Abstract
Background
Bipolar disorder is a heterogeneous mood disorder associated with several important clinical comorbidities, such as eating disorders. This clinical heterogeneity complicates the identification of genetic variants contributing to bipolar susceptibility. Here we investigate comorbidity of eating disorders as a subphenotype of bipolar disorder to identify genetic variation that is common and unique to both disorders.
Methods
We performed a genome-wide association analysis contrasting 184 bipolar subjects with eating disorder comorbidity against both 1,370 controls and 2,006 subjects with bipolar disorder only from the Bipolar Genome Study (BiGS).
Results
The most significant genome-wide finding was observed bipolar with comorbid eating disorder vs. controls within SOX2-OT (p=8.9×10−8 for rs4854912) with a secondary peak in the adjacent FXR1 gene (p=1.2×10−6 for rs1805576) on chromosome 3q26.33. This region was also the most prominent finding in the case-only analysis (p=3.5×10−7 and 4.3×10−6, respectively). Several regions of interest containing genes involved in neurodevelopment and neuroprotection processes were also identified.
Limitations
While our primary finding did not quite reach genome-wide significance, likely due to the relatively limited sample size, these results can be viewed as a replication of a recent study of eating disorders in a large cohort.
Conclusions
These findings replicate the prior association of SOX2-OT with eating disorders and broadly support the involvement of neurodevelopmental/neuroprotective mechanisms in the pathophysiology of both disorders. They further suggest that different clinical manifestations of bipolar disorder may reflect differential genetic contributions and argue for the utility of clinical subphenotypes in identifying additional molecular pathways leading to illness.
Keywords: eating disorders, bipolar disorder, genome-wide association (GWAS), comorbidity, SOX2-OT
INTRODUCTION
Bipolar disorder is a severe mood disorder with an estimated heritability of 60–93% (Kieseppa et al., 2004; Lichtenstein et al., 2009; McGuffin et al., 2003; Taylor et al., 2002). Genome-wide association (GWA) studies of large samples have recently identified several strong candidates for susceptibility genes, including ADCY2, ANK3, CACNA1C, NCAN, ODZ4, and TRANK1 (Cichon et al., 2011; Ferreira et al., 2008; Green et al., 2013; Chen et al., 2013; Muhleisen et al., 2014; Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011), although the pathways by which genetic variants impact risk are complex and remain largely unknown. Bipolar disorder also presents with complex, highly variable clinical manifestations, including several important comorbidities that constitute a wide range of disorder subtypes (MacQueen et al., 2005). This phenotypic heterogeneity impedes the clarification of genetic variants contributing to susceptibility, since a given sampling of bipolar patients likely consists of multiple different subtypes, each with a unique genetic architecture (Alda, 2004; Alda et al., 2009). The use of subphenotypes derived from clinical factors known to be associated with the disorder may establish more homogeneous subgroups of patients with distinct underlying genetic risk factors (Saunders et al., 2008). While several potentially important subphenotypes of bipolar disorder have been identified as part of the characteristic symptomatology or comorbidity (MacQueen et al., 2005; Saunders et al., 2008), few GWA analyses have utilized clinical subphenotypes for bipolar disorder (Greenwood and Kelsoe, 2013; Swaminathan et al., 2015; Winham et al., 2014).
Mounting evidence suggests a strong connection between the etiology of bipolar disorder and that of eating disorders. Patients with bipolar disorder have elevated rates of eating disorders (McElroy et al., 2013; McElroy et al., 2006; McElroy et al., 2005), with eating disorder comorbidity being more commonly observed among female than male bipolar patients (Kawa et al., 2005; McElroy et al., 2011), consistent with observations in the general population (Hudson et al., 2007). While rates of binge eating behaviors range from 13% to 38% in bipolar disorder (Kruger et al., 1996; Ramacciotti et al., 2005), eating disorder comorbidity appears to not be limited to the behavioral features of aberrant eating (i.e., binge eating, purging, dietary restriction) and may represent a marker for increased symptom load and illness burden (Wildes et al., 2007). Finally, epidemiological studies suggest an association between eating disorders and subthreshold bipolar symptoms, including affective temperaments, as well as between hypomania and binge eating behaviors, and the two disorders show considerable overlap in terms of phenomenology, course, comorbidity, family history, and pharmacologic treatment response (Lunde et al., 2009; McElroy et al., 2005).
This strong link between bipolar disorder and eating disorders may suggest a partially overlapping pathogenesis (McElroy et al., 2005), or it may imply that eating disorder comorbidity forms a specific subphenotype of bipolar disorder with a unique genetic architecture. Herein, we aimed to detect the genetic variants associated with increased risk for eating disorders in individuals with bipolar disorder through a GWA analysis.
METHODS
Subjects
Patients for this study were derived from the Bipolar Genome Study (BiGS). For genotyping as part of the BiGS, bipolar I subjects of European Ancestry were selected from those collected by the National Institute of Mental Health (NIMH) Genetics Initiative for Bipolar Disorder in five waves at 11 sites across the United States, as described elsewhere in detail (Dick et al., 2003; Smith et al., 2009). All subjects were assessed using the Diagnostic Interview for Genetic Studies (DIGS), which was combined with family informant data and medical records to arrive at best-estimate diagnoses according to DSM-III-R or DSM-IV criteria (Nurnberger et al., 1994). Control subjects were selected from those ascertained through an NIMH-supported contract mechanism between Dr. Pablo Gejman and Knowledge Networks, Inc. (Sanders et al., 2010). The selected controls were matched for gender and ethnicity (i.e., European Ancestry) with the bipolar patients, and all control subjects who endorsed a history of bipolar disorder, psychosis, or major depression on the medical questionnaire were excluded from this study.
Genotyping and Cleaning
The initial sample was genotyped at the Broad Institute as part of the as part of the Genetic Association Information Network (GAIN) using the Affymetrix 6.0 (1 M SNP) array. A total of 1,001 bipolar cases, 1,033 controls, and 724,067 SNPs were available for analysis following an extensive quality control (QC) process to eliminate subjects with ≥5% missing data and SNPs with ≥5% missing data, minor allele frequencies (MAF) <0.01, and Hardy-Weinberg Equilibrium p<10−6 (Smith et al., 2009). A second sample of 1,198 bipolar cases and 403 controls was similarly genotyped at the Translational Genomics Institute (TGEN) and underwent a comparable QC process that resulted in 728,187 SNPs available for analysis (Smith et al., 2011). An additional round of QC performed on the merged GAIN and TGEN samples resulted in 703,012 passing SNPs. Identity by state (IBS) was used to identify and remove individuals with cryptic relatedness. The genetic homogeneity of the sample was assured by multidimensional scaling (MDS). All individuals were reported to be of European ancestry, and no population outliers were detected. The final bipolar cohort (N=2190) is 58.7% female with an average age of 42.6±12.7, and the control cohort (N=1370) is 46.4% female with an average age of 52.2±17.1.
Phenotypes
Phenotypes for analysis were derived from the Phenome Database, which compiles data across the DIGS 2, 3, and 4 to arrive at a common set of variables for each subject and allow for diagnostic consistency among the different versions of the DIGS used for this sample (Potash et al., 2007). As part of the complete DIGS interview, all bipolar subjects were queried as to their eating habits and weight, and best estimate diagnoses of anorexia and bulimia nervosa were assigned. Bipolar patients were divided into the comorbid eating disorder (BD+ED) and bipolar disorder only (BDO) groups according to these diagnoses. Diagnostic crossover is very common within and among eating disorder diagnoses. Studies suggest that 20% to 50% of patients with a diagnosis of anorexia will develop bulimia over time (Eckert et al., 1995), 10% to 20% of those with bulimia will develop anorexia (Tozzi et al., 2005), and 62% of those diagnosed with the restricting-type of anorexia will switch to the binge-eating/purging-type (Eddy et al., 2002). The prevalence of these transitions suggests that anorexia and bulimia share genetic risk factors (Kendler et al., 1991; Schweiger and Fichter, 1997). Anorexia and bulimia are also cross transmitted in families, along with subthreshold forms of eating disorders, providing further evidence to suggest that these disorders are transmitted as a continuum of liability (Lilenfeld et al., 1998; Strober et al., 2000). We have thus combined anorexia and bulimia nervosa, as well as their subtypes, for an evaluation of the genetic determinants of eating disorder comorbidity within bipolar disorder.
