency alone showed only modest effect (Fig. 1C, 1D). However, fMLF-induced activation of Rac1 and Rac2 was reduced in DKO neutrophils to 12.3 and 19.9% of the WT levels at 15 s after stimulation (Fig. 1C, 1D). Similar defects were observed when these neutrophils were stimulated with chemokine CXCL2 (data not shown). Because the expression level of DOCK2 in DOCK5−/− neutrophils or DOCK5 in DOCK2−/− neutrophils was comparable to that of the WT control (Fig. 1E), it is clear that the exacerbated phenotype observed in DKO neutrophils does not reflect loss of compensatory upregulation of the other member in single-deficient neutrophils. Additionally, we found that deficiency of DOCK2 and DOCK5 does not affect the expression level of Dbl-GEFs such as P-Rex1 and Vav (Fig. 1E). These results indicate that DOCK2 and DOCK5 act additively to regulate Rac activation in response to G protein–coupled receptor (GPCR) stimulation.
FIGURE 1.
DOCK2 and DOCK5 act additively in regulation of GPCR-mediated Rac activation. (A) Expression of DOCK-A family proteins in BM neutrophils, splenocytes, and mouse embryonic fibroblasts (MEFs). Cell lysates were subjected to immunoblotting using anti-DOCK1, -DOCK2, and -DOCK5 Abs. Data are representative of two independent experiments. (B) Flow cytometric analysis for the expression of Gr-1 and CD11b on BM neutrophils from WT, DOCK2−/−, DOCK5−/−, and DKO mice. Numbers indicate the percentages of Gr-1+CD11b+ cells in purified BM neutrophils. Data are representative of more than three independent experiments. (C and D) Activation of Rac1 and Rac2 in BM neutrophils from WT, DOCK2−/−, DOCK5−/−, and DKO mice stimulated with fMLF (10 μM) for the indicated times. Cell lysates were subjected to pull-down assays using GST-fusion Rac-binding domain of PAK1 before immunoblotting with anti-Rac1 and -Rac2 Abs. Results were quantified by densitometry and are expressed as the ratio of the GTP-bound form to total protein after normalization of the 15 s value of WT neutrophils to an arbitrary value of 1. Data are indicated as means ± SEM of three separate experiments. *p < 0.05, **p < 0.01. (E) Expression of DOCK2, DOCK5, P-Rex1, and Vav proteins in BM neutrophils from WT, DOCK2−/−, DOCK5−/−, and DKO mice. Cell lysates were subjected to immunoblotting using anti-DOCK2, -DOCK5, –P-Rex1, and -Vav Abs. Data are representative of two independent experiments. (F and G) Phosphorylations of ERK, Akt, and p38 in BM neutrophils from WT, DOCK2−/−, DOCK5−/−, and DKO mice stimulated with fMLF (10 μM) for the indicated times. Cell lysates were subjected to immunoblotting using phosphorylation-specific Abs against ERK, Akt, and p38. Results were quantified by densitometry and are expressed as the ratio of phosphorylated form to total protein after normalization of the WT value (2 min value for ERK and Akt, 5 min value for p38) to an arbitrary value of 1. Data are indicated as means ± SEM of three separate experiments. *p < 0.05, **p < 0.01.
It has been reported that chemoattractant-induced phosphorylations of ERK and Akt are severely impaired in neutrophils lacking both Rac1 and Rac2, as compared with those in single-deficient neutrophils (6, 7). To further examine the effect of DOCK2/ DOCK5 double deficiency on GPCR signaling, we compared phosphorylation status of these molecules among WT, DOCK2−/−, DOCK5−/−, and DKO neutrophils. Upon stimulation with fMLF, ERK, Akt, and also p38 were phosphorylated in WT neutrophils (Fig. 1F). These phosphorylations were diminished to 46.2, 76.3, and 53.4% of the WT level, respectively, but were still observed in DOCK2−/− neutrophils (Fig. 1F, 1G). However, consistent with the defect in Rac activation, phosphorylations of ERK, Akt, and p38 were almost completely abolished in DKO neutrophils (Fig. 1F, 1G). These results further indicate that DOCK5 and DOCK2 act additively in the GPCR-mediated signal transduction pathway.
