Abstract
Introduction
Preeclampsia (PE) is a major complication of pregnancy that could lead to maternal and fetal morbidity and mortality. The pathophysiological mechanisms of PE are not completely understood, but recent research has begun to unravel some of the potential mechanisms.
Areas covered
Genetic polymorphisms and altered maternal immune response may cause impaired remodeling of the spiral arteries; a potential early defect in PE. Inadequate invasion of cytotrophoblasts into the decidua leads to reduced uteroplacental perfusion pressure (RUPP) and placental ischemia/hypoxia. Placental ischemia causes the release of biologically active factors such as anti-angiogenic factors, inflammatory cytokines, reactive oxygen species, hypoxia-inducible factors, and angiotensin II receptor autoantibodies. These vasoactive factors could cause systemic vascular endotheliosis and consequent increase in vascular resistance and blood pressure, glomerular endotheliosis causing proteinuria, cerebrovascular endotheliosis causing cerebral edema, seizures and visual disturbances, and hepatic endotheliosis which may contribute to the manifestations of HELLP syndrome. PE-associated vascular endotheliosis causes a decrease in vasodilator mediators such as nitric oxide, prostacyclin and endothelium-derived hyperpolarizing factor, an increase in vasoconstrictors such as endothelin-1, angiotensin II and thromboxane A2, and enhanced mechanisms of vascular smooth muscle contraction such as intracellular Ca2+, protein kinase C and Rho-kinase. Changes in matrix metalloproteinase activity and extracellular matrix cause vascular remodeling and further vasoconstriction.
Expert opinion
Some of the genetic, immune and vasoactive factors involved in vascular endotheliosis could be used as biomarkers for early detection, and as potential targets for prevention and treatment of PE.
Keywords: pregnancy, preeclampsia, blood pressure, endothelium, vascular smooth muscle
INTRODUCTION
Normal pregnancy (Norm-Preg) is associated with significant changes in the hemodynamics in order to meet the nutrient and metabolic demands of the mother and fetus. Pregnancy-associated hemodynamic changes include increased plasma volume, heart rate and cardiac output, vasodilation of the uterine and systemic circulation, and decreased vascular resistance and blood pressure (BP) particularly during mid-gestation. Hypertension in pregnancy (HTN-Preg) occurs in 5–8% of pregnancies and may take one of 4 forms: chronic HTN that predates pregnancy, preeclampsia (PE)-eclampsia, chronic HTN with superimposed PE, and nonproteinuric gestational HTN [1] (* = of importance, a very informative review on PE). PE is clinically manifested as increased BP, often proteinuria, and occasionally edema and increased platelet aggregation. PE may also be a part of HELLP syndrome manifested as hemolysis, elevated liver enzymes and low platelet count. PE may also be associated with fetal intrauterine growth restriction (IUGR) and reduced birth weight. If untreated, PE can lead to eclampsia with severe neurovascular complications including severe HTN and convulsions [2].
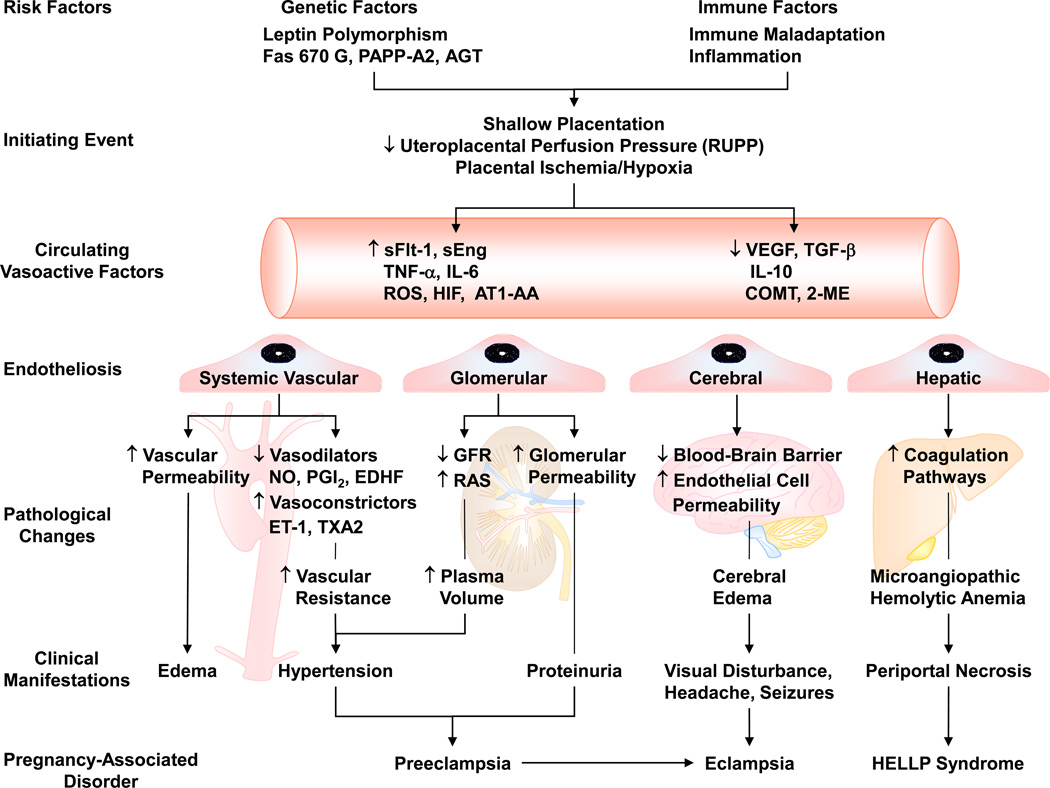
Because the etiology of PE is largely unclear, it has been difficult to develop effective preventive or therapeutic measures, and currently the only definitive treatment for PE is delivery of the infant and placenta. Risk factors for PE have been suggested including genetic polymorphism and immune maladaptation. These risk factors are believed to cause abnormal placental development, reduction of uteroplacental perfusion pressure (RUPP), and placental ischemia/hypoxia. Placental ischemia is thought to trigger the release of vasoactive factors that cause imbalance in circulating angiogenic and anti-angiogenic factors and lead to generalized endotheliosis in the systemic, glomerular, cerebral and/or hepatic circulation [3] (* = of importance, describes the role of endotheliosis in PE). Systemic endotheliosis could cause increased vasoconstriction, vascular resistance and BP. Glomerular endotheliosis is the likely cause of proteinuria. Cerebrovascular endotheliosis could affect the blood-brain barrier and lead to severe headaches, visual disturbances and seizures. Endotheliosis in the hepatic vessels could lead to changes in liver enzymes and other manifestations of HELLP syndrome (Fig. 1). Because it is difficult to perform mechanistic studies in pregnant women, animal models of HTN-Preg have been developed in order to delineate some of the potential mechanisms of PE. Studies in these experimental models have demonstrated significant changes in the renal control mechanisms of BP, and highlighted glomerular injury and altered kidney function as possible causes of the increased BP [4]. Also, genetic, immune and bioactive vascular factors have been suggested to play a role in the pathogenesis of PE. In this review, we will discuss the current knowledge of the genetic and immunologic factors implicated in PE. We will also discuss how impaired placentation could cause an imbalance in angiogenic and anti-angiogenic factors and other vasoactive factors resulting in the generalized endotheliosis and the changes in vascular endothelial cells (ECs), vascular smooth muscle (VSM) and extracellular matrix (ECM) associated with HTN-Preg. We will also discuss potential biomarkers for early prediction and diagnosis, and future directions for prevention and treatment of PE.
Fig. 1.
Risk factors and pathogenesis of preeclampsia. Genetic and immune factors cause shallow placentation and RUPP during late pregnancy and trigger the release of circulating bioactive factors. Bioactive factors could affect the systemic blood vessels and lead to generalized vasoconstriction, increased vascular resistance and hypertension, or cause increased vascular permeability and edema. The bioactive factors could injure the kidney leading to increased plasma volume and severe HTN, as well as glomerular endotheliosis and proteinuria. Increased cerebral vascular permeability and edema lead to seizures and life-threatening eclampsia. Endotheliosis of the hepatic vessels could lead to activation of coagulation pathways and manifest as HELLP syndrome. GFR: glomerular filtration rate, RAS: Renin Angiotensin System.
1. Genes and Genetic Imprinting in PE
According to the genetic-conflict theory, fetal genes function to enhance the transfer of nutrients to the fetus, while maternal genes function to restrict transfer that exceeds maternal optimum. Hence, a possible conflict between fetal and maternal genes may occur in PE. With genetic imprinting, a similar conflict may occur within fetal cells between maternally- and paternally-derived genes leading to genetic diseases such as Angelman’s syndrome. The gene-conflict theory predicts that while fetal genes and placental factors act to raise maternal BP to increase blood supply to the fetus, maternal factors act to reduce BP so that the maternal blood supply is not seriously impaired. Thus, the EC dysfunction associated with PE may represent a fetal-rescue strategy when the uteroplacental blood supply is inadequate in order to increase the non-placental vascular resistance and BP and provide adequate blood supply to the fetus [5]. Several maternal, fetal and paternal genes may be involved in PE.
1.1. Maternal Genes in PE
Maternal genes may play a role in PE. Female offspring of a pregnancy complicated by PE may have a higher risk of developing PE in their own pregnancies, suggesting inheritance of PE susceptibility genes [2]. Genome-wide linkage studies have identified more than one gene locus on chromosomes 1, 2p13, 2q, 3p, 4q, 9, 10q, 11q23–24, 12q, 15q, 18 and 22q, suggesting that PE is a multigene disorder [2] (Table 1). When the global gene expression was compared in placentas of PE and Norm-Preg women in the first trimester, about 36 major genes were identified in PE placentas, 31 of them were downregulated [6]. Some of the genes implicated in PE include ACVR2A, STOX1 and those of VEGF, hypoxia-inducible factor (HIF), Fas and leptin (Fig. 2) as well as angiotensinogen and cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-10 (IL-10).
Table 1.
Representative Maternal Genes Associated with Preeclampsia
| Gene symbol |
Full Name | Gene Map Locus |
Function | Polymorphism | MIM Number |
Ref |
|---|---|---|---|---|---|---|
| AGT | Angiotensinogen | 1q42-q43 | Converted to AngI then AngII, which maintains BP and is linked to HTN |
M235T | 106150 | [78] |
| F5 | Coagulation Factor V | 1q23 | Produces hemostasis factors; inactivated by protein C |
R485K, M385T | 612309 | [79] |
| MTHFR | 5,10-methylene- tetrahydrofolate reductase |
1p36.3 | Produces enzyme that converts homocysteine to methionine |
T677C | 607093 | [80] |
| NOS3 | Nitric Oxide Synthase 3 |
7q36 | Synthesizes NO in endothelium; maintains vascular homeostasis |
C786T, T894G (linked to metabolic syndrome) |
163729 | [80, 81] |
| PAPP-A2 | Pregnancy- Associated Plasma Protein A2, Pappalysin-2 |
1q23-q25 | Encodes proteases that cleave insulin-like growth factor (IGF)-binding protein-5, thus affecting IGF signaling |
n/a | 211918 228237 213332 |
[14] |
|
PEE1 PEE2 PEE3 |
PE/eclampsia-1 PE/eclampsia-2 PE/eclampsia-3 |
2p13 2p25 9p13 |
Genome scans in Australia, Iceland and Finland suggest linkage to PE; function unknown |
n/a | 189800 609402 609403 |
[82] |
| Siglec-6 | Sialic acid binding Ig-like lectin 6 |
19q13.3 | Receptor for leptin; rate of expression is related to progression of labor |
n/a | 604405 | [14, 83] |
MIM, Mendelian Inheritance in Man; n/a, not available.
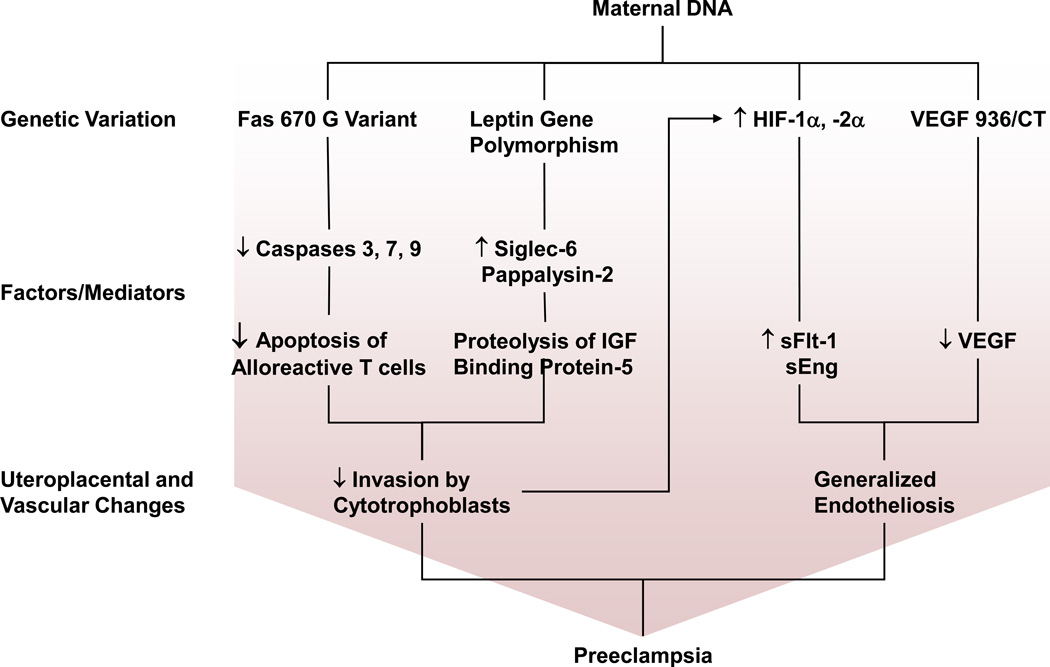
Fig 2.
