Abstract
Clinical studies demonstrate that scopolamine, a nonselective muscarinic acetycholine receptor (mAchR) antagonist, produces rapid therapeutic effects in depressed patients, and preclinical studies report that the actions of scopolamine require glutamate receptor activation and the mechanistic target of rapamycin complex 1 (mTORC1) in the medial prefrontal cortex (mPFC). The present study extends these findings to determine the role of the mPFC and specific muscarinic acetylcholine receptor (M-AchR) subtypes in the actions of scopolamine. Administration of scopolamine increases the activity marker Fos in the mPFC, including the infralimbic (IL) and prelimbic (PrL) subregions. Microinfusions of scopolamine into either the IL or PrL produced significant antidepressant responses in the forced swim test, and neuronal silencing of IL or PrL blocked the antidepressant effects of systemic scopolamine. The results also demonstrate that systemic administration of a selective M1-AChR antagonist, VU0255035 produced an antidepressant response and stimulated mTORC1 signaling in the PFC, similar to the actions of scopolamine. Finally, we used a chronic unpredictable stress model as a more rigorous test of rapid antidepressant actions, and found that scopolamine or VU0255035 administration blocked the anhedonic response caused by CUS, an effect that requires chronic administration of typical antidepressants. Taken together, these findings indicate that mPFC is a critical mediator of the behavioral actions of scopolamine, and identify the M1-AChR as a therapeutic target for the development of novel and selective rapid-acting antidepressants.
Keywords: glutamate, neuronal silencing, depression, stress, anhedonia
Introduction
Major depressive disorder (MDD) is a recurring, debilitating illness characterized by depressed mood, diminished self-esteem, decreased motivation, and inability to experience pleasure. Moreover, MDD is one of the leading causes of morbidity and mortality worldwide leading to a significant socioeconomic impact on health care systems (Kessler et al, 2003). Given the unmet needs in the treatment of this condition, including tolerability, slow onset of action, and low rates of efficacy (Fournier et al, 2010; Papakostas, 2007), the search for new therapies that can overcome these limitations has been a high priority of drug development programs. Recent clinical findings have provided several novel treatments that address these issues, including the NMDA receptor antagonist ketamine (Berman et al, 2000; Zarate et al, 2006) and the non-selective muscarinic acetylcholine receptor (M-AChRs) antagonist scopolamine (Drevets and Furey, 2010; Furey and Drevets, 2006), both of which produce rapid antidepressant actions in depressed patients, including patients considered treatment resistant (i.e., have failed to respond to two or more typical antidepressant agents).
Recent mechanistic studies in rodent models have demonstrated similarities in the downstream cellular actions underlying the rapid antidepressant effects of ketamine and scopolamine (Duman and Aghajanian, 2012; Li et al, 2010; Li et al, 2011; Voleti et al, 2013). We have reported that a single dose of scopolamine, like ketamine, rapidly increases the mechanistic target of rapamycin complex 1 (mTORC1) signaling in the medial prefrontal cortex (mPFC), and that the antidepressant behavioral actions of scopolamine are blocked by infusion of rapamycin, a selective inhibitor of mTORC1 (Voleti et al, 2013). These studies also show that the behavioral actions of scopolamine, like ketamine, are blocked by pretreatment with a glutamate AMPA receptor antagonist, consistent with evidence that scopolamine and ketamine cause a rapid, transient burst of glutamate in the mPFC (Li et al, 2010; Moghaddam et al, 1997; Voleti et al, 2013). This burst of glutmate is thought to occur via blockade of muscarinic or NMDA receptors by scopolamine or ketamine, respectively, on GABA interneurons, resulting in disinhibition of glutamate transmission (see supplementary Figure 1). Glutamate-AMPA receptor activation leads to stimulation of voltage dependent calcium channels and release of BDNF, which then stimulates mTORC1 signaling and synaptogenesis (Duman and Aghajanian, 2012; Voleti et al., 2013). These studies also demonstrate that a single dose of scopolamine or ketamine rapidly increases the number and function of spine synapses on layer V pyramidal neurons in the mPFC (Li et al, 2010; Voleti et al, 2013). We have also reported that a single dose of ketamine rapidly reverses the anhedonia caused by exposure to chronic unpredictable stress (CUS) (Li et al, 2011). The effect of CUS and the resulting anhedonia, a core symptom of depression, is only blocked by chronic (2 to 3 weeks) administration of a typical antidepressant (Willner, 1997), and therefore provides a rigorous test for putative rapid-acting antidepressants, although the effects of scopolamine have not been tested.
Based on our findings that scopolamine increases mTORC1 and spine synapses in the mPFC, the current studies were undertaken to examine the role of the mPFC in the antidepressant actions of scopolamine and to better understand the M-AChR subtype underlying these actions. We also determine if scopolamine administration can reverse the anhedonia caused by CUS exposure. We use a combination of scopolamine microinfusion, neuronal silencing, and selective M-AChR antagonist approaches to address these questions. Taken together, these findings highlight a role for M1-AChR in the mPFC as an integral mediator of the antidepressant effects following scopolamine.
Material and Methods
Animals
Male Sprague–Dawley rats weighing 175–250 g were pair-housed and maintained in standard conditions with a 12-h light/dark cycle and ad libitum access to food and water. Animal use and procedures were in accordance with the National Institutes of Health guidelines and approved by the Yale University Animal Care and Use Committees.
Drug Administration and Surgical Procedure
Animals received a single acute injection of vehicle, scopolamine (25 µg/kg, intra-peritoneal, i.p.), or the preferential M1-AChR antagonist VU0255035 (N-[3-oxo-3-[4-(4-pyridinyl)-1-piper azinyl]propyl]-2,1,3- benzothiadiazole-4-sulfonamide) (1 or 3 mg/kg, i.p.). In a separate series of studies the influence of 3 doses of scopolamine or VU0255035 on alternate days was also examined. Tissue was collected from separate groups of animals for molecular or immunohistochemical studies, and separate cohorts were also used in behavioral experiments as described below. For experiments involving central administration of scopolamine (30–100 pg, intra-cerebral-ventricular, i.c.v.), muscimol or the selective M3-AChR antagonist 4-Diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP) (100 or 200 pmol, i.c.v.), rats were implanted with either intracortical or intracerebral ventricular (i.c.v.) guide cannulas under anesthesia with pentobarbital (50 mg/kg i.p.) as previously reported (Cota et al, 2006; Li et al, 2010). Coordinates for prelimbic (PrL) and infralimbic (IL) cortex were determined according to a stereotaxic atlas (Paxinos and Watson, 2007) and were +3.2 rostral-caudal (RC), ±1.0 medial-lateral (ML), −2.8 dorsal-ventral (DV) or 2.8 RC, 3.1 ML, 3.8 DV for PL or IL respectively, using bilateral cannulas. For IL, cannulas were implanted with a medial-lateral angulation of 30° to avoid destruction of PrL due to cannula placement. After recovery for at least 7 days, PBS or scopolamine were infused in the PrL or IL cortex at 0.1 µl/min rate. For neuronal silencing prior to systemic scopolamine, muscimol was administered (1.25 µg in 0.2 µl) bilaterally using the same coordinates described for IL and PrL. Coordinates for i.c.v. infusion of 4-DAMP (unilateral) were −0.9 RC, ±1.5 ML, −3.5 DV; vehicle (DMSO) or 4-DAMP were delivered i.c.v. at 0.25 µl/min rate.
