Abstract
Abnormal high frequency oscillations (HFOs) in EEG recordings are thought to be reflections of mechanisms responsible for focal seizure generation in the temporal lobe and neocortex. HFOs have also been recorded in patients and animal models of infantile spasms. If HFOs are important contributors to infantile spasms then anticonvulsant drugs that suppress these seizures should decrease the occurrence of HFOs. In experiments reported here, we used long-term video/EEG recordings with digital sampling rates capable of capturing HFOs. We tested the effectiveness of vigabatrin (VGB) in the TTX animal model of infantile spasms. VGB was found to be quite effective in suppressing spasms. In 3 of 5 animals, spasms ceased after a daily two week treatment. In the other 2 rats, spasm frequency dramatically decreased but gradually increased following treatment cessation. In all animals, hypsarrhythmia was abolished by the last treatment day. As VGB suppressed the frequency of spasms, there was a decrease in the intensity of the behavioral spasms and the duration of the ictal EEG event. Analysis showed that there was a burst of high frequency activity at ictal onset, followed by a later burst of HFOs. VGB was found to selectively suppress the late HFOs of ictal complexes. VGB also suppressed abnormal HFOs recorded during the interictal periods. Thus VGB was found to be effective in suppressing both the generation of spasms and hypsarrhythmia in the TTX model. Vigabatrin also appears to preferentially suppress the generation of abnormal HFOs, thus implicating neocortical HFOs in the infantile spasms disease state.
Keywords: epilepsy, West syndrome, HFOs, hypsarrhythmia, anticonvulsant
INTRODUCTION
Over the past 15 years, there has been a growing appreciation of the importance of the high frequency content of EEGs in epilepsy research (Bragin et al., 2010, Jacobs et al., 2012, Zijlmans et al., 2012). Brief bursts of high frequency oscillations (HFOs) in the range of 200–600 Hz have been recorded during interictal periods in both humans and animals models (Bragin et al., 2002a, Bragin et al., 2002b, Jirsch et al., 2006, Urrestarazu et al., 2007, Worrell et al., 2008, Schevon et al., 2009, Crepon et al., 2010, Jiruska et al., 2010). While HFOs in the frequency range of 80–200 Hz are recorded in normal brains, abnormal HFOs at these and higher frequencies have been shown to be closely associated with suspected sites of seizure generation. They have also been reported to occur at the onset of seizures (Bragin et al., 2005, Jacobs et al., 2008). Thus abnormal HFOs have been suggested to be a biomarker of sites of seizure generation and are currently beginning to be used to guide epilepsy surgery (Zijlmans et al., 2012).
If abnormal HFOs are important contributors to epilepsy, then one would predict that the occurrence of these events should decrease upon treatment with anticonvulsant drugs. To our knowledge such studies have not been performed in humans or animal models. However, one study has reported that HFOs become more frequent in patients after a reduction in antiepileptic drug treatment (Zijlmans et al., 2009).
Neurophysiological studies of epileptic spasms in humans and an animal model have reported the presence of abnormal HFOs in EEG recordings (Panzica et al., 2007, Frost et al., 2011, Nariai et al., 2011a, Nariai et al., 2011b, Frost et al., 2012). Infantile spasms is the most common form of epileptic spasms. The neurobiological basis for these brief seizures is unknown but results from clinical studies have implicated a possible interaction between abnormal neocortical and subcortical circuits in their generation (Chugani et al., 1992, Dulac et al., 2002, Lado and Moshe, 2002, Hrachovy and Frost, 2003). A neocortical contribution of abnormal HFOs to spasm generation is supported by recent recordings in both humans and animals (Frost et al., 2011, Nariai et al., 2011a, Nariai et al., 2011b, Frost et al., 2012).
Children with infantile spasms are often treated with vigabatrin (VGB) (Willmore et al., 2009, Carmant, 2011). VGB is an irreversible, suicide substrate inhibitor of GABA transaminase, the major catabolic enzyme of the inhibitory neurotransmitter, GABA (Jung et al., 1977, Sarhan and Seiler, 1979, Ben-Menachem, 2011). Once bound to the active site of the enzyme, it results in irreversible inhibition and a rapid increase in the levels of GABA in the brain. Only with the synthesis of additional enzyme molecules do GABA levels return to normal.
In studies reported here, we examined the effectiveness of VGB in the TTX model of infantile spasms (Lee et al., 2008). This model shares many of the neurophysiological features of the human condition. Our recent studies have documented neocortical HFOs throughout the ictal complex of spasms (Frost et al., 2011). In addition, high amplitude HFOs are observed interictally (Frost et al., 2012). We show here that VGB is highly effective in suppressing spasms and hypsarrhythmia in the TTX model and markedly suppresses the generation of both ictal and interictal HFOs.
METHODS
TTX infusion
The methods used to chronically infuse Tetrodotoxin (TTX, Alomone Labs) intracortically have been described previously (Galvan et al., 2000, Lee et al., 2008). Briefly, 11 or 12 day-old male rat pups were anesthetized with ketamine/xylazine and an osmotic mini pump (Alzet Model 2004) was implanted under the skin along the animals back. The day before implantation, the minipump was filled with 200 μL of 12 μM TTX, which was dissolved in phosphate buffered saline. Control rats were implanted with osmotic minipumps that contained only the vehicle. Pumps were connected to a 28 gauge stainless steel cannula (Plastics One 3280P-SPC) that was stereotaxically implanted into the right somatosensory cortex (AP: 2.2, ML: 2.0 from bregma and 0.8 mm below the surface of cortex) and anchored with dental acrylic. The pumps constantly infused TTX into the cortex over a 28 day period. All procedures used in this study were approved by the Baylor College of Medicine animal welfare committee and were in keeping with NIH guidelines.
Treatment with Vigabatrin
In the TTX model, behavioral spasms are first observed between postnatal day (P) 16 and 20. Over time, spasm frequency increases and persists for several months. Thus we implanted EEG electrodes after weaning, usually between P32 to P35. On the following day, video/EEG recordings were initiated and following 5 days of baseline recordings VGB treatment was initiated. To test the effectiveness of VGB, we treated rats with 250 mg/kg/day, 325 mg/kg/day or 400 mg/kg/day (i.p.) for 2 weeks.
Long-term video/EEG recordings
Video/EEGs were recorded continuously 24 hours a day, 7 days a week. Animals were housed individually in large, autoclavable Plexiglas recording cages and had free access to food and water throughout this time. The floor of the cages was constructed from stainless steel wire mesh (McMaster Carr) and the ceiling from Plexiglas that was designed to allow free passage of the cable (Plastics One: 363/2–000) from the rat headpost to a Plastics One commutator (SL12C/5B) mounted above each cage. This commutator allowed unhindered movement of animals within the cage. Wires from the commutator were fed to the head board of a Nicolet/Viasys instrument. The behavior of each animal was captured with a separate black and white camera (Supercircuit PC88WR) mounted just outside the recording cage and streamed to a Nicolet/Viasys instrument via a video splitter (Supercircuit QS22).
EEG electrode construction and recording methods were very similar to those reported previously (Frost et al., 2011, Frost et al., 2012). Recording electrodes were constructed from Teflon-coated stainless steel wire (0.005 inches in diameter −127 μm – bare diameter). Teflon was stripped 0.3–0.5 mm from the wire’s tip. Recording electrodes were implanted 0.8 mm below the cortical surface at 6 sites in neocortex (See Figure 4C), most commonly three weeks after pump implantation. Reference electrodes were placed in the cerebellum and electrodes in neck musculature served as isolated system grounds. The recording sites were chosen in relation to the TTX infusion site. Three electrodes were placed ipsilateral to the infusion site 3 mm anterior, posterior and lateral to the cannula implantation site. These electrodes were denoted as right anterior (RA), right posterior (RP) and right lateral (RL). Three additional electrodes were placed homotopically in the contralateral cortex and were referred to as left anterior (LA), left posterior (LP) and left lateral (LL). Recordings made on the Nicolet/Viasys instrument used a digital sampling rate of 2048 Hz. An anti-aliasing filter provided an attenuation of −6 dB at 500 Hz and −16 dB at 900 Hz prior to digitization. While the antialiasing filter removed most high frequency components above 500 Hz, low-pass digital filters used post-sampling (see below) were set to 900 Hz in order to maximize the ability to detect any high frequency components that might be present.
