Cirrhosis is a result of advanced liver disease and is characterized by fibrosis of liver tissue and conversion of normal architecture into regenerative nodules,1 2 leading to a loss of liver function. According to the Centers for Disease Control, cirrhotic liver disease is responsible for 15,000 deaths per year in the United States.3 4 Portal hypertension (defined as elevation of hepatic venous pressure gradient ≥ 5 mm Hg) is a hallmark of cirrhosis, which is the result of a complicated process of inflammation, necrosis, collagen deposition, and regenerating nodules in the liver parenchyma.5 6 Increased portal pressure ultimately reduces portal blood flow, leading to the release of vasodilators and blood pooling in the splanchnic circulation. This results in renal hypoperfusion, with consequent activation of the renal–angiotensin–aldosterone system and fluid retention.7 Combined with decreased oncotic forces from hypoalbuminemia, fluid leakage from the splanchnic circulation begins to exceed the capacity of the lymphatic drainage system, and ascites, a characteristic complication of decompensated cirrhosis, develops.5 6 7
In extreme cases, the maladaptive vasodilatory response can lead to hepatorenal syndrome (HRS), a rapidly progressive form of acute renal failure that occurs in patients with cirrhosis and ascites in the absence of other causes of renal failure. It is characterized by a marked reduction in glomerular filtration rate (GFR) and renal plasma flow. The hallmark of HRS is intense renal vasoconstriction with predominant peripheral arterial vasodilation. Tubular function is preserved with the absence of proteinuria or histologic changes in the kidney. HRS exists on a spectrum with milder forms of acute kidney injury in patients with cirrhosis.8 It has been estimated that approximately 40% of patients with cirrhosis and ascites will develop HRS within 5 years, and 50% of patients hospitalized for a complication of cirrhosis will develop renal impairment, generally within a few days of admission.9 10 The prognosis for HRS is poor, with a mortality rate as high as 80% within 2 weeks.11
Diagnostic Criteria
HRS-like attributes, such as a marked decreased in urine output and increased intra-abdominal pressure, in patients with ascites were first described in 1923,12 and HRS was formally described as a distinct disease entity in 1963.13 In 1996, the International Ascites Club formulated diagnostic criteria for HRS, which were revised in 2007.9 14 The criteria are summarized as follows:
Major Criteria
Chronic or acute liver disease with advanced hepatic failure and portal hypertension
Low GFR as indicated by a serum creatinine level > 1.5 mg/dL or 24-hour creatinine clearance < 40 mL/minute
Absence of shock, ongoing bacterial infection, and current or recent treatment with nephrotoxic drugs, absence of gastrointestinal fluid losses (repeated vomiting or intense diarrhea), or renal fluid losses (weight loss > 500 g/day for several days in patients with ascites without peripheral edema or weight loss > 1,000 g/day in patients with peripheral edema)
No sustained improvement in renal function (a decrease in the serum creatinine level to 1.5 mg/dL or less, or an increase in the 24-hour creatinine clearance to 40 mL/minute or more) after diuretic withdrawal and plasma volume expansion with 1.5 L of isotonic saline
Lack of significant proteinuria < 500 mg/dL and no ultrasonographic evidence of obstructive uropathy or parenchymal renal disease
There are two subtypes of HRS, which are differentiated by their time course and the presence of precipitating factors: Type 1 HRS has a rapid decline of renal function following a precipitating event, whereas Type 2 HRS has a slower time course and does not have a trigger.11 15 Other authors have argued in favor of expanding the definition; Munoz proposed four subtypes, including classifications for HRS superimposed on kidney disease of a different etiology or on fulminant liver failure.14 A recent working group composed of representatives from the International Ascites Club and the Acute Dialysis Quality Initiative recommended a modification of the current standard; this includes moving away from using serum creatinine as a measure of renal function, and adding classifications for other types of acute or chronic kidney disease that may be superimposed on cirrhosis, all under the heading of an overall disease classification called hepatorenal disorder.13
Pathogenesis
Both Type 1 and Type 2 HRS are distinct expressions of a common underlying disorder.16 The pathogenesis of the disease is complex and not wholly understood, but the hallmark features are splanchnic vasodilation resulting in effective central hypovolemia leading to cardiovascular dysfunction with vasoconstriction and renal hypoperfusion (Fig. 1).
Fig. 1.
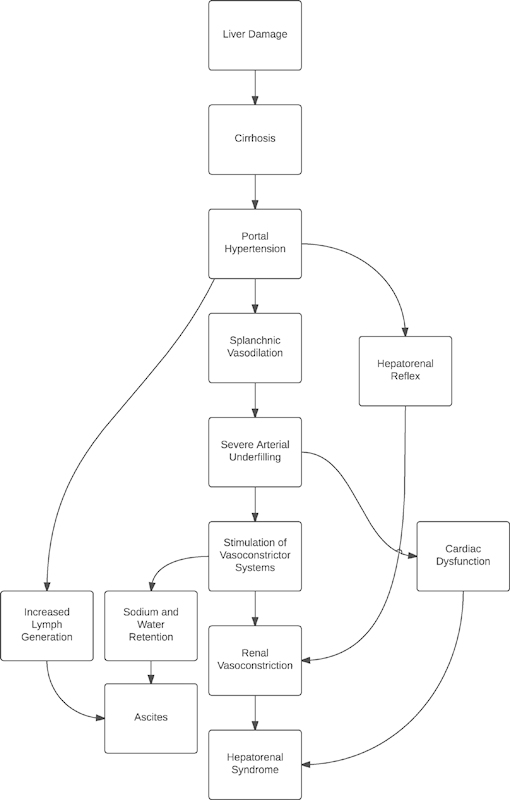
Pathogenesis and treatment targets of hepatorenal syndrome.