DOCK2 and DOCK5 additively regulate GPCR-mediated neutrophil functions in mice
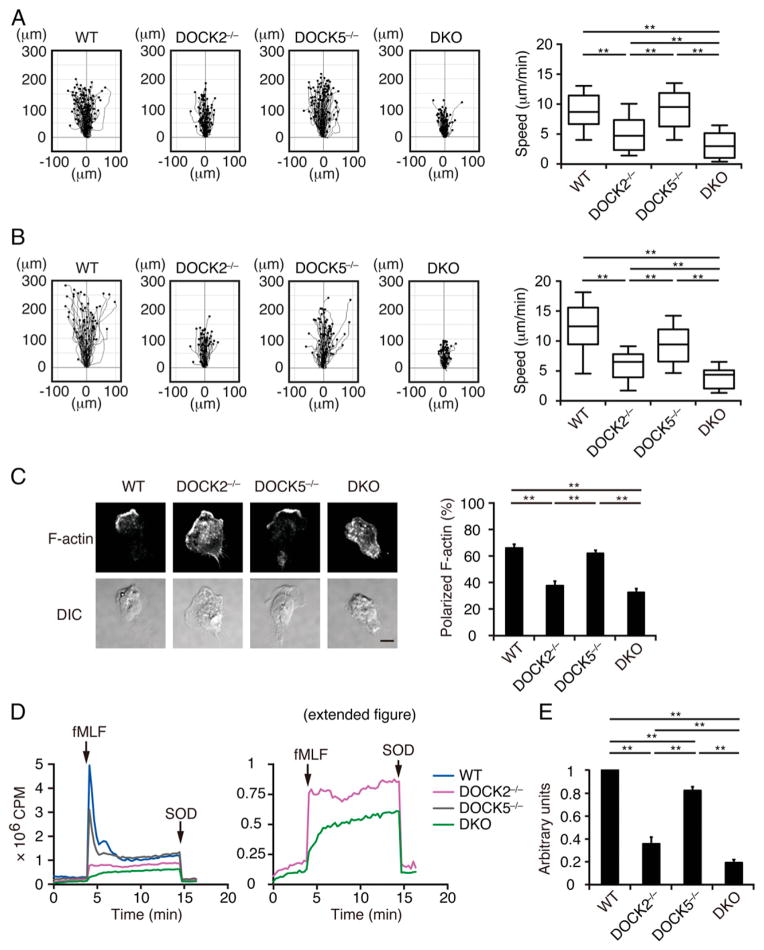
Having found that DOCK2 and DOCK5 regulate GPCR-mediated Rac activation in mouse BM neutrophils, we examined the effect of DOCK2 and/or DOCK5 deficiency on neutrophil chemotaxis. In a chemotaxis assay using an EZ-TAXIScan chamber, WT neutrophils actively migrated toward the fMLF source at the speed of 8.7 μm/min (Fig. 2A). Although the average speed of DOCK2−/− neutrophils was reduced to <61% of the WT level, DOCK5 deficiency alone did not significantly affect motility (Fig. 2A). However, DKO neutrophils clearly showed a more severe defect than did DOCK2−/− neutrophils, and they could hardly migrate toward the fMLF source (Fig. 2A). Similar results were obtained when BM neutrophils chemotaxing along the CXCL2 gradient were analyzed (Fig. 2B). Consistent with this finding, most DKO neutrophils undergoing chemotaxis exhibited abnormal morphology with poorly focused distribution of F-actin (Fig. 2C).
FIGURE 2.
DOCK2 and DOCK5 additively regulate neutrophil chemotaxis and ROS production. (A) Mouse BM neutrophils chemotaxing under fMLF gradient (0–10 μM) were analyzed using an EZ-TAXIScan chamber. Data were collected at 30-s intervals for 20 min. Data are representative of three independent experiments (n = 51–100/group). Each box exhibits the median (central line within each box), the 25th and 75th percentile values (box end), and the 10th and 90th percentile values (error bar). **p < 0.01. (B) BM neutrophils chemotaxing under CXCL2 gradient (0–1 μg/ml) were similarly analyzed as in (A). (C) BM neutrophils chemotaxing under the fMLF gradient were stained with phalloidin, and the percentages of neutrophils with polarized F-actin localization were compared. DIC, differential interference contrast. Scale bar, 10 μm. Data are indicated as means ± SEM of five separate experiments. **p < 0.01. Cells are judged to be positive when F-actin staining is confined to less than one third of the circumference. (D and E) ROS production was compared among WT, DOCK2−/−, DOCK5−/−, and DKO neutrophils stimulated with fMLF (10 μM). In (D), the right panel indicates the magnified view of the graph to show the difference between DOCK2−/− and DKO neutrophils. In (E), results are expressed as the ratio after normalization of the WT value to an arbitrary value of 1. Data are indicated as means ± SD of triplicate samples. **p < 0.01.
