Abstract
The Amplatzer Vascular Plug (AVP) was created for peripheral embolization as a modification of the family of Amplatz septal occluders used in the treatment of congenital heart malformations. The device has evolved over the years and multiple versions have been launched into the market. Each of the versions of the device has some important modifications in terms of the size of the introducer's system, number of layers, and resultant thrombogenicity. It is very important for the operator to become familiar with the unique features of the AVP, and to understand the advantages and limitations of each model in the AVP family to achieve an optimal embolic result. The purpose of this article is to review the evolution and current clinical applications of the AVP in the field of interventional radiology, with emphasis on the advantages and limitations of this device in comparison with other embolization agents.
Keywords: Amplatzer Vascular Plug, embolization, coils, arteriovenous fistulas, interventional radiology
Objectives: Upon completion of this article, the reader will be able to discuss the indications and limitations of the Amplatzer Vascular Plug in different clinical scenarios, and identity the differences between the multiple versions of the plug family.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Device Description and Mechanism of Action
The Amplatzer Vascular Plug (AVP; St. Jude Medical, St. Paul, MN) is a disk made of a mesh of braided nitinol. The disk is attached to a 155-cm-long, PTFE-coated delivery wire with a stainless-steel micro screw, which allows the operator to release the plug into the final position by rotating the cable in a counter clockwise fashion using a supplied torque device. The plug can be retrieved and readjusted as needed before final release. It has platinum marker bands at the ends to increase radiopacity. The AVP is compatible with magnetic resonance imaging (MRI), within a static magnetic field of ≤ 3 T. The different types of AVP are listed and shown in Table 1.
Table 1. The AVP family.
| Version | Details | Comments |
|---|---|---|
AVP I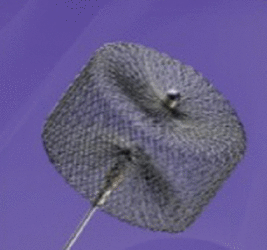
|
Provides rapid occlusion with precise positioning in short landing zones
Single-layered cylindrical disk Available in diameters ranging from 4 to 16 mm Requires 4–6F sheaths |
Not very thrombogenic in high flow situations, many times requiring placement of other embolics Easy placement |
AVP II
|
Provides faster occlusion and can adjust to the variable landing zones
Made out of a densely braided multilayer nitinol mesh with three components generating six barrier planes for acceleration of vascular occlusion Sizes: 3–22 mm Requires 4–7F sheaths |
Faster occlusion Longer segment needed The 3 lobes can be shortened by compression, allowing for better sealing and fitting into a shorter landing zone |
AVP III
|
Provides even faster occlusion, enhanced performance in high flow situations and improved wall apposition in challenging cases Sizes: 4–14 mm Requires 4–7F sheaths |
Not available in the United States |
AVP IV
|
Reaches distal vasculature through tortuous anatomy and is compatible with select diagnostic 0.038-in inner lumen catheters Sizes: 4–8 mm |
Difficult to inject contrast using the provided valve For occlusion of medium-sized (3–6 mm) vessels |
Abbreviation: AVP, Amplatzer Vascular Plug.
Source: Images courtesy of Saint Jude Medical.
The AVP acts as an embolic agent by promoting clot formation. The resistance to blood flow created by the nitinol mesh facilitates fibrin coverage during the clotting process. Thrombogenicity is increased by adding more braid layers or having more wire struts with smaller openings; the increased cross-sectional area coverage accelerates thrombus formation and embolization after plug placement. To increase the thrombogenicity of the device and prevent migration, it is recommended to oversize the device by 30 to 50% of the estimated diameter of the vessel (or 20–30% with the AVP IV). Large vessel size, high-flow situations, and abnormal coagulation factors can prolong the occlusion time with the AVP.1 The number of bodies of the plug and the final diameter of the vessel are important factors to determine the final length of the device. It is possible to some extent to overlap the bodies of the device in cases of shorter landing zones, or to elongate an oversize device in longer landing zones before final release.
The advantages of the AVP over coils are numerous in many clinical situations that require exact agent placement during embolization. The AVP has minimal risk of migration even in high-flow situations or in short landing zones, where coils may not be stable. The delivery mechanism also allows repositioning. It is possible to achieve occlusion of a large-diameter vessel with a single device rather than multiple coils. It can also be used when coil placement does not achieve complete occlusion.
Potential Challenges of Using the AVP
Device advancement: The AVP I and II require larger guiding catheters or delivery sheaths, which may be difficult to place in excessive vessel tortuosity and anatomic complexity. Flexible sheaths with different distal shape configurations offer both support and flexibility. The Flexor Check-Flo Introducers (Cook Medical, Bloomington, IN) and the Destination sheaths (Terumo Medical, Somerset, NJ) work well in most cases. Nonbraided sheaths such as the Brite Tip Sheath (Cordis, Bridgewater, NJ) have a tendency to kink. Sheaths with metallic spirals such as the Super Arrow-Flex sheaths (Arrow International Inc., Reading, PA) may increase resistance when advancing the plug and are not recommended by the manufacturer.1
The introduction of the AVP IV allows the operator to embolize vessels up to 6 mm in diameter using a diagnostic 5F delivery catheter, which greatly facilitates placing the device in tortuous vessels. A braided diagnostic catheter with a 0.038″ inner lumen is recommended. The manufacturer has tested specific catheters, such as 5F Boston Scientific Imager II, 4F-5F Cordis Tempo, and the 5F Merit Impress. In very tortuous situations, however, advancing the device through the catheter may result in dislodgement of the catheter tip from the intended embolization area, or the plug can get lodged inside the lumen of the catheter. Some techniques that have been described to overcome this problem include placing a sheath very distally and then using a guiding catheter inside the sheath to deploy the plug, or using a larger sheath and a buddy wire.1 2 3 Also, for the larger 7- and 8-mm AVP IVs, the use of a 5F. guiding catheter with a 0.056″ inner lumen such as the Envoy (Cordis) could be very helpful. The continuous injection of normal saline through the sheath or flushing port of the AVP IV can ease the advancement of the device by reducing the friction of the plug against the catheter wall.4
Persistent patency: Incomplete embolization could be related to a vessel that never occludes or to a vessel that was occluded initially and then later recanalizes.5 The problem of lack of thrombosis was more common with the earlier versions of the AVP, but can be also encountered with the newer versions (Fig. 1). The manufacturer increased the number of layers or lobes of the later generations of the plug to prevent this problem. The occlusion time has been highly variable depending on the caliber of the vessel, the coagulation status, the flow dynamics, size of the plug, and number of layers. The reported occlusion times for proximal splenic embolization have improved from 26 minutes with the AVP I to 15 minutes using the AVP II.2 Despite these advancements, the occlusion time is still unpredictable.1 6 The manufacturer recommends repeating angiograms every 5 minutes until complete stasis of the injected contrast is noted; however, this is problematic due to the prolonged radiation, contrast dose, and lengthened procedural time. Although there is not a fixed threshold amount of time, if thrombosis is not achieved within 10 to 15 minutes, many operators prefer to place a second plug or use additional coils, glue, or large gelatin sponge particles.7 The close mesh of the plug usually traps the coils or other embolization agents with minimal risk of distal embolization. It is very important to avoid the use of liquid embolics or small particles in critical areas such as the carotid, vertebral, or pulmonary artery circulation, as any distal embolization through the struts of the plug is still possible with potentially catastrophic results.7
Fig. 1.
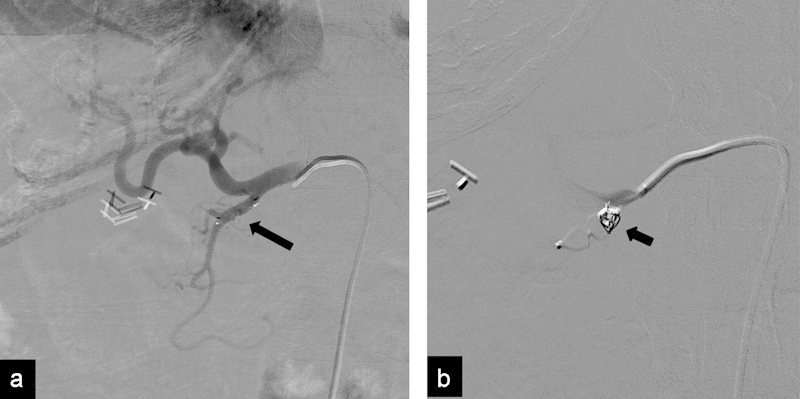
(a) Hepatic angiogram 2 weeks after embolization of the GDA with an AVP IV (arrow) for radioembolization shows persistent patency of the GDA. (b) Angiogram after placement of an additional proximal coil (arrow) shows occlusion of the GDA.
Another potential source of incomplete embolization is the presence of a side branch that originates near the proximal end of the plug. This situation is noted more frequently in the gastroduodenal artery (GDA), where small pancreaticoduodenal branches commonly originate very close to the origin of the GDA. This problem also occurs with coil embolization; however, the ability to place the plug very close to the origin seems to help alleviate this problem; occasionally, placement of a microcoil in the proximal branch may be required, and traversing the struts of the proximal portion of the plug can be very difficult or impossible. Another potential reason for incomplete embolization is using a plug that is not at least 20% oversized compared with the diameter of the larger vessel, where the plug configuration makes the device less likely to cause thrombosis (Fig. 2).
Fig. 2.
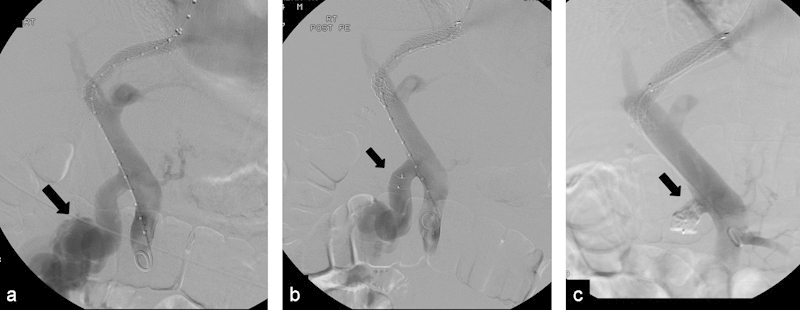
(a) Patient with cirrhosis and portal hypertension with bleeding duodenal varices. Portogram after TIPS shows large portomesenteric shunt (arrow) causing the ectopic varices; an AVP 1 was placed. (b) Patient had recurrent bleeding 2 weeks after initial embolization procedure. Portogram demonstrates that the portomesenteric shunt is still patent; note that the AVP 1 is about the same size as the vein. (c) Portogram shows occlusion of the shunt after injection of NBCA glue proximal to the plug (arrow).
Vessel recanalization: A vessel that was initially occluded can later recanalize, and although this is rare, it can have serious consequences.8 9 In most series where follow-up angiograms have been obtained, the incidence of vessel recanalization seems to be much lower than that reported with coils. For example, following embolization of pulmonary arteriovenous malformations (PAVMS), the overall recanalization rate of PAVMS using the plug has been reported to be 1 to 7%, while the recanalization rate with coils is 8 to 15%.1
Plug detachment: The configuration of the AVP requires a relatively straight segment of target vessel with a fairly constant diameter. In cases with many angulated branching points, jamming of the screw thread has resulted in early detachment.10
Contrast injection: In most cases, contrast can be injected through the delivery sheath to confirm plug position before final release. With the AVP IV, especially with the 7 and 8 mm sizes, it is very difficult to inject contrast through the proximal hemostatic valve due to the tight seal. The excessive pressure required to inject contrast frequently results in damage to the seal of the valve, and checking final position may be difficult or impossible.10
Device cost: The device is significantly more expensive compared with regular pushable and most retrievable coils. In the United States, the price of the plug varies from ∼$800 for the AVP I to ∼$1,000 for the AVP IV. Despite the high initial cost of the device, cost savings in many clinical applications could be significant compared with using multiple coils, as a single device is adequate in most cases. In some cases, where more than one plug or the use of additional embolic agents may be required, the cost savings could be minimal to nonexistent. In most clinical applications, however, the use of the AVP has resulted in cost savings not only from the cost of the embolization supplies but also from the reduction in overall procedural times.
Reconfiguration: Reconfiguration of the AVP was reported by Sheridan et al, where the device, due to its natural tendency to achieve its nominal diameter, resulted in shorter and wider devices in follow-up imaging.11 As the device is deployed, elongated, and oversized in most instances, reconfiguration is probably an unreported imaging finding of little clinical significance in most peripheral applications.
Device repositioning: In the majority of the cases, the device can be retracted and repositioned as needed. Removing the device can sometimes cause vessel spasm and potential vascular injury. It is recommended not to retract the plug into the guide catheter or sheath, but rather to push the catheter or sheath over the fixed plug to collapse it. Retracting the AVP IV using nonbraided catheters is not recommended, as the tip of the catheter can be damaged.
Device migration: Migration of the AVP is very rare. Potential causes include placing a plug that is not oversized for the vessel, or using the plug in very short landing zones, especially in high-flow situations such as arteriovenous fistulas (AVF). Surgical manipulation of a vessel into which an AVP has been placed has resulted in device migration.12 Device migration with embolization seems to be a relatively common complication (up to 12% incidence) after the treatment of aortic pseudoaneurysms. These extreme cases present unique challenges such as very high flows, large necks, and very short landing zones.13 Migration of the cardiac plugs has also been reported. Migrated devices can be removed by engaging one of the short tips with a snare or by engaging the mesh itself using biopsy forceps, and then collapsing the plug inside a larger sheath for removal.14 15 Endothelialization of the migrated device can make the retrieval very difficult and potentially traumatic.16
Clinical Applications
Vascular Uses
Pulmonary Arteriovenous Malformations Embolization
One of the most accepted applications of the AVP is in the embolization of PAVMs. Extensive experience has been acquired with the use of the different versions of the plug.17 18 19 20 21 The advantages of the device in these patients are multiple: usually only one device is sufficient to treat each lesion; the risk of distal embolization is minimal in this particular area where any distal embolization could lead to a stroke; and operators can achieve a very distal occlusion of the feeding vessel at the neck of the venous sac, thereby reducing the risk of embolizing branches to adjacent normal lung and the likelihood of persistent perfusion of the venous sac by bronchial collaterals or pulmonary artery recanalization (Fig. 3).17 The mean vessel occlusion time is just over 3 minutes for AVPs used in the treatment of simple PAVMs, which also could minimize the opportunity for systemic embolization of microthrombi from the device surface.18
Fig. 3.
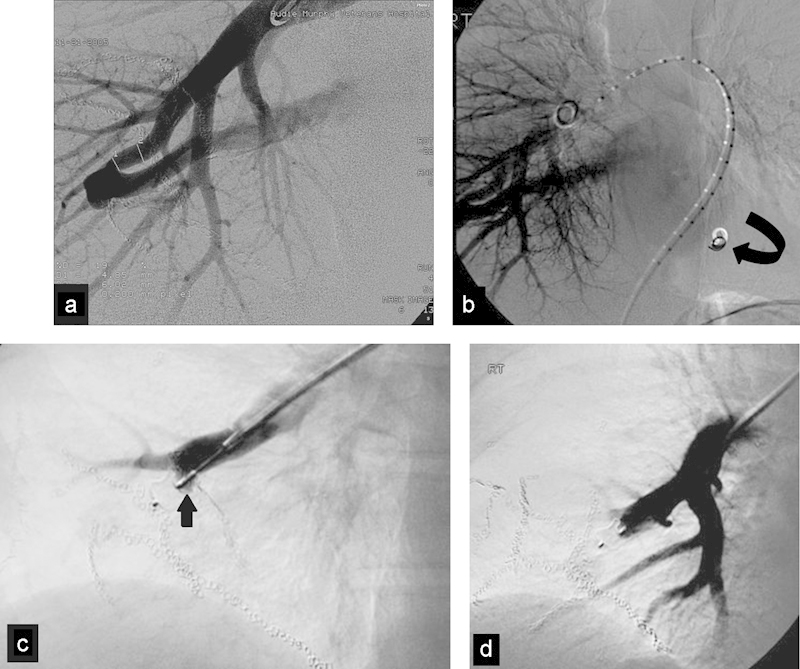
(a) DSA shows a pulmonary AVF in a patient with remote gunshot wound to the chest. (b) DSA after coil placement shows migration of the coil into the left ventricle (curved arrow). The coil migrated to the femoral artery where it was retrieved. (c) DSA confirming adequate position of the plug (arrow) before final release. (d) DSA after AVP I placement shows successful exclusion of the AVF with a single plug.
Hart et al treated 69 patients with 161 PAVMS, with a total of 120 lesions treated with a single AVP 1 (75%); 27 complex PAVMs also required coils to occlude smaller additional feeding arteries. Fourteen PAVMs with small tortuous feeding arteries were treated with coils alone. Although reinterventions were required in some patients, all vessels previously treated by AVPs remained occluded during follow-up angiography.17 The overall recanalization rate of the PAVMS using the plug has been around 1 to 7%; in comparison, coil embolization is associated with a PAVM recanalization rate of 8 to 15%.1 Some authors suggest combining the AVP I with coils to decrease the recanalization rates.22 More recent limited experience with the AVP IV also has shown promising results with no recanalization of the aneurysmal sac after a mean follow-up of 21 months.23
There is controversy in the literature if embolization of the venous sac of the PAVM is superior to the traditional embolization of the feeding artery alone. Embolization of the sac eliminates the risk of recurrence but requires the use of multiple soft detachable coils, as some of the sacs are of considerable size. Some authors have performed combined embolization of the sac with detachable coils and the feeding artery with an AVP, an advantageous approach in complex PAVMs with short feeding arteries and large outflow vessels.24
The AVP has been used also in cases of Hughes-Stovin syndrome, a rare condition characterized by peripheral deep venous thrombosis accompanied by single or multiple pulmonary arterial aneurysms.25
Splenic Artery Embolization
Proximal splenic artery (SA) embolization is an ideal indication for the AVP because the anatomy tends to be favorable with long landing zones (Fig. 4). The AVP facilitates this procedure that usually requires placement of multiple coils (with a risk of distal migration of up to 30%), and the potential for splenic infarction.2 26 The AVP has been used for SA embolization in cases of trauma, hypersplenism, the treatment of portal hypertension complications, and SA steal syndrome.2 6 7 10 26 27 28 The main challenge occurs in those patients with very tortuous SA where advancing the delivery sheath can be very difficult.2 The mean occlusion time was on average 10 minutes for patients with SA syndrome and trauma.6 27 A significant difference in the occlusion time has been described, with patients with portal hypertension and SA steal syndrome requiring more coils or AVPs to achieve complete occlusion due to their hyperdynamic circulation and larger size vessels than trauma patients.2 In the study by Zhu et al comparing AVP I and II versus coils, there was no statistically significant difference in average procedural time, mean occlusion time, or average cost of the embolic material; there was, however, a statistically significant difference in the radiation dose in each group, with an average dose of 842 mGy in the AVP group and 1,309 mGy in the coil group.2
Fig. 4.
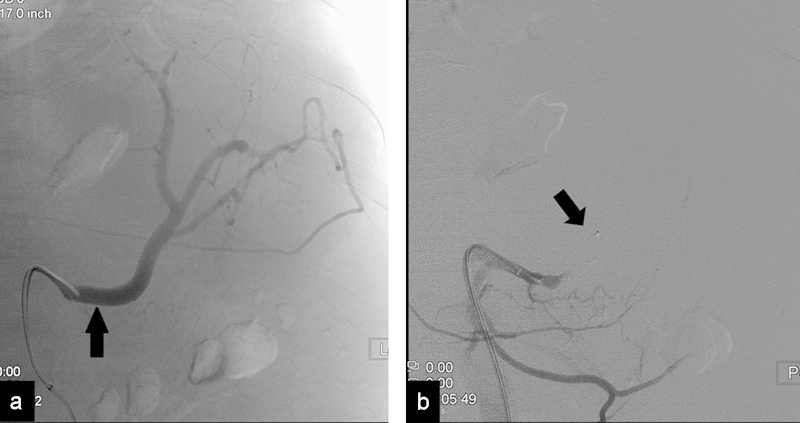
(a) Angiogram in a patient with hypersplenism shows patent splenic artery (arrow). (b) Angiogram after proximal embolization shows occlusion of the splenic artery with the plug (arrow).
Embolization of Aortoiliac Segments
The AVP has been used extensively to prevent endoleaks occluding the internal iliac artery (IIA), inferior mesenteric artery, and accessory renal arteries prior to EVAR, as well as the subclavian artery before or during thoracic aneurysm endovascular repair.29 30 31 The main advantage of the AVP in IIA embolization is that very proximal occlusion is possible with a single device, which may be associated with a lower incidence of pelvic ischemia that can occur with distal embolization following embolization with coils30 (Fig. 5). However, even proximal IIA embolization can be associated with buttock claudication, and the utility of IIA embolization in the prevention of type II endoleaks has been questioned, with recent studies favoring placing bifurcated iliac stents to preserve flow into the IIA.32 33 34
Fig. 5.
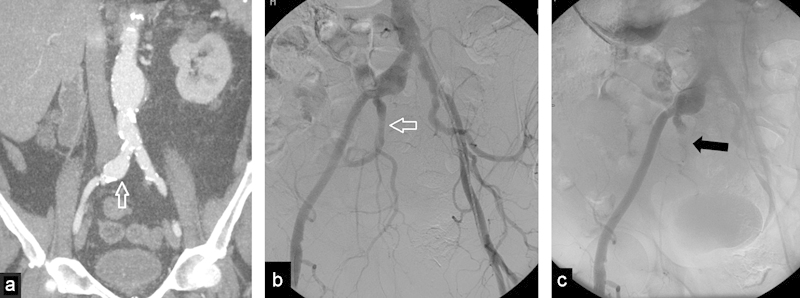
(a) Coronal image of a computed tomography with contrast, shows an abdominal aortic aneurysm with extension into the right common iliac artery (arrow). (b) Angiogram before embolization shows right common iliac artery aneurysm with patent right internal iliac artery (arrow). (c) Angiogram after internal iliac artery embolization with an AVP (arrow). Note very proximal embolization.
The AVP can also be used to treat internal iliac aneurysms by placing the plug proximal and distal to the aneurysm. It can also be used in the endovascular occlusion of common iliac aneurysms by combining it with a femoro-femoral bypass.35 36
The AVP has been used to occlude the abdominal aorta in patients with ruptured AAA who have no other surgical options available.37 The AVP, but mainly the septal occluder, is used in extreme cases of complex pseudoaneurysms of the aorta and its major branches. However, only very selected cases are candidates for this procedure, which require a sufficient rim of tissue to prevent plug migration and a relatively narrow neck. In 25 reported cases, there was a 12% (3/25) device embolization rate and an 8% (2/25) failure rate necessitating conversion to open repair.13 38 39 40 41 Embolization of the device has been reported even after 12 months.13 The AVP also has been successfully used in the treatment of aortic branch artery pseudoaneurysms associated with intramural hematoma.42
Arteriovenous Fistulas Embolization
The AVP is especially useful in the treatment of high-flow situations such as AVF, where the risk of coil migration is high (Fig. 3). Thanks to the plug, the need to use adjunct techniques to prevent coil migration, such as placement of occlusion balloons in the venous and arterial side (“stop flow technique”), or using a constrained Wallstent bridging the arterial and venous sides of the AVF, are rarely required.43 44 The AVP has been used to occlude renal, mesenteric, subclavian, carotid, and coronary AVFs.45 46 47 48 49 50 Due to the lower thrombogenicity of the AVP I or AVP IV and higher flow of rates through these fistulas, occlusion times over 10 to 35 minutes have been reported as well as the need for additional coil or glue embolization (Fig. 6).51
Fig. 6.
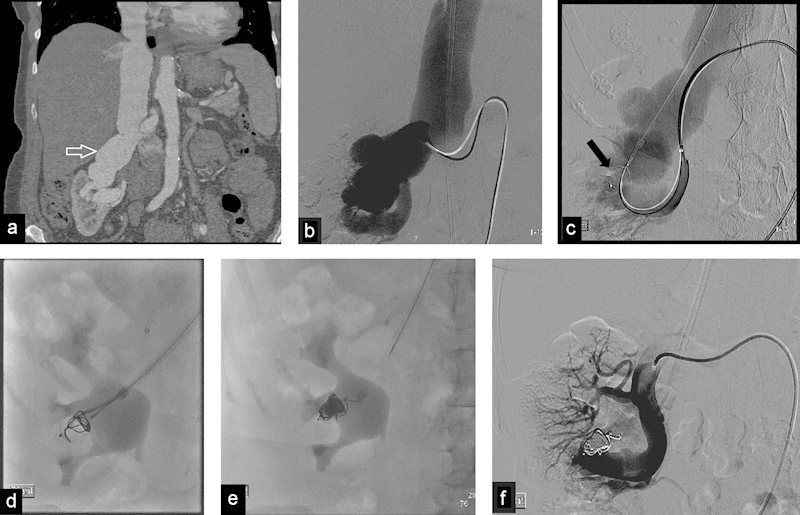
(a) Coronal contrast enhanced CT image shows massive dilatation of the right renal vein (arrow) due to long standing renal AVF. (b) DSA shows a high flow renal AVF. The arterial branches are barely visualized due to the enlarged renal veins. (C) DSA shows use of a combined transarterial and transvenous approach with precise placement of the AVP 1 at the site of the fistula (arrow). (d) Radiograph shows that with the plug still attached to the cable, a micro catheter is inserted inside the plug and metallic coils are placed to increase thrombogenicity. (e) Radiograph after plug release shows the AVP filled with coils. (f) DSA after plug placement shows successful exclusion of the AVF.
The AVP I is a very useful device in the treatment of AVF with short necks. A “stuffing technique” to make the plug even shorter in very short landing zones has been described. After the plug is deployed to the midpoint, the sheath is advanced over the AVP, causing the proximal tip of the AVP (still in the guiding sheath) to invaginate into the device already deployed distal half before final release.52 If the anatomy is favorable, using an arterial approach alone is possible to embolize the AVF. The use of a combined arterial and transvenous approach to deploy the device precisely into the fistula itself has been described, providing maximum stiffness and stability with minimal risk of device migration (Fig. 6).45
Radioembolization
The administration of yttrium-90 particles (radioembolization) has become an integral part of the management of patients with advanced primary liver cancer and liver-dominant metastatic disease. A very important component of this procedure is the embolization of any potential gastrointestinal branches to prevent accidental migration of the radioactive particles into the gastrointestinal tract that could lead to very serious and difficult-to-treat ulcerations. This is usually accomplished by preemptive embolization of the GDA, right or left gastric artery, and any supraduodenal or retroduodenal branches. Due to the small size of these branches, the AVP has primarily been used to embolize the larger GDAs; in this vessel, the AVP has the advantage of allowing very proximal embolization at the origin of the vessel instead of using multiple coils.53 In theory, a shorter stump may decrease the incidence of perfused proximal side branches as well as development of hepatoenteric anastomotic vessels, which may serve as pathways for nontarget deposition of Y-90 particles.53 It has been shown that a mean of 6.2 standard pushable coils are needed for GDA occlusion, while usually only one AVP is required.54 Also, trying to place a coil very proximally in the origin of the GDA has a significant risk of coil migration into the hepatic artery, which in some cases may necessitate retrieval of the migrated coil. Initial reports with the AVP II showed that despite the limitations of placing the large-lumen catheter or sheaths into the GDA, the fluoroscopy time (7.8 minutes for coils vs. 2.6 minutes for the AVP II) and embolization time (23.1 minutes for coils vs. 8.8 minutes for the AVP II) were significantly shorter with the plug. However, in patients with short or angulated celiac origins, it was not possible to advance the device into place.55 The AVP IV has been used recently to embolize the GDA. This smaller device is easy to use in most patients, but persistent patency and the presence of small duodenal branches in the proximal GDA are potential causes of incomplete embolization requiring additional coils (Fig. 2).
Portal and Mesenteric Venous Circulation
The relatively large size of the left coronary vein and other varices in the portal vein territory makes them ideal territories for the use of the AVP in the embolization of bleeding varices in patients with portal hypertension (Fig. 7). Owing to the tendency to have distal recanalization with potential variceal rebreeding, some authors have combined the use of the plug with injection of sclerosing agents distally. The unique design of the AVP allows the operator to place a microcatheter distally first, and then use the plug proximally to prevent reflux of the sclerosing agent into the main portal system. After the distal varices are sclerosed, the plug is released to obtain proximal embolization. Usually the esophageal varices (EV) are embolized after the transjugular portosystemic shunt (TIPS) is created; one of the limitations of the system is the need to advance the delivery sheath through the freshly placed stent graft, with a minor but real risk of TIPS stent migration. A longer sheath (> 65 cm) is usually required and due to the limited flexibility of these systems, a very good support with adequate distal purchase is needed, as the advancement of the relatively rigid larger plugs can be very difficult and may result in dislodgement from the intended embolization area. Varices can also be embolized using a transhepatic approach, but due to usually large size of these veins, the larger AVP II with the need for relatively large 6 or 7F sheaths is required most of the time; this represents a significant disadvantage of this approach due to the risk of intraperitoneal bleeding, and many operators embolize the transhepatic access route to ameliorate the risk of bleeding.56
Fig. 7.
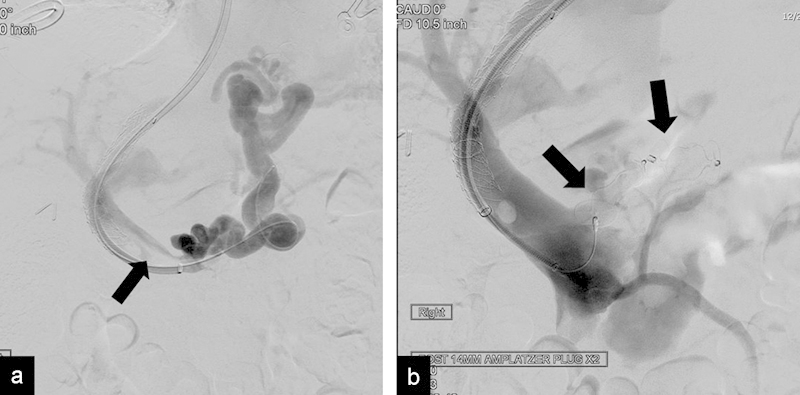
(a) Portogram shows a TIPS with partial thrombosis of the main portal vein (arrow) and large esophageal varices. (b) Portogram shows verification of the final position of the AVPs (arrows) before final release. Minimal residual thrombus in the main portal vein is also seen after mechanical thrombectomy.
In patients with severe hepatic encephalopathy or severe liver failure after TIPS placement, the plug has been used to occlude the TIPS. Acute closure of the TIPS can be poorly tolerated, however, as the acute increase in portal venous pressure can lead to recurrence of variceal bleeding and ascites as well as severe acute hemodynamic changes.56 57 In patients with acute complications of such TIPS closures, it is possible to reopen the TIPS by removing the plug (Fig. 8).
Fig. 8.
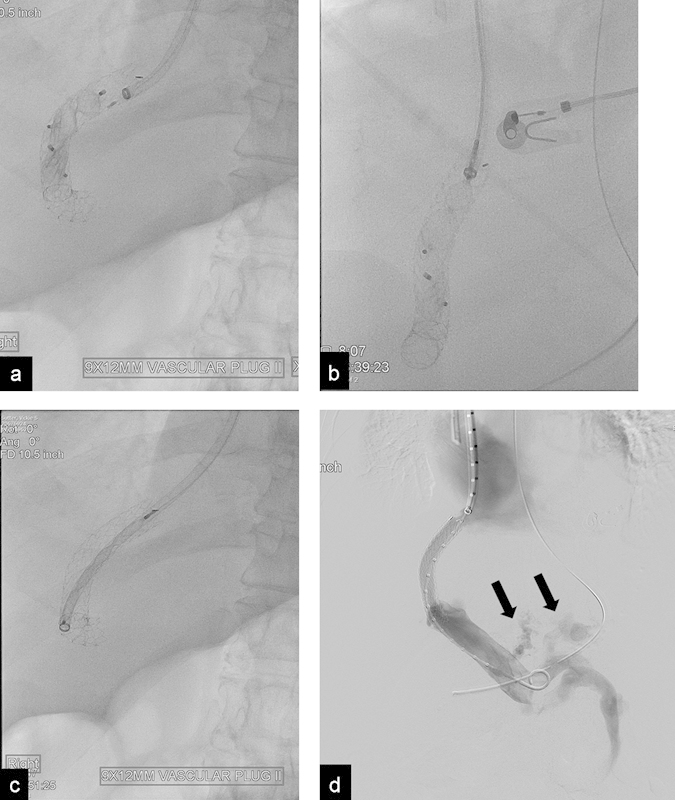
(a) Radiograph shows two AVPs used to close a TIPS in a patient with severe encephalopathy. Patient developed severe variceal bleeding after shunt closure. (b) Radiograph shows the proximal end of the plug being collapsed after it was grabbed with a snare. (c) Radiograph shows the plug completely collapsed inside a 10 Fr. sheath. (d) Portogram after plug retrieval and balloon dilatation of the shunt shows patent TIPS; the esophageal varices (arrows) were subsequently embolized.
The plug is also very useful in the occlusion of large portosystemic shunts that may cause refractory hepatic encephalopathy or bleeding from ectopic varices.58 59 60 They can also be used in the treatment of congenital portocaval shunts such as the type 2 Abernethy malformations.61 62 63 In the treatment of these congenital shunts that may produce congestive heart failure, another advantage of the device is that it can be deployed temporally and the hemodynamic effects of the shunt closure is evaluated, to see if acute shunt closure is well tolerated before final deployment. This use is similar to a temporary occlusion performed with an open technique.
Gastric Varices and Balloon-Assisted Retrograde Transvenous Obliteration
Balloon-assisted retrograde transvenous obliteration (BRTO) is a procedure that has been used for over two decades in Asia for the treatment of gastric varices (GV). Typically, an occlusion balloon is placed in the left adrenal vein or gastrorenal shunt and is left in place overnight, while the GV are treated after injection of a liquid sclerosing agent. The main complication of BRTO is nontarget embolization due to balloon deflation or migration. The plug has been successfully used instead of the occlusion balloon for the BRTO and in the treatment of other portosystemic shunts; this technique has been termed the plug-assisted retrograde transvenous obliteration (PARTO). Gwon et al reported 20 cases where the AVP II was deployed using a sheath at the most dilated gastrorenal shunt, then a 4F angled-tip catheter was advanced between the vascular plug and the shunt wall. The vascular plug was then pulled down to the narrowest gastrorenal shunt, where contrast material stasis at the gastrorenal shunt was achieved; additional embolization was performed with gelatin sponges to embolize the gastrorenal shunt, GV, afferent veins, and efferent veins. After embolization, the catheter was removed and the vascular plug was detached.64 Preliminary results using this technique show the technique to be highly effective without the need to keep the occlusion balloon in place for many hours (Fig. 9).
Fig. 9.
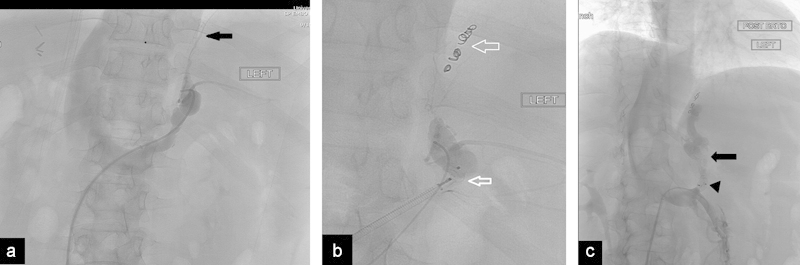
(a) Use of the plug for BRTO. Left adrenal venogram demonstrates a gastrorenal shunt with a left pericardiophrenic vein (arrow). (b) Radiograph shows the a microcatheter placed distally with the attached plug deployed proximally (black arrow). The pericardiophrenic vein was embolized with coils (white arrow). (c) Left renal venogram shows the plug released in the proximal shunt (arrowhead). The opacified sclerosing agent is visualized inside the gastrorenal shunt (arrow).
Preoperative Portal Vein Embolization
Portal vein embolization (PVE), performed to produce occlusion of right portal vein branches to induce hypertrophy of the left lobe prior to right hemihepatectomy, is an accepted procedure in many major cancer centers. Multiple embolic agents have been described including gelatin sponge, polyvinyl alcohol particles, Histoacryl glue, coils, and a combination of the above. The main potential risk of many of these agents is the nontarget embolization of the unaffected liver that could jeopardize left lobe hypertrophy and subsequent suitability for surgery. The use of the AVP for PVE was first described by Ringe et al, to produce proximal embolization after the distal portal branches were embolized with particles65 (Fig. 10) . A very effective technique to embolize the portal vein was described by Bent et al, using an ipsilateral approach through which the AVP I was placed 1 cm distal to the portal bifurcation; the distal portal branches were subsequently embolized with a combination with Histoacryl glue and iodized oil in 16 patients with a mean procedure time of 30 minutes. In this technique, the few minutes it takes the plug to completely embolize the portal vein allows the portal flow to carry the liquid embolics distally, which in theory prevents the development of intrahepatic portoportal collateral veins from the left to the right lobe that could potentially reduce hypertrophy levels.66 This technique has been very successful in reducing nontarget embolization. Also, the use of ipsilateral technique decreases the risk of complications of the remnant lobe. Yoo et al used the plug in combination with gelatin foam for a success rate of 97.6% (40/41), with one case of liver abscess and one extensive portal vein thrombosis.67
Fig. 10.
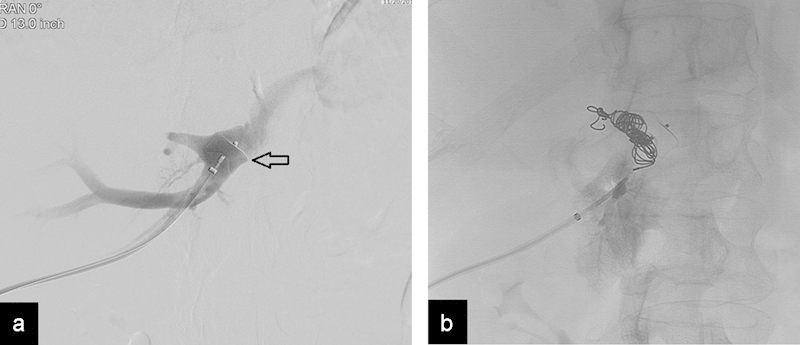
(a) Use of the AVP for preoperative portal vein embolization. Radiograph shows AVP I (arrow) placed 1 cm distal to the portal bifurcation using a percutaneous access. (b) Radiograph shows the deployed AVP; the right portal vein was embolized with particles and additional coils.
Solid-Organ Percutaneous Access Closure
The plug can be used for the embolization of the percutaneous access after transhepatic or transplenic portal vein access, both to prevent bleeding, and to occlude the biliary tract after percutaneous biliary interventions to prevent bile leakage (Fig. 11). The AVP has been very successful in preventing percutaneous access complications, but the cost of the device compared with that of other embolic agents and the risk of infectious complications, as a permanent foreign body is left in place, needs to be considered before its routine use.68 69
Fig. 11.
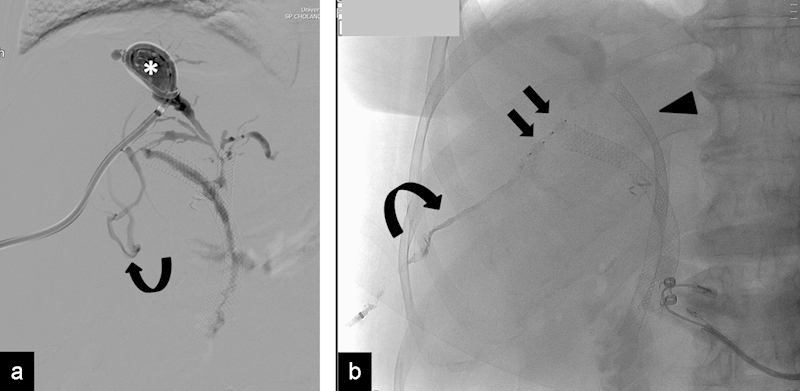
(a) Cholangiogram shows a biloma with multiple strictures after biliary drainage in a patient with cholangiocarcinoma and previous metallic stents, presenting with cholangitis (*). Communication with hepatic veins (curved arrow) is also seen. (b) Radiograph shows placement of an additional metallic stent (arrowhead); after stent deployment, two AVP IV plugs were used to close the tract (arrows). Histoacryl glue was then placed to further close the tract and the skin entry site to control ascites leakage. The plugs prevented glue migration into the biliary ducts.
Gonadal Vein Embolization
Embolizations of the spermatic vein in the treatment of varicocele and the ovarian vein for pelvic congestion syndrome are minimally invasive procedures that slowly are gaining wider acceptance.70 The use of the plug is relatively straightforward in these applications, but due to the long segment of these veins, multiple devices may be required which could be cost prohibitive.71 The plug can also be used in this scenario to prevent proximal reflux of liquid sclerosing agents. For this technique, a microcatheter is deployed distally in the ovarian and pelvic veins, after which the plug is deployed proximally in the ovarian vein. After the sclerosing agent is injected, the microcatheter is removed and the plug is deployed.
Dialysis Fistulas Complications
The AVP has been used to treat many complications of dysfunctional dialysis AVF. The AVP has been an excellent alternative to surgical ligation of the fistula in patients with severe arm swelling and untreatable central venous occlusion, ischemic steal syndrome, or hyperdynamic heart failure, with a reported technical success rate of 100%.72 73
The plug is also frequently used for the occlusion of competitive collaterals in immature dialysis fistulas, and in selected patients for the occlusion of the radial artery immediately proximal to the fistula in patients with distal ischemia.74 75
Emergency Embolization and Neurovascular Applications
The AVP can be used in many acute situations that needed rapid embolization, including ruptured aortic, renal, iliac, and other large vessel aneurysms; ruptured tumors; and embolization of traumatic injuries of the spleen, liver, kidneys, and pelvis.76 77
The AVP has been used in a wide variety of conditions that require sacrifice of the carotid or vertebral circulations including trauma, fusiform giant cerebral aneurysms, caroticocavernous fistulae, and to control massive bleeding in patients with advanced head, neck, or skull base tumors with vascular invasion.76 78 79 80 The rapid occlusion time prevents the potentially devastating complication of thrombus formation within the affected vessel and distal embolization into the intracranial circulation. There is a very low risk of distal migration of the device itself due to its radial expansion.
Nonvascular Uses
Genitourinary Applications
Permanent occlusion of the ureters is indicated in patients with anorectal, vaginal, and/or perineal fistulae that continue to leak urine despite placement of bilateral nephrostomy tubes for urinary diversion. Multiple materials have been described to occlude the ureters, with mixed results, including coils with or without gelatin sponge or glue; detachable balloons; constrained covered stents; nylon or silicon plugs; and electrocautery or fulguration. Direct ureteral occlusions can also be achieved by ureteral clipping.81 82 The ureteral closure rates combining coils with gelatin sponge or tissue adhesive have been mixed, with success rates of 50 to 100%.81 82 A high incidence of partial or complete migration of the embolization material with need for reintervention has been reported.82 The use of the AVP alone has been also insufficient for the permanent occlusion of the ureter. Modifications to the use of the AVP include adding latex layers by placing a surgical glove finger over the plug,83 84 and using the plug as a scaffold for additional coil- and tissue-adhesive application. The success rates for these combined techniques range from 80 to 100%.85 86
Occlusion of Gastrointestinal and Respiratory Fistulas
Bronchopleural and esophagorespiratory fistulas are serious problems after pulmonary resection and esophagectomy. The AVP has been reported to be very successful when used alone or combined with glue and coils.87 88 89 More recently, one-way endobronchial valves have also been very useful for the treatment of bronchopleural fistulas.90
Conclusion
The AVP is a very versatile embolization agent that allows the operator to treat a variety of conditions including very challenging vascular lesions, such as high-flows AVF and vessels with short landing zones, with minimal risk of device distal embolization or migration. Variable and sometimes unpredictable occlusion times remain a major shortcoming of this device. Becoming familiar with the different versions of the device within the AVP family and the utility of combining the AVP with other embolization therapies is very important. As in all embolization therapies, meticulous technique, modified according to the architecture of the individual lesion to be treated, and with the appropriate choice of the occlusive agent, are key ingredients to a successful embolization therapy.
References
- 1.Wang W, Li H, Tam M D, Zhou D, Wang D X, Spain J. The Amplatzer Vascular Plug: a review of the device and its clinical applications. Cardiovasc Intervent Radiol. 2012;35(4):725–740. doi: 10.1007/s00270-012-0387-z. [DOI] [PubMed] [Google Scholar]
- 2.Zhu X, Tam M D, Pierce G. et al. Utility of the Amplatzer Vascular Plug in splenic artery embolization: a comparison study with conventional coil technique. Cardiovasc Intervent Radiol. 2011;34(3):522–531. doi: 10.1007/s00270-010-9957-0. [DOI] [PubMed] [Google Scholar]
- 3.Rimon U, Heldenberg E, Golan G, Shinfeld A, Garniek A. Amplatzer Vascular Plug: expanding the applications spectrum. Cardiovasc Intervent Radiol. 2008;31 02:S84–S87. doi: 10.1007/s00270-007-9042-5. [DOI] [PubMed] [Google Scholar]
- 4.Ferro C, Rossi U G, Bovio G, Petrocelli F, Seitun S. The Amplatzer Vascular Plug 4: preliminary experience. Cardiovasc Intervent Radiol. 2010;33(4):844–848. doi: 10.1007/s00270-009-9749-6. [DOI] [PubMed] [Google Scholar]
- 5.White H A, Travis S J. The Amplatzer vascular plug. Cardiovasc Intervent Radiol. 2008;31(2):448–449. doi: 10.1007/s00270-007-9259-3. [DOI] [PubMed] [Google Scholar]
- 6.Maurer M H, Mogl M T, Podrabsky P. et al. Splenic artery syndrome after orthotopic liver transplantation: treatment with the Amplatzer vascular plug. Cardiovasc Intervent Radiol. 2011;34(6):1208–1213. doi: 10.1007/s00270-010-0083-9. [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Tam M D, Spain J, Quintini C. Gelfoam-assisted Amplatzer vascular plug technique for rapid occlusion in proximal splenic artery embolization. AJR Am J Roentgenol. 2013;200(3):677–681. doi: 10.2214/AJR.12.8949. [DOI] [PubMed] [Google Scholar]
- 8.Dorenberg E J, Hafsahl G, Andersen R, Krohg-Sørensen K. Recurrent rupture of a hypogastric aneurysm caused by spontaneous recanalization of an Amplatzer vascular plug. J Vasc Interv Radiol. 2006;17(6):1037–1041. doi: 10.1097/01.RVI.0000222821.56922.03. [DOI] [PubMed] [Google Scholar]
- 9.Gómez-Martínez P, Ciampi Dopazo J J, González Fejás A, Lanciego C. Spontaneous recanalization after embolization of the renal artery with an Amplatzer vascular plug 4 [in Spanish] Radiologia. 2014;56(4):357–360. doi: 10.1016/j.rx.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Pech M, Mohnike K, Wieners G. et al. Advantages and disadvantages of the Amplatzer Vascular Plug IV in visceral embolization: report of 50 placements. Cardiovasc Intervent Radiol. 2011;34(5):1069–1073. doi: 10.1007/s00270-011-0150-x. [DOI] [PubMed] [Google Scholar]
- 11.Sheridan B, Ward C, Justo R. Reconfiguration of the Amplatzer Vascular Plug II 5 months after occlusion of venovenous collateral in a bidirectional cavopulmonary circulation. Catheter Cardiovasc Interv. 2010;75(6):857–860. doi: 10.1002/ccd.22459. [DOI] [PubMed] [Google Scholar]
- 12.Maleux G, Rega F, Heye S, Troost E, Budts W. Asymptomatic migration of a first-generation AMPLATZER vascular plug into the abdominal aorta: conservative management may be an option. J Vasc Interv Radiol. 2011;22(4):569–570. doi: 10.1016/j.jvir.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 13.Patel A V, Gupta S, Laffin L J, Retzer E M, Dill K E, Shah A P. One size does not fit all: case report of two percutaneous closures of aortic pseudoaneurysm and review of the literature. Cardiovasc Revasc Med. 2014;15(3):160–164. doi: 10.1016/j.carrev.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Guimaraes M, Denton C E, Uflacker R, Schonholz C, Selby B Jr, Hannegan C. Percutaneous retrieval of an Amplatzer septal occluder device that had migrated to the aortic arch. Cardiovasc Intervent Radiol. 2012;35(2):430–433. doi: 10.1007/s00270-011-0139-5. [DOI] [PubMed] [Google Scholar]
- 15.Chan N Y, Choy C C, Lau C L. Successful percutaneous retrieval of a dislodged left atrial appendage occlusion device with double transseptal sheaths and biopsy bioptome. Catheter Cardiovasc Interv. 2015;85(2):328–331. doi: 10.1002/ccd.25647. [DOI] [PubMed] [Google Scholar]
- 16.Obeid S, Nietlispach F, Lüscher T F, Alibegovic J. Percutaneous retrieval of an endothelialized AMPLATZER cardiac plug from the abdominal aorta 6 months after embolization. Eur Heart J. 2014;35(47):3387. doi: 10.1093/eurheartj/ehu361. [DOI] [PubMed] [Google Scholar]
- 17.Hart J L, Aldin Z, Braude P, Shovlin C L, Jackson J. Embolization of pulmonary arteriovenous malformations using the Amplatzer vascular plug: successful treatment of 69 consecutive patients. Eur Radiol. 2010;20(11):2663–2670. doi: 10.1007/s00330-010-1851-2. [DOI] [PubMed] [Google Scholar]
- 18.Abdel Aal A K, Hamed M F, Biosca R F, Saddekni S, Raghuram K. Occlusion time for Amplatzer vascular plug in the management of pulmonary arteriovenous malformations. AJR Am J Roentgenol. 2009;192(3):793–799. doi: 10.2214/AJR.08.1534. [DOI] [PubMed] [Google Scholar]
- 19.Kucukay F, Özdemir M, Şenol E, Okten S, Ereren M, Karan A. Large pulmonary arteriovenous malformations: long-term results of embolization with AMPLATZER vascular plugs. J Vasc Interv Radiol. 2014;25(9):1327–1332. doi: 10.1016/j.jvir.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 20.Tapping C R, Ettles D F, Robinson G J. Long-term follow-up of treatment of pulmonary arteriovenous malformations with AMPLATZER Vascular Plug and AMPLATZER Vascular Plug II devices. J Vasc Interv Radiol. 2011;22(12):1740–1746. doi: 10.1016/j.jvir.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 21.Letourneau-Guillon L, Faughnan M E, Soulez G. et al. Embolization of pulmonary arteriovenous malformations with Amplatzer vascular plugs: safety and midterm effectiveness. J Vasc Interv Radiol. 2010;21(5):649–656. doi: 10.1016/j.jvir.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 22.Trerotola S O, Pyeritz R E. Does use of coils in addition to Amplatzer vascular plugs prevent recanalization? AJR Am J Roentgenol. 2010;195(3):766–771. doi: 10.2214/AJR.09.3953. [DOI] [PubMed] [Google Scholar]
- 23.Rabellino M, Serra M, Peralta O. et al. Early experience with the AMPLATZER vascular plug IV for the occlusion of pulmonary arteriovenous malformations. J Vasc Interv Radiol. 2014;25(9):1333–1337. doi: 10.1016/j.jvir.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Hundt W, Kalinowski M, Kiessling A. et al. Novel approach to complex pulmonary arteriovenous malformation embolization using detachable coils and Amplatzer vascular plugs. Eur J Radiol. 2012;81(5):e732–e738. doi: 10.1016/j.ejrad.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 25.Tzilalis V D, Vourliotakis G, Tsironis I A, Tsiligiris V D, Brountzos E N. Use of an Amplatzer vascular plug in embolization of a pulmonary artery aneurysm in a case of Hughes-Stovin syndrome: a case report. J Med Case Reports. 2011;5:425. doi: 10.1186/1752-1947-5-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogl T J, Pegios W, Balzer J O, Lobo M, Neuhaus P. Arterial steal syndrome in patients after liver transplantation: transarterial embolization of the splenic and gastroduodenal arteries [in German] Rofo. 2001;173(10):908–913. doi: 10.1055/s-2001-17592. [DOI] [PubMed] [Google Scholar]
- 27.Widlus D M, Moeslein F M, Richard H M III. Evaluation of the Amplatzer vascular plug for proximal splenic artery embolization. J Vasc Interv Radiol. 2008;19(5):652–656. doi: 10.1016/j.jvir.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 28.Ng E H, Comin J, David E, Pugash R, Annamalai G. AMPLATZER Vascular Plug 4 for proximal splenic artery embolization in blunt trauma. J Vasc Interv Radiol. 2012;23(7):976–979. doi: 10.1016/j.jvir.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Burbelko M, Kalinowski M, Heverhagen J T. et al. Prevention of type II endoleak using the AMPLATZER vascular plug before endovascular aneurysm repair. Eur J Vasc Endovasc Surg. 2014;47(1):28–36. doi: 10.1016/j.ejvs.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Chun J Y, Mailli L, Abbasi M A. et al. Embolization of the internal iliac artery before EVAR: is it effective? Is it safe? Which technique should be used? Cardiovasc Intervent Radiol. 2014;37(2):329–336. doi: 10.1007/s00270-013-0659-2. [DOI] [PubMed] [Google Scholar]
- 31.Kickuth R, Dick F, Triller J, Ludwig K, Schmidli J, Do D D. Internal iliac artery embolization before endovascular repair of aortoiliac aneurysms with a nitinol vascular occlusion plug. J Vasc Interv Radiol. 2007;18(9):1081–1087. doi: 10.1016/j.jvir.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Kouvelos G N, Koutsoumpelis A, Peroulis M, Matsagkas M. In endovascular aneurysm repair cases, when should you consider internal iliac artery embolization when extending a stent into the external iliac artery? Interact Cardiovasc Thorac Surg. 2014;18(6):821–824. doi: 10.1093/icvts/ivu042. [DOI] [PubMed] [Google Scholar]
- 33.Wong S, Greenberg R K, Brown C R, Mastracci T M, Bena J, Eagleton M J. Endovascular repair of aortoiliac aneurysmal disease with the helical iliac bifurcation device and the bifurcated-bifurcated iliac bifurcation device. J Vasc Surg. 2013;58(4):861–869. doi: 10.1016/j.jvs.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 34.Fernández-Alonso L, Fernández-Alonso S, Grijalba F U. et al. Endovascular treatment of abdominal aortic aneurysms involving iliac bifurcation: role of iliac branch graft device in prevention of buttock claudication. Ann Vasc Surg. 2013;27(7):851–855. doi: 10.1016/j.avsg.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Coupe N J, Ling L, Cowling M G, Asquith J R, Hopkinson G B. Treatment of a common iliac aneurysm by endovascular exclusion using the Amplatzer vascular plug and femorofemoral crossover graft. Cardiovasc Intervent Radiol. 2009;32(4):772–775. doi: 10.1007/s00270-009-9520-z. [DOI] [PubMed] [Google Scholar]
- 36.Ratnam L A, Walkden R M, Munneke G J, Morgan R A, Belli A M. The Amplatzer vascular plug for large vessel occlusion in the endovascular management of aneurysms. Eur Radiol. 2008;18(9):2006–2012. doi: 10.1007/s00330-008-0967-0. [DOI] [PubMed] [Google Scholar]
- 37.Zander T, Baldi S, Rabellino M. et al. Successful occlusion of a ruptured aortic aneurysm using the Amplatzer Vascular Plug: a technical note. Cardiovasc Intervent Radiol. 2011;34(2) 02:S136–S141. doi: 10.1007/s00270-010-9872-4. [DOI] [PubMed] [Google Scholar]
- 38.Verma H, Hiremath N, Maiya S, George R K, Tripathi R K. Endovascular exclusion of complex postsurgical aortic arch pseudoaneurysm using vascular plug devices and a review of vascular plugs. Perspect Vasc Surg Endovasc Ther. 2012;24(4):193–197. doi: 10.1177/1531003513501203. [DOI] [PubMed] [Google Scholar]
- 39.Kanani R S, Neilan T G, Palacios I F, Garasic J M. Novel use of the Amplatzer septal occluder device in the percutaneous closure of ascending aortic pseudoaneurysms: a case series. Catheter Cardiovasc Interv. 2007;69(1):146–153. doi: 10.1002/ccd.20794. [DOI] [PubMed] [Google Scholar]
- 40.Jolly N Garg R K Raman J Hijazi Z M Amplatzer septal occluder device for closure of aortic pseudoaneurysms Catheter Cardiovasc Interv 2007704619–620., author reply 621 [DOI] [PubMed] [Google Scholar]
- 41.Scholtz W, Jategaonkar S, Haas N A. Successful interventional treatment of a retrosternal pseudoaneurysm of the ascending aorta with an Amplatzer Vascular Plug II. J Invasive Cardiol. 2010;22(3):E44–E46. [PubMed] [Google Scholar]
- 42.Ferro C, Rossi U G, Seitun S, Scarano F, Passerone G, Williams D M. Aortic branch artery pseudoaneurysms associated with intramural hematoma: when and how to do endovascular embolization. Cardiovasc Intervent Radiol. 2013;36(2):422–432. doi: 10.1007/s00270-012-0512-z. [DOI] [PubMed] [Google Scholar]
- 43.Mansueto G, D'Onofrio M, Minniti S, Ferrara R M, Procacci C. Therapeutic embolization of idiopathic renal arteriovenous fistula using the “stop-flow” technique. J Endovasc Ther. 2001;8(2):210–215. doi: 10.1177/152660280100800218. [DOI] [PubMed] [Google Scholar]
- 44.Resnick S Chiang A Transcatheter embolization of a high-flow renal arteriovenous fistula with use of a constrained Wallstent to prevent coil migration J Vasc Interv Radiol 200617(2, Pt 1):363–367. [DOI] [PubMed] [Google Scholar]
- 45.Brountzos E N, Ptohis N, Grammenou-Pomoni M. et al. High-flow renal arteriovenous fistula treated with the Amplatzer vascular plug: implementation of an arterial and venous approach. Cardiovasc Intervent Radiol. 2009;32(3):543–547. doi: 10.1007/s00270-008-9383-8. [DOI] [PubMed] [Google Scholar]
- 46.Taneja M, Lath N, Soo T B. et al. Renal artery stump to inferior vena cava fistula: unusual clinical presentation and transcatheter embolization with the Amplatzer vascular plug. Cardiovasc Intervent Radiol. 2008;31 02:S92–S95. doi: 10.1007/s00270-007-9232-1. [DOI] [PubMed] [Google Scholar]
- 47.Perkov D, Novačić K, Novosel L, Knežević N. Percutaneous embolization of idiopathic renal arteriovenous fistula using Amplatzer vascular plug II. Int Urol Nephrol. 2013;45(1):61–68. doi: 10.1007/s11255-012-0358-y. [DOI] [PubMed] [Google Scholar]
- 48.Campbell J E, Davis C, Defade B P, Tierney J P, Stone P A. Use of an Amplatzer vascular plug for transcatheter embolization of a renal arteriovenous fistula. Vascular. 2009;17(1):40–43. doi: 10.2310/6670.2008.00071. [DOI] [PubMed] [Google Scholar]
- 49.Hodgkinson J D, Cheshire N, Bicknell C, Hamady M. A novel endovascular treatment for long-standing high-flow arteriovenous fistula. J Vasc Surg. 2015;61(5):1321–1323. doi: 10.1016/j.jvs.2013.10.082. [DOI] [PubMed] [Google Scholar]
- 50.Fischer G, Apostolopoulou S C, Rammos S, Kiaffas M, Kramer H H. Transcatheter closure of coronary arterial fistulas using the new Amplatzer vascular plug. Cardiol Young. 2007;17(3):283–287. doi: 10.1017/S1047951107000510. [DOI] [PubMed] [Google Scholar]
- 51.Koc O, Cil B E, Peynircioglu B, Emlik D, Ozbek O. Complementary use of NBCA with the Amplatzer vascular plug for embolization of a high-flow traumatic hepatic arteriovenous fistula. Cardiovasc Intervent Radiol. 2009;32(5):1105–1107. doi: 10.1007/s00270-009-9505-y. [DOI] [PubMed] [Google Scholar]
- 52.Peynircioglu B, Cil B. Amplatzer stuffing technique in the treatment of an iatrogenic mesenteric arteriovenous fistula. Cardiovasc Intervent Radiol. 2009;32(6):1247–1251. doi: 10.1007/s00270-009-9614-7. [DOI] [PubMed] [Google Scholar]
- 53.Bulla K, Hubich S, Pech M, Löwenthal D, Ricke J, Dudeck O. Superiority of proximal embolization of the gastroduodenal artery with the Amplatzer vascular plug 4 before yttrium-90 radioembolization: a retrospective comparison with coils in 134 patients. Cardiovasc Intervent Radiol. 2014;37(2):396–404. doi: 10.1007/s00270-013-0684-1. [DOI] [PubMed] [Google Scholar]
- 54.Dudeck O, Bulla K, Wieners G. et al. Embolization of the gastroduodenal artery before selective internal radiotherapy: a prospectively randomized trial comparing standard pushable coils with fibered interlock detachable coils. Cardiovasc Intervent Radiol. 2011;34(1):74–80. doi: 10.1007/s00270-010-9845-7. [DOI] [PubMed] [Google Scholar]
- 55.Pech M, Kraetsch A, Wieners G. et al. Embolization of the gastroduodenal artery before selective internal radiotherapy: a prospectively randomized trial comparing platinum-fibered microcoils with the Amplatzer Vascular Plug II. Cardiovasc Intervent Radiol. 2009;32(3):455–461. doi: 10.1007/s00270-008-9498-y. [DOI] [PubMed] [Google Scholar]
- 56.Pattynama P M, Wils A, van der Linden E, van Dijk L C. Embolization with the Amplatzer vascular plug in TIPS patients. Cardiovasc Intervent Radiol. 2007;30(6):1218–1221. doi: 10.1007/s00270-007-9089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paz-Fumagalli R, Crain M R, Mewissen M W, Varma R R. Fatal hemodynamic consequences of therapeutic closure of a transjugular intrahepatic portosystemic shunt. J Vasc Interv Radiol. 1994;5(6):831–834. doi: 10.1016/s1051-0443(94)71616-x. [DOI] [PubMed] [Google Scholar]
- 58.Kessler J, Trerotola S O. Use of the Amplatzer Vascular Plug for embolization of a large retroperitoneal shunt during transjugular intrahepatic portosystemic shunt creation for gastric variceal bleeding. J Vasc Interv Radiol. 2006;17(1):135–140. doi: 10.1097/01.rvi.0000186958.59457.10. [DOI] [PubMed] [Google Scholar]
- 59.Park J K, Cho S K, Kee S, Lee E W. Vascular plug-assisted retrograde transvenous obliteration of portosystemic shunts for refractory hepatic encephalopathy: a case report. Case Rep Radiol. 2014;2014:391420. doi: 10.1155/2014/391420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Power A H, Bjarnason H. Large spontaneous intrahepatic portal-systemic venous shunt treated with coil and Amplatzer vascular plug embolization. Perspect Vasc Surg Endovasc Ther. 2012;24(2):90–94. doi: 10.1177/1531003512455223. [DOI] [PubMed] [Google Scholar]
- 61.Evans W N, Galindo A, Acherman R J, Rothman A, Berthoty D P. Congenital portosystemic shunts and AMPLATZER vascular plug occlusion in newborns. Pediatr Cardiol. 2009;30(8):1083–1088. doi: 10.1007/s00246-009-9501-7. [DOI] [PubMed] [Google Scholar]
- 62.Guneyli S, Cinar C, Bozkaya H, Parildar M, Oran I, Akin Y. Successful transcatheter closure of a congenital high-flow portosystemic venous shunt with the Amplatzer vascular plug II. Perspect Vasc Surg Endovasc Ther. 2012;24(4):202–205. doi: 10.1177/1531003513496850. [DOI] [PubMed] [Google Scholar]
- 63.Passalacqua M, Lie K T, Yarmohammadi H. Congenital extrahepatic portosystemic shunt (Abernethy malformation) treated endovascularly with vascular plug shunt closure. Pediatr Surg Int. 2012;28(1):79–83. doi: 10.1007/s00383-011-2944-y. [DOI] [PubMed] [Google Scholar]
- 64.Gwon D I, Ko G Y, Yoon H K. et al. Gastric varices and hepatic encephalopathy: treatment with vascular plug and gelatin sponge-assisted retrograde transvenous obliteration—a primary report. Radiology. 2013;268(1):281–287. doi: 10.1148/radiol.13122102. [DOI] [PubMed] [Google Scholar]
- 65.Ringe K I, Weidemann J, Rosenthal H. et al. Transhepatic preoperative portal vein embolization using the Amplatzer Vascular Plug: report of four cases. Cardiovasc Intervent Radiol. 2007;30(6):1245–1247. doi: 10.1007/s00270-007-9158-7. [DOI] [PubMed] [Google Scholar]
- 66.Bent C L, Low D, Matson M B, Renfrew I, Fotheringham T. Portal vein embolization using a nitinol plug (Amplatzer vascular plug) in combination with histoacryl glue and iodinized oil: adequate hypertrophy with a reduced risk of nontarget embolization. Cardiovasc Intervent Radiol. 2009;32(3):471–477. doi: 10.1007/s00270-009-9515-9. [DOI] [PubMed] [Google Scholar]
- 67.Yoo H, Ko G Y, Gwon D I. et al. Preoperative portal vein embolization using an Amplatzer vascular plug. Eur Radiol. 2009;19(5):1054–1061. doi: 10.1007/s00330-008-1240-2. [DOI] [PubMed] [Google Scholar]
- 68.Dollinger M, Goessmann H, Mueller-Wille R, Wohlgemuth W A, Stroszczynski C, Heiss P. Percutaneous transhepatic and transsplenic portal vein access: embolization of the puncture tract using Amplatzer vascular plugs. Rofo. 2014;186(2):142–150. doi: 10.1055/s-0033-1350514. [DOI] [PubMed] [Google Scholar]
- 69.Dionello R, Warakaulle D, Liong W C. A novel use of the AMPLATZER vascular plug: preventing bile leak following inadvertent subcapsular deployment of a biliary stent. Cardiovasc Intervent Radiol. 2010;33(1):213–214. doi: 10.1007/s00270-009-9605-8. [DOI] [PubMed] [Google Scholar]
- 70.Bittles M A, Hoffer E K. Gonadal vein embolization: treatment of varicocele and pelvic congestion syndrome. Semin Intervent Radiol. 2008;25(3):261–270. doi: 10.1055/s-0028-1085927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Basile A, Marletta G, Tsetis D, Patti M T. The Amplatzer vascular plug also for ovarian vein embolization. Cardiovasc Intervent Radiol. 2008;31(2):446–447. doi: 10.1007/s00270-007-9235-y. [DOI] [PubMed] [Google Scholar]
- 72.Bui J T, Gaba R C, Knuttinen M G, West D L, Owens C A. Amplatzer vascular plug for arteriovenous hemodialysis access occlusion: initial experience. J Vasc Access. 2009;10(1):5–10. doi: 10.1177/112972980901000102. [DOI] [PubMed] [Google Scholar]
- 73.Gumus B. Percutaneous embolization of hemodialysis fistulas by AMPLATZER vascular plug with midterm follow-up. J Vasc Interv Radiol. 2011;22(11):1581–1585. doi: 10.1016/j.jvir.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 74.Ozyer U, Aytekin C, Yildirim U M. et al. Use of the Amplatzer vascular plug II in endovascular occlusion of dialysis shunts with tributary veins. J Vasc Access. 2011;12(1):76–77. doi: 10.5301/jva.2010.5984. [DOI] [PubMed] [Google Scholar]
- 75.Bourquelot P, Karam L, Raynaud A, Beyssen B, Ricco J B. Amplatzer vascular plug for occlusion or flow reduction of hemodialysis arteriovenous access. J Vasc Surg. 2014;59(1):260–263. doi: 10.1016/j.jvs.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 76.Mangini M, Laganà D, Fontana F. et al. Use of Amplatzer Vascular Plug (AVP) in emergency embolisation: preliminary experience and review of literature. Emerg Radiol. 2008;15(3):153–160. doi: 10.1007/s10140-007-0696-8. [DOI] [PubMed] [Google Scholar]
- 77.Teichgräber U K, De Bucourt M. Massive retroperitoneal hemorrhage from a giant renal angiomyolipoma treated by selective arterial embolization with an Amplatzer Vascular Plug II. Acta Radiol Short Rep. 2012;1(2):1–2. doi: 10.1258/arsr.2012.110029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mihlon F, Agrawal A, Nimjee S M. et al. Enhanced, rapid occlusion of carotid and vertebral arteries using the AMPLATZER Vascular Plug II device: the Duke Cerebrovascular Center experience in 8 patients with 22 AMPLATZER Vascular Plug II devices. World Neurosurg. 2015;83(1):62–68. doi: 10.1016/j.wneu.2013.07.084. [DOI] [PubMed] [Google Scholar]
- 79.Eller J L, Hopkins L N. Use of vascular plug devices in the management of neurovascular emergencies. World Neurosurg. 2015;83(1):9–10. doi: 10.1016/j.wneu.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 80.Macht S, Mathys C, Schipper J, Turowski B. Initial experiences with the Amplatzer Vascular Plug 4 for permanent occlusion of the internal carotid artery in the skull base in patients with head and neck tumors. Neuroradiology. 2012;54(1):61–64. doi: 10.1007/s00234-010-0823-1. [DOI] [PubMed] [Google Scholar]
- 81.Farrell T A, Wallace M, Hicks M E. Long-term results of transrenal ureteral occlusion with use of Gianturco coils and gelatin sponge pledgets. J Vasc Interv Radiol. 1997;8(3):449–452. doi: 10.1016/s1051-0443(97)70587-6. [DOI] [PubMed] [Google Scholar]
- 82.Schild H H, Günther R, Thelen M. Transrenal ureteral occlusion: results and problems. J Vasc Interv Radiol. 1994;5(2):321–325. doi: 10.1016/s1051-0443(94)71494-9. [DOI] [PubMed] [Google Scholar]
- 83.Schild H H, Meyer C, Möhlenbroch M, Mueller S C, Simon B, Kuhl C K. Transrenal ureter occlusion with an Amplatzer vascular plug. J Vasc Interv Radiol. 2009;20(10):1390–1392. doi: 10.1016/j.jvir.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 84.Pieper C C, Meyer C, Hauser S, Wilhelm K E, Schild H H. Transrenal ureteral occlusion using the Amplatzer vascular plug II: a new interventional treatment option for lower urinary tract fistulas. Cardiovasc Intervent Radiol. 2014;37(2):451–457. doi: 10.1007/s00270-013-0662-7. [DOI] [PubMed] [Google Scholar]
- 85.Shabrang C, Kelbach S M, Hsu D P, Zippe C D, Lie K T. Therapeutic ureteral occlusion with n-butyl cyanoacrylate glue and an AMPLATZER plug scaffold. J Vasc Interv Radiol. 2012;23(3):428–430. doi: 10.1016/j.jvir.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 86.Saad W E, Kalagher S, Turba U C. et al. Ureteric embolization for lower urinary tract fistulae: use of two amplatzer vascular plugs and N-butyl cyanoacrylate employing the “sandwich” technique. Cardiovasc Intervent Radiol. 2013;36(4):1068–1072. doi: 10.1007/s00270-012-0510-1. [DOI] [PubMed] [Google Scholar]
- 87.Akulian J, Pathak V, Lessne M. et al. A novel approach to endobronchial closure of a bronchial pleural fistula. Ann Thorac Surg. 2014;98(2):697–699. doi: 10.1016/j.athoracsur.2013.09.105. [DOI] [PubMed] [Google Scholar]
- 88.Fruchter O, Bruckheimer E, Raviv Y, Rosengarten D, Saute M, Kramer M R. Endobronchial closure of bronchopleural fistulas with Amplatzer vascular plug. Eur J Cardiothorac Surg. 2012;41(1):46–49. doi: 10.1016/j.ejcts.2011.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koo J H, Park K B, Choo S W, Kim K, Do Y S. Embolization of postsurgical esophagopleural fistula with AMPLATZER vascular plug, coils, and Histoacryl glue. J Vasc Interv Radiol. 2010;21(12):1905–1910. doi: 10.1016/j.jvir.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 90.Giddings O, Kuhn J, Akulian J. Endobronchial valve placement for the treatment of bronchopleural fistula: a review of the current literature. Curr Opin Pulm Med. 2014;20(4):347–351. doi: 10.1097/MCP.0000000000000063. [DOI] [PubMed] [Google Scholar]