Splanchnic vasodilation is one of the key causative factors of HRS and is caused by a constellation of vasodilatory responses. This splanchnic vasodilation is temporally antecedent to HRS.16 In cirrhotic patients, nitrous oxide production is increased in the splanchnic bed and reduced in the liver sinusoids, resulting in increased portal gradient pressures.17 Other vasodilators, including calcitonin gene-related peptide and adrenomedullin, are present in increased concentrations in HRS patients due to increased production and decreased hepatic clearance.18
Increased inflammation may be precipitated by spontaneous bacterial peritonitis (SBP), the most frequent infectious precipitant of HRS. One-third of patients with cirrhosis and ascites who develop SBP eventually develop renal failure.19 Other infections commonly precipitate HRS as well, including urinary tract infections and biliary/gastrointestinal infections.20 A low-grade systemic proinflammatory state exists in HRS, with an elevation of cytokines, including IL-6 and TNF-α, which may be associated with translocation of enteric bacteria.21
Central hypovolemia in HRS is secondary to a decrease in systemic vascular resistance.22 This vasodilation combined with restricted portal blood flow causes blood to pool in the splanchnic circulation, creating an effective hypovolemia in the central circulation.21 23 Additionally, both the baroreflex and cardiovascular responses to angiotensin II, norepinephrine, and vasopressin are abnormal, causing further blood pressure dysregulation.24
Further exacerbating the circulatory dysfunction in HRS is cirrhotic cardiomyopathy, a phenomenon that affects both systolic and diastolic function. Electrophysiologic changes occur, including prolonged QT intervals and electromechanical dyssynchrony, and the ability of the heart to respond to inotropic and chronotropic stimuli is reduced.17 While cardiac output is often high in absolute terms because of the decreased systemic vascular resistance, the impaired function becomes apparent when either the resistance is normalized or there is a physiologic stress stimulus.17 25
The result of this multifactorial circulatory dysfunction reduces renal perfusion pressure and renal blood flow.24 The kidneys autoregulate blood flow at blood pressures above 70 mm Hg; however, overactivation of the sympathetic system increases the kidneys' reliance on adequate blood pressure to maintain perfusion at lower pressures.22 Constriction of the afferent and efferent arterioles is caused by stimulation of α-adrenergic receptors and renin release both by reduced blood flow and β-adrenergic receptors.24 Stimulation of the renin–angiotensin–aldosterone axis is increased because of the inability of the cirrhotic liver to degrade renin.26
Crucially, renal failure in HRS is almost entirely of a prerenal nature; the kidneys are, at least initially, intrinsically healthy. For this reason, recovery of kidney function in HRS is possible after liver transplantation, and kidneys from deceased HRS patients have even been successfully used as donor organs.27
There are other mechanisms theorized to contribute to the development of HRS. There is some empirical support for a hepatorenal reflex, whereby abnormal liver blood flow directly alters kidney hemodynamics. Canine studies have shown that an acute increase in portal pressure causes an increase in renal nerve activity. This phenomenon is not observed when the liver is denervated.28 In human studies, occlusion of the portal vein immediately reduces renal blood flow in excess of the change in cardiac output.29 Finally, a lumbar sympathetic block has been shown to improve aspects of renal function, including sodium excretion, renal blood flow, and GFR in eight patients with HRS.30 Such a reflex may help explain why a procedure such as transjugular intrahepatic portosystemic shunt (TIPS), which reduces the portal pressure gradient, improves HRS in many patients.
A third possible contributing factor, along with effective central hypovolemia and a hepatorenal reflex, is the direct effect of increased intra-abdominal pressure from ascites. Large-volume ascites and infection have been identified in 70 to 100% of cases of Type 1 HRS.11 Increased intra-abdominal pressure can cause venous congestion and a decline in GFR.31 In addition, organ compression can stimulate activation of the renin–angiotensin–aldosterone system, exacerbating the renal pathology.32
Treatment
Medical Management
Historically, medical management of Type 1 HRS has been generally ineffective, as reflected in the high mortality rate of the disease if there is no liver transplantation.25 33 Most patients who die of HRS succumb to the consequences of hyperkalemia, metabolic acidosis, or uremic intoxication, with an increased risk for coagulopathies or infection. Hypotension makes hemodialysis difficult and dangerous, and rapid fluid and electrolyte shifts can cause cerebral edema.34 Nevertheless, medical management of HRS should be initiated as soon as the diagnosis is suspected, including treating the causative infection (if present). Additionally, the use of any nephrotoxic drugs (such as NSAIDs and intravenous contrast agents) should be avoided. Some success has been achieved with the combination of vasoconstrictors and albumin infusion.9 15
The cornerstone of modern medical treatment for both forms of HRS is expanding central blood volume by increasing total plasma volume while reducing peripheral vasodilation.9 Plasma volume support most commonly involves infusion of intravenous albumin.15 The resulting rise in central blood volume also may increase preload, helping to correct some of the cardiac dysfunction, while improving kidney perfusion by increasing mean arterial blood pressure.24 25
While vasoconstrictors are not curative, they greatly increase the chances of a patient surviving to receive a liver transplant. Vasoconstrictor therapy consists of three classes of drugs: vasopressin analogs, α-adrenergic agonists, and somatostatin analogs (Table 1).35 By reversing splanchnic vasodilation, vasoconstrictor therapy increases central blood volume and mean arterial pressure, correcting some of the problems that lead to kidney dysfunction.36
Table 1. Medications commonly used in the treatment of HRS.
| Class | Drug | Action | Comment |
|---|---|---|---|
| Albumin | Intravascular volume expansion | Complimentary component of most vasoconstrictor therapies | |
| Vasopressin analogs | Vasopressin, ornipressin | Vasopressin receptor agonist | Some improvement in HRS: serious side effects |
| Terlipressin | V1 receptor agonist | Most well-validated vasopressin analog: unavailable in United States | |
| Alpha-adrenergic agonists | Noradrenaline | Adrenergic agonist | Benefits comparable to terlipressin: greater availability |
| Midodrine | Selective alpha-1 adrenergic agonist | Frequently paired with octreotide: less effective than noradrenaline or terlipressin | |
| Somatostatin analog | Octreotide | Inhibits splanchnic blood flow | Improves renal blood flow: decreases GFR |
| Dopamine agonist | Dopamine | Improves renal blood flow: no improvement in renal function |
Abbreviations: GFR, glomerular filtration rate; HRS, hepatorenal syndrome.