Maternal genes involved in preeclampsia. Potential genes that are altered in preeclampsia include Fas 670 variant, leptin gene polymorphism, HIF and VEGF 936C/T. Fas 670 G variant causes a decrease in activity of caspases 3, 7 and 9 and hence decreased apoptosis of alloreactive T cells. Leptin gene polymorphism leads to an increase in Siglec-6 and Pappalysin-2, thus affecting insulin growth factor (IGF) signaling. Both Fas 670 G Variant and leptin gene polymorphism cause inadequate uterine invasion by cytotrophoblasts and placental ischemia/hypoxia. Placental hypoxia causes increases in HIF expression and HIF-1α and −2α proteins which in turn cause an increase in sFlt-1 and sEng. VEGF 936C/T variant causes a decrease in VEGF levels. Increased sFlt-1/VEGF ratio leads to generalized endotheliosis and preeclampsia.
ACVR2A located on chromosome 2q22 and STOX1 on chromosome 10q22 are two of the first PE susceptibility genes identified within confirmed regions with significant genome-wide linkage, and both involving normal variations single nucleotide polymorphisms (SNPs) [7] (* = of importance, describes the first PE susceptibility genes identified). STOX1 Y153H common polymorphism and transmission of the maternal susceptibility allele have been observed in families where several generations of women exhibited familial severe early-onset PE. STOX1 encodes a transcription factor and has been linked to trophoblast dysfunction and IUGR [7]. Interestingly, STOX1 overexpression in choriocarcinoma cells mimics the transcriptional consequences of PE in the human placenta [8, 9]. Also, wild-type female mice crossed with transgenic male mice overexpressing human STOX1 gene show characteristics of PE including HTN, proteinuria, and increased plasma levels of soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sEng) [10].
Subjects carrying the T allele VEGF 936C/T genotype have lower plasma VEGF levels than subjects carrying the VEGF 936C/C genotype. Changes in VEGF gene and plasma levels could play a role in the vascular endotheliosis associated with PE [11].
HIF-1α and −2α proteins and regulated genes are increased in PE placenta. Upregulation of placental HIF-1α may induce the expression of sFlt-1 and sEng, which are secreted into the maternal circulation, and cause disruption of the endothelium and other manifestations of PE. Elevated levels of HIF-1α protein in PE placenta may be due to both increased formation secondary to ischemia/hypoxia and reduced degradation after reperfusion/oxygenation as a result of proteasomal dysfunction [12].
The Fas ligand-Fas interaction is a known pathway of apoptosis. The binding of Fas ligand to Fas triggers the activation of caspases 3, 7 and 9, which promote apoptosis. In Norm-Preg, activated T lymphocytes, which recognize paternal antigens, express Fas and interact with the cytotrophoblasts expressing the Fas ligand, leading to apoptosis of T cells and reduction in their ability to recognize and destroy the cytotrophoblasts invading the myometrium and spiral arteries. Polymorphisms of Fas and Fas ligand genes may play a role in PE. In PE, the expression of Fas 670 G variant is associated with reduced Fas production in activated T lymphocytes, leading to reduced T cell apoptosis, increased destruction of cytotrophoblasts, and inadequate invasion of spiral arteries [13].
Leptin gene expression may also be involved in PE. Leptin gene expression is increased in the basal plate of PE compared with Norm-Preg placenta. Also, genes encoding sialic acid binding Ig-like lectin 6 (Siglec-6), a potential leptin receptor, and pappalysin-2 (PAPP-A2), a protease that cleaves insulin-like growth factor (IGF) binding protein-5 (IGFBP-5), are over-expressed in PE. In fibroblasts, increased IGFBP-5 proteolysis may attenuate its stimulatory effects on cell migration. Placental cytotrophoblasts may respond in a similar fashion such that an increase in PAPP-A2 could inhibit cytotrophoblast invasion by mechanisms involving increased IGFBP-5 proteolysis. Because IGF and insulin signaling act via the same pathways, it is likely that alterations in leptin, Siglec-6, and PAPP-A2 levels may work in concert to inhibit cytotrophoblast invasion, resulting in PE [14].
Studies have suggested that homozygous T 235 coding angiotensinogen genotype is associated with reduced plasma volume in nulligravid women, which might provide the basis for the “intolerance to plasma volume expansion” theory of PE and contribute to IUGR in PE [15, 16]. Studies have also shown possible association between clinical and laboratory manifestations of PE and the TNF-α gene G308A (rs1800629) polymorphism [17]. Other studies have shown specific IL-10 gene promoter polymorphisms in women with early-onset PE [18].
1.2. Fetal Genes in PE
Fetal genes are also an important factor in the development of PE. Women homozygous for the inhibitory killer immunoglobulin-like receptor (KIR) haplotypes (AA) have higher risk of PE than women homozygous for the stimulator KIR halpotypes (BB), and the effect is strongest if the fetus is homozygous for the human leukocyte antigen class II (HLA-C2) haplotype [19]. Also, gene-to-gene interaction between fetal HLA-G and maternal KIR2DL4 is associated with PE risk in multigravid pregnancy [20]. In mice, mutations in the cyclin-dependent kinase inhibitor cdkn1c (p57Kip2), a regulator of embryonic growth, induces changes in the placental architecture indicative of the RUPP found in HTN-Preg. Also, pregnant mice expressing wild-type levels of p57Kip2 develop a PE-like condition when carrying p57kip2-deficient pups, supporting a role for fetal genes in the development of PE [2].
1.3. Paternal Genes in PE
Paternal genes could play a role in the development of PE. In a study which compared men whose mothers had PE to men whose mothers did not have PE, a paternal family history of PE was associated with more than 2-fold increase in risk of PE [21]. PE is also common in hydatid form mole pregnancies in which all the fetal chromosomes originate from the father [2].
1.4. Ethnic Background and PE
Maternal ethnicity may be a factor in the development of PE. African-American women have the highest incidence rate of PE (5.2%), while Asian women have the lowest rate (3.5%) [2]. Paternal ethnicity may also play a role in the incidence of PE. Among African-American women, the incidence of PE is greater if paternal ethnicity is different from maternal ethnicity (5.8%) than if the paternal and maternal ethnicities are the same (5.2%). Also, among Asian women, the incidence of PE is greater if paternal ethnicity is different (4.6%) than if it is the same as maternal ethnicity (3.2%). In contrast, among Native-American women, the incidence of PE is greater if the paternal ethnicity is the same (9.7%) than if it is different from maternal ethnicity (3.3%) [22].
2. Immune Maladaptation in PE
Maternal immune maladaptation to fetal or paternal factors could play a role in PE. PE is more common during a first conception, after the mother has switched partners, in women who use barrier contraception, and in cases of donated gametes. Among nulliparous women, a previous spontaneous or induced abortion may have a protective effect against PE, but nulliparous women who had an abortion with a different partner are at the same risk as primigravidas. Also, while multiparous women may have a lower risk of PE than nulliparous women, and the protective effect of multiparty is lost with a change of partner. These observations suggest that tolerance to paternal antigens develops during the first pregnancy, and that memory T cells, which induce paternal antigen-specific tolerance, quickly increase in the next pregnancy and lower the risk of maladaptation during pregnancy [23]. Also, in mice, maternal T cells acquire a transient state of tolerance to specific paternal alloantigens during pregnancy. Importantly, conditions associated with suppressed immune response such as HIV-related T-cell immune deficiency are associated with a low rate of PE [19].
Prolonged exposure of the female reproductive system to seminal fluid may alter the immune response and decrease the risk of PE. Vaginal or uterine antigen presenting cells (APC) may take up soluble major histocompatibility complex (MHC) class I antigens present in the seminal fluid leading to specific tolerance to paternal MHC class I antigens [23]. Alloreactive maternal T cells may also undergo apoptosis when they come in contact with soluble MHC Class I antigens. Also, transforming growth factor-β (TGF-β) present in the seminal fluid may initiate inflammatory response and increase pro-inflammatory cytokines and chemokines in uterine tissues, leading to deviation of the immune activity of T cells towards the inflammatory reaction, and thereby decreased activity against paternal sperm alloantigens [23]. This is supported by experiments in rodents demonstrating that exposure of the female reproductive tract to seminal TGF-β initiates an influx of APC that process ejaculate antigens and subsequently activate the lymphocytes in lymph nodes draining the uterus and, as a consequence, induce systemic tolerance to paternal MHC class I antigens [5]. Thus prolonged exposure to soluble MHC class I antigens may be important for the induction of tolerance to paternal antigens, while limited exposure or low levels of soluble MHC-class I antigens in sperms may be a contributing factor in PE [23].
Natural killer (NK) cells may play a role in induction of MHC class I tolerance. Uterine NK cells produce angiogenic factors such as VEGF, placental growth factor (PlGF) and TGF-β [23]. During Norm-Preg, activation of decidual uterine NK cells by paternal MHC class I antigens causes an increase in VEGF, which reacts with FIt-1 receptors on human intermediate and extravillous trophoblasts (EVT) and thereby causes efficient EVT invasion. The failure of interplay between growth factors from decidual uterine NK cells and trophoblast receptors may be responsible for the decreased EVT invasion in PE [24].
During Norm-Preg, T cells which react with autoantigens are removed by Fas ligand/Fas-mediated apoptosis, a process known as ‘clonal deletion’, leading to tolerance. HLA-G and the Fas ligand expressed on trophoblasts may play a role in clonal deletion [23]. Soluble HLA-G1 is produced by syncytiotrophoblasts and probably EVT, and may exert immunotolerance to fetal or paternal antigens by inducing apoptosis of activated maternal CD8+ T cells via the Fas ligand/Fas system. Lack of or decreased soluble HLA-G1 expression in trophoblasts may cause inadequate tolerance at the feto-maternal interface resulting in PE [23].
There may be a relation between inflammation or infection and PE. Anti-cytomegalovirus (CMV) and anti-Chlamydia pneumonia antibodies are higher in early than late onset PE or Norm-Preg [25]. Also, women sero-negative for antibodies against viral agents such as CMV, herpes-simplex virus type 2, and Epstein-Barr virus are at higher risk of PE than sero-positive women because sero-negative women are more susceptible to primary infection during pregnancy [26].
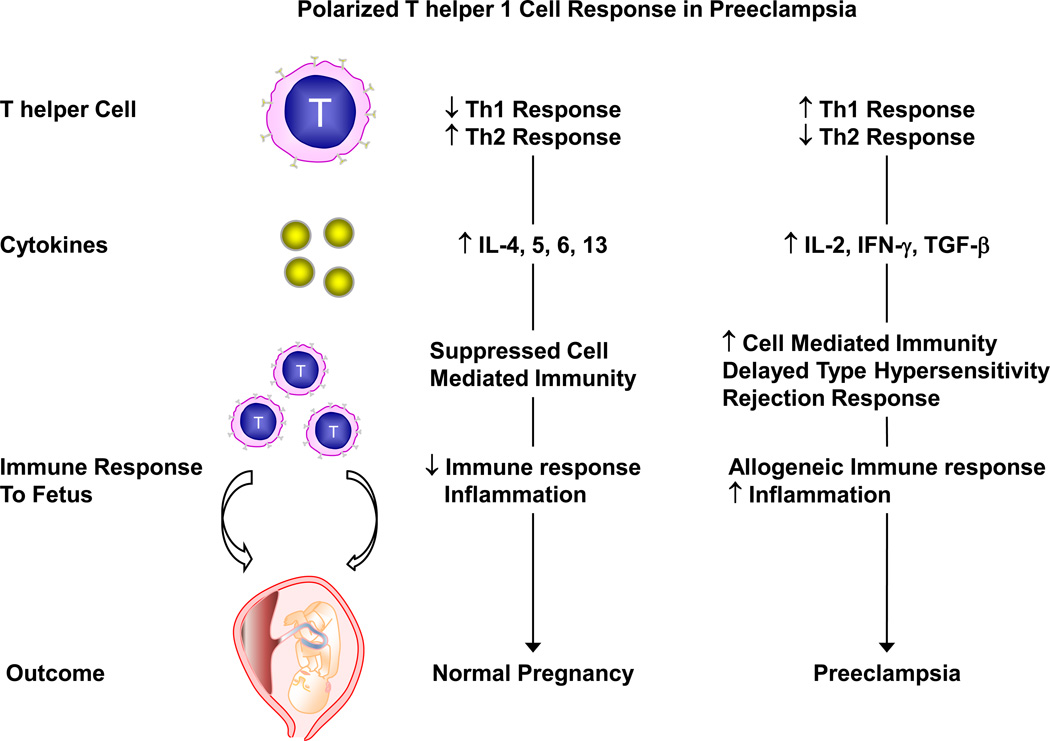
Cytokine-secreting cells could also be involved in the immunologic response associated with PE. The CD4-positive T helper cells are classified into Th1 and Th2 cells. Th1 cells produce IL-2, IFN-γ and TGF-β, which acts on effector cells to enhance cell-mediated immunity, delayed type hypersensitivity and rejection response. Th2 cells secrete IL-4, IL-5, IL-6 and IL-13, and are involved in antibody production and suppression of cell-mediated immunity. In Norm-preg, the predominance of Th2 over Th1 cells causes downregulation of the allogeneic immune responses to the fetus and reduces inflammation. In PE, the balance between Th1 and Th2 cells may shift toward Th1 cells with a polarized Th1 immune response and a decrease in Th2 response (Fig. 3) [23].
Fig 3.
Polarized T helper 1 cell response during PE. During Norm-Preg, T helper cell 2 (Th2) response is predominant over T helper 1 (Th1) cell response. Th2 cell secretes cytokines like IL-4, −5, −6, −13 leading to the suppression of cell-mediated immunity and decreased immune response. In preeclampsia, Th1 cell response may increase and dominate the Th2 cell response. Th1 cells secrete cytokines like IL-2, IFN-γ, TGF-β leading to increased cell-mediated immunity, delayed type hypersensitivity and rejection response. This leads to increased allogeneic immune response and inflammation, and may contribute to the pathogenesis and manifestations of preeclampsia.