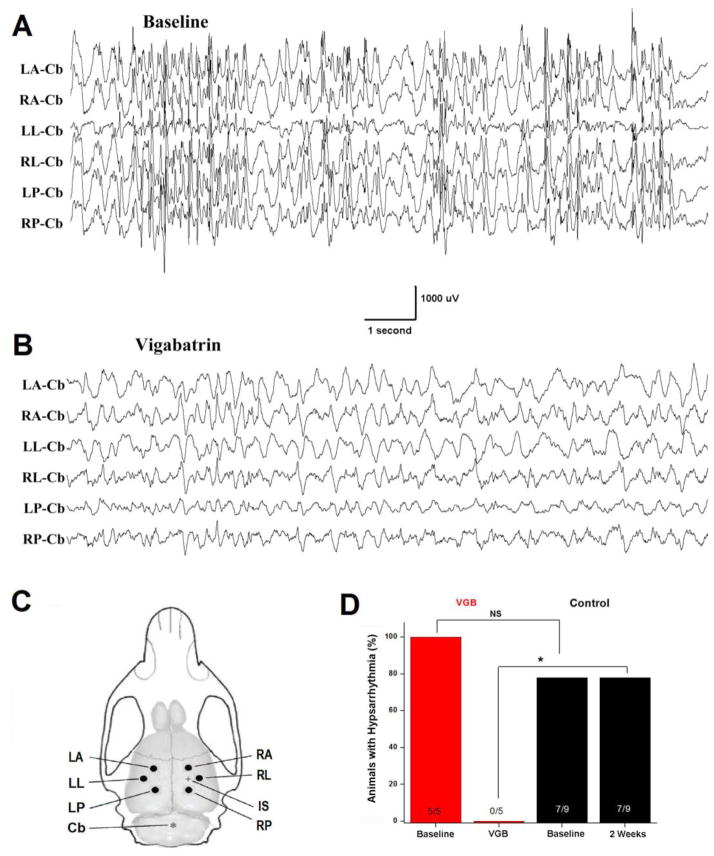
Figure 4.
VGB suppresses hypsarrhythmia. (A) Baseline recordings illustrate a representative example of hypsarrhythmia in an epileptic rat. Recordings were made during non-REM sleep. (B) On the final day of VGB treatment, hypsarrhythmia was not present during non-REM sleep recordings from this same animal. (C) Drawing of rat brain and skull depicting the location of recording electrodes. TTX infusion site is marked with + and reference electrode with *. Recording electrodes were implanted 3.0 mm from the infusion site and homotopically in contralateral cortex. LA = left anterior, RA = right anterior, LL = left lateral, RL = right lateral, LP = left posterior, RP = right posterior, IS = infusion site, CB = cerebellar reference electrode. (D) Plotted are percent of animals displaying hypsarrhythmia. All rats (5 of 5) had hypsarrhythmia prior to VGB and none had it 2 weeks later. In contrast, 7 of 9 TTX-infused control rats (i.e. not treated with VGB) had hypsarrhythmia during baseline recordings and all 7 had hypsarrhythmia 2 weeks later. * P ≤ 0.05, Fisher Exact Test. NS non-significant.
Analysis of EEG Recordings
The results reported here were based on analysis of EEGs recorded from 14 TTX-infused rats. Five were treated with VGB and the remaining 9 served as untreated controls. In addition, 1 saline-infused and 3 non-infused controls were treated with VGB. Recordings typically began between P35 and P40 and in most instances continued for 5–10 weeks. When quantifying alterations in either ictal or interictal HFOs, recordings from the left anterior (LA) or right anterior (RA) recording electrodes in our arrays were analyzed. Each experimental group had both RA and LA recordings included in their analysis. Electrode positions are illustrated in Figure 4C.
When analyzing high frequency activity during interictal EEGs, we restricted our analysis to prolonged periods of non-REM (NREM) sleep. Likewise, hypsarrhythmia was most prominent during this sleep state. However, a comparison to hysarrhythmia during waking was made in Supplemental Figure 3. Behavioral spasms and accompaning ictal events often occurred during transitions from sleep to wake states (Lee et al. 2008). Thus ictal events were analyzed primarily during waking.
High frequency EEG activity was characterized using digital bandpass filtering and compressed spectral arrays (Frost et al., 2011, Frost et al., 2012). The filters were designed to provide an attenuation of 31 db/octave beyond the cut-off point. Compressed spectral arrays (Bickford et al., 1972) were derived from EEG segments of specified durations, using a 100 msec sliding analysis window which was advanced through the digitized sample in increments of 10 data points (equivalent to 5 msec). The 100 msec data samples were multiplied by a cosine-tapered (Tukey) window with a 20% taper-to-constant ratio prior to computation of the fast Fourier transform (FFT). The compressed FFT arrays may be displayed as contour plots with frequency on the vertical axis and time on the horizontal axis, or subjected to further quantification (see below). This procedure permitted a frequency range of 10 to 900 Hz with a frequency resolution of 10 Hz (although components above 500 Hz were markedly attenuated by the antialiasing filter) and a temporal resolution of 100 msec. Analysis software was developed using the Matlab programming language. The spectral displays used here are similar in concept to those used in published human studies (Kobayashi et al., 2004, Panzica et al., 2007).
To provide discrete quantitative measures of the high frequency activity present interictally and in association with ictal events, compressed spectral arrays were processed further by summating the spectral component values between 50 and 900 Hz, in each of the overlapping 100 msec sections of the array. The summated amplitude values (square root of spectral power) were then displayed as a time plot, permitting visualization of temporal alterations in the amount of HFO activity in the specified frequency range (50–900 Hz). Further quantification of the total amount of HFO activity occurring within a specified time segment was achieved by integration of the time plots over the selected time period.
Histology
Cresyl violet (Nissl) staining techniques were used to examine the impact of TTX infusion and VGB treatment on the neocortex. Following long-term video/EEG recordings, rats were initially perfused with PBS, pH 7.4 (38°C) followed by 4% paraformaldehyde that was dissolved in 0.1 M PBS, pH 7.4 (4°C) that contained 4% sucrose. The brains were fixed overnight and cryoprotected with 30% sucrose before sectioning with a cryostat. Fifty micrometer serial sections were cut and stained with cresyl violet acetate. Twenty-four-bit digital bright-field images were captured from a Zeiss upright Imager Discovery.V8 dissection microscope under consistent light conditions, using an Axiocam MRm camera with AxioVision Rel. 4.8 software, and a single Plan Apo S 1.0X objective with a FWD 60 mm range.
Statistics
Data are summarized as mean ± SEM. When data were normally distributed a one-way or two-way ANOVA was performed to determine statistical significance. A post-hoc Holm-Sidak test was used to correct for multiple comparisons. For non-normally distributed data a Kruskal-Wallis ANOVA was employed followed by a Mann-Whitney U non-parametric test. Origin (Version 8 Pro) or Sigma Stat (Version 3.5) was used to perform all statistical tests.
RESULTS
Vigabatrin Suppresses Spasm Generation
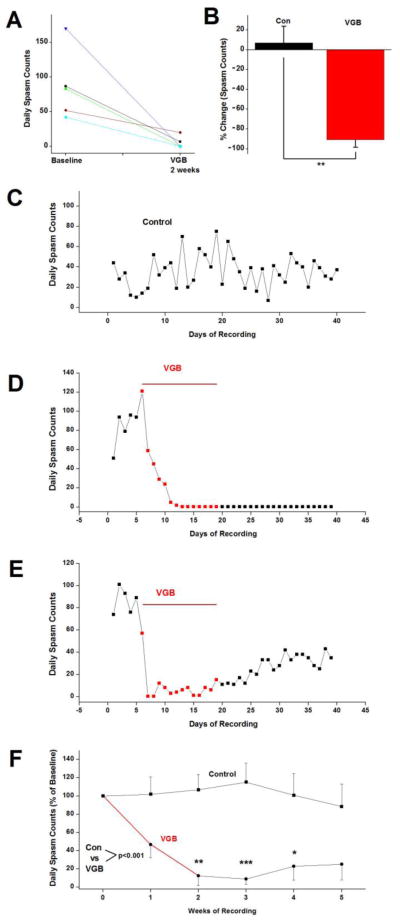
Following 5 days of baseline video/EEG recordings, we treated rats with VGB for 2 weeks with a dosage of 325 mg/kg/day (i.p.), which was divided equally between an early morning and a late afternoon injection. Results in Figures 1A and B summarize the effects of VGB treatment on daily spasm counts in 5 animals at the end of the 2 week treatment. There was substantial variability among animals in spasm frequency during a 5 day baseline recording period. In this instance, there was more than a 4 fold difference in daily spasm counts – on average from 42 to 170 spasms per day. Nonetheless, after two weeks of treatment spasm counts in every rat were decreased. Three rats became seizure free and the other 2 had a 92% and a 61% reduction in spasm frequency. Figure 1B shows that on average there was a 90.8% decrease in daily spasm counts in VGB treated rats, which was statistically different from the 7.1% increase in spasm counts in 9 control rats over a similar 2 week period (1-way ANOVA, p ≤ 0.01).