The vasopressin drugs used for HRS include vasopressin, ornipressin, and terlipressin.37 These drugs act on the V1 vasopressin receptors found in the systemic, splanchnic, renal, and coronary circulations, the activation of which causes vasoconstriction.37 38 Both vasopressin and ornipressin have shown mixed results in improving renal function, either alone or when combined with dopamine.39 40 41 Furthermore, the use of each has been limited by serious side effects, including mesenteric and myocardial ischemia and ventricular arrhythmias.37
Terlipressin is the best-studied vasopressin analog, and it has shown excellent efficacy in improving renal function.38 A prohormone of lysine-vasopressin, when combined with albumin, is the most effective medical therapy for Type 1 HRS.42 It has been used for several decades overseas, but had not been approved by the FDA for use in the United States until it was recently granted orphan drug designation, a pathway for accelerated clinical trials and approval.42 43 Between 34 and 75% of patients have a good response to terlipressin, recovering some or all renal function.44 45 46 47 A 2010 meta-analysis of randomized controlled trials with a total of 223 patients found that patients receiving terlipressin had a 1.85 times greater likelihood of transplant-free survival after 90 days when compared with placebo.48
Norepinephrine is the other drug that has been shown to be effective in the treatment of HRS. It has the benefit of greater availability and lower cost compared with terlipressin.49 50 Norepinephrine with albumin has been shown to have a response rate of up to 83% and have a favorable effect on renin and aldosterone levels.51 In two small trials (n = 22, n = 40), the effects of terlipressin and norepinephrine were found to be similar in mixed groups of patients with either Type 1 or Type 2 HRS.49 50
The α-agonist midodrine is generally coadministered with the somatostatin agonist octreotide, based on studies conducted several decades ago. There is a lack of large-scale data concerning mortality, although GFR and hormonal profile have been shown to benefit from this therapy.52
Other pharmacotherapies have also been explored. The endothelin antagonist tezosentan was investigated in the hopes that it would reverse the renal vasoconstriction of the disease. However, a trial of six patients with Type 2 HRS resulted in worsening renal function, along with systemic hypotension in one patient.53 Dopamine infusion improves the angiographic appearance of renal blood flow but does not result in improved urine production or GFR.54 A similar lack of efficacy was observed with the selective dopamine agonist fenoldopam.55
Recently, it was suggested that antileukotriene drugs should be investigated for their ability to block leukotriene-mediated renal hemodynamic disturbances. Several antileukotriene drugs are readily available and have well-understood safety profiles.56 However, no reports of human or animal trials of these drugs are available.
Hemodialysis is generally ineffective in patients with HRS, and data on the efficacy of hemodialysis are limited. Keller et al studied 100 patients with cirrhosis and acute renal failure, including 26 with HRS, and showed that dialysis could be effective in some patients, providing they did not have thrombocytopenia, encephalopathy, or malignancy.57 Witzke et al prospectively studied 30 patients with HRS (of unspecified type) and found that hemodialysis was not effective in mechanically ventilated patients but could slightly improve short-term survival in nonventilated patients.58
A possible future therapeutic option is the molecular adsorbent recirculating system (MARS), a liver support system that combines the functions of a dialysis machine and a closed-loop albumin circuit that serves to remove albumin-bound toxins from dialyzed blood.58 Some small trials have found that MARS improves renal function and survival in Type 1 HRS, in addition to decreasing hepatic encephalopathy and improving hepatic protein synthesis.59 However, this treatment modality is not widely used and is not supported by any large studies.
Transjugular Intrahepatic Portosystemic Shunt and Peritoneovenous Shunt
TIPS is used to reduce portal hypertension in cirrhosis and treat ascites by decreasing transvascular filtration into the peritoneal space.60 However, knowledge of the effects of the procedure on HRS is limited by a lack of large-scale studies. Further complicating matters, patients with HRS often have contraindications for TIPS.61
TIPS procedures reduce portal pressure by establishing a shunt between the portal and hepatic veins, reducing the portal gradient by a mean of 10 mm Hg.62 This allows blood formerly pooled in the splanchnic circulation to reenter the systemic circulation.60 However, far from improving circulatory dynamics, TIPS actually worsens the hyperdynamic circulation of HRS. There is an immediate decrease in systemic vascular resistance and an increase in cardiac output.25 63 64 65 These circulatory changes may in part be due to an increase in vasodilation.22
Nevertheless, there is good evidence that TIPS may have a beneficial effect on renal function in cirrhosis patients with Type 1 HRS, with less evidence available for Type 2 HRS.9 To date, the largest study of the effects of TIPS on patients with impaired renal function is a 7-year retrospective analysis, which included 65 patients with a serum creatinine greater than 1.2 mg/dL. These patients demonstrated a significantly lowered serum creatinine level postprocedure. Unfortunately, this study did not identify a specific cohort of patients with HRS.66 Other studies have found similar results, demonstrating that sodium excretion and serum creatinine can improve within hours, and renal hemodynamics can normalize within 6 to 12 months in the majority of patients.65 67 Curiously, the magnitude of these improvements did not correlate with the change in portal gradient postprocedure.68
TIPS may improve the hormonal profile of patients. Renin, angiotensin, vasopressin, and norepinephrine levels decrease toward normal levels following the procedure, even in patients with Type 1 HRS.22 62 67 68 69 70 71 72 One study investigated the efficacy of TIPS following a 2-week treatment of patients with Type 1 HRS with midodrine, octreotide, and albumin; in these patients, renal function returned to normal with a transient decrease in systemic vascular resistance. Crucially, this study demonstrated that TIPS could still be effective following a preliminary course of vasoconstrictor therapy. Additionally, patients who received a TIPS were either still living with their TIPS or had received a liver transplant after 6-month follow-up.72
Nevertheless, no study has yet conclusively shown that TIPS improves survival compared with other treatment options for HRS. Complications of using a TIPS are well known, including procedure-related bleeding and hepatic encephalopathy.
A surgical peritoneovenous shunt has been observed to improve renal function in patients with HRS but did not prolong survival. The shunt may support intravascular volume with additional fluid or possibly decrease intra-abdominal pressure.73 Because it needs high maintenance with a high complication rate, the peritoneovenous shunt has been largely supplanted by the TIPS for the treatment of refractory ascites. In addition, because it does not decrease the portal pressure gradient, it is not effective in the treatment or prevention of variceal bleeding.74 The high rates of serious complications, including SBP, have largely relegated the peritoneovenous shunt to instances where neither TIPS nor liver transplant is available.
