Abstract
Image-guided high-intensity focused ultrasound (HIFU) is an innovative therapeutic technology, permitting extracorporeal or endocavitary delivery of targeted thermal ablation while minimizing injury to the surrounding structures. While ultrasound-guided HIFU was the original image-guided system, MR-guided HIFU has many inherent advantages, including superior depiction of anatomic detail and superb real-time thermometry during thermoablation sessions, and it has recently demonstrated promising results in the treatment of both benign and malignant tumors. HIFU has been employed in the management of prostate cancer, hepatocellular carcinoma, uterine leiomyomas, and breast tumors, and has been associated with success in limited studies for palliative pain management in pancreatic cancer and bone tumors. Nonthermal HIFU bioeffects, including immune system modulation and targeted drug/gene therapy, are currently being explored in the preclinical realm, with an emphasis on leveraging these therapeutic effects in the care of the oncology patient. Although still in its early stages, the wide spectrum of therapeutic capabilities of HIFU offers great potential in the field of image-guided oncologic therapy.
Keywords: HIFU, interventional oncology, focused ultrasound surgery, image guided, ablation, interventional radiology
Objectives: Upon completion of this article, the reader will be able to describe the physical properties of high-intensity focused ultrasound (HIFU) as well as its utility in oncologic therapy.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
High-intensity focused ultrasound (HIFU) therapy, also known as focused ultrasound surgery (FUS), is a noninvasive technology that delivers ultrasound (US) waves via an extracorporeal or endocavitary approach to specific targets within the human body. HIFU therapy results in targeted thermal ablation of tissue without injury to the surrounding structures, and over the past 10 years it has increasingly gained clinical interest as a novel tool for image-guided ablation.
The safety and feasibility of HIFU have been tested for the ablation of both benign and malignant tumors, including uterine leiomyomas as well as tumors of the prostate, pancreas, liver, breast, kidney, brain, and bone. Additional HIFU bioeffects beyond thermoablation include US-mediated tissue sensitization to radiation and chemotherapy, and US-mediated local drug and gene delivery to tumors. These areas of ongoing research remain of great interest in terms of their oncologic treatment potential.
History of HIFU Therapy
In 1927, Wood and Loomis first described the thermal properties of high-intensity US. Subsequently, in 1942, Lynn et al described the use of a focused US generator capable of producing focal thermal injury to ex vivo liver specimens as well as the brains of animals through the intervening scalp, skull, and meninges without incidental injury to the skin.1 2 In the 1950s, the Fry brothers developed a transcranial HIFU system that could be used after craniotomy to target deep-seated areas of the brain in primates, furthering interest in HIFU ablation to treat movement disorders like Parkinson syndrome.3 Several of the earliest studies on HIFU ablation therapy in humans, under US guidance, were performed on the prostate and were reported in the early 1990s by Marberger and coworkers and Madersbacher et al; with subsequent advancements in imaging guidance, both US and magnetic resonance imaging (MRI), having allowed for treatment of a wide range of benign and malignant tumors.4 5
Ultrasound Thermal Ablation: Physical Properties
The physical properties of high-intensity US waves permit precisely targeted energy deposition. As long as a medium is present, sound can transport energy in the form of waves. US is a form of sound at higher frequency (>20,000 Hz) than the human ear can detect. Although radiofrequency and microwave ablation require an electrode or antenna for energy delivery, HIFU may traverse biologic tissues for delivery to the target, with energy accumulation maximized in the target area and minimized within intervening tissues. High-intensity US generally refers to US with an intensity of greater than 5 W/cm2, which is capable of producing coagulation necrosis of tissue and is most often utilized for HIFU ablation. In contrast, low-intensity US (0.125–3 W/cm2) leads to nondestructive heating and is in the range used for physiotherapy.6 US may result in various reactions when insonated into biological tissue. Among these reactions, thermal ablation and acoustic cavitation are, at present, the most clinically relevant.
HIFU thermal effects are secondary to the absorption of US waves. Frictional heat results from vibrations and/or rotations of molecules secondary to the US waves. Protein denaturation and coagulation necrosis typically occur at 56°C with an exposure length of 1 second, whereas a temperature of greater than 43°C for 1 hour may render the tissue more susceptible to chemotherapy and radiation.7 8 9 The temperature elevation of biological tissue secondary to US absorption is linearly proportional to the sonic intensity.10
HIFU as an Ablation Tool
HIFU, possessing average spatial intensities in the range of 100 to 10,000 W/cm2, is utilized for local ablation of tumors. When HIFU is deposited in a focal area with the intention of coagulation necrosis, the induced thermal lesions are well circumscribed with an intermediate zone between the intact and destroyed cells, which is only several cell layers thick. The surrounding tissue typically remains unaffected due to the low acoustic energy density in these areas. It is this surgical-like precision that led to the name, “focused ultrasound surgery.”
The classic thermal lesion has a cigar shape that parallels the direction of the US propagation (Fig. 1). These lesions are typically 1.5 to 2 mm in width and 1.5 to 2 cm in length when produced by a typical 1.5 MHz HIFU field.9 10 11 Given the small size of these thermal lesions relative to most tumors undergoing treatment, individual thermal lesions must be closely juxtaposed without intervening viable tissue to form a complete ablation zone; this process can be quite time consuming. Simultaneous development of multiple ablative focal points as well as fast electronic scanning of the focus can lead to an increased size of the focal region, allowing for a decreased overall treatment time (Fig. 2).12 This technique will be further described later. As sonic attenuation is low at lower US frequencies and energy is more sharply focused at higher US frequencies, frequencies may be adjusted to tailor therapy.
Fig. 1.
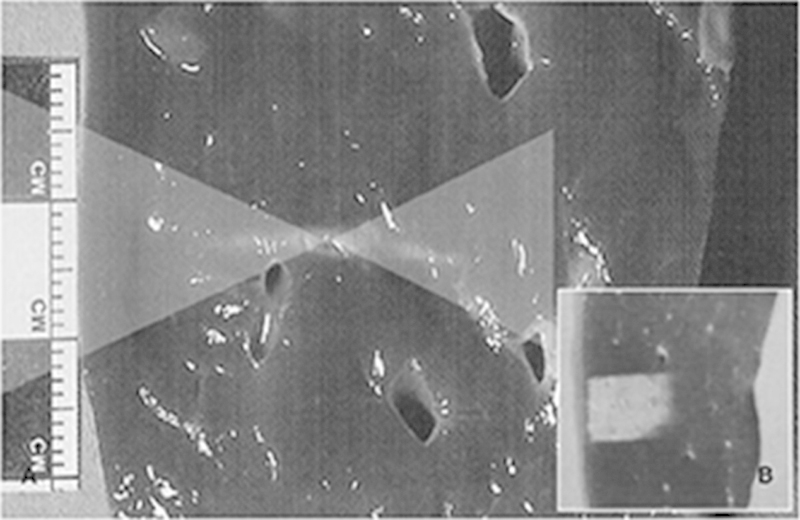
Classical thermal lesion formed by focused US surgery (US absorption only) on porcine liver specimen. (a) Cigar-shaped thermal lesion is formed at focal zone of US wave pathway (two overlaid triangles) following HIFU single exposure. (b) Final thermal lesion after stacking each single lesion. Single lesions are much smaller than clinically common tumors and therefore each thermal lesion should be stacked compactly without leaving intervening viable tissue. This lesion can cover the entire pathological lesion as well as having a very sharp margin that could be controlled easily. (Reprinted with permission from Kim YS, Rhim H, Choi MJ, Lim HK, Choi D. High-intensity focused ultrasound therapy: an overview for radiologists. Korean J Radiol 2008; 9(4):291–302.)
Fig. 2.
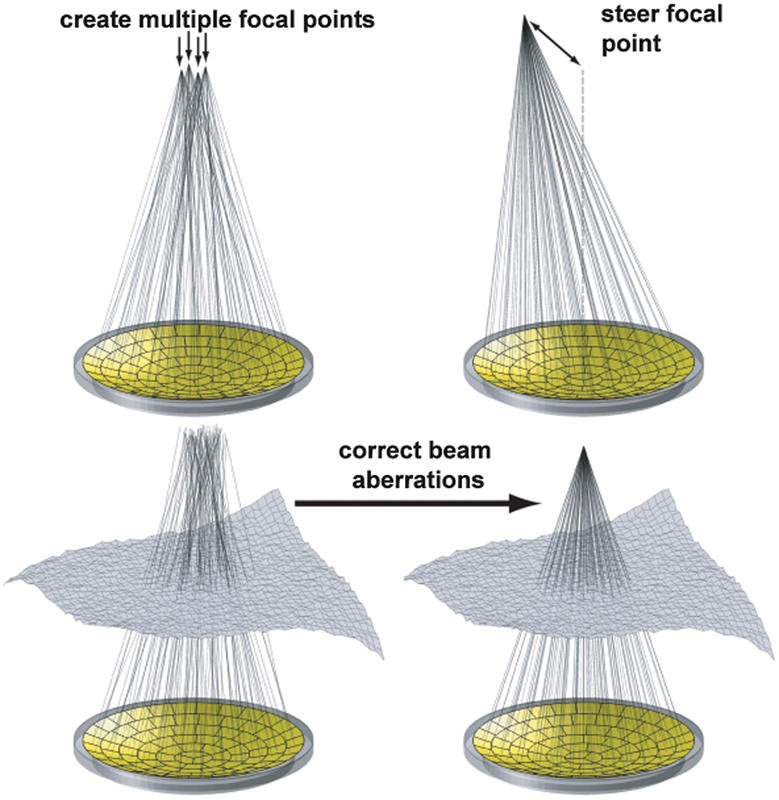
Ways a phased-array transducer can be used include producing multiple focal spots to increase the ablated volume per sonication, steering the focal point to different locations, and correcting for aberrations caused by tissue structures in the ultrasound beam path. (Reprinted with permission from Tempany et al.12)
Transducer Technology
Specialized transducers composed of piezo-active materials with specific acoustic properties designed for high-power US application are utilized for HIFU thermoablation. Typically, HIFU transducers produce acoustic intensities in the target tissue of 100 to 10,000 W/cm2, whereas diagnostic transducers deliver intensities in the range of 0.0001 to 0.1000 W/cm2. The piezo-active materials oscillate upon the application of an alternating voltage, thereby generating US waves.13
The simplest and cheapest transducer design is a self-focusing spherical-shaped piezo-ceramic transducer with a defined aperture and focal length. More sophisticated phased-array transducers consist of a large number of transducer elements for which the electrical signals applied to each element can be varied.14 With these more complex systems, beam steering and beam-forming capabilities are feasible, with the acoustic fields produced by individual elements coalesced to produce a single focus maneuvered through a clinically relevant volume, or with the ability to create several foci simultaneously. Both techniques increase the overall ablation volume compared with simpler transducer systems.
Specific designs for phased-array transducers range from 256-element phased-arrays for treatment of large deep-seated tissue volumes to 1,000 elements in endorectal transducers for high-resolution treatment of the prostate.15 16
Imaging Guidance
A reliable imaging method for image-guided therapies is mandated for safe, accurate thermoablation. Diagnostic US and MRI are the two imaging modalities utilized to guide HIFU therapy. Both methods have unique advantages and limitations.
Ultrasound
The majority of HIFU treatments used worldwide are performed under US guidance.17 US-guided HIFU (US-HIFU) provides real-time image-guided therapy, as the diagnostic and therapeutic US fields are superimposed. Additionally, this option is economically favorable, as small flexible devices with distinctly lower relative purchase and maintenance costs are available.
Disadvantages of US-HIFU are the inability to adequately assess and control lesion formation. Hyperechoic changes on B-mode US that occur during heating can obscure both the treated target and surrounding anatomy. Additional disadvantages of US-HIFU include relatively poor tissue-contrast, a limited field of view, and deterioration of the image quality as treatment progresses.
