Abstract
Background
Regimen selection for highly treatment-experienced patients is complicated.
Methods
Using a web-based utility, study team members reviewed antiretroviral (ARV) history and resistance data and recommended individual ARV regimens and nucleoside reverse transcriptase inhibitor (NRTI) options for treatment-experienced participants consisting of 3–4 of the following agents: raltegravir (RAL), darunavir (DRV)/ritonavir, tipranavir (TPV)/ritonavir, etravirine (ETR), maraviroc (MVC), and enfuvirtide (ENF). We evaluated team recommendations and site selection of regimen and NRTIs. Associations between baseline factors and the selection of a complex regimen (defined as including four ARV agents or ENF) were explored with logistic regression.
Results
A total of 413 participants entered the study. Participants initiated the first or second recommended regimen 86% of the time and 21% of participants started a complex regimen. In a multivariable model, ARV resistance to NRTI (odds ratio [OR]=2.2), non-nucleoside reverse transcriptase inhibitor (NNRTI, OR=6.2) or boosted protease inhibitor (PI, OR=6.6), prior use of integrase strand transfer inhibitor (INSTI, OR=25), and race–ethnicity (all P≤0.01) were associated with selection of a complex regimen. Black non-Hispanic (OR=0.5) and Hispanic participants from the continental US (OR=0.2) were less likely to start a complex regimen, compared to white non-Hispanics.
Conclusions
In this multi-center trial, we developed a web-based utility that facilitated treatment recommendations for highly treatment-experienced patients. Drug resistance, prior INSTI use, and race–ethnicity were key factors in decisions to select a more complex regimen.
Keywords: HIV drug resistance, Treatment-experienced, Phenotypic susceptibility score, Tropism, cPSS, Antiretroviral therapy
Introduction
For patients experiencing virologic failure due to HIV drug resistance, the Department of Health and Human Services (DHHS) Panel on Antiretroviral (ARV) Guidelines for Adults and Adolescents recommends using at least 2, and optimally 3, fully active drugs to construct a new treatment regimen in order to achieve durable suppression of HIV-1 RNA levels.1 Guidelines recommend resistance testing to inform selection of new treatment regimens for patients with drug-resistant virus.1–3 Incorporating prior treatment history and patient preference such as willingness to use enfuvirtide (ENF, a fusion inhibitor), a medication self-administered by injection, are also important considerations when deciding on new treatment regimens.1–3 The decision of how many and which agents to use in the next regimen, for patients with substantial resistance or treatment experience to the first three classes of ARV agents (NRTI, NNRTI, and PI), is complicated. Current guidelines offer only general advice. Retrospective studies show that ARV activity, as measured by phenotype assays, or phenotypic susceptibility score (PSS), predicts virologic outcomes.4,5 Historically, clinicians add previously used, or “recycled”, NRTIs to the new regimen in an attempt to increase therapeutic effect,6–8 but this approach has not been tested in the setting of newer ARVs.
Between 2005 and 2007, several new ARV medications received accelerated FDA approval increasing treatment options for patients with drug resistant virus. These new medications were the protease inhibitors darunavir (DRV) and tipranavir (TPV), etravirine (ETR), an NNRTI, maraviroc (MVC), a chemokine receptor 5 (CCR5) entry inhibitor, and raltegravir (RAL), an integrase strand transfer inhibitor (INSTI). In the OPTIONS (Optimized Treatment that Includes or Omits NRTIs, AIDS Clinical Trials Group A5241) study9 we tested the hypothesis of whether it was beneficial and safe to add or omit NRTIs to an optimized regimen based upon PSSs.
The objectives of this report are to describe the study design and baseline characteristics of study participants; the process by which the recommendations were developed and communicated to the clinical trial sites, how often the research sites followed the team’s treatment recommendations, and baseline factors associated with selection of regimens and NRTIs. We hypothesized that there would be a high rate of selection of one of the first two treatment recommendations.
Methods
Study design
Study participants were recruited from 62 sites across the continental US and Puerto Rico from 31 January 2008 to 6 June 2011 in the OPTIONS study. Sites obtained local institutional review board approval and written informed consent was obtained from all study participants.
Study participants were eligible if they were ≥16 years old, had chronic HIV-1 infection, had ARV experience or resistance to NRTI and NNRTI, were taking a PI-containing regimen for at least 8 weeks prior to study entry, and had plasma HIV RNA ≥1000 copies/ml. Participants had a calculated creatinine clearance (CrCl) at least 50 ml/min, and no active hepatitis B infection. (See Supplement 1 for complete inclusion and exclusion criteria). Combined phenotype/genotype resistance (PhenoSense® GT, Monogram Biosciences) and co-receptor tropism (Trofile®, Monogram Biosciences, South San Francisco, CA, USA) testing were performed on plasma HIV isolates derived from participants at the screening visit.