As Rac is a cytosolic component of NADPH oxidase (1, 2), we next compared ROS production among WT, DOCK2−/−, DOCK5−/−, and DKO neutrophils. When WT neutrophils were stimulated with fMLF, they produced ROS in a superoxide dismutase–inhibitable manner (Fig. 2D). Although ROS production by DOCK2−/− neutrophils was decreased to 35.7% of that of WT neutrophils, DOCK5 deficiency alone showed only modest effect (Fig. 2D, 2E). In the absence of both DOCK2 and DOCK5, however, fMLF-induced ROS production was further reduced to 19.4% of the WT level (Fig. 2D, 2E). Collectively, these results indicate that DOCK2 and DOCK5 act additively in mouse neutrophils to regulate the GPCR-mediated chemotactic response and ROS production.
A small-molecule inhibitor of DOCK2/DOCK5 suppresses human neutrophil functions
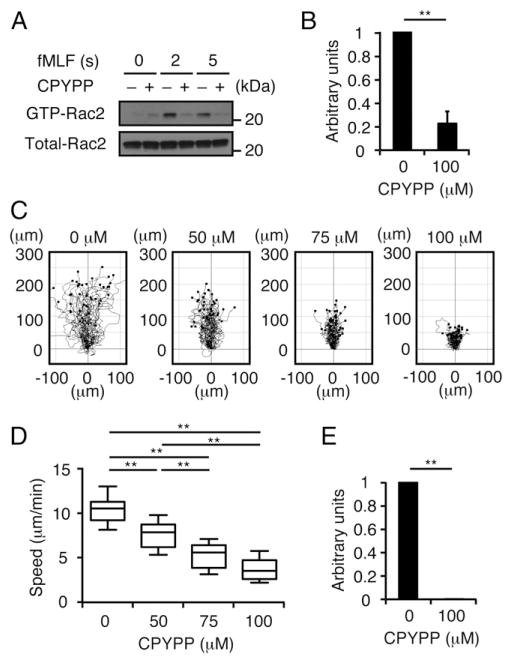
To examine whether the role of DOCK2 and DOCK5 could be extended to human neutrophils, we used CPYPP, a small-molecule inhibitor that binds to the DHR-2 domain of DOCK-A subfamily members and inhibits their Rac GEF activity (25–27). As expected, treatment of human peripheral blood neutrophils with CPYPP at 100 μM reduced fMLF-induced Rac2 activation to 22.5% of the vehicle (DMSO)-treated samples (Fig. 3A, 3B). Consistent with this finding, CPYPP inhibited chemotactic response in a dose-dependent manner (Fig. 3C, 3D). Additionally, fMLF-induced ROS production was almost completely lost when human peripheral blood neutrophils were treated with CPYPP at 100 μM (Fig. 3E). These results indicate that DOCK2 and DOCK5 regulate GPCR-mediated neutrophil functions in humans through their Rac GEF activity.
FIGURE 3.
Treatment of human neutrophils with CPYPP inhibits Rac2 activation, chemotaxis, and ROS production. (A and B) Following treatment with CPYPP (100 μM) or vehicle (DMSO) alone for 1 h, human peripheral blood neutrophils were stimulated with fMLF (10 μM) for the indicated times. Cell lysates were subjected to pull-down assays using the GST-fusion Rac-binding domain of PAK1 before immunoblotting using anti-Rac2 Ab. Results were quantified by densitometry and are expressed as the ratio after normalization of the 5 s value of vehicle-treated sample to an arbitrary value of 1. Data are indicated as means ± SEM of three separate experiments. **p < 0.01. (C and D) Human peripheral blood neutrophils were treated with CPYPP at the indicated concentrations and their chemotactic responses under fMLF gradient (0–10 μM) were analyzed using an EZ-TAXIScan chamber. Data were collected at 30-s intervals for 20 min. Data are representative of three separate experiments (n = 40/group). Each box exhibits the median (central line within each box), the 25th and 75th percentile values (box end), and the 10th and 90th percentile values (error bar). **p < 0.01. (E) ROS production in response to fMLF (10 μM) stimulation was compared between human neutrophils treated with CPYPP (100 μM) and vehicle alone. Results are expressed as the ratio after normalization of the value of vehicle-treated sample to an arbitrary value of 1. Data are indicated as means ± SD of triplicate samples. **p < 0.01.