The Childhood Life Event Scale (CLES) was administered at the time of interview for a portion of the cohort to document various traumatic events that may occur between the ages of 3–12 using a 12-point scale across 9 events. We rated this instrument as a cumulative total score and further evaluated subjects experiencing ≥2 events, per established methods (Anand et al., 2015). We also separately evaluated trauma in three categories: 1) death of a parent or sibling, having to leave home unexpectedly, and other serious life changing events; 2) beginning of a chronic illness, extended hospitalization (≥1 month), and permanent injury or disability; and 3) physical abuse and violent life threatening experiences. We note that some responses for the second category are confounded by the onset of mood symptoms in this cohort. The mean total CLES score across the 1,396 bipolar patients with valid data, including 131 patients with eating disorder comorbidity, was 1.4 ± 1.6 (range 0–10).
Statistical Analyses
An initial analysis comparing patients with comorbid eating disorders (BD+ED, N=184) to the healthy controls (CTL, N=1370) was performed to identify genetic factors that are unique to this subtype. In a complementary case-only analysis, the comorbid group was compared to the non-comorbid bipolar only group (BDO, N=2006) to identify genetic factors that may modify the expression of eating disorders within the context of bipolar disorder. These results were contrasted with those from an analysis comparing the non-comorbid group with controls to evaluate the specificity of the resultant associations for eating disorder comorbidity. Female sex-specific analyses for the 3q36.33 region included 158 BD+ED, 1127 BDO, and 636 CTL subjects. All association analyses were performed using logistic regression in PLINK (Purcell et al., 2007). As genomic inflation factors for the analyses ranged from 1.00 to 1.016, no correction for population stratification was deemed necessary. Label-switching permutations were performed to assess the empirical significance and stability of the results.
RESULTS
All bipolar patients were first evaluated for a variety of clinical characteristics related to binging and purging behaviors and patterns of dieting, exercise, and weight loss. Among the 2190 total bipolar patients, 272 (12.4%) had intentionally lost a lot of weight, and 320 (14.6%) had frequent eating binges as often as twice a week for at least three months. However, only 184 (8.4%) met full diagnostic criteria for an eating disorder, which included 66 (3%) with anorexia nervosa, 109 (5%) with bulimia nervosa, and 9 (0.4%) with eating disorder not otherwise specified. Among those with comorbid eating disorders, 15.8% were restricting type anorexia, 20.1% were binge eating/purging type anorexia, 39.7% were purging type bulimia, and 19.6% were non-purging type bulimia. As data suggests that anorexia and bulimia nervosa share genetic risk factors and exist on a continuum of liability (Kaye, 2008), we combined these diagnoses and their subtypes for an assessment of eating disorder comorbidity in bipolar disorder.
Given the evidence suggesting that eating disorder comorbidity may represent a marker for increased symptom load and illness severity, additional clinical characteristics of bipolar patients with and without eating disorder comorbidity were examined and are summarized in Table 1. Patients from the comorbid eating disorders (BD+ED) group had a significantly earlier age at onset of bipolar disorder than those from the non-comorbid bipolar only (BDO) group (14.4 vs. 19.3, p<0.001) and were much more likely to be female (85.9% vs. 56.2%, p<0.001). Rapid cycling was also present at a significantly elevated rate in the BD+ED group (71.7% vs. 55.0%, p<0.001). Furthermore, a significantly higher proportion of patients from the BD+ED group had experienced suicidal ideation (85.9% vs. 69.4%, p<0.001) and had attempted suicide (62.5% vs. 43.2%, respectively, p<0.001) than those without this comorbidity. While alcohol abuse was only moderately elevated in the BD+ED group (54.9% vs. 47.0%, p=0.040), significantly elevated comorbidity rates of panic disorder, agoraphobia, social phobia, and obsessive-compulsive disorder (OCD) were observed for the BD+ED group, as compared with the BDO group (p≤0.001). Overall, anxiety spectrum disorders were found to co-occur with eating disorders in bipolar patients at a rate of 62.5% compared with a rate of 36.4% in the absence of eating disorders (p<0.001). Finally, patients of the BD+ED group appeared to have experienced more traumatic life events during their early childhood years, particularly events related to physical abuse or violence (p<0.05).
Table 1.
Clinical characteristics of bipolar patients with and without comorbid eating disorders.
| BD comorbid ED (N=184) | BD only (N=2006) | Statistics | |
|---|---|---|---|
| Female Gender | 158 (85.9%) | 1127 (56.2%) | χ2(1)=61.3, p<0.001 |
| Age at Onset of Bipolar Disorder | 14.4 ± 6.6 | 19.3 ± 9.5 | t=9.0, p<0.001 |
| Global Assessment of Functioning | 61.7 ± 14.6 | 64.0 ± 16.0 | t=1.8, p=0.072 |
|
| |||
| Panic Disorder | 75 (40.8%) | 481 (24.0%) | χ2(1)=25.1, p<0.001 |
| Agoraphobia | 47 (25.5%) | 280 (14.0%) | χ2(1)=17.8, p<0.001 |
| Social Phobia | 38 (20.7%) | 240 (11.9%) | χ2(1)=11.5, p=0.001 |
| Obsessive-Compulsive Disorder (OCD) | 50 (27.2%) | 190 (9.5%) | χ2(1)=54.1, p<0.001 |
| Alcohol Abuse | 101 (54.9%) | 943 (47.0%) | χ2(1)=4.2, p=0.040 |
| Substance Abuse | 29 (15.8%) | 328 (16.4%) | χ2(1)=0.04, p=0.836 |
| Rapid Cycling | 132 (71.7%) | 1104 (55.0%) | χ2(1)=15.9, p<0.001 |
| Suicidal Ideation | 158 (85.9%) | 1393 (69.4%) | χ2(1)=16.8, p<0.001 |
| Suicide Attempt | 115 (62.5%) | 871 (43.2%) | χ2(1)=24.7, p<0.001 |
|
| |||
| Number of Traumatic Events in Childhood a | 1.7 ± 1.6 | 1.4 ± 1.6 | t=−2.1, p=0.040 |
| Experience of ≥2 Traumatic Events in Childhood b | 62 (47.3%) | 473 (37.4%) | χ2(1)=5.1, p=0.023 |
| Experience of a Life Changing Event in Childhood c | 61 (46.6%) | 472 (37.3%) | χ2(1)=4.3, p=0.038 |
| Experience of a Chronic Illness, Hospitalization, or Injury in Childhood d | 25 (19.1%) | 217 (17.2%) | χ2(1)=0.3, p=0.579 |
| Experience of Physical Abuse or Violence in Childhood e | 34 (26.0%) | 222 (17.5%) | χ2(1)=5.6, p=0.018 |
Total number of traumatic events experienced between the ages of 3 and 12, as defined by the Childhood Life Events Scale (CLES). Note that 131 subjects with BD+ED and 1265 subjects with BD only have CLES data.
Experience of 2 or more traumatic events across the entire CLES.
Experience of trauma related to the death of a parent or sibling, leaving home unexpectedly, and other serious life changing events.
Experience of trauma related to the beginning of a chronic illness, extended hospitalization (≥1 month), or permanent injury or disability. Note that this category is confounded by the report of onset of mood symptoms for many patients.
Experience of trauma related to physical abuse or violent life threatening experiences.
The results of the clinical comparisons suggest that eating disorder comorbidity may form a specific subphenotype of bipolar disorder. In order to differentiate genetic factors that are unique to the comorbid eating disorder subtype from those that modify the expression of eating disorders within bipolar disorder, the comorbid group (BD+ED) was compared to the healthy controls (CTL) and to the bipolar only group (BDO), respectively. These results were then contrasted with the analysis of BDO vs. CTL to evaluate specificity for eating disorder comorbidity. The results of the genome-wide analyses are displayed in Figure 1. Genomic regions of interest were defined as those with at least two SNPs with p<10−4 with additional support for association (i.e., p<10−3) provided by surrounding SNPs within 100kb. A comprehensive list of regions meeting these criteria is provided in Supplementary Table S1 with a comparison of the statistics from all analyses.
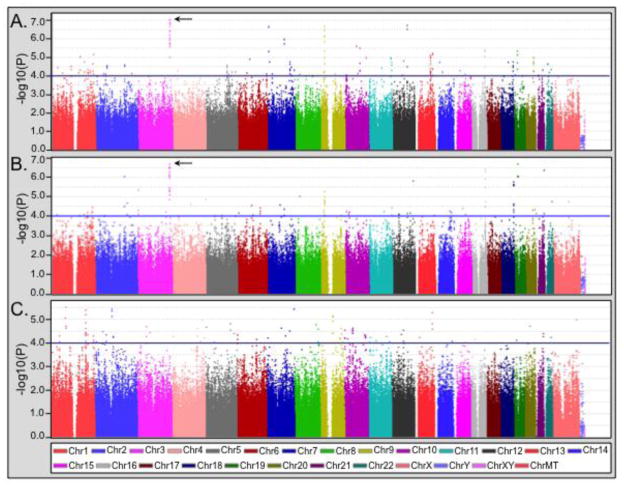
Figure 1.