Real-time thermometry, lesion detection, and focal spot corrections in the setting of target obscuration are not as sophisticated compared with MR-guided techniques. There are, however, recent studies demonstrating promising results for US-based thermometry and US focal spot corrections. Integration of a focused US therapy transducer for hyperthermia with a microwave array for noninvasive temperature monitoring has been described in phantoms, with self-registering dual mode US arrays providing immediate and spatially accurate temperature feedback in ex vivo porcine liver models.18 19 20 Indirect temperature monitoring techniques capitalizing on the temperature dependence of backscattered US power during sonications have also been described.18 19 20 Jensen et al demonstrated passive acoustic mapping of broadband and harmonic emissions to significantly outperform conventional B-mode imaging, as both a detector of lesion occurrence and as a method of mapping the position of the ablated tissue, in freshly excised bovine livers.21
Magnetic Resonance Imaging
MR-guided HIFU (MR-HIFU) was developed, in part, due to the need for three-dimensional treatment planning and continuous real-time monitoring of thermal damage in the target zone. This modality has demonstrated success in the treatment of various benign and malignant tumors.22
MRI monitoring is superior to US as a guidance tool for HIFU therapy for several reasons. MRI provides morphological images for planning and targeting with exquisite anatomical resolution. This modality also provides excellent tissue contrast. Additionally, MRI offers superb real-time thermometry, allowing for measurement of temperature changes and cumulative thermal dose, enabling predictions of the extent of tissue damage.23
The most commonly employed MR thermometry technique is the proton resonance frequency (PRF) shift method, which takes advantage of the linear relationship between temperature elevations in tissue and MR phase shifts. This permits temperature mapping utilizing phase differences.24 Limitations of conventional MR thermometry are its susceptibility to motion artifacts due to the need for image subtraction and its inability to monitor temperature changes in fat.25 Recent MR-HIFU technical developments include acoustic radiation force imaging for focal spot identification and motion correction as well as multi-echo hybrid PRF/T1 pulse sequences that can measure temperature simultaneously in fat- and water-based tissues.26 27
At the end of treatment, contrast-enhanced T1 imaging can be performed to evaluate for a lack of enhancement in the area of treatment.
Ongoing challenges to the widespread implementation of MR-HIFU for thermoablation include the need for an MR-compatible HIFU unit, the price of the MR scanner, and the duration of treatment sessions.
HIFU Clinical Platforms
Extracorporeal US-HIFU systems were the first devices utilized for HIFU thermoablation and are therefore the devices associated with the most longstanding HIFU experience. The most widely used clinical extracorporeal device is the Model JC focused US system (Haifu Technology Co. Ltd., Chongqing, China), which has been used in East Asia since 1997.28 This device consists of the following: a real-time diagnostic US device; integrated therapeutic transducers; a six-direction movement system allowing movement of the integrated transducer along the X, Y, and Z planes; a moving table allowing the patient's body to be moved over the therapeutic transducer for better tumor localization and targeting; computer units for automated control; an US generator that produces the high-intensity US; and a degassed water circulation unit. The HIFU-2001 (Sumo Corporation Ltd., Kowloon, Hong Kong) and the FEP-BY system are alternative extracorporeal US-HIFU systems that are also more popular in Asia than in Europe and North America. These devices are being used for the treatment of several different types of cancer, including liver, renal, and pancreatic tumors.29
Given that prostate cancer is the most frequently diagnosed cancer in men in the United States and Europe, specialized HIFU devices have been developed for the treatment of prostate cancer and other pelvic pathologies. Two commercially available intracavitary US-HIFU clinical devices are Ablatherm (EDAP TMS, Lyon, France) and Sonablate 500 (Focus Surgery, Indianapolis, IN), both developed in the 1990s. While Ablatherm possesses a robot-controlled treatment probe with dual US transducers, Sonablate utilizes a single transducer with split beam technology, thereby increasing the size of the focal zone and allowing simultaneous treatment and imaging. Both of these devices are US guided and utilize a single movable probe. While both have been employed in trials for the treatment of prostate cancer, each has the potential to be used in the management of other pelvic malignancies.30
MR-HIFU platforms are recently developed systems. Commercially available MR-HIFU systems include the ExAblate (InSightec, Ltd., Tirat Carmel, Israel) and the Sonalleve (Philips Medical Systems, Vantaa, Finland) systems.
The ExAblate (InSightec, Ltd.) is the system currently approved for clinical use in the United States. This system has been primarily employed for ablation of symptomatic uterine leiomyoma.31 It consists of an MR-compatible US transducer delivering therapy via point-by-point sonication under real-time MR imaging guidance. The MR guidance permits treatment planning and temperature monitoring at treatment sites based on PRF shift MR thermometry as described earlier.24
With increasing clinical experience and expertise, technical limitations of MR-HIFU ablation were recognized. Procedural times have typically been long, especially for the treatment of large lesions. This is secondary to the small size of the individual focal points implemented for point-by-point sonication and the cooling times required between individual sonications. Untreated viable tissue between adjacent ablation points may also result as a consequence of this point-by-point methodology of ablation.32 33 Recently developed techniques of electronic steering of the US focus along predefined trajectories, and the use of phased-array transducers and driving electronics that allow rapid temporal switching of the focal point location, address this limitation and enable rapid volumetric sonications.34 35 36 Additionally, local tissue characteristics, including US attenuation and tissue perfusion, can lead to unpredictable over- or undertreatment of target tissue.37 Real-time MR thermometry with thermal feedback algorithms permit intraprocedural modification of sonication parameters, thereby leading to a spatiotemporally controlled temperature profile, demonstrated both in phantoms and in vivo.38 39 40 41 These technical advancements in MR-HIFU therapy may improve safety and the accuracy of intraprocedural treatment monitoring.33 42
The Sonalleve MR-HIFU system (Philips Medical Systems) implements volumetric thermal feedback, providing ablation control, and MR thermometry monitoring of the anatomic target region comprising both the near and far field. With volumetric ablation, the HIFU transducer transmits continuous energy in concentric circles using various sized treatment cells (Fig. 3).43 When volumetric heating is performed with thermal feedback, each volumetric ablation is programmed to achieve ablative temperatures, and the duration of sonication is determined by the system and subsequently modified according to the mean cell temperature.43 The system also displays real-time temperature data with a colorized temperature scale superimposed on gray-scale MR anatomic images, allowing thermal monitoring of the target and anatomic near and far fields (Fig. 4). Accurate and predictable thermoablation with this MR-HIFU system has been demonstrated in the treatment of uterine leiomyoma.43
Fig. 3.
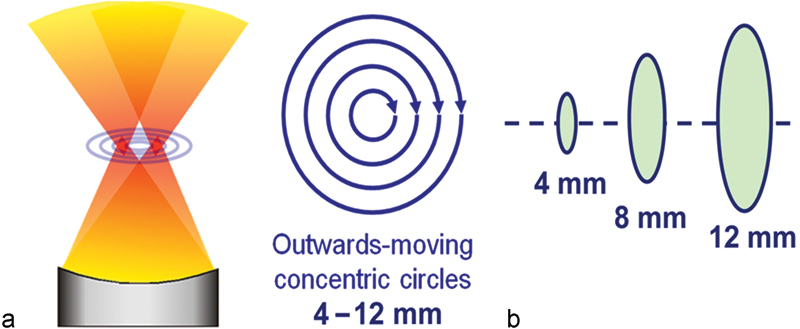
MR-guided HIFU volumetric ablation. (a) Schematic of HIFU transducer and beam, applying focused acoustic energy in concentric circles within a treatment cell. (b) Treatment cells 4, 8, or 12 mm in diameter, with a ratio of cell diameter to length of ∼1:2.5. (Reprinted with permission from Venkatesan et al.43)
Fig. 4.
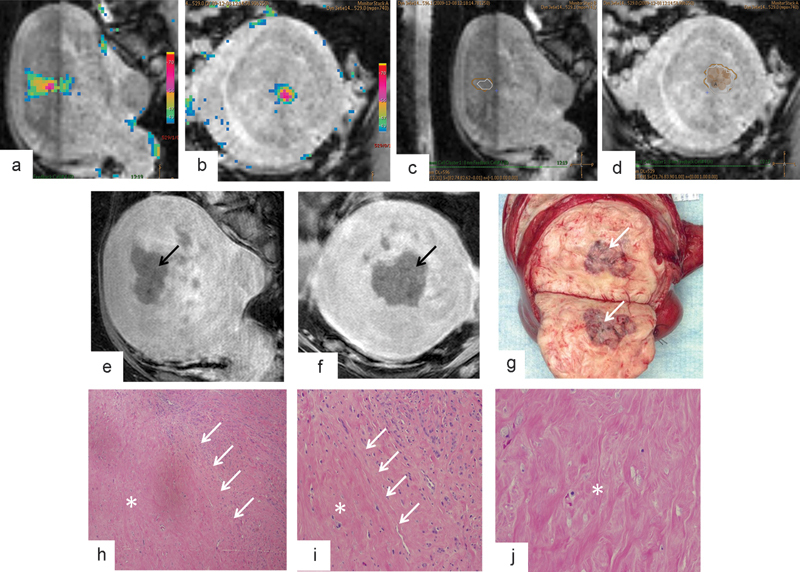
Intraprocedural MR-guided HIFU monitoring and MR imaging and histopathologic findings after leiomyoma ablation with MR-guided HIFU. (a–d) Graphic user interface displays multiplanar three-dimensional T2-weighted imaging and overlaid temperature maps (a–b) and overlaid thermal dose estimates (c–d) during sonication of an anterior intramural leiomyoma within the body of the uterus. Accumulated thermal dose information in the treated volume is displayed at the end of each sonication as a thermal dose estimate. These thermal doses are reported in CEM43, with 30 CEM43 (beige polygon, c–d) corresponding to onset of tissue alteration and 240 CEM43 (white polygon, c–d) representing predicted territory of complete necrosis. Both 30 CEM43 and 240 CEM43 thermal dose estimates are updated after each sonication. (e–f) Sagittal (e) and coronal (f) contrast-enhanced MR images after HIFU show nonenhancing treated region (black arrows). (g) Bivalved gross uterine specimen shows hemorrhagic necrosis in the area of treatment (white arrow). (h–j) Low-magnification (4 × ) histologic images of margin (h), high-magnification (10 × ) images of margin (j), and high-magnification images of the center of the ablation zone (j) confirm necrosis (asterisk) and narrow zone of transition (white arrows) between viable and necrotic HIFU-treated tissue. (Reprinted with permission from Venkatesan et al.43)
Clinical Experience with HIFU Ablation to Date
Prostate
Prostate tumors are, worldwide, the most common entity treated with HIFU. Two decades ago, benign prostate hyperplasia was the major pathology managed with HIFU, whereas today increasing attention is focused on the treatment of prostate cancer. Aside from skin cancer, prostate cancer is the most frequently diagnosed cancer in American men, and remains the second most common cause of cancer-related death.44 Given the prostate's proximity to the rectal surface at a depth of 1 to 4 cm from the anus, and the fact that its position is minimally affected by respiratory motion, it is in many ways an ideal target for HIFU.
Prior studies have demonstrated that up to 29% of patients undergoing radical prostatectomy for screening-detected prostate cancer have indolent disease at pathological examination.45 46 Similarly, up to 16.4% of patients diagnosed with prostate cancer may be candidates for more conservative management based on the widely used modified “Epstein” criteria: prostate-specific antigen (PSA) level ≤ 10 ng/mL and/or PSA density ≤ 0.15 ng/mL/g; clinical stage cT1 or cT2a; one-third or less of biopsy cores positive for cancer; and absence of Gleason pattern 4 or 5 tumor on biopsy.45 As definitive methods of treatment including radical prostatectomy and radiation are associated with substantial morbidity (incontinence, impotence, anorectal dysfunction), low-risk patients may be better managed with more conservative therapy, such as active surveillance, or focal therapy including HIFU.47 In a study assessing 310 men with prostate cancer and modified “Epstein” criteria placing them in the “very low-risk disease” group, only 9.0% of the men opted for active surveillance; 61% underwent radical prostatectomy, 23% underwent radiation therapy, and 7% underwent androgen-deprivation therapy.45 Invasive thermoablation with HIFU has the potential to be an attractive alternative for these patients.