DOCK2 and DOCK5 additively regulate PMA-induced Rac activation and ROS production
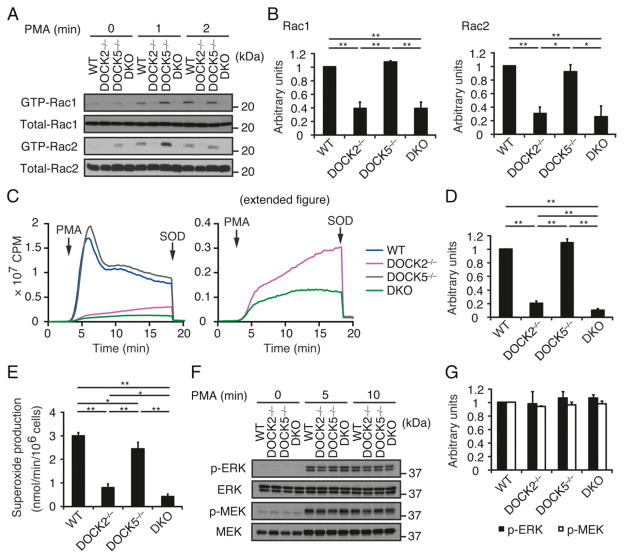
PMA, an activator of protein kinase C, induces a slow but sustained activation of Rac, independently of PI3K activation (29). To examine how DOCK2 and/or DOCK5 deficiency affects PMA-induced Rac activation, we stimulated mouse BM neutrophils with 100 nM PMA. Although activated Rac1 and Rac2 were readily detected in WT and DOCK5−/− neutrophils at 1 and 2 min after stimulation, such activation was severely impaired in both DOCK2−/− and DKO neutrophils (Fig. 4A, 4B). It is likely that DOCK2 and DOCK5 also act additively in this pathway, because PMA-induced ROS production was further reduced in DKO neutrophils, as compared with that in DOCK2−/− neutrophils (10.4 versus 20.2% of the WT level; Fig. 4C, 4D). Similar results were obtained when ROS production was measured by the cytochrome c assay (Fig. 4E). In contrast, unlike the GPCR-mediated stimulation, PMA-induced activation of ERK and MEK, which were measured with phosphorylation-specific Abs, occurred normally even in the DKO neutrophils (Fig. 4F, 4G).
FIGURE 4.
PMA-induced Rac activation and ROS production are also defective in DKO neutrophils. (A and B) Activation of Rac1 and Rac2 in BM neutrophils from WT, DOCK2−/−, DOCK5−/−, and DKO mice stimulated with PMA (100 nM) for the indicated times. Cell lysates were subjected to pull-down assays using GST-fusion Rac-binding domain of PAK1 before immunoblotting with anti-Rac1 and -Rac2 Abs. Results were quantified by densitometry and are expressed as the ratio of GTP-bound form to total protein after normalization of the 2 min value of WT neutrophils to an arbitrary value of 1. Data are indicated as means ±SEM of three separate experiments. *p <0.05, **p <0.01. (C and D) ROS production was compared among WT, DOCK2−/−, DOCK5−/−, and DKO neutrophils stimulated with PMA (324.24 nM). In (C), the right panel indicates the magnified view of the graph to show the difference between DOCK2−/− and DKO neutrophils. In (D), results are expressed as the ratio after normalization of the WT value to an arbitrary value of 1. Data are indicated as means ±SD of triplicate samples. **p < 0.01. (E) ROS production by WT, DOCK2−/−, DOCK5−/−, and DKO neutrophils stimulated with PMA (324.24 nM) was measured by means of a cytochrome c reduction assay. Data are indicated as means ± SD of triplicate samples. *p < 0.05, **p < 0.01. (F and G) Phosphorylations of ERK and MEK in BM neutrophils from WT, DOCK2−/−, DOCK5−/−, and DKO mice stimulated with PMA (100 nM) for the indicated times. Cell lysates were subjected to immunoblotting using phosphorylation-specific Abs against ERK and MEK. Results were quantified by densitometry and are expressed as the ratio of phosphorylated form to total protein after normalization of the 5 min value of WT neutrophils to an arbitrary value of 1. Data are indicated as means ± SEM of three separate experiments.