Results of the genome-wide association analysis of bipolar with comorbid eating disorder (BD+ED) vs. healthy controls (CTL) and the noncomorbid bipolar only group (BDO). (A) Analysis of BD+ED (N=184) vs. CTL (N=1370). (B) Case-only analysis of BD+ED vs. BDO (N=2006). (C) The analysis of BDO vs. CTL is provided for comparison. In each panel, a blue line indicates p<10−4, and an arrow indicates the 3q26.33 region.
The most significant genome-wide finding was observed for rs4854912 within the SOX2-OT region (p=8.9×10−8, odds ratio (OR)=1.9) on chromosome 3q26.33. While the overall best result was obtained for the BD+ED vs. CTL analysis, this region also produced the best finding in the BD+ED vs. BDO case-only analysis for a neighboring SNP (rs13086738, p=2.1×10−7, OR=1.8). Further support for this region derived from 13 SNPs in linkage disequilibrium that were associated in both analyses with p values <10−5 (see Table S1). These SNPs spanned 235kb from SOX2-OT and extended into FXR1 (see Figure 2). Since the associated SNPs display an extremely high degree of linkage disequilibrium, we performed analyses conditional on rs4854912 and rs13086738 as appropriate. No other SNP in the 3q26.33 region remained significant, suggesting that this is a single association signal. Given the significantly larger proportion of females in the BD+ED group compared to the BDO and CTL groups, we repeated the analysis of this region both covarying for gender and in females only. The results remained similar, albeit slightly weaker, when gender was included as a covariate in the model for the BD+ED vs. CTL (rs4854912, p=1.6×10−6, OR=1.9) and BD+ED vs. BDO comparisons (rs13086738, p=1.7×10−6, OR=1.8). Still, the same 13 SNPs displayed evidence of association with p values<10−4. The gender stratified analyses further confirmed that the size and direction of effect were consistent between females only and the complete sample for the BD+ED vs. CTL (rs4854912, p=4.3×10−5, OR=1.8) and BD+ED vs. BDO comparisons (rs13086738, p=4.3×10−5, OR=1.7), despite substantially reduced sample sizes.
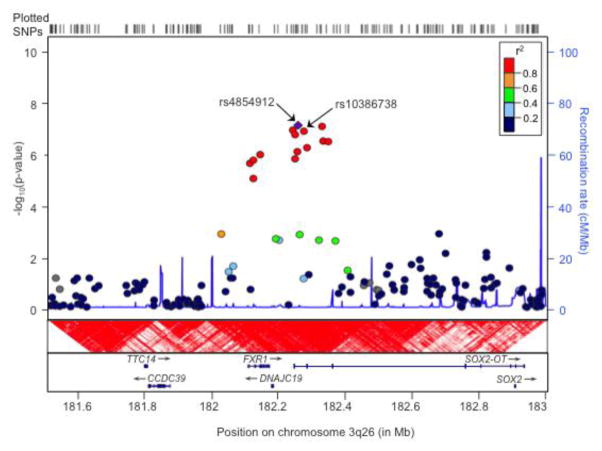
Figure 2.
Details of the 3q26 region from LocusZoom (Pruim et al., 2010), displaying the chromosomal context, linkage disequilibrium structure, and patterns of recombination surrounding the peak SNP, rs4854912, which is shown as a purple diamond. All locations are based on the hg 18 assembly, and linkage disequilibrium patterns across the region are shown according to the CEPH reference population from the HapMap release 22 with red indicating complete disequilibrium (D′=1). The association results shown correspond to the BD+ED vs. CTL analysis (see Figure 1A), with a peak p value of 8.9×10−8 observed for rs4854912. Nearby genes SOX2-OT and FXR1 are also shown. The BD+ED vs. BDO analysis produced a very similar regional association plot, and the peak SNP from that analysis, rs10386738, is also indicated.
Several other genomic regions of interest were also detected in these analyses (see Table S1 and Figure S1). Association to NALCN on chromosome 13q33.1 was observed most prominently for the BD+ED vs. CTL comparison (peak p=6.0×10−6, OR=1.7 for rs9554752), and the region of significance extended into the adjacent NALCN-AS1 gene, which encodes the antisense RNA of NALCN, with p values <10−4. Other genes of interest that were most prominent in the BD+ED vs. CTL analysis included NRF1 on 7q32.2, NRG3 on 10q23.1, and ADNP on 20q13.13. The ABCG1 gene on chromosome 21q22.3 was most prominent in the BD+ED vs. BDO analysis (peak p=4.3×10−7). Genes of interest in the analyses of BD+ED vs. BDO and vs. CTL included CADM3 on chromosome 1q23.2, ATP2B4 on 1q32.1, and RYR2 on 1q43.
DISCUSSION
Bipolar disorder shows significant clinical phenotypic heterogeneity, which may reflect differences in the underlying genetic architecture. The use of clinical features to refine the diagnosis and reduce the phenotypic heterogeneity may provide additional power to detect genetic risk variants (Manchia et al., 2013). Based on prior evidence suggesting a partially overlapping pathogenesis, we investigated bipolar disorder with eating disorder comorbidity through GWA analyses towards the identification of variants contributing either uniquely to this subphenotype or to risk for both bipolar and eating disorders (McElroy et al., 2005).
The lifetime prevalence rates of 3% for anorexia nervosa and 5% for bulimia nervosa observed in our sample are consistent with prior estimates of these eating disorders in bipolar disorder and are significantly elevated in comparison with the rates of 0.3–0.7% and 1.5–2.5% observed for these disorders, respectively, in the general population (Kaye, 2008; McElroy et al., 2011). Consistent with the findings of others, presence of a lifetime comorbid eating disorder in our study was associated with female gender, an earlier age at onset of bipolar disorder, and a history of rapid cycling and suicide, all of which predict a more severe course of illness (Brietzke et al., 2011; McElroy et al., 2011). While anxiety disorders often show comorbidity with bipolar disorder (McElroy et al., 2001), we find a dramatic increase in the comorbidity of panic disorder, OCD, and social phobia in bipolar patients with eating disorder comorbidity, consistent with prior observations (Jen et al., 2013). However, this may not be unexpected given the relationship between anxiety and eating disorders aside from the context of bipolar disorder (Kaye, 2008). Finally, childhood trauma has been implicated as a precursor to both bipolar disorder and eating disorders, with a particular emphasis on physical abuse as a trigger (Jen et al., 2013; Leverich et al., 2002; Post and Leverich, 2006; Rayworth et al., 2004; Rodriguez et al., 2005). Our data provides further evidence to support this connection, with an increased incidence of traumatic events in childhood for bipolar patients with eating disorder comorbidity, an effect that seems to be driven primarily by experiences of physical abuse and violence. Further evaluation of the interaction of trauma exposure as an early environmental risk factor and particular genetic risk factors will be necessary to understand the relationship between trauma, bipolar disorder, and eating disorder comorbidity.
The most significant genome-wide finding was observed for the SOX2-OT/FXR1 region on chromosome 3q26.33 in the BD+ED vs. CTL analysis with a peak p value of 8.9×10−8. This region also featured prominently the BD+ED vs. BDO comparison with a peak p value of 2.1×10−7. For both analyses, a total of 13 SNPs in high linkage disequilibrium spanning 235kb provided support for association with p values <10−5. The chromosome 3q26-27 region has previously been implicated in linkage studies of bipolar disorder (Badenhop et al., 2002; Kelsoe et al., 2001). A recent large GWA study of 2,907 cases and 14,860 controls also reported a significant association between SOX2-OT and anorexia nervosa (peak p=3.0×10−7, OR=1.2 for rs9839776) (Boraska et al., 2014). Although located within the same large gene, rs9839776 is approximately 500kb from the associated region in our analyses. Still, given the exclusive association of SOX2-OT with the BD+ED group in our study, these combined results may either suggest a role for this gene in eating disorders or provide evidence for a commonality of genes underlying bipolar disorder and eating disorders. Interestingly, a recent very large study of schizophrenia produced two genome-wide significant hits to the 3q26.33 region, one for an insertion/deletion polymorphism in FXR1 (OR=0.91, p=1.3×10–11) and another for a SNP in SOX2-OT (rs9841616, OR=0.92, p=1.65×10–8), with many SNPs providing additional support for association (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). This region thus warrants further investigation as harboring one or more genes common to the etiology of several psychiatric disorders.