Blana et al reported results among 140 patients who underwent whole gland ablation with US-HIFU, with 8-year overall survival and cancer-specific survival rates of 83 and 98%, respectively.48 Ahmed et al employed a focal therapy approach for the treatment of prostate cancer with US-HIFU and demonstrated a decreased rate of complications, although with a variable degree of oncological control.49
Given the added advantages of ablation control and thermometry monitoring, there are several reasons to hypothesize that MR-HIFU will achieve equally, if not more, efficacious focal tumor therapy while reducing the risk of complications. As it is often difficult to identify critical structures including the urethra and neurovascular bundles with US guidance, MR-HIFU for prostatic cancer has recently been pursued as an alternative form of therapy. As MRI is able to readily identify critical structures, it may potentially avoid injury to these structures. Additional advantages of real-time thermometry are its ability to confirm the adequacy of thermal dose delivered to the target, in contrast to US-guided therapy for which treatment effects at the target site may only demonstrate vague hyperechoic changes.
Napoli et al described the treatment of patients with biopsy-proven unifocal prostate cancer who underwent transrectal MR-HIFU; resultant pathologic specimens demonstrated extensive coagulative necrosis at the ablation site with surrounding healthy tissue demonstrating only inflammatory changes (Fig. 5).50 Two systems are commercially available. The ExAblate system (InSightec, Ltd) utilizes an endorectal probe and combines a phased-array US transducer, an imaging coil, and a cooling system, whereas the Sonalleve system (Philips Medical Systems) utilizes a transurethral applicator. While the transrectal approach may offer a greater degree of flexibility and shaping of the target volume, the transurethral approach is less likely to result in rectal injury and may lead to a faster procedure overall.
Fig. 5.
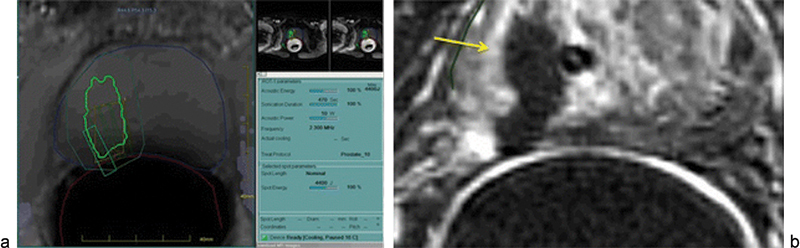
A 68-year-old man with low-risk organ confined prostate cancer (prostate-specific antigen nadir, 8; Gleason score, 6–3 + 3) indicated to radical prostatectomy was included in a phase I trial for MR-guided HIFU treatment before surgery. (a) At treatment time, prostate cancer was visible at 3 T MR images that were used for treatment planning. The system automatically generates a lesion-specific sonication program that spares normal prostate parenchyma for focal ablation. More importantly, the system spares the rectal wall, preventing local parietal damage through active intrarectal cooling and real-time temperature mapping at treatment. (b) Immediately after treatment, gadolinium-enhanced T1-weighted image was acquired for treatment efficacy and safety control. The ablated volume appears as a nonperfusing area (yellow arrow) with intact adjacent rectal wall. Surgery after MR-guided FUS treatment was performed without treatment-related complications or operator difficulties. (Reprinted with permission from Napoli et al.50)
Preliminary experience with salvage HIFU for local recurrence following radiation therapy has demonstrated success. In 2008, Zacharakis el al reported that at a mean follow-up of 7 months after salvage HIFU for local recurrence, 71% of patients had no evidence of disease.51 Uchida et al demonstrated that in patients with recurrence after external beam radiation therapy, brachytherapy, or proton therapy, salvage HIFU was associated with biochemical disease-free survival rates in low- and intermediate-risk groups of 100 and 86%, respectively.52
Long-term cancer recurrence rates following radical prostatectomy range from 17 to 29%.53 While radiation therapy is widely utilized for the management of recurrence, two studies demonstrated success with HIFU, with patients able to return home earlier following treatment.54 55
The most common complications associated with HIFU ablation of prostate cancer include urinary tract infections, urethral strictures, rectourethral fistula, erectile dysfunction, and urinary incontinence.56 57 58 59 Continued maturation of experience with US- and MR-HIFU may decrease the rate of these complications in the future.
Uterus
Uterine leiomyomas, also known as fibroids, may result in pelvic pain and pressure, mass effect on the bladder or bowel leading to frequent urination and/or constipation, and excessive menstrual bleeding. Fibroids may be present in as many as 70 to 80% of women by the age of 50.60 61 Surveys have established that as many as 79% of women desire treatments that avoid invasive surgery. Additionally, 43% of women younger than 40 years desire preserved fertility. As many as 28% of patients with fibroids have symptoms severe enough to warrant treatment.62
Many studies evaluating fibroid treatments have examined improvements in symptom severity. The Uterine Fibroid Symptom and health-related Quality of Life questionnaire (UFS-QOL) is a validated instrument to quantify bleeding, bulk, and other symptoms arising from fibroids.62 It consists of an 8-item symptom severity scale, and 29 health-related quality of life items; the score can be used to evaluate symptom severity and appropriateness for MR-HIFU treatment.
Studies have also described radiological endpoints. A primary measurement of treatment success is nonenhancement within the fibroid on postgadolinium MR images obtained immediately after the therapeutic portion of the procedure. This nonperfused volume (NPV) ratio correlates with symptomatic improvement and fibroid involution.63
Recent clinical studies describe clinical experience with MR-HIFU. The ExAblate system (InSightec, Ltd) was approved by the FDA in 2004 for use in the United States, and obtained CE mark approval in Europe for treatment of symptomatic uterine fibroids. Conditions of use of the ExAblate system include a requirement that patients be pre- or peri-menopausal, and that “patients should have completed child bearing.”64 This reflects the unknown effect of MR-HIFU on pregnancy and concerns that the procedure might weaken or damage the uterine wall and predispose to uterine rupture.
Nevertheless, studies evaluating pregnancy outcomes following MR-HIFU have been promising. One study demonstrated 54 pregnancies in 51 women after MR-HIFU.65 In a series of 22 pregnancies after previous MR-HIFU, 14 patients underwent subsequent uncomplicated vaginal deliveries and 8 underwent uncomplicated cesarean sections.65
Several factors make some fibroids more amenable to treatment than others. Anatomic location is critical; anteriorly located fibroids are preferable, while posteriorly located fibroids (more than 12 cm from the anterior abdominal wall) are difficult to treat, as they are positioned beyond the range at which heating from HIFU can be successfully achieved.64 It has been recommended that treatment is pursued for fibroids under 10 cm in size.66 However, Kim et al suggested a one-layer ablation strategy for fibroids over 10 cm, by placing all the treatment cells in one coronal plane, and using larger 16-mm cells to treat the central portions of the tumor and smaller cells to treat the outer portions of the tumor.67 Generally, treatment volume should not exceed 500 cm3, as it will necessitate an excessively long single-session treatment.64 However, a fibroid greater than 500 cm3 could be treated during multiple sessions. Peripherally calcified fibroids reflect the US beam and are therefore typically not treatable.64 Fibroids that are T2-hyperintense at baseline are difficult to heat, which may be considered a relative contraindication.68 Overall, pre-procedure imaging offers critical information on leiomyoma treatment eligibility as well as the likelihood of successful posttreatment outcomes.
Several studies have indicated that HIFU treatment results in significant improvement in bulk and bleeding symptoms. In 2003, Stewart et al performed one of the first feasibility studies of MR-HIFU for uterine fibroids. Fifty-five women were treated and the authors reported sufficient necrosis volume with no major complications.69 One of the largest clinical studies reported to date involved a cohort of 359 women with symptomatic fibroids who underwent MR-HIFU at seven sites worldwide.69 70 Targeted symptom reduction rates were reported to be 71% at 6 months and 51% at 12 months, with results from this study leading to subsequent regulatory approval of the device.69
Several studies have replicated these impressive results. Funaki et al examined 91 patients with fibroids treated with MR-HIFU and demonstrated significantly decreased symptom severity during a 24-month follow-up period.71 Napoli et al reported their experience with the treatment of 75 symptomatic patients with a total of 89 fibroids. Contrast-enhanced MRIs were obtained at 3, 6, and 12 months to evaluate reduction in the NPV ratio, fibroid volume, and diameter. At 12 months, fibroid size decreased to a mean diameter of 41.2 mm (28.4%) and a volume of 54.6 mm3 (45.9%) (Fig. 6). All patients displayed mild to moderate reduction in leiomyoma size with significant reduction in fibroid-related symptoms and a reported higher quality of life.50 In contrast, Hindley et al reported volume reduction rates of tumors of 13.5% at 6 months, associated with nonenhancing volume that remained within the treated fibroid.72
Fig. 6.
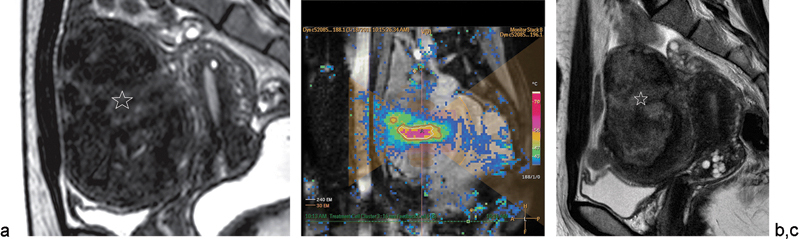
Uterine fibroid in a 37-year-old woman who reported aggravated urinary frequency for 1 year treated with volumetric MR-guided HIFU ablation. (a) Baseline sagittal T2-weighted MR image shows large subserosal uterine fibroid (star) (11.6 cm, 446.7 mL) with signal intensity higher than that of skeletal muscle but lower than that of myometrium. (b) Sagittal MR thermometric image obtained during sonication with a 16-mm treatment cell (sonication frequency, 1.2 MHz; acoustic power, 140 W). Temperature maps and automatically drawn 240 EM thermal dose contours (white lines) that were formed by one sonication are shown. Thermal dose contours were shifted slightly anteriorly, presumably because of a near-field heating effect; however, contours were well within the fibroid margin. Low-temperature color pixels outside the heating area represent noise. A = center of treatment cell, yellow lines = 30 EM thermal dose contours. (c) Sagittal T2-weighted MR image at 3-month follow-up shows obvious volume shrinkage of fibroid tumor (star) (42.5% baseline). (Reprinted with permission from Kim et al.67)
The reintervention rate for MR-HIFU after 2 years of follow-up has been reported at 17.6%.71 This is in contrast to the cumulative 5-year probability of reoperation for recurrent leiomyoma after myomectomy, which ranges from 6.7 to 9.0%.73 74 While this may suggest greater technical success with myomectomy, it should be noted that populations and methods were different between these two studies.