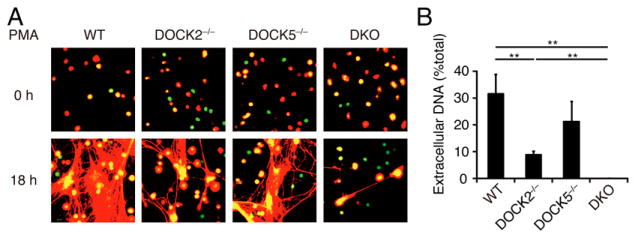
DOCK2 and DOCK5 additively regulate NET formation
Neutrophils stimulated with microbes or proinflammatory agents release their own DNA, histones, proteases, and other antimicrobial molecules, forming a web-like extracellular network designated as NETs (23). Although NETs play important roles in trapping and killing microorganisms to prevent spread of infection, NET formation has been also implicated in vascular inflammation and autoimmune diseases (30–33). To examine the effect of DOCK2 and/or DOCK5 deficiency on NET formation, we stimulated mouse BM neutrophils with PMA, a potent inducer of NETosis in vitro. WT and DOCK5−/− neutrophils produced massive NETs at 18 h after PMA stimulation (Fig. 5A). However, such NET formation was reduced with DOCK2−/− neutrophils, and it was almost completely lost with DKO neutrophils (Fig. 5A, 5B), which is consistent with the defects of ROS production in these neutrophils (Fig. 4C–E). We also found that treatment with CPYPP almost totally abolished PMA-induced Rac activation and NET formation (data not shown). Thus, DOCK2 and DOCK5 are also important for NET formation.
FIGURE 5.
DKO neutrophils exhibit a severe defect in NET formation. (A) Following stimulation with PMA (20 nM) for 18 h at 37°C, WT, DOCK2−/−, DOCK5−/−, and DKO neutrophils were stained with Sytox Green and Sytox Orange for visualization of NETs (original magnification ×200). (B) NET formation was quantitatively compared among PMA (20 nM)-stimulated neutrophils from WT, DOCK2−/−, DOCK5−/−, and DKO mice. Data are indicated as means ± SEM of five separate experiments. **p < 0.01.
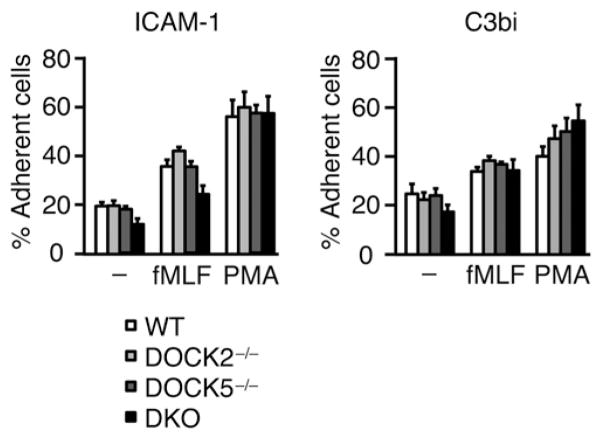
DOCK2 and DOCK5 are dispensable for integrin-dependent neutrophil adhesion
Integrin-dependent neutrophil adhesion is necessary for neutrophils to extravasate the endothelial barrier and to migrate into target tissues. Therefore, we examined whether DOCK2 and DOCK5 contribute to the adhesive response of neutrophils. For this purpose, we stimulated WT or mutant BM neutrophils with fMLF and PMA and compared their binding to ICAM-1 and C3bi. As shown in Fig. 6, even DKO neutrophils mediated normal adhesion to these integrin ligands. So far, it has been reported that neutrophils lacking Vav1 and Vav3 exhibit reduced adhesion with multiple defects in integrin-mediated signaling cascades, including activation of Rac, Cdc42, and RhoA (34). Therefore, this process is likely to be mediated by Vav1 and Vav3, independently of DOCK2/DOCK5-mediated Rac activation.
FIGURE 6.
Neutrophils lacking DOCK2 and DOCK5 are capable of normal adhesion. BM neutrophils from WT, DOCK2−/−, DOCK5−/−, and DKO mice were incubated on the ICAM-1 or C3bi-coated plates for 30 min at 37°C in the presence or absence of fMLF (10 μM) or PMA (162.12 nM). After being washed twice with HBSS containing 20 mM HEPES (pH 7.4) and 0.1% BSA, the percentage of adherent cells was compared among WT, DOCK2−/−, DOCK5−/−, and DKO neutrophils. Data are indicated as means ± SEM of three independent experiments.