The study team considered 20 potential ARV regimens (not including NRTIs) using the six study-provided medications (DRV, ENF, ETR, MVC, RAL, and TPV). Ritonavir (r) was prescribed with DRV and TPV and was not provided by the study. Each potential regimen contained between three and five drugs, for which either clinical data or drug–drug interactions supported use of the specific combination (Supplement 2). The study team asked about any prior intolerance to ARVs, prior non-adherence and willingness to use ENF on study. For each participant, individual drugs were assessed for predicted activity based on phenotype, genotype and co-receptor tropism test results, and past history of ARV use. RAL and ENF were designated as either susceptible or resistant (PSS 1 or 0) based on prior use (any prior use imputed to be resistant and PSS=0, see statistical analysis below). For MVC, if there was a history of prior MVC use or prior or current tropism test with non-CCR5 virus, the PSS was set at 0, while for DRV, ETR, and TPV a continuous PSS (cPSS) was calculated (Supplement 3). The drug PSS values were added together to create regimen-specific scores (regimen cPSS). See Supplement 4 for an example of calculation of regimen PSS for all regimens for a potential participant. Participants with at least one potential regimen with a cPSS >2.0 (without including the contribution from NRTIs) were randomized to either add or omit NRTIs to their new regimen. If no regimen with a cPSS >2.0 could be identified, the participant entered a non-randomized arm of the study and added NRTIs. Identification of at least two NRTIs for potential use on the study was required for each participant prior to randomization. If abacavir (ABC) was recommended by the study team, sites were instructed to confirm a negative HLA-B*5701 test result. Participants randomized to omit NRTIs did not take NRTIs during the study.
Regimen and NRTI recommendation and selection process
A web-based utility was developed for team review on conference calls of individual study participant’s plasma HIV-1 phenotype/genotype and HIV-1 co-receptor tropism assay results, cPSS for each potential regimen option for that study participant, and selected elements of the medical history such as willingness to use ENF. ARV regimens and NRTI options were recommended by team members who had expertise in ARV therapy, drug resistance interpretation, and/or pharmacology. Antiretroviral regimen and NRTI options were ranked by the team; primary and secondary reasons were recorded to communicate the rationale for the prioritization.
Regimen options were communicated to each site by an emailed report. If there was more than one option for an ARV study regimen or NRTI combination, the site investigator and participant were selected from among the potential options. The study site also recorded their reason(s) for ARV regimen and NRTI selection.
Statistical analyses
Analyses include all participants randomized or enrolled in the study (participants with cPSS <2.0 were not randomized). Baseline characteristics, ARV drug susceptibility, and regimen and NRTI selections are presented with descriptive statistics. Pre-planned outcomes included (a) selection of first or second choice recommendation for ARV regimen and NRTIs; (b) selection of a complex regimen, defined as a regimen with four or five ARV agents or inclusion of ENF; and (c) selection of an NRTI combination other than TDF plus either FTC or lamivudine (3TC). Pre-specified covariates included: age, sex, race/ethnicity, geographic region, ARV experience, CD4 cell count, HIV-1 RNA, HIV-1 co-receptor tropism, calculated creatinine clearance (CrCl; for the NRTI outcome only), hepatitis C virus (HCV) status, injection drug use (IDU) history, drug class resistance, and calendar year of enrollment. Drug resistance was defined using “net assessment” (susceptible, partially susceptible, or resistant) from PhenoSense® GT. For RAL and ENF, previous drug class experience was inferred as having resistance. Resistance testing to INSTIs and entry inhibitors was not used in the study. For MVC, previous drug experience, history of or current non-CCR5 tropic virus, was inferred as having resistance to MVC since those participants were not candidates for MVC therapy.
Univariate and multivariable logistic regression analyses, stratified by cPSS ≤ or >2, explored cross-sectional associations between baseline covariates and each outcome, with no adjustment for multiplicity. Multivariable models were derived using forward selection and covariates with univariate P≤0.10, and scientific input was used to define categories for continuous variables.
Results
Demographics and entry regimens
A total of 413 participants entered the OPTIONS study between 31 January 2008 and 6 June 2011. Baseline characteristics of this diverse, highly treatment-experienced population are shown in Table 1. Median CD4 cell count was 196 cells/μl and median HIV RNA was 4.2 log10 copies/ml. The most frequently taken PI at study entry was lopinavir/ritonavir (LPVr, 33%), followed by atazanavir (ATV, 28%), DRVr (18%), fosamprenavir (FPV, 13%), and TPVr (6%). NRTIs taken at study entry were as follows: tenofovir (TDF) (70%), emtricitabine (FTC) (47%), or lamivudine (3TC) (34%), or ABC (28%); Nine percent were also taking an NNRTI.
Table 1.