Liver Transplant
Liver transplant is the only treatment for HRS that improves long-term survival.25 Replacement of the cirrhotic liver immediately results in resolution of splanchnic vasodilation and improvement of kidney perfusion, with kidney recovery occurring over the course of several days. Hemodialysis may only be necessary as a temporary measure.33 While pretransplant renal function is correlated with liver transplant outcomes, patients with HRS have better-than-expected survival compared with transplant patients without HRS75; approximately 80% of Type 1 HRS patients survive for 5 years posttransplant.25 Perhaps contributing to these positive outcomes is the fact that HRS patients have a high Model for End-stage Liver Disease (MELD) score because of their elevated serum creatinine, as opposed to having more elevated bilirubin or international normalized ratio (INR) due to more severe liver damage.61
In cases where deceased-donor liver transplant is not available, HRS patients may be candidates for living donor transplant. While a high MELD score would seem to be a contraindication for a living donor transplant, HRS patients have had good outcomes.75 76 77 Because of the often-emergent nature of the procedure, workup must be completed within a few days; this is technically feasible, but the main concern is that this may increase the psychological hazard of donation.25 75
Treatment with vasoconstrictors does not appear to affect outcomes of liver transplant. A study examining the effects of terlipressin with albumin versus a placebo with albumin on transplant outcomes found no difference in survival of transplant recipients while improving survival of nonrecipients.78
Combined liver–kidney transplant has been utilized for some patients with HRS.79 In cases where HRS is complicated by underlying renal parenchymal injury or chronic kidney disease, transplantation of both organs may be necessary. HRS is the primary diagnosis in 2% of combined liver–kidney transplants.80 However, the procedure has the effect of giving some of the highest-risk recipients higher quality organs, presenting an ethical challenge to organ allocation.37 Because of the high likelihood of renal recovery—a phenomenon that has been documented for over 40 years—a kidney transplant following a liver transplant, in patients with insufficient renal function recovery, may be a better option.25 A 60-day waiting period could be a reasonable watchful waiting period post–liver transplant that would allow time for renal recovery without jeopardizing patient health.81
Practice Guidelines
The AASLD and EASL have both issued practice guidelines for the treatment of HRS, although their recommendations are not very specific and are somewhat contradictory. The 2012 AASLD guidelines recommend the use of either octreotide plus midodrine or norepinephrine with albumin infusion for patients with HRS, as well as an expedited transplant referral.82 They also cite TIPS as an investigatory treatment for HRS, noting the lack of controlled trials comparing the procedure with medical management.83 The EASL recommends terlipressin with albumin, except for patients with ischemic cardiovascular disease, and describes norepinephrine and midodrine plus octreotide as potential alternatives with limited evidence. These guidelines also cite a lack of evidence for TIPS as a treatment option, as there have been no sufficient data to support use in HRS.84
Prognostic Factors
Prognostic factors have been sought to predict both patients who are likely to develop HRS and those who are likely to be responsive to treatment. Renal function monitoring, both in patients at risk of HRS and those being treated for the disease, is by far the most effective means of predicting the likelihood and course of the disease. Multifactorial metrics such as MELD and Child-Pugh score can also predict all-cause mortality from liver disease, including HRS. Hormonal measures can also be used.
Serum creatinine is the most commonly used measure of renal function. Calculated GFR using the MDRD equation is a good predictor of survival in patients with cirrhotic ascites, and high serum creatinine is predictive of a poor response to vasoconstrictor therapy in HRS.11 44 Cystatin C is a more accurate estimate of GFR than serum creatinine or MDRD calculations.85 It has been found to be a better predictor of HRS and survival than serum creatinine.86 However, the assays are expensive and require more testing and standardization to become a universally practicable measure of renal function.87 Another measure of kidney function is urinary neutrophil gelatinase-associated lipocalin (UNGAL), a protein expressed by the tubular epithelia-injured kidneys. It is a sensitive test for noninvasive differentiation etiologies of kidney disease, able to distinguish between intrinsic acute injury, HRS, and other prerenal disease, and is recommended by the AASLD.82 88 However, like Cystatin C, UNGAL is both expensive and lacks standardization, and neither is likely to become part of standard clinical practice in the short term.
The MELD score and the Child-Pugh score were developed as prognostic tools for liver disease.89 90 The MELD score uses the INR, serum bilirubin, serum creatinine, and etiology of cirrhosis to stratify patients based on risk. It has the advantage over Child-Pugh of not including subjective clinical variables and having a specific measure of a renal variable.91 MELD scores are 75 to 80% accurate in predicting survival in HRS patients but are less effective in predicting renal disease.92
Hormonal measures such as renin, aldosterone, and norepinephrine can predict survival in patients with HRS.91 For example, adrenal insufficiency increases the risk of HRS developing secondary to a bacterial infection; this may be due to an exaggerated inflammatory response and poor circulatory function.93
Prevention
Prevention of HRS involves identification of those patients at risk of developing the disease and avoiding states that adversely affect renal blood perfusion, especially hypovolemic states.94 Diuretic use should be closely monitored; if diuresis exceeds ascites absorption, effective hypovolemia may develop.24 The response to loop diuretics is blunted in patients with cirrhosis and ascites, and spironolactone dose should be limited. In general, patients on diuretics should be followed with electrolyte and creatinine monitoring.95 Adequate hydration is also an important concern, and paracentesis should be accompanied by albumin infusion to minimize circulatory disturbances.24 95 Nephrotoxic drugs, including aminoglycosides and NSAIDs, should be avoided as much as possible.94 Patients should be monitored for signs of infection that may precipitate HRS, especially SBP. Large fluid shifts, such as during paracentesis, should be avoided. Clinicians should also be alert for early signs of renal impairment, such as electrolyte abnormalities, increased serum creatinine, and decreased urine output.
Prophylactic medications have received some investigation. Beta-blockers were investigated to decrease portal pressure, and the adrenergic blockade resulted in improved α-adrenergic tone.24 Norfloxacin has been administered as a preventative measure for SBP and decreased the rate of either type of HRS while improving survival at 3 months and 1 year when compared with placebo.96 Pentoxifylline, a phosphodiesterase inhibitor and anti-inflammatory agent, has been shown to be effective in reducing portal hypertension and reducing the risk of developing HRS in one placebo-controlled trial of 70 patients.96
Conclusion
HRS is a serious complication of cirrhosis of the liver and carries a high mortality. The pathogenesis of HRS is complex and incompletely understood, but it is postulated that splanchnic vasodilation reduces effective circulating volume, which in turn leads to renal hypoperfusion. While liver transplant remains the only long-term treatment for HRS, more effective medical treatment options and a greater understanding of the potential benefits of TIPS, MARS, and other interventions promise improved outcomes for patients affected by this serious complication of cirrhotic liver disease.