SOX2-OT encodes for the SRY-box containing gene 2 (SOX2) overlapping transcript and was identified as a spliced, long non-protein-coding RNA (lncRNA) with an intron overlapping the SOX2 gene in the same transcriptional orientation. SOX2-OT may regulate the expression of SOX2, the product of which plays a key role in both embryonic and adult neurogenesis (Amaral et al., 2009). SOX2-OT also appears to represent a biomarker for the early and late stages of neurodegeneration (Arisi et al., 2011), and an isoform of SOX2-OT transcribed from a distal, highly conserved element located within the region of our association peak is exclusively expressed in the brain with enrichment in regions of adult neurogenesis (Amaral et al., 2009). FXR1 is an autosomal homolog of Fragile X mental retardation-related protein 1 (FMR1), which is responsible for the Fragile-X syndrome in humans (Ashley et al., 1993; Siomi et al., 1993; Siomi et al., 1995). During embryonic development, the expression of FXR1 is restricted to early stages of proliferation and differentiation in regions of the central nervous system, suggesting that FXR1 may play an important role in nerve cell proliferation and early brain development (Coy et al., 1995).
Several other genomic regions with p values ranging from 10−5 to 10−7 were identified in the BD+ED vs. CTL analysis, which may suggest variants that are unique to a comorbid eating disorder subtype of BD. These included NALCN and its antisense RNA on chromosome 13q32-33. NALCN encodes a nonselective sodium leak channel that plays an important role in the regulation of neuronal excitability (Cahoy et al., 2008; Lu et al., 2007). Suggestive evidence for linkage to bipolar disorder has been observed for chromosome 13q31-34 (Kelsoe et al., 2001; Potash et al., 2003; Shaw et al., 2003), and GWA further implicates NALCN in bipolar disorder (Ollila et al., 2009; Wang et al., 2010). Several of the identified genes are involved in the regulation of neurodevelopment and neuroplasticity. NRF1 on chromosome 7q32.2 encodes nuclear respiratory factor 1 and has been shown to regulate neurite outgrowth (Wang et al., 2009; Wang et al., 2013). NRG3 (neuregulin 3) on chromosome 10q23.1 plays a critical role in the development of the embryonic cerebral cortex (Meier et al., 2013), and variants of this gene are associated with cognitive deficits in bipolar disorder and schizophrenia (Meier et al., 2013). ADNP on chromosome 20q13.13 encodes an activity-dependent neuroprotective protein that appears essential for neuronal differentiation, neurodevelopment, and neuroprotection, with abundant expression in hippocampus, hypothalamus, cerebral cortex, and cerebellum (Aboonq et al., 2012; Oz et al., 2014; Pinhasov et al., 2003; Yang et al., 2012). Finally, several genes spanning chromosome 1q23-43 are involved in the regulation of calcium homeostasis and neurodevelopment, including CADM3 (cell adhesion molecule 3 isoform 1), ATP2B4 (plasma membrane calcium ATPase isoform 4), and RYR2 (ryanodine receptor 2) (Galeotti et al., 2008; Kakunaga et al., 2005; Tempel and Shilling, 2007; Zalk et al., 2007). Calcium is a ubiquitous signaling molecule that plays a crucial role in the regulation of neurotransmitter release, synaptic plasticity, neurite outgrowth, and neurodegeneration (Berridge, 1998; Ciccolini et al., 2003), and several studies have suggested an altered calcium homeostasis in the pathophysiology of bipolar disorder (Emamghoreishi et al., 2000; Yoon et al., 2001).
The case only analysis, which was intended to identify genes modifying the expression of eating disorder comorbidity in bipolar disorder, implicated ABCG1 on chromosome 21q22. This region has previously been implicated in genetic linkage studies of bipolar disorder (Aita et al., 1999; Kaneva et al., 2004; Straub et al., 1994), and ABCG1 has been suggested as both a positional and functional candidate gene for bipolar disorder (Kirov et al., 2001).. ABCG1 encodes the ATP-binding cassette sub-family G member 1, a transporter protein involved in the cellular uptake of tryptophan, the precursor for serotonin, which is involved in the pathophysiology of mood disorders. Serotonin is also a key regulator of eating behavior, and genetic variants contributing to serotonergic dysfunction impact risk for eating disorders (Kaye, 2008; Lucki, 1998).
The regions of interest in the present study did not include genes identified by previous GWA studies of bipolar disorder, such as ADCY2, ANK3, CACNA1C, NCAN, ODZ4, and TRANK1 (Cichon et al., 2011; Ferreira et al., 2008; Green et al., 2013; Chen et al., 2013; Muhleisen et al., 2014; Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011). These regions also did not include genes that have been reported to be associated with anorexia nervosa, such as CTNNA2, CNTNAP2, EPHX2, GABRG1, HTR1D, OPRD1, and PPP3CA (Bloss et al., 2011; Boraska et al., 2014; Scott-Van Zeeland et al., 2014; Wang et al., 2011). This, in combination with the identification of genes in the BD+ED analyses that were not significant in the BDO vs. CTL analysis, suggests that bipolar disorder with eating disorder comorbidity may represent a unique clinical phenotype that is distinct from both bipolar disorder and eating disorders. Our analyses also appear to broadly support the involvement genes related to neurodevelopment and neuroprotective mechanisms in pathophysiology of bipolar disorder and the modification of the expression of this clinical subtype (Harwood, 2003; Rowe and Chuang, 2004; Sanches et al., 2008; Soeiro-de-Souza et al., 2012).
There are limitations to the present study related primarily to sample size. The BD+ED group was relatively small with 184 subjects, although the comparison groups were substantially larger. Still, we may lack sufficient power to detect smaller genetic effects with confidence. The estimation of p values may also be distorted in smaller samples, although the permutation analyses confirmed the stability of our results and provided comparable estimates of association. Additionally, the use of all available subjects from the GAIN and TGEN cohorts combined to increase the sample size of the BD+ED group precluded a replication effort. However, we view our study as a replication and confirmation of the recent findings of Boraska and colleagues (Boraska et al., 2014), which implicated SOX2-OT in a GWA of anorexia nervosa with a similar p value (3.01×10−7) in a much larger cohort. Given that our p values were comparable to those of Boraska and that the direction of effect was the same for our BD+ED analyses as for anorexia vs. controls, we feel our study substantially strengthens the confidence of the involvement of SOX2-OT in the etiology of eating disorders. Finally, it must be noted that a prior study of the GAIN portion of this sample by Winham and colleagues reported an association for the APOB gene with binge eating behavior in bipolar disorder that we were unable to confirm (Winham et al., 2014). This discrepancy is likely due to several factors. First, we have added the TGEN cohort, nearly doubling the overall sample size, although possibly introducing genetic heterogeneity due to differences in ascertainment methods between the bipolar cohorts (Greenwood and Kelsoe, 2013). Second, we relied on a more stringent definition of eating disorders, requiring a confirmed diagnosis for categorization as comorbidity, whereas the Winham study categorized subjects according to a broader binge eating phenotype via a single DIGS question (Winham et al., 2014). As an example, our study identified only 60 patients with BD+ED from the GAIN cohort with the remaining 124 derived from TGEN, whereas the Winham study identified 206 bipolar patients with binge eating behavior in the GAIN cohort alone. Lastly, we used a combined eating disorder diagnosis that included both anorexia and bulimia to increase the sample size, which may have introduced genetic heterogeneity and divergence from the prior study. We thus expect different results from these studies employing alternative phenotype definitions in overlapping yet distinct cohorts.
In summary, we confirmed the association of a recently identified candidate gene for anorexia nervosa in bipolar patients with eating disorder comorbidity. We also identified several genomic regions of interest containing genes involved in neurodevelopment and neuroprotection processes that may be relevant to the specific pathophysiology of eating disorder comorbidity in bipolar disorder or may represent part of the shared pathophysiology underlying both bipolar disorder and eating disorders. Although these findings require confirmation in larger datasets, they support the concept that different clinical manifestations of bipolar disorder may reflect differences in the underlying genetic architecture.
Supplementary Material
HIGHLIGHTS.
Genome-wide association of eating disorders within bipolar disorder was evaluated.
These results replicate the prior association of SOX2-OT with eating disorders.
Genes involved in neurodevelopment and neuroprotection were also identified.