Neoadjuvant use of gonadotropin-releasing hormone (GnRH) analogs is an effective adjuvant therapy in the treatment of fibroids with HIFU. GnRH agonists reduce estrogen levels, resulting in leiomyoma volume reduction.75 Additionally, this reduces vascular flow, proposed to enhance thermoablative coagulative necrosis due to diminished heat conduction.76 77 78 In a 2006 study by Smart et al, a 3-month course of a GnRH agonist followed by MR-HIFU in 49 women showed improvements in both symptom control (83% at 6 months, 89% at 12 months) and volume reduction rates (21% at 6 months, 37% at 12 months).78
Several complications have been reported following the treatment of uterine fibroids with HIFU thermoablation. In a study examining 45 patients treated with US-HIFU of uterine fibroids, gross hematuria was noted in 19 (42.2%) of the patients. Presumably, this was secondary to unintended or unappreciated bladder heating.79 Skin burns in the near field may occur secondary to targeting a site too close to the skin surface, improper coupling due to the presence of an intervening scar, or improperly shaved and cleaned skin.64 69 In the focal zone, pain or cramping may occur during the procedure; however, this is typically minimal and reduced with moderate sedation. In the far field, nerve stimulation may lead to back or leg pain, thought to be due to heat absorption by the pelvic bones with secondary conduction and damage to adjacent nerves.64 One series demonstrated a 7% overall rate of leg pain persisting more than 10 days following the procedure.69
HIFU has also shown promising results in the treatment of adenomyosis. In 2006, Rabinovici and Stewart reported outcomes of nine patients with adenomyosis treated with MR-HIFU at several centers. The procedure is thought to work via hyperthermic destruction of “islands” of aberrant endometrium and through precise targeting of adenomyotic tissue.80 In this series, one patient had 40% of focal uterine adenomyosis ablated: three menstrual cycles later, she successfully conceived and had an uneventful pregnancy.80 Fukunishi et al described 20 patients treated for adenomyosis and followed up for 6 months, and found improvements in symptom severity scale while reporting no serious complications.81 Based on these early studies, MR-HIFU may be considered safe and effective for management of adenomyosis, as it permits large ablative volumes and pain relief. Further trials are needed to evaluate long-term efficacy, as some studies have demonstrated increased adenomyoma size 3 to 4 months following ablation.82
Liver
As few as 25% of patients with hepatocellular carcinoma (HCC) are amenable to surgical resection upon presentation, often due to marginal liver function reserve secondary to cirrhosis.83 HIFU has been used in the treatment of both unresectable, advanced HCC as well as liver metastases. It has been shown to be well tolerated in HCC patients with Child-Pugh A and B cirrhosis, and in select Child-Pugh C cirrhotic patients (Fig. 7).84
Fig. 7.
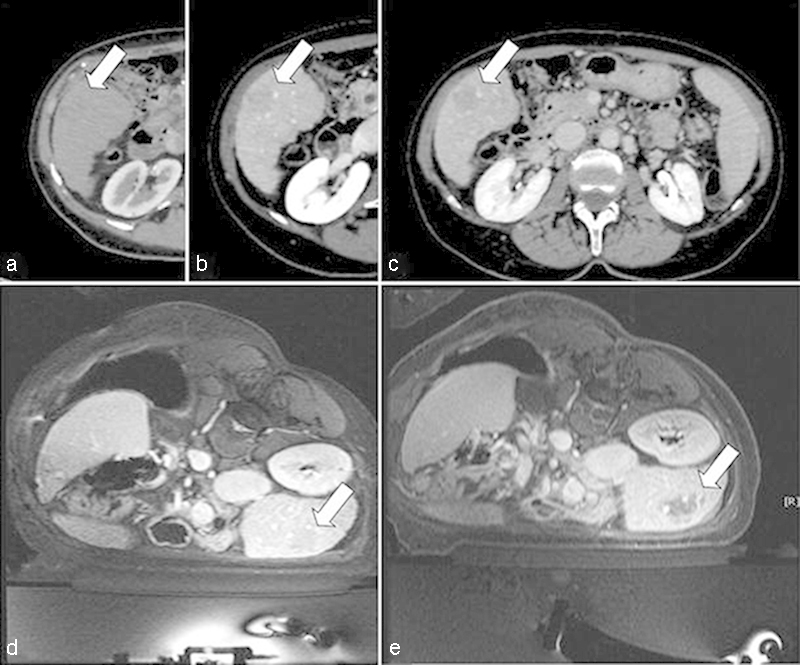
A 68-year-old woman with hypovascular HCC in the VI hepatic segment, previously resected for a single nodule at the left lobe, refused another surgery. It was proposed that she undergo MR-guided FUS treatment of an acoustically accessible lesion. Contrast-enhanced axial CT image shows a hypovascular hepatocellular carcinoma (white arrow) in the VI segment during arterial (a), portal venous (b), and late venous phase (c). The treatment was performed under general anesthesia, with the patient positioned the right lateral decubitus to reduce liver movement and to achieve wider contact between the abdominal wall and the transducer surface. Pretreatment localization of the tumor is demonstrated with contrast-enhanced acquisition (d), with posttreatment visualization of the nonperfused area (e). (Reprinted with permission from Napoli et al.50)
Obstacles to US-directed therapy include technical challenges with effective beam propagation through the ribs, long ablation times secondary to large tumor size, and respiratory motion of the liver. In particular, high acoustic reflection and attenuation caused by the ribs provides a significant barrier to US waves. This can be associated with significant reductions in energy deposition at the focal point, as well as unwanted energy deposition in the intercostal muscles and subcutaneous tissue leading to injury.85 Although rib resections have been performed in prior preclinical studies to create an acoustic window, advances in US technology have permitted less invasive techniques.28 These techniques include selective activation of individual transducer elements of a phased-array design, as well as detection of the reflected US waves of the obstructed elements directly or identification of the obstructed elements on anatomical 3D MR imaging, permitting disabling of these elements with reduced risks of nontarget thermal injury or suboptimal treatment of the target.85
Safety and short-to-intermediate term efficacy of HIFU for HCC ablation have been described.86 In patients with unresectable HCC receiving HIFU, Ng et al demonstrated 1- and 3-year survival rates of 87.7 and 62.4%, respectively.87 Chan et al evaluated the feasibility of HIFU and patient survival in 27 patients with recurrent HCC (average tumor size of 1.8 cm) after first-line therapy with either hepatectomy or radiofrequency ablation at a median follow-up of 28 months. At 1-month follow-up MRI, complete tumor ablation was obtained in 85.2% of the patients. The 1-, 2-, and 3-year overall survival rates were 96.3, 81.5, and 69.8%, respectively, and there were no in-hospital mortalities.88 In patients with large HCC (average tumor size of 8.1 cm), Wu et al evaluated the safety and efficacy of HIFU treatment in 55 patients. These authors demonstrated a complete ablation rate as high as 69.2%, no major complications, and overall survival rates of 61.5% at 12 months and 35.3% at 18 months.89
HIFU has also been used in combination with transarterial chemoembolization (TACE) in the treatment of HCC. Jin et al demonstrated complete tumor ablation in 45.2% of patients with tumor size and ablation response demonstrating significant prognostic factors in predicting therapeutic response.90 In a prospective randomized controlled trial enrolling 89 consecutive patients with Child-Pugh A or B cirrhosis and unresectable HCC larger than 5 cm in diameter with no previous treatment for HCC, TACE + HIFU demonstrated significantly greater tumor response (72.8 vs. 44.5%, p < 0.05) as well as significantly higher 1-, 2-, 3-, and 5-year overall survival rates compared with TACE alone (p < 0.01).91
As HIFU therapy and technology continue to evolve, it is possible that HIFU may serve as a bridging therapy to liver transplant for patients with HCC. While TACE has demonstrated excellent outcomes in bridging therapy, only patients with preserved liver function and asymptomatic multinodular tumors without vascular invasion or extrahepatic spread are currently eligible.92 Radiofrequency ablation is currently employed as one means of bridging patients to transplant; however, it is limited by the number of nodules that may be treated as well as the maximal tumor diameter of the nodules. Cheung et al evaluated 49 consecutive HCC patients listed for liver transplantation, 29 of which received TACE, 16 received no additional therapy, and 5 patients received HIFU. Additionally, five more patients with the same tumor staging (within the UCSF criteria) who received HIFU but were not on the actual transplant list were included for comparison. There was no difference in terms of tumor size and number between the HIFU and TACE groups. Within the HIFU group, nine patients (90%) had a complete response and one patient (10%) had a partial response to treatment. In the TACE group, only one patient (3%) had a response to the treatment, while 14 patients (48%) had stable disease and 14 patients (48%) had progressive disease. No patient in the HIFU group dropped off the transplant list, while seven patients in the TACE group dropped off (six for local progression of disease, one for development of extrahepatic metastasis).93
Clinical data suggest that HIFU is both safe and effective in treating tumors adjacent to major vessels. In a study of 39 patients (42 total lesions) with HCC, all treated tumors (average greatest dimension of 7.4 cm) had a distance between the tumor and a main blood vessel (portal vein, inferior vena cava, main hepatic veins) of less than 1 cm, and no major blood vessel injury occurred.94 Twenty-one of the 42 tumors (50%) were completely ablated, while the remainder of the tumors sustained ablation of more than 50% of their volume after one session of HIFU.
Serious complications reported in a minority of HCC patients treated with HIFU include rib fractures, pneumothorax, pleural effusion, biliary obstruction, and fistula formation.95
Pancreas
The majority of patients with pancreatic cancer are ineligible for curative surgery.96 There are, therefore, many opportunities for palliative applications of HIFU in pancreatic cancer. The majority of studies to date have involved small sample sizes and focused on safety and efficacy.
Several preliminary studies have examined HIFU treatment in patients with unresectable pancreatic cancer.97 98 99 100 These studies report promising rates of palliative pain relief in 87 to 100% of patients, and median survival ranging from 7 to 12.4 months.97 98 99 100 Follow-up studies have demonstrated tumor shrinkage, with a mean of 49% regression and a lack of blood supply to the tumor (Fig. 8).97
Fig. 8.
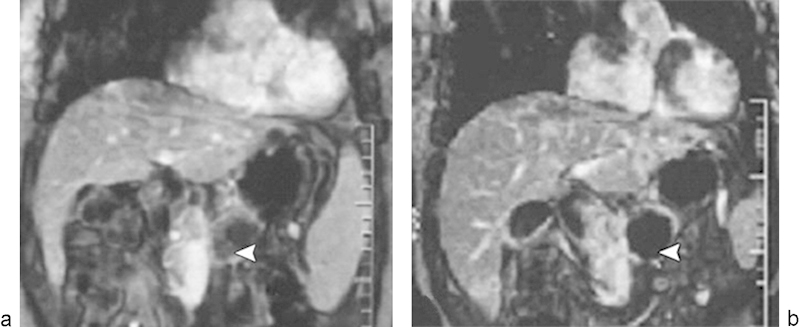
Dynamic contrast-enhanced gradient-echo T1-weighted MR images (180/6.0, 90-degree flip angle, 128 × 256 matrix, 10-mm-thick sections, 2-mm intersection gap, one signal acquired, and 18-second acquisition time) obtained with breath holding from a 48-year-old man who underwent high-intensity focused ultrasound ablation for advanced pancreatic cancer. The tumor was 4.5 × 4.5 cm in diameter and located in the body of the pancreas. (a) Image obtained before high-intensity focused ultrasound shows the blood supply in the pancreatic lesion (arrowhead). (b) Image obtained 2 weeks after high-intensity focused ultrasound shows no evidence of contrast enhancement in the treated lesion (arrowhead), which is indicative of complete coagulation necrosis in the pancreatic cancer. (Reprinted with permission from Wu et al.97)
Postprocedural mild abdominal pain has been reported in 34% and skin burns in 4% of patients.100 The largest safety and efficacy study to date examined 224 patients with advanced pancreatic cancer treated with HIFU monotherapy; this study reported abdominal distention/anorexia/nausea in 4%, vertebral injury in 1%, and obstructive jaundice in 1% of patients.101
Studies have also examined combining HIFU with chemotherapy agents. In one trial, 37 patients were treated with gemcitabine and concurrent HIFU.102 A response rate of 43.6% and pain relief in 78.6% of patients were reported; overall survival was 12.6 months.102 Another trial compared 14 patients receiving HIFU alone with 25 patients who underwent HIFU plus gemcitabine, reporting pain relief in 79% and median survival of 11 months in this patient cohort.103
Renal
Limited clinical studies to date employing HIFU in the treatment of renal tumors have focused on safety and efficacy, with results being inferior in comparison to those using existing probe-based ablative therapies. Two extracorporeal systems have been described: one produced by Storz Medical (Storz, Schaffhausen, Switzerland) and the other manufactured by Chongqing Haifu Co (Chongqing Haifu Co. Ltd., Chongqing, China).104
A 2003 study by Wu et al described their preliminary experience with the Chongqing system. Thirteen patients were treated with HIFU; partial ablation was achieved in 10 patients and complete ablation in 3 patients.105 The same device was used in another study in which four of six patients demonstrated no tumor enhancement on follow-up imaging.106
A 2006 study by Häcker et al, examining 19 patients with renal cell carcinoma undergoing HIFU prior to surgical resection, reported thermal damage in 15 of 19 kidneys.107 Interestingly, the effects did not correspond to the quantity of HIFU pulses implemented.107 A 2008 phase 2 trial assessing the Storz system (Storz, Schaffhausen, Switzerland) evaluated the treatment of 14 tumors with HIFU prior to resection and demonstrated sufficient coagulative necrosis in only 9 of 14 kidneys, with residual tumor in all 14 cases.108 Grade 3 skin burns were described by Häcker et al in 2 of 19 patients, and in another case, accidental thermal injury to the small intestine was observed.107
Studies examining the use of laparoscopic probes have reported comparable findings. A 2008 study by Klingler et al examined seven renal tumors treated with a 4.0-MHz laparoscopic HIFU probe prior to nephrectomy.109 Four of the seven tumors demonstrated 100% ablation.109 A 2011 study by Ritchie et al examined 12 patients with renal tumors treated with a new laparoscopic probe. No evidence of ablation was seen in the first five tumors, but in the following seven patients, ablation was apparent, suggesting a learning curve inherent to the procedure.110
Breast
Given its superficial location and ability to be readily immobilized for targeted therapy, the breast reflects an amenable target for HIFU treatment. One of the earliest studies to explore HIFU treatment for breast cancer was performed in 2001 by Hynynen et al. The authors examined MR-HIFU under local anesthesia for the treatment of 11 fibroadenomas in 9 patients.111 Of the eleven lesions, eight (72%) were partially to nearly completely treated (Fig. 9).