Discussion
Previous studies have indicated that DOCK2 is a major Rac GEF acting downstream of GPCRs in neutrophils (14, 15). However, as DOCK2 deficiency does not completely abolish chemoattractant-induced Rac activation (14), other Rac GEFs are likely to be involved in this process. In this study, we have shown that by deleting both DOCK2 and DOCK5 in mouse BM neutrophils, activation of Rac1 and Rac2 in response to fMLF is markedly reduced. Consistent with this finding, DKO neutrophils exhibited more severe defects in chemotaxis and ROS production than did DOCK2−/− neutrophils. Additionally, we found that fMLF-induced phosphorylations of ERK, Akt, and p38, which are closely associated with Rac activation in neutrophils (3, 6, 7), are severely impaired in DKO neutrophils. The importance of DOCK2 and DOCK5 could be extended to human neutrophils, because fMLF-induced Rac activation, chemotaxis, and ROS production were almost completely lost when human peripheral blood neutrophils were treated with CPYPP (25–27). Our results thus identify DOCK5 as a Rac GEF that acts additively with DOCK2 in the GPCR-mediated signaling pathway in humans and mice.
Several lines of evidence indicate that DH domain–containing GEFs such as P-Rex1 and Vav1 also contribute to GPCR-mediated neutrophil functions (12, 13, 35). Indeed, it was recently reported that fMLF-induced ROS production and migration are reduced to 50% of the WT levels in neutrophils simultaneously lacking both P-Rex1 and Vav1 (36). Because P-Rex1 and Vav1 are normally expressed in DKO neutrophils, it is clear that DOCK2/DOCK5 deficiency does not affect the expression levels of these classical GEFs. The relationship between these DH domain–containing GEFs and DOCK family GEFs is currently unknown. Interestingly, however, a recent study indicated that P-Rex1 acts as a GEF for RhoG in vitro and its deficiency leads to a severe defect in fMLF-induced RhoG activation in neutrophils (37). Activated RhoG binds to ELMO, which then regulates localization and activation of DOCK-A subfamily members by interacting with their N-terminal regions (38–40). Therefore, there may a cross-talk between DH domain–containing GEF-mediated and DOCK family GEF-mediated signaling cascades. In contrast, although P-Rex1 and Vav family proteins do not play major roles in PMA-mediated signal transduction (12, 13, 36), PMA-induced Rac activation and ROS production were severely impaired in DKO neutrophils. Thus, in neutrophils, DOCK2 and DOCK5 are universal Rac GEFs acting in both PI3K-dependent and -independent pathways.
Although NET formation plays a key role in trapping and killing microorganisms, this process also contributes to development of vascular inflammation and autoimmune diseases (30–33). In this study, we have shown that PMA-induced NET formation is almost completely lost for DKO neutrophils. Although the Raf/MEK/ERK pathway has been show to be important for PMA-induced NET formation (41), phosphorylations of ERK and MEK occurred normally in DKO neutrophils stimulated with PMA. Therefore, it is suggested that DOCK2 and DOCK5 regulate NET formation without affecting the Raf/MEK/ERK pathway, but probably through Rac activation and ROS production.
In conclusion, in this study we have shown that DOCK2 and DOCK5 are the Rac GEFs critical for neutrophil chemotaxis, ROS production, and NET formation in mice and humans. Our results suggest that the Rac GEF activity of DOCK2 and DOCK5 may be a therapeutic target for controlling NET-mediated vascular inflammation and autoimmune diseases.
Acknowledgments
This work was supported by the Core Research for Evolutional Science and Technology program (to Y.F.) and the Strategic Japanese–Swiss Cooperative Program (to Y.F.) of the Japan Science and Technology Agency; Grants-in-Aid for Scientific Research (to Y.F.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and Grants-in-Aid for Scientific Research (to Y.F.) from the Japan Society for the Promotion of Science; and by the Canadian Institute of Health Research (to J.-F.C.). M.W. is a Research Fellow of the Japan Society for the Promotion of Science. J.-F.C. is a Senior Investigator from the Fonds de Recherche-Santé du Québec.
We thank Ayumi Inayoshi, Arisa Aosaka, and Siqinbala (Kyushu University) for technical support and assistance.