Clinical subtypes of bipolar disorder may reflect distinct genetic contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboonq MS, Vasiliou SA, Haddley K, Quinn JP, Bubb VJ. Activity-dependent neuroprotective protein modulates its own gene expression. Journal of molecular neuroscience : MN. 2012;46:33–39. doi: 10.1007/s12031-011-9562-y. [DOI] [PubMed] [Google Scholar]
- Aita VM, Liu J, Knowles JA, Terwilliger JD, Baltazar R, Grunn A, Loth JE, Kanyas K, Lerer B, Endicott J, Wang Z, Penchaszadeh G, Gilliam TC, Baron M. A comprehensive linkage analysis of chromosome 21q22 supports prior evidence for a putative bipolar affective disorder locus. Am J Hum Genet. 1999;64:210–217. doi: 10.1086/302185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alda M. The phenotypic spectra of bipolar disorder. Eur Neuropsychopharmacol. 2004;14(Suppl 2):S94–99. doi: 10.1016/j.euroneuro.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Alda M, Hajek T, Calkin C, O’Donovan C. Treatment of bipolar disorder: new perspectives. Ann Med. 2009;41:186–196. doi: 10.1080/07853890802409489. [DOI] [PubMed] [Google Scholar]
- Amaral PP, Neyt C, Wilkins SJ, Askarian-Amiri ME, Sunkin SM, Perkins AC, Mattick JS. Complex architecture and regulated expression of the Sox2ot locus during vertebrate development. Rna. 2009;15:2013–2027. doi: 10.1261/rna.1705309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Koller DL, Lawson WB, Gershon ES, Nurnberger JI BiGS Collaborative. Genetic and childhood trauma interaction effect on age of onset in bipolar disorder: An exploratory analysis. J Affect Disord. 2015;179:1–5. doi: 10.1016/j.jad.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arisi I, D’Onofrio M, Brandi R, Felsani A, Capsoni S, Drovandi G, Felici G, Weitschek E, Bertolazzi P, Cattaneo A. Gene expression biomarkers in the brain of a mouse model for Alzheimer’s disease: mining of microarray data by logic classification and feature selection. J Alzheimers Dis. 2011;24:721–738. doi: 10.3233/JAD-2011-101881. [DOI] [PubMed] [Google Scholar]
- Ashley CT, Jr, Wilkinson KD, Reines D, Warren ST. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993;262:563–566. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- Badenhop RF, Moses MJ, Scimone A, Mitchell PB, Ewen-White KR, Rosso A, Donald JA, Adams LJ, Schofield PR. A genome screen of 13 bipolar affective disorder pedigrees provides evidence for susceptibility loci on chromosome 3 as well as chromosomes 9, 13 and 19. Mol Psychiatry. 2002;7:594–603. doi: 10.1038/sj.mp.4001025. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Bloss CS, Berrettini W, Bergen AW, Magistretti P, Duvvuri V, Strober M, Brandt H, Crawford S, Crow S, Fichter MM, Halmi KA, Johnson C, Kaplan AS, Keel P, Klump KL, Mitchell J, Treasure J, Woodside DB, Marzola E, Schork NJ, Kaye WH. Genetic association of recovery from eating disorders: the role of GABA receptor SNPs. Neuropsychopharmacology. 2011;36:2222–2232. doi: 10.1038/npp.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraska V, Franklin CS, Floyd JA, Thornton LM, Huckins LM, Southam L, Rayner NW, Tachmazidou I, Klump KL, Treasure J, Lewis CM, Schmidt U, Tozzi F, Kiezebrink K, Hebebrand J, Gorwood P, Adan RA, Kas MJ, Favaro A, Santonastaso P, Fernandez-Aranda F, Gratacos M, Rybakowski F, Dmitrzak-Weglarz M, Kaprio J, Keski-Rahkonen A, Raevuori A, Van Furth EF, Slof-Op ‘t Landt MC, Hudson JI, Reichborn-Kjennerud T, Knudsen GP, Monteleone P, Kaplan AS, Karwautz A, Hakonarson H, Berrettini WH, Guo Y, Li D, Schork NJ, Komaki G, Ando T, Inoko H, Esko T, Fischer K, Mannik K, Metspalu A, Baker JH, Cone RD, Dackor J, DeSocio JE, Hilliard CE, O’Toole JK, Pantel J, Szatkiewicz JP, Taico C, Zerwas S, Trace SE, Davis OS, Helder S, Buhren K, Burghardt R, de Zwaan M, Egberts K, Ehrlich S, Herpertz-Dahlmann B, Herzog W, Imgart H, Scherag A, Scherag S, Zipfel S, Boni C, Ramoz N, Versini A, Brandys MK, Danner UN, de Kovel C, Hendriks J, Koeleman BP, Ophoff RA, Strengman E, van Elburg AA, Bruson A, Clementi M, Degortes D, Forzan M, Tenconi E, Docampo E, Escaramis G, Jimenez-Murcia S, Lissowska J, Rajewski A, Szeszenia-Dabrowska N, Slopien A, Hauser J, Karhunen L, Meulenbelt I, Slagboom PE, Tortorella A, Maj M, Dedoussis G, Dikeos D, Gonidakis F, Tziouvas K, Tsitsika A, Papezova H, Slachtova L, Martaskova D, Kennedy JL, Levitan RD, Yilmaz Z, Huemer J, Koubek D, Merl E, Wagner G, Lichtenstein P, Breen G, Cohen-Woods S, Farmer A, McGuffin P, Cichon S, Giegling I, Herms S, Rujescu D, Schreiber S, Wichmann HE, Dina C, Sladek R, Gambaro G, Soranzo N, Julia A, Marsal S, Rabionet R, Gaborieau V, Dick DM, Palotie A, Ripatti S, Widen E, Andreassen OA, Espeseth T, Lundervold A, Reinvang I, Steen VM, Le Hellard S, Mattingsdal M, Ntalla I, Bencko V, Foretova L, Janout V, Navratilova M, Gallinger S, Pinto D, Scherer SW, Aschauer H, Carlberg L, Schosser A, Alfredsson L, Ding B, Klareskog L, Padyukov L, Courtet P, Guillaume S, Jaussent I, Finan C, Kalsi G, Roberts M, Logan DW, Peltonen L, Ritchie GR, Barrett JC, Estivill X, Hinney A, Sullivan PF, Collier DA, Zeggini E, Bulik CM Wellcome Trust Case Control C. A genome-wide association study of anorexia nervosa. Mol Psychiatry. 2014;19:1085–1094. doi: 10.1038/mp.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brietzke E, Moreira CL, Toniolo RA, Lafer B. Clinical correlates of eating disorder comorbidity in women with bipolar disorder type I. J Affect Disord. 2011;130:162–165. doi: 10.1016/j.jad.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DT, Jiang X, Akula N, Shugart YY, Wendland JR, Steele CJ, Kassem L, Park JH, Chatterjee N, Jamain S, Cheng A, Leboyer M, Muglia P, Schulze TG, Cichon S, Nothen MM, Rietschel M, BiGs, McMahon FJ. Genome-wide association study meta-analysis of European and Asian-ancestry samples identifies three novel loci associated with bipolar disorder. Mol Psychiatry. 2013;18:195–205. doi: 10.1038/mp.2011.157. [DOI] [PubMed] [Google Scholar]
- Ciccolini F, Collins TJ, Sudhoelter J, Lipp P, Berridge MJ, Bootman MD. Local and global spontaneous calcium events regulate neurite outgrowth and onset of GABAergic phenotype during neural precursor differentiation. J Neurosci. 2003;23:103–111. doi: 10.1523/JNEUROSCI.23-01-00103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichon S, Muhleisen TW, Degenhardt FA, Mattheisen M, Miro X, Strohmaier J, Steffens M, Meesters C, Herms S, Weingarten M, Priebe L, Haenisch B, Alexander M, Vollmer J, Breuer R, Schmal C, Tessmann P, Moebus S, Wichmann HE, Schreiber S, Muller-Myhsok B, Lucae S, Jamain S, Leboyer M, Bellivier F, Etain B, Henry C, Kahn JP, Heath S, Hamshere M, O’Donovan MC, Owen MJ, Craddock N, Schwarz M, Vedder H, Kammerer-Ciernioch J, Reif A, Sasse J, Bauer M, Hautzinger M, Wright A, Mitchell PB, Schofield PR, Montgomery GW, Medland SE, Gordon SD, Martin NG, Gustafsson O, Andreassen O, Djurovic S, Sigurdsson E, Steinberg S, Stefansson H, Stefansson K, Kapur-Pojskic L, Oruc L, Rivas F, Mayoral F, Chuchalin A, Babadjanova G, Tiganov AS, Pantelejeva G, Abramova LI, Grigoroiu-Serbanescu M, Diaconu CC, Czerski PM, Hauser J, Zimmer A, Lathrop M, Schulze TG, Wienker TF, Schumacher J, Maier W, Propping P, Rietschel M, Nothen MM. Genome-wide association study identifies genetic variation in neurocan as a susceptibility factor for bipolar disorder. Am J Hum Genet. 2011;88:372–381. doi: 10.1016/j.ajhg.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochet-Bissuel M, Lory P, Monteil A. The sodium leak channel, NALCN, in health and disease. Frontiers in cellular neuroscience. 2014;8:132. doi: 10.3389/fncel.2014.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coy JF, Sedlacek Z, Bachner D, Hameister H, Joos S, Lichter P, Delius H, Poustka A. Highly conserved 3′ UTR and expression pattern of FXR1 points to a divergent gene regulation of FXR1 and FMR1. Hum Mol Genet. 1995;4:2209–2218. doi: 10.1093/hmg/4.12.2209. [DOI] [PubMed] [Google Scholar]