Fig. 9.
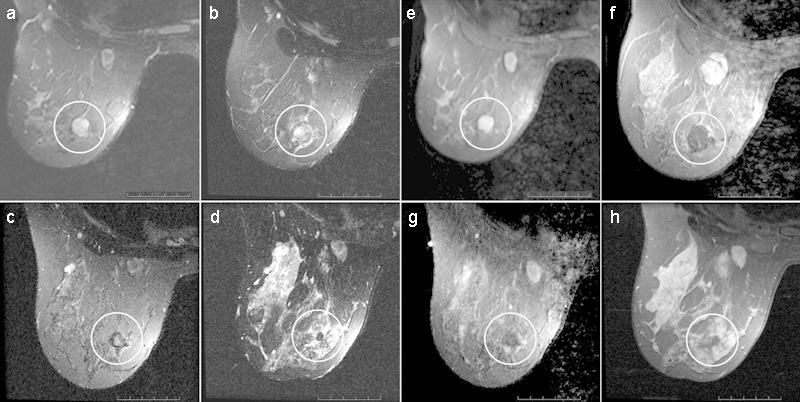
MR images show complete response at long-term follow-up of a breast fibroadenoma (circled area) treated with MR imaging-guided FUS (a–d), T2-weighted fat-suppressed fast SE images (a–c: 2,500/100; d: 3,850/100) and (e–h), T1-weighted fat-suppressed postcontrast images (e, f: 600/12; g: 400/12; h: 517/12) obtained 2 months before therapy and at 7 days, 6 months, and 3 years after therapy, respectively. (Reprinted with permission from Hynynen et al.111)
In 2003, Wu et al performed another early feasibility study in which 48 women with breast cancer were randomized to receive either modified radical mastectomy or HIFU followed by modified radical mastectomy. No severe adverse effects were reported in the HIFU group, and the HIFU-treated tissue showed evidence of coagulative necrosis.112
A 2006 study by Furusawa examined 30 women who underwent MR-HIFU for breast cancer followed by wide excision or mastectomy.113 Tumors showed an average of 96% necrosis of tumor volume postablation.113 This study was followed up by a 2007 study in which 21 patients with invasive/noninvasive ductal carcinoma, with a median diameter of 15 mm, underwent HIFU ablation. Seventeen patients underwent a single course of treatment while 4 patients underwent two courses. The authors reported only one case of recurrence during 14 months of follow-up.114 In a 2003 study by Gianfelice et al, 12 patients with invasive breast cancer were treated with MR-HIFU prior to surgery, with a wide range of percentage necrosis observed histopathologically in treated lesions (46–88%).115
Napoli et al reported results on 10 patients with an average of 48 sonications required to cover each lesion and an average treatment time of 2 hours 20 minutes. Nine out of ten patients demonstrated no residual enhancement of the ablated lesions. All patients underwent routine breast-conserving surgery within 21 days, and surgical pathology demonstrated an absence of residual cancer in nine out of ten patients with a margin of at least 5 mm of normal breast tissue around the necrotic area.50
Challenges to the implementation of HIFU therapy for breast cancer include favorable local cure and overall survival rates, particularly for early-stage disease, with standard of care lumpectomy and radiation. When diagnosed at a localized stage without spread to lymph nodes, nearby structures, or other locations outside the breast, the 5-year survival rate is >95%.44
HIFU may have utility in treating women who do not desire surgery or who are nonsurgical candidates. Wu et al studied long-term clinical results in a group of 22 patients with biopsy-proven breast cancer refusing surgical resection (4 patients with stage 1, 9 patients with stage 2a, 8 patients with stage 3b, and 1 patient with stage 4). All 22 patients underwent chemotherapy and radiotherapy after MR-HIFU. Tumors disappeared on contrast-enhanced MRI in 8 of the patients (36.4%) and regressed in 14 (63.6%). Local recurrence was noted in only two patients (9.2%) (at 18 and 22 months after treatment). Five-year disease-free survival and recurrence-free survival rates were 95 and 85%, respectively.116
Few complications with HIFU therapy in this setting have yet to be reported, although clinical experience remains limited to date. Hynynen et al described a single patient experiencing transient edema111; however, Wu et al reported no severe adverse effects.112
Spleen
The use of HIFU for splenic pathology has an emerging, recently described role. A preliminary study in nine patients evaluated the use of HIFU to treat HCC complicated by hypersplenism. The authors found a mean percent splenic ablation volume of 28.8% ± 6.1%.117
Novel HIFU Bioeffects
Nonthermal HIFU bioeffects are currently being explored in the preclinical realm, with an emphasis on leveraging such therapeutic effects to the care of the oncology patient.
Acoustic Cavitation
Acoustic cavitation results in the formation and the activity of a gas or vapor-filled cavity within biological tissue. When US wave intensity exceeds a specific threshold, the resultant negative pressure due to rarefaction may be large enough to extract gas from the tissue, leading to bubble formation. Stable cavitation results when this bubble undergoes repeat radial oscillations to a resonant size at the insonated frequency. Unstable cavitation occurs when a bubble oscillates but expands gradually above its resonant size secondary to net influxes of vapor in the bubble, ultimately undergoing violent and asymmetrical collapse and disintegration that has been associated with cell death and tissue damage.8 118
Given that the thermal effects by US absorption are linearly proportional to the sonic intensity, this effect is easier to predict and control and has therefore been traditionally employed. While the addition of mechanical cavitation is less predictable and may be associated with a higher rate of complications, it can increase the overall tissue volume ablated, thereby effectively increasing ablation efficiency.119
In HIFU, intravenous microbubble agent injection may enhance the effects of many different therapeutic responses when acoustic cavitation is known to be involved. Essentially, the microbubbles serve to act as cavitation nuclei, lowering the threshold for acoustic cavitation. In in vivo animal models, microbubble administration delivered concomitantly with HIFU thermoablation has demonstrated further enhancement of the tissue temperature with shortening of the sonication time required for treating the same sized tumors.120 121 122
Microstreaming
Microstreaming—a phenomenon that results when fluid movement leads to the production of shear forces causing cell membrane disruption—has also been associated with cell damage in in vitro and animal studies. Radiation forces cause additional destructive bioeffects including cell membrane deformation, microstreaming, and organelle rotation.123
Hyperthermia
While coagulation necrosis typically occurs at 56°C with an exposure length of 1 second, hyperthermic temperatures of approximately 40 to 45°C for 1 hour may render the tissue more susceptible to chemotherapy and radiation7 8 9 (Fig. 10). MR-HIFU can noninvasively heat solid tumors with resulting low temperature-sensitive liposomes, releasing their drug cargo in response to such temperatures and improving drug delivery to solid tumors.
Fig. 10.
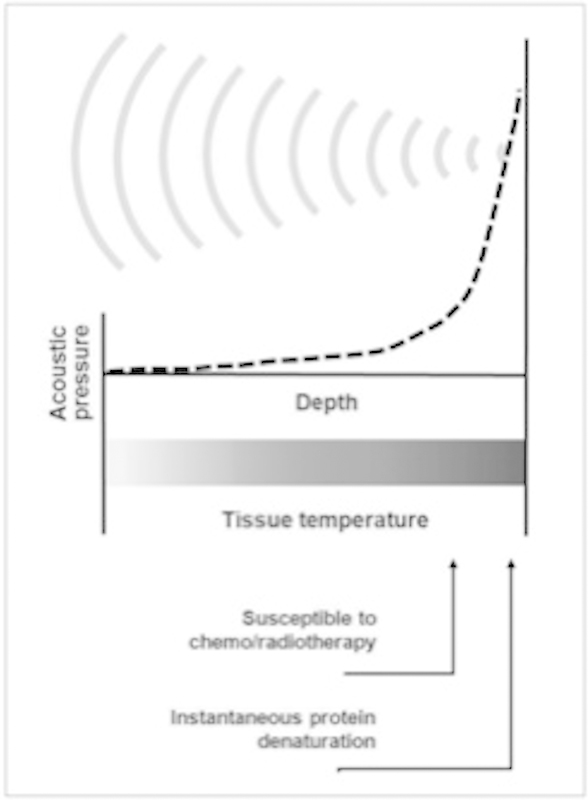
Basic concept of HIFU-induced tissue change by hyperthermia. As US waves are focused onto small spot, acoustic pressure is rapidly elevated near focus where tissue temperatures are also raised to level that is sufficient for thermotherapeutic effects, resulting in coagulation necrosis. (Reprinted with permission from Kim YS, Rhim H, Choi MJ, Lim HK, Choi D. High-intensity focused ultrasound therapy: an overview for radiologists. Korean J Radiol 2008;9(4):291–302.)
Partanen et al described a binary control algorithm for real-time mild hyperthermia feedback control, and found accurate and homogenous heating within the targeted region in vitro and in vivo. This makes this technique suitable for applications in drug delivery.124 Several studies have demonstrated that concurrent chemotherapy and HIFU is safe and feasible as an oncologic treatment modality in the preclinical setting, with future clinical investigation needed.102 103 125
Mechanical Histotripsy
Histotripsy utilizes US to produce mechanical lesions. The mechanical effect of highly vibrating microbubbles created at a focal region of histotripsy immediately ablates tissue by liquefaction. Histotripsy offers rapid tissue debulking, a nonthermal mode of tissue injury, and highly accurate tissue ablation.
There have recently been several preclinical studies assessing the use of histotripsy to treat benign prostatic hypertrophy, liver tumors, and kidney tumors. Khokhlova et al described an approach they referred to as “boiling histotripsy,” in which a millimeter-sized bubble has been shown to rapidly fractionate liver tissue in a porcine model.126 This rapid, focal tissue destruction may allow for treatment of lesions adjacent to vital structures.
Immune System Modulation
It has been suggested that HIFU may produce an antitumor immune response.127 In a study of 15 patients undergoing HIFU for solid malignancies, serum levels of immunosuppressive cytokines decreased after HIFU.128 The authors of this study proposed that this may be a secondary benefit of HIFU, which may restore antitumor immunity.128
Breast tumors treated with HIFU have been found to have reduced expression of PCNA, MMP-9, and CD44v6. This may correlate with a decreased ability of a malignancy to proliferate, invade, and metastasize.112 In a related study, CD3, CD4, CD8, CD4/CD8, B lymphocytes, and natural killer cells were able to better infiltrate HIFU-ablated regions.129 Ongoing investigation is underway to evaluate the mechanisms of HIFU-related tumor immune modulation, to determine how these may be harnessed for antitumor therapy.