Abbreviations used in this article
- BM
bone marrow
- DH
Dbl homology
- DHR
DOCK homology region
- DKO
double knockout
- GEF
guanine nucleotide exchange factor
- GPCR
G protein–coupled receptor
- NET
neutrophil extracellular trap
- ROS
reactive oxygen species
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Bokoch GM. Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 2005;15:163–171. doi: 10.1016/j.tcb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 3.Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, et al. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 4.Williams DA, Tao W, Yang F, Kim C, Gu Y, Mansfield P, Levine JE, Petryniak B, Derrow CW, Harris C, et al. Dominant negative mutation of the hematopoietic-specific Rho GTPase, Rac2, is associated with a human phagocyte immunodeficiency. Blood. 2000;96:1646–1654. [PubMed] [Google Scholar]
- 5.Li S, Yamauchi A, Marchal CC, Molitoris JK, Quilliam LA, Dinauer MC. Chemoattractant-stimulated Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac2 and Rac2 gene dosage effect on neutrophil functions. J Immunol. 2002;169:5043–5051. doi: 10.4049/jimmunol.169.9.5043. [DOI] [PubMed] [Google Scholar]
- 6.Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- 7.Sun CX, Downey GP, Zhu F, Koh AL, Thang H, Glogauer M. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood. 2004;104:3758–3765. doi: 10.1182/blood-2004-03-0781. [DOI] [PubMed] [Google Scholar]
- 8.Filippi MD, Harris CE, Meller J, Gu Y, Zheng Y, Williams DA. Localization of Rac2 via the C terminus and aspartic acid 150 specifies superoxide generation, actin polarity and chemotaxis in neutrophils. Nat Immunol. 2004;5:744–751. doi: 10.1038/ni1081. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 10.Côté J-F, Vuori K. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J Cell Sci. 2002;115:4901–4913. doi: 10.1242/jcs.00219. [DOI] [PubMed] [Google Scholar]
- 11.Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, Tempst P, Hawkins PT, Stephens LR. P-Rex1, a PtdIns(3,4,5)P3- and Gβγ-regulated guanine-nucleotide exchange factor for Rac. Cell. 2002;108:809–821. doi: 10.1016/s0092-8674(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 12.Welch HC, Condliffe AM, Milne LJ, Ferguson GJ, Hill K, Webb LM, Okkenhaug K, Coadwell WJ, Andrews SR, Thelen M, et al. P-Rex1 regulates neutrophil function. Curr Biol. 2005;15:1867–1873. doi: 10.1016/j.cub.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 13.Dong X, Mo Z, Bokoch G, Guo C, Li Z, Wu D. P-Rex1 is a primary Rac2 guanine nucleotide exchange factor in mouse neutrophils. Curr Biol. 2005;15:1874–1879. doi: 10.1016/j.cub.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Kunisaki Y, Nishikimi A, Tanaka Y, Takii R, Noda M, Inayoshi A, Watanabe K, Sanematsu F, Sasazuki T, Sasaki T, Fukui Y. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J Cell Biol. 2006;174:647–652. doi: 10.1083/jcb.200602142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishikimi A, Fukuhara H, Su W, Hongu T, Takasuga S, Mihara H, Cao Q, Sanematsu F, Kanai M, Hasegawa H, et al. Sequential regulation of DOCK2 dynamics by two phospholipids during neutrophil chemotaxis. Science. 2009;324:384–387. doi: 10.1126/science.1170179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, Tosello-Trampont AC, Macara IG, Madhani H, Fink GR, Ravichandran KS. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol. 2002;4:574–582. doi: 10.1038/ncb824. [DOI] [PubMed] [Google Scholar]
- 17.Meller N, Merlot S, Guda C. CZH proteins: a new family of Rho-GEFs. J Cell Sci. 2005;118:4937–4946. doi: 10.1242/jcs.02671. [DOI] [PubMed] [Google Scholar]
- 18.Côté J-F, Motoyama AB, Bush JA, Vuori K. A novel and evolutionarily conserved PtdIns(3,4,5)P3-binding domain is necessary for DOCK180 signalling. Nat Cell Biol. 2005;7:797–807. doi: 10.1038/ncb1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T, Hanawa-Suetsugu K, Kukimoto-Niino M, Nishizaki T, Shirouzu M, et al. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood. 2012;119:4451–4461. doi: 10.1182/blood-2012-01-407098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurin M, Fradet N, Blangy A, Hall A, Vuori K, Côté J-F. The atypical Rac activator Dock180 (Dock1) regulates myoblast fusion in vivo. Proc Natl Acad Sci USA. 2008;105:15446–15451. doi: 10.1073/pnas.0805546105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vives V, Laurin M, Cres G, Larrousse P, Morichaud Z, Noel D, Côté J-F, Blangy A. The Rac1 exchange factor Dock5 is essential for bone resorption by osteoclasts. J Bone Miner Res. 2011;26:1099–1110. doi: 10.1002/jbmr.