- Dick DM, Foroud T, Flury L, Bowman ES, Miller MJ, Rau NL, Moe PR, Samavedy N, El-Mallakh R, Manji H, Glitz DA, Meyer ET, Smiley C, Hahn R, Widmark C, McKinney R, Sutton L, Ballas C, Grice D, Berrettini W, Byerley W, Coryell W, DePaulo R, MacKinnon DF, Gershon ES, Kelsoe JR, McMahon FJ, McInnis M, Murphy DL, Reich T, Scheftner W, Nurnberger JI., Jr Genomewide linkage analyses of bipolar disorder: a new sample of 250 pedigrees from the national institute of mental health genetics initiative. Am J Hum Genet. 2003;73:107–114. doi: 10.1086/376562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert ED, Halmi KA, Marchi P, Grove W, Crosby R. Ten-year follow-up of anorexia nervosa: clinical course and outcome. Psychol Med. 1995;25:143–156. doi: 10.1017/s0033291700028166. [DOI] [PubMed] [Google Scholar]
- Eddy KT, Keel PK, Dorer DJ, Delinsky SS, Franko DL, Herzog DB. Longitudinal comparison of anorexia nervosa subtypes. Int J Eat Disord. 2002;31:191–201. doi: 10.1002/eat.10016. [DOI] [PubMed] [Google Scholar]
- Emamghoreishi M, Li PP, Schlichter L, Parikh SV, Cooke R, Warsh JJ. Associated disturbances in calcium homeostasis and G protein-mediated cAMP signaling in bipolar I disorder. Biol Psychiatry. 2000;48:665–673. doi: 10.1016/s0006-3223(00)00884-2. [DOI] [PubMed] [Google Scholar]
- Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, Fan J, Kirov G, Perlis RH, Green EK, Smoller JW, Grozeva D, Stone J, Nikolov I, Chambert K, Hamshere ML, Nimgaonkar VL, Moskvina V, Thase ME, Caesar S, Sachs GS, Franklin J, Gordon-Smith K, Ardlie KG, Gabriel SB, Fraser C, Blumenstiel B, Defelice M, Breen G, Gill M, Morris DW, Elkin A, Muir WJ, McGhee KA, Williamson R, MacIntyre DJ, MacLean AW, St Clair D, Robinson M, Van Beck M, Pereira AC, Kandaswamy R, McQuillin A, Collier DA, Bass NJ, Young AH, Lawrence J, Ferrier IN, Anjorin A, Farmer A, Curtis D, Scolnick EM, McGuffin P, Daly MJ, Corvin AP, Holmans PA, Blackwood DH, Gurling HM, Owen MJ, Purcell SM, Sklar P, Craddock N. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti N, Vivoli E, Bartolini A, Ghelardini C. A gene-specific cerebral types 1, 2, and 3 RyR protein knockdown induces an antidepressant-like effect in mice. J Neurochem. 2008;106:2385–2394. doi: 10.1111/j.1471-4159.2008.05581.x. [DOI] [PubMed] [Google Scholar]
- Green EK, Hamshere M, Forty L, Gordon-Smith K, Fraser C, Russell E, Grozeva D, Kirov G, Holmans P, Moran JL, Purcell S, Sklar P, Owen MJ, O’Donovan MC, Jones L, Wtccc, Jones IR, Craddock N. Replication of bipolar disorder susceptibility alleles and identification of two novel genome-wide significant associations in a new bipolar disorder case-control sample. Mol Psychiatry. 2013;18:1302–1307. doi: 10.1038/mp.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Kelsoe JR. Genome-wide association study of irritable vs. elated mania suggests genetic differences between clinical subtypes of bipolar disorder. PLoS One. 2013;8:e53804. doi: 10.1371/journal.pone.0053804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood AJ. Neurodevelopment and mood stabilizers. Current molecular medicine. 2003;3:472–482. doi: 10.2174/1566524033479672. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen A, Saunders EF, Ornstein RM, Kamali M, McInnis MG. Impulsivity, anxiety, and alcohol misuse in bipolar disorder comorbid with eating disorders. International journal of bipolar disorders. 2013;1:13. doi: 10.1186/2194-7511-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakunaga S, Ikeda W, Itoh S, Deguchi-Tawarada M, Ohtsuka T, Mizoguchi A, Takai Y. Nectin-like molecule-1/TSLL1/SynCAM3: a neural tissue-specific immunoglobulin-like cell-cell adhesion molecule localizing at non-junctional contact sites of presynaptic nerve terminals, axons and glia cell processes. Journal of cell science. 2005;118:1267–1277. doi: 10.1242/jcs.01656. [DOI] [PubMed] [Google Scholar]
- Kaneva RP, Chorbov VM, Milanova VK, Kostov CS, Nickolov KI, Chakarova CF, Stoyanova VS, Nikolova-Hill AN, Krastev SK, Onchev GN, Kremensky IM, Kalaydjieva LV, Jablensky AV. Linkage analysis in bipolar pedigrees adds support for a susceptibility locus on 21q22. Psychiatr Genet. 2004;14:101–106. doi: 10.1097/01.ypg.0000128766.92096.ad. [DOI] [PubMed] [Google Scholar]
- Kawa I, Carter JD, Joyce PR, Doughty CJ, Frampton CM, Wells JE, Walsh AE, Olds RJ. Gender differences in bipolar disorder: age of onset, course, comorbidity, and symptom presentation. Bipolar Disord. 2005;7:119–125. doi: 10.1111/j.1399-5618.2004.00180.x. [DOI] [PubMed] [Google Scholar]
- Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiology & behavior. 2008;94:121–135. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsoe JR, Spence MA, Loetscher E, Foguet M, Sadovnick AD, Remick RA, Flodman P, Khristich J, Mroczkowski-Parker Z, Brown JL, Masser D, Ungerleider S, Rapaport MH, Wishart WL, Luebbert H. A genome survey indicates a possible susceptibility locus for bipolar disorder on chromosome 22. Proc Natl Acad Sci U S A. 2001;98:585–590. doi: 10.1073/pnas.011358498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, MacLean C, Neale M, Kessler R, Heath A, Eaves L. The genetic epidemiology of bulimia nervosa. Am J Psychiatry. 1991;148:1627–1637. doi: 10.1176/ajp.148.12.1627. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, Partonen T, Haukka J, Kaprio J, Lonnqvist J. High concordance of bipolar I disorder in a nationwide sample of twins. Am J Psychiatry. 2004;161:1814–1821. doi: 10.1176/ajp.161.10.1814. [DOI] [PubMed] [Google Scholar]
- Kirov G, Lowry CA, Stephens M, Oldfield S, O’Donovan MC, Lightman SL, Owen MJ. Screening ABCG1, the human homologue of the Drosophila white gene, for polymorphisms and association with bipolar affective disorder. Mol Psychiatry. 2001;6:671–677. doi: 10.1038/sj.mp.4000899. [DOI] [PubMed] [Google Scholar]
- Kruger S, Shugar G, Cooke RG. Comorbidity of binge eating disorder and the partial binge eating syndrome with bipolar disorder. Int J Eat Disord. 1996;19:45–52. doi: 10.1002/(SICI)1098-108X(199601)19:1<45::AID-EAT6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Leverich GS, McElroy SL, Suppes T, Keck PE, Jr, Denicoff KD, Nolen WA, Altshuler LL, Rush AJ, Kupka R, Frye MA, Autio KA, Post RM. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51:288–297. doi: 10.1016/s0006-3223(01)01239-2. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilenfeld LR, Kaye WH, Greeno CG, Merikangas KR, Plotnicov K, Pollice C, Rao R, Strober M, Bulik CM, Nagy L. A controlled family study of anorexia nervosa and bulimia nervosa: psychiatric disorders in first-degree relatives and effects of proband comorbidity. Arch Gen Psychiatry. 1998;55:603–610. doi: 10.1001/archpsyc.55.7.603. [DOI] [PubMed] [Google Scholar]