Targeted Drug/Gene Delivery
Studies have suggested that HIFU may allow disruption of the blood–brain barrier to enhance drug delivery.130 Microbubbles serve as vehicles for drug or plasmid DNA delivery, either in an encapsulated or an attached form. Microbubbles pass through vessels, and US is used to selectively rupture the microbubbles for release of the drugs or genes. Additionally, the microbubbles act as nuclei, promoting acoustic cavitation. Violent microstreaming from the rupture of the microbubbles enhances the uptake of drugs/genes into the cells by sonoporation (transient alteration of cell membrane structures due to the mechanical force of US) (Fig. 11).
Fig. 11.
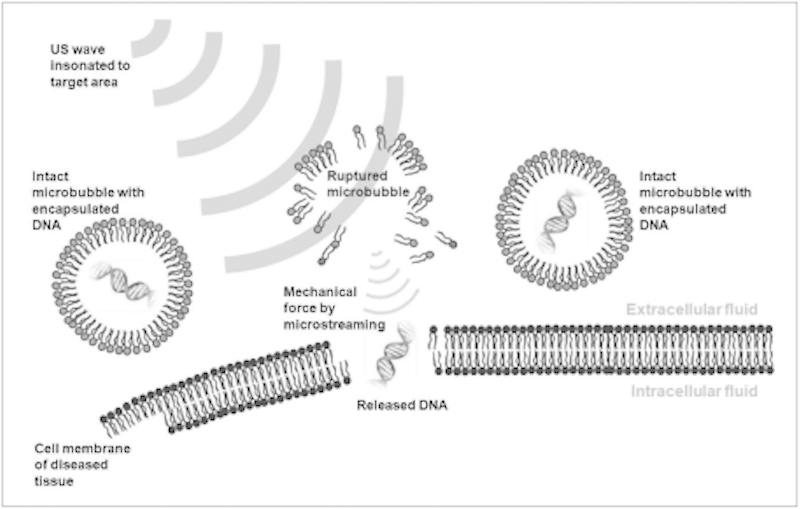
Schematic drawing of US-induced gene therapy. When plasmid DNA-containing microbubbles are passed through blood vessels adjacent to diseased cells, insonated US waves rupture microbubbles and release plasmid DNA. Released DNA penetrates into cell through membranes by means of sonoporation. (Reprinted with permission from Kim YS, Rhim H, Choi MJ, Lim HK, Choi D. High-intensity focused ultrasound therapy: an overview for radiologists. Korean J Radiol 2008;9(4):291–302.)
A key advantage of US-enhanced gene therapy is that it targets delivery only to the diseased area, and can thereby increase the concentration of therapeutic agents at a focused area with a lower probability of systemic complications. Staruch et al reported that thermosensitive liposomes may be used for targeted drug delivery and doxorubicin release in rabbit models.131 132
Conclusion
HIFU therapy has demonstrated clinical success in the treatment of both benign and malignant tumors. With the advent of MR guidance and the continued advancements in intraprocedural ablation monitoring, tumor control may be improved while adverse events are minimized. Additionally, HIFU therapy appears to be promising in the investigational setting in combination with other therapies including chemotherapy and radiation, and in the preclinical setting enhanced targeted drug/gene delivery and immune system modulation. While still in its early stages of development compared with other probe-based ablative therapies, the multifaceted therapeutic capabilities of HIFU present great potential in the field of image-guided therapy.
Acknowledgments
The authors wish to thank Megan Griffiths for her assistance in organizing the references for this article.
References
- 1.Lynn J G, Zwemer R L, Chick A J, Miller A E. A new method for the generation and use of focused ultrasound in experimental biology. J Gen Physiol. 1942;26(2):179–193. doi: 10.1085/jgp.26.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood R W, Loomis A L. The physical and biological effects of high frequency sound waves of great intensity. Philos Mag and J of Science. 1927;4(22):417–436. [Google Scholar]
- 3.Fry W J, Barnard J W, Fry F J, Brennan J F. Ultrasonically produced localized selective lesions in the central nervous system. Am J Phys Med. 1955;34(3):413–423. [PubMed] [Google Scholar]
- 4.Susani M, Madersbacher S, Kratzik C, Vingers L, Marberger M. Morphology of tissue destruction induced by focused ultrasound. Eur Urol. 1993;23 01:34–38. doi: 10.1159/000474677. [DOI] [PubMed] [Google Scholar]
- 5.Madersbacher S, Pedevilla M, Vingers L, Susani M, Marberger M. Effect of high-intensity focused ultrasound on human prostate cancer in vivo. Cancer Res. 1995;55(15):3346–3351. [PubMed] [Google Scholar]
- 6.ter Harr G R. West Sussex: John Wiley & Sons; 2004. Therapeutic and surgical applications; pp. 407–456. [Google Scholar]
- 7.Kennedy J E, Ter Haar G R, Cranston D. High intensity focused ultrasound: surgery of the future? Br J Radiol. 2003;76(909):590–599. doi: 10.1259/bjr/17150274. [DOI] [PubMed] [Google Scholar]
- 8.ter Haar G R. West Sussex: John Wiley & Sons; 2004. Ultrasonic biophysics; pp. 348–406. [Google Scholar]
- 9.ter Haar G. Therapeutic applications of ultrasound. Prog Biophys Mol Biol. 2007;93(1–3):111–129. doi: 10.1016/j.pbiomolbio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Crocker M J. New York: John Wiley & Sons; 1997. Encyclopedia of Acoustics. 1st ed. [Google Scholar]
- 11.ter Haar G R. High intensity focused ultrasound for the treatment of tumors. Echocardiography. 2001;18(4):317–322. doi: 10.1046/j.1540-8175.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 12.Tempany C M, McDannold N J, Hynynen K, Jolesz F A. Focused ultrasound surgery in oncology: overview and principles. Radiology. 2011;259(1):39–56. doi: 10.1148/radiol.11100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubinsky T J, Cuevas C, Dighe M K, Kolokythas O, Hwang J H. High-intensity focused ultrasound: current potential and oncologic applications. AJR Am J Roentgenol. 2008;190(1):191–199. doi: 10.2214/AJR.07.2671. [DOI] [PubMed] [Google Scholar]
- 14.Gavrilov L R, Hand J W. Two-dimensional phased arrays for surgery: movement of a single focus. Acoust Phys. 2000;46:390–399. [Google Scholar]
- 15.Daum D R, Hynynen K. A 256-element ultrasonic phased array system for the treatment of large volumes of deep seated tissue. IEEE Trans Ultrason Ferroelectr Freq Control. 1999;46(5):1254–1268. doi: 10.1109/58.796130. [DOI] [PubMed] [Google Scholar]
- 16.McKenna E C More Sites and Patients Join Clinical Trial Assessing New MR-Guided FUS System for Prostate Cancer Focused Ultrasound Foundation; 2011
- 17.Vaezy S, Shi X, Martin R W. et al. Real-time visualization of high-intensity focused ultrasound treatment using ultrasound imaging. Ultrasound Med Biol. 2001;27(1):33–42. doi: 10.1016/s0301-5629(00)00279-9. [DOI] [PubMed] [Google Scholar]
- 18.Meaney P M, Zhou T, Fanning M W, Geimer S D, Paulsen K D. Microwave thermal imaging of scanned focused ultrasound heating: phantom results. Int J Hyperthermia. 2008;24(7):523–536. doi: 10.1080/02656730801944922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebbini E S, Yao H, Shrestha A. Dual-mode ultrasound phased arrays for image-guided surgery. Ultrason Imaging. 2006;28(2):65–82. doi: 10.1177/016173460602800201. [DOI] [PubMed] [Google Scholar]
- 20.Straube W L, Arthur R M. Theoretical estimation of the temperature dependence of backscattered ultrasonic power for noninvasive thermometry. Ultrasound Med Biol. 1994;20(9):915–922. doi: 10.1016/0301-5629(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 21.Jensen C R, Ritchie R W, Gyöngy M, Collin J R, Leslie T, Coussios C C. Spatiotemporal monitoring of high-intensity focused ultrasound therapy with passive acoustic mapping. Radiology. 2012;262(1):252–261. doi: 10.1148/radiol.11110670. [DOI] [PubMed] [Google Scholar]
- 22.Jolesz F A, Hynynen K. Magnetic resonance image-guided focused ultrasound surgery. Cancer J. 2002;8 01:S100–S112. [PubMed] [Google Scholar]
- 23.Rieke V, Butts Pauly K. MR thermometry. J Magn Reson Imaging. 2008;27(2):376–390. doi: 10.1002/jmri.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hindman J C. Proton resonance shift of water in the gas and liquid states. J Chem Phys. 1966;44:4582–4592. [Google Scholar]
- 25.Hynynen K, McDannold N. Singapore: World Scientific Publishing; 2006. MRI-guided focused ultrasound for local tissue ablation and other image-guided interventions; pp. 167–218. [Google Scholar]
- 26.Kaye E A, Chen J, Pauly K B. Rapid MR-ARFI method for focal spot localization during focused ultrasound therapy. Magn Reson Med. 2011;65(3):738–743. doi: 10.1002/mrm.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Todd N, Diakite M, Payne A, Parker D L. In vivo evaluation of multi-echo hybrid PRF/T1 approach for temperature monitoring during breast MR-guided focused ultrasound surgery treatments. Magn Reson Med. 2014;72(3):793–799. doi: 10.1002/mrm.24976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu F, Wang Z B, Chen W Z. et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason Sonochem. 2004;11(3–4):149–154. doi: 10.1016/j.ultsonch.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Shehata I A. Treatment with high intensity focused ultrasound: secrets revealed. Eur J Radiol. 2012;81(3):534–541. doi: 10.1016/j.ejrad.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 30.Gardner T A, Koch M O. Prostate cancer therapy with high-intensity focused ultrasound. Clin Genitourin Cancer. 2005;4(3):187–192. doi: 10.3816/CGC.2005.n.031. [DOI] [PubMed] [Google Scholar]
- 31.Tempany C M, Stewart E A, McDannold N, Quade B J, Jolesz F A, Hynynen K. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology. 2003;226(3):897–905. doi: 10.1148/radiol.2271020395. [DOI] [PubMed] [Google Scholar]
- 32.McDannold N, Tempany C M, Fennessy F M. et al. Uterine leiomyomas: MR imaging-based thermometry and thermal dosimetry during focused ultrasound thermal ablation. Radiology. 2006;240(1):263–272. doi: 10.1148/radiol.2401050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enholm J K, Köhler M O, Quesson B, Mougenot C, Moonen C T, Sokka S D. Improved volumetric MR-HIFU ablation by robust binary feedback control. IEEE Trans Biomed Eng. 2010;57(1):103–113. doi: 10.1109/TBME.2009.2034636. [DOI] [PubMed] [Google Scholar]
- 34.Daum D R, Hynynen K. Thermal dose optimization via temporal switching in ultrasound surgery. IEEE Trans Ultrason Ferroelectr Freq Control. 1998;45(1):208–215. doi: 10.1109/58.646926. [DOI] [PubMed] [Google Scholar]
- 35.Fan X, Hynynen K. Control of the necrosed tissue volume during noninvasive ultrasound surgery using a 16-element phased array. Med Phys. 1995;22(3):297–306. doi: 10.1118/1.597603. [DOI] [PubMed] [Google Scholar]
- 36.Liu H L, Lin W L, Chen Y Y. A fast and conformal heating scheme for producing large thermal lesions using a 2D ultrasound phased array. Int . J Hyperthermia. 2007;23(1):69–82. doi: 10.1080/02656730601087518. [DOI] [PubMed] [Google Scholar]
- 37.McDannold N J, King R L, Jolesz F A, Hynynen K H. Usefulness of MR imaging-derived thermometry and dosimetry in determining the threshold for tissue damage induced by thermal surgery in rabbits. Radiology. 2000;216(2):517–523. doi: 10.1148/radiology.216.2.r00au42517. [DOI] [PubMed] [Google Scholar]
- 38.Mougenot C, Quesson B, de Senneville B D. et al. Three-dimensional spatial and temporal temperature control with MR thermometry-guided focused ultrasound (MRgHIFU) Magn Reson Med. 2009;61(3):603–614. doi: 10.1002/mrm.21887. [DOI] [PubMed] [Google Scholar]
- 39.Mougenot C, Salomir R, Palussière J, Grenier N, Moonen C T. Automatic spatial and temporal temperature control for MR-guided focused ultrasound using fast 3D MR thermometry and multispiral trajectory of the focal point. Magn Reson Med. 2004;52(5):1005–1015. doi: 10.1002/mrm.20280. [DOI] [PubMed] [Google Scholar]