Figure 1.
VGB suppresses epileptic spasms. (A) The number of daily spasms was reduced in all 5 of the animals treated with VGB (325 mg/kg/day) over a 2 week period. (B) On average, spasm frequency was reduced by 91% in the 5 rats treated with VGB. In contrast, over the same time period spasm frequency in TTX-infused control rats was increased 7.1%. (C) Daily spasm counts in a control rat demonstrate the variations in spasm frequency over 40 days of recordings. (D) Daily spasm counts from a VGB-treated rat where spasms ceased during the 2 week treatment and did not recur after cessation of treatment. Three of the 5 rats treated responded in this way. (E) For the remaining 2 rats, spasm frequency transiently decreased but thereafter increased back towards baseline levels. Plotted are daily spasm counts from one of these rats. (F) Daily spasm counts averaged over weekly intervals in control and VGB-treated rats and normalized to 5 day pretreatment baseline. Overall, compared to control rats, VGB-treatment resulted in a significant reduction in spasm frequency (P ≤ 0.001, 2-way ANOVA). Plotted are mean ± SEM. Comparing VGB- treated animals to controls at the same time point, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001.
Figures 1C–F compare the time course of changes in spasm counts in control and VGB-treated rats. Panels C – E plot the daily spasm counts in 3 rats over 40 days of recordings. Panel C is from a control rat and demonstrates the substantial variability in spasm counts from day-to-day over 40 days. This is due in part to spasm clustering, a recognized feature of this epileptic syndrome (Kellaway et al., 1979, Lee et al., 2008). In neither vehicle treated nor untreated control rats did spasm counts show a consistent trend of increasing or decreasing over this period of time. On the other hand, in the VGB-treated rats shown in Panels D and E, effects were rapid and within 2–3 days spasm frequency was below baseline levels. In panel D, spasms ceased after 7 days of treatment and did not recur even 3 weeks after cessation of treatment. Three of the 5 VGB treated rats shared a similar response to VGB in that spasms were completely eliminated and once stopped did not recur. In panel E, the rat responded very rapidly to VGB and spasms ceased on the second and third day of treatment. However despite continued treatment with the drug, spasm counts gradually increased and increased further once treatment ended but did not return to baseline levels. Another animal had a similar time course of response to VGB but after 2 weeks spasm counts were decreased only 61%. Once treatment stopped, spasm counts gradually increased and returned to pretreatment baseline level within 3 weeks.
Figure 1F compares the time course of alterations in spasm counts for the 5 VGB-treated rats to changes in spasm counts for the 9 control rats over a 5 week period. Due to the substantial animal to animal variability in basal spasm counts illustrated earlier (Figure 1A and see below); we normalized daily spasm counts in every animal to the average counts recorded during the 5 day baseline. This graph clearly demonstrates that on average rats treated with VGB had a marked reduction in spasm frequency after 2 weeks of treatment.
We also analyzed our data sets without normalizing to baseline counts. The analysis of the raw data (Supplemental Figure 1A) supports the conclusion that VGB is highly effective in suppressing spasms. The graph also reflects the variability in basal spasm counts between animals, which is shown directly in Supplemental Figure 1B. It is interesting to note that the baseline spasm counts for the 2 animals in which VGB did not completely eliminate spasms did not differ substantially from that of the 3 responders. Thus, differences in spasm frequency are unlikely to be an explanation for the failure of these 2 animals to fully respond to this anticonvulsant.
Due to the heterogeneity across our data sets, the data were found not to be normally distributed. This is a common finding in both anticonvulsant clinical trials as well as in drug testing in animal models of chronic epilepsy (French, 2001, Grabenstatter et al., 2005, Eastman et al., 2010, Siddiqui and Hershkowitz, 2010). Due to this, we first used a Kruskal-Wallis ANOVA to examine the overall effect of VGB treatment and then a Mann-Whitney U non-parametric test to determine if VGB’s effects on spasm frequency at particular points in time were statistically significant. For both the normalized to baseline data in Figure 1F and the raw spasm counts in Supplementary Figure 1A, VGB treatment overall produced a highly significant effect on spasm counts (p ≤ 0.001). When examining individual weeks of recordings, spasm counts in the VGB-treated animals were found to be statistically different from that of control rats at 2, 3 and 4 weeks after initiating treatment for the normalized data and at 2 and 3 weeks for the raw spasm counts. In addition, compared to baseline counts for the VGB-treated group in Supplemental Figure 1A, counts at weeks 2, 3, 4, and 5 were significantly different (p ≤ 0.05). Baseline counts between controls and VGB-treated animals were not significantly different.
Another strategy often used in the analysis of drug efficacy in epilepsy is to use a log10 transformation of the seizure counts, which frequently converts data to a normal distribution (Grabenstatter et al., 2005, Eastman et al., 2010, Siddiqui and Hershkowitz, 2010). We also used this approach in analyzing our results. The log10 transformation did result in a normal distribution of our data. A 2-way ANOVA of the transformed data confirmed that VGB treatment had a significant effect on spasm frequency and that differences in spasm counts at 2, 3 and 4 weeks after initiating treatment differed from those in the control group at these same times (results not shown).
In addition to the VGB dose of 325 mg/kg/day, we examined the effectiveness of 250 mg/kg/day in suppressing spasms. This is a dose that has been used by others in examining VGB’s effectiveness in other animal models of epilepsy (Nissinen and Pitkanen, 2007). We treated 2 rats with this dose. Both animals appeared to respond since one had a 46% decrease in spasm frequency during the second week of treatment and the other had a 53% decrease (See Supplemental Figure 2A). However, unlike the effects of 325 mg/kg/day (e.g. Supplemental Figure 2B) neither rat became seizure free on any day of treatment. Once treatment stopped, spasm counts returned to pretreatment baseline levels. We also treated other animals with a higher dose - 400mg/kg/day in equally divided doses of 200mg/kg in the early morning and late afternoon. However, this dose resulted in significant weight loss and treatment was terminated.
Vigabatrin Suppresses the Late High Frequency Oscillations of Ictal Complexes
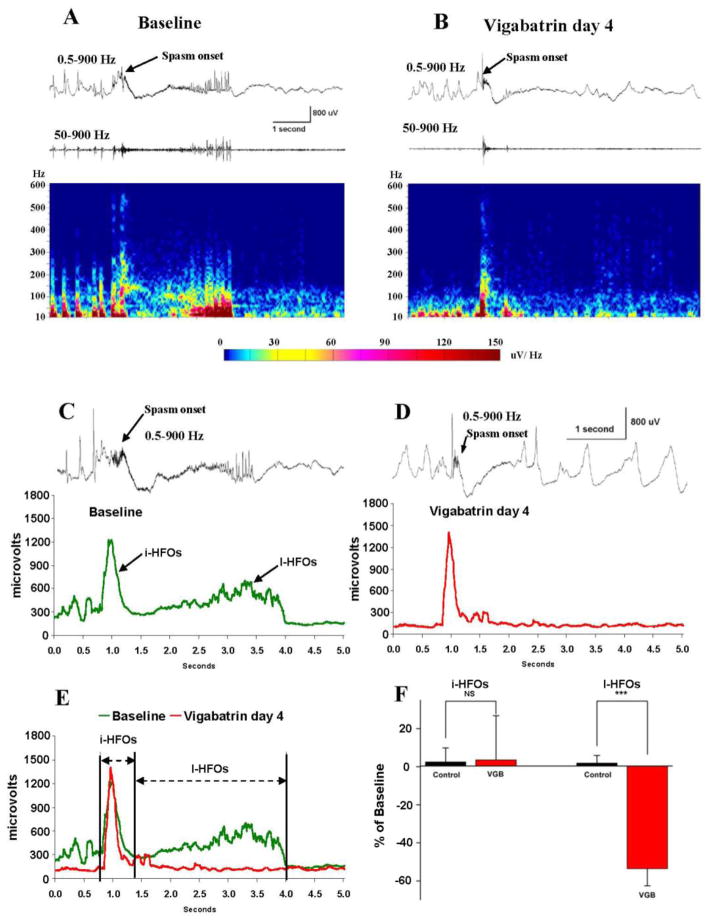
As we evaluated the effects of VGB on spasm generation, it quickly became apparent that during the first few days of treatment the drug appeared to have consistent effects on the duration of the ictal EEG events of the spasms and their behavioral accompaniments. The behavioral spasms became briefer and less intense and at the same time the duration of ictal events appeared to be shortened. An example of this effect on EEG recordings is shown in Figure 2A and B where 2 ictal events are compared. An ictal event (single channel recording site shown and bandpass filtered at 0.5–900 Hz) that was recorded on the last day of baseline recordings is compared to one on the fourth day of VGB treatment. Prior to treatment in this animal, frequent HFOs occurred, which resulted in brief bursts of high frequency activity in baseline recordings. The onset of the ictal event and behavioral spasm is denoted by an arrow. Immediately noticeable in these traces is that, while the initial slow wave is clearly present, high-frequency activity during the electrodecremental phase and subsequent fast spiking is markedly diminished after VGB treatment. This is even more apparent in the 50–900 Hz band pass filtered traces of the same events. Similarly, spectral contour arrays in the lower panel of Figures 2A and B show that prior to treatment intense high frequency activity up to 150–200 Hz persists for 3 sec following the initial burst of activity. This is almost completely absent after VGB treatment.