Baseline characteristics of study participants (n=413)
| Characteristic | Median (Q1, Q3) or n (%) |
|---|---|
| Age, years | 46 (40, 51) |
| Sex | |
| Male | 314 (76%) |
| Female | 99 (24%) |
| Race–ethnicity | |
| Black, non-Hispanic | 167 (40%) |
| White, non-Hispanic | 132 (32%) |
| Hispanic, Puerto Rico | 37 (9%) |
| Hispanic, Continental United States | 60 (15%) |
| Othera | 17 (4%) |
| Geographic region | |
| Northeast | 145 (35%) |
| Midwest | 98 (24%) |
| South | 79 (19%) |
| West | 54 (13%) |
| Puerto Rico | 37 (9%) |
| Number of years taking HAARTb | 10 (7, 12) |
| Number of years taking PIsb | 9 (6, 11) |
| Prior ENF use | 93 (23%) |
| Prior INSTI use | 30 (7%) |
| Baseline CD4 (cells/μl) | 196 (90, 348) |
| Baseline HIV-1 RNA (log10 copies/ml) | 4.2 (3.6, 4.6) |
| HIV-1 tropism | |
| CCR5 only | 187 (45%) |
| Dual mixed or CXCR4 | 200 (48%) |
| Not reportedc | 26 (6%) |
| Willing to use ENF | 203 (49%) |
| Calculated creatinine clearance (ml/min) | 104 (87, 128) |
| Hepatitis C statusd | |
| Positive | 58 (14%) |
| Negative | 349 (85%) |
| Indeterminate | 6 (1%) |
| Injection drug use (IDU) history | |
| Never | 374 (91%) |
| Previously | 39 (9%) |
| Enrollment year | |
| 2008 | 238 (58%) |
| 2009 | 101 (24%) |
| 2010–2011 | 74 (18%) |
| Lack of susceptibility to any: | |
| NRTI | 111 (27%) |
| NNRTI | 66 (16%) |
| Boosted PI | 86 (21%) |
| Number of drugs to which the virus was resistant: | |
| NRTIs (range: 0–6) | 3 (1, 5) |
| 0–1 NRTI | 107 (26%) |
| 2–6 NRTIs | 306 (74%) |
| NNRTIs (range: 0–3) | 2 (0, 2) |
| Boosted PIs (range: 0–6) | 2 (0, 4) |
| Continuous phenotypic susceptibility | |
| Score of selected regimen | |
| ≤2 | 53 (13%) |
| >2 (subsequently randomized) | 360 (87%) |
Other race–ethnicity: Asian, Pacific Islander (n=6), American Indian, Alaskan native (4), More than one race (3), Unknown (4).
One participant with missing data.
Unable to obtain tropism result.
Hepatitis C antibody result, with historical chronic hepatitis C diagnosis inferred as positive.
Q1, Q3: first and third quartiles; n: number; HAART: highly active antiretroviral therapy; PI: protease inhibitor; INSTI: integrase strand transfer inhibitor; CCR5: chemokine receptor 5; CXCR4: chemokine receptor 4; ENF: enfuvirtide; NRTI: nucleoside/tide reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor.
ARV drug resistance at study entry
Resistance to individual agents
Figure 1 shows baseline ARV drug susceptibility to the individual ARV agents in the NRTI, NNRTI, and PI classes within the study population using the net resistance assessment algorithm. Seventy-seven percent of participants had no prior exposure to ENF and 93% had no prior exposure to INSTI; 45% of participants had exclusively CCR5-tropic virus (Table 1).
Figure 1.
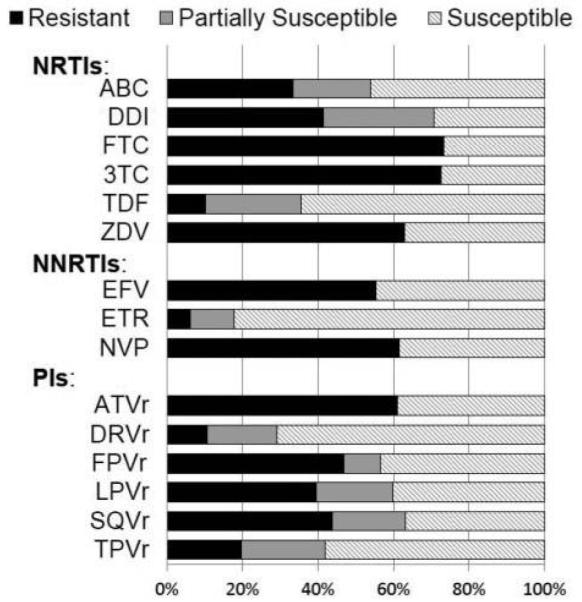
Percent of study participants (n = 413) with resistant, partially susceptible or susceptible virus to each ARV agent at study entry based on the Monogram Pheno-Sense® GT net assessment which incorporates phenotype and genotype information. NRTI: Nucleoside reverse transcriptase inhibitor, NNRTI: non-nucleoside reverse transcriptase inhibitor, PI: protease inhibitor, ABC: abacavir, ddI: didanosine, FTC: emtricitabine, 3TC: lamivudine; TDF: tenofovir, ZDV: zidovudine, EFV: efavirenz, ETR: etravirine, NVP: nevirapine, r: ritonavir boosting, ATV: atazanavir, DRV: darunavir, FPV: fosamprenavir, LPV: lopinavir, SQV: saquinavir, TPV: tipranavir. The percent of viral variants fully susceptible were as follows: (NRTIs) TDF = 64%, ABC = 46%, ZDV = 36%, and 27% to 3TC or FTC = 27%; (NNRTIs) ETR = 82%; and (PIs) DRV/r = 71%, TPV/r = 58%.
Resistance within ARV classes
Resistance to at least one agent within an ARV drug class was observed in 80% of participants for the NRTI, 62% for the NNRTI, and 67% for the PI class. Although all participants had history of drug exposure or resistance to NRTI, NNRTI, and PI classes, screening resistance tests showed no resistance to the NRTIs and PIs in 16% of participants, and no resistance to any drug in the three classes in 8% of participants. During the three-year accrual to the study, the percent of screening resistance tests showing susceptibility to NRTIs, NNRTIs, and PIs increased (data not shown), reflecting enrollment of a less heavily ARV-treated population as the trial progressed.