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanematsu F, Nishikimi A, Watanabe M, Hongu T, Tanaka Y, Kanaho Y, Côté J-F, Fukui Y. Phosphatidic acid-dependent recruitment and function of the Rac activator DOCK1 during dorsal ruffle formation. J Biol Chem. 2013;288:8092–8100. doi: 10.1074/jbc.M112.410423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 24.Fukui Y, Hashimoto O, Sanui T, Oono T, Koga H, Abe M, Inayoshi A, Noda M, Oike M, Shirai T, Sasazuki T. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412:826–831. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- 25.Nishikimi A, Uruno T, Duan X, Cao Q, Okamura Y, Saitoh T, Saito N, Sakaoka S, Du Y, Suenaga A, et al. Blockade of inflammatory responses by a small-molecule inhibitor of the Rac activator DOCK2. Chem Biol. 2012;19:488–497. doi: 10.1016/j.chembiol.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Laurin M, Huber J, Pelletier A, Houalla T, Park M, Fukui Y, Haibe-Kains B, Muller WJ, Côté J-F. Rac-specific guanine nucleotide exchange factor DOCK1 is a critical regulator of HER2-mediated breast cancer metastasis. Proc Natl Acad Sci USA. 2013;110:7434–7439. doi: 10.1073/pnas.1213050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakai Y, Tanaka Y, Yanagihara T, Watanabe M, Duan X, Terasawa M, Nishikimi A, Sanematsu F, Fukui Y. The Rac activator DOCK2 regulates natural killer cell-mediated cytotoxicity in mice through the lytic synapse formation. Blood. 2013;122:386–393. doi: 10.1182/blood-2012-12-475897. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akasaki T, Koga H, Sumimoto H. Phosphoinositide 3-kinase-dependent and -independent activation of the small GTPase Rac2 in human neutrophils. J Biol Chem. 1999;274:18055–18059. doi: 10.1074/jbc.274.25.18055. [DOI] [PubMed] [Google Scholar]
- 30.Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, Gröne H-J, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med. 2011;17:1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gakidis MA, Cullere X, Olson T, Wilsbacher JL, Zhang B, Moores SL, Ley K, Swat W, Mayadas T, Brugge JS. Vav GEFs are required for β2 integrin-dependent functions of neutrophils. J Cell Biol. 2004;166:273–282. doi: 10.1083/jcb.200404166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim C, Marchal CC, Penninger J, Dinauer MC. The hemopoietic Rho/Rac guanine nucleotide exchange factor Vav1 regulates N-formyl-methionyl-leucyl-phenylalanine-activated neutrophil functions. J Immunol. 2003;171:4425–4430. doi: 10.4049/jimmunol.171.8.4425. [DOI] [PubMed] [Google Scholar]
- 36.Lawson CD, Donald S, Anderson KE, Patton DT, Welch HC. P-Rex1 and Vav1 cooperate in the regulation of formyl-methionyl-leucyl-phenylalanine-dependent neutrophil responses. J Immunol. 2011;186:1467–1476. doi: 10.4049/jimmunol.1002738. [DOI] [PubMed] [Google Scholar]
- 37.Damoulakis G, Gambardella L, Rossman KL, Lawson CD, Anderson KE, Fukui Y, Welch HC, Der CJ, Stephens LR, Hawkins PT. P-Rex1 directly activates RhoG to regulate GPCR-driven Rac signalling and actin polarity in neutrophils. J Cell Sci. 2014;127:2589–2600. doi: 10.1242/jcs.153049. [DOI] [PubMed] [Google Scholar]
- 38.Katoh H, Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature. 2003;424:461–464. doi: 10.1038/nature01817. [DOI] [PubMed] [Google Scholar]
- 39.Sanui T, Inayoshi A, Noda M, Iwata E, Stein JV, Sasazuki T, Fukui Y. DOCK2 regulates Rac activation and cytoskeletal reorganization through interaction with ELMO1. Blood. 2003;102:2948–2950. doi: 10.1182/blood-2003-01-0173. [DOI] [PubMed] [Google Scholar]
- 40.Hanawa-Suetsugu K, Kukimoto-Niino M, Mishima-Tsumagari C, Akasaka R, Ohsawa N, Sekine S, Ito T, Tochio N, Koshiba S, Kigawa T, et al. Structural basis for mutual relief of the Rac guanine nucleotide exchange factor DOCK2 and its partner ELMO1 from their autoinhibited forms. Proc Natl Acad Sci USA. 2012;109:3305–3310. doi: 10.1073/pnas.1113512109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, Waldmann H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011;7:75–77. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]