Figure 2.
As VGB reduces the frequency of spasms, the late HFOs of ictal events are abolished. (A and B) Comparison of ictal events before and after 4 days of VGB treatment. Top trace: single channel recordings bandpass filtered at 0.5–900 Hz. The initial slow wave (arrows) is relatively unaltered but the high frequency activity during the subsequent electrodecrement and fast spiking is greatly diminished. This is more apparent in 50–900 Hz filtered lower traces and the spectral contour arrays below. (C and D) To quantify this effect across animals, the amplitude of 50–900 Hz activity was summated during ictal events. The summations of eight randomly selected ictal events were averaged for each animal. Plots from one rat before and during day 4 of VGB treatment are shown. For reference, one representative ictal event is shown above each plot. Two peaks of high frequency activity are apparent in the baseline plot. We have called these the initial (i) and late (l) HFOs of ictal events. VGB appears to selectively suppress the l-HFOs. (E) Superimposed plots of summated ictal high frequency activity before and during VGB treatment denoting times during which the amplitudes of the i-HFOs and l-HFOs were selectively integrated to permit comparison across animals. (F) Results of these integrations show that VGB suppresses the l-HFOs when compared to recordings at very similar times from control TTX-infused rats that were not treated with VGB. During these early times of treatment, the i-HFOs appear unaltered by VGB treatment. *** P ≤ 0.001, ns - non-significant.
To quantify these effects across animals, we summated the amplitude of 50–900 Hz activity during the course of ictal events (see methods). In each animal, we averaged the results of summations from 8 randomly selected ictal events obtained during baseline recordings and 8 random ictal events during the early phase of VGB treatment. Because of animal to animal variations in the timing of effectiveness of VGB in suppressing spasms (e.g. see Figures 1D and 1E), the VGB-treatment samples were collected at either day 3, 4, 5 or 7 days after initiating VGB therapy. The graphs in Figure 2C and D show the results of this analysis from one rat. Here, the summated amplitude is plotted against time during which events occurred. For reference, one representative ictal event is shown above each plot. During baseline recordings there are 2 clear peaks of high frequency activity. The first is at the onset of the ictal event and coincides with the generation of the initial slow wave. The second is coincident with the HFOs of the electrodecrement and the subsequent run of fast spikes. We refer to these 2 peaks of high frequency activity as the “initial HFOs” and “late HFOs” of ictal events or i-HFOs and l-HFOs. During VGB treatment in Figure 2D, the i-HFOs appear relatively unaltered by the treatment but the l-HFOs are essentially abolished.
In Figure 2E, the plots in 2C and D are superimposed for direct comparison. In addition, we demarcate the regions identified as the i-HFOs and l-HFOs, which were used to further quantify data. To compare the intensity of HFOs across animals we integrated the spectral plots for the times when the i-HFOs and l-HFOs were generated. Results from 8 animals (4 VGB-treated and 4 control rats) are summarized in Figure 2F. Consistent with results from the animal analyzed in Figures 2C–E, there was on average a small change (6% increase) in the i-HFO activity during VGB treatment and that was very similar to a 2.5% increase seen in untreated TTX-infused control rats whose EEGs were analyzed at similar times during long-term EEG recordings. In contrast, while the l-HFO activity in control rats increased 1.8% in our recordings, during VGB treatment the l-HFO activity decreased 54% (P ≤ 0.001).
Vigabatrin Suppresses Interictal High Frequency EEG Activity
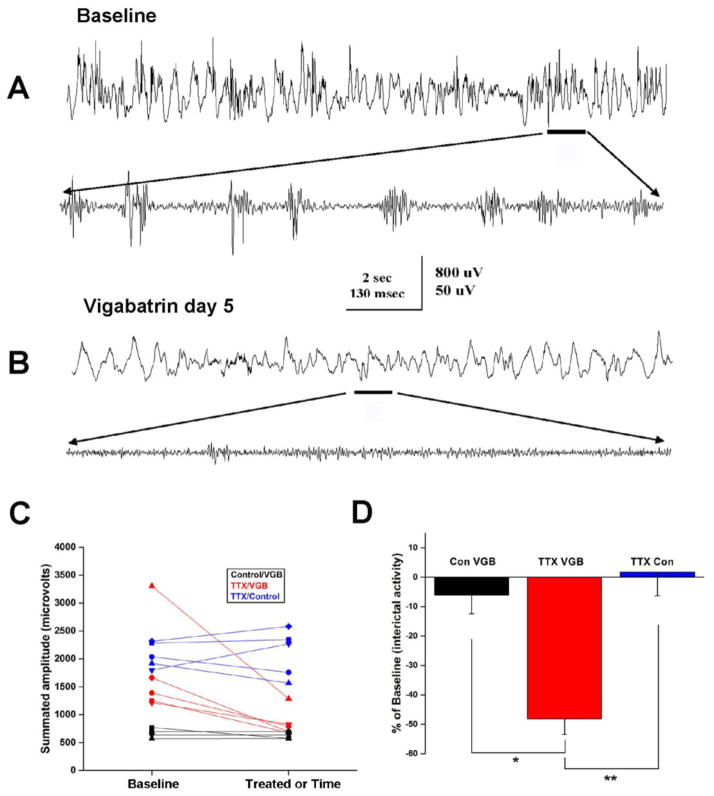
A close inspection of the plots in Figure 2E suggests that VGB might reduce EEG high frequency activity prior to the onset of the ictal event. Notice that in baseline recordings prior to the ictal event, high frequency activity varied between 200 and 500 μV in amplitude but after treatment it was closer to 100 μV. This led us to analyze the effects VGB treatment might have on interictal HFOs. Previously, we have described the occurrence of large and very frequent HFOs in the TTX model of infantile spasms (Frost et al., 2012). The upper trace of Figure 3A, illustrates the neurophysiological features of these events. Individual events from the segment denoted by a line are further illustrated in faster time base recordings in the lower trace. Following 5 days of VGB treatment, recordings in Figure 3B indicate that the frequency and amplitude of these HFO events are greatly diminished. To examine this possibility in detail, we analyzed 8 randomly selected 14 second epochs of interictal EEG recordings from 5 animals during both baseline periods and several days after initiating VGB treatment. Like our analysis of the high frequency activity of ictal events, we summated the amplitude of all high frequency activity in the 50–900 Hz range.
Figure 3.
VGB suppresses interictal high frequency activity. (A and B) Recordings demonstrating interictal high frequency activity during baseline recordings and the suppression of this activity after 4 days of VGB treatment. Selected segments of slow time-base recordings in the upper traces (band pass filtered: 0.5–900 Hz) are expanded in time in the lower traces in each panel and band pass filtered at 250–900 Hz to illustrate high frequency activity. (C) To quantify these results across animals, 8 randomly selected 14 second epochs of interictal EEG recordings were analyzed from 5 animals during baseline periods and during VGB treatment. We summated the amplitude of all high frequency activity in the 50–900 Hz range and calculated averages for the 8 epochs from each rat. Results plotted in red show that interictal high frequency activity was suppressed in all epileptic animals treated with VGB. In comparison, VGB did not impact high frequency activity recorded in 4 control rats (not infused with TTX: plotted in black). Note: as expected HFO activity in non-epileptic control rats was much lower than in the TTX-infused rats; also VGB treatment appears to normalize HFO activity (i.e. brings it into the range recorded in control rats in 4 of the 5 epileptic rats). Over similar time periods, interictal HFO activity in 5 untreated TTX-infused rats was on average unchanged (plotted in blue). (D) Comparison of alterations in interictal high frequency activity from baseline in control and TTX-infused rats by VGB treatment. The effects were much greater in the TTX-infused rats. In addition, over nearly identical time periods there was essentially no change in HFOs in TTX-infused rats that were not treated with VGB.