3. Abnormal Placentation as an Initiating Event in PE
Although the pathophysiology of PE remains undefined, placental ischemia/hypoxia is considered an important initiating event. The vascular development of the placenta involves first, vasculogenesis and formation of a primitive vascular network from endothelial progenitor cells; second, branching angiogenesis; and third, non-branching angiogenesis [27]. During early pregnancy, cytotrophoblasts invade the uterine spiral arteries, replacing the endothelial layer and destroying the medial elastic, muscular, and neural tissue. Cytotrophoblasts initially express adhesion molecules characteristic of epithelial cells such as integrins α6/β4 and α6/β1 and E-Cadherin. As cytotrophoblasts take the invasive pathway, epithelial cell-like adhesion molecules cease to be expressed, and expression of integrins α1/β1 and αv/β3 is observed; a process referred to as vascular mimicry or pseudovasculogenesis [28] (** = of considerable importance, describes the changes in integrins during placentation). By the end of the second trimester, the uterine spiral arteries are lined exclusively by cytotrophoblasts, and ECs are no longer present in the endometrial or superficial myometrial regions. The remodeling of the spiral arteries results in a low resistance arteriolar system with a dramatic increase in blood supply to the growing fetus. In PE, abnormal expression of epithelial cell-like adhesion molecules and apoptosis of cytotrophoblasts leads to limited invasion of the spiral arteries to only the superficial layers of the decidua, with 30% to 50% of the spiral arteries escaping endovascular trophoblast remodeling [28]. The failure of trophoblast invasion results in RUPP and placental ischemia. Animal models of RUPP in late pregnant sheep, dogs, rabbits and rats exhibit a HTN state that resembles PE (Table 2). Studies in the RUPP model have supported that the ischemic placenta may induce the release of biologically active factors that contributes to the maternal EC dysfunction associated with PE [4, 29].
Table 2.
Representative Animal Models of HTN-Preg
| Model | Technique | Manifestations Experimental Observations |
Norm-Preg | HTN-Preg | Ref |
|---|---|---|---|---|---|
| RUPP Rat |
On gestation day 14, silver clip (0.203 mm ID) on abdominal aorta, another clip (0.1 mm ID) on main uterine branches of ovarian arteries. |
↑ BP (mmHg) Proteinuria (mg/24 h) ↓ Pup weight (g) ↓ Litter size (# of pups) ↑ Cardiac output (ml/min) ↓ Cardiac index (CI, ml/min) ↑ TPR (mmHg/ml/min) ↑ Plasma sFlt-1 (pg/ml) ↑ Plasma sEng (apu) ↑ Plasma TNF-α (pg/ml) ↑ HIF-1α (apu) ↓ Heme oxygenase-1 (HO-1, apu) ↓ Bradykinin vascular relaxation (%) ↓ ACh vascular relaxation (%) |
99±3 15±2 3.2±0.1 12.7±0.5 65±5 348±19 0.98±0.08 82±26 0.05±0.01 14.8±3.3 0.68±0.09 2.5±0.1 62±2 92±1 |
132±4 108±15 1.76±0.08 7.7±1.3 104±7 246±20 2.15±0.02 660±270 0.10±0.02 40±7.6 1.42±0.25 1.4±0.3 40±2 70±3 |
[4, 52, 84– 86] |
| TNF-α or IL-6 infused Rat |
On gestation day 14, pregnant rats are infused iv with TNF- α or IL-6 200ng/kg/day for 5 days. |
↑ BP (mmHg) Little change in pup weight (g) Little change in litter size (pups) ↓ GFR (ml/min) ↓ RPF (ml/min) ↑ RVR (mmHg/ml/min) ↑ Vascular responses to vasoconstrictors ↓ Endothelium-dependent NO- cGMP vascular relaxation |
96±3 4.0±0.1 12±0.8 2.6±0.4 9.2±1.6 7.7±1.0 |
123±3 3.7±.2 11±0.0 1.7±0.4 5.4±1.2 21.6±5.7 |
[37, 87– 89] |
| L-NAME Treated Pregnant Rat |
On gestation day 15, pregnant rats received L-NAME 4 mg/kg/day in drinking water. |
↑ BP (mmHg) Proteinuria (mg/24 h) ↓ Pup weight (g) ↓ Litter size (pups) ↓ Cardiac output (ml/min) ↑ TPR (mm Hg/ml/min) ↑ VSM contraction and [Ca2+]i |
96±2 ~2.5 4.9±0.1 8.7±0.9 113.3±11.1 2.90±0.44 |
137±6 ~10 3.9±0.1 4.8±1.7 87.4±8.4 5.08±0.58 |
[53, 90, 91] |
| 2-ME Deficient Pregnant Mouse |
Compare COMT+/+ COMT+/+ + COMT inhibitor RO41–0960 SQ 25 mg/kg/day) Compare COMT−/− COMT−/− + 2-ME SQ 10 ng/day |
↑ BP (mmHg) ↑ Albumin/Creatinine ↓ Placenta weight # Dead embryos ↑ BP (mmHg) ↑ Albumin/Creatinine ↓ Placenta weight # Dead embryos |
COMT+/+ 105 1.0 0.094 1 +2-ME 108 1.20 0.090 1 |
COMT+/++ inhibitor 122 1.48 0.085 COMT−/− 121 1.50 0.085 2.5 |
[49] |
apu, arbitrary pixel unit; n/a, not available; 2-ME, 2-methoxy estradiol, COMT, catechol-O-methyl transferase; GFR, glomerular filtration rate; RPF, renal plasma flow; TPR, total peripheral resistance; RVR, renal vascular resistance. Numbers are adapted from the respective references and presented as Means±SEM.
4. Biologically Active Factors in PE
Placental ischemia/hypoxia induce the release of various bioactive factors. Increased plasma and vascular tissue levels of these bioactive factors could cause EC and VSM dysfunction, severe vasoconstriction and PE in humans (Table 3) or HTN-Preg in rats (Table 4). The EC dysfunction in PE may result from anti-angiogenic/angiogenic imbalance with high circulating levels of the anti-angiogenic factors sFlt-1 and sEng and low circulating levels of the proangiogenic factors VEGF and PlGF [3]. Other biologically active factors in PE include cytokines, ROS, HIFs, AngII type 1 receptor autoantibodies (AT1-AA), and sex hormone metabolites (Fig. 4).
Table 3.
Plasma Levels and Effects of Bioactive Factors and Vascular Mediators during Normal Pregnancy (Norm-Preg) and Preeclampsia in Humans
| Bioactive Factor | Norm-Preg | Preeclampsia | Site of Action |
Effect | Ref |
|---|---|---|---|---|---|
| VEGF (pg/ml) | 184±18 | 396±35 | Endothelium | Angiogenesis | [92] |
| sFlt-1 (pg/ml) | 339.4 | 7247 | Endothelium | Anti VEGF | [93] |
| sEng (ng/ml) | 9.8 | 46.4 | Endothelium | Anti TGF-β | [29] |
|
TNF-α (pg/ml) IL-6 (pg/ml) |
3.31 4.9±1.1 |
4.68 16.5±2.1 |
Endothelium Endothelium |
EC dysfunction EC dysfunction |
[94, 95] |
|
ROS (Neutrophils O2•− release nmol/106 cells per 5 min) |
3.63±0.91SD | 6.20±0.92SD | Endothelium | ↓ NO bioavailability, ↑ vasoconstrictors |
[41] |
| HIF (apu) | 116483±16374 | 134287±13346 | DNA | Gene Transcription |
[12] |
|
MMP-2 (ng/ml) MMP-9 (ng/ml) TIMP-1 (ng/mL) TIMP-2 (ng/mL) |
669 (560–760) 390 (277–569) 148 (121–188) 228 (207–267) |
834 (656–1002) 290 (280–470) 213 (212–220) 232 (225–245) |
ECM | Vascular Remodeling |
[67] |
| 2-ME (ng/ml) | ~1.5 | ~0.9 | Estrogen receptor |
Vasodilation | [49] |
|
NO (plasma nitrate/nitrite µmol/l) |
13.0±4.3SD | 18.1±6.2SD ↑, --, ↓ |
VSM | Vasorelaxation | [96] |
|
PGI2 (urinary PGF1α pg/mg Creatinine) |
1452 | 1354 | VSM | Vasorelaxation | [97] |
|
EDHF Bradykinin relaxation (%) +L-NAME+Indo MEGJ-mediated |
↑ 60±4 19±3 |
↓ 51±2.0 15±3 |
VSM | Vasorelaxation | [59] |
| AngII (fmol/L) | 53.3±10.1 | 30.15±2.3 | VSM AT1R | Vasoconstriction | [98] |
L-NAME, Nω-nitro-L-arginine methyl ester; Indo, indomethacin; MEGJ, myoendothelial gap junction. Numbers are adapted from the respective references and represent Means±SEM. Standard deviation values are marked with SD.
Table 4.
Plasma and Vascular Tissue Levels, and Effects of Bioactive Factors and Vascular Mediators in Norm-Preg and HTN-Preg in Rats
| Bioactive Factor | Norm-Preg | HTN-Preg | Site of Action | Effect | Ref |
|---|---|---|---|---|---|
| VEGF (pg/ml) | 830±33 | 594±34 | Endothelium | Angiogenesis | [30, 85] |
| sFlt-1 (pg/ml) | 82±26 | 660±270 | Endothelium | Anti VEGF | [85] |
| sEng (apu) | 0.05±0.01 | 0.10±0.02 | Endothelium | Anti TGF-β | [4] |
|
TNF-α (pg/ml) IL-6 (pg/ml) |
14.8±3.3 287±12 |
40±7.6 778±29 |
Endothelium Endothelium |
EC dysfunction EC dysfunction |
[86] [99] |
|
ROS (placental 8- isoprostane ng/g tissue) |
0.8±0.1 | 1.9 ± 0.4 | Endothelium | ↓ NO bioavailability, ↑ vasoconstrictors |
[100] |
| HO-1 (apu) | 2.5±0.1 | 1.4±0.3 | Most Cells | Anti-oxidant | [4] |
| HIF-1α (apu) | 0.68±0.09 | 1.42±0.25 | DNA | Gene Transcription | [4] |
| AT1-AA (U) | 0.6±0.3 | 15.3±1.6 | AT1R | Vasoconstriction | [48] |
|
NO (urinary nitrite/nitrate, µmol/24hr) |
46.4±5.3 | 49.8±6.4 ↑, --, ↓ |
VSM | Vasorelaxation | [52] |
| PGI2 (ng/mL) | 2.78±0.05 | 1.44±0.65 | VSM | Vasorelaxation | [101] |
| ET-1 (pg/ml) | 1.7±0.3 | 2.2±0.3 | VSM | Vasoconstriction | [102] |
|
VSM [Ca2+]i (nM) Basal Phe (10−5 M) AngII (10−7 M) |
63±5 149±8 149±8 |
109±8 234±1 225±9 |
Myofilaments Myofilaments Myofilaments |
Basal Tone Contraction Contraction |
[63, 103] |
apu, arbitrary pixel unit. Numbers are adapted from the respective references and represent Means±SEM.
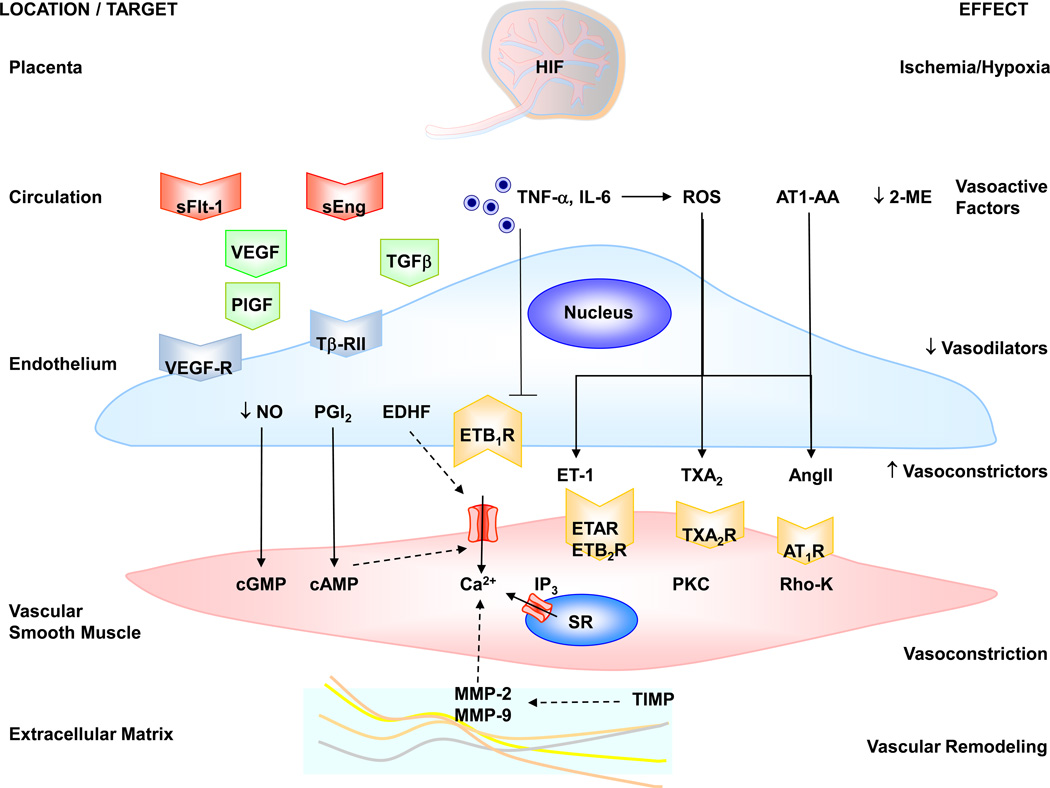
Fig. 4.