Recommended regimens and rationale
For 21% of participants, only one ARV regimen was recommended; 29% had 2–3 options and the remaining 50% had at least four options. The most frequently recommended first choice regimen was RAL+DRVr+ETR (52%, Fig. 2) followed by four-drug regimens adding either ENF (15%) or MVC (14%) to these drugs. The fourth most frequently recommended first choice regimen was MVC+RAL+DRVr (7%) (Fig. 2).
Figure 2.
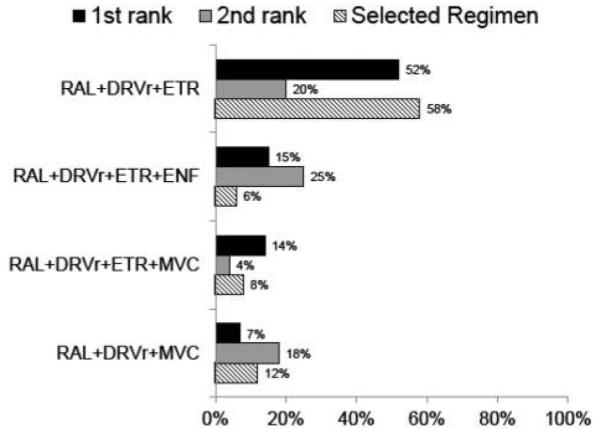
The top four recommended ARV regimens from the study team including the first and second study team ranked choices (designated first and second rank). The top four of 20 regimens are shown, the remaining 16 regimens were either never recommended as first rank (three cases) or recommended as first rank <2% of the time (data not shown). The selected regimen is the regimen the site and participant chose to start among the options that were recommended (n = 413). RAL: raltegravir; DRV: darunavir; ETR: etravirine; ENF: enfuvirtide; MVC: maraviroc.
The most frequently recommended second choice regimen option was an ENF-containing regimen (ENF+RAL+DRVr+ETR, 25% of 328), followed by RAL+DRVr+ETR (20%) and MVC+RAL+DRVr (18%). Three other MVC-containing regimens (without ENF) were recommended for 15%, and ENF-containing regimens were recommended as the second choice option for 46%. PI-sparing regimens were recommended as second choice for 12% of 328 participants (such as MVC+RAL+ETR or other ENF-containing regimens).
The most common reason for the recommended first choice regimen was the regimen activity score (regimen cPSS, 58%) followed by regimen simplicity (three-drug or ENF-sparing regimens, 17%) or there was only one regimen recommended (11%). Secondary reasons cited included whether the participant was willing to use ENF (36%), tropism result (28%), and cPSS (18%).
NRTI recommendations and rationale
The team offered more than one NRTI combination to 93% of the study population; the median number of combinations offered was 3. Two combinations of NRTIs were recommended in a majority of participants: TDF+FTC (96%) and TDF+FTC+ZDV (79%). ABC-containing NRTI combinations were recommended for 34% of subjects. ZDV was less frequently recommended as part of the first choice NRTI combination over time: 74% in 2008, 66% in 2009, and 38% in 2010–2011 (post hoc Fisher’s exact P<0.001). Reasons for NRTI combination recommendations were as follows: (1) potential interaction between TDF- and ZDV-associated mutations that may impair viral fitness and increase activity of the NRTI (65%); (2) phenotype result (16%); and (3) ARV history (6%).
Site/participant regimen selection
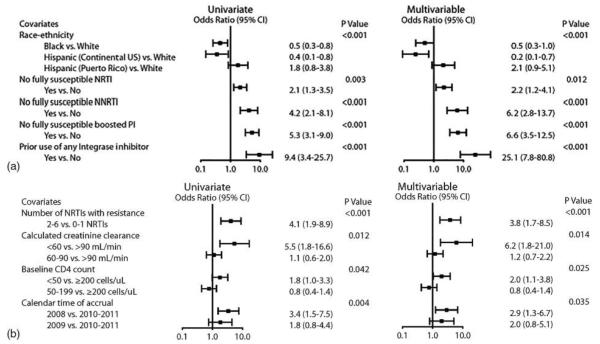
Among cases with multiple ARV regimen options (328/413, 79%), the regimen chosen by the site investigator and study participant was the first or second most highly recommended regimen in 86% of participants. The most frequently chosen regimen was RAL+DRVr+ETR. RAL-containing regimens were chosen in 98% of cases. Overall, 89% chose a regimen containing DRVr and 82% chose an ETR-containing regimen, whereas ENF-containing regimens were chosen by only 12%. The reasons sites listed for regimen selection included the following: highest ranked recommendation (33%), regimen simplicity (24%), avoidance of ENF (16%), and highest cPSS regimen (9%). In multivariable analysis the likelihood of accepting the first or second regimen was related to the presence of dual-mixed (odds ratio (OR)=3.5, 95% confidence interval (CI) 1.7–7.0, P<0.001) or non-reportable virus (OR=9.3, 95% CI 1.2–71.5, P=0.033) compared to CCR5 containing virus on tropism assay; a higher viral load (50 000 to <100 000 copies/ml) at screening compared to <50 000 copies/ml (OR=6.4, 95% CI 1.5–28.0, P=0.013); and five or more regimen options compared to only one option offered (OR=0.2, 95% CI 0.1–0.6, P=0.005).