Acknowledgments
The authors greatly appreciate the support of the Imaging Institute of the Cleveland Clinic in preparing this manuscript.
Footnotes
Funding No financial support was provided for this article.
References
- 1.Crawford J M. Philadelphia, PA: Elsevier Saunders; 2005. Liver and biliary tract; pp. 877–938. [Google Scholar]
- 2.Asrani S K Larson J J Yawn B Therneau T M Kim W R Underestimation of Liver-Related Mortality in the United States [Internet] Gastroenterology. 2013 [cited June 24, 2013];(0): Available at: http://www.sciencedirect.com/science/article/pii/S0016508513004988. Accessed October 13, 2015 [DOI] [PMC free article] [PubMed]
- 3.Jepsen P, Vilstrup H, Andersen P K, Lash T L, Sørensen H T. Comorbidity and survival of Danish cirrhosis patients: a nationwide population-based cohort study. Hepatology. 2008;48(1):214–220. doi: 10.1002/hep.22341. [DOI] [PubMed] [Google Scholar]
- 4.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Oliver J A, Verna E C. Afferent mechanisms of sodium retention in cirrhosis and hepatorenal syndrome. Kidney Int. 2010;77(8):669–680. doi: 10.1038/ki.2010.4. [DOI] [PubMed] [Google Scholar]
- 6.Moore C M, Van Thiel D H. Cirrhotic ascites review: Pathophysiology, diagnosis and management. World J Hepatol. 2013;5(5):251–263. doi: 10.4254/wjh.v5.i5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong F, O'Leary J G, Reddy K R. et al. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology. 2013;145(6):1280–12880. doi: 10.1053/j.gastro.2013.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Postgrad Med J. 2008;84(998):662–670. doi: 10.1136/gut.2006.107789. [DOI] [PubMed] [Google Scholar]
- 9.Ginès P, Guevara M, Arroyo V, Rodés J. Hepatorenal syndrome. Lancet. 2003;362(9398):1819–1827. doi: 10.1016/S0140-6736(03)14903-3. [DOI] [PubMed] [Google Scholar]
- 10.Olivera-Martinez M, Sayles H, Vivekanandan R, D' Souza S, Florescu M C. Hepatorenal syndrome: are we missing some prognostic factors? Dig Dis Sci. 2012;57(1):210–214. doi: 10.1007/s10620-011-1861-1. [DOI] [PubMed] [Google Scholar]
- 11.Thorington J M, Scimidt C F. A study of urinary output and blood-pressure changes resulting in experimental ascites. Am J Med Sci. 1923;165:880–889. [Google Scholar]
- 12.Wong F, Nadim M K, Kellum J A. et al. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60(5):702–709. doi: 10.1136/gut.2010.236133. [DOI] [PubMed] [Google Scholar]
- 13.Arroyo V, Ginès P, Gerbes A L. et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23(1):164–176. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 14.Munoz S J The hepatorenal syndrome Med Clin North Am 2008924813–837., viii–ix v iii–ix. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-del-Arbol L, Monescillo A, Arocena C. et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42(2):439–447. doi: 10.1002/hep.20766. [DOI] [PubMed] [Google Scholar]
- 16.Zardi E M, Abbate A, Zardi D M. et al. Cirrhotic cardiomyopathy. J Am Coll Cardiol. 2010;56(7):539–549. doi: 10.1016/j.jacc.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 17.Møller S, Henriksen J H, Bendtsen F. Pathogenetic background for treatment of ascites and hepatorenal syndrome. Hepatol Int. 2008;2(4):416–428. doi: 10.1007/s12072-008-9100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruiz-del-Arbol L, Urman J, Fernández J. et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38(5):1210–1218. doi: 10.1053/jhep.2003.50447. [DOI] [PubMed] [Google Scholar]
- 19.Fasolato S, Angeli P, Dallagnese L. et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45(1):223–229. doi: 10.1002/hep.21443. [DOI] [PubMed] [Google Scholar]
- 20.Albillos A, de la Hera A, González M. et al. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37(1):208–217. doi: 10.1053/jhep.2003.50038. [DOI] [PubMed] [Google Scholar]
- 21.Stadlbauer V, Wright G A, Banaji M. et al. Relationship between activation of the sympathetic nervous system and renal blood flow autoregulation in cirrhosis. Gastroenterology. 2008;134(1):111–119. doi: 10.1053/j.gastro.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 22.Møller S, Iversen J S, Henriksen J H, Bendtsen F. Reduced baroreflex sensitivity in alcoholic cirrhosis: relations to hemodynamics and humoral systems. Am J Physiol Heart Circ Physiol. 2007;292(6):H2966–H2972. doi: 10.1152/ajpheart.01227.2006. [DOI] [PubMed] [Google Scholar]
- 23.Møller S Henriksen J H Review article: pathogenesis and pathophysiology of hepatorenal syndrome—is there scope for prevention? Aliment Pharmacol Ther 2004200331–41., discussion 42–43 [DOI] [PubMed] [Google Scholar]
- 24.Ginès P, Guevara M, Perez-Villa F. Management of hepatorenal syndrome: another piece of the puzzle. Hepatology. 2004;40(1):16–18. doi: 10.1002/hep.20313. [DOI] [PubMed] [Google Scholar]
- 25.Iwatsuki S, Popovtzer M M, Corman J L. et al. Recovery from “hepatorenal syndrome” after orthotopic liver transplantation. N Engl J Med. 1973;289(22):1155–1159. doi: 10.1056/NEJM197311292892201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epstein M, Berk D P, Hollenberg N K. et al. Renal failure in the patient with cirrhosis. The role of active vasoconstriction. Am J Med. 1970;49(2):175–185. doi: 10.1016/s0002-9343(70)80073-0. [DOI] [PubMed] [Google Scholar]
- 27.Koyama S, Kanai K, Aibiki M, Fujita T. Reflex increase in renal nerve activity during acutely altered portal venous pressure. J Auton Nerv Syst. 1988;23(1):55–62. doi: 10.1016/0165-1838(88)90166-x. [DOI] [PubMed] [Google Scholar]