Circulating bioactive factors and vascular mediators in preeclampsia. Placental hypoxia/ischemia and the increase in HIF trigger the release of circulating factors such as sFlt-1, sEng, TNF-α, IL-6, ROS and AT1-AA. sFlt-1 binds VEGF and prevents it from activating its natural VEGF-R and as a result prevents the angiogenic effects of VEGF and PlGF. sEng binds TGF-β and prevents it from activating its natural TβRII receptor and producing its angiogenic effects. sFlt-1 and sEng may also cause systemic endotheliosis and decreased release of endothelial vasodilators such as NO, PGI2 and EDHF. Other vasoactive factors such as TNF-α and IL-6 could decrease the endothelial ETB1R vasodilator activity or increase ROS production. ROS decrease the bioactivity of NO, and stimulate the release of vasoconstrictors such as ET-1, TXA2 and AngII, which increase [Ca2+]i, PKC, Rho-K and stimulate VSM contraction. AT1-AA acts on AT1R and further stimulates VSM Ca2+ and PKC activity. Decreased 2-ME could lead to further reduction in vasodilator mechanisms. MMPs are involved in pregnancy-associated vascular remodeling and their activity may be reduced by tissue inhibitors of MMPs (TIMPs). Solid arrows indicate stimulation. Interrupted arrows indicate inhibition.
4.1. Vascular Endothelial Growth Factor (VEGF)
VEGF is a major angiogenic factor and a prime regulator of EC proliferation, vasculogenesis and vascular permeability. VEGF binds to Flt-1 (VEGFR-1) and flk1 (KDR, VEGFR2), both are tyrosine kinase receptors present on EC membrane. VEGF may also have vasodilator effects by stimulating the NO-cGMP vascular relaxation pathway. VEGF increases EC [Ca2+]i, which promotes calmodulin binding to eNOS and thereby stimulates NO production [2].
While the circulating total VEGF levels are elevated in PE, the free unbound VEGF concentration is reduced possibly due to the elevated levels of sFlt-1, which captures free VEGF [30]. This is supported by the observation that patients receiving VEGF antagonists such as bevacizumab for renal cell carcinoma develop HTN, proteinuria and endothelial activation resembling PE [31]. Also, mice lacking one VEGF allele in renal podocytes develop the typical renal pathology found in PE. In non-pregnant mice, infusion of VEGF antibodies to cause a 50% reduction of VEGF leads to glomerular endotheliosis and proteinuria similar to what is seen in PE [31]. Also, in vitro angiogenesis studies have shown that exogenous VEGF or an sFlt-1 antibody can reverse the anti-angiogenic effects of PE plasma [32]. These observations support that the VEGF levels and signaling pathways may be altered during PE.
4.2. Placental Growth Factor (PlGF)
PlGF is a pro-angiogenic factor of the VEGF family that may play a role in EC growth and the control of vasculogenesis, angiogenesis, and placental development [30, 32]. Circulating PlGF levels exceed those of VEGF by >40-fold during Norm-Preg, although its affinity for VEGFR-1 is only 1/10th of the affinity of VEGF. During PE, circulating PlGF levels decrease while the levels of its antagonist protein sFlt-1 increase [30], and the sFlt-1/PlGF ratio is higher in PE than Norm-Preg women. Because the sFlt-1/PlGF ratio could be elevated as early as the second trimester, it can be used as an early biomarker of the onset of PE [29]. However, the use of sFlt-1 and sEng as predictors of PE should be viewed with caution as their levels may not be altered in some women or experimental animals with PE/HTN-Preg.
4.3. Soluble fms-like tyrosine Kinase-1 (sFlt-1)
sFlt-1 is a splice variant of the VEGF receptor Flt-1 that lacks the transmembrane and cytoplasmic domains and acts as a potent VEGF and PlGF antagonist by preventing their interaction with their endogenous receptor (Fig. 4). sFlt-1 is made in large amounts by the PE placenta and released into the maternal circulation [30]. Circulating sFlt-1 levels are increased in women with established PE and may begin to rise before the onset of clinical symptoms [29]. Consistent with the antagonistic effect of sFlt-1, free (or unbound) VEGF and PlGF concentrations are decreased in PE women. Increases in maternal sFlt-1 are observed in several conditions that are considered risk factors for PE [29, 30]. For example, in twin pregnancy, there is a 2- to 3-fold increase in the incidence of PE [33] and the circulating sFlt-1 levels and sFlt-1/PlGF ratios are twice as high as those in singleton pregnancy [34]. Also, in trisomy 13 an extra copy of the Flt-1/sFlt-1 gene (present on 13q12) may lead to excess circulating sFlt-1, reduced free PlGF level, and hence increased sFlt-1/PlGF ratio, and increased risk of PE [34]. Exogenous sFlt-1 given to pregnant rats results in HTN, proteinuria and glomerular endotheliosis, akin to the PE phenotype observed in human [30]. Also, in tissue culture, exposure of EC to conditioned medium containing PE plasma results in reduced angiogenesis, while removal of sFlt-1 by immunoprecipitation restores EC function and angiogenesis to normal levels [32].
4.4 Endoglin
Endoglin (Eng), a co-receptor for TGF-β1 and TGF-β3, is highly expressed on cell membranes of vascular ECs and syncytiotrophoblasts [29]. Placental endoglin is up-regulated in PE, releasing soluble endoglin (sEng) into the maternal circulation [35]. sEng is an anti-angiogenic protein that inhibits TGF-β1 signaling in the vasculature [35] (Fig. 4). Circulating sEng levels increase in the beginning 2 to 3 months before the onset of PE and more steeply in women who develop PE, and peak at the onset of clinical manifestations [29]. sEng may act in concert with sFlt-1 to cause EC dysfunction in severe PE and HELLP syndrome.
sEng disrupts formation of endothelial tubes in human umbilical vein endothelial cells (HUVECs) in culture and induces vascular permeability and HTN in pregnant rat in vivo [35]. Mutations in endoglin result in loss of capillaries, arteriovenous malformations, and hereditary hemorrhagic telangiectasia [33]. Coadministration of sEng and sFlt-1 in pregnant rats is associated with proteinuria, severe HTN, HELLP syndrome, and IUGR, and histological analysis of tissues from these rats reveals severe glomerular endotheliosis, placental infarction, liver necrosis, and fragmented red blood cells (schistocytes) [35]. Also, placental ischemia in the RUPP rat model is associated with increased expression of sEng and HIF-α and shifts the balance of angiogenic factors in the maternal circulation toward an angiostatic state [4].
4.5 Cytokines
During Norm-Preg, women may show an inflammatory response and increased plasma levels of plasminogen activator inhibitor-1 (PAI-1), TNF-α and C-reactive protein. In PE, increased production and release of TNF-α, IL-6, and IL-8 from ischemic placenta may contribute to the maternal EC dysfunction. TNF-α reduces acetylcholine-induced vasodilatation and enhances the release of ET-1 from ECs. TNF-α induces oxidative damage as it destabilizes electron flow in mitochondria, resulting in the release of oxidizing free radicals and formation of lipid peroxides that damage ECs. The plasma level of TNF-α and IL-6 increases 2- to 3-fold in women with PE compared with third-trimester Norm-Preg and women with gestational HTN [36]. In contrast, IL-10, which may be involved in the maintenance of pregnancy by inducing corpus luteum maturation and progesterone production, is decreased in PE [2]. RUPP during pregnancy and the ensuing placental ischemia are thought to increase the release of TNF-α and IL-6 into the maternal circulation, leading to generalized EC dysfunction and HTN. Also, chronic infusion of TNF-α or IL-6 in late pregnant rats causes significant increases in BP, enhanced vascular contraction and reduced endothelium-dependent vascular relaxation possibly due to inhibition of the endothelial NO-cGMP pathway [37] (* = of importance, provides evidence for a role of cytokines in endothelial dysfunction in HTN-Preg).
It has been suggested that PE may represent an excess maternal inflammatory response to pregnancy. PE is characterized by leukocyte activation, which may account for the elevated cytokine levels [38]. In particular, maternal neutrophils are activated during their passage in the decidua of women with PE [38]. Also, conditioned medium of cultured placental villi taken from PE women causes activation of neutrophils [39].
4.6. Reactive Oxygen Species (ROS)
Pregnancy is a state of oxidative stress characterized by placental production of ROS such as superoxide (O2•−), hydrogen peroxide (H2O2), hydroxyl radicals (−OH) and peroxynitrite (ONOO−). In Norm-Preg, ROS are counterbalanced by abundant antioxidant defenses. However, in PE excessive ROS production overpowers antioxidant defenses, leading to increased oxidative stress and ROS activity. Increased levels of lipid peroxides and loss of glutathione peroxidase (GPx) activity have been demonstrated in placentas from PE women. Also, mRNA levels of ROS modulators such as heme oxygenase (HO)-1, (HO)-2, copper/zinc superoxide dismutase (SOD), GPx and catalase are decreased in blood cells of PE women, and the reduction in the amount of HO-1 and HO-2 correlates with the severity of PE [40].
Neutrophils represent an important source of ROS and EC damage in PE. Stimulated neutrophils from women with PE produce more O2•− and cause more EC damage compared to those from Norm-Preg women. Of note, neutrophils also produce NO, which can protect cells from O2•−-induced damage during Norm-Preg. However, in PE, excess O2•− scavenge the NO produced by neutrophils to form peroxynitrite (ONOO–), effectively reducing NO bioavailability and causing EC injury [41].
O2•− and ONOO¯ may promote vascular dysfunction in PE through stimulation of MMP-1, −2, and −9 in VSM. ROS increase VSM [Ca2+]i by stimulating Ca2+ influx, inhibiting Ca2+-ATPase activity, and promoting inositol trisphosphate (IP3)-induced Ca2+ release from the intracellular stores. ROS may also promote vasoconstriction by activating protein kinase C (PKC) and Rho-A/Rho-kinase signaling pathways [2].
4.7. Hypoxia Inducible Factors (HIF)
HIF-1 is a transcriptional factor that plays a role in the physiologic responses to hypoxia and the pathophysiology of ischemic cardiovascular disease, and may be involved in PE. HIF-1 is a heterodimer consisting of an oxygen-regulated HIF1-α and HIF2-α subunits and a constitutively expressed HIF1-β subunit. To date, more than 100 HIF-1 downstream genes with varying functions have been identified including VEGF, leptin, TGF-β3, and NOS. Also, DNA microarrays have shown that more than 2% of all human genes are regulated directly or indirectly by HIF-1 in arterial ECs. HIF-1 activates the expression of these genes by binding to a 50-base pair cis-acting hypoxia response element located in their enhancer and promoter regions. Under normoxic conditions, the α-subunit of HIF-1 is rapidly degraded by ubiquitination and proteosomal degradation. The amount of HIF-1α and HIF-2α, but not HIF-1β, is increased in PE placenta. The increase in HIF-1α protein occurs as a result of both increased formation secondary to ischemia/hypoxia and reduced degradation after reperfusion/oxygenation due to proteosomal dysfunction [12]. The molecular mechanisms underlying the changes in HIF expression and the genes affected in PE are unclear. However, upregulation of HIF-1 has been shown to enhance VEGF expression. Also, a HIF-1α binding site exists in the promoter region of the ET-1 gene and may regulate ET-1 expression and consequently vasoconstriction [2].
4.8. Angiotensin II (AngII), and AT1 Receptor Autoantibodies (AT1-AA)
AngII is an important circulating hormone for BP regulation and electrolyte homeostasis. Also, ~40% of AngII is produced locally in the placenta by chymase, a chymotrypsin-like serine protease produced mainly from villous syncytiotrophoblast [42]. AngII, via AT1R, causes vascular remodeling by promoting vasoconstriction, vascular growth, and inflammation. AngII increases VSM [Ca2+]i and activates Rho/Rho-kinase, a major regulator of VSM contraction, the cytoskeleton and vascular remodeling. Inflammatory cytokines such as TNF-α stimulate AngII production in the female reproductive tract and IL-6 increases AT1R expression of VSM cells [2]. On the other hand, AngII, via endothelial AT2R, stimulates the release of NO through activation of eNOS and the synthesis of PGI2, which oppose AngII-induced vasoconstriction.
Norm-Preg is associated with increased plasma renin and AngII levels, but decreased sensitivity and blunted response to AngII. The reduction in AngII sensitivity during Norm-Preg could be related to the relative expression of AT1R and AT2R, and the absence of adaptive changes in AngII receptor expression could contribute to PE [43]. During Norm-Preg monomeric AT1R are inactivated by ROS leading to lower AngII sensitivity. In PE, AT1R forms a heterodimer with bradykinin B2 receptor and becomes resistant to ROS inactivation and therefore remains active and hyper-responsive to AngII [44]. AngII peptide levels, renin, and angiotensin converting enzyme (ACE) mRNA are also higher in the uterine placental bed of PE compared with Norm-Preg controls [45].
Plasma hemopexin activity increases during Norm-Preg from 10 weeks onward and active hemopexin downregulates AT1R in human monocytes and ECs in vitro, and in aortic rings of Norm-Preg rats. Decreased hemopexin activity during PE may enhance AT1R expression and promote vascoconstriction [46].
AT1R agonistic autoantibodies (AT1-AA) may provide a link between placental ischemia and HTN-Preg (Fig. 4). AT1-AA induces signaling in vascular cells and activates protein-1, calcineurin, and nuclear factor kappa B (NFκB). Also, activation of AT1R by AT1-AA in human trophoblasts may increase PAI-1 production and cause shallow trophoblast invasion [47]. Administration of AT1-AA to pregnant rats increases ET-1 levels in renal cortex and placenta. Also, AT1-AA can additively contribute, alongside AngII and local placental hypoxia, to the excess sFlt-1 secretion in PE [48].