- 40.Arora D, Minor M A, Skliar M, Roemer R B. Control of thermal therapies with moving power deposition field. Phys Med Biol. 2006;51(5):1201–1219. doi: 10.1088/0031-9155/51/5/011. [DOI] [PubMed] [Google Scholar]
- 41.Vanne A, Hynynen K. MRI feedback temperature control for focused ultrasound surgery. Phys Med Biol. 2003;48(1):31–43. doi: 10.1088/0031-9155/48/1/303. [DOI] [PubMed] [Google Scholar]
- 42.Köhler M O, Mougenot C, Quesson B. et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys. 2009;36(8):3521–3535. doi: 10.1118/1.3152112. [DOI] [PubMed] [Google Scholar]
- 43.Venkatesan A M, Partanen A, Pulanic T K. et al. Magnetic resonance imaging-guided volumetric ablation of symptomatic leiomyomata: correlation of imaging with histology. J Vasc Interv Radiol. 2012;23(6):786–7.94E6. doi: 10.1016/j.jvir.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American Cancer Society . Atlanta, GA: American Cancer Society; 2015. Cancer Facts and Figures 2015. [Google Scholar]
- 45.Epstein J I, Walsh P C, Carmichael M, Brendler C B. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271(5):368–374. [PubMed] [Google Scholar]
- 46.Carter H B, Sauvageot J, Walsh P C, Epstein J I. Prospective evaluation of men with stage T1C adenocarcinoma of the prostate. J Urol. 1997;157(6):2206–2209. [PMC free article] [PubMed] [Google Scholar]
- 47.Miller D C, Sanda M G, Dunn R L. et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772–2780. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- 48.Blana A Rogenhofer S Ganzer R et al. Eight years' experience with high-intensity focused ultrasonography for treatment of localized prostate cancer Urology 20087261329–1333., discussion 1333–1334 [DOI] [PubMed] [Google Scholar]
- 49.Ahmed H U, Hindley R G, Dickinson L. et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol. 2012;13(6):622–632. doi: 10.1016/S1470-2045(12)70121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Napoli A, Anzidei M, Ciolina F. et al. MR-guided high-intensity focused ultrasound: current status of an emerging technology. Cardiovasc Intervent Radiol. 2013;36(5):1190–1203. doi: 10.1007/s00270-013-0592-4. [DOI] [PubMed] [Google Scholar]
- 51.Zacharakis E, Ahmed H U, Ishaq A. et al. The feasibility and safety of high-intensity focused ultrasound as salvage therapy for recurrent prostate cancer following external beam radiotherapy. BJU Int. 2008;102(7):786–792. doi: 10.1111/j.1464-410X.2008.07775.x. [DOI] [PubMed] [Google Scholar]
- 52.Uchida T, Shoji S, Nakano M. et al. High-intensity focused ultrasound as salvage therapy for patients with recurrent prostate cancer after external beam radiation, brachytherapy or proton therapy. BJU Int. 2011;107(3):378–382. doi: 10.1111/j.1464-410X.2010.09518.x. [DOI] [PubMed] [Google Scholar]
- 53.Han M, Partin A W, Zahurak M, Piantadosi S, Epstein J I, Walsh P C. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169(2):517–523. doi: 10.1097/01.ju.0000045749.90353.c7. [DOI] [PubMed] [Google Scholar]
- 54.Hayashi M, Shinmei S, Asano K. Transrectal high-intensity focused ultrasound for treatment for patients with biochemical failure after radical prostatectomy. Int J Urol. 2007;14(11):1048–1050. doi: 10.1111/j.1442-2042.2007.01880.x. [DOI] [PubMed] [Google Scholar]
- 55.Murota-Kawano A, Nakano M, Hongo S, Shoji S, Nagata Y, Uchida T. Salvage high-intensity focused ultrasound for biopsy-confirmed local recurrence of prostate cancer after radical prostatectomy. BJU Int. 2010;105(12):1642–1645. doi: 10.1111/j.1464-410X.2009.08990.x. [DOI] [PubMed] [Google Scholar]
- 56.Tsakiris P, Thüroff S, de la Rosette J, Chaussy C. Transrectal high-intensity focused ultrasound devices: a critical appraisal of the available evidence. J Endourol. 2008;22(2):221–229. doi: 10.1089/end.2007.9849. [DOI] [PubMed] [Google Scholar]
- 57.Lee H M, Hong J H, Choi H Y. High-intensity focused ultrasound therapy for clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 2006;9(4):439–443. doi: 10.1038/sj.pcan.4500901. [DOI] [PubMed] [Google Scholar]
- 58.Blana A, Walter B, Rogenhofer S, Wieland W F. High-intensity focused ultrasound for the treatment of localized prostate cancer: 5-year experience. Urology. 2004;63(2):297–300. doi: 10.1016/j.urology.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 59.Vallancien G Prapotnich D Cathelineau X Baumert H Rozet F Transrectal focused ultrasound combined with transurethral resection of the prostate for the treatment of localized prostate cancer: feasibility study J Urol 2004171(6, Pt 1):2265–2267. [DOI] [PubMed] [Google Scholar]
- 60.Baird D D, Dunson D B, Hill M C, Cousins D, Schectman J M. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 61.Borah B J, Nicholson W K, Bradley L, Stewart E A. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209(4):3190–3.19E22. doi: 10.1016/j.ajog.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harding G, Coyne K S, Thompson C L, Spies J B. The responsiveness of the uterine fibroid symptom and health-related quality of life questionnaire (UFS-QOL) Health Qual Life Outcomes. 2008;6:99. doi: 10.1186/1477-7525-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dobrotwir A, Pun E. Clinical 24 month experience of the first MRgFUS unit for treatment of uterine fibroids in Australia. J Med Imaging Radiat Oncol. 2012;56(4):409–416. doi: 10.1111/j.1754-9485.2012.02376.x. [DOI] [PubMed] [Google Scholar]
- 64.Coakley F V, Foster B R, Farsad K. et al. Pelvic applications of MR-guided high intensity focused ultrasound. Abdom Imaging. 2013;38(5):1120–1129. doi: 10.1007/s00261-013-9999-2. [DOI] [PubMed] [Google Scholar]
- 65.Rabinovici J David M Fukunishi H Morita Y Gostout B S Stewart E A; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids Fertil Steril 2010931199–209. [DOI] [PubMed] [Google Scholar]
- 66.Behera M A, Leong M, Johnson L, Brown H. Eligibility and accessibility of magnetic resonance-guided focused ultrasound (MRgFUS) for the treatment of uterine leiomyomas. Fertil Steril. 2010;94(5):1864–1868. doi: 10.1016/j.fertnstert.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 67.Kim Y S, Kim J H, Rhim H. et al. Volumetric MR-guided high-intensity focused ultrasound ablation with a one-layer strategy to treat large uterine fibroids: initial clinical outcomes. Radiology. 2012;263(2):600–609. doi: 10.1148/radiol.12111707. [DOI] [PubMed] [Google Scholar]
- 68.Lénárd Z M, McDannold N J, Fennessy F M. et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery—imaging predictors of success. Radiology. 2008;249(1):187–194. doi: 10.1148/radiol.2491071600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stewart E A, Rabinovici J, Tempany C M. et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril. 2006;85(1):22–29. doi: 10.1016/j.fertnstert.2005.04.072. [DOI] [PubMed] [Google Scholar]
- 70.Stewart E A Gostout B Rabinovici J Kim H S Regan L Tempany C M Sustained relief of leiomyoma symptoms by using focused ultrasound surgery Obstet Gynecol 2007110(2, Pt 1):279–287. [DOI] [PubMed] [Google Scholar]
- 71.Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol. 2009;34(5):584–589. doi: 10.1002/uog.7455. [DOI] [PubMed] [Google Scholar]
- 72.Hindley J, Gedroyc W M, Regan L. et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. AJR Am J Roentgenol. 2004;183(6):1713–1719. doi: 10.2214/ajr.183.6.01831713. [DOI] [PubMed] [Google Scholar]
- 73.Yoo E H, Lee P I, Huh C Y. et al. Predictors of leiomyoma recurrence after laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(6):690–697. doi: 10.1016/j.jmig.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 74.Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol. 2005;105(4):877–881. doi: 10.1097/01.AOG.0000156298.74317.62. [DOI] [PubMed] [Google Scholar]
- 75.Lethaby A, Vollenhoven B, Sowter M. Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. BJOG. 2002;109(10):1097–1108. doi: 10.1111/j.1471-0528.2002.01225.x. [DOI] [PubMed] [Google Scholar]
- 76.Weeks A D, Wilkinson N, Arora D S, Duffy S R, Wells M, Walker J J. Menopausal changes in the myometrium: an investigation using a GnRH agonist model. Int J Gynecol Pathol. 1999;18(3):226–232. doi: 10.1097/00004347-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 77.Rutgers J L, Spong C Y, Sinow R, Heiner J. Leuprolide acetate treatment and myoma arterial size. Obstet Gynecol. 1995;86(3):386–388. doi: 10.1016/0029-7844(95)00191-S. [DOI] [PubMed] [Google Scholar]
- 78.Smart O C, Hindley J T, Regan L, Gedroyc W G. Gonadotrophin-releasing hormone and magnetic-resonance-guided ultrasound surgery for uterine leiomyomata. Obstet Gynecol. 2006;108(1):49–54. doi: 10.1097/01.AOG.0000222381.94325.4f. [DOI] [PubMed] [Google Scholar]
- 79.Ren X L, Zhou X D, Yan R L. et al. Sonographically guided extracorporeal ablation of uterine fibroids with high-intensity focused ultrasound: midterm results. J Ultrasound Med. 2009;28(1):100–103. doi: 10.7863/jum.2009.28.1.100. [DOI] [PubMed] [Google Scholar]
- 80.Rabinovici J, Stewart E A. New interventional techniques for adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20(4):617–636. doi: 10.1016/j.bpobgyn.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Fukunishi H, Funaki K, Sawada K, Yamaguchi K, Maeda T, Kaji Y. Early results of magnetic resonance-guided focused ultrasound surgery of adenomyosis: analysis of 20 cases. J Minim Invasive Gynecol. 2008;15(5):571–579. doi: 10.1016/j.jmig.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 82.Dong X, Yang Z. High-intensity focused ultrasound ablation of uterine localized adenomyosis. Curr Opin Obstet Gynecol. 2010;22(4):326–330. doi: 10.1097/GCO.0b013e32833bea2e. [DOI] [PubMed] [Google Scholar]
- 83.Fan S T. Surgical therapy of hepatocellular carcinoma in the cirrhotic liver. Swiss Surg. 1999;5(3):107–110. doi: 10.1024/1023-9332.5.3.107. [DOI] [PubMed] [Google Scholar]
- 84.Cheung T T, Chu F S, Jenkins C R. et al. Tolerance of high-intensity focused ultrasound ablation in patients with hepatocellular carcinoma. World J Surg. 2012;36(10):2420–2427. doi: 10.1007/s00268-012-1660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quesson B, Merle M, Köhler M O. et al. A method for MRI guidance of intercostal high intensity focused ultrasound ablation in the liver. Med Phys. 2010;37(6):2533–2540. doi: 10.1118/1.3413996. [DOI] [PubMed] [Google Scholar]
- 86.Xu G, Luo G, He L. et al. Follow-up of high-intensity focused ultrasound treatment for patients with hepatocellular carcinoma. Ultrasound Med Biol. 2011;37(12):1993–1999. doi: 10.1016/j.ultrasmedbio.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 87.Ng K K, Poon R T, Chan S C. et al. High-intensity focused ultrasound for hepatocellular carcinoma: a single-center experience. Ann Surg. 2011;253(5):981–987. doi: 10.1097/SLA.0b013e3182128a8b. [DOI] [PubMed] [Google Scholar]