Results of these analyses from the 5 TTX/VGB treated rats are summarized in Figure 3C where the graphs (in red) clearly show that VGB reduced the high frequency activity during interictal recordings in all 5 epileptic rats. In comparison, a similar analysis done from recordings in 5 TTX/control rats (i.e. not treated with VGB – in blue) over a very similar time period showed that interictal HFO activity either increased or decreased slightly in amplitude and for the group HFO activity did not appear to change appreciably in amplitude. Since results in our previous analysis of interictal high frequency activity showed that such activity was less frequent and of lower amplitude in normal control animals (Frost et al., 2012), we next wondered if VGB might preferentially suppress abnormal interictal HFOs. To address this we performed similar recordings from 4 control rats (3 untreated, 1 intracortically infused with saline) and after 5 days of baseline recordings we treated each animal with VGB for 5 days. On the fifth day, we examined the effects of the VGB treatment on high frequency EEG activity. The Control/VGB graphs in Figure 3C (in black) show that as expected the summated amplitude of high frequency activity during baseline periods was less than that in TTX treated rats. Moreover VGB had little impact on the high frequency activity that was present in these recordings. The graph also shows that VGB treatment of the epileptic rats not only reduced interictal high frequency activity in all rats but in 4 of the 5 rats the high frequency activity was indistinguishable from that seen in non-epileptic control rats. This supports the idea that VGB may preferentially eliminate the abnormal HFOs recorded in this model of infantile spasms.
The bar graphs in Figure 3D summarize the results from our analysis of HFOs in the 3 treatment groups. Data are normalized to baseline HFO interictal activity. Results support the hypothesis that VGB preferentially suppresses HFOs in TTX-infused rats compared to control rats. In addition the results indicate that over the same time period the intensity of HFO activity in untreated TTX rats undergoes only minor changes in intensity when compared to the effects observed during VGB treatment.
Overall, our analysis of ictal and interictal HFO activity shows that both the l-HFOs of ictal events and interictal HFOs are suppressed soon after initiating VGB treatment, but at these times the i-HFOs of ictal events are spared (Figure 2). In wondering what may be a distinguishing property of iHFOs, we compared the peak amplitudes of these 3 events. Our analysis showed that iHFOs are uniformly larger in amplitude than either the l-HFOs (see also Figure 2C) or interictal HFOs. Indeed in baseline recordings from 7 rats, the amplitude of interictal HFOs were 45.8 ± 0.05% and the l-HFOs 61.1 ± 0.11% of the iHFOs. Differences in discharge intensity may be an important contributing factor to this differential drug sensitivity (see Discussion).
Vigabatrin suppresses hypsarrhythmia
Previous analyses of EEG recordings in this animal model have shown that in addition to the ictal events of spasms, rats also routinely display the highly abnormal interictal EEG pattern, called hypsarrhythmia (Lee et al., 2008, Frost et al., 2012). As in humans, hypsarrhythmia in the TTX model consists of unusually large slow waves which can occur asynchronously across the cortex and can exceed 1 mV in amplitude. Large and frequent multifocal interictal spikes are intermixed with the slower events. In our analysis of our long-term monitoring recordings we checked for the presence of hypsarrhythmia. In the 5 rats treated with VGB, hypsarrhythmia was present during baseline recordings in all 5 rats and was always most prominent during NREM sleep. We then checked on the final day of VGB treatment. While in some instances background EEG recordings only approached those of normal control rats, in every VGB-treated rat, hypsarrhythmia had been eliminated. Examples of recordings during NREM sleep during baseline and after VGB treatment are shown in Figures 4A and B and illustrate the effectiveness of VGB in this regard. When EEGs were examined 2 weeks after cessation of VGB treatment, the 3 rats in which spasms did not recur still did not display hypsarrhythmia. However, for one of the rats in which spasms were only transiently suppressed (and whose spasms counts are plotted in Figure 1E), hypsarrhythmia reemerged 2 weeks after stopping treatment.
In EEG recordings, hypsarrhythmia is most commonly and prominently observed during NREM sleep in both a clinical setting (Hrachovy et al., 1981, Hrachovy et al., 1984) and in our animal model. However, hypsarrhythmia can also be observed in awake states. Supplemental Figure 3A and B compares the features of hypsarrhythmia in these two behavioral states. While sharing many features, the amplitude of slow waves of hypsarrhythmia during NREM sleep were generally larger than during waking and interictal spike frequency greater. Despite these differences, VGB was equally effective in suppressing hypsarrhythmia during NREM sleep and waking (Supplemental Figure 3C and 3D).
When EEGs from control TTX-infused rats (i.e. not treated with VGB) were examined, all rats that had hypsarrhythmia during baseline recordings (7 of the 9 rats) had hypsarrhythmia two weeks later. The differences in outcomes between the control and VGB treated animals are plotted in Figure 4D.
It should be noted that the original description of hypsarrhythmia included asynchrony of large slow waves across the cortex as an important feature which contributed significantly to the “chaotic” appearance of the EEG (Gibbs et al., 1954). However, subsequently many investigators have described numerous variations from this prototypic pattern that are now referred to as “modified” hypsarrhythmia (Hrachovy et al., 1984, Kramer et al., 1997, Hussain et al., 2015). One such EEG pattern is hypsarrhythmia with interhemispheric synchronization. This common variation is still considered hypsarrhythmia since it retains many features of the original EEG pattern (e.g. high amplitude slow waves, frequent interictal spikes) and is associated with clinical spasms and ictal EEG events in the same subject. While recordings of hypsarrhythmia in the TTX model can demonstrate the chaotic and asynchronous slow wave activity originally described in children (Lee et al. 2008), the EEG slow wave activity in some animals such as in Figure 4 and Supplemental Figure 3A and 3B is synchronized across channels. Thus the EEG pattern in this animal would be considered modified hypsarrhythmia. In support of this EEG pattern being hypsarrhythmia is the fact that the slow waves during sleep and awake are much larger than those in recordings from saline-infused control rats shown in Supplemental Figure 4. (See also Frost et al. 2012 for a quantitative comparison of EEG amplitude spectrums of hypsarrhythmia and NREM sleep in control animals). Furthermore, the abnormal slow waves in our epileptic animals are greatly reduced in amplitude or eliminated by VGB treatment (Figure 4 and Supplemental Figures 3C and 3D). However, VGB treatment had no effect on the EEG recordings from control rats (Supplemental Figures 4C and D) and did not suppress the slow waves of NREM sleep in these rats (Supplemental Figures 4B vs 4D). The latter results are in keeping with earlier studies that showed VGB has little or no effect on normal EEG activity in humans (Hammond and Wilder, 1985, Mervaala et al., 1989, Marciani et al., 1997).
TTX Produces a Focal Neocortical Lesion That Is Not Reversed by VGB
We next undertook a histological examination of the brains of both control and VGB-treated TTX-infused animals. We wondered if a frank neuropathology could be identified and if so would it be observed in all TTX-treated rats and would VGB treatment restore normal brain anatomy. If it did, this could help explain how VGB suppresses spasms and hypsarrhythmia. Results from this analysis are illustrated in Supplemental Figure 5. Unexpectedly, we found that TTX did produce a localized lesion at the infusion site. The images in Supplemental Figures 5A, C and E compare the appearance of the brain of 3 rats after fixation. One was from a saline-infused control rat (panel A) illustrating normal brain anatomy. The second was from a TTX-infused control rat (panel C) and the third was from a second epileptic rat treated with VGB (panel E). The lesions in the 2 TTX-infused rats have a similar appearance. Indeed, every epileptic rat in this study displayed a focal lesion whether they were treated with VGB or not. Histological examination of brains with Nissl staining revealed a thinning of the neocortex near the TTX infusion site (see dashed red lines in Supplemental Figures 5D and F) but not in the saline-infused control rat (Supplemental Figure 5B). A disruption of the neocortical lamination was also apparent following TTX infusion. Thus, while TTX routinely induced a neocortical neuropathology, VGB treatment did not appear to interfere with the underlying pathological process responsible for the lesion.