- Lu B, Su Y, Das S, Liu J, Xia J, Ren D. The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm. Cell. 2007;129:371–383. doi: 10.1016/j.cell.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Lunde AV, Fasmer OB, Akiskal KK, Akiskal HS, Oedegaard KJ. The relationship of bulimia and anorexia nervosa with bipolar disorder and its temperamental foundations. J Affect Disord. 2009;115:309–314. doi: 10.1016/j.jad.2008.10.012. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Hajek T, Alda M. The phenotypes of bipolar disorder: relevance for genetic investigations. Mol Psychiatry. 2005;10:811–826. doi: 10.1038/sj.mp.4001701. [DOI] [PubMed] [Google Scholar]
- Manchia M, Cullis J, Turecki G, Rouleau GA, Uher R, Alda M. The impact of phenotypic and genetic heterogeneity on results of genome wide association studies of complex diseases. PLoS One. 2013;8:e76295. doi: 10.1371/journal.pone.0076295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SL, Altshuler LL, Suppes T, Keck PE, Jr, Frye MA, Denicoff KD, Nolen WA, Kupka RW, Leverich GS, Rochussen JR, Rush AJ, Post RM. Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 patients with bipolar disorder. Am J Psychiatry. 2001;158:420–426. doi: 10.1176/appi.ajp.158.3.420. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Crow S, Biernacka JM, Winham S, Geske J, Cuellar Barboza AB, Prieto ML, Chauhan M, Seymour LR, Mori N, Frye MA. Clinical phenotype of bipolar disorder with comorbid binge eating disorder. J Affect Disord. 2013;150:981–986. doi: 10.1016/j.jad.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SL, Frye MA, Hellemann G, Altshuler L, Leverich GS, Suppes T, Keck PE, Nolen WA, Kupka R, Post RM. Prevalence and correlates of eating disorders in 875 patients with bipolar disorder. J Affect Disord. 2011;128:191–198. doi: 10.1016/j.jad.2010.06.037. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Kotwal R, Keck PE., Jr Comorbidity of eating disorders with bipolar disorder and treatment implications. Bipolar Disord. 2006;8:686–695. doi: 10.1111/j.1399-5618.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Kotwal R, Keck PE, Jr, Akiskal HS. Comorbidity of bipolar and eating disorders: distinct or related disorders with shared dysregulations? J Affect Disord. 2005;86:107–127. doi: 10.1016/j.jad.2004.11.008. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- Meier S, Strohmaier J, Breuer R, Mattheisen M, Degenhardt F, Muhleisen TW, Schulze TG, Nothen MM, Cichon S, Rietschel M, Wust S. Neuregulin 3 is associated with attention deficits in schizophrenia and bipolar disorder. Int J Neuropsychopharmacol. 2013;16:549–556. doi: 10.1017/S1461145712000697. [DOI] [PubMed] [Google Scholar]
- Muhleisen TW, Leber M, Schulze TG, Strohmaier J, Degenhardt F, Treutlein J, Mattheisen M, Forstner AJ, Schumacher J, Breuer R, Meier S, Herms S, Hoffmann P, Lacour A, Witt SH, Reif A, Muller-Myhsok B, Lucae S, Maier W, Schwarz M, Vedder H, Kammerer-Ciernioch J, Pfennig A, Bauer M, Hautzinger M, Moebus S, Priebe L, Czerski PM, Hauser J, Lissowska J, Szeszenia-Dabrowska N, Brennan P, McKay JD, Wright A, Mitchell PB, Fullerton JM, Schofield PR, Montgomery GW, Medland SE, Gordon SD, Martin NG, Krasnow V, Chuchalin A, Babadjanova G, Pantelejeva G, Abramova LI, Tiganov AS, Polonikov A, Khusnutdinova E, Alda M, Grof P, Rouleau GA, Turecki G, Laprise C, Rivas F, Mayoral F, Kogevinas M, Grigoroiu-Serbanescu M, Propping P, Becker T, Rietschel M, Nothen MM, Cichon S. Genome-wide association study reveals two new risk loci for bipolar disorder. Nat Commun. 2014;5:3339. doi: 10.1038/ncomms4339. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863–844. [DOI] [PubMed] [Google Scholar]
- Ollila HM, Soronen P, Silander K, Palo OM, Kieseppa T, Kaunisto MA, Lonnqvist J, Peltonen L, Partonen T, Paunio T. Findings from bipolar disorder genome-wide association studies replicate in a Finnish bipolar family-cohort. Mol Psychiatry. 2009;14:351–353. doi: 10.1038/mp.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz S, Kapitansky O, Ivashco-Pachima Y, Malishkevich A, Giladi E, Skalka N, Rosin-Arbesfeld R, Mittelman L, Segev O, Hirsch JA, Gozes I. The NAP motif of activity-dependent neuroprotective protein (ADNP) regulates dendritic spines through microtubule end binding proteins. Mol Psychiatry. 2014;19:1115–1124. doi: 10.1038/mp.2014.97. [DOI] [PubMed] [Google Scholar]
- Pinhasov A, Mandel S, Torchinsky A, Giladi E, Pittel Z, Goldsweig AM, Servoss SJ, Brenneman DE, Gozes I. Activity-dependent neuroprotective protein: a novel gene essential for brain formation. Brain research Developmental brain research. 2003;144:83–90. doi: 10.1016/s0165-3806(03)00162-7. [DOI] [PubMed] [Google Scholar]
- Post RM, Leverich GS. The role of psychosocial stress in the onset and progression of bipolar disorder and its comorbidities: the need for earlier and alternative modes of therapeutic intervention. Development and psychopathology. 2006;18:1181–1211. doi: 10.1017/S0954579406060573. [DOI] [PubMed] [Google Scholar]
- Potash JB, Toolan J, Steele J, Miller EB, Pearl J, Zandi PP, Schulze TG, Kassem L, Simpson SG, Lopez V, MacKinnon DF, McMahon FJ. The bipolar disorder phenome database: a resource for genetic studies. Am J Psychiatry. 2007;164:1229–1237. doi: 10.1176/appi.ajp.2007.06122045. [DOI] [PubMed] [Google Scholar]
- Potash JB, Zandi PP, Willour VL, Lan TH, Huo Y, Avramopoulos D, Shugart YY, MacKinnon DF, Simpson SG, McMahon FJ, DePaulo JR, Jr, McInnis MG. Suggestive linkage to chromosomal regions 13q31 and 22q12 in families with psychotic bipolar disorder. Am J Psychiatry. 2003;160:680–686. doi: 10.1176/appi.ajp.160.4.680. [DOI] [PubMed] [Google Scholar]
- Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, Boehnke M, Abecasis GR, Willer CJ. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychiatric GWAS Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43:977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramacciotti CE, Paoli RA, Marcacci G, Piccinni A, Burgalassi A, Dell’Osso L, Garfinkel PE. Relationship between bipolar illness and binge-eating disorders. Psychiatry Res. 2005;135:165–170. doi: 10.1016/j.psychres.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Rayworth BB, Wise LA, Harlow BL. Childhood abuse and risk of eating disorders in women. Epidemiology. 2004;15:271–278. doi: 10.1097/01.ede.0000120047.07140.9d. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Perez V, Garcia Y. Impact of traumatic experiences and violent acts upon response to treatment of a sample of Colombian women with eating disorders. Int J Eat Disord. 2005;37:299–306. doi: 10.1002/eat.20091. [DOI] [PubMed] [Google Scholar]
- Rowe MK, Chuang DM. Lithium neuroprotection: molecular mechanisms and clinical implications. Expert reviews in molecular medicine. 2004;6:1–18. doi: 10.1017/S1462399404008385. [DOI] [PubMed] [Google Scholar]
- Sanches M, Keshavan MS, Brambilla P, Soares JC. Neurodevelopmental basis of bipolar disorder: a critical appraisal. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1617–1627. doi: 10.1016/j.pnpbp.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Sanders AR, Levinson DF, Duan J, Dennis JM, Li R, Kendler KS, Rice JP, Shi J, Mowry BJ, Amin F, Silverman JM, Buccola NG, Byerley WF, Black DW, Freedman R, Cloninger CR, Gejman PV. The Internet-based MGS2 control sample: self report of mental illness. Am J Psychiatry. 2010;167:854–865. doi: 10.1176/appi.ajp.2010.09071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders EH, Scott LJ, McInnis MG, Burmeister M. Familiality and diagnostic patterns of subphenotypes in the National Institutes of Mental Health bipolar sample. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:18–26. doi: 10.1002/ajmg.b.30558. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger U, Fichter M. Balliere’s Clinical Psychiatry. Balliere’s Tindall; London: 1997. Eating disorders: clinical presentation, classification and etiologic models; pp. 199–216. [Google Scholar]
- Scott-Van Zeeland AA, Bloss CS, Tewhey R, Bansal V, Torkamani A, Libiger O, Duvvuri V, Wineinger N, Galvez L, Darst BF, Smith EN, Carson A, Pham P, Phillips T, Villarasa N, Tisch R, Zhang G, Levy S, Murray S, Chen W, Srinivasan S, Berenson G, Brandt H, Crawford S, Crow S, Fichter MM, Halmi KA, Johnson C, Kaplan AS, La Via M, Mitchell JE, Strober M, Rotondo A, Treasure J, Woodside DB, Bulik CM, Keel P, Klump KL, Lilenfeld L, Plotnicov K, Topol EJ, Shih PB, Magistretti P, Bergen AW, Berrettini W, Kaye W, Schork NJ. Evidence for the role of EPHX2 gene variants in anorexia nervosa. Mol Psychiatry. 2014;19:724–732. doi: 10.1038/mp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SH, Mroczkowski-Parker Z, Shekhtman T, Alexander M, Remick RA, Sadovnick AD, McElroy SL, Keck PE, Jr, Kelsoe JR. Linkage of a bipolar disorder susceptibility locus to human chromosome 13q32 in a new pedigree series. Mol Psychiatry. 2003;8:558–564. doi: 10.1038/sj.mp.4001267. [DOI] [PubMed] [Google Scholar]
- Siomi H, Siomi MC, Nussbaum RL, Dreyfuss G. The protein product of the fragile X gene, FMR1, has characteristics of an RNA-binding protein. Cell. 1993;74:291–298. doi: 10.1016/0092-8674(93)90420-u. [DOI] [PubMed] [Google Scholar]
- Siomi MC, Siomi H, Sauer WH, Srinivasan S, Nussbaum RL, Dreyfuss G. FXR1, an autosomal homolog of the fragile X mental retardation gene. The EMBO journal. 1995;14:2401–2408. doi: 10.1002/j.1460-2075.1995.tb07237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EN, Bloss CS, Badner JA, Barrett T, Belmonte PL, Berrettini W, Byerley W, Coryell W, Craig D, Edenberg HJ, Eskin E, Foroud T, Gershon E, Greenwood TA, Hipolito M, Koller DL, Lawson WB, Liu C, Lohoff F, McInnis MG, McMahon FJ, Mirel DB, Murray SS, Nievergelt C, Nurnberger J, Nwulia EA, Paschall J, Potash JB, Rice J, Schulze TG, Scheftner W, Panganiban C, Zaitlen N, Zandi PP, Zollner S, Schork NJ, Kelsoe JR. Genome-wide association study of bipolar disorder in European American and African American individuals. Mol Psychiatry. 2009;14:755–763. doi: 10.1038/mp.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EN, Koller DL, Panganiban C, Szelinger S, Zhang P, Badner JA, Barrett TB, Berrettini WH, Bloss CS, Byerley W, Coryell W, Edenberg HJ, Foroud T, Gershon ES, Greenwood TA, Guo Y, Hipolito M, Keating BJ, Lawson WB, Liu C, Mahon PB, McInnis MG, McMahon FJ, McKinney R, Murray SS, Nievergelt CM, Nurnberger JI, Jr, Nwulia EA, Potash JB, Rice J, Schulze TG, Scheftner WA, Shilling PD, Zandi PP, Zollner S, Craig DW, Schork NJ, Kelsoe JR. Genome-Wide Association of Bipolar Disorder Suggests an Enrichment of Replicable Associations in Regions near Genes. PLoS Genet. 2011;7:e1002134. doi: 10.1371/journal.pgen.1002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro-de-Souza MG, Dias VV, Figueira ML, Forlenza OV, Gattaz WF, Zarate CA, Jr, Machado-Vieira R. Translating neurotrophic and cellular plasticity: from pathophysiology to improved therapeutics for bipolar disorder. Acta Psychiatr Scand. 2012;126:332–341. doi: 10.1111/j.1600-0447.2012.01889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, Lehner T, Luo Y, Loth JE, Shao W, Sharpe L, Alexander JR, Das K, Simon R, Fieve RR, et al. A possible vulnerability locus for bipolar affective disorder on chromosome 21q22.3. Nat Genet. 1994;8:291–296. doi: 10.1038/ng1194-291. [DOI] [PubMed] [Google Scholar]
- Strober M, Freeman R, Lampert C, Diamond J, Kaye W. Controlled family study of anorexia nervosa and bulimia nervosa: evidence of shared liability and transmission of partial syndromes. Am J Psychiatry. 2000;157:393–401. doi: 10.1176/appi.ajp.157.3.393. [DOI] [PubMed] [Google Scholar]
- Swaminathan S, Koller DL, Foroud T, Edenberg HJ, Xuei X, Niculescu AB, 3rd, Nurnberger JI, Jr Bipolar Genome Study Consortium. Characteristics of Bipolar I patients grouped by externalizing disorders. J Affect Disord. 2015;178:206–214. doi: 10.1016/j.jad.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Faraone SV, Tsuang MT. Family, twin, and adoption studies of bipolar disease. Curr Psychiatry Rep. 2002;4:130–133. doi: 10.1007/s11920-002-0046-1. [DOI] [PubMed] [Google Scholar]
- Tempel BL, Shilling DJ. The plasma membrane calcium ATPase and disease. Sub-cellular biochemistry. 2007;45:365–383. doi: 10.1007/978-1-4020-6191-2_13. [DOI] [PubMed] [Google Scholar]
- Tozzi F, Thornton LM, Klump KL, Fichter MM, Halmi KA, Kaplan AS, Strober M, Woodside DB, Crow S, Mitchell J, Rotondo A, Mauri M, Cassano G, Keel P, Plotnicov KH, Pollice C, Lilenfeld LR, Berrettini WH, Bulik CM, Kaye WH. Symptom fluctuation in eating disorders: correlates of diagnostic crossover. Am J Psychiatry. 2005;162:732–740. doi: 10.1176/appi.ajp.162.4.732. [DOI] [PubMed] [Google Scholar]
- Wang JL, Chang WT, Tong CW, Kohno K, Huang AM. Human synapsin I mediates the function of nuclear respiratory factor 1 in neurite outgrowth in neuroblastoma IMR-32 cells. Journal of neuroscience research. 2009;87:2255–2263. doi: 10.1002/jnr.22059. [DOI] [PubMed] [Google Scholar]
- Wang JL, Tong CW, Chang WT, Huang AM. Novel genes FAM134C, C3orf10 and ENOX1 are regulated by NRF-1 and differentially regulate neurite outgrowth in neuroblastoma cells and hippocampal neurons. Gene. 2013;529:7–15. doi: 10.1016/j.gene.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Wang K, Zhang H, Bloss CS, Duvvuri V, Kaye W, Schork NJ, Berrettini W, Hakonarson H Price Foundation Collaborative G. A genome-wide association study on common SNPs and rare CNVs in anorexia nervosa. Mol Psychiatry. 2011;16:949–959. doi: 10.1038/mp.2010.107. [DOI] [PubMed] [Google Scholar]
- Wang KS, Liu XF, Aragam N. A genome-wide meta-analysis identifies novel loci associated with schizophrenia and bipolar disorder. Schizophr Res. 2010;124:192–199. doi: 10.1016/j.schres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Wildes JE, Marcus MD, Fagiolini A. Eating disorders and illness burden in patients with bipolar spectrum disorders. Compr Psychiatry. 2007;48:516–521. doi: 10.1016/j.comppsych.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winham SJ, Cuellar-Barboza AB, McElroy SL, Oliveros A, Crow S, Colby CL, Choi DS, Chauhan M, Frye MA, Biernacka JM. Bipolar disorder with comorbid binge eating history: a genome-wide association study implicates APOB. J Affect Disord. 2014;165:151–158. doi: 10.1016/j.jad.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MH, Yang YH, Lu CY, Jong SB, Chen LJ, Lin YF, Wu SJ, Chu PY, Chung TW, Tyan YC. Activity-dependent neuroprotector homeobox protein: A candidate protein identified in serum as diagnostic biomarker for Alzheimer’s disease. Journal of proteomics. 2012;75:3617–3629. doi: 10.1016/j.jprot.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Yoon IS, Li PP, Siu KP, Kennedy JL, Cooke RG, Parikh SV, Warsh JJ. Altered IMPA2 gene expression and calcium homeostasis in bipolar disorder. Mol Psychiatry. 2001;6:678–683. doi: 10.1038/sj.mp.4000901. [DOI] [PubMed] [Google Scholar]
- Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annual review of biochemistry. 2007;76:367–385. doi: 10.1146/annurev.biochem.76.053105.094237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.