4.9. 2-Methoxy Estradiol (2-ME) Deficiency
2-ME, a natural metabolite of estradiol, is generated by catechol-O-methyltransferase (COMT) in the placenta. Plasma levels of 2-ME are elevated during the third trimester of Norm-Preg, but are markedly lower in women with severe PE. Pregnant mice deficient in COMT demonstrate a PE-like phenotype resulting from the absence of 2-ME. 2-ME ameliorates PE-like features in COMT-deficient pregnant mice and suppresses placental hypoxia, HIF-1α expression and sFlt-1 elevation. Measurement of 2-ME in plasma and urine may be used as a diagnostic marker for PE, and 2-ME may prevent or treat PE [49] (* of importance = describes the changes in estrogen metabolism in PE).
5. Generalized Endotheliosis in PE
One of the major targets of the circulating vasoactive factors in PE is ECs. Women with PE may develop glomerular endotheliosis, swelling of glomerular ECs and display fibrin deposit within EC and mesangial cells that could cause renal injury. Microalbuminuria is an important manifestation of renal endothelial dysfunction, and two third of women with PE may show microalbuminuria 2 to 4 months after delivery [50]. In PE, the glomerular endotheliosis may be caused by decreased VEGF levels. VEGF, produced by podocytes in the glomerulus, is essential for the preservation of general endothelial wellness. VEGF also plays a role in the formation of fenestrae, the small pores in ECs that allow the transfer of molecules from the glomerular capillaries into the urinary space. In women with PE, the excessive production of sFlt-1 inhibits VEGF signaling, and the lack of fenestrae causes reduction in glomerular filtration rate and eventually leads to proteinuria [51]. Endotheliosis could also affect the blood vessels of the brain leading to seizures or visual disturbances, or the hepatic vessels of the liver leading to HELLP syndrome. Also, vascular tone is regulated by both vasodilator and vasoconstrictor mediators, and generalized endotheliosis in PE could cause an imbalance in vascular mediators and lead to increased systemic vascular resistance and HTN.
5.1. Decreased Endothelium-Derived Vasodilators in PE
5.1.1. Nitric Oxide (NO)
NO is a potent vasodilator and relaxant of VSM. NO is produced by endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS). The NOS isoforms share a common effect leading to oxidation of L-arginine to L-citrulline and the release of NO. NOS expression and activity are increased in human uterine artery, and NO production is enhanced during Norm-Preg. Also, plasma concentration and urinary excretion of cGMP, a second messenger of NO and cellular mediator of VSM relaxation, are increased in Norm-Preg. Because NO is an important vasodilator in Norm-Preg, NO deficiency has been suggested to play a role in PE. However, whether NO production decreases during PE is unclear because it is difficult to accurately assess NO in humans. For example, measurement of whole-body NO in clinical settings has shown variable results caused by difficulties in controlling for nitrate intake. Also, whole-body NO may not be an accurate measure of the NO system activity in specific tissues such as the vasculature or the kidneys [52].
Experimental studies have shown that NOS blockade with Nω-nitro-L-arginine methyl ester (L-NAME) during mid- to late gestation in rats produces pathological changes similar to those observed in women with PE including increased BP, renal vasoconstriction, proteinuria, thrombocytopenia and IUGR [53]. Also, chronic RUPP in pregnant rats causes significant increase in BP, proteinuria, decreased GFR and renal plasma flow (RPF), and IUGR. Although the RUPP rat may not show reduction in whole-body NO synthesis as assessed by urinary excretion of nitrate/nitrite, specific reduction in nNOS expression in the kidneys may contribute to the changes in renal hemodynamics and the HTN in this experimental model of PE [52]. Also, acetylcholine induced relaxation and NO production are reduced in RUPP compared with Norm-Preg rats, supporting specific reduction in NO synthesis in the vasculature [54].
5.1.2. Prostacyclin (PGI2)
PGI2 is produced from the metabolism of arachidonic acid by cyclooxygenase 2 (COX)-2 and COX-1, and is a potent vasodilator and inhibitor of platelet aggregation. During Norm-Preg, the synthesis of 6-keto-PGF1α (a stable metabolite of PGI2) is increased in fetoplacental and umbilical tissues and may play role in the regulation of the maternal and fetal circulation [55, 56]. Plasma and urinary concentrations of PGF1α are lower in severe PE than in Norm-Preg, suggesting that the overall PGI2 synthesis is diminished. Endothelial PGI2 production may also decrease in PE [57]. Also, while the release of PGI2 may not be different in apical and basal trophoblasts of PE compared with Norm-Preg women, the release of thromboxane A2 (TXA2), another COX product, from basal trophoblast cells is increased in PE women, and may contribute to increased placental vasoconstriction [58].
5.1.3. Endothelium-Derived Hyperpolarizing Factor (EDHF)
EDHF is an important relaxing factor particularly in the small resistance vessels, and is believed to be involved in the control of local organ blood flow, peripheral vascular resistance and BP, all of which are disturbed in PE. Studies in subcutaneous arteries from Norm-Preg and PE women have shown similar overall relaxation responses to bradykinin, but reduced EDHF-mediated relaxation component in PE. Also, while myoendothelial gap junctions could be the main pathway for EDHF in Norm-Preg, PE vessels show heterogeneous contribution of EDHF, and involved either myoendothelial gap junctions alone or in combination with H2O2 or cytochrome P-450 epoxygenase products of arachidonic acid [59]. Also, studies on the uterine vessels of pregnant rats have suggested that EDHF activates a delayed rectifier type of voltage-sensitive K+ channel. Although NO, PGI2 and EDHF have different vasodilator mechanisms, they may affect each other. For example, PGI2 may upregulate eNOS and NO production in ECs, and PGI2 also activates the inward rectifier K+ channels through the release of EDHF [2].
5.2. Increased Endothelium-Derived Vasoconstrictors in PE
5.2.1. Endothelin-1 (ET-1)
ET-1 is a potent vasoconstrictor synthesized by ECs. Hypoxia induces the synthesis and secretion of ET-1 from ECs. Also, ET-1 secretion is 4- to 8-fold higher in umbilical cord ECs of PE than Norm-Preg women. The elevated plasma levels of ET-1 in PE women return to normal levels within 48 h of delivery, suggesting that ET-1 may contribute to the vasoconstriction and HTN in PE. Typically, plasma levels of ET-1 are highest during the later stage of the disease, suggesting that ET-1 may not be involved in the initiation of PE, but rather in its progression into a malignant phase [2].
Although the circulating levels of ET-1 may not dramatically increase during PE, the role of ET-1 as a paracrine or autocrine agent in the vasculature should be considered. ET-1 interacts with ETAR and ETB2R in VSM leading to vascular contraction. ET-1 stimulates Ca2+ release from the intracellular stores, Ca2+ influx through Ca2+ channels, and PKC-mediated Ca2+-sensitization pathways of VSM contraction [2]. ET-1 also activates ETB1R in ECs and causes the release of vasodilator substances such as NO, PGI2 and EDHF. ET-1, via activation of ETB1R, may mediate the reduced myogenic reactivity and vasodilation of renal arteries as well as hyperfiltration during pregnancy in rats [60]. Downregulation of ETBR may predispose women to PE by impairing trophoblast invasion. This is supported by recent report that the expression and activity of microvascular endothelial ETBR are downregulated in RUPP rat model of HTN-Preg [61] (** = of considerable importance, first evidence of a role of decreased ETBR in HTN-Preg). Also, excessive expression of ET-1, ETAR or ETB2R on VSM of renal arterioles may overwhelm the vasodilation promoted by relaxin [60].
Studies have shown that increasing AT1-AA in pregnant rats to the levels observed in PE women increases BP, and that the HTN is attenuated by oral administration of the AT1R antagonist losartan or an ETAR antagonist, supporting a role of ET-1 in PE [48].
5.2.2. Thromboxane A2 (TXA2)
TXA2 is a potent stimulator of platelet aggregation, vasoconstriction, and VSM cell proliferation and mitogenesis. PE is associated with decreased vascular production of PGI2 and excessive production of TXA2, and this imbalance might explain some of the manifestations of PE such as HTN and platelet aggregation. Measurements of urinary metabolites of TXB2, markers of TXA2 synthesis, and PGI2 have shown an imbalance in their levels that predates the clinical symptoms of PE [57]. These observations have led to the suggestion that antiplatelet agents such as low-dose aspirin and other thromboxane modulators might be effective in preventing PE. However, clinical trials on women at high risk for PE showed no benefit of low dose aspirin when used as a preventive measure [2]. Another study has shown that ozagrel, a thromboxane modulator, reduces the occurrence of PE, HTN-Preg and proteinuria [62], making it important to further examine the role of TXA2 in PE.
6. Increased Vascular Smooth Muscle Mediators in HTN-Preg
6.1. VSM Ca2+
Ca2+ is a major determinant of VSM contraction and growth. Increases in [Ca2+]i due to Ca2+ release from the intracellular stores and Ca2+ entry from the extracellular space trigger VSM contraction. Ca2+ binds calmodulin to form a complex, which activates myosin light chain (MLC) kinase, causes MLC phosphorylation, initiates actin-myosin interaction and produces VSM contraction. Endothelium-derived relaxing factors act on VSM to inhibit phospholipase C, open K+ channels or stimulate Ca2+ extrusion, and thereby decrease [Ca2+]i. EC dysfunction is associated with decreased release of relaxing factors, and decreased VSM Ca2+ extrusion mechanisms. EC dysfunction also causes an increase in vasoconstrictor substances, which stimulate Ca2+ mobilization in VSM. Studies in myometrial and subcutaneous resistance microvessels have shown that the vascular reactivity to high KCl depolarizing solution, phenylephrine (Phe) and AngII is not increased in PE compared with Norm-Preg women, suggesting that this is an unlikely mechanism of the increased vascular resistance in PE [2]. On the other hand, basal and AngII- and KCl-induced maintained [Ca2+]i is greater in renal arterial VSM cells from RUPP than Norm-Preg rats [63], suggesting that the Ca2+ entry mechanisms of VSM contraction are enhanced in animal models of HTN-Preg.
6.2. VSM Protein Kinase C (PKC)
PKC is one of the second messengers involved in VSM contraction. Direct activation of PKC by phorbol esters causes sustained contraction of VSM with no significant change in [Ca2+]I, suggesting a role for PKC in increasing the Ca2+ sensitivity of the contractile proteins. Vascular PKC activity does not change during early and mid-gestation, but is reduced in late pregnant compared with virgin rats, ewes and gilts [64]. The changes in vascular PKC activity during late pregnancy could be related to the increased NO and cGMP production. Vascular PKC activity is greater in L-NAME treated pregnant rats than virgin rats, suggesting that L-NAME not only inhibits NO synthesis, but may also increase the synthesis of vasoactive compounds that increase PKC activity [64]. Increased VSM PKC expression/activity has been shown in HTN. Also, the Ca2+ sensitivity of VSM contractile myofilaments is increased in women with PE, and PKC activation may be involved in the vascular changes observed in PE [2]. PKC may also play a role in the changes in AngII and AT1R-mediated signaling associated with PE. IgG in plasma from PE women enhances AT1R-mediated chronotropic response in cultured neonatal rat cardiomyocytes, and treatment of the cardiomyocytes with the PKC inhibitor calphostin C prevented the stimulatory effect of PE IgG. Also, examination of VSM cells with confocal microscopy have shown colocalization of purified IgG from PE women and AT1-AA, suggesting that PE women may develop stimulatory AT1-AA via a PKC-mediated process [65]. The expression and activity of α- and δ-PKC are also enhanced in the aorta of L-NAME treated pregnant compared with Norm-Preg rats, supporting a role of PKC in the increased vasoconstriction and vascular resistance in HTN-Preg [64].
6.3. VSM Rho/Rho-Kinase
Rho-kinase is activated by interaction with the small GTP-binding protein RhoA, and it participates in a variety of important physiological functions in the vasculature, including VSM contraction, cell proliferation, cell adhesion, migration, and other aspects of the inflammatory response. Rho-kinase may play a role in the increased Ca2+ sensitivity of the contractile proteins observed in subcutaneous resistance arteries from PE compared with Norm-Preg and nonpregnant women. Also, stimulation of AT1R induces upregulation of RhoA/Rho-kinase activity in hypertensive rats, and an increase in AngII activity during PE may activate Rho-kinase and promote vasoconstriction [66]. Other studies have shown that Rho-kinase mRNA expression is downregulated in umbilical arteries of PE women [2], making it important to further investigate the role of Rho-kinase in the vascular changes associated with PE.
7. Extracellular Matrix and Matrix Metalloproteinases in PE
The extracellular matrix (ECM) provides the architectural framework of the arterial wall, and provides a medium that could regulate the behavior of the vascular cells and their ability to migrate, proliferate and survive injury. Matrix metalloproteinases (MMPs) are a family of zinc- and Ca2+-dependent endopeptidases involved in vascular ECM turnover and remodeling. MMPs include collagenases, gelatinases, stromelysins, and other subtypes. MMPs may play a role in the vascular dysfunction in PE. MMP-2 is the main collagenolytic enzyme in umbilical cord artery (UCA) wall. MMP-2 cleaves big ET-1 into ET-11-32, which is a potent vasodilator. MMP-2 and −9 may also decrease vascular [Ca2+]i, through reduction of Ca2+ entry from the extracellular space [2]. MMP activity is modulated by tissue inhibitors of MMPs (TIMPs). A study has shown that both serum MMP-2 and TIMP-1 levels are increased in PE [67], while other studies suggest that decreased levels of MMP-2 in UCA reduce the breakdown of collagen in the arterial wall [2]. Other studies have shown changes in MMP-2 and −9 protein amount and activity in uterine, placental and aortic tissue of RUPP rats [68]. MMPs may have a bi-directional relationship with VEGF, whereby VEGF upregulates MMP expression, while membrane type-1 MMP promotes VEGF expression [2]. Studies have also shown that during pregnancy and chronic relaxin administration in nonpregnant rats for days, increased vascular MMP-2 may mediate renal vasodilation, hyperfiltration, and decreased myogenic activity of renal arterioles. In contrast, vascular MMP-9 may play a role in the vasodilation and decreased myogenic activity of renal arterioles after short-term administration of relaxin for only several hours [69]. Hence, further studies are needed to determine the role of MMPs in Norm-preg and PE.