As might be expected from an infusion of the type employed in our studies, there was some variation between animals in terms of the extent of the lesion. This is apparent comparing Supplemental Figures 5D and F. We also wondered if variations in pathology between animals could help explain why some rats had higher spasm counts (Figure 1A, C and D), more intense interictal HFOs (Figure 3C) and responded more fully to VGB treatment (Figure 1 and 3C). However, visual inspections have revealed no clear correlation between lesion size or alterations in brain histology and spasm counts or responsiveness to VGB.
DISCUSSION
The results reported here show that VGB is highly effective in not only suppressing spasms but also eliminating them in a majority of animals. This latter effect persisted for weeks after the cessation of treatment and is likely a permanent alteration in the propensity for seizure generation. Prior reviews and commentaries have proposed a number of criteria for an “ideal” animal model of the human condition (Stafstrom et al., 2006, Swann and Moshe, 2012). One of these has been that animals should respond to drugs that are effective in eliminating spasms in humans. Since VGB is effective in humans, our results extend the clinical relevance of the TTX model. In all experiments, we employed continuous, high sampling rate (2048 Hz) digital EEG/video recordings over periods of weeks to months to analyze the impact of an anticonvulsant on neuronal activity. To our knowledge, this has never been attempted previously and we believe our results demonstrate that additional insights into seizure mechanisms and how anticonvulsant drugs work can be obtained using these techniques. For instance, our results clearly show that as VGB suppresses the generation of spasms it simultaneously suppresses the generation of HFOs not only during ictal events but interictally as well. Indeed, our analysis of interictal HFOs suggests that, in eliminating abnormal high frequency activity in epileptic animals, the high frequency content of the EEG closely approaches that of control rats (Figure 3C). This coupled with the fact that VGB has little impact on the high frequency content of EEGs in control rats leads us to suspect that VGB may be acting to selectively eliminate abnormal HFOs in animals with spasms.
Insights into preclinical drug trial strategies for infantile spasms
Continuous video/EEG recordings provide an unprecedented wealth of data on the natural history of a seizure disorder. They provide a detailed description of the frequency, patterns (e.g. clustering) and progression of a disorder, which can only be guessed at when intermittent sampling techniques are used. However, our long-term monitoring results also have revealed the challenges in drug testing in this animal model. Spasm clustering and marked differences in basal spasm frequency from animal to animal introduce substantial heterogeneity in data sets. In order for a drug to be shown effective either its effects must be quite large or a large number of animals would have to be treated to obtain statistically significant results. The latter is likely unrealistic given that long-term monitoring is so time, labor and resource (e.g. requiring terabytes of data storage) intensive. However, the way infants respond when a drug treatment is successful is similar to how the majority of rats responded to VGB in this study – i.e. for unknown reasons the effects could be considered “all-or-none” (Stafstrom et al., 2011) (see discussion below). That is, over the course of treatment the infant or animal becomes completely free of seizures and often they do not recur even upon cessation of drug treatment. In this regard, only the total elimination of spasms is considered a successful treatment in clinical practice and if any spasms persist, it is considered a treatment failure. Thus, given the large, dramatic response to a successful treatment, it may be realistic to use animal models like this to screen for new drugs to treat infantile spasms.
Why 2 of the 5 treated rats in this study only transiently responded to VGB is unknown. However this is not unlike the clinical situation where – depending on the clinical trial - between 14% and 84% of patients were judged unresponsive to VGB, i.e. they were not seizure free (Carmant, 2011). Contributing factors in our study could be: 1) an inadequate dose of VGB - although a slightly higher dose (400 vs 325 mg/kg/day) produced dramatic weight loss; 2) differences in the age of rats when treatment began – this seems unlikely since the average age of the fully responsive rats was 37 days and of partial responders was 39 days; 3) differences in spasms counts – this also seems unlikely since the rats with the highest and lowest baseline seizure counts (42 and 170 per day) both became seizure free after 2 weeks of treatment (see Supplemental Figure 2B) or 4) differences in prior seizure history. The latter is potentially an explanation since we have no detailed information of earlier seizure burden in these animals. This would require long-term monitoring from infant rats which is technically impossible at this time due to the need to keep rat pups with their dam. However, the development of implantable microtelemetry devices may permit such studies in the future.
HFOs and insights into the mechanisms of the infantile spasms generation
In recent years, evidence from numerous research groups has supported the notion that abnormal interictal HFOs may be a biomarker for epileptogenic regions in hippocampus and neocortex (Bragin et al., 2010, Jacobs et al., 2012). Indeed HFOs have been proposed to be superior to interictal spikes in mapping epileptic foci for surgical resection (Jacobs et al., 2008, Zijlmans et al., 2012). The abnormal HFOs of epilepsy are often referred to as “fast ripples” and are thought to have spectral power between 200 and 600 Hz. They are distinguished from “ripples” that have a lower frequency content (80–200 Hz) and can be recorded in normal humans and animals. However, the distinction between ripples and fast ripples in epilepsy is currently a matter of discussion since abnormal HFOs in epilepsy have been reported to span both the ripple and fast ripple bands (Engel et al., 2009). This is also the case in our recordings of HFOs in the TTX model – e.g. see Figure 2 (Frost et al., 2011, Frost et al., 2012). Nonetheless, the neuronal mechanisms underlying ripples and fast ripples have been suggested to be quite distinct. Ripples have been proposed to be summated inhibitory postsynaptic potentials generated in the cell bodies of pyramidal cells (Ylinen et al., 1995). Fast ripples are thought to be generated by spatially restricted populations of hyperexcitable and synaptically connected pyramidal cells (Bragin et al., 2002a). However, even this distinction has become clouded by a recent study that concluded that ripples are dependent on the activity of networks of pyramidal cells since optogenetic silencing of these cells abolished ripples. Interneuron activity appears to be important in the pacing of pyramidal cell activity at ripple frequencies (Stark et al., 2014).
GABA-mediated inhibitory synaptic transmission has been hypothesized to control the generation of abnormal HFOs and restrict the spread from their sites of origin. In keeping with this idea are results showing that a local blockade of synaptic inhibition results in an expansion of sites of HFO generation (Bragin et al., 2002a). The effects of VGB treatment reported here are fully consistent with these results, since one would predict that the increase in GABA content of synapses following irreversible GABA transaminase inactivation would lead to an increased effectiveness of synaptic inhibition which should in turn suppress the hyperexcitability of HFO-generating pyramidal cells. Our previous microwire recordings in the TTX model show that robust bursts of multiunit activity are recorded simultaneously with generation of neocortical HFOs (Frost et al., 2012). Thus, the effects of VGB reported here are consistent with a hyperexcitable neocortical network hypothesis as a mechanism contributing to infantile spasm generation.
Consistent with a neocortical contribution to spasms are analyses of neocortical HFOs during the generation of epileptic spasms in both humans and the TTX model. In humans, regional increases in HFOs up to 300 Hz have been reported to occur in neocortex during the ictal discharges of spasms (Nariai et al., 2011b). Interestingly, surgical removal of these sites was shown to eliminate spasms in a majority of patients. Our previous recordings of HFOs during ictal events in the TTX model showed similar increases in high frequency activity during ictal events (Frost et al., 2012). This is confirmed in Figure 2A, where a burst of high frequency activity is seen at seizure onset concurrent with the generation of the initial slow wave. This is followed by runs of HFOs, which are lower in amplitude than the preceding burst of high frequency activity. However, towards the end of the ictal event they often increase in intensity concurrent with repetitive spikes/sharp wave complexes. Here we refer to the two components of ictal HFOs as the i-HFOs and l-HFOs of ictal complexes and show that VGB selectively eliminated the l-HFOs. Why there is a difference in the pharmacological sensitivity of the i-HFOs and l-HFOs is unknown. One possibility is that the underlying mechanisms of the i-HFOs and l-HFOs are different.
Alternatively, the blockade of the l-HFO could be a reflection of a VGB-induced reduction in the size of the neocortical networks generating ictal events. As discussed earlier, suppression of GABAergic synaptic transmission in a model of temporal lobe epilepsy has been shown to result in an expansion of sites of HFO generation (Bragin et al., 2002a) which would reflect an increase in the size of the neuronal network generating the HFOs. In keeping with this idea, enhanced inhibitory synaptic transmission produced by daily treatment with VGB could gradually reduce the size of the network generating the HFOs. Since the intensity of l-HFOs is less than that of i-HFOs (e.g. see Figure 2C and Results), these events could be more susceptible to increases in inhibitory synaptic transmission. This could be a reason why they are eliminated early during VGB treatment. However, additional studies will be required to determine if this is the case.
With continued VGB treatment, the i-HFOs are eventually abolished since at these times animals can become seizure free and thus ictal complexes and i-HFOs are no longer present. These results along with our previous recordings of multiunit activity throughout ictal complexes favor a neocortical origin for spasms. However, as discussed earlier previous clinical studies have proposed a subcortical (possibly brain stem) contribution to infantile spasms. It remains a possibility that subcortical regions also contribute in important ways to the generation of infantile spasms.
Additional Insights into the TTX Model
We were surprised to find that infusion of TTX resulted in a focal neocortical lesion (Supplemental Figure 5). The molecular mechanism underlying this injury is unknown. However, it is well known that neuronal survival in the developing central nervous system is dependent on neuronal activity (Hagenston and Bading, 2011). Intracellular calcium entry mediated by synaptic transmission and action potentials has been shown to signal to the nucleus and promote the transcription of antiapoptotic genes. Thus a TTX-mediated suppression of these mechanisms could lead to neuronal loss and result in the lesions reported here. Importantly, every VGB-treated rat in this study had a focal neocortical lesion. Thus we are unable to explain VGB anticonvulsant actions via a rescue of normal brain anatomy. We were also unable to explain animal to animal variability in spasm frequency, interictal HFO activity or VGB responsivity based on a visual inspection of whole brain mounts or Nissl stained sections. However, a more detailed quantitative histological analysis may reveal neuroanatomical changes that do correlate with one of these clinical endpoints. However, our experimental design precluded such an analysis.
Our previous studies reported that interictal HFOs and HFOs during ictal events are more prominent contralateral to the TTX infusion site (Frost et al. 2011, Frost et al. 2012). This is perhaps not surprising when TTX infusion is on-going since this sodium channel antagonist would be expected to block neuronal activity at and near its site of application. Indeed, our earlier 2-deoxyglucose studies and EEG recordings from the infusion site showed this was the case (Galvan et al., 2000). However, the discovery of a TTX-induced lesion likely helps to explain the long term suppression of EEG activity ipsilateral to the site of TTX infusion. The potential loss of neurons and disruption of neuronal circuitry in the lesion would be expected to prevent those neocortical regions from fully participating in the generation of seizures and could explain the predominance of HFOs contralateral to TTX infusion.
Clinically, infantile spasms has been divided into 2 major subgroups: 1) symptomatic – where there is an identifiable neurological disorder that likely causes the spasms and 2) cryptogenic – where no specific cause can be identified. Recently, a revised terminology has been suggested where the term symptomatic infantile spasms is replaced with infantile spasms with a structural/metabolic etiology (Berg et al., 2010). Given that there is a neocortical lesion in the TTX model; one could then classify it as an animal model of infantile spasms with a structural etiology or using the older term symptomatic infantile spasms.
Insights into the infantile spasms disease state
Commonly when anticonvulsant drugs are used successfully to stop seizures, continuous treatment is required. Otherwise upon withdrawal of the drug, seizures recur. However, this is often not the case for infantile spasms although relapses can occur in some patients. In the 3 animals of our study that became seizure free after 2 weeks of VGB treatment, spasms did not recur even 2–3 weeks after cessation of treatment. This was also true for hypsarrhythmia. Since the recovery time for GABA transaminase after VGB treatment has been shown to be approximately 5 days (Jung et al., 1977), it is very likely that GABA levels have returned to pretreatment levels by this time. Yet neither spasms nor hypsarrhythmia returned. This suggests that there are fundamental differences in the neuronal and network mechanisms that underlie this form of epilepsy compared to other seizure disorders. It suggests that the neuronal networks that are responsible for this disease state are in some way able to remodel themselves or reset their state of excitability for extended periods of time. Clinically, this often turns out to be a lifetime. Investigating this phenomenon in animal models would be an important step in understanding the neuronal mechanisms of infantile spasms and identifying targets for new therapies.
Conclusion
While results reported here provide new insights into the mechanisms underlying spasms and hypsarrhythmia, there are potentially broader implications for our studies. Despite a large and growing interest in the importance of HFOs in various forms of focal epilepsy, a demonstration that abnormal HFOs are actually causative in seizure generation has been a challenge for the field. One way to address this issue would be to examine the effectiveness of clinically relevant anticonvulsant drugs on HFO generation and seizures. Surprisingly, such studies have never been performed. Thus our experiments are the first to show that as seizures abate during anticonvulsant therapy the generation of HFOs is markedly suppressed, thus implicating abnormal HFOs in the genesis of one type of epilepsy.
Supplementary Material
Highlights.
Vigabatrin suppressed spasms and hypsarrhythmia in the TTX animal model.
An initial and late burst of HFOs were identified during ictal events.
As vigabatrin suppressed spasms, it preferentially blocked the late HFOs.
The sensitivity of late HFOs may reflect differences in the network generating them.
Vigabatrin also suppressed the generation of abnormal interictal HFOs.
Acknowledgments
We would like to thank Dr. Ed Bertram for his advice on establishing long-term Video/EEG recordings in our lab. We thank Trang Lam for her help with histology. We thank Dr. Sunita Misra for comments on an early draft and final version of this paper. We thank Lundbeck LLC for supplying vigabatrin.
Funding. This work was supported by grants from the CURE’s Infantile Spasms Initiative and NIH-NINDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ben-Menachem E. Mechanism of action of vigabatrin: correcting misperceptions. Acta neurologica Scandinavica Supplementum. 2011;(192):5–15. doi: 10.1111/j.1600-0404.2011.01596.x. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51(4):676–85. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Bickford RG, Billinger TW, Fleming NI, Steward F. The compressed spectral array (CSA). a pictorial EEG. ProcSan Diego BiomedSymp. 1972;11:365–70. [Google Scholar]
- Bragin A, Azizyan A, Almajano J, Wilson CL, Engel J., Jr Analysis of chronic seizure onsets after intrahippocampal kainic acid injection in freely moving rats. Epilepsia. 2005;46(10):1592–8. doi: 10.1111/j.1528-1167.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Staba RJ. High-frequency oscillations in epileptic brain. Current opinion in neurology. 2010;23(2):151–6. doi: 10.1097/WCO.0b013e3283373ac8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. JNeurosci. 2002a;22(5):2012–21. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel J., Jr Interictal high-frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. AnnNeurol. 2002b;52(4):407–15. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- Carmant L. Vigabatrin therapy for infantile spasms: review of major trials in Europe, Canada, and the United States; and recommendations for dosing. Acta neurologica Scandinavica Supplementum. 2011;(192):36–47. doi: 10.1111/j.1600-0404.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Shewmon DA, Sankar R, Chen BC, Phelps ME. Infantile spasms: II. Lenticular nuclei and brain stem activation on positron emission tomography. AnnNeurol. 1992;31(2):212–9. doi: 10.1002/ana.410310212. [DOI] [PubMed] [Google Scholar]
- Crepon B, Navarro V, Hasboun D, Clemenceau S, Martinerie J, Baulac M, et al. Mapping interictal oscillations greater than 200 Hz recorded with intracranial macroelectrodes in human epilepsy. Brain. 2010;133(Pt 1):33–45. doi: 10.1093/brain/awp277. [DOI] [PubMed] [Google Scholar]
- Dulac O, Soufflet C, Chiron C, Kaminska A. What is West syndrome? IntRevNeurobiol. 2002;49:1–22. doi: 10.1016/s0074-7742(02)49003-4. [DOI] [PubMed] [Google Scholar]
- Eastman CL, Verley DR, Fender JS, Temkin NR, D’Ambrosio R. ECoG studies of valproate, carbamazepine and halothane in frontal-lobe epilepsy induced by head injury in the rat. ExpNeurol. 2010;224(2):369–88. doi: 10.1016/j.expneurol.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50(4):598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- French JA. Proof of efficacy trials: endpoints. Epilepsy Res. 2001;45(1–3):53–6. doi: 10.1016/s0920-1211(01)00216-9. [DOI] [PubMed] [Google Scholar]
- Frost JD, Jr, Lee CL, Hrachovy RA, Swann JW. High frequency EEG activity associated with ictal events in an animal model of infantile spasms. Epilepsia. 2011;52(1):53–62. doi: 10.1111/j.1528-1167.2010.02887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JD, Jr, Lee CL, Le JT, Hrachovy RA, Swann JW. Interictal high frequency oscillations in an animal model of infantile spasms. NeurobiolDis. 2012;46(2):377–88. doi: 10.1016/j.nbd.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan CD, Hrachovy RA, Smith KL, Swann JW. Blockade of neuronal activity during hippocampal development produces a chronic focal epilepsy in the rat. JNeurosci. 2000;20(8):2904–16. doi: 10.1523/JNEUROSCI.20-08-02904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs EL, Fleming MM, Gibbs FA. Diagnosis and prognosis of hypsarhythmia and infantile spasms. Pediatrics. 1954;13(1):66–73. [PubMed] [Google Scholar]
- Grabenstatter HL, Ferraro DJ, Williams PA, Chapman PL, Dudek FE. Use of chronic epilepsy models in antiepileptic drug discovery: the effect of topiramate on spontaneous motor seizures in rats with kainate-induced epilepsy. Epilepsia. 2005;46(1):8–14. doi: 10.1111/j.0013-9580.2005.13404.x. [DOI] [PubMed] [Google Scholar]
- Hagenston AM, Bading H. Calcium signaling in synapse-to-nucleus communication. Cold Spring Harbor perspectives in biology. 2011;3(11):a004564. doi: 10.1101/cshperspect.a004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond EJ, Wilder BJ. Effects of gamma-vinyl-GABA on the human electroencephalogram. Neuropharmacology. 1985;24(10):975–84. doi: 10.1016/0028-3908(85)90125-x. [DOI] [PubMed] [Google Scholar]
- Hrachovy RA, Frost JD., Jr Infantile epileptic encephalopathy with hypsarrhythmia (infantile spasms/West syndrome) J ClinNeurophysiol. 2003;20(6):408–25. doi: 10.1097/00004691-200311000-00004. [DOI] [PubMed] [Google Scholar]
- Hrachovy RA, Frost JD, Jr, Kellaway P. Sleep characteristics in infantile spasms. Neurology. 1981;31(6):688–93. doi: 10.1212/wnl.31.6.688. [DOI] [PubMed] [Google Scholar]
- Hrachovy RA, Frost JD, Jr, Kellaway P. Hypsarrhythmia: variations on the theme. Epilepsia. 1984;25(3):317–25. doi: 10.1111/j.1528-1157.1984.tb04195.x. [DOI] [PubMed] [Google Scholar]
- Hussain SA, Kwong G, Millichap JJ, Mytinger JR, Ryan N, Matsumoto JH, et al. Hypsarrhythmia assessment exhibits poor interrater reliability: a threat to clinical trial validity. Epilepsia. 2015;56(1):77–81. doi: 10.1111/epi.12861. [DOI] [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49(11):1893–907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Staba R, Asano E, Otsubo H, Wu JY, Zijlmans M, et al. High-frequency oscillations (HFOs) in clinical epilepsy. Prog Neurobiol. 2012;98(3):302–15. doi: 10.1016/j.pneurobio.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129(Pt 6):1593–608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Jiruska P, Finnerty GT, Powell AD, Lofti N, Cmejla R, Jefferys JG. Epileptic high-frequency network activity in a model of non-lesional temporal lobe epilepsy. Brain. 2010;133(Pt 5):1380–90. doi: 10.1093/brain/awq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MJ, Lippert B, Metcalf BW, Bohlen P, Schechter PJ. gamma-Vinyl GABA (4-amino-hex-5-enoic acid), a new selective irreversible inhibitor of GABA-T: effects on brain GABA metabolism in mice. J Neurochem. 1977;29(5):797–802. doi: 10.1111/j.1471-4159.1977.tb10721.x. [DOI] [PubMed] [Google Scholar]
- Kellaway P, Hrachovy RA, Frost JD, Jr, Zion T. Precise characterization and quantification of infantile spasms. AnnNeurol. 1979;6(3):214–8. doi: 10.1002/ana.410060306. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Oka M, Akiyama T, Inoue T, Abiru K, Ogino T, et al. Very fast rhythmic activity on scalp EEG associated with epileptic spasms. Epilepsia. 2004;45(5):488–96. doi: 10.1111/j.0013-9580.2004.45703.x. [DOI] [PubMed] [Google Scholar]
- Kramer U, Sue WC, Mikati MA. Hypsarrhythmia: frequency of variant patterns and correlation with etiology and outcome. Neurology. 1997;48(1):197–203. doi: 10.1212/wnl.48.1.197. [DOI] [PubMed] [Google Scholar]
- Lado FA, Moshe SL. Role of subcortical structures in the pathogenesis of infantile spasms: what are possible subcortical mediators? IntRevNeurobiol. 2002;49:115–40. doi: 10.1016/s0074-7742(02)49010-1. [DOI] [PubMed] [Google Scholar]
- Lee CL, Frost JD, Jr, Swann JW, Hrachovy RA. A new animal model of infantile spasms with unprovoked persistent seizures. Epilepsia. 2008;49(2):298–307. doi: 10.1111/j.1528-1167.2007.01377.x. [DOI] [PubMed] [Google Scholar]
- Marciani MG, Stanzione P, Maschio M, Spanedda F, Bassetti MA, Mattia D, et al. EEG changes induced by vigabatrin monotherapy in focal epilepsy. Acta Neurol Scand. 1997;95(2):115–20. doi: 10.1111/j.1600-0404.1997.tb00080.x. [DOI] [PubMed] [Google Scholar]
- Mervaala E, Partanen J, Nousianinen U, Sivenius J, Riekkinen P. Electrophysiologic effects of gamma-vinyl GABA and carbamazepine. Epilepsia. 1989;30(2):189–93. doi: 10.1111/j.1528-1157.1989.tb05453.x. [DOI] [PubMed] [Google Scholar]
- Nariai H, Matsuzaki N, Juhasz C, Nagasawa T, Sood S, Chugani HT, et al. Ictal high-frequency oscillations at 80–200 Hz coupled with delta phase in epileptic spasms. Epilepsia. 2011a;52(10):e130–e4. doi: 10.1111/j.1528-1167.2011.03263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nariai H, Nagasawa T, Juhasz C, Sood S, Chugani HT, Asano E. Statistical mapping of ictal high-frequency oscillations in epileptic spasms. Epilepsia. 2011b;52(1):63–74. doi: 10.1111/j.1528-1167.2010.02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissinen J, Pitkanen A. Effect of antiepileptic drugs on spontaneous seizures in epileptic rats. Epilepsy Res. 2007;73(2):181–91. doi: 10.1016/j.eplepsyres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Panzica F, Binelli S, Canafoglia L, Casazza M, Freri E, Granata T, et al. ICTAL EEG fast activity in West syndrome: from onset to outcome. Epilepsia. 2007;48(11):2101–10. doi: 10.1111/j.1528-1167.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- Sarhan S, Seiler N. Metabolic inhibitors and subcellular distribution of GABA. Journal of neuroscience research. 1979;4(5–6):399–421. doi: 10.1002/jnr.490040508. [DOI] [PubMed] [Google Scholar]
- Schevon CA, Trevelyan AJ, Schroeder CE, Goodman RR, McKhann G, Jr, Emerson RG. Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain. 2009;132(Pt 11):3047–59. doi: 10.1093/brain/awp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui O, Hershkowitz N. Primary Efficacy Endpoint in Clinical Trials of Antiepileptic Drugs: Change or Percent Change. Drug Information Journal. 2010;44:343–50. [Google Scholar]
- Stafstrom CE, Arnason BG, Baram TZ, Catania A, Cortez MA, Glauser TA, et al. Treatment of infantile spasms: emerging insights from clinical and basic science perspectives. J Child Neurol. 2011;26(11):1411–21. doi: 10.1177/0883073811413129. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Moshe SL, Swann JW, Nehlig A, Jacobs MP, Schwartzkroin PA. Models of pediatric epilepsies: strategies and opportunities. Epilepsia. 2006;47(8):1407–14. doi: 10.1111/j.1528-1167.2006.00674_1.x. [DOI] [PubMed] [Google Scholar]
- Stark E, Roux L, Eichler R, Senzai Y, Royer S, Buzsaki G. Pyramidal cell-interneuron interactions underlie hippocampal ripple oscillations. Neuron. 2014;83(2):467–80. doi: 10.1016/j.neuron.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann JW, Moshe SL. On the Basic Mechanisms of Infantile Spasms. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4. Bethesda (MD): 2012. [PubMed] [Google Scholar]
- Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130(Pt 9):2354–66. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- Willmore LJ, Abelson MB, Ben-Menachem E, Pellock JM, Shields WD. Vigabatrin: 2008 update. Epilepsia. 2009;50(2):163–73. doi: 10.1111/j.1528-1167.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131(Pt 4):928–37. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, et al. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. JNeurosci. 1995;15(1 Pt 1):30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72(11):979–86. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M, Jiruska P, Zelmann R, Leijten FS, Jefferys JG, Gotman J. High-frequency oscillations as a new biomarker in epilepsy. Ann Neurol. 2012;71(2):169–78. doi: 10.1002/ana.22548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.