- 28.Jalan R, Forrest E H, Redhead D N, Dillon J F, Hayes P C. Reduction in renal blood flow following acute increase in the portal pressure: evidence for the existence of a hepatorenal reflex in man? Gut. 1997;40(5):664–670. doi: 10.1136/gut.40.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solis-Herruzo J A, Duran A, Favela V. et al. Effects of lumbar sympathetic block on kidney function in cirrhotic patients with hepatorenal syndrome. J Hepatol. 1987;5(2):167–173. doi: 10.1016/s0168-8278(87)80569-x. [DOI] [PubMed] [Google Scholar]
- 30.Mohmand H, Goldfarb S. Renal dysfunction associated with intra-abdominal hypertension and the abdominal compartment syndrome. J Am Soc Nephrol. 2011;22(4):615–621. doi: 10.1681/ASN.2010121222. [DOI] [PubMed] [Google Scholar]
- 31.de Laet I E, Malbrain M. Current insights in intra-abdominal hypertension and abdominal compartment syndrome. Med Intensiva. 2007;31(2):88–99. doi: 10.1016/s0210-5691(07)74781-2. [DOI] [PubMed] [Google Scholar]
- 32.Chok K S, Fung J Y, Chan S C. et al. Outcomes of living donor liver transplantation for patients with preoperative type 1 hepatorenal syndrome and acute hepatic decompensation. Liver Transpl. 2012;18(7):779–785. doi: 10.1002/lt.23401. [DOI] [PubMed] [Google Scholar]
- 33.Hasper D, Jörres A. New insights into the management of hepato-renal syndrome. Liver Int. 2011;31 03:27–30. doi: 10.1111/j.1478-3231.2011.02586.x. [DOI] [PubMed] [Google Scholar]
- 34.Leung W, Wong F. Hepatorenal syndrome: do the vasoconstrictors work? Gastroenterol Clin North Am. 2011;40(3):581–598. doi: 10.1016/j.gtc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Gluud L L, Christensen K, Christensen E, Krag A. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology. 2010;51(2):576–584. doi: 10.1002/hep.23286. [DOI] [PubMed] [Google Scholar]
- 36.Memon I, Klein C L. Impact of hepatorenal syndrome and liver transplantation. Curr Opin Organ Transplant. 2011;16(3):301–305. doi: 10.1097/MOT.0b013e328346576c. [DOI] [PubMed] [Google Scholar]
- 37.Sharman A, Low J. Vasopressin and its role in critical care. Continuing education in anaesthesia. Crit Care Pain. 2008;8:134–137. [Google Scholar]
- 38.Guevara M, Ginès P, Fernández-Esparrach G. et al. Reversibility of hepatorenal syndrome by prolonged administration of ornipressin and plasma volume expansion. Hepatology. 1998;27(1):35–41. doi: 10.1002/hep.510270107. [DOI] [PubMed] [Google Scholar]
- 39.Gülberg V, Bilzer M, Gerbes A L. Long-term therapy and retreatment of hepatorenal syndrome type 1 with ornipressin and dopamine. Hepatology. 1999;30(4):870–875. doi: 10.1002/hep.510300430. [DOI] [PubMed] [Google Scholar]
- 40.Saló J, Ginès A, Quer J C. et al. Renal and neurohormonal changes following simultaneous administration of systemic vasoconstrictors and dopamine or prostacyclin in cirrhotic patients with hepatorenal syndrome. J Hepatol. 1996;25(6):916–923. doi: 10.1016/s0168-8278(96)80297-2. [DOI] [PubMed] [Google Scholar]
- 41.Krag A, Borup T, Møller S, Bendtsen F. Efficacy and safety of terlipressin in cirrhotic patients with variceal bleeding or hepatorenal syndrome. Adv Ther. 2008;25(11):1105–1140. doi: 10.1007/s12325-008-0118-7. [DOI] [PubMed] [Google Scholar]
- 42.Newswire P R FDA Grants Orphan-Drug Designation for Novel Terlipressin Formulation for the Treatment of Ascites [updated 2013 Jan 4, cited 2013 Jun 23] Available at: http://www.prnewswire.com/news-releases/fda-grants-orphan-drug-designation-for-novel-terlipressin-formulation-for-the-treatment-of-ascites-185655002.html. Accessed October 13, 2015
- 43.Nazar A, Pereira G H, Guevara M. et al. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2010;51(1):219–226. doi: 10.1002/hep.23283. [DOI] [PubMed] [Google Scholar]
- 44.Alessandria C, Venon W D, Marzano A, Barletti C, Fadda M, Rizzetto M. Renal failure in cirrhotic patients: role of terlipressin in clinical approach to hepatorenal syndrome type 2. Eur J Gastroenterol Hepatol. 2002;14(12):1363–1368. doi: 10.1097/00042737-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Narahara Y, Kanazawa H, Sakamoto C. et al. The efficacy and safety of terlipressin and albumin in patients with type 1 hepatorenal syndrome: a multicenter, open-label, explorative study. J Gastroenterol. 2012;47(3):313–320. doi: 10.1007/s00535-011-0485-8. [DOI] [PubMed] [Google Scholar]
- 46.Sanyal A J, Boyer T, Garcia-Tsao G. et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134(5):1360–1368. doi: 10.1053/j.gastro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sagi S V, Mittal S, Kasturi K S, Sood G K. Terlipressin therapy for reversal of type 1 hepatorenal syndrome: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2010;25(5):880–885. doi: 10.1111/j.1440-1746.2009.06132.x. [DOI] [PubMed] [Google Scholar]
- 48.Alessandria C, Ottobrelli A, Debernardi-Venon W. et al. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. J Hepatol. 2007;47(4):499–505. doi: 10.1016/j.jhep.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 49.Sharma P, Kumar A, Shrama B C, Sarin S K. An open label, pilot, randomized controlled trial of noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. Am J Gastroenterol. 2008;103(7):1689–1697. doi: 10.1111/j.1572-0241.2008.01828.x. [DOI] [PubMed] [Google Scholar]
- 50.Duvoux C, Zanditenas D, Hézode C. et al. Effects of noradrenalin and albumin in patients with type I hepatorenal syndrome: a pilot study. Hepatology. 2002;36(2):374–380. doi: 10.1053/jhep.2002.34343. [DOI] [PubMed] [Google Scholar]