8. Management and Biomarkers of PE
Currently, delivery of the fetus and placenta is the most effective measure to cure PE. Adequate prenatal care is most important in the management of PE and includes bed rest and anti-HTN drugs such as oral nifedipine or i.v. hydralazine or diazoxide, depending on the severity of HTN. Angiotensin receptor blockers (ARBs) and angiotensin converting enzyme (ACE) inhibitors are contraindicated in PE possibly due to potential teratogenic effects. If PE worsens into eclampsia, maintenance of airway patency and prevention of fluid aspiration should be the first measure, then anticonvulsants should be given, with Mg2+ sulfate infusion being the drug of choice.
Because PE has a relatively long preclinical phase before clinically manifesting in late gestation, the identification of women at risk, early diagnosis using various biomarkers, and prompt management could improve the maternal and perinatal outcome. Measurement of plasma levels of biomarkers such as VEGF, sFlt-1 and sEng might allow the stratification of PE patients in different categories according to the severity of symptoms [70] (Table 5, Table 6). Biomarkers may also allow early disease assessment in asymptomatic pregnant women, in particular among target groups at increased risk based on their clinical history e.g. PE or HTN in a previous pregnancy, or pre-pregnancy state e.g. HTN, obesity, or autoimmune disease.
Table 5.
Biomarkers Levels in Maternal Peripheral Blood for the Prediction (1st, 2nd and 3rd Trimester) and Detection (Manifest Disorder) of Preeclampsia (PE)
| Biomarker | Norm-Preg Trimester | PE Trimester | Manifest PE |
Ref | ||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 1st | 2nd | 3rd | |||
| sFlt-1 (pg/ml) | 1537±812SD | 2788.2±1329.4SD | n/a | 1764±757SD | 4945.1±3329.4SD | n/a | ↑ | [104, 105] |
| sEng (pg/ml) | 5010±1010SD | 4905.9±1332.1SD | 12900 | 5570±1180SD | 11509.2±6108.2SD | 26500 | ↑ | [29, 104, 105] |
| PlGF (pg/ml) | 144±43SD | 175.4±109.6SD | n/a | 153±60SD | 99.6±52.2SD | n/a | ↓ | [104, 105] |
|
TGF-β1 (pg/ml) |
n/a | 4718.6±2371.9SD | n/a | n/a | 2901.1±1308.4SD | n/a | ↓ | [104] |
|
sP-Selectin (ng/ml) |
n/a | 82.5±1.2 | 80.0±1.3 | n/a | 91.2±2.0 | 101±1.3 | ↑ | [106] |
|
sL-Selectin (ng/ml) |
n/a | 1761±31.3 | 1662±34.6 | n/a | 1194±43.2 | 994±32.9 | ↓ | [106] |
|
sE-Selectin (ng/ml) |
n/a | 36.8±1.0 | 54.6±0.9 | n/a | 42.7±0.9 | 65.9±0.8 | ↑ | [106] |
|
sICAM-1 (ng/ml) |
n/a | 165±3.1 | 136±4.4 | n/a | 203±6.1 | 275±7.3 | ↑ | [106] |
| s-Met (ng/ml) | n/a | 259.1±13.3 | 293.7±20.6 | n/a | 182.5±6.8 | 235.1±11.7 | ↓ | [77] |
|
Inhibin-A (µg/ml) |
180±05SD | n/a | n/a | 220±113SD | n/a | n/a | ↑ | [105] |
|
Cell-free mRNA FLT-1 (copies/ml) |
n/a | 1.90±0.32SD | n/a | n/a | 2.39±0.32SD | n/a | ↑ | [71] |
sE-Selectin, soluble E-selectin, sICAM-1, soluble Intercellular Adhesion Molecule-1; sL-Selectin, soluble L-selectin, s-Met, soluble c-Met; sP-Selectin, soluble P-selectin; TGF-β1, transforming growth factor-β1. Numbers are adapted from the respective references and represent Means±SEM. Standard deviation values are marked with SD
Table 6.
Genetic, Immune and Bioactive Factors in Preeclampsia
| Factors | Increased | Decreased |
|---|---|---|
| Genetic | HIF-1α and −2α proteins Leptin, Siglec-6, Pappalysin-2 |
Fas 670 G variant |
| Immune Response | T helper cell 1 response Neutrophil activity |
HLA-G1 Expression |
| Biological Factors | Total VEGF sFlt-1, sEng TNF-α, IL-6, IL-1β C-reactive protein, PAI-1 ROS HIF |
Free VEGF PlGF GPx activity Heme oxygenase 1 & 2 Cu/Zn SOD, Catalase 2-Methoxy Estradiol (2-ME) |
|
Endothelium Derived Factors |
ET-1 TXA2 → TXB2 |
NO PGI2 → PGF1α EDHF |
|
Vascular Smooth Muscle Mediators |
Ca2+ PKC Rho/Rho-kinase |
MMP activity |
| Biomarkers | ||
| mRNA levels | PAI-1, t-PA FLT-1 Endoglin Placenta-specific 1 Selectin P |
|
| Other markers | Mean platelet volume Uric acid Insulin-like factor 3 Inhibin A, Activin-A sFlt1–14 P-selectin |
Platelet count GRP78 (C-terminal/Full length) soluble c-Met TGF-β1 L-selectin PAI-2 |
PAI-1, Plasminogen activator inhibitor-1; t-PA, tissue-type plasminogen activator; FLT-1, fms-like tyrosine Kinase-1; s-Met: soluble c-Met; GPx, glutathione peroxidase; GRP78, glucose-regulated protein 78
Microarray analysis may help to screen the placental transcriptome for upregulated and downregulated genes in PE. In one study, mRNA levels of plasminogen activator inhibitor-1 (PAI-1), tissue-type plasminogen activator (t-PA), VEGF receptor 1 (Flt-1), endoglin, placenta-specific 1 and P-selectin were increased in plasma from pregnant women who later developed PE in comparison with Norm-Preg women. Also, Flt-1 had the highest detection rate while placenta-specific protein 1 (PLAC1) had the lowest detection rate (Table 5). The best multivariable model was obtained by the combination of all markers [71].
Assessment of the immune system could be useful in predicting the risk of PE in early pregnancy. The alternate complement pathway may be upregulated in PE and plasma levels of factor B-derived Bb fragment are higher in PE than Norm-Preg women [2]. An increase in inflammatory factors in PE may represent an abnormal maternal immunological response, with a change in the role of monocytes and natural killer cells, altered release of circulating cytokines, and increased AT1-AA and activation of pro-inflammatory AT1R [19].
The mean platelet volume (MPV) could be an important predictor of PE. Longitudinal studies have shown that women who develop PE have higher MPV 4.6 weeks prior to the appearance of symptoms than Norm-Preg women [2]. Also, thrombocytopenia is a common abnormality in PE, and progresses with the severity of the condition [24].
Uric Acid is a marker of oxidative stress, tissue injury and renal dysfunction, and may help in predicting PE. During Norm-Preg, uric acid levels decrease initially, but then gradually increase over gestational time. Hypoxia and ischemia of the placenta and cytokines such as interferon induce the expression of xanthine oxidase which increases the production of uric acid and ROS. During PE, hyperuricemia may develop as early as 10th week of gestation. Increased circulating uric acid may attenuate normal trophoblast invasion and spiral artery remodeling, stimulate monocytes to produce TNF-α, IL-6 and IL-1β, and contribute to the reduced NO production and endothelial dysfunction [72].
Amniotic fluid derived by amniocentesis in the second trimester may be useful in predicting maternal complications and PE. Insulin-like factor 3 (INSL3) is a member of the insulin/relaxin family of peptide hormones made by the fetal testis and is responsible for the first trans-abdominal phase of testicular descent. In the presence of a male fetus, INSL3 is elevated in amniotic fluid samples of women who subsequently developed PE [2]. The amniotic fluid levels of inhibin A, a glycoprotein produced by syncytiotrophoblast, are higher in pregnant women who subsequently developed severe PE than in Norm-Preg women [73]. Also, the level of sFlt-1 is higher in the amniotic fluid of PE than Norm-Preg women [74].
Uterine artery Doppler screening at 23 weeks of pregnancy may predict PE as an abnormal early diastolic bilateral uterine artery notching in the waveform, although ~50% of women with uterine artery notching do not develop PE. PE women with uterine artery notching have altered levels of fibrinolytic activators and inhibitors such as tissue-type plasminogen activator (t-PA), PAI-1, PAI-2, plasmin-α2-antiplasmin (PAP) and D-dimers. The increase in t-PA levels in women with PE appears to be related to EC activation/dysfunction [75].
Recent studies identified a human-specific splicing variant of VEGF type 1 receptor (designated sFlt1–14) that is qualitatively different from sFlt-1 and functions as a potent VEGF inhibitor. sFlt1–14 is generated primarily in nonendothelial cells such as VSM. The expression of sFlt1–14 is elevated in placenta of women with PE, and is specifically induced in abnormal clusters of degenerative syncytiotrophoblasts known as syncytial knots. Other studies have shown that the ratio of circulating C-terminal glucose-regulated protein 78 (GRP78) over full length GRP78, an endoplasmic reticulum stress protein present on the cell surface of invasive cytotrophoblast, is lower in plasma of women who later develop PE [76]. Also, the circulating levels of soluble c-Met (sMet), a receptor for hepatocyte growth factor, are markedly lower at the early second trimester of severe PE than Norm-Preg women, and may serve as an early predictor of severe PE [77].
Despite the existence of several potential markers for PE, their reliability in predicting PE has not been consistent and their predictive value needs to be further assessed in order to identify the best marker combinations for use in clinical settings.
9. Conclusions
PE is major complication of pregnancy and a complex disorder that may involve maternal, fetal and paternal genes, and the maternal immune response. Genetic predisposition and immune maladaptation affect the normal development of the placenta and lead to placental ischemia/hypoxia and the release of anti-angiogenic factors, cytokines, ROS, HIF-1 and AT1-AA. These bioactive factors cause vascular endotheliosis of the systemic, glomerular, cerebral and hepatic vessels, and decreased vasodilator mediators and increased vasoconstrictors leading to severe vasoconstriction, increased vascular resistance and HTN.
Expert Opinion.
Recent research has provided significant information regarding the causes and mechanisms of HTN-Preg and PE. Clinical and observational studies have suggested that certain genetic predispositions and immune factors could cause inadequate development of the placenta and placental ischemia/hypoxia. Experimental studies in animal models of HTN-Preg such as the RUPP rat model have shown that placental ischemia could trigger the release of several bioactive factors that target blood vessels and lead to endothelial dysfunction, increased vasoconstriction, vascular resistance and HTN-Preg.
Identification of bioactive factors such as sFlt-1, TNF-α, HIF, ROS and the role of endothelial dysfunction and vascular mediators such as ET-1 in the pathogenesis of PE could provide potential biomarkers for early detection of PE. Changes in the levels of these factors could also help to determine the severity of PE and the proper time for initiation of treatment.
An important question is whether the observed changes in bioactive factors and vascular mediators are the cause or the consequence of PE. PE may not be caused by a single factor, and it is likely that concertive effects of more than one factor may be involved. Also, whether these factors act synergistically or affect other pathogenic mechanisms is unclear.
Future studies should further define the specific genes and immunologic factors involved, and how they could cause inadequate development of the placenta. Also, further research is needed to determine how placental ischemia/hypoxia could lead to the release of vasoactive factors and generalized vascular dysfunction.
Using specific and sensitive biomarkers should help in reducing the incidence of PE and early detection of the disorder before it progresses to severe stages.
Current management of PE includes antihypertensive therapy and infusion of Mg2+ sulfate. The identification of new bioactive factors and vascular mediators could provide novel approaches and more specific therapeutic options to target the cause of PE rather than general symptomatic treatment of the manifestations.
Targeting cytokines such as TNF-α using the TNF-α antagonist etanercept, or changing the angiogenic/antiangiogenic balance using PlGF could help in reducing the severity of PE and prolongation of pregnancy until full maturity of the fetus and thereby reduce the potential of IUGR. Additional experimental in vivo interventions and clinical studies should help to introduce these potential targets as new options in the management of PE.
Article highlights box.
Preeclampsia (PE) is a pregnancy-associated disorder with adverse consequences to the health of mother and fertus, but the pathophysiological mechanisms involved are unclear.
Genetic polymorphisms and altered maternal immune response may cause inadequate invasion of cytotrophoblasts into the decidua, impaired remodeling of the uterine and spiral arteries, and reduced uteroplacental perfusion pressure (RUPP).
Placental ischemia causes the release of various anti-angiogenic factors, inflammatory cytokines, reactive oxygen species, hypoxia-inducible factors, and angiotensin II receptor autoantibodies into the maternal circulation.
Circulating vasoactive factors could cause systemic vascular endotheliosis and hypertension; glomerular endotheliosis and proteinuria; cerebrovascular endotheliosis and seizures; hepatic endotheliosis and the HELLP syndrome.
Vascular endotheliosis during PE could be manifested as decreased vasodilators such as nitric oxide, prostacyclin and hyperpolarizing factor, increased vasoconstrictors such as endothelin-1 and thromboxane A2, enhanced mechanisms of vascular smooth muscle contraction such as Ca2+, protein kinase C and Rho-kinase, and changes in matrix metalloproteinases, extracellular matrix and vascular remodeling.
Identification of the genetic, immune and vasoactive factors involved in vascular endotheliosis should provide useful biomarkers for early detection and potential new approaches for prevention and management of PE.
ACKNOWLEDGEMENTS
This work was supported by grants from National Heart, Lung, and Blood Institute (HL-65998, HL-98724, HL-111775) and The Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD-60702). We thank Daniel Lukason for assistance in revising and proof-reading the manuscript.
List of Abbreviations
- AngII
angiotensin II
- ARB
angiotensin receptor blocker
- AT1R
angiotensin type 1 receptor
- AT1-AA
angiotensin AT1 receptor autoantibodies
- BP
blood pressure
- [Ca2+]i
intracellular free Ca2+ concentration
- cGMP
cyclic guanosine monophosphate
- COMT
catechol-O-methyltransferase
- COX
cyclooxygenase
- EC
endothelial cell
- ECM
extracellular matrix
- EDHF
endothelium-derived hyperpolarizing factor
- ET-1
endothelin-1
- EVT
extravillous trophoblasts
- GPx
glutathione peroxidase
- HELLP
hemolysis elevated liver enzymes low platelets
- HIF
hypoxia-inducible factor
- HO
heme oxygenase
- HTN-Preg
hypertension in pregnancy
- HUVECs
human umbilical vein endothelial cells
- IL
interleukin
- IGF
insulin-like growth factor
- IGFBP-5
IGF binding protein-5
- IUGR
intrauterine growth restriction
- L-NAME
Nω-nitro-L-arginine methyl ester
- 2-ME
2-methoxy estradiol
- MHC
major histocompatibility complex
- MMP
matrix metalloproteinase
- NFκB
nuclear factor kappa B
- NO
nitric oxide
- NOS
nitric oxide synthase
- Norm-Preg
normal pregnancy
- O2•−
superoxide anion
- PAI
plasminogen activator inhibitor
- PE
preeclampsia
- PGI2
prostacyclin
- Phe
phenylephrine
- PKC
protein kinase C
- PlGF
placental growth factor
- ROS
reactive oxygen species
- RUPP
reduced uterine perfusion pressure
- sEng
soluble endoglin
- sFlt-1
soluble fms-like tyrosine kinase-1
- SOD
superoxide dismutase
- TGF-β
transforming growth factor-β
- TIMP
tissue inhibitor of MMP
- TNF-α
tumor necrosis factor-α
- TXA2
thromboxane A2
- VEGF
vascular endothelial growth factor
- VSM
vascular smooth muscle
REFERENCES
- 1.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46(6):1243–1249. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 2.Sheppard SJ, Khalil RA. Risk factors and mediators of the vascular dysfunction associated with hypertension in pregnancy. Cardiovasc Hematol Disord Drug Targets. 2010;10(1):33–52. doi: 10.2174/187152910790780096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and angiogenic imbalance. Annu Rev Med. 2008;59:61–78. doi: 10.1146/annurev.med.59.110106.214058. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension. 2009;53(2):399–403. doi: 10.1161/HYPERTENSIONAHA.108.123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dekker G, Sibai B. Primary, secondary, and tertiary prevention of pre-eclampsia. Lancet. 2001;357(9251):209–215. doi: 10.1016/S0140-6736(00)03599-6. [DOI] [PubMed] [Google Scholar]
- 6.Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009;30(1):15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dijk M, Oudejans CB. STOX1: Key player in trophoblast dysfunction underlying early onset preeclampsia with growth retardation. J Pregnancy. 2011;2011:521826. doi: 10.1155/2011/521826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenstad MH, Johnson MP, Loset M, et al. STOX2 but not STOX1 is differentially expressed in decidua from pre-eclamptic women: data from the Second Nord-Trondelag Health Study. Mol Hum Reprod. 2010;16(12):960–968. doi: 10.1093/molehr/gaq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rigourd V, Chauvet C, Chelbi ST, et al. STOX1 overexpression in choriocarcinoma cells mimics transcriptional alterations observed in preeclamptic placentas. PLoS One. 2008;3(12):3905. doi: 10.1371/journal.pone.0003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doridot L, Passet B, Mehats C, et al. Preeclampsia-like symptoms induced in mice by fetoplacental expression of STOX1 are reversed by aspirin treatment. Hypertension. 2013;61(3):662–668. doi: 10.1161/HYPERTENSIONAHA.111.202994. [DOI] [PubMed] [Google Scholar]
- 11.Papazoglou D, Galazios G, Koukourakis MI, Kontomanolis EN, Maltezos E. Association of −634G/C and 936C/T polymorphisms of the vascular endothelial growth factor with spontaneous preterm delivery. Acta Obstet Gynecol Scand. 2004;83(5):461–465. doi: 10.1111/j.0001-6349.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 12.Rajakumar A, Michael HM, Daftary A, Jeyabalan A, Gilmour C, Conrad KP. Proteasomal activity in placentas from women with preeclampsia and intrauterine growth restriction: implications for expression of HIF-alpha proteins. Placenta. 2008;29(3):290–299. doi: 10.1016/j.placenta.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Ciarmela P, Boschi S, Bloise E, et al. Polymorphisms of FAS and FAS ligand genes in preeclamptic women. Eur J Obstet Gynecol Reprod Biol. 2010;148(2):144–146. doi: 10.1016/j.ejogrb.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Winn VD, Gormley M, Paquet AC, et al. Severe preeclampsia-related changes in gene expression at the maternal-fetal interface include sialic acid-binding immunoglobulin-like lectin-6 and pappalysin-2. Endocrinology. 2009;150(1):452–462. doi: 10.1210/en.2008-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernstein IM, Ziegler W, Stirewalt WS, Brumsted J, Ward K. Angiotensinogen genotype and plasma volume in nulligravid women. Obstet Gynecol. 1998;92(2):171–173. doi: 10.1016/s0029-7844(98)00206-3. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein IM, Meyer MC, Osol G, Ward K. Intolerance to volume expansion: a theorized mechanism for the development of preeclampsia. Obstet Gynecol. 1998;92(2):306–308. doi: 10.1016/s0029-7844(98)00207-5. [DOI] [PubMed] [Google Scholar]
- 17.Zubor P, Dokus K, Zigo I, Skerenova M, Pullmann R, Danko J. TNF alpha G308A gene polymorphism has an impact on renal function, microvascular permeability, organ involvement and severity of preeclampsia. Gynecol Obstet Invest. 2014;78(3):150–161. doi: 10.1159/000364865. [DOI] [PubMed] [Google Scholar]
- 18.Sowmya S, Sri Manjari K, Ramaiah A, et al. Interleukin 10 gene promoter polymorphisms in women with early-onset pre-eclampsia. Clin Exp Immunol. 2014;178(2):334–341. doi: 10.1111/cei.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward K. Searching for genetic factors underlying pre-eclampsia: recent progress and persistent challenges. Minerva Ginecol. 2008;60(5):399–419. [PubMed] [Google Scholar]
- 20.Tan CY, Chong YS, Loganath A, et al. Possible gene-gene interaction of KIR2DL4 with its cognate ligand HLA-G in modulating risk for preeclampsia. Reprod Sci. 2009;16(12):1135–1143. doi: 10.1177/1933719109342280. [DOI] [PubMed] [Google Scholar]
- 21.Esplin MS, Fausett MB, Fraser A, et al. Paternal and maternal components of the predisposition to preeclampsia. N Engl J Med. 2001;344(12):867–872. doi: 10.1056/NEJM200103223441201. [DOI] [PubMed] [Google Scholar]
- 22.Caughey AB, Stotland NE, Washington AE, Escobar GJ. Maternal ethnicity, paternal ethnicity, and parental ethnic discordance: predictors of preeclampsia. Obstet Gynecol. 2005;106(1):156–161. doi: 10.1097/01.AOG.0000164478.91731.06. [DOI] [PubMed] [Google Scholar]
- 23.Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y. The role of the immune system in preeclampsia. Mol Aspects Med. 2007;28(2):192–209. doi: 10.1016/j.mam.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 25.von Dadelszen P, Magee LA, Krajden M, et al. Levels of antibodies against cytomegalovirus and Chlamydophila pneumoniae are increased in early onset pre-eclampsia. BJOG. 2003;110(8):725–730. [PubMed] [Google Scholar]
- 26.Trogstad LI, Eskild A, Bruu AL, Jeansson S, Jenum PA. Is preeclampsia an infectious disease? Acta Obstet Gynecol Scand. 2001;80(11):1036–1038. doi: 10.1034/j.1600-0412.2001.801112.x. [DOI] [PubMed] [Google Scholar]
- 27.Geva E, Ginzinger DG, Zaloudek CJ, Moore DH, Byrne A, Jaffe RB. Human placental vascular development: vasculogenic and angiogenic (branching and nonbranching) transformation is regulated by vascular endothelial growth factor-A, angiopoietin-1, and angiopoietin-2. J Clin Endocrinol Metab. 2002;87(9):4213–4224. doi: 10.1210/jc.2002-020195. [DOI] [PubMed] [Google Scholar]
- 28.McMaster MT, Zhou Y, Fisher SJ. Abnormal placentation and the syndrome of preeclampsia. Semin Nephrol. 2004;24(6):540–547. doi: 10.1016/s0270-9295(04)00124-x. [DOI] [PubMed] [Google Scholar]
- 29.Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355(10):992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 30.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358(11):1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95(9):884–891. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 33.Maynard SE, Moore Simas TA, Solitro MJ, et al. Circulating angiogenic factors in singleton vs multiple-gestation pregnancies. Am J Obstet Gynecol. 2008;198(2):200, e1–e7. doi: 10.1016/j.ajog.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 34.Bdolah Y, Lam C, Rajakumar A, et al. Twin pregnancy and the risk of preeclampsia: bigger placenta or relative ischemia? Am J Obstet Gynecol. 2008;198(4):428, e1–e6. doi: 10.1016/j.ajog.2007.10.783. [DOI] [PubMed] [Google Scholar]
- 35.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 36.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40(2):102–111. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 37.Orshal JM, Khalil RA. Reduced endothelial NO-cGMP-mediated vascular relaxation and hypertension in IL-6-infused pregnant rats. Hypertension. 2004;43(2):434–444. doi: 10.1161/01.HYP.0000113044.46326.98. [DOI] [PubMed] [Google Scholar]
- 38.Mellembakken JR, Aukrust P, Olafsen MK, Ueland T, Hestdal K, Videm V. Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension. 2002;39(1):155–160. doi: 10.1161/hy0102.100778. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Gu Y, Philibert L, Lucas MJ. Neutrophil activation induced by placental factors in normal and pre-eclamptic pregnancies in vitro. Placenta. 2001;22(6):560–565. doi: 10.1053/plac.2001.0691. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura M, Sekizawa A, Purwosunu Y, et al. Cellular mRNA expressions of anti-oxidant factors in the blood of preeclamptic women. Prenat Diagn. 2009;29(7):691–696. doi: 10.1002/pd.2278. [DOI] [PubMed] [Google Scholar]
- 41.Tsukimori K, Fukushima K, Tsushima A, Nakano H. Generation of reactive oxygen species by neutrophils and endothelial cell injury in normal and preeclamptic pregnancies. Hypertension. 2005;46(4):696–700. doi: 10.1161/01.HYP.0000184197.11226.71. [DOI] [PubMed] [Google Scholar]
- 42.Yang G, Chang L, Alexander JS, Groome LJ, Yuping W. Chymotrypsin-like protease (chymase) mediates endothelial activation by factors derived from preeclamptic placentas. Reprod Sci. 2009;16(9):905–913. doi: 10.1177/1933719109337333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anguiano-Robledo L, Reyes-Melchor PA, Bobadilla-Lugo RA, Perez-Alvarez VM, Lopez-Sanchez P. Renal angiotensin-II receptors expression changes in a model of preeclampsia. Hypertens Pregnancy. 2007;26(2):151–161. doi: 10.1080/10641950701252827. [DOI] [PubMed] [Google Scholar]
- 44.AbdAlla S, Abdel-Baset A, Lother H, el Massiery A, Quitterer U. Mesangial AT1/B2 receptor heterodimers contribute to angiotensin II hyperresponsiveness in experimental hypertension. J Mol Neurosci. 2005;26(2–3):185–192. doi: 10.1385/JMN:26:2-3:185. [DOI] [PubMed] [Google Scholar]
- 45.Anton L, Merrill DC, Neves LA, et al. The uterine placental bed Renin-Angiotensin system in normal and preeclamptic pregnancy. Endocrinology. 2009;150(9):4316–4325. doi: 10.1210/en.2009-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bakker WW, Henning RH, van Son WJ, et al. Vascular contraction and preeclampsia: downregulation of the Angiotensin receptor 1 by hemopexin in vitro. Hypertension. 2009;53(6):959–964. doi: 10.1161/HYPERTENSIONAHA.108.127951. [DOI] [PubMed] [Google Scholar]
- 47.Bobst SM, Day MC, Gilstrap LC, 3rd, Xia Y, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human mesangial cells and induce interleukin-6 and plasminogen activator inhibitor-1 secretion. Am J Hypertens. 2005;18(3):330–336. doi: 10.1016/j.amjhyper.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 48.LaMarca B, Parrish M, Ray LF, et al. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension. 2009;54(4):905–909. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanasaki K, Palmsten K, Sugimoto H, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453(7198):1117–1121. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- 50.Bar J, Kaplan B, Wittenberg C, et al. Microalbuminuria after pregnancy complicated by pre-eclampsia. Nephrol Dial Transplant. 1999;14(5):1129–1132. doi: 10.1093/ndt/14.5.1129. [DOI] [PubMed] [Google Scholar]
- 51.Stillman IE, Karumanchi SA. The glomerular injury of preeclampsia. J Am Soc Nephrol. 2007;18(8):2281–2284. doi: 10.1681/ASN.2007020255. [DOI] [PubMed] [Google Scholar]
- 52.Alexander BT, Kassab SE, Miller MT, et al. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37(4):1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 53.Khalil RA, Crews JK, Novak J, Kassab S, Granger JP. Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats. Hypertension. 1998;31(5):1065–1069. doi: 10.1161/01.hyp.31.5.1065. [DOI] [PubMed] [Google Scholar]
- 54.Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension. 2000;35(1 Pt 2):367–372. doi: 10.1161/01.hyp.35.1.367. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki Y, Hattori T, Kajikuri J, Yamamoto T, Suzumori K, Itoh T. Reduced function of endothelial prostacyclin in human omental resistance arteries in pre-eclampsia. J Physiol. 2002;545(Pt 1):269–277. doi: 10.1113/jphysiol.2002.022384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Majed BH, Khalil RA. Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn. Pharmacol Rev. 2012;64(3):540–582. doi: 10.1124/pr.111.004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bowen RS, Zhang Y, Gu Y, Lewis DF, Wang Y. Increased phospholipase A2 and thromboxane but not prostacyclin production by placental trophoblast cells from normal and preeclamptic pregnancies cultured under hypoxia condition. Placenta. 2005;26(5):402–409. doi: 10.1016/j.placenta.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Zhao S, Gu Y, Lewis DF, Wang Y. Predominant basal directional release of thromboxane, but not prostacyclin, by placental trophoblasts from normal and preeclamptic pregnancies. Placenta. 2008;29(1):81–88. doi: 10.1016/j.placenta.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luksha L, Nisell H, Luksha N, Kublickas M, Hultenby K, Kublickiene K. Endothelium-derived hyperpolarizing factor in preeclampsia: heterogeneous contribution, mechanisms, and morphological prerequisites. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):510–519. doi: 10.1152/ajpregu.00458.2007. [DOI] [PubMed] [Google Scholar]
- 60.Martin D, Conrad KP. Expression of endothelial nitric oxide synthase by extravillous trophoblast cells in the human placenta. Placenta. 2000;21(1):23–31. doi: 10.1053/plac.1999.0428. [DOI] [PubMed] [Google Scholar]
- 61.Mazzuca MQ, Li W, Reslan OM, Yu P, Mata KM, Khalil RA. Downregulation of microvascular endothelial type B endothelin receptor is a central vascular mechanism in hypertensive pregnancy. Hypertension. 2014;64(3):632–643. doi: 10.1161/HYPERTENSIONAHA.114.03315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seki H, Kuromaki K, Takeda S, Kinoshita K, Satoh K. Trial of prophylactic administration of TXA2 synthetase inhibitor, ozagrel hydrochloride, for preeclampsia. Hypertens Pregnancy. 1999;18(2):157–164. doi: 10.3109/10641959909023075. [DOI] [PubMed] [Google Scholar]
- 63.Murphy JG, Herrington JN, Granger JP, Khalil RA. Enhanced [Ca2+]i in renal arterial smooth muscle cells of pregnant rats with reduced uterine perfusion pressure. Am J Physiol Heart Circ Physiol. 2003;284(1):393–403. doi: 10.1152/ajpheart.00247.2002. [DOI] [PubMed] [Google Scholar]
- 64.Kanashiro CA, Cockrell KL, Alexander BT, Granger JP, Khalil RA. Pregnancy-associated reduction in vascular protein kinase C activity rebounds during inhibition of NO synthesis. Am J Physiol Regul Integr Comp Physiol. 2000;278(2):295–303. doi: 10.1152/ajpregu.2000.278.2.R295. [DOI] [PubMed] [Google Scholar]
- 65.Wallukat G, Homuth V, Fischer T, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103(7):945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kataoka C, Egashira K, Inoue S, et al. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 2002;39(2):245–250. doi: 10.1161/hy0202.103271. [DOI] [PubMed] [Google Scholar]
- 67.Montagnana M, Lippi G, Albiero A, et al. Evaluation of metalloproteinases 2 and 9 and their inhibitors in physiologic and pre-eclamptic pregnancy. J Clin Lab Anal. 2009;23(2):88–92. doi: 10.1002/jcla.20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li W, Mata KM, Mazzuca MQ, Khalil RA. Altered matrix metalloproteinase-2 and −9 expression/activity links placental ischemia and anti-angiogenic sFlt-1 to uteroplacental and vascular remodeling and collagen deposition in hypertensive pregnancy. Biochem Pharmacol. 2014;89(3):370–385. doi: 10.1016/j.bcp.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeyabalan A, Novak J, Doty KD, et al. Vascular matrix metalloproteinase-9 mediates the inhibition of myogenic reactivity in small arteries isolated from rats after short-term administration of relaxin. Endocrinology. 2007;148(1):189–197. doi: 10.1210/en.2006-0989. [DOI] [PubMed] [Google Scholar]
- 70.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22(2):143–148. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 71.Purwosunu Y, Sekizawa A, Okazaki S, et al. Prediction of preeclampsia by analysis of cell-free messenger RNA in maternal plasma. Am J Obstet Gynecol. 2009;200(4):386, e1–e7. doi: 10.1016/j.ajog.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 72.Bainbridge SA, Roberts JM, von Versen-Hoynck F, Koch J, Edmunds L, Hubel CA. Uric acid attenuates trophoblast invasion and integration into endothelial cell monolayers. Am J Physiol Cell Physiol. 2009;297(2):440–450. doi: 10.1152/ajpcell.00593.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim SY, Ryu HM, Yang JH, et al. Maternal serum and amniotic fluid inhibin A levels in women who subsequently develop severe preeclampsia. J Korean Med Sci. 2006;21(3):452–456. doi: 10.3346/jkms.2006.21.3.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2005;122(1):33–39. doi: 10.1016/j.ejogrb.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 75.Hunt BJ, Missfelder-Lobos H, Parra-Cordero M, et al. Pregnancy outcome and fibrinolytic, endothelial and coagulation markers in women undergoing uterine artery Doppler screening at 23 weeks. J Thromb Haemost. 2009;7(6):955–961. doi: 10.1111/j.1538-7836.2009.03344.x. [DOI] [PubMed] [Google Scholar]
- 76.Laverriere A, Landau R, Charvet I, et al. GRP78 as a marker of pre-eclampsia: an exploratory study. Mol Hum Reprod. 2009;15(9):569–574. doi: 10.1093/molehr/gap037. [DOI] [PubMed] [Google Scholar]
- 77.Zeng X, Sun Y, Yang HX, et al. Plasma level of soluble c-Met is tightly associated with the clinical risk of preeclampsia. Am J Obstet Gynecol. 2009;201(6):618, e1–e7. doi: 10.1016/j.ajog.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 78.Zafarmand MH, Franx A, Sabour S, et al. The M235T variant of the angiotensinogen gene is related to development of self-reported hypertension during pregnancy: the Prospect-EPIC cohort study. Hypertens Res. 2008;31(7):1299–1305. doi: 10.1291/hypres.31.1299. [DOI] [PubMed] [Google Scholar]
- 79.Duckers C, Simioni P, Spiezia L, et al. Low plasma levels of tissue factor pathway inhibitor in patients with congenital factor V deficiency. Blood. 2008;112(9):3615–3623. doi: 10.1182/blood-2008-06-162453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buurma AJ, Turner RJ, Driessen JH, et al. Genetic variants in pre-eclampsia: a meta-analysis. Hum Reprod Update. 2013;19(3):289–303. doi: 10.1093/humupd/dms060. [DOI] [PubMed] [Google Scholar]
- 81.Piccoli JC, Gottlieb MG, Castro L, et al. Association between 894G>T endothelial nitric oxide synthase gene polymorphisms and metabolic syndrome. Arq Bras Endocrinol Metabol. 2008;52(8):1367–1373. doi: 10.1590/s0004-27302008000800027. [DOI] [PubMed] [Google Scholar]
- 82.Fitzpatrick E, Goring HH, Liu H, et al. Fine mapping and SNP analysis of positional candidates at the preeclampsia susceptibility locus (PREG1) on chromosome 2. Hum Biol. 2004;76(6):849–862. doi: 10.1353/hub.2005.0017. [DOI] [PubMed] [Google Scholar]
- 83.Brinkman-Van der Linden EC, Hurtado-Ziola N, Hayakawa T, et al. Human-specific expression of Siglec-6 in the placenta. Glycobiology. 2007;17(9):922–931. doi: 10.1093/glycob/cwm065. [DOI] [PubMed] [Google Scholar]
- 84.Chen W, Khalil RA. Differential [Ca2+]i signaling of vasoconstriction in mesenteric microvessels of normal and reduced uterine perfusion pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):1962–1972. doi: 10.1152/ajpregu.90523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50(6):1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 86.LaMarca B, Speed J, Fournier L, et al. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. 2008;52(6):1161–1167. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davis JR, Giardina JB, Green GM, Alexander BT, Granger JP, Khalil RA. Reduced endothelial NO-cGMP vascular relaxation pathway during TNF-alpha-induced hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2002;282(2):390–399. doi: 10.1152/ajpregu.00270.2001. [DOI] [PubMed] [Google Scholar]
- 88.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension. 2005;46(4):1022–1025. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 89.Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens. 2002;15(2 Pt 1):170–175. doi: 10.1016/s0895-7061(01)02255-5. [DOI] [PubMed] [Google Scholar]
- 90.Kassab S, Miller MT, Hester R, Novak J, Granger JP. Systemic hemodynamics and regional blood flow during chronic nitric oxide synthesis inhibition in pregnant rats. Hypertension. 1998;31(1 Pt 2):315–310. doi: 10.1161/01.hyp.31.1.315. [DOI] [PubMed] [Google Scholar]
- 91.Souza CO, Peracoli MT, Weel IC, et al. Hepatoprotective and anti-inflammatory effects of silibinin on experimental preeclampsia induced by L-NAME in rats. Life Sci. 2012;91(5–6):159–165. doi: 10.1016/j.lfs.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 92.Shaarawy M, Al-Sokkary F, Sheba M, Wahba O, Kandil HO, Abdel-Mohsen I. Angiogenin and vascular endothelial growth factor in pregnancies complicated by preeclampsia. Int J Gynaecol Obstet. 2005;88(2):112–117. doi: 10.1016/j.ijgo.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 93.Stepan H, Geide A, Faber R. Soluble fms-like tyrosine kinase 1. N Engl J Med. 2004;351(21):2241–2242. doi: 10.1056/NEJM200411183512123. [DOI] [PubMed] [Google Scholar]
- 94.Hayashi M, Ueda Y, Yamaguchi T, et al. Tumor necrosis factor-alpha in the placenta is not elevated in pre-eclamptic patients despite its elevation in peripheral blood. Am J Reprod Immunol. 2005;53(3):113–119. doi: 10.1111/j.1600-0897.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 95.Casart YC, Tarrazzi K, Camejo MI. Serum levels of interleukin-6, interleukin-1beta and human chorionic gonadotropin in pre-eclamptic and normal pregnancy. Gynecol Endocrinol. 2007;23(5):300–303. doi: 10.1080/09513590701327638. [DOI] [PubMed] [Google Scholar]
- 96.Ranta V, Viinikka L, Halmesmaki E, Ylikorkala O. Nitric oxide production with preeclampsia. Obstet Gynecol. 1999;93(3):442–445. doi: 10.1016/s0029-7844(98)00465-7. [DOI] [PubMed] [Google Scholar]
- 97.Mills JL, DerSimonian R, Raymond E, et al. Prostacyclin and thromboxane changes predating clinical onset of preeclampsia: a multicenter prospective study. JAMA. 1999;282(4):356–362. doi: 10.1001/jama.282.4.356. [DOI] [PubMed] [Google Scholar]
- 98.Brosnihan KB, Neves LA, Anton L, Joyner J, Valdes G, Merrill DC. Enhanced expression of Ang-(1–7) during pregnancy. Braz J Med Biol Res. 2004;37(8):1255–1262. doi: 10.1590/s0100-879x2004000800017. [DOI] [PubMed] [Google Scholar]
- 99.Wallace K, Richards S, Dhillon P, et al. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension. 2011;57(5):949–55. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294(2):541–550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 101.Verlohren S, Niehoff M, Hering L, et al. Uterine vascular function in a transgenic preeclampsia rat model. Hypertension. 2008;51(2):547–553. doi: 10.1161/HYPERTENSIONAHA.107.103176. [DOI] [PubMed] [Google Scholar]
- 102.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46(1):82–86. doi: 10.1161/01.HYP.0000169152.59854.36. [DOI] [PubMed] [Google Scholar]
- 103.Murphy JG, Fleming JB, Cockrell KL, Granger JP, Khalil RA. [Ca(2+)](i) signaling in renal arterial smooth muscle cells of pregnant rat is enhanced during inhibition of NOS. Am J Physiol Regul Integr Comp Physiol. 2001;280(1):87–99. doi: 10.1152/ajpregu.2001.280.1.R87. [DOI] [PubMed] [Google Scholar]
- 104.Lim JH, Kim SY, Park SY, Yang JH, Kim MY, Ryu HM. Effective prediction of preeclampsia by a combined ratio of angiogenesis-related factors. Obstet Gynecol. 2008;111(6):1403–1409. doi: 10.1097/AOG.0b013e3181719b7a. [DOI] [PubMed] [Google Scholar]
- 105.Baumann MU, Bersinger NA, Mohaupt MG, Raio L, Gerber S, Surbek DV. First-trimester serum levels of soluble endoglin and soluble fms-like tyrosine kinase-1 as first-trimester markers for late-onset preeclampsia. Am J Obstet Gynecol. 2008;199(3):266, e1–e6. doi: 10.1016/j.ajog.2008.06.069. [DOI] [PubMed] [Google Scholar]
- 106.Chavarria ME, Lara-Gonzalez L, Garcia-Paleta Y, Vital-Reyes VS, Reyes A. Adhesion molecules changes at 20 gestation weeks in pregnancies complicated by preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2008;137(2):157–164. doi: 10.1016/j.ejogrb.2007.06.014. [DOI] [PubMed] [Google Scholar]