- 88.Chan A C, Cheung T T, Fan S T. et al. Survival analysis of high-intensity focused ultrasound therapy versus radiofrequency ablation in the treatment of recurrent hepatocellular carcinoma. Ann Surg. 2013;257(4):686–692. doi: 10.1097/SLA.0b013e3182822c02. [DOI] [PubMed] [Google Scholar]
- 89.Wu F, Wang Z B, Chen W Z. et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of patients with large hepatocellular carcinoma. Ann Surg Oncol. 2004;11(12):1061–1069. doi: 10.1245/ASO.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 90.Jin C B, Wu F, Wang Z B, Chen W Z, Zhu H. High intensity focused ultrasound therapy combined with transcatheter arterial chemoembolization for advanced hepatocellular carcinoma [in Chinese] Zhonghua Zhong Liu Za Zhi. 2003;25(4):401–403. [PubMed] [Google Scholar]
- 91.Li C, Zhang W, Zhang R, Zhang L, Wu P, Zhang F. Therapeutic effects and prognostic factors in high-intensity focused ultrasound combined with chemoembolisation for larger hepatocellular carcinoma. Eur J Cancer. 2010;46(13):2513–2521. doi: 10.1016/j.ejca.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 92.Vogl T J, Naguib N N, Nour-Eldin N E. et al. Review on transarterial chemoembolization in hepatocellular carcinoma: palliative, combined, neoadjuvant, bridging, and symptomatic indications. Eur J Radiol. 2009;72(3):505–516. doi: 10.1016/j.ejrad.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 93.Cheung T T, Fan S T, Chan S C. et al. High-intensity focused ultrasound ablation: an effective bridging therapy for hepatocellular carcinoma patients. World J Gastroenterol. 2013;19(20):3083–3089. doi: 10.3748/wjg.v19.i20.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang L, Zhu H, Jin C. et al. High-intensity focused ultrasound (HIFU): effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur Radiol. 2009;19(2):437–445. doi: 10.1007/s00330-008-1137-0. [DOI] [PubMed] [Google Scholar]
- 95.Jung S E, Cho S H, Jang J H, Han J Y. High-intensity focused ultrasound ablation in hepatic and pancreatic cancer: complications. Abdom Imaging. 2011;36(2):185–195. doi: 10.1007/s00261-010-9628-2. [DOI] [PubMed] [Google Scholar]
- 96.Siegel R, DeSantis C, Virgo K. et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 97.Wu F, Wang Z B, Zhu H. et al. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology. 2005;236(3):1034–1040. doi: 10.1148/radiol.2362041105. [DOI] [PubMed] [Google Scholar]
- 98.Orsi F, Zhang L, Arnone P. et al. High-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locations. AJR Am J Roentgenol. 2010;195(3):W245-52. doi: 10.2214/AJR.09.3321. [DOI] [PubMed] [Google Scholar]
- 99.Wang K, Chen Z, Meng Z. et al. Analgesic effect of high intensity focused ultrasound therapy for unresectable pancreatic cancer. Int J Hyperthermia. 2011;27(2):101–107. doi: 10.3109/02656736.2010.525588. [DOI] [PubMed] [Google Scholar]
- 100.Sung H Y, Jung S E, Cho S H. et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas. 2011;40(7):1080–1086. doi: 10.1097/MPA.0b013e31821fde24. [DOI] [PubMed] [Google Scholar]
- 101.Wang K, Zhu H, Meng Z. et al. Safety evaluation of high-intensity focused ultrasound in patients with pancreatic cancer. Onkologie. 2013;36(3):88–92. doi: 10.1159/000348530. [DOI] [PubMed] [Google Scholar]
- 102.Zhao H, Yang G, Wang D. et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs. 2010;21(4):447–452. doi: 10.1097/CAD.0b013e32833641a7. [DOI] [PubMed] [Google Scholar]
- 103.Gao H F, Wang K, Meng Z Q. et al. High intensity focused ultrasound treatment for patients with local advanced pancreatic cancer. Hepatogastroenterology. 2013;60(128):1906–1910. doi: 10.5754/hge13498. [DOI] [PubMed] [Google Scholar]
- 104.Nabi G, Goodman C, Melzer A. High intensity focused ultrasound treatment of small renal masses: Clinical effectiveness and technological advances. Indian J Urol. 2010;26(3):331–337. doi: 10.4103/0970-1591.70561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu F Wang Z B Chen W Z Bai J Zhu H Qiao T Y Preliminary experience using high intensity focused ultrasound for the treatment of patients with advanced stage renal malignancy J Urol 2003170(6, Pt 1):2237–2240. [DOI] [PubMed] [Google Scholar]
- 106.Illing R O, Kennedy J E, Wu F. et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer. 2005;93(8):890–895. doi: 10.1038/sj.bjc.6602803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Häcker A, Michel M S, Marlinghaus E, Köhrmann K U, Alken P. Extracorporeally induced ablation of renal tissue by high-intensity focused ultrasound. BJU Int. 2006;97(4):779–785. doi: 10.1111/j.1464-410X.2006.06037.x. [DOI] [PubMed] [Google Scholar]
- 108.Marberger M, Schatzl G, Cranston D, Kennedy J E. Extracorporeal ablation of renal tumours with high-intensity focused ultrasound. BJU Int. 2005;95 02:52–55. doi: 10.1111/j.1464-410X.2005.05200.x. [DOI] [PubMed] [Google Scholar]
- 109.Klingler H C Susani M Seip R Mauermann J Sanghvi N Marberger M J A novel approach to energy ablative therapy of small renal tumours: laparoscopic high-intensity focused ultrasound Eur Urol 2008534810–816., discussion 817–818 [DOI] [PubMed] [Google Scholar]
- 110.Ritchie R W, Leslie T A, Turner G D. et al. Laparoscopic high-intensity focused ultrasound for renal tumours: a proof of concept study. BJU Int. 2011;107(8):1290–1296. doi: 10.1111/j.1464-410X.2010.09620.x. [DOI] [PubMed] [Google Scholar]
- 111.Hynynen K, Pomeroy O, Smith D N. et al. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology. 2001;219(1):176–185. doi: 10.1148/radiology.219.1.r01ap02176. [DOI] [PubMed] [Google Scholar]
- 112.Wu F, Wang Z B, Cao Y D. et al. A randomised clinical trial of high-intensity focused ultrasound ablation for the treatment of patients with localised breast cancer. Br J Cancer. 2003;89(12):2227–2233. doi: 10.1038/sj.bjc.6601411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Furusawa H, Namba K, Thomsen S. et al. Magnetic resonance-guided focused ultrasound surgery of breast cancer: reliability and effectiveness. J Am Coll Surg. 2006;203(1):54–63. doi: 10.1016/j.jamcollsurg.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 114.Furusawa H, Namba K, Nakahara H. et al. The evolving non-surgical ablation of breast cancer: MR guided focused ultrasound (MRgFUS) Breast Cancer. 2007;14(1):55–58. doi: 10.2325/jbcs.14.55. [DOI] [PubMed] [Google Scholar]
- 115.Gianfelice D, Khiat A, Amara M, Belblidia A, Boulanger Y. MR imaging-guided focused ultrasound surgery of breast cancer: correlation of dynamic contrast-enhanced MRI with histopathologic findings. Breast Cancer Res Treat. 2003;82(2):93–101. doi: 10.1023/B:BREA.0000003956.11376.5b. [DOI] [PubMed] [Google Scholar]
- 116.Wu F, Wang Z B, Zhu H. et al. Extracorporeal high intensity focused ultrasound treatment for patients with breast cancer. Breast Cancer Res Treat. 2005;92(1):51–60. doi: 10.1007/s10549-004-5778-7. [DOI] [PubMed] [Google Scholar]
- 117.Zhu J, Zhu H, Mei Z. et al. High-intensity focused ultrasound ablation for treatment of hepatocellular carcinoma and hypersplenism: preliminary study. J Ultrasound Med. 2013;32(10):1855–1862. doi: 10.7863/ultra.32.10.1855. [DOI] [PubMed] [Google Scholar]
- 118.Dalecki D. Mechanical bioeffects of ultrasound. Annu Rev Biomed Eng. 2004;6:229–248. doi: 10.1146/annurev.bioeng.6.040803.140126. [DOI] [PubMed] [Google Scholar]
- 119.Clement G T. Perspectives in clinical uses of high-intensity focused ultrasound. Ultrasonics. 2004;42(10):1087–1093. doi: 10.1016/j.ultras.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 120.Yu T, Wang G, Hu K, Ma P, Bai J, Wang Z. A microbubble agent improves the therapeutic efficiency of high intensity focused ultrasound: a rabbit kidney study. Urol Res. 2004;32(1):14–19. doi: 10.1007/s00240-003-0362-x. [DOI] [PubMed] [Google Scholar]
- 121.Kaneko Y, Maruyama T, Takegami K. et al. Use of a microbubble agent to increase the effects of high intensity focused ultrasound on liver tissue. Eur Radiol. 2005;15(7):1415–1420. doi: 10.1007/s00330-005-2663-7. [DOI] [PubMed] [Google Scholar]
- 122.Hanajiri K, Maruyama T, Kaneko Y. et al. Microbubble-induced increase in ablation of liver tumors by high-intensity focused ultrasound. Hepatol Res. 2006;36(4):308–314. doi: 10.1016/j.hepres.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 123.Hynynen K H. New York, NY: Informa Healthcare USA Inc.; 2008. Fundamental principles of therapeutic ultrasound; pp. 5–18. [Google Scholar]
- 124.Partanen A, Yarmolenko P S, Viitala A. et al. Mild hyperthermia with magnetic resonance-guided high-intensity focused ultrasound for applications in drug delivery. . Int J Hyperthermia. 2012;28(4):320–336. doi: 10.3109/02656736.2012.680173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dromi S, Frenkel V, Luk A. et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res. 2007;13(9):2722–2727. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khokhlova T D, Wang Y N, Simon J C. et al. Ultrasound-guided tissue fractionation by high intensity focused ultrasound in an in vivo porcine liver model. Proc Natl Acad Sci U S A. 2014;111(22):8161–8166. doi: 10.1073/pnas.1318355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu F. High intensity focused ultrasound ablation and antitumor immune response. J Acoust Soc Am. 2013;134(2):1695–1701. doi: 10.1121/1.4812893. [DOI] [PubMed] [Google Scholar]
- 128.Zhou Q, Zhu X Q, Zhang J, Xu Z L, Lu P, Wu F. Changes in circulating immunosuppressive cytokine levels of cancer patients after high intensity focused ultrasound treatment. Ultrasound Med Biol. 2008;34(1):81–87. doi: 10.1016/j.ultrasmedbio.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 129.Lu P, Zhu X Q, Xu Z L, Zhou Q, Zhang J, Wu F. Increased infiltration of activated tumor-infiltrating lymphocytes after high intensity focused ultrasound ablation of human breast cancer. Surgery. 2009;145(3):286–293. doi: 10.1016/j.surg.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 130.Etame A B, Diaz R J, Smith C A, Mainprize T G, Hynynen K, Rutka J T. Focused ultrasound disruption of the blood-brain barrier: a new frontier for therapeutic delivery in molecular neurooncology. Neurosurg Focus. 2012;32(1):E3. doi: 10.3171/2011.10.FOCUS11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Staruch R M, Ganguly M, Tannock I F, Hynynen K, Chopra R. Enhanced drug delivery in rabbit VX2 tumours using thermosensitive liposomes and MRI-controlled focused ultrasound hyperthermia. Int J Hyperthermia. 2012;28(8):776–787. doi: 10.3109/02656736.2012.736670. [DOI] [PubMed] [Google Scholar]
- 132.Staruch R, Chopra R, Hynynen K. Localised drug release using MRI-controlled focused ultrasound hyperthermia. Int J Hyperthermia. Group. 2011;27(2):156–171. doi: 10.3109/02656736.2010.518198. [DOI] [PubMed] [Google Scholar]