- 51.Karwa R, Woodis C B. Midodrine and octreotide in treatment of cirrhosis-related hemodynamic complications. Ann Pharmacother. 2009;43(4):692–699. doi: 10.1345/aph.1L373. [DOI] [PubMed] [Google Scholar]
- 52.Wong F, Moore K, Dingemanse J, Jalan R. Lack of renal improvement with nonselective endothelin antagonism with tezosentan in type 2 hepatorenal syndrome. Hepatology. 2008;47(1):160–168. doi: 10.1002/hep.21940. [DOI] [PubMed] [Google Scholar]
- 53.Bennett W M, Keeffe E, Melnyk C, Mahler D, Rösch J, Porter G A. Response to dopamine hydrochloride in the hepatorenal syndrome. Arch Intern Med. 1975;135(7):964–971. [PubMed] [Google Scholar]
- 54.Hadengue A, Moreau R, Bacq Y, Gaudin C, Braillon A, Lebrec D. Selective dopamine DA1 stimulation with fenoldopam in cirrhotic patients with ascites: a systemic, splanchnic and renal hemodynamic study. Hepatology. 1991;13(1):111–116. [PubMed] [Google Scholar]
- 55.Capella G L. Anti-leukotriene drugs in the prevention and treatment of hepatorenal syndrome. Prostaglandins Leukot Essent Fatty Acids. 2003;68(4):263–265. doi: 10.1016/s0952-3278(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 56.Keller F, Heinze H, Jochimsen F, Passfall J, Schuppan D, Büttner P. Risk factors and outcome of 107 patients with decompensated liver disease and acute renal failure (including 26 patients with hepatorenal syndrome): the role of hemodialysis. Ren Fail. 1995;17(2):135–146. doi: 10.3109/08860229509026250. [DOI] [PubMed] [Google Scholar]
- 57.Witzke O, Baumann M, Patschan D. et al. Which patients benefit from hemodialysis therapy in hepatorenal syndrome? J Gastroenterol Hepatol. 2004;19(12):1369–1373. doi: 10.1111/j.1440-1746.2004.03471.x. [DOI] [PubMed] [Google Scholar]
- 58.Mitzner S R, Stange J, Klammt S. et al. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl. 2000;6(3):277–286. doi: 10.1002/lt.500060326. [DOI] [PubMed] [Google Scholar]
- 59.Busk T M, Bendtsen F, Møller S. Cardiac and renal effects of a transjugular intrahepatic portosystemic shunt in cirrhosis. Eur J Gastroenterol Hepatol. 2013;25(5):523–530. doi: 10.1097/MEG.0b013e32835d09fe. [DOI] [PubMed] [Google Scholar]
- 60.Rajekar H, Chawla Y. Terlipressin in hepatorenal syndrome: evidence for present indications. J Gastroenterol Hepatol. 2011;26 01:109–114. doi: 10.1111/j.1440-1746.2010.06583.x. [DOI] [PubMed] [Google Scholar]
- 61.Guevara M, Ginès P, Bandi J C. et al. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: effects on renal function and vasoactive systems. Hepatology. 1998;28(2):416–422. doi: 10.1002/hep.510280219. [DOI] [PubMed] [Google Scholar]
- 62.Azoulay D, Castaing D, Dennison A, Martino W, Eyraud D, Bismuth H. Transjugular intrahepatic portosystemic shunt worsens the hyperdynamic circulatory state of the cirrhotic patient: preliminary report of a prospective study. Hepatology. 1994;19(1):129–132. [PubMed] [Google Scholar]
- 63.Lotterer E, Wengert A, Fleig W E. Transjugular intrahepatic portosystemic shunt: short-term and long-term effects on hepatic and systemic hemodynamics in patients with cirrhosis. Hepatology. 1999;29(3):632–639. doi: 10.1002/hep.510290302. [DOI] [PubMed] [Google Scholar]
- 64.Saugel B, Phillip V, Gaa J. et al. Advanced hemodynamic monitoring before and after transjugular intrahepatic portosystemic shunt: implications for selection of patients—a prospective study. Radiology. 2012;262(1):343–352. doi: 10.1148/radiol.11110043. [DOI] [PubMed] [Google Scholar]
- 65.Anderson C L, Saad W E, Kalagher S D. et al. Effect of transjugular intrahepatic portosystemic shunt placement on renal function: a 7-year, single-center experience. J Vasc Interv Radiol. 2010;21(9):1370–1376. doi: 10.1016/j.jvir.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 66.Rössle M, Gerbes A L. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: a critical update. Gut. 2010;59(7):988–1000. doi: 10.1136/gut.2009.193227. [DOI] [PubMed] [Google Scholar]
- 67.Jalan R, Redhead D N, Thomas H W. et al. Mechanisms of changes in renal handling of sodium following transjugular intrahepatic portal systemic stent-shunt (TIPSS) Eur J Gastroenterol Hepatol. 1996;8(11):1111–1116. doi: 10.1097/00042737-199611000-00015. [DOI] [PubMed] [Google Scholar]
- 68.Gerbes A L, Gülberg V, Waggershauser T, Holl J, Reiser M. Renal effects of transjugular intrahepatic portosystemic shunt in cirrhosis: comparison of patients with ascites, with refractory ascites, or without ascites. Hepatology. 1998;28(3):683–688. doi: 10.1002/hep.510280313. [DOI] [PubMed] [Google Scholar]
- 69.Wong F, Sniderman K, Liu P, Allidina Y, Sherman M, Blendis L. Transjugular intrahepatic portosystemic stent shunt: effects on hemodynamics and sodium homeostasis in cirrhosis and refractory ascites. Ann Intern Med. 1995;122(11):816–822. doi: 10.7326/0003-4819-122-11-199506010-00002. [DOI] [PubMed] [Google Scholar]
- 70.Quiroga J, Sangro B, Núñez M. et al. Transjugular intrahepatic portal-systemic shunt in the treatment of refractory ascites: effect on clinical, renal, humoral, and hemodynamic parameters. Hepatology. 1995;21(4):986–994. [PubMed] [Google Scholar]
- 71.Wong F, Pantea L, Sniderman K. Midodrine, octreotide, albumin, and TIPS in selected patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2004;40(1):55–64. doi: 10.1002/hep.20262. [DOI] [PubMed] [Google Scholar]
- 72.Linas S L, Schaefer J W, Moore E E, Good J T Jr, Giansiracusa R. Peritoneovenous shunt in the management of the hepatorenal syndrome. Kidney Int. 1986;30(5):736–740. doi: 10.1038/ki.1986.249. [DOI] [PubMed] [Google Scholar]
- 73.Martin L G. Percutaneous placement and management of the Denver shunt for portal hypertensive ascites. AJR Am J Roentgenol. 2012;199(4):449–453. doi: 10.2214/AJR.12.9203. [DOI] [PubMed] [Google Scholar]
- 74.Teperman L. Living donation for the very ill patient with type I hepatorenal syndrome: are we ready? Liver Transpl. 2012;18(7):753–754. doi: 10.1002/lt.23455. [DOI] [PubMed] [Google Scholar]
- 75.Demirbas B T, Piskin T, Dayangac M. et al. Successful treatment of severe hepatorenal syndrome with living donor liver transplantation. Hepatogastroenterology. 2012;59(119):2305–2306. doi: 10.5754/hge10791. [DOI] [PubMed] [Google Scholar]
- 76.Lee J P, Kwon H Y, Park J I. et al. Clinical outcomes of patients with hepatorenal syndrome after living donor liver transplantation. Liver Transpl. 2012;18(10):1237–1244. doi: 10.1002/lt.23493. [DOI] [PubMed] [Google Scholar]
- 77.Boyer T D, Sanyal A J, Garcia-Tsao G. et al. Impact of liver transplantation on the survival of patients treated for hepatorenal syndrome type 1. Liver Transpl. 2011;17(11):1328–1332. doi: 10.1002/lt.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nadim M K, Kellum J A, Davenport A. et al. Hepatorenal syndrome: the 8th International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2012;16(1):R23. doi: 10.1186/cc11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eason J D, Gonwa T A, Davis C L, Sung R S, Gerber D, Bloom R D. Proceedings of consensus conference on simultaneous liver kidney transplantation (SLK) Am J Transplant. 2008;8(11):2243–2251. doi: 10.1111/j.1600-6143.2008.02416.x. [DOI] [PubMed] [Google Scholar]
- 80.Ruiz R, Barri Y M, Jennings L W. et al. Hepatorenal syndrome: a proposal for kidney after liver transplantation (KALT) Liver Transpl. 2007;13(6):838–843. doi: 10.1002/lt.21149. [DOI] [PubMed] [Google Scholar]
- 81.Runyon B A; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update Hepatology 20094962087–2107. [DOI] [PubMed] [Google Scholar]
- 82.Boyer T D Haskal Z J; American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: Update 2009 Hepatology 2010511306. [DOI] [PubMed] [Google Scholar]
- 83.European Association for the Study of the Liver . EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53(3):397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 84.Sharawey M A, Shawky E M, Ali L H, Mohammed A A, Hassan H A, Fouad Y M. Cystatin C: a predictor of hepatorenal syndrome in patients with liver cirrhosis. Hepatol Int. 2011;5(4):927–933. doi: 10.1007/s12072-011-9266-y. [DOI] [PubMed] [Google Scholar]
- 85.Seo Y S, Jung E S, An H. et al. Serum cystatin C level is a good prognostic marker in patients with cirrhotic ascites and normal serum creatinine levels. Liver Int. 2009;29(10):1521–1527. doi: 10.1111/j.1478-3231.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- 86.Davenport A, Cholongitas E, Xirouchakis E, Burroughs A K. Pitfalls in assessing renal function in patients with cirrhosis—potential inequity for access to treatment of hepatorenal failure and liver transplantation. Nephrol Dial Transplant. 2011;26(9):2735–2742. doi: 10.1093/ndt/gfr354. [DOI] [PubMed] [Google Scholar]
- 87.Verna E C, Brown R S, Farrand E. et al. Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci. 2012;57(9):2362–2370. doi: 10.1007/s10620-012-2180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Londoño M C, Cárdenas A, Guevara M. et al. MELD score and serum sodium in the prediction of survival of patients with cirrhosis awaiting liver transplantation. Gut. 2007;56(9):1283–1290. doi: 10.1136/gut.2006.102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fidelman N, Kwan S W, LaBerge J M, Gordon R L, Ring E J, Kerlan R K Jr. The transjugular intrahepatic portosystemic shunt: an update. AJR Am J Roentgenol. 2012;199(4):746–755. doi: 10.2214/AJR.12.9101. [DOI] [PubMed] [Google Scholar]
- 90.Nazal L, Cárdenas A. Prognostic markers in patients with ascites and hepatorenal syndrome. Dis Markers. 2011;31(3):139–146. doi: 10.3233/DMA-2011-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Al Sibae M R, Cappell M S. Accuracy of MELD scores in predicting mortality in decompensated cirrhosis from variceal bleeding, hepatorenal syndrome, alcoholic hepatitis, or acute liver failure as well as mortality after non-transplant surgery or TIPS. Dig Dis Sci. 2011;56(4):977–987. doi: 10.1007/s10620-010-1390-3. [DOI] [PubMed] [Google Scholar]
- 92.Acevedo J, Fernández J, Prado V. et al. Relative adrenal insufficiency in decompensated cirrhosis: Relationship to short-term risk of severe sepsis, hepatorenal syndrome, and death. Hepatology. 2013;58(5):1757–1765. doi: 10.1002/hep.26535. [DOI] [PubMed] [Google Scholar]
- 93.McCormick P A, Donnelly C. Management of hepatorenal syndrome. Pharmacol Ther. 2008;119(1):1–6. doi: 10.1016/j.pharmthera.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 94.Wong F, Blendis L. New challenge of hepatorenal syndrome: prevention and treatment. Hepatology. 2001;34(6):1242–1251. doi: 10.1053/jhep.2001.29200. [DOI] [PubMed] [Google Scholar]
- 95.Fernández J, Navasa M, Planas R. et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133(3):818–824. doi: 10.1053/j.gastro.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 96.Tyagi P, Sharma P, Sharma B C, Puri A S, Kumar A, Sarin S K. Prevention of hepatorenal syndrome in patients with cirrhosis and ascites: a pilot randomized control trial between pentoxifylline and placebo. Eur J Gastroenterol Hepatol. 2011;23(3):210–217. doi: 10.1097/MEG.0b013e3283435d76. [DOI] [PubMed] [Google Scholar]