Brain Slice Preparation and Immunohistochemistry
Brain slices were prepared as previously described (Peng et al, 1995). Briefly, rats were deeply anesthetized (Chloral hydrate 400 mg/kg IP) and perfused transcardially with phosphate buffered saline (PBS) followed by 4% phosphate-buffered paraformaldehyde (pH 7.4). The brains were then cut into serial 40 µm thick coronal sections that were then incubated for 36–40 h in anti-c-Fos rabbit polyclonal primary antibody (Calbiochem® PC38T) diluted 1:20,000 in blocking solution (5% normal goat serum and 0.2% Triton X-100 in PBS). Secondary anti-rabbit antibody (Vector Laboratories, BA-1000) was applied at 1:1.000 dilution and a standard avidin-biotin peroxidase protocol (ABC kit, Vector), using a heavy metal-intensification of the 3-3' diaminobenzidine (DAB Peroxidase Substrate Kit, Vector) was used to visualize c-Fos immunoreactivity.
Behavioral analysis: Forced Swim Test (FST) and Novelty Suppressed Feeding Test (NSFT)
Behavioral responses in the FST were conducted as previously described (Li et al, 2010). Rats were exposed to a pre-swim 24 h prior to the treatment with scopolamine or selective M-AChR antagonists, for 15 min in a clear cylinder filled with water (24±1C, 45cm depth). Rats were administered scopolamine, 4-DAMP or VU0255035, and 24 h later were tested for immobilization in the FST. In the blocking studies, muscimol was administered (0.2 nmol in 2 µl, i.c.v.) 1 h prior to scopolamine. Video-recorded sessions were scored for total immobility time during minutes 2–6 of the test by a blinded experimenter. Brains were collected, and cannula placement was determined by histology; only rats with correct cannula placement were included for analysis. NSFT was conducted as previously described (Elsayed et al, 2012). Rats were food-deprived for 24 h and placed in an open field (76.5 cm × 76.5 cm × 40 cm, plexiglass) with a small amount of food in the center. The latency to feed (time to approach and take first bite of food) was recorded; home cage food intake was measured right after the test as a control.
Chronic unpredictable stress and sucrose preference test
Chronic unpredictable stress (CUS) is an experimental procedure used to mimic re-occurring uncontrollable stressors that may lead to the pathophysiology of depression. In brief, animals were exposed to a variable sequence of mild and unpredictable stressors, 2 per day for 28 days. The stressors included 45° cage tilt, wet bedding, lights on overnight, lights off for 3 hours, food and water deprivation overnight, isolation overnight, odor, 4°C cold stress, 18°C cold swim stress, stroboscope overnight, crowded housing and cage rotation. Control animals were handled daily but otherwise left undisturbed in their home cage. On day 21 the animals received 4°C cold and then cage rotation, and 4 hours after the last stress rats received either 1 or 3 doses of scopolamine, VU0255035, or saline/vehicle; 24 hr after the first or third dose rats were subjected to the sucrose preference test (SPT). Prior to the SPT rats were habituated to a palatable solution containing sucrose (1%; Sigma, St Louis, MO, USA) and water for 72 h. The day of the test rats were water deprived for 4 h and then the animals were exposed to two identical bottles, one containing the sucrose solution and the other containing water, for 1 h. This procedure has been adapted from previous studies executed in our lab (Li et al, 2011). Sucrose and water consumption were determined by measuring the change in the volume of fluid consumed. Sucrose preference was defined as the ratio of the volume of sucrose versus total volume of sucrose and water consumed during the 1-h test.
Statistical Analysis
Statistical analysis was conducted by Student’s t-test (two groups) or Oneway ANOVA (more than two groups) followed by Newman-Keuls or Fishers multiple comparisons test. Significance was determined at p < 0.05 or less, where specified.
Results
Scopolamine rapidly increases Fos immunoreactivity in the medial PFC
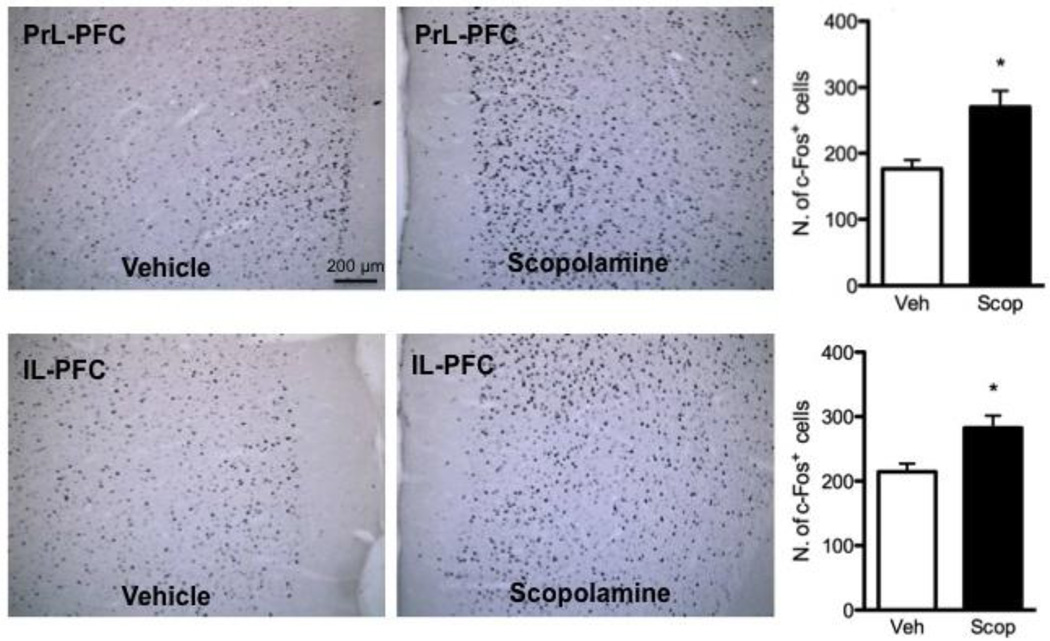
In a previous study we found that scopolamine increases extracellular glutamate, mTORC1 signaling, and spine synapses in the mPFC (Voleti et al, 2013). The burst of glutamate indicates that scopolamine stimulates neuronal activity, and here we examined the influence of scopolamine on levels of Fos immunoreactivity as a marker to map the mPFC subregions that are activated by scopolamine. We used a dose of scopolamine, 25 µg/kg (i.p.) found to be optimal in our previous study (Voleti et al, 2013) and examined Fos+ immunoreactivity one hour after scopolamine administration. Figure 1 shows examples of Fos immunoreactivity in PrL and IL PFC of rats administered saline or scopolamine. Fos+ neurons were counted across three coronal sections from bregma (+2.20, +3.20, +4.20 mm) for PrL and IL and the results are shown on the right panels of Figure 1. Robust induction of Fos+ neurons was observed in both PrL and IL subregions of the mPFC after a single dose of scopolamine (Figure 1a & 1b; p = 0.002 and p = 0.007 respectively, Student’s t-test). Values represent the result of an average of Fos+ cells from both the left and right hemispheres from the three different sections relative to bregma. These findings indicate that systemic scopolamine administration rapidly increases neuronal activity in the PrL and IL subregions of the mPFC. We also found significant scopolamine-induction of Fos+ neurons in other brain regions, including the lateral habenula, nucleus accumbens shell, and medial amygdala, and trends in the nucleus accumbens core and central nucleus of the amygdala (Supplemental Figure 1).
Figure 1. Scopolamine increases Fos+ immunoreactivity in the mPFC.
The influence of scopolamine administration (25 µg/kg, i.p.) on Fos+ immunoreactivity in mPFC of rat was determined 1 hr after drug treatment. Shown in the panels to the left are representative images from vehicle and scopolamine treated animals demonstrating induction of Fos+ neurons in the PrL and IL. The number of Fos+ neurons was analyzed and the results are show in the bar graphs to the right for each region. The results are the mean ± S.E.M. of 6 rats per group. * p < 0.01 compared to vehicle (Student’s t-test).
Direct infusions of scopolamine into mPFC produce antidepressant behavioral responses
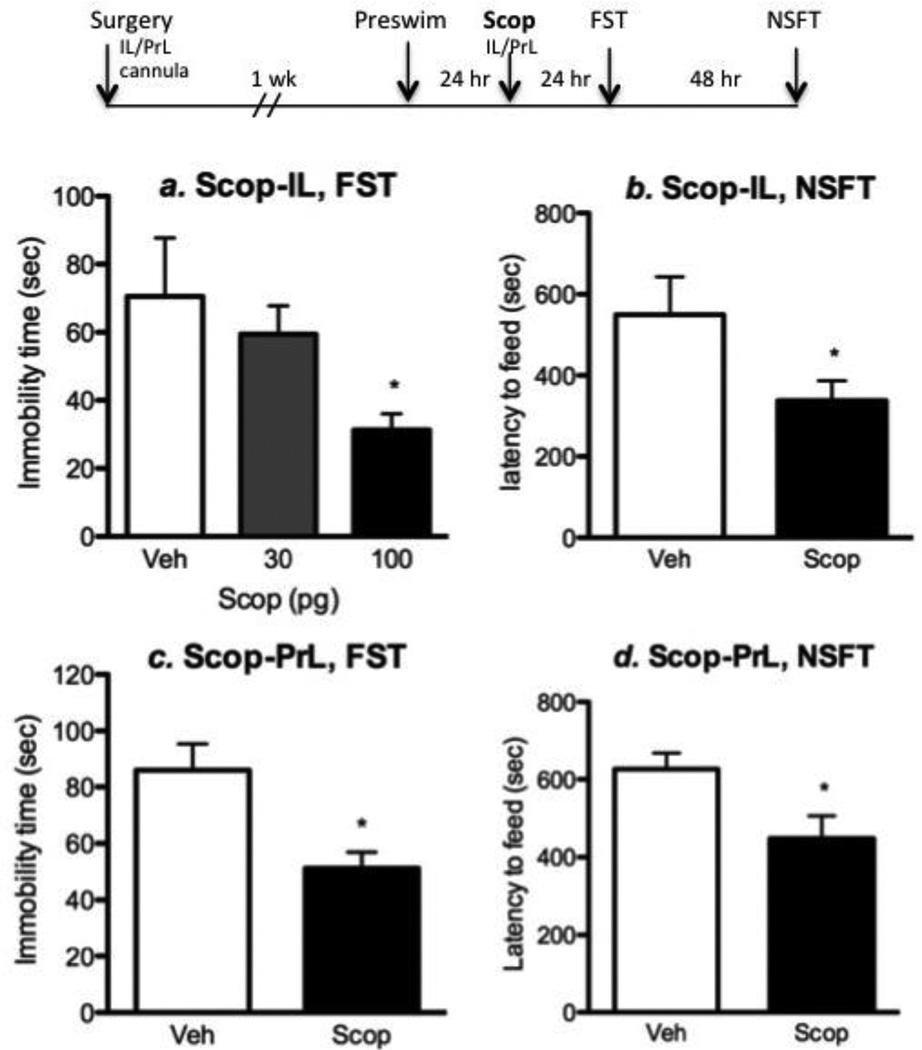
To directly test the role of mPFC in the behavioral actions of scopolamine, animals were infused with scopolamine or vehicle into the IL or PrL subregions and tested in the FST and NSFT. Preliminary dose finding studies were conducted with infusions into the IL. Rats were first exposed to a pre-swim and 24 hr later received infusions (bilateral) of scopolamine at doses of 30 and 100 pg into the IL, and then tested 24 hr later for immobility in the FST. The lower dose had no effect but the 100 pg dose of scopolamine resulted in a significant reduction of immobility time in the FST (Figure 2a) (F2, 17 = 3.605; p = 0.04). IL infusion of scopolamine (100 pg) also produced a trend for an anxiolytic-like effect in the NSFT, as it reduced the latency to feed in an open field (Figure 2b). The higher dose (100 pg) was used for the PrL infusion experiment, and was found to produce a similar reduction of immobility time in the FST (p = 0.02, Student’s t-test) and decreased latency to feed in the NSFT (Figure 2c, d).
Figure 2. Microinfusions of scopolamine into the mPFC produce antidepressant behavioral responses.
The influence of scopolamine microinfusions into mPFC subregions of rat on behavior in the FST and NSFT was determined (see diagram at the top for experimental design). (a) For initial studies we examined microinfusions of two doses of scopolamine, 30 and 100 pg, into the IL and determined immobility in the FST 24 hr later. These studies demonstrate that the 100 pg dose of scopolamine significantly decreases immobility in the FST. (b) Only the 100 pg dose of scopolamine was used for subsequent studies, and infusions into IL and were found to decrease the latency to feed in the NSFT. (c, d) Microinfusions of scopolamine into the PrL also decreased immobility in the FST and latency to feed in the NSFT. Results are the mean ± S.E.M., n = 7–9 per group. *p < 0.05 compared to controls (ANOVA and Newman-Keuls post-hoc test for results in A, and Student’s t-test for B, C, and D).
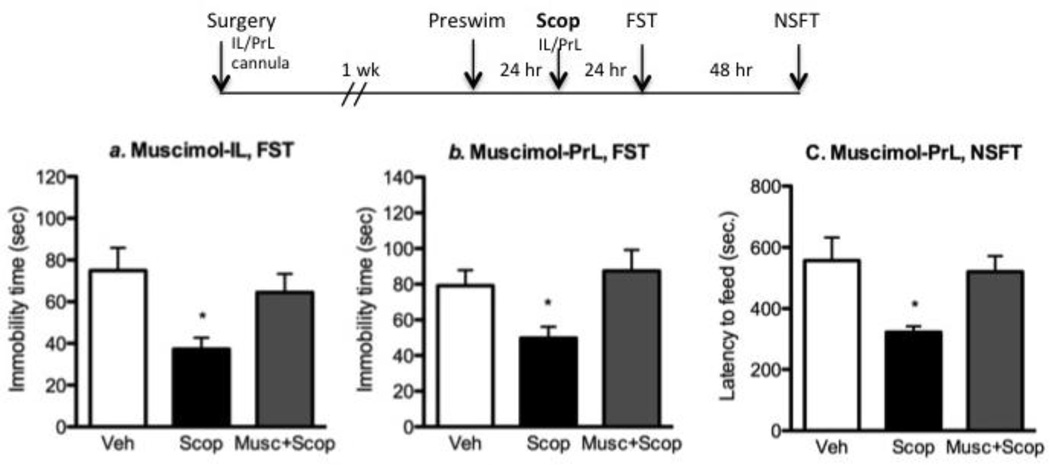
To determine if neuronal activation of mPFC is required for the antidepressant actions of scopolamine, we tested whether the local infusion of muscimol, a gamma-amino-butyric acid type A (GABA-A) receptor agonist that silences neurons, blocks the effects of systemic scopolamine. The infusion sites and doses used for these studies were based on previous reports demonstrating restricted spread and subregion-specific inactivation following muscimol infusion in the IL or PrL (Laurent and Westbrook, 2009; Sierra-Mercado et al, 2011). Muscimol (1.25 µg/side) or vehicle (PBS) was infused into the PrL or IL sub-regions one hr prior to systemic administration of scopolamine (25 µg/kg) injection (i.p.). Behavioral tests were performed 24 h after scopolamine injection, a time when the acute actions of muscimol and scopolamine have subsided but the more long-lasting antidepressant actions are observed (Voleti et al, 2013). Systemic administration of scopolamine produced a significant antidepressant response in the FST, and this effect was completely blocked by infusion of muscimol into either the IL or PrL (Figure 3a & 3b, respectively, F2, 26 = 4.552, p = 0.02; F2, 18 = 5.382, p = 0.01). We have recently demonstrated that infusions of muscimol alone do not influence behavior in the FST at the time point examined, 25 hr after infusions (Fuchikami et al, 2015); this data is included in supplementary Figure 2. Analysis of NFST conducted 72 hr after scopolamine administration showed that muscimol infusion into the PrL prevented the decrease in latency to feed (F2, 18 = 5.529, p = 0.01). These data demonstrate that scopolamine infusions into the IL or PrL are sufficient to promote antidepressant behavioral effects, and that inactivation of these subregions can block the antidepressant effects of systemic scopolamine.
Figure 3. Infusion of muscimol into mPFC blocks the antidepressant actions of systemic scopolamine.
The influence of the neuronal silencing agent, muscimol, infused into mPFC subregions of rat on the antidepressant response to systemic scopolamine (i.p.) was determined. The experimental design is shown at the top. Muscimol was infused 1 hr prior to scopolamine administration and immobility in the FST was determined 24 hr later. (A) Infusion of muscimol into the IL (A) or PrL (B) significantly blocked the decrease in immobility resulting from scopolamine administration. Results are the mean ± S.E.M., n = 10 per group. * p < 0.05 compared to controls (ANOVA and Newman-Keuls post hoc test).
Selective M1-AChR antagonist administration produces antidepressant responses
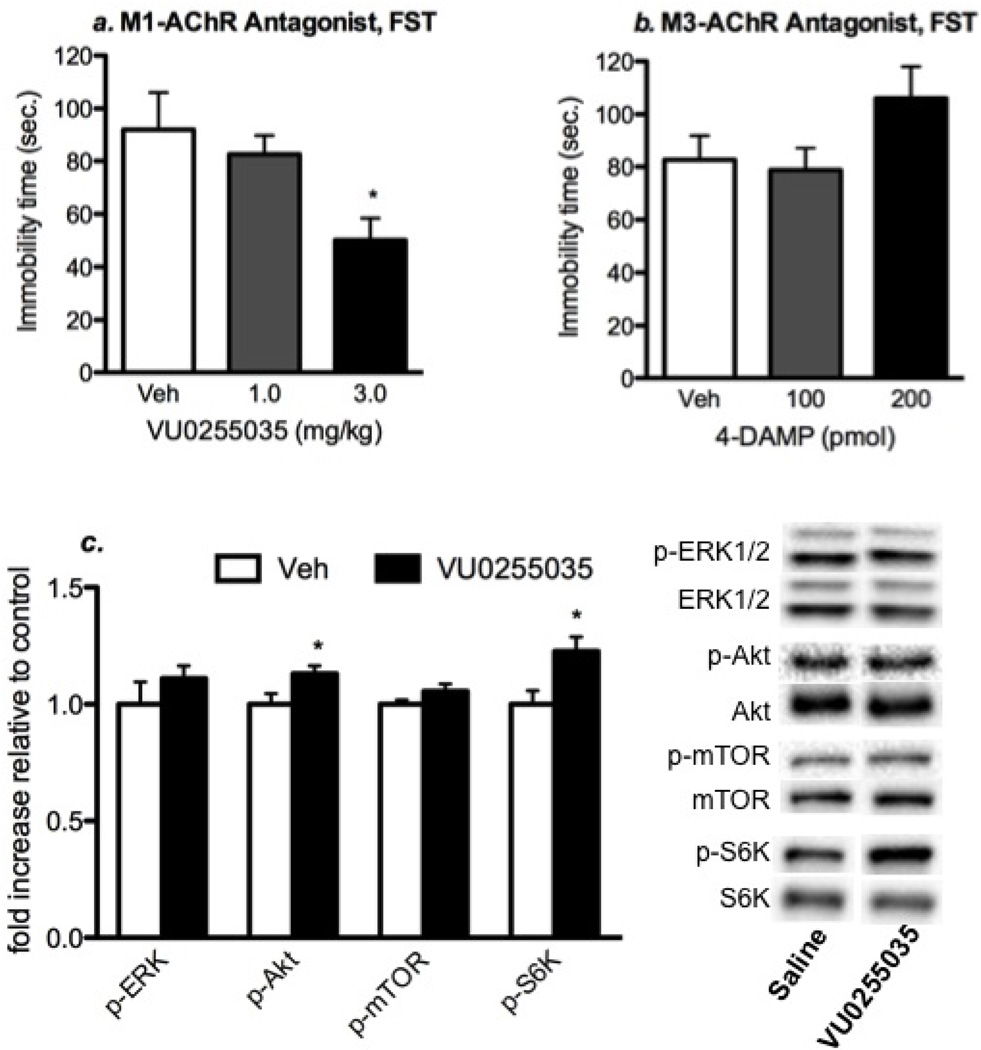
Scopolamine is a non-selective M-AChR antagonist and may produce antidepressant effects through interactions with one of the five different M-AChR subtypes. In our previous study we found that telenzepine, an antagonist with limited selectivity for M1-AChR (7-fold relative to other M-AChR subtypes), produced antidepressant effects in the FST and increased mTORC1 signaling (Voleti et al, 2013). Here we extend these studies by testing VU0255035, another M1-AChR antagonist that has approximately 70-fold selectivity for M1-AChR (Sheffler et al, 2009). We examined two doses, 1 and 3 mg/kg (i.p.), and found that administration of VU0255035 at the higher dose significantly reduced immobility time compared to saline group (Figure 4A) (p < 0.02, one-way ANOVA and Newman-Keuls post-hoc test). For comparison, we also examined an M3-AChR selective antagonist, 4-DAMP (Moriya et al, 1999). Infusion of 4-DAMP (100 or 200 pmol, i.c.v.) had no effect on immobility in the FST (Figure 4b).
Figure 4. Administration of a selective M1-AChR antagonist produces antidepressant-like effects in the FST and increases mTORC1 signaling.
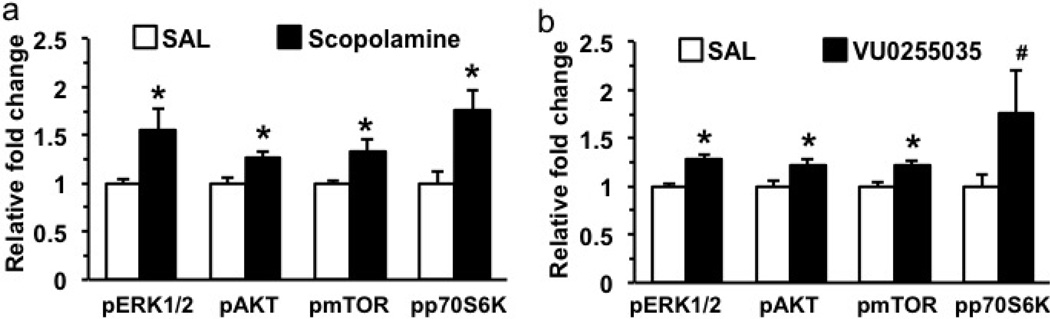
The influence of a selective M1-AChR antagonist, VU0255035 on immobility in the FST and mTORC1 signaling in the PFC was determined. (A) Rats were administered (i.p.) VU0255035 at two different doses, 1 and 3 mg/kg VU0255035 and 24 hr later were tested in the FST. We found that the 3 mg/kg dose of VU0255035 significantly decreased immobility in the FST (ANOVA and Newman-Keuls post hoc test). (B) For comparison, the influence of a M3-AChR antagonist, 4-DAMP, infused (i.c.v.) at two different doses was examined, but no significant effects were observed. (C) The influence of VU0255035 (3 mg/kg, i.p.) on mTORC1 signaling in the PFC was determined by western blot analysis. Shown on the right are representative immunoblots for each phosphorylated kinase, as well as total levels of the corresponding nonphosphorylated protein. Results are relative fold change and are the mean ± S.E.M., n = 7–9 per group. p < 0.05 compared to controls (Student’s t-test).
We also examined the ability of VU0255035 administration (3 mg/kg, i.p.) to activate the mTORC1 signaling pathway 1 hr after drug treatment. Levels of the phosphorylated and activated forms of mTOR and the downstream target p70 S6 kinase (S6K), and two upstream kinases ERK and Akt were analyzed 1 hr after VU0255035 administration. The results demonstrate that VU0255035 administration produces a significant increase in levels of phospho-Akt and phospho-S6K (Figure 4C); there were no significant effects on levels of phospho-mTOR or phospho-ERK. Together these findings demonstrate that M1-AChR antagonism produces antidepressant behavioral and molecular signaling responses similar to scopolamine.
Scopolamine and VU0225035 attenuate CUS-induced deficits in sucrose preference
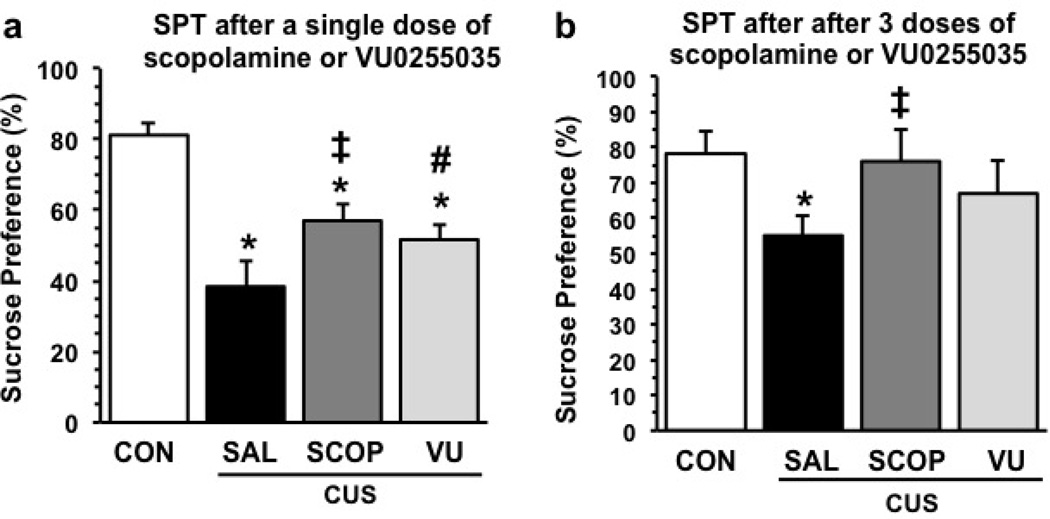
FST has been extensively used as a screen for the identification of putative antidepressant drugs, but has limited validity since this test is responsive to acute or short-term administration of typical antidepressants that take weeks or months to produce a response in depressed patients. To more rigorously test the rapid antidepressant actions of scopolamine and VU0255035 we used a chronic unpredictable stress (CUS) model of depression. Exposure of rats to CUS produces anhedonia, a core symptom of depression that can be measured in a sucrose preference test (SPT) (Willner, 1997). The reduction in the SPT is only reversed after 2 to 3 weeks of treatment with a typical antidepressant (Willner et al, 1987), but we have reported that a single dose of ketamine produces a rapid reversal of anhedonia resulting from CUS exposure (Li et al, 2011). Here we tested the influence of scopolamine or VU0255035 in the CUS/SPT model. Rats exposed to CUS demonstrated a significant decrease in sucrose preference, and administration of a single dose of scopolamine (25 µg/kg) or VU0255035 (3 mg/kg) 24 hr before testing partially blocked this effect (Figure 5a) (F3,50 = 12.0, p < 0.0001; * p < 0.005 compared to control; ‡ p < 0.05 compared to Sal/CUS; # p = 0.08 compared to Sal/CUS). CUS exposure resulted in a small decrease in body weight as expected after 3 weeks of stress exposure; this decrease was not influenced by either scopolamine or VU0255035 administration (Supplemental Figure 3).
Figure 5. Administration of scopolamine or VU0255035 blocks the influence of CUS exposure on sucrose preference.
Rats were exposed to CUS (21 d) and were then received either one or three doses of vehicle, scopolamine (25 µg/kg), or VU0255035 (3 mg/kg) and levels of sucrose preference were determined 24 hr later. Results are the mean ± S.E.M., n = 8 to 16 per group. (a) Results of the SPT after a single dose of scopolamine or VU0255035 (F3,50 = 12.0, p<0.0001). * p < 0.005 compared to control; ‡ p < 0.05 compared to Sal/CUS; # p = 0.08 compared to Sal/CUS (Fisher’s LSD). (b) Results of the SPT after three doses of scopolamine or VU0255035 (F3,20 = 3.81, p < 0.02). * p < 0.005 compared to control; ‡ p < 0.02 compared to Sal/CUS (Fisher’s LSD).
Because a single dose of scopolamine or VU0255035 only partially blocked the effects of CUS, we also tested the influence of a 3-times dosing regimen. The choice of this regimen is based on clinical findings demonstrating a more efficacious antidepressant response to a second and third dose of scopolamine administered over 5 to 7 days (Furey and Drevets, 2006; Drevets and Furey, 2010). Preliminary studies demonstrate that 3 doses of scopolamine or VU0255035 administered every other day for 5 days results in robust induction of mTORC1 signaling in the PFC (Figure 6) that is greater than observed with a single dose of VU0255035 (Figure 4) or scopolamine (Voleti et al., 2013). We also observed modest effects on mTORC1 signaling in the hippocampus (Supplemental Figure 4). This 3-times dosing regimen of scopolamine also produced a significant antidepressant response in the FST and NSFT, but had no effect on locomotor activity (Supplemental Figure 5). Based on these findings we tested the 3-times dosing regimen in the CUS/SPT paradigm. The results demonstrate that 3 doses of scopolamine completely blocked the effects of CUS in the SPT (Figure 5b) (F3,20 = 3.81, p < 0.02; * p < 0.005 compared to control; ‡ p < 0.02 compared to Sal/CUS). The 3-times VU0255035 dosing regimen partially reversed the effects of CUS in the SPT but the CUS+VU0255035 was not significantly different from CUS±Saline. Together, these findings are consistent with the hypothesis that scopolamine and VU0255035 produce rapid antidepressant actions in a rodent model that requires chronic administration of a typical antidepressant.
Figure 6. Three-times dosing of scopolamine or VU0255035 causes robust stimulation of mTORC1 signaling in the PFC.
Scopolamine (25 µg/kg) or VU0255035 (3 mg/kg) were administered in 3 doses over 5 days and PFC levels of the phosphorylated forms of ERK, Akt, mTOR, and p70S6K was determined by western blot. The levels of each phospho-protein were compared to total levels of the unphosphorylated kinase as shown in Figure 4. Results are presented as relative fold change and are the mean ± S.E.M., n = 5–7 per group. * p < 0.05 compared to controls (Student’s t-test).
Discussion
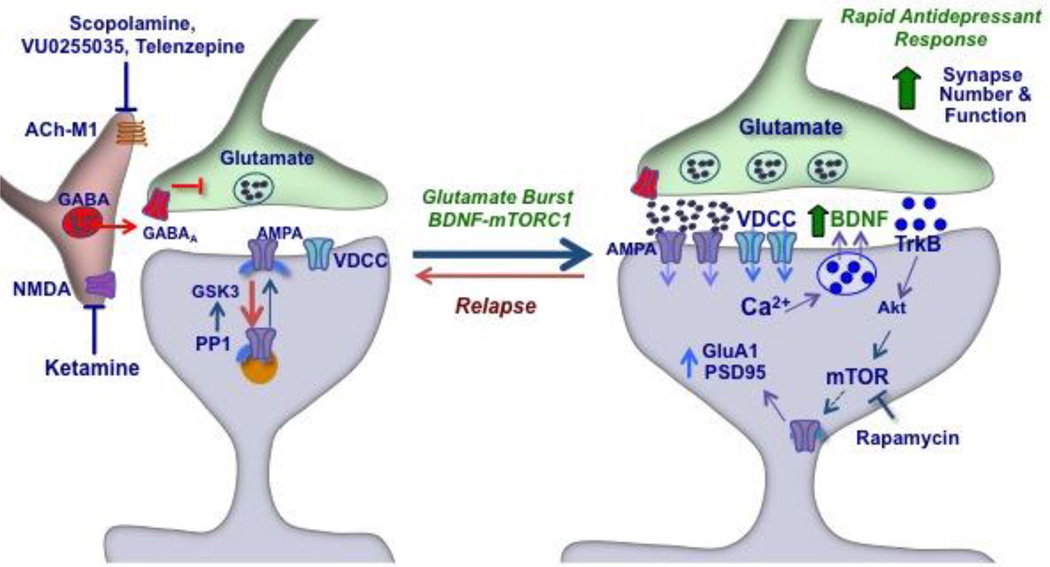
Previous studies demonstrating that scopolamine increases extracellular glutamate, mTORC1 signaling, and spine synapse number and function in the mPFC indicate that scopolamine increases neuronal activity and synaptogenesis (Voleti et al, 2013). This is thought to occur via blockade of muscarinic receptors on GABAergic interneurons, resulting in disinhibition of neuronal pyramidal cell activity and a burst of glutamate transmission (Figure 7). This possibility is supported by the current finding that scopolamine rapidly increases the neuronal activation marker Fos in the mPFC (Ceccatelli et al, 1989; Hoffman and Lyo, 2002; Sagar et al, 1988). A role for neuronal activation is further supported by studies demonstrating that local infusion of a neuronal silencing agent blocks the effects of systemic scopolamine. The rapid, transient burst of glutamate and neuronal activation then lead to more long-term changes in synapse number that may underlie the antidepressant behavioral responses to scopolamine.
Figure 7. Mechanism for scopolamine to cause a burst of glutamate, activation of mTORC1 signaling, and increased synapse formation.
Scopolamine blockade of Ach-M1 receptors located on GABAergic interneurons results in disinhibition and a burst of glutamate transmission, activation of AMPA receptors, and depolarization of postsynaptic pyramidal neurons. This leads to activation of voltage dependent calcium channels (VDCC), which activates release of BDNF and the subsequent stimulation of TrkB receptors and the Akt-mTORC1 signaling pathway. This pathway controls the translation and synthesis of synaptic proteins, such as GluA1 and PSD95, that are required for the formation of new synapses that occur and are associated with the rapid antidepressant actions of scopolamine. Administration of rapamycin, a selective mTORC1 inhibitor, blocks the antidepressant actions scopolamine.
The possibility that mPFC subregions are an important target region is supported by neuroimaging studies showing that depressed patients have reduced gray matter volume and altered blood flow in PFC (Drevets et al, 2008; MacQueen et al, 2008), and rodent chronic stress studies reporting reductions in dendrite complexity and spine synapse number and function in mPFC (Liu and Aghajanian, 2008; Radley et al, 2006; Radley et al, 2004). Conversely, scopolamine and other rapid acting antidepressants (i.e., ketamine) increase spine synapse number and function in the mPFC (Li et al., 2010; Voleti et al., 2013), and ketamine rapidly reverses the synaptic deficits caused by chronic stress exposure (Li et al., 2011). Here we directly test the importance of mPFC subregions, and show that microinfusions of scopolamine produce antidepressant and anxiolytic responses similar to systemic scopolamine. Blockade of the antidepressant actions of systemic scopolamine by local infusions of a neuronal silencing agent provide further support for mPFC. The lack of differential effects of infusions into IL or PrL could be related to the diffusion of scopolamine, but this is less likely to explain the blocking effects of neuronal silenxing as previous studies using the same dose and infusion coordinates for muscimol have reported differential effects of these subregions (Laurent and Westbrook, 2009; Sierra-Mercado et al, 2011; Fuckikami et al., 2015). Further studies will be needed to examine the role of these and other regions, as well as circuits underlying the actions of scopolamine. For example, we also observed increased Fos+ immunoreactivity in several other regions of interest that could be either directly or indirectly activated by scopolamine, and that could contribute to the antidepressant behavioral responses.
Scopolamine is a non-selective antagonist of the five known muscarinic receptor subtypes (Golding and Stott, 1997). We previously demonstrated that telenzepine, an M1-AChR antagonist with limited selectivity produced antidepressant behavioral and mTORC1 signaling effects similar to scopolamine. Here we extend these findings and demonstrate that VU0255035, a muscarinic receptor antagonist with much higher selectivity for M1-AChR (~70 fold over M2) (Sheffler et al, 2009; Xiang et al, 2012), produces antidepressant behavioral responses in the FST. This is consistent with a recent report in mice demonstrating that scopolamine produces an antidepressant response in the FST, and that this effect is blocked in M1-AChR knockout mice (Witkin et al, 2014). In contrast, infusions of a selective M3-AChR antagonist, 4-DAMP did not influence behavior in the FST consistent with the results of Witkin et al. (2014) in which scopolamine caused antidepressant responses in M3-AChR knockout mice. In the present study, we also demonstrate that VU0255035 blockade of M1-AChR rapidly stimulates mTORC1 signaling in the mPFC, which has not been tested in previous studies. These studies support the possibility that a selective M1-AChR antagonist could be effective for the treatment of depression. Although M1-AChR blockade could have side effects, notably memory impairment reported in rats (Roldan et al, 1997), there is evidence that VU0255035 does not cause cognitive deficits (Sheffler et al, 2009). Finally, the study by Witkin and colleagues also reports that the antidepressant response to scopolamine is blocked in M2-AChR knockout mice. Thus, further studies will be required to examine the role of M2-AChR in the behavioral and mTORC1 signaling responses to scopolamine.
Although scopolamine is reported to produce antidepressants actions in rodent models, these studies have been limited to tests that are considered drug-screening paradigms, such as the FST and do not have good validity as models of depression. An additional limitation is that the FST is responsive to acute administration of a typical antidepressant and therefore cannot distinguish drugs that have rapid acting efficacy. To address these issues we used a CUS model that results in anhedonia a core symptom of depression, that is only responsive to chronic administration of a typical antidepressant (Willner, et al, 1987, Willner, 1997). The results demonstrate that a single dose of scopolamine or VU0255035 partially reverse the effects of CUS on sucrose preference. Moreover, using a 3-times dosing paradigm over 5 days (based on the clinical dosing regimen), we found that scopolamine completely reversed the anhedonic effects of CUS. Using the same 3-times dosing regimen, VU0255035 produced a strong trend for a complete reversal, and further studies will be needed to determine if a higher dose of VU0255035 or additional doses produce a complete reversal. Three-times dosing of scopolamine or VU0255035 also produced robust increases in levels of mTORC1 signaling in the PFC, providing further evidence of the efficacy of this dosing regimen.
In conclusion, the results of this study further elucidate the rapid antidepressant properties of scopolamine, demonstrating that mPFC plays a key role in the behavioral responses to scopolamine and that the M1-AChR subtype strongly contributes to the behavioral and mTORC1 signaling actions of scopolamine. The antidepressant actions of scopolamine, and importantly the selective M1-AChR antagonist are further strengthened by the results demonstrating reversal of the anhedonic effects of CUS exposure Nevertheless, the role of other M-AChR subtypes still need to be investigated as some have modulatory activity over the cholinergic system in the brain (Medalla et al, 2012). Moreover, characterization of the circuits connected with mPFC, as well as other primary targets of scopolamine will require further investigation.
Supplementary Material
Highlights.
Medial PFC is a critical site for the antidepressant actions of scopolamine.
Scopolamine blocks the anhedonia caused by exposure to chronic stress.
Selective blockade of muscarinic M1 receptors produces antidepressant actions.
Acknowledgements
Supported by National Institutes of Health Grant MH093897 and the State of Connecticut.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: There are no competing financial interests in relation to the work described.
References
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biological psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Villar MJ, Goldstein M, Hokfelt T. Expression of c-Fos immunoreactivity in transmitter-characterized neurons after stress. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(23):9569–9573. doi: 10.1073/pnas.86.23.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312(5775):927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Furey ML. Replication of scopolamine's antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biological psychiatry. 2010;67(5):432–438. doi: 10.1016/j.biopsych.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain structure & function. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsayed M, Banasr M, Duric V, Fournier NM, Licznerski P, Duman RS. Antidepressant effects of fibroblast growth factor-2 in behavioral and cellular models of depression. Biological psychiatry. 2012;72(4):258–265. doi: 10.1016/j.biopsych.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA : the journal of the American Medical Association. 2010;303(1):47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchikami M, Thomas AM, Liu RJ, Wohleb ES, Land BB, DiLeone RJ, Aghajanian GK, Duman RS. Optogenetic stimulation of infralimbic PFC reproduces ketamine's rapid and sustained actions. PNAS USA. 2015 doi: 10.1073/pnas.1414728112. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Archives of general psychiatry. 2006;63(10):1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding JF, Stott JR. Comparison of the effects of a selective muscarinic receptor antagonist and hyoscine (scopolamine) on motion sickness, skin conductance and heart rate. British journal of clinical pharmacology. 1997;43(6):633–637. doi: 10.1046/j.1365-2125.1997.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Lyo D. Anatomical markers of activity in neuroendocrine systems: are we all 'fos-ed out'? Journal of neuroendocrinology. 2002;14(4):259–268. doi: 10.1046/j.1365-2826.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA : the journal of the American Medical Association. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biological psychiatry. 2013;73(12):1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learning & memory. 2009;16(9):520–529. doi: 10.1101/lm.1474609. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biological psychiatry. 2011;69(8):754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Aghajanian GK. Stress blunts serotonin-and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(1):359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Meunier E, Fossier P, Baux G, Amar M. Cholinergic modulation of the cortical neuronal network. Pflugers Archiv : European journal of physiology. 2003;446(1):17–29. doi: 10.1007/s00424-002-0999-2. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biological psychiatry. 2008;64(10):880–883. doi: 10.1016/j.biopsych.2008.06.027. [DOI] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Muscarinic receptor activity induces an afterdepolarization in a subpopulation of hippocampal CA1 interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(14):5703–5710. doi: 10.1523/JNEUROSCI.19-14-05703.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalla M, Barbas H. The anterior cingulate cortex may enhance inhibition of lateral prefrontal cortex via m2 cholinergic receptors at dual synaptic sites. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(44):15611–15625. doi: 10.1523/JNEUROSCI.2339-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17(8):2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya H, Takagi Y, Nakanishi T, Hayashi M, Tani T, Hirotsu I. Affinity profiles of various muscarinic antagonists for cloned human muscarinic acetylcholine receptor (mAChR) subtypes and mAChRs in rat heart and submandibular gland. Life sciences. 1999;64(25):2351–2358. doi: 10.1016/s0024-3205(99)00188-5. [DOI] [PubMed] [Google Scholar]
- Papakostas GI. Limitations of contemporary antidepressants: tolerability. The Journal of clinical psychiatry. 2007;68(Suppl 10):11–17. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th edn. Amsterdam ; Boston: Academic Press/Elsevier; 2007. [Google Scholar]
- Peng ZC, Chen S, Bentivoglio M. A sensitive double immunostaining protocol for Fos-immunoreactive neurons. Brain research bulletin. 1995;36(1):101–105. doi: 10.1016/0361-9230(94)00125-k. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Cholinergic excitation of GABAergic interneurons in the rat hippocampal slice. The Journal of physiology. 1992;450:127–142. doi: 10.1113/jphysiol.1992.sp019119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebral cortex. 2006;16(3):313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125(1):1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Roldan G, Bolanos-Badillo E, Gonzalez-Sanchez H, Quirarte GL, Prado-Alcala RA. Selective M1 muscarinic receptor antagonists disrupt memory consolidation of inhibitory avoidance in rats. Neuroscience letters. 1997;230(2):93–96. doi: 10.1016/s0304-3940(97)00489-8. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240(4857):1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Sheffler DJ, Williams R, Bridges TM, Xiang Z, Kane AS, Byun NE, et al. A novel selective muscarinic acetylcholine receptor subtype 1 antagonist reduces seizures without impairing hippocampus-dependent learning. Molecular pharmacology. 2009;76(2):356–368. doi: 10.1124/mol.109.056531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(2):529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biological psychiatry. 2013;74(10):742–749. doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(48):12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134(4):319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93(3):358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Overshiner C, Li X, Catlow JT, Wishart GN, Schober DA, et al. M1 and m2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. The Journal of pharmacology and experimental therapeutics. 2014;351(2):448–456. doi: 10.1124/jpet.114.216804. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Thompson AD, Jones CK, Lindsley CW, Conn PJ. Roles of the M1 muscarinic acetylcholine receptor subtype in the regulation of basal ganglia function and implications for the treatment of Parkinson's disease. The Journal of pharmacology and experimental therapeutics. 2012;340(3):595–603. doi: 10.1124/jpet.111.187856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of general psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.