Abstract
Post-natal osteogenesis after mechanical trauma or stimulus occurs through either endochondral healing, intramembranous healing or lamellar bone formation. Bone morphogenetic protein 2 (BMP2) is up-regulated in each of these osteogenic processes and is expressed by a variety of cells including osteoblasts and vascular cells. It is known that genetic knockout of Bmp2 in all cells or in osteo-chondroprogenitor cells completely abrogates endochondral healing after full fracture. However, the importance of BMP2 from differentiated osteoblasts and endothelial cells is not known. Moreover, the importance of BMP2 in non-endochondral bone formation such as intramembranous healing or lamellar bone formation is not known. Using inducible and tissue-specific Cre-lox mediated targeting of Bmp2 in adult (10–24 week old) mice, we assessed the role of BMP2 expression globally, by osteoblasts, and by vascular endothelial cells in endochondral healing, intramembranous healing and lamellar bone formation. These three osteogenic processes were modeled using full femur fracture, ulnar stress fracture, and ulnar non-damaging cyclic loading, respectively. Our results confirmed the requirement of BMP2 for endochondral fracture healing, as mice in which Bmp2 was knocked out in all cells prior to fracture failed to form a callus. Targeted deletion of Bmp2 in osteoblasts (osterix-expressing) or vascular endothelial cells (vascular endothelial cadherin-expressing) did not impact fracture healing in any way. Regarding non-endochondral bone formation, we found that BMP2 is largely dispensable for intramembranous bone formation after stress fracture and also not required for lamellar bone formation induced by mechanical loading. Taken together our results indicate that osteoblasts and endothelial cells are not a critical source of BMP2 in endochondral fracture healing, and that non-endochondral bone formation in the adult mouse is not as critically dependent on BMP2.
Keywords: Bone Homeostasis, BMP2, Bone Injury, Facture Healing, Orthopedics, Osteoblasts
1. Introduction
Post-natal osteogenesis after mechanical trauma or stimulus occurs through one of three well-described processes: endochondral healing, intramembranous healing, or lamellar bone formation [1–4]. Endochondral healing occurs after a complete fracture that is initially mechanically unstable [5]. First, a hematoma forms which is then replaced by a large cartilaginous callus that surrounds the fracture gap and adjacent bone. Woven bone forms directly at the margins of the healing region and also, with time, replaces the central cartilage callus; the whole bone is stabilized when woven bone bridges the fracture gap. The woven bone callus eventually remodels into stronger, more compact bone that is almost indistinguishable from the pre-injured bone [1,2,6]. Intramembranous healing occurs after stress fracture or stable complete fracture [2,7]. This healing process has some similarities to endochondral healing except it lacks the cartilage callus phase. A smaller woven bone callus directly forms around the fracture line, stabilizes the bone and is remodeled over time [3,8]. Lamellar bone formation occurs as part of normal bone modeling (or re-modeling). It is different from both endochondral and intramembranous healing as it is not a repair response. Lamellar bone forms slowly in response to mild or moderate anabolic stimuli such as non-damaging mechanical loading [4]. Many factors are involved in these three bone forming modalities, and there are differences in the cells types, signaling pathways, and cytokines necessary for successful bone formation in each [6–10].
Vascular cells are activated in both endochondral and intramembranous healing. In the initial stages of healing the vascular network dilates to increase the blood flow to the injury site [11]. Vasodilatation facilitates the release of cytokines locally and systemically to initiate the inflammation response and to recruit and activate cells to start the repair process. Later the vascular network increases through angiogenesis to supply cells with the oxygen and nutrients needed for new tissue formation and to remove carbon dioxide and tissue-breakdown products. Eventually, like the bone callus, the vascular network remodels to approximately pre-injury state [1,2,6,11]. Inhibition of vasodilatation or angiogenesis significantly decreases the amount of new woven bone formed during endochondral and intramembranous healing [12–16]. Likewise, application of angiogenic agonists significantly increases the amount of new bone formed [15]. On the other hand, lamellar bone formation in response to anabolic stimuli, in particular non-damaging mechanical loading, does not depend on vasodilatation or angiogenesis [9,10,16].
Bone morphogenetic protein 2 (BMP2) is up-regulated in each of these osteogenic processes [8–10,17–19]. In endochondral healing, BMP2 is expressed in pre-hypertrophic chondrocytes, osteoblasts, osteocytes, and vascular cells [17,19]. Knockout of BMP2 in all cells (using an inducible ubiquitously expressed Cre) or in osteo-chondroprogenitor cells (using the limb-specific Prx1-Cre) completely abrogates endochondral fracture healing. Cells fail to form a cartilage callus, and a persistent granulation tissue fills the defect area [20,21]. Even when bone grafts from knockout mice are placed into a wild type host, the cells lacking BMP2 neither undergo differentiation nor contribute to the healing response, indicating that the actions of endogenous BMP2 are largely autocrine [21,22]. While these seminal results establish the general requirement of BMP2 expression in osteo-chondral cells at the time of injury, it remains unclear if expression in any single cell type is critical. Also, it is uncertain which stages of repair are BMP2-dependent (i.e. inflammation, cartilaginous callus formation, or later bone formation). BMP2 modulates the activity of many different cell types and could play a different role during each healing phase. During intramembranous healing, BMP2 is also expressed in many cell types, i.e., activated periosteal progenitor cells, osteoblasts, osteocytes, and vascular cells [7,9,12,23]. The effect of BMP2 knockout, either globally or tissue-specifically, on the intramembranous healing process has not been reported. Lastly, after non-damaging mechanical loading that stimulates lamellar bone formation, BMP2 expression is up-regulated [9]. Taken together with findings that BMP2 is critical for post-natal bone formation [20] and that deletion of BMP2 in osteoblast lineage cells results in osteopenia and reduced bone strength [24,25], this result suggests that BMP2 may be critical in loading-induced bone formation.
Our objective was to further examine the importance of BMP2 in these osteogenic processes, with a focus on narrowing down the cell types that may be critical sources of BMP2. To address this objective, we used three bone formation models, one that is cartilage dependent (endochondral healing) and two that are cartilage independent (intramembranous healing and loading-induced lamellar bone formation). Using inducible and tissue-specific knockout of Bmp2 in mice, we assessed the role of global BMP2 expression and BMP2 expressed by vascular endothelial cells and osteoblasts as critical sources of BMP2. Based on the outcomes of previous studies we hypothesized that BMP2 is necessary for both types of bone repair and that knockout from osteoblasts and vascular-endothelial cells, two cell types that upregulate BMP2 after fracture and stress fracture, impairs healing. We further hypothesized that BMP2 expressed by osteoblasts is necessary for the loading-induced lamellar bone formation.
2. Methods
2.1 Animals
This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the IACUC of Washington University of St. Louis (Protocol Number: 20110209). Mice were maintained in standard housing with 12 hr light/dark cycles and food and water ad libitum. Transgenic mice with exon 3 of the Bmp2 gene floxed [20] (Bmp2fl/fl, gift of Dr. Vicki Rosen via material transfer agreement with Harvard University) were crossed with one of three Cre lines to target different cell populations: all cells (inducible), osteoblasts and osteocytes (inducible), and vascular-endothelial cells (constitutive). Experimental groups were comprised of either all male, all female, or male and female mice as reported in the result tables. The choice of sex was based simply on availability; we did not observe any sex-genotype interactions in this study’s results or our previous phenotyping studies [24]. The conditional knockout (cKO) mice carried two floxed Bmp2 alleles and were Cre-positive (Bmp2fl/fl; Cre-positive). Control mice carried two floxed Bmp2 alleles but lacked Cre (Bmp2fl/fl;Cre-negative) and were from the same breeding colony as the respective cKO mice.