In 21% (85/413) of participants, a complex ARV regimen was selected (n=51 included ENF and n=34 containing four drugs not including ENF or NRTIs); a 5-drug regimen was not selected for any participant. Factors significantly associated with increased odds of selecting a complex ARV regimen included the following: prior use of INSTIs and lack of any susceptible NRTI, NNRTI, or PI. For example, participants with no susceptible PI option had 6.6 times greater odds (multivariable adjusted estimate) of choosing a complex ARV regimen than those with at least one susceptible PI (95% CI 3.5–12.5). Factors significantly associated with decreased odds of selecting a complex ARV regimen included black race and continental US Hispanic ethnicity (compared to white non-Hispanic). Despite similar rates of complex ARV regimen recommendation, Hispanic participants from the continental US had an OR of 0.2 (95% CI: 0.1–0.7) and black participants had an OR of 0.5 (95% CI: 0.3–1.0), whereas Hispanic participants from Puerto Rico had about two-fold greater odds (95% CI: 0.9–5.1) of selecting a complex ARV regimen compared to white non-Hispanic participants in a multivariable analysis adjusted for baseline resistance (Fig. 3A).
Figure 3.
(A) Factors associated with accepting a complex regimen defined as using four ARV agents or inclusion of ENF in the regimen. Higher odds ratio favors selection of a complex regimen, n = 396 for race–ethnicity and multivariable model (n = 17 other race–ethnicity excluded); n = 413 otherwise. The square boxes represent the estimated odds ratio and horizontal lines represent the Wald 95% CI plotted on a logarithmic scale. Additional covariates that were not statistically significant (univariate P-value): age (P=0.4), sex (P=0.8), prior use of ENF (P=0.9), CD4 cell count (P=0.2), HIV-1 RNA (P=0.8), HIV-1 tropism (P=0.4), hepatitis C status (P=0.8), IDU history (P=0.7), and calendar year of enrollment (P=0.4). All analyses were stratified by cPSS >2.0 (Arm A/B) versus ≤2.0 (Arm C). (B) Factors associated with selecting an NRTI combination other than TDF + FTC (3TC), n = 413. The square boxes represent the estimated odds ratio and horizontal lines represent the Wald 95% CI plotted on a logarithmic scale. Additional covariates that were not significant in multivariable model selection (univariate result): age (<40 years OR = 0.5 [95% CI, 0.2–0.9], 40 to <45 years OR = 0.6 [0.3–1.2], 45 to <50 years OR = 0.4 [0.2–0.8], ≥50 years OR = 1.0 [reference], P=0.03), sex (female OR = 0.6 [0.3–1.1] versus male, P=0.08), race–ethnicity (P=0.2), geographic region (P=0.5), prior use of ENF (P=0.3), prior use of INSTI (P=0.6), HIV-1 RNA (P=0.4), HIV-1 tropism (P=0.14), hepatitis C status (P=0.7), IDU history (P=0.11), number of boosted PIs with resistance (2–6 PIs, OR = 1.8 [1.1–3.0] versus 0–1 PIs [reference], P=0.02), and NNRTI resistance (P=0.7). All analyses were stratified by cPSS > 2.0 (Arm A/B) versus ≤2.0 (Arm C).
Site/participant selection of an NRTI combination
The top two NRTI combinations chosen were TDF+FTC (or TDF+3TC) in 78% and TDF+FTC+ZDV in 15% of participants. A total of 12 unique NRTI combinations were chosen among study participants, all contained FTC or 3TC, most contained TDF (96%), followed by ZDV (19%), ABC (4%), and ddI or d4T very infrequently (<1%). A 92% acceptance rate of the recommended first or second NRTI combinations was observed. Among 385 participants with >1 NRTI combination option, simplicity (38%) and highest ranked NRTI combination (31%) were primary reasons listed by the sites.
The number of NRTIs to which the virus was resistant (2–6 vs 0–1) and CD4 count <50 cells/μl was associated with selection of an NRTI combination other than TDF+FTC (3TC) (Figure 3B). Lower creatinine clearance and earlier calendar year of enrollment were also associated with this outcome.
Discussion
The OPTIONS study evaluated the process of expert-guided regimen design and selection utilizing a web-based tool with real-time feedback to study sites. The first- or second-ranked ARV regimens and NRTI options were selected by sites in 86 and 92% of cases, respectively. This suggests that the process and recommendations were acceptable. The RAL+DRVr+ETR regimen and the four-drug regimen adding ENF were the most frequently recommended and highest preferred regimens by the study team. The primary reason for these specific regimen recommendations was the expected high levels of activity as measured by the regimen activity score. In addition, the study team considered RAL+DRVr+ETR to be a regimen with proven activity for treatment-experienced patients.10 However, sites and participants were less likely to choose ENF-containing regimens. Thus, MVC+DRVr+RAL became the second highest site-selected regimen. Although the study team often ranked (58%) the NRTI combination of TDF+FTC+ZDV highly (for participants with extensive cross-resistance, in order to take advantage of NRTI mutational interactions), the sites/participants were less enthusiastic about this choice, citing simplicity as an important consideration in their decision (sites chose the combination of TDF+FTC 78% of the time).
The choice of a more complicated ARV regimen was driven by viral resistance, prior experience and race–ethnicity. Not surprisingly, resistance to a greater number of ARVs within a class was associated with selection of a complex regimen. For example, if a participant’s virus was not susceptible to any NNRTI or any PI, the odds of selecting a complex regimen were increased by sixfold. Since DRVr was the PI most often used and ETR the only NNRTI option, retained susceptibility to these drugs was key to regimen simplicity in this population. Prior use of an INSTI was also associated with selecting a complex regimen. Thus, prior INSTI use and lack of susceptibility to ETR and DRVr may be important factors in selecting a more complex regimen in clinical practice. The odds of selecting a complex ARV regimen were approximately 50–80% lower for black and continental US Hispanic participants, even after accounting for drug resistance. The explanation for this latter finding is unclear. More black participants proportionally joined the study over time which correlated with the recommendation of less complex ARV regimens by the study team but this was not statistically significant (data not shown). Furthermore, there was no change in the proportion of Hispanic participants over time. Also, the study team recommendations did not vary by race. Thus, it appears that local investigator/participant selection of less complicated ARV regimens for blacks and Hispanics may have been driving the observed difference. Fewer Hispanic participants were willing to use ENF (25 vs 55% for other races). Whites had a longer median prior use of ARVs (14 years) compared to blacks (10 years) and Hispanics (11 years). There was one study that documented differences in patterns of ARV use with blacks being more likely to use NNRTI-based regimens compared to whites that used more PI-based regimens.11 This was also an unexpected finding in this study without clear explanation. Future analyses of treatment-experienced patients will need to examine patterns of types of ARV regimens while accounting for prior ARV experience, viral resistance patterns, CD4 counts, and viral load. Although web-based tools to guide ARV selection have existed for more than a decade12 this is the first randomized trial to utilize a web utility in combination with centralized expert opinion to guide selection of therapy. In the current study, the study team offered expert guidance to site investigators in the selection of a new regimen among ARVs approved for patients with drug-resistant virus. We followed treatment guidelines, i.e., included a combination of fully or partially active agents, defined by the cPSS, to achieve a regimen phenotypic score greater than 2.0 and recommended regimen options to sites. Selection of one of the top-ranked regimens was high, indicating that centralization of regimen recommendation was successful. Prior studies testing novel drugs typically randomly assign participants to new drugs or control in the context of an optimized background regimen utilizing resistance testing.10,13–20 In most studies, local study investigators chose the optimized background regimen, guided by parameters set by the protocol (such as “no more than two active or three partially active drugs”). The most common regimen selected in our study was DRVr, RAL, and ETR similar to the regimen tested in the TRIO study.10 In that trial 103 subjects were assigned to the above regimen and local investigators were allowed to choose an optimized background (88% used NRTIs and 11.7% used ENF). The design of that study also differed in that subjects were required to have susceptibility to DRV and at least three NRTI mutations. Thus, we believe our trial had a novel approach by ensuring that all randomized participants in the study had more than two active (based on PSS) agents utilizing a web-based regimen selection tool that calculated the cPSS for each of 20 possible regimens and assigned drugs with partial drug susceptibility a score between 0 and 1. Prior treatments and intolerances were included in the web tool. The study team made recommendations in real time to the sites and the best regimen for each study participant was then selected by the local study investigator and the study participant.
Designing regimens for patients who are highly treatment experienced is complicated and DHHS guidelines strongly recommend expert involvement.1 Unlike treatment-naïve patients, where HIV ARV guideline panels provide multiple specific regimen options, the selection of regimens for a more treatment-experienced patient relies on a careful consideration of treatment history, current and past resistance tests, and patient willingness to take a complex regimen with multiple drugs, twice daily dosing and sometimes an injectable agent. Decisions are even more difficult if there is a history of poor adherence. Many patients who need new regimens, due to virologic failure and resistance, have failed to adhere to simpler, once-daily therapies. While it is clear that including two, and preferably three, fully “active” agents are optimal, the number of drugs needed to achieve optimal ARV activity is uncertain when fewer than three fully active agents are available, and likely varies for each patient due to cross-resistance within classes, archived or very low level resistance, and the unavailability of regimens that could be constructed entirely from new, presumably resistance-free, classes. Since the study was completed, dolutegravir, an INSTI with activity against some INSTI-resistant HIV isolates, was approved for use in the US; dolutegravir can be used for patients who previously received RAL and might have been useful to simplify regimens for participants in our study.9
In this multi-center randomized trial, we developed a web-based utility that facilitated treatment recommendations for highly treatment-experienced patients. Drug resistance, prior ARV experience, and race–ethnicity were key factors in decisions to select a more complex regimen. The OPTIONS study demonstrates a successful approach to centrally implementing recommendations for treatment that are acceptable to local investigators and patients.
Supplementary Material
Acknowledgements
We thank the study participants for their invaluable contributions. We thank Eric Buckley and Bruce Dejaiffee at Frontier Science and Technology Research Foundation who developed the web utility. In addition to the authors, the study team included the following members: Evelyn Hogg, BA, Social & Scientific Systems, Silver Spring, MD, USA; Dave Rusin, MT (ASCP), Frontier Science and Technology Research Foundation Inc., Amherst, NY, USA; Debra Payne, PharmD, McKesson Specialty, Toronto, ON, Canada; Lynette Purdue, NIAID Division of AIDS, Bethesda, MD, USA; Beatriz Grinsztejn, MD, PhD, Instituto de Pesquisa Clinica Evandro Chagas Fiocruz, Rio de Janeiro, Brazil; Juan Guanira, MD Asociacion Civil Impacta Salud y Educacion, Lima, Peru; Loren Miller, MD, MPH, UCLA School of Medicine, Harbor-UCLA Medical Center, Torrance, CA, USA; Fred Sattler, MD, University of Southern California Keck School of Medicine, Los Angeles, CA, USA; Bret Rudy, MD, Children’s Hospital of Philadelphia, Philadelphia, PA, USA; Rolando Viani, MD, University of California, San Diego, La Jolla, CA, USA; George Siberry, MD, MPH, National Institute of Child Health and Human Development, Pediatric, Adolescent and Maternal AIDS (PAMA) Branch, Bethesda, MD, USA; Amy Sbrolla, ACRN, BSN, Massachusetts General Hospital, Boston, MA, USA; Patricia Anthony, BS, CLS, Maternal Child Adolescent Virology Research Lab, University of Southern California, Los Angeles, CA, USA; Eric Stets, BS, Frontier Science and Technology Research Foundation, Amherst, NY, USA; Adam Manzella, Frontier Science and Technology Research Foundation, Amherst, NY, USA; Robert Levaro, Tucson, AZ, USA; Lauren Petrella, Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA; Peter Piliero, MD, Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT, USA; Carol Jean Guittari, MS, RPh, Hoffmann-La Roche Inc., Nutley, NJ, USA; Miklos Salgo, MD, PhD, Hoffmann-La Roche Inc., Nutley, NJ, USA; Randi Leavitt, MD, Merck and Company Inc., North Wales, PA, USA; Eoin Coakley, MD (formerly at Monogram Biosciences) Abbott Laboratories, Abbott Park, IL, USA; Charles Walworth, MD, Monogram Biosciences, South San Francisco, CA, USA; Karina DeFaria, formerly at Monogram Biosciences, South San Francisco, CA, USA; Pamela Clax, DPM, Pfizer, Inc., New York, NY, USA; Joseph Mrus, MD, MSc, Janssen Services, LLC, Titusville, NJ, USA; David Anderson, MD, Janssen Services, LLC, Titusville, NJ, USA.
Funding
The project described was supported by Award Number UM1AI068636 from the National Institute of Allergy and Infectious Diseases (NIAID) and supported by National Institute of Mental Health (NIMH), National Institute of Dental and Craniofacial Research (NIDCR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the National Institutes of Health. The work was also supported by the Statistical and Data Management Center (SDMC) grant [AI68634] from the NIAID. Boehringer-Ingelheim, Hoffmann-La Roche, Janssen, Merck, and Pfizer provided study medications and Monogram Biosciences provided HIV genotype and phenotype resistance and HIV tropism testing. KTT: UM1 AI 069412 and P30 AI042853 (Lifespan/Tufts/Brown CFAR), P30 AI50410 (UNCKM), AI 064086 (K24 to RH), AI 069432 (UCSD ACTU), AI 36214 (UCSD CFAR Clinical Investigation and Biostatistics Core) RTG is supported by National Institutes of Health (NIH) [R01 AI066992-04A1] and by grants to the AIDS Clinical Trials Group (NIH U01 AI 694722) and the Harvard University Center for AIDS Research (NIH 2P30 AI060354-06). CJF is supported by UM1 AI069501 from the AIDS Clinical Trials Group.
Footnotes
Prior presentation: This work was presented at the CROI 2013, 20th Conference on Retroviruses and Opportunistic Infections (March 3–6, 2013, Abstract #557) in Atlanta, Georgia.
ClinicalTrials.gov Identifier: NCT00537394
Contributors
In the acknowledgments section, we list other team members who contributed to the study. The acknowledgments appendix lists two research staff at each site that enrolled participants.
Conflicts of interest
KTT’s institution has received research grants from BristolMyers Squibb, Gilead, GlaxoSmithKline/ViiV, Merck, and Tibotec. KRM has received research support to University of North Carolina at Chapel Hill from Merck, AbbVie, and Gilead. RTG’s institution has received educational grants from Janssen, GlaxoSmithKline/ViiV, and Abbott. CJF’s institution has received research grants from Gilead, Janssen, and Pfizer. VAJ has received research virology laboratory equipment and training support to the University of Alabama at Birmingham School of Medicine from Roche. JJE has received research support to University of North Carolina at Chapel Hill from Bristol Myers Squibb, GlaxoSmithKline/ViiV, and Merck and consulting fees from BristolMyers Squibb, Gilead, GlaxoSmithKline/ViiV, Janssen, and Merck. RHH has received research support to University of California at San Diego from Abbott, BristolMyers Squibb, GlaxoSmithKline/ViiV, Gilead, Janssen, Merck and Roche, and consulting fees from Abbott, BristolMyers Squibb, Gilead, GlaxoSmithKline/ViiV, Janssen, and Merck. He is currently employed at Gilead Sciences.
Ethics approval
Study participants were recruited from 64 sites across the continental US and Puerto Rico. Sites obtained local institutional IRB approval and written informed consent was obtained from all study participants.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, Management of the Treatment-Experienced Patient; [November 13, 2014]. p. H1. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Google Scholar]
- 2.Thompson MA, Aberg JA, Hoy FJ, Telenti A, Benson C, Cahn P, et al. Antiretroviral treatment of adult HIV infection: recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch MS, Gunthard HF, Schapiro JM, Brun-Vezinet F, Clotet B, Hammer SM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: recommendations of an International AIDS Society-USA panel. Clin Infect Dis. 2008;47(2):266–285. doi: 10.1086/589297. [DOI] [PubMed] [Google Scholar]
- 4.Katzenstein DA, Bosch RJ, Hellmann N, Wang N, Bacheler L, Albrecht M, et al. Phenotypic susceptibility and virological outcome in nucleoside-experienced patients receiving three or four antiretroviral drugs. AIDS. 2003;17(6):821–830. doi: 10.1097/00002030-200304110-00007. [DOI] [PubMed] [Google Scholar]
- 5.Swanstrom R, Bosch RJ, Katzenstein D, Cheng H, Jiang H, Hellmann N, et al. Weighted phenotypic susceptibility scores are predictive of the HIV-1 RNA response in protease inhibitor-experienced HIV-1-infected subjects. J Infect Dis. 2004;190(5):886–893. doi: 10.1086/422692. [DOI] [PubMed] [Google Scholar]
- 6.Campbell TB, Shulman NS, Johnson SC, Zolopa AR, Young RK, Bushman L, et al. Antiviral activity of lamivudine in salvage therapy for multidrug-resistant HIV-1 infection. Clin Infect Dis. 2005;41(2):236–242. doi: 10.1086/430709. [DOI] [PubMed] [Google Scholar]
- 7.Castagna A, Danise A, Menzo S, Galli L, Gianotti N, Carini E, et al. Lamivudine monotherapy in HIV-1-infected patients harbouring a lamivudine-resistant virus: a randomized pilot study (E-184V study) AIDS. 2006;20(6):795–803. doi: 10.1097/01.aids.0000218542.08845.b2. [DOI] [PubMed] [Google Scholar]
- 8.Fox Z, Dragsted UB, Gerstoft J, Phillips AN, Kjaer J, Mathiesen L, et al. A randomized trial to evaluate continuation versus discontinuation of lamivudine in individuals failing a lamivudine-containing regimen: the COLATE trial. Antivir Ther. 2006;11(6):761–770. [PubMed] [Google Scholar]
- 9.Tashima K, Smeaton L, Andrade A, Eron J, Fichtenbaum C, Gandhi R, et al. Omitting NRTI from ARV regimens is not inferior to adding NRTI in treatment-experienced HIV+ subjects failing a protease inhibitor regimen: the ACTG OPTIONS study. Presented at: CROI 2013, 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA, US. March 3–6, 2013; [Oral 153LB] [Google Scholar]
- 10.Yazdanpanah Y, Fagard C, Descamps D, Taburet AM, Colin C, Roquebert B, et al. High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: results of the ANRS 139 TRIO trial. Clin Inf Dis. 2009;49:1441–1449. doi: 10.1086/630210. [DOI] [PubMed] [Google Scholar]
- 11.Oramasionwu CU, Brown CM, Lawson KA, Ryan L, Skinner J, Frei CR. Differences in national antiretroviral prescribing patterns between black and white patients with HIV/AIDS, 1996-2006. South Med J. 2011;104(12):794–800. doi: 10.1097/SMJ.0b013e318236c23a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis. 2006;42(11):1608–1618. doi: 10.1086/503914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lalezari JP, Henry K, O’Hearn M, Montaner JS, Piliero PJ, Trottier B, et al. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med. 2003;348(22):2175–2185. doi: 10.1056/NEJMoa035026. [DOI] [PubMed] [Google Scholar]
- 14.Gathe J, Cooper DA, Farthing C, Jayaweera D, Norris D, Pierone G, Jr, et al. Efficacy of the protease inhibitors tipranavir plus ritonavir in treatment-experienced patients: 24-week analysis from the RESIST-1 trial. Clin Infect Dis. 2006;43(10):1337–1346. doi: 10.1086/508353. [DOI] [PubMed] [Google Scholar]
- 15.Cahn P, Villacian J, Lazzarin A, Katlama C, Grinsztejn B, Arasteh K, et al. Ritonavir-boosted tipranavir demonstrates superior efficacy to ritonavir-boosted protease inhibitors in treatment-experienced HIV-infected patients: 24-week results of the RESIST-2 trial. Clin Infect Dis. 2006;43(10):1347–1356. doi: 10.1086/508352. [DOI] [PubMed] [Google Scholar]
- 16.Clotet B, Bellos N, Molina JM, Cooper D, Goffard JC, Lazzarin A, et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomized trials. Lancet. 2007;369(9568):1169–1178. doi: 10.1016/S0140-6736(07)60497-8. [DOI] [PubMed] [Google Scholar]
- 17.Steigbigel RT, Cooper DA, Kumar PN, Eron JE, Schechter M, Markowitz M, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359(4):339–354. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- 18.Gulick RM, Lalezari J, Goodrich J, Clumeck N, DeJesus E, Horban A, et al. Maraviroc for previously treated patients with R5 HIV-1 infection; MOTIVATE Study Teams. N Engl J Med. 2008;359(14):1429–1441. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katlama C, Haubrich R, Lalezari J, Lazzarin A, Madruga JV, Molina JM, et al. Efficacy and safety of etravirine in treatment-experienced HIV-1 patients: pooled 48-week analysis of two randomized, controlled trials. AIDS. 2009;23(17):2289–2300. doi: 10.1097/QAD.0b013e3283316a5e. [DOI] [PubMed] [Google Scholar]
- 20.Eron JJ, Clotet B, Durant J, Katlama C, Kumar P, Lazzarin A, et al. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING Study. J Infect Dis. 2013;207(5):740–748. doi: 10.1093/infdis/jis750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.