The first Cre, a ubiquitin-C promoted Cre (UBC-Cre, B6;129S-Tg(UBC-cre/ERT2)1Ejb/J, from Jackson Labs, Bar Harbor, ME), causes global deletion following induction with tamoxifen (Sigma, St. Louis, MO). Tamoxifen was dissolved in corn oil at a concentration of 20 mg/mL and injected in 2 doses (100 ug/g body weight) 8 and 7 days before loading. Both knockout (Bmp2fl/fl;UBC-Cre, referred to as “UBC-Cre cKO”) and control (Bmp2fl/fl, “UBC-Cre control”) animals were injected. Dosing scheme was validated by reporter mice [26] and qPCR (approximately 50–55% knockout efficiency for muscle and bone respectively, supplemental Figures S1–S2). The second Cre, an osterix promoted Cre (OSX-Cre, B6.Cg-Tg(Sp7-tTA,tetO-EGFP/Cre)1Amc/J, from Jackson Labs) under the control of tetracycline [27] targeted osteogenic lineage cells starting from the pre-osteoblast stage. We recently reported the phenotype of Bmp2fl/fl;OSX-Cre mice when recombination was not suppressed (no tetracycline). At 12- and 24-weeks of age, bones from Bmp2fl/fl;OSX-Cre mice are smaller and more brittle than controls, and the mice develop malocclusions [24]. We were concerned that baseline skeletal geometric differences could confound the outcomes of the current study; to eliminate these issues the knockout was delayed until weaning by administering the tetracycline analogue doxycycline (Sigma, St. Louis, MO) in water in all breeding cages (1 ug/mL in 3–5% sugar water) to both knockout (Bmp2fl/fl;OSX-Cre, “OSX-Cre cKO”) and control (Bmp2fl/fl, “OSX-Cre control”) mice. Knockout delay until weaning eliminated the skeletal size and malocclusion phenotypes and reduced the severity of the brittle phenotype (supplemental Tables S1–S5). By the time of experimental loading the efficiency of BMP2 knockout in doxycycline treated animals was similar to that of knockout animals from our previous studies when mice were not treated with doxycycline [24] (knockout efficiency in bone approximately 60%, supplemental Figure S2). The third Cre, vascular-endothelial-cadherin promoted Cre (VEC-Cre, B6.Cg-Tg(Cdh5-Cre)7Mlia/J, from Jackson Labs) targeted deletion in vascular-endothelial cells [28]. Knockout validation by immunohistochemistry and phenotyping studies have been performed by our group previously for this mouse model [24]. There are no phenotypic differences between knockouts (Bmp2fl/fl;VEC-Cre, “VEC-Cre cKO”) and littermate controls (Bmp2fl/fl, “VEC-Cre control”). For this study we also performed validation via qPCR of CD31+ and CD11a− cells sorted from heart and lung samples (knockout efficiency 90%, supplemental Figure S2).
2.2 Endochondral Healing
2.2.1 Fracture Model
A closed femoral fracture was created in the right hindlimb based on the method of Hiltunen et al. [29] adapted with modification to the femur. Each mouse was anesthetized using isofluorane (2–3% inhalation). The fur was shaved around the knee area and the skin disinfected with Betadine. A small incision (1 cm) was made at the knee lateral and parallel to the patellar tendon to dislocate the patella. A hole was made in the distal end of the femur to provide access to the intramedullary canal. A thin tungsten guidewire (0.125 mm diameter) was placed into the femur. The mouse was then secured in a three-point bending fixture connected to a materials testing machine (Dynamight 8841, Instron, Norwood, MA) wherein the femur was broken at the mid-diaphysis by application of a displacement controlled ramp (30 mm/s) with displacement limited to prevent excessive angulation of the femur. The fracture was then stabilized with a 24 gauge stainless steel rod placed over the guidewire. The guidewire was subsequently removed, and the wound sutured closed. Animals were given Buprenex (0.05 mg/kg, subcutaneous) for pain treatment immediately post-surgery and after as needed. Animals were 10 weeks old at time of fracture (n=10–14/group for OSX-Cre and VEC-Cre microCT, n=7–8 for OSX-Cre gene expression). Mice allocated for dynamic histomorphometry were given calcein (10 mg/kg BW, IP, Sigma) and alizarin red fluorochrome injections (25 mg/kg BW, IP, Sigma) 7 and 12 days post fracture, respectively. Global, inducible knockout of Bmp2 has been previously reported in an endochondral healing model [21] so only a small set of UBC-Cre cKO and control mice (n=4–6) were included for purposes of model validation.
2.2.2 MicroCT and Histology
Mice were euthanized 14 days after fracture. At this timepoint in normal mice, the callus is at maximal size and is typically partially bridged with bone, with small amounts of unmineralized cartilage remaining at the fracture midline [29]. Fractured hindlimbs were dissected with surrounding soft tissues removed and fixed overnight in 10% neutral buffered formalin. Fractured femurs were scanned in their entirety using microCT (vivaCT, Scanco Medical, Wayne, PA; X-ray tube potential 55 kVp, integration time 300 ms, X-ray intensity 145 μA, isotropic voxel size 21 um, frame averaging 1, projections 500, medium resolution scan). The midpoint of the fracture was found and analysis included 200 slices (4.2 mm) centered on the midpoint; this region encompassed the majority of the callus. A contour was drawn around the margin of the entire callus. Then we applied a lower threshold of 183 per mille to segment mineralized tissue (all bone inside the callus) and a higher threshold of 460 per mille to segment the original cortical bone inside the callus volume. The following parameters were determined: total callus volume (TV), total bone volume (BV), tissue mineral density (TMD) of all bone inside the callus, and callus bone mineral density (BMD; excluding the original cortical bone, calculated using equation 1).
| [1] |
Where,
ρcallus =Callus Density
ρt= Total Tissue Mineral Density (Callus, Cortical Bone, & Marrow)
Vt = Total Tissue Volume
ρct = Cortical Bone Mineral Density
Vct = Cortical Bone Volume
Following microCT scanning, samples were either decalcified (14% EDTA) and processed for histologic analysis (paraffin sections, 5 microns) or dehydrated and embedded in poly-methyl methacrylate (plastic) for longitudinal sectioning (100 microns, SP1600, Leica, Buffalo Grove, IL). Paraffin sections were stained with hematoxylin and eosin, or picrosirius red and Alcian blue (n=3–5/group). Sections were visualized with nanozoomer (NanoZoomer HT, Olympus, Center Valley, PA). Fluorescent images of the plastic sections were taken (Olympus IX51 with Olympus DP70 camera, Waltham, MA) to visualize the calcein (green) and alizarin (red) labels (n=5–7/group). These images were used to qualitatively confirm the microCT findings, so no quantitative measures were taken.
2.2.3 Gene Expression
Mice were euthanized 7 days after fracture, when the callus in normal mice is mostly unmineralized cartilage [29]. Both femora were harvested for analysis (n=7–8/group). The overlying soft tissues and muscle were stripped carefully to preserve the callus on the fractured limb. After the articulating ends were cut off and the marrow removed by centrifugation, the remaining bone and callus tissues were frozen in liquid nitrogen. The samples were pulverized in a liquid nitrogen cooled microdismembranator (B Braun, Melsungen, Germany), suspended in 1 mL of Trizol (Life Technologies, Grand Island, NY), briefly vortexed, and mixed with chloroform (200 uL, Sigma). The trizol/chloroform mix was transferred to a pre-spun phase lock gel separator (5PRIME, Gaithersburg, MD) and spun for 7 min at 13000 rpm. The upper aqueous phase was transferred to a new tube and then processed according to the manufacturer’s instructions to isolate the RNA (RNeasy mini kit with DNAse away, Qiagen, Valencia, CA). RNA quality was verified for each sample (RIN>7, 2100 Bioanalyzer, Agilent Technologies, Santa Clara, CA). After quantification of the RNA concentration (Nanodrop, Thermo Scientific, Wilmington, DE), 500 ng of cDNA was created for each sample using iScript (Bio-Rad, Hercules, CA). Finally, qPCR for genes related to chondrogenesis, osteogenesis, and angiogenesis as well as possible compensatory BMPs and BMP antagonists (Table 1, Integrated DNA Technologies, Coralville, IA) was performed using 8.3 ng of cDNA template and SYBR green chemistry (StepOnePlus, Life technologies).
Table 1.
Primers used for qPCR of day 7 fracture callus
| Gene of Interest | Abbreviation | Associated Process | Oligo Sequence or Primer Set |
|---|---|---|---|
| Aggrecan | Acan | Chondrogenesis | Mm.PT.58.23585796 |
| Bone Morphogenetic Protein 2 (exon 3) | Bmp2 | Osteo- & Chondrogenesis | F: TGG AAG TGG CCC ATT TAG AG, R: TGA CGC TTT TCT CGT TTG TG |
| Bone Morphogenetic Protein 3 (exons 1–2) | Bmp3 | Osteogenesis Antagonist | Mm.PT.58.8861659 |
| Bone Morphogenetic Protein 4 (exons 1–2) | Bmp4 | Osteogenesis | Mm.PT.58.7116776 |
| Bone Morphogenetic Protein 7 (exons 2–3) | Bmp7 | Osteogenesis | Mm.PT.58.30464128 |
| Bone Sialoprotein | Bsp | Osteogenesis | F: CCG GCC ACG CTA CTT TCT T, R: GGA CTG GAA ACC GTT TC |
| Chordin (exons 21–23) | Chrd | BMP Antagonist | Mm.PT.58.30333725 |
| Collagen type 1a1 | Col1 | Osteogenesis | F: GCTCCT CTT AGG GGC CAC T, R: CCA CGT CTC ACC ATT GGG G |
| Collagen type 2a1 (exons 48–50) | Col2 | Chondrogenesis | Mm.PT.58.28573530 |
| Gremlin1 (exons 1–2) | Grem1 | BMP Antagonist | Mm.PT.58.11631114 |
| Noggin (exon 1) | Nog | BMP Antagonist | Mm.PT.58.30936710.g |
| Osterix | Osx | Osteogenesis | F: CCC TTC TCA AGC ACC AAT GG, R: AAG GGT GGG TAG TCA TTT GCA TA |
| Sox9 | Sox9 | Osteo- & Chondrogenesis | F : GAG CCG GAT CTG AAG AGG GA, R: GCT TGA CGT GTG GCT TGT TC |
| Vascular Endothelial Growth Factor | Vegf | Angiogenesis | Mm.PT.58.31754187 |
| Importin 8 (exons 7–9) | Ipo8 | Reference Gene | Mm.PT.58.6409954 |
| TATA Binding Protein (exons 2–3) | Tbp | Reference Gene | Mm.PT.58.42394711 |
2.3 Intramembranous Healing
2.3.1 Stress Fracture (Fatigue) Model
To create a non-displaced fracture that heals via direct intramembranous bone formation, a previously established non-invasive, axial forelimb fatigue loading model was used [30]. First, a group of calibration mice (n=3–4 per group) was used to determine the loading parameters (loading force and displacement to fracture). Under isofluorane inhalation anesthesia the mouse carpus and olecranon were immobilized in two loading cups such that a compressive load could be applied along the bone axis causing combined compression-plus-bending of the ulna. The top cup was displaced at a rate of 0.5 mm/s until the entire limb fractured (DynaMight 8841). During displacement loading the resulting force was recorded and the ultimate compressive force determined (LabVIEW, National Instruments, Austin, TX). The mouse, still under anesthesia, was repositioned so that the contralateral limb was similarly aligned between the two loading cups. A cyclic load was then applied as a haversine wave at a rate of 2 Hz from −0.3 N to 75% of the contralateral limb’s ultimate compressive force. Loading continued until whole limb fracture. Animals were euthanized immediately following cyclic calibration loading. Analysis was performed on the recorded displacement data to find the difference between the 10th cycle minimum displacement and displacement at fracture (average displacement to fracture).
Survival loading was similar to the cyclic loading of calibration mice in that the loading force cycled from −0.3 N to 75% of the average ultimate compressive load for the calibration mice (Table 2). However, to create a non-displaced stress fracture, loading stopped when the displacement difference from the 10th-cycle reached 50% of the average displacement to fracture determined from the calibration group (Table 2). Only right forelimbs were loaded. Animals were given Buprenex (0.05 mg/kg, subcutaneous) for pain treatment immediately post-injury. Calcein and alizarin labels were given at 3 and 8 days post loading, respectively, to mark new bone formation. UBC-Cre and OSX-Cre groups were 12–14 weeks old (n=8–9/group); VEC-Cre groups were 24 weeks old (n=5/group). Both of these ages are considered young-adult, and we did not observe differences in the healing response between them.
Table 2.
Mechanical loading parameters
| BMP2 Genotype | Stress Fracture Loading (Intramembranous Healing) | Non-Damaging Loading (Lamellar Bone Formation) | |||
|---|---|---|---|---|---|
| Sex | Peak Cyclic Force (N) | Stopping Displacement (mm) | Sex | Peak Cyclic Force (N) | |
| UBC Control | M | −4.40 | −0.5 | F | −3.7 |
| UBC cKO | M | −4.40 | −0.5 | F | −3.7 |
| OSX Control | M | −4.00 | −0.4 | F & M | −3.4 (F) or −3.6 (M) |
| OSX cKO | M | −3.70 | −0.4 | F & M | −3.4 (F) or −3.6 (M) |
| VEC Control | M | −4.95 | −0.4 | N/A | |
| VEC cKO | M | −4.95 | −0.4 | N/A | |
2.3.2 MicroCT & Dynamic Histomorphometry
Mice were euthanized 10 days after loading, when the woven bone callus is well mineralized. Forelimbs were dissected with surrounding soft tissues intact and fixed overnight in 10% neutral buffered formalin. Loaded limbs (right) were scanned in their entirety using microCT (μCT40, Scanco Medical; X-ray tube potential 55 kVp, integration time 200 ms, X-ray intensity 145 μA, isotropic voxel size 16 um, frame averaging 1, projections 500, medium resolution scan). Then the scans were analyzed to find the following woven bone callus parameters: extent along the bone axis, woven bone volume, and woven bone mineral density (threshold 180 per mille). All measures were for the newly formed woven bone callus alone and did not include the original cortical bone or marrow cavity (density calculated using equation 1, where Vct = Cortical Bone Volume + Marrow Volume and ρCt = Cortical Bone Mineral Density + Marrow Mineral Density).
Following microCT scanning, all samples were dehydrated and embedded in poly-methyl methacrylate (plastic). Two 100 micron thick cross-sections were cut from each bone (SP1600) at the callus midpoint, mounted to glass slides (Superfrost, Fisher, Pittsburg, PA), and polished with increasingly fine grades of sand paper until they reached a 30 micron thickness. Fluorescent images were taken (Olympus IX51 with Olympus DP70 camera) to visualize the calcein and alizarin labels. Sections were analyzed (BIOQUANT OSTEO, BIOQUANT Image Analysis Corporation, Nashville, TN) for woven bone area.
2.4 Lamellar Bone Formation
2.4.1 Non-Damaging Loading Model
To induce lamellar bone formation in the absence of damage, a forelimb loading model based on our previous work with rats was used [31]. The placement of the mouse in the loading machine was the same as the stress fracture model, but the loading regime was different. One hundred cycles of a triangular wave form were applied from −0.3 N to 70% of the average maximal compressive force at 2 Hz with a 9.5 second rest inserted (Table 2). Animals were given Buprenex (0.05 mg/kg, subcutaneous) for pain treatment immediately post-loading and after as needed. Similar to the stress fracture model, mice were given calcein and alizarin at 3 and 8 days post loading to mark new bone formation. Only the UBC-Cre and OSX-Cre lines were tested (12–14 weeks old, n=9–13/Group); VEC-Cre mice were not included because of the lack of phenotype of the VEC-Cre cKO mice either basally or in the fracture or stress fracture models (see Results).
2.4.2 Dynamic Histomorphometry
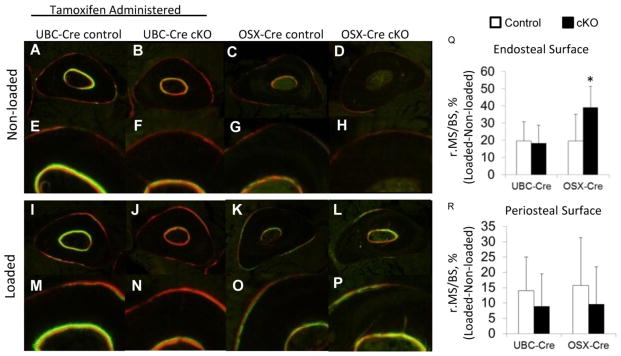
Ten days after loading mice were euthanized. Right and left ulnae from each mouse were processed as described above and 30 micron thick plastic sections were obtained at the mid-shaft. Fluorescent images were taken (Olympus) to visualize the calcein and alizarin labels. Transverse sections were analyzed on the periosteal and endosteal surfaces (BIOQUANT OSTEO) for mineralizing surface per bone surface (MS/BS), mineral apposition rate (MAR) and bone formation rate per bone surface (BFR/BS). The relative mineralizing surface per bone surface (r.MS/BS) was calculated for each mouse by subtracting the MS/BS of the non-loaded limb (left) from that of the loaded limb (right).
2.4 Statistics
Data are presented as mean ± standard deviation. Conditional knockouts (Bmp2fl/fl;_____-Cre, “cKO”) were compared to their respective controls (Bmp2fl/fl from the same strain, “control”). For microCT and dynamic histology outcomes groups were compared using either unpaired t-tests when groups were single sexed or two-way ANOVA when males and females were included in the groups (factors: group and sex) (StatView v. 5.0, SAS Institute, Cary, NC). When two-way ANOVA proved significant differences between groups (p<0.05) the data was further tested with Fisher’s Least Significant Difference (FLSD) for post-hoc analysis. The mean and standard deviation presented for these groups is pooled males and female data. For gene expression data, two-way ANOVA with post-hoc FLSD analysis was initially used to compare non-normalized outcomes (factors: group and limb/treatment). If group was significant for both tests, each limb’s expression was compared between groups using unpaired t-tests. If limb/treatment was significant for both tests, that outcome’s data for each group was compared between limbs using paired t-tests. Unpaired t-tests were used for all outcomes already normalized to the control limb. A p-value of less than 0.05 was considered significant for all tests and outcomes.
3. Results
3.1 Endochondral Bone Healing is Not Impaired by Reduced Expression of Osteoblast or Endothelial Bmp2
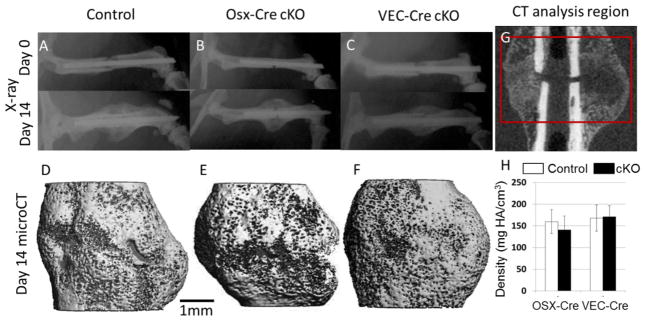
Two-weeks following complete fracture, healing differed greatly between the global and targeted knockout groups. UBC-Cre cKO mice failed to show any healing response (Supplemental Figure S3), consistent with a previous report [21]. This result indicated that the tamoxifen-inducible deletion of Bmp2 using the UBC-Cre cKO mice was effective and confirmed the requirement of normal BMP2 expression levels for callus formation [20,21]; no further analysis of these mice was done. In stark contrast to the lack of fracture callus in femora of UBC-Cre cKO mice, both OSX-Cre and VEC-Cre cKO mice formed mineralized calluses of normal size and density in comparison to their respective controls (Figures 1–3 and Table 3).
Figure 1.
Representative post-fracture images from control (Bmp2fl/fl) and cKO Bmp2fl/fl;____-Cre) from OSX-Cre and VEC-Cre lines. (A–C) Serial X-ray images at day 0 (immediately post fracture) and day 14 (prior to sacrifice) illustrate stabilized mid-femoral fracture. A dense callus can be seen on X-ray at day 14. (D–F) Representative 3D microCT reconstructions of callus show similar bridging of the external callus by mineralized tissue. (G) A representation of the 200-slice analysis region on CT. (H) Callus mineral density is similar in all knockouts relative to their respective controls. *p<0.05 vs. control
Figure 3.
Dynamic histomorphometry of fractured femora. Labels were given at day 7 (calcein, green) and day 12 (alizarin, red) post-fracture before sacrifice at day 14. Both controls (A–B) and knockouts (C–D) illustrated good callus bridging with mineralization occurring at days 12–13 throughout a majority of the callus and across the fracture line. Some unmineralized cartilage pockets remain. Longitudinal sections (100 um) were cut through the femur.
Table 3.
Fracture healing (endochondral) results from microCT
| Genotype | No. mice | Sex | Total Volume (TV, mm3) | Bone Volume (BV, mm3) | Tissue Mineral Density (mg HA/cm3) |
|---|---|---|---|---|---|
| BMP2 OSX Control | 14 | M&F | 52.6 ± 13.8 | 17.4 ± 3.96 | 755 ± 45.3 |
| BMP2 OSX cKO | 13 | M&F | 54.8 ± 9.10 | 16.6 ± 4.15 | 747 ± 32.5 |
| BMP2 VEC Control | 14 | M&F | 50.5 ± 15.1 | 17.2 ± 3.29 | 810 ± 31.1 |
| BMP2 VEC cKO | 10 | M&F | 47.8 ± 9.47 | 17.0 ± 2.47 | 785 ± 26.8 |
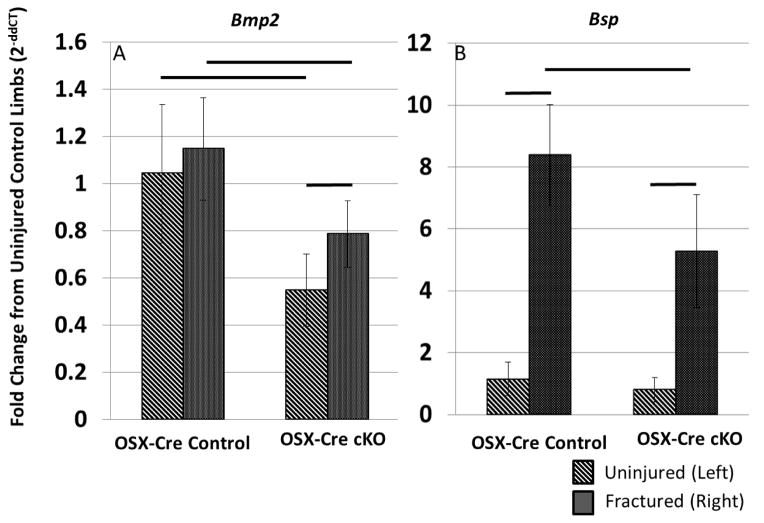
Gene expression within the OSX-Cre calluses 7 days after injury was mostly consistent with the microCT and dynamic histology findings. For all genes, the fold changes in comparison to the contralateral, uninjured limb were similar in knockout and control groups (Table 4). In both OSX-Cre cKO and Control groups, chondrogenic and osteogenic genes were upregulated in the injured limb compared to non-injured control. Cartilage related genes showed the greatest increase of expression followed by the bone related genes, which is consistent with the relative amounts of each tissue type being formed during this healing phase. Vegf, which is a pro-angiogenic factor, was similar between limbs. The candidate compensatory BMPs, Bmp4 and Bmp7, were down regulated, while the anti-osteogenic and BMP antagonists had mixed results. Bmp3 and Grem1 were down regulated; Chrd and Nog were upregulated. Bmp2 and Bsp were the only two genes that were differently upregulated between groups. Baseline Bmp2 expression in uninjured femora was 55% less in knockouts compared to controls, which is similar to the findings of our validation study (Figure S2). Although Bmp2 expression was modestly increased by injury in both groups relative to the contralateral limb (25–50%), the level of expression in the injured knockout bones remained significantly lower than the controls (35%, Figure 4 and Table 4). Post-hoc analysis of Bsp showed that expression was similar in the uninjured limbs between cKO and control. However, Bsp expression in the injured limb reached a higher fold change in the control group (approximately 1000%) than in the cKO group (approximately 730%) (Figure 4 and Table 4). Together these results demonstrate that although Bmp2 expression is significantly diminished in osteoblasts and osteocytes of OSX-Cre cKO mice, there is little effect on the expression of other genes related to cartilage or bone formation.
Table 4.
Relative changes in gene expression 7 days after fracture in the OSX-Cre line
| Gene of Interest | Fold Change from Uninjured Limb (2^(dCTLeft- dCTRight)) | |
|---|---|---|
| BMP2 OSX-Cre Control | BMP2 OSX-Cre cKO | |
| n = 8 | n= 7 | |
| Acan | 1094 ± 968^ | 1293 ± 1928^ |
| Bmp2 | 1.3 ± 0.6 | 1.5 ± 0.3^“* |
| Bmp3 | 0.25 ± 0.09^ | 0.30 ±0.11^ |
| Bmp4 | 0.33 ± 0.14^ | 0.37 ±0.12^ |
| Bmp7 | 0.33 ± 0.19^ | 0.30 ±0.08^ |
| Bsp | 10.1 ±7.7^ | 7.3± 2.5^* |
| Chrd | 3.5 ±1.9^ | 5.3 ± 3.9^ |
| Col1 | 7.0 ± 7.5^ | 11.5 ± 7.8^ |
| Col2 | 259 ± 357^ | 169 ± 119^ |
| Grem1 | 0.43 ±0.22^ | 0.75 ±0.46 |
| Nog | 5.6 ±4.6^ | 7.6 ± 6.7^ |
| Osx | 2.3 ± 1.4^ | 2.4 ± 1.0^ |
| Sox9 | 79.1 ± 68.2^ | 95.9 ±53.6^ |
| Vegf | 1.4 ± 0.6 | 1.3 ± 0.8 |
p<0.05 Uninjured (Left) vs Fractured (Right), ANOVA with Paired Post-hoc Analysis
p<0.05 Uninjured Control vs Uninjured cKO, ANOVA with Unpaired Post-hoc Analysis
p<0.05 Fractured Control vs Fractured cKO, ANOVA with Unpaired Post-hoc Analysis
Figure 4.
Changes in gene expression 7 days after fracture in the OSX-Cre line. The values are normalized to the OSX-Cre control group’s uninjured (left) femora data. (A) Bmp2 is modestly upregulated during fracture repair in both control (Bmp2fl/fl) and cKO (Bmp2fl/fl;OSX-Cre) animals. However, since the baseline expression in knockout animals is approximately half that of control animals, the increase in cKO fractured limbs does not reach the same level as that of fractured controls. (B) Bsp is similarly expressed in both groups’ uninjured limbs. However, the increased expression caused by fracture is blunted in cKO animals. Lines indicate p<0.05 between groups.
3.2 Bmp2 Reduction Does Not Impair Intramembranous Healing
Using a model of stress fracture that elicits a robust periosteal intramembranous woven bone healing response [3,30], we tested the requirement of BMP2 in this process. In contrast to the complete lack of endochondral fracture callus in UBC-Cre mice, where Bmp2 is inducibly reduced in all cell types, these mice formed a normal intramembranous woven bone callus after stress fracture. In fact, microCT and histology on ulnae after 10 days of healing showed that all mouse groups had mineralized calluses of comparable size surrounding the stress fracture site (Figure 5). The woven bone callus extent, woven bone callus volume (microCT) and woven bone area (dynamic histomorphometry) were unchanged between UBC-Cre, OSX-Cre and VEC-Cre cKO versus their respective controls (Table 5). Callus bone mineral density was modestly decreased in OSX-Cre cKO (11%, p=0.03) but unaffected in UBC-Cre or VEC-Cre cKO (Table 5). In sum, reduced expression of Bmp2 by cKO had a negligible effect on intramembranous woven bone formation.
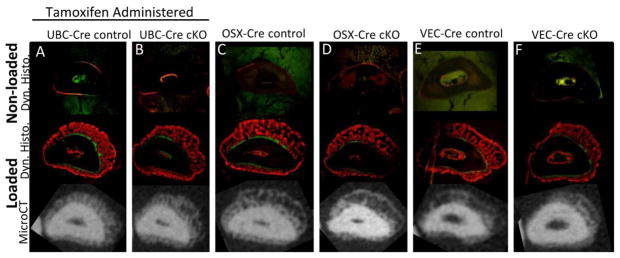
Figure 5.
Dynamic histomorphometry and microCT cross sections of ulnae 10 days after fatigue loading. Labels were given at day 3 (calcein, green) and day 8 (alizarin, red) post fracture before sacrifice at day 10. As expected contralateral, non-loaded limbs (top panel) demonstrated little to no baseline bone formation in both control (A, C, E) and cKO (B, D, F) animals. Following stress fracture woven bone was formed which was mainly mineralized on days 8–9. The amount of woven bone was not altered with BMP2 cKO as illustrated by both representative dynamic histology (middle panel) and microCT cross sections (bottom panel). (n=5–9/group, 2 sections per sample)
Table 5.
Stress fracture healing (intramembranous woven bone) results from microCT and dynamic histomorphometry
| BMP2 Genotype | No. Mice | Sex | Woven Bone Extent (mm) | Woven Bone Volume (mm3) | Woven Bone BMD (mg HA/cm3) | Woven Bone Area (mm2) |
|---|---|---|---|---|---|---|
| UBC Control | 9 | M | 2.92 ± 0.72 | 0.625 ± 0.185 | 370 ± 58.6 | 0.025 ± 0.003 |
| UBC cKO | 7 | M | 2.70 ± 0.72 | 0.559 ± 0.134 | 397 ± 43.7 | 0.026 ± 0.002 |
| OSX Control | 8 | M | 2.97 ± 0.86 | 0.555 ± 0.378 | 383 ± 39.9 | 0.015 ± 0.007 |
| OSX cKO | 8 | M | 2.62 ± 1.08 | 0.490 ± 0.409 | 339 ± 34.9* | 0.019 ± 0.010 |
| VEC Control | 5 | M | 2.56 ± 0.95 | 0.554 ± 0.498 | 296 ± 148 | 0.026 ± 0.039 |
| VEC cKO | 5 | M | 2.91 ± 1.01 | 0.729 ± 0.983 | 378 ± 81.0 | 0.021 ± 0.017 |
p<0.05 vs. Cre negative controls
3.3 BMP2 Reduction Does Not Inhibit Loading-Induced Lamellar Bone Formation
Ten days following non-damaging mechanical loading, all mice responded with increased lamellar bone formation in the loaded limb versus contralateral non-loaded limb (Figure 6, Table 6). Woven bone was not present on any loaded or non-loaded limbs. At the periosteal surface there were no differences in the loading-induced increase in bone formation parameters between UBC-Cre and OSX-Cre cKO versus their respective controls (Figure 6). On the endocortical surface, both UBC-Cre and OSX-Cre cKO had in increases in bone formation compared to their respective controls, although they were not in consistent measures. UBC-Cre cKOs had significant increases in mineral apposition rate and bone formation rate per bone surface area, while OSX-Cre cKOs had a greater increase in mineralizing surface than controls. These results indicate that loading-induced lamellar bone formation is not inhibited by a reduction in BMP2. BMP2 may in fact act to limit lamellar bone formation on the endocortical surface.
Figure 6.
Dynamic histomorphometry cross sections of ulnae after non-damaging loading. Labels were given at day 3 (calcein, green) and day 8 (alizarin, red) post loading before sacrifice at day 10. (A–H) Representative images from contralateral, non-loaded ulnae demonstrated little to no baseline labeling on the periosteal and endocortical surfaces. (I–P) Non-damaging mechanical loading stimulated increased bone formation compared to non-loaded controls. (Q) The relative amount of endosteal mineralizing surface was increased in the OSX-Cre cKO animals compared to controls. (R) There were no differences in periosteal bone mineralizing surface changes between either UBC-Cre cKO or OSX-Cre cKO and their respective controls.
Table 6.
Non-damaging mechanical loading (lamellar bone) results from dynamic histomorphometry
| BMP2 Genotype | No. mice | Sex | Mineralizing Surface (MS/BS, %) | Mineral Apposition Rate (MAR, um/day) | Bone Formation Rate per Bone Surface (BFR/BS, um/day) | |||
|---|---|---|---|---|---|---|---|---|
| Periosteal Bone Analysis | Non-loaded (Left) | Loaded (Right) | Non-Loaded (Left) | Loaded (Right) | Non-Loaded (Left) | Loaded (Right) | ||
| UBC Control | 12 | F | 19.1 ± 11.2 | 33.1^ ± 10.5 | 0.776 ± 0.089 | 1.30^ ± 0.279 | 0.175 ± 0.023 | 0.443^ ± 0.193 |
| UBC cKO | 13 | F | 21.7 ± 9.73 | 30.6^ ± 9.33 | 0.610 ± 0.187 | 1.21 ± 0.365 | 0.184 ± 0.078 | 0.425 ± 0.225 |
| OSX Control | 9 | M&F | 33.5 ± 9.4 | 49.3^ ± 16.8 | 0.915 ± 0.264 | 1.18 ± 0.280 | 0.351 ± 0.159 | 0.593 ± 0.291 |
| OSX cKO | 12 | M&F | 26.3 ± 9.87 | 36.0^ ± 15.2 | 1.03 ± 0.329 | 1.10 ± 0.388 | 0.371 ± 0.147 | 0.444 ± 0.324 |
| Endocortical Bone Analysis | ||||||||
| UBC Control | 12 | F | 37.4 ± 19.5 | 57.0^ ± 23.6 | 1.18 ± 0.442 | 1.29 ± 0.242 | 0.540 ± 0.372 | 0.856 ± 0.269 |
| UBC cKO | 13 | F | 46.2 ± 22.0 | 64.5^ ± 19.2 | 1.65 ± 0.516 | 1.81* ± 0.343 | 0.856 ± 0.446 | 1.21^* ± 0.329 |
| OSX Control | 9 | M&F | 21.8 ± 16.9 | 41.7^ ± 13.6 | 1.18 ± 0.244 | 1.13 ± 0.358 | 0.417 ± 0.128 | 0.542 ± 0.275 |
| OSX cKO | 12 | M&F | 18.3 ± 13.3 | 57.5^* ± 18.2 | 0.987 ± 0.214 | 1.15 ± 0.415 | 0.349 ± 0.116 | 0.733 ± 0.497 |
p<0.05 Non-loaded vs. Loaded within a group
p<0.05 Control Loaded vs. cKO Loaded
4. Discussion
BMP2 has been widely studied for its therapeutic value as a bone-inducing protein, but its role in skeletal healing and homeostasis is less well studied. It has been well established that cellular BMP2 expression at the time of injury is a requirement for endochondral healing [20–22]. However, previous studies knocked out BMP2 in multiple cell populations at once making it impossible to determine which cell types are critical BMP2 sources or which endochondral healing stages are BMP2-dependent (initiation vs cartilage formation vs bone formation). Our objective was to examine the importance of BMP2 produced by different cell types in the post-natal osteogenic processes of endochondral healing, intramembranous healing and loading-induced lamellar bone formation. We used adult mice in which Bmp2 was conditionally targeted in one of three cell populations: all cells (inducibly), osteoblasts, or vascular endothelial cells (Table 7). Our results confirmed the requirement of normal BMP2 expression at the time of injury for endochondral fracture healing, as mice in which Bmp2 was reduced in all cells prior to fracture failed to form a callus. Similar reduction of Bmp2 in vascular endothelial cells (vascular endothelial cadherin-expressing) or osteoblasts (osterix-expressing) did not impact fracture healing in any way. Regarding non-endochondral bone formation, we found that BMP2 reduction did not impair woven bone formation after stress fracture or lamellar bone formation induced by mechanical loading. Taken together our results indicate that osteoblasts and endothelial cells are not a critical source of BMP2 in early endochondral fracture healing, and that non-endochondral bone formation in the adult mouse does not require normal BMP2 expression.
Table 7.
Summary of the osteogenic response to Bmp2 conditional knockout
| Bmp2 conditional knockout | |||
|---|---|---|---|
| Osteogenic Condition | All cells, inducible; UBC-Cre-ERT | Osteoblasts/Osteocytes after P21; Osx-Cre-TetO | Vascular endothelial cells; VEC-Cre |
| Fracture healing (endochondral) | Strongly Affected no callus |
Normal | Normal |
| Stress fracture healing (intramembranous woven bone) | Normal | Mildly Affected slightly reduced expression of Bsp, woven bone BMD |
Normal |
| Non-damaging mechanical loading (lamellar bone) | Normal | Normal | (not tested) |
BMP2 is expressed by multiple cell types during endochondral fracture healing, including cells of the activated periosteum, chondrocytes and osteoblasts [17,19]. The functional significance of BMP2 from different cell types in fracture healing is incompletely understood, but a clear requirement of BMP2 in osteochondral progenitors has been described. Tsuji et al. [20] reported that mice lacking BMP2 in limb osteochondral progenitors (Prx1-expressing cells) failed to form calluses after fracture. Wang et al. reported that inducible deletion of Bmp2 in all cells prevented endochondral healing in a bone graft model [21]. Our results, using a similar approach as Wang et al. to inducibly delete Bmp2, also revealed a lack of callus formation after femoral fracture consistent with previous studies. Importantly, this result indicates that the tamoxifen-inducible Bmp2fl/fl;UBC-CreERT2 model was working in our laboratory. But, when Bmp2 was reduced by a similar amount only in cells that were committed to the osteoblast lineage using OSX-Cre, we observed no defect in callus formation. Thus, normal BMP2 expression by pre-osteoblasts, osteoblasts and osteocytes does not appear to be required for fracture healing. Of note, because we targeted Bmp2 post-natally using doxycycline repression of Cre until day P21, our strategy should target primarily (pre)osteoblasts [32] and not the non-osseous cells types that express osterix transiently earlier in development [33].
Stress fractures heal in part by rapid formation of periosteal woven bone [3], a process similar to intramembranous bone formation that occurs at the margins of a fracture callus [7]. We and others have documented BMP2 upregulation [7,8], with expression localized to endothelial cells of the expanded periosteum as well as in osteoblasts in the nascent woven bone in the first 3 days after stress fracture [7]. Similarly, upregulation and expression of BMP2 in vascular and bone cells has been reported during distraction osteogenesis, another bone formation process that is largely intramembranous [23]. Despite the clear increase in localized expression of BMP2 in these settings, the results of the current study indicate that normal levels of BMP2 are not critical for intramembranous bone healing. Periosteal woven bone formation was unimpaired either by inducible deletion in all cells (UBC-Cre) or by conditional deletion in osteoblast lineage cells (OSX-Cre) or vascular endothelial cells (VEC-Cre). The only effect we observed was an 11% decrease in woven bone BMD in the OSX-Cre cKO mice, suggesting a minor delay in mineralization. Our findings appear to be at odds with two reports describing a critical role for BMP2 in intramembranous bone formation. Alam et al. reported that mice heterozygous for Prx1-Cre driven Bmp2 deletion have modestly impaired bone formation in a distraction osteogenesis model [34], although the authors did note the presence of chondrocytes and fibrocartilage in the distraction gap suggesting that healing was at least in part through an endochondral mechanism. Bais et al. [35] knocked down expression of BMP2 using shRNA and reported diminished endosteal bone formation in mice after marrow ablation. One difference in these studies versus the current study is that the intramembranous bone formation in the stress fracture model is periosteal, compared to endosteal in distraction osteogenesis and marrow ablation. The requirement for BMP2-mediated bone formation may differ between these two environments as evidenced in our lamellar results showing site-specific differences in periosteal and endocortical bone formation between cKO models.
The lack of effect of Bmp2 reduction on periosteal intramembranous bone formation that we observed suggests a compensatory effect by other osteogenic genes. Notably, the stress fracture model we employed here does not lead to an increase in other osteogenic BMP ligands. In unpublished data we observed either no change or downregulation of BMP4, 6 and 7 (Supplemental Table S6). On the other hand, we also observed downregulation of BMP3, a negative regulator of bone formation [36], and downregulation of several BMP antagonists (e.g., chordin, gremlin) [10]. Apart from BMP, other osteogenic mediators are activated in the stress fracture model. Most notably, we have observed strong downregulation of Sost and Dkk1 [10], negative regulators of Wnt signaling in bone, and a recent report describes reduced sclerostin expression in the region of the stress fracture [37]. Thus, it is possible that altered expression of other regulators of osteogenesis is sufficient to overcome a reduction in BMP2.
BMP2 expression is modestly upregulated (~1.8-fold) after non-damaging mechanical loading that leads to lamellar bone formation [10], suggesting it may be important to this post-natal bone modeling process. However, contrary to our hypothesis, we observed no inhibition of increased formation of lamellar bone stimulated by a bout of non-damaging mechanical loading in Bmp2 cKO mice. Measures of periosteal bone formation were not different between cKO and control mice. In fact, endocortical MS/BS and BFR/BS were increased by a greater extent in cKO than controls in the OSX-Cre and UBC-Cre lines, respectively. This finding may seem at odds with the important role of BMP2 for post-natal bone formation reported by us and others. Deletion of Bmp2 in osteochondral progenitors (Prx1-Cre [20]) or in osteoblasts (3.6Col1-Cre [25], OSX-Cre[24]) leads to reduced cortical bone width and area indicating reduced periosteal apposition. However, the timing of deletion in these models was early, during embryonic development. We hypothesize that early deletion of Bmp2 influences bone modeling during post-natal growth, even if the skeleton is relatively normal at birth. In support of this, the bone phenotype was largely prevented by using doxycycline to suppress OSX-mediated Bmp2 deletion until after P21 in the current study, whereas when doxycycline was not administered the OSX-Cre cKO mice were osteopenic with slender bones [24]. Thus, our findings indicate that BMP2 is not critical for normal and loading-induced periosteal apposition in the adult skeleton.
Angiogenesis is required for bone formation during skeletal repair, including endochondral and intramembranous bone healing [12–14]. Because of the pattern of expression of BMP2 in vascular cells, we and others [7,23] have hypothesized that BMP2 is a critical mediator of vascular-bone crosstalk during osteogenesis. The results of the current study do not support this hypothesis, as deletion of BMP2 in cells expressing vascular endothelial cadherin (VECad) did not interfere with bone formation either in full fracture or stress fracture settings. Moreover, these mice have no skeletal phenotype [24]. It is possible that subtle changes in vascularity were present in these mice, as global BMP2 is required for normal vascular development [38], and Yang et al. [25] recently reported decreased vasculature in 3.6Col1-Cre;Bmp2 cKO mice. Nonetheless, given the lack of important effects on skeletal traits we elected not to characterize vascular morphology.
For both of our tissue specific BMP2 knockout mouse lines, OSX-Cre and VEC-Cre, we cannot exclude the possibility that BMP2 from other cell types was abundant enough to exert a compensatory paracrine effect. This is particularly applicable in endochondral healing, whereBMP2 is expressed strongly by proliferating and pre-hypertrophic chondrocytes 5 to 14 days after fracture [17,39]. However, our interpretation of the available evidence is that paracrine BMP2 effects are not likely, and chondrocyte BMP2 would not significantly affect knockout cells. First, developmental studies have shown that BMP2 on its own does not have a large effect radius [40–43]. Only cells within a 500um radius of a BMP2 eluting bead or cells modified to constitutively release BMP2 show phosphorylated SMAD activity or abnormal behavior [40,41,43]. Also, any matrix-bound BMP2 liberated following fracture is not sufficient to rescue healing when cells are not capable of producing adequate BMP2 as shown by the global knockouts used here (UBC-Cre) and by Wang et al [21]. In both models, cKO bones should have normal levels of matrix-bound BMP2 since the knockout was not initiated until shortly before injury. Furthermore, lineage tracing studies showed that cells from BMP2 knockout grafts failed to differentiate correctly even when they were immediately adjacent to wildtype cells [21,22]. Together, these studies suggest that BMP2 mainly acts through an autocrine mechanism.
Several limitations should be considered when interpreting our findings. First, while we are confident in the localization of deletion to the target tissues, as described in our previous report [24] and in the Supplemental Figure S1, the efficiency of deletion was less than complete. Based on mRNA expression of Bmp2 from heart and lung tissue FACS sorted to enrich for endothelial cells we observed a reduction of 90% in VEC-Cre cKO; based on expression of Bmp2 from homogenized bone tissue (without FACS sorting), we observed a reduction of 60% with OSX-Cre and UBC-Cre (Figure S2). Thus, our models represent reductions in the normal levels of BMP2 rather than complete absence. Importantly, despite the imperfect models, we observed that the UBC-Cre inducible deletion resulted in no callus formation after full fracture. This indicates that a 60% reduction in BMP2 was functionally significant. On the other hand, the same inducible deletion did not functionally impact intramembranous or periosteal lamellar bone formation, suggesting that these processes do not depend critically on normal levels of BMP2. It is possible that a more efficient global or osteoblast knockout would have shown an effect. A second limitation is that there may be some cartilage in the periosteal callus formed after stress fracture in the mouse ulnae, as we noted in a report describing the model [30]. Importantly, in the current study we lowered the level of fatigue displacement to generate a less severe stress fracture and thereby minimize cartilage formation. Examination of microCT and dynamic histomorphometric images confirms that regions of cartilage, if present, were small and surrounded intramembranous woven bone.
In conclusion, we confirmed that normal BMP2 expression is required for callus formation after fracture. On the other hand, the formation of periosteal intramembranous bone, either at the margins of the fracture callus or at the site of a stress fracture, was not affected by a reduction in BMP2 expression from conditional deletion either ubiquitously or in osteoblasts or vascular endothelial cells. Finally, loading-induced lamellar bone formation was not impaired by a Bmp2 reduction either ubiquitously or in osteoblasts. Taken together, our findings support the requirement of BMP2 for endochondral bone healing, but indicate that BMP2 is less critical for non-endochondral bone healing. Also, our results also support the hypothesis that the critical role of BMP2 in endochondral healing is associated with the initial steps of cartilage callus formation. In the two mouse lines that did not affect osteo-chondral progenitors or matrix producing chondrocytes (OSX-Cre and VEC-Cre), the callus volume and density in cKO mice was normal for endochondral healing. This suggests that healing proceeded normally through the initial inflammatory and cartilage phases. On the other hand, the one mouse line that affected progenitors and chondrocytes (UBC-Cre) had no fracture callus. Furthermore, when mice from the same global knockout line were given stress fractures, which have little or no cartilage formation, healing was essentially normal. Altogether our results indicate through process of elimination that BMP2 expression in progenitor cells or chondrocytes is necessary for formation of the cartilaginous callus during endochondral healing and not critical for the later stages of bone formation. Targeted deletion in these cell populations is needed conclusively test this hypothesis.
Supplementary Material
Figure 2.
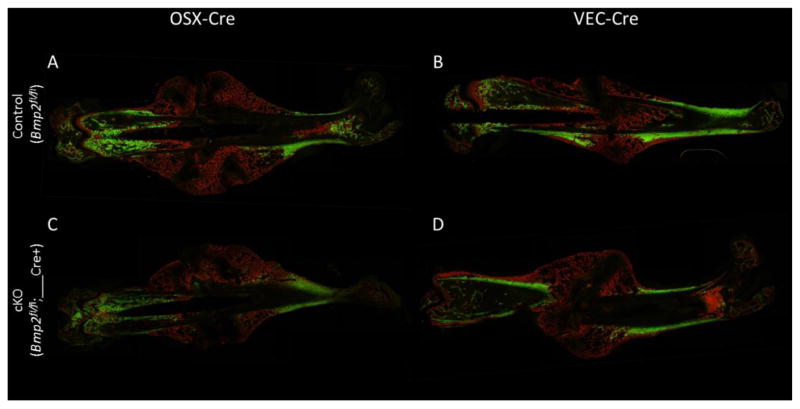
Histological images of callus healing at 14 days post-fracture. Sections are stained with picrosirius red and Alcian blue to mark bone and cartilage, respectively. (A–B) Littermate controls for both OSX-Cre and VEC-Cre demonstrate the range in normal callus healing, e.g., in the size of cartilage callus remaining at 14 days. (C) Despite the variations in normal healing cKO calluses do not differ from their controls.
Highlights.
Bmp2 expression in Osteogenic Linage Cells or Vascular Endothelial Cells is not required for endochondral healing
Bmp2 expression is largely dispensable for cartilage independent bone formation processes (i.e. intramembranous healing or periosteal lamellar modeling).
This study’s results support the hypothesis that BMP2’s critical role in endochondral healing is associated with cartilage callus formation.
Acknowledgments
Grant Supporters:
SM: Washington University T32 Metabolic Skeletal Disorders Training Program (NIH 1T32AR060719-01).
SM, JM, EB, MS: NIH / NIAMS AR050211
SM, JM, EB, MG, MS: Washington University Musculoskeletal Research Center (NIH P30 AR057235).
This work was funded by the Washington University T32 Metabolic Skeletal Disorders Training Program (NIH 1T32AR060719-01, SM), NIH / NIAMS AR050211, the Washington University Musculoskeletal Research Center (NIH P30 AR057235), and the Hope Center Alafi Neuroimaging Lab at Washington University (NIH P30 NS057105).
Abbreviations
- BMP2
Bone Morphogenetic Protein 2
- OSX-Cre
Osterix Promoted Cre
- VEC-Cre
Vascular Endothelial Cadherin Promoted Cre
- UBC-Cre
Ubiquitin C promoted Cre
- BW
body weight
- IP
Intaperitoneal
- qPCR
Quantitative reverse transcription polymerase chain reaction
- TV
Total Volume
- BV
Bone Volume
- BMD
Bone mineral density or Apparent Mineral Density
- TMD
Mineralized Tissue mineral density
- MS/BS
mineralizing surface per bone surface
- MAR
mineral apposition rate
- BFR/BS
bone formation rate per bone surface
- r.MS/BS
Relative mineralizing surface per bone
- S
indicates supplemental information
Footnotes
Supplemental Information: Included.
Author’s Roles: Study Design: SM, JM, MG, and MS. Study Conduct: SM, JM, EB, and MG. Data Collection: SM, JM, EB. Data Interpretation: SM, JM, EB, and MS. Drafting Manuscript: SM & JM. Revising Manuscript Content: SM, JM, EB, MG, and MS. Approving final version of manuscript: SM, JM, EB, MG, and MS. SM, JM, and MS take responsibility for the integrity of the data analysis.
Disclosures:
SM: Travel Support: International Bone and Mineral Society
JM: None
EB: None
MG:
Consultant: DePuy-Synthes, Stryker, RTI Biologics, Pacira Pharmaceuticals, Institutional Research Support: DePuy-Synthes
MS:
Speakers Bureau: Amgen
Institutional Research Support: Merck
Royalties: Springer
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sarah Howe McBride-Gagyi, Email: SMcBrid9@slu.edu.
Jennifer A. McKenzie, Email: McKenzieJ@wudosis.wustl.edu.
Evan G. Buettmann, Email: BuettmannE@wustl.edu.
Michael J. Gardner, Email: GardnerM@wudosis.wustl.edu.
Matthew J. Silva, Email: SilvaM@wudosis.wustl.edu.
References
- 1.Zuscik M. Skeletal Healing. In: Rosen C, editor. Prim Metab Bone Dis Disord Miner Metab. 8. John Wiley & Sons, Inc; 2013. pp. 90–8. [Google Scholar]
- 2.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551–5. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uthgenannt BA, Kramer MH, Hwu JA, Wopenka B, Silva MJ. Skeletal self-repair: stress fracture healing by rapid formation and densification of woven bone. J Bone Miner Res. 2007;22:1548–56. doi: 10.1359/jbmr.0070614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McBride SH, Silva MJ. Adaptive and Injury Response of Bone to Mechanical Loading. Bonekey Osteovision. 2012:1. doi: 10.1038/bonekey.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epari DR, Duda GN, Thompson MS. Mechanobiology of bone healing and regeneration: in vivo models. Proc Inst Mech Eng H. 2010;224:1543–53. doi: 10.1243/09544119JEIM808. [DOI] [PubMed] [Google Scholar]
- 6.Abou-Khalil R, Colnot C. Cellular and molecular bases of skeletal regeneration: What can we learn from genetic mouse models? Bone. 2014;64C:211–21. doi: 10.1016/j.bone.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 7.Wohl GR, Towler DA, Silva MJ. Stress fracture healing: fatigue loading of the rat ulna induces upregulation in expression of osteogenic and angiogenic genes that mimic the intramembranous portion of fracture repair. Bone. 2009;44:320–30. doi: 10.1016/j.bone.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidd LJ, Stephens AS, Kuliwaba JS, Fazzalari NL, Wu ACK, Forwood MR. Temporal pattern of gene expression and histology of stress fracture healing. Bone. 2010;46:369–78. doi: 10.1016/j.bone.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 9.McKenzie JA, Silva MJ. Comparing histological, vascular and molecular responses associated with woven and lamellar bone formation induced by mechanical loading in the rat ulna. Bone. 2011;48:250–8. doi: 10.1016/j.bone.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenzie JA, Bixby EC, Silva MJ. Differential gene expression from microarray analysis distinguishes woven and lamellar bone formation in the rat ulna following mechanical loading. PLoS One. 2011;6:e29328. doi: 10.1371/journal.pone.0029328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hankenson KD, Dishowitz M, Gray C, Schenker M. Angiogenesis in bone regeneration. Injury. 2011;42:556–61. doi: 10.1016/j.injury.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobsen KA, Al-Aql ZS, Wan C, Fitch JL, Stapleton SN, Mason ZD, et al. Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J Bone Miner Res. 2008;23:596–609. doi: 10.1359/jbmr.080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomlinson RE, McKenzie JA, Schmieder AH, Wohl GR, Lanza GM, Silva MJ. Angiogenesis is required for stress fracture healing in rats. Bone. 2013;52:212–9. doi: 10.1016/j.bone.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hausman MR, Schaffler MB, Majeska RJ. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone. 2001;29:560–4. doi: 10.1016/s8756-3282(01)00608-1. [DOI] [PubMed] [Google Scholar]
- 15.Pountos I, Panteli M, Panagiotopoulos E, Jones E, Giannoudis PV. Can we enhance fracture vascularity: What is the evidence? Injury. 2014;45 (Suppl 2):S49–57. doi: 10.1016/j.injury.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Tomlinson RE, Schmieder AH, Quirk JD, Lanza GM, Silva MJ. Antagonizing the αvβ3 Integrin Inhibits Angiogenesis and Impairs Woven but Not Lamellar Bone Formation Induced by Mechanical Loading. J Bone Miner Res. 2014;29:1970–80. doi: 10.1002/jbmr.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu YY, Lieu S, Lu C, Miclau T, Marcucio RS, Colnot C. Immunolocalization of BMPs, BMP antagonists, receptors, and effectors during fracture repair. Bone. 2010;46:841–51. doi: 10.1016/j.bone.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Rosen V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 20:475–80. doi: 10.1016/j.cytogfr.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Kloen P, Lauzier D, Hamdy RC. Co-expression of BMPs and BMP-inhibitors in human fractures and non-unions. Bone. 2012;51:59–68. doi: 10.1016/j.bone.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 20.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–9. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Huang C, Xue M, Zhang X. Expression of endogenous BMP-2 in periosteal progenitor cells is essential for bone healing. Bone. 2011;48:524–32. doi: 10.1016/j.bone.2010.10.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chappuis V, Gamer L, Cox K, Lowery JW, Bosshardt DD, Rosen V. Periosteal BMP2 activity drives bone graft healing. Bone. 2012;51:800–9. doi: 10.1016/j.bone.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Matsubara H, Hogan DE, Morgan EF, Mortlock DP, Einhorn TA, Gerstenfeld LC. Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone. 2012;51:168–80. doi: 10.1016/j.bone.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McBride SH, McKenzie JA, Bedrick BS, Kuhlmann P, Pasteris JD, Rosen V, et al. Long Bone Structure and Strength Depend on BMP2 from Osteoblasts and Osteocytes, but Not Vascular Endothelial Cells. PLoS One. 2015;9:e96862. doi: 10.1371/journal.pone.0096862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang W, Guo D, Harris MA, Cui Y, Gluhak-Heinrich J, Wu J, et al. Bmp2 gene in osteoblasts of periosteum and trabecular bone links bone formation to vascularization and mesenchymal stem cells. J Cell Sci. 2013 doi: 10.1242/jcs.118596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 27.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–44. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 28.Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, et al. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–67. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 29.Hiltunen A, Vuorio E, Aro HT. A standardized experimental fracture in the mouse tibia. J Orthop Res. 1993;11:305–12. doi: 10.1002/jor.1100110219. [DOI] [PubMed] [Google Scholar]
- 30.Martinez MD, Schmid GJ, McKenzie JA, Ornitz DM, Silva MJ. Healing of non-displaced fractures produced by fatigue loading of the mouse ulna. Bone. 2010;46:1604–12. doi: 10.1016/j.bone.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh Y-F, Silva MJ. In vivo fatigue loading of the rat ulna induces both bone formation and resorption and leads to time-related changes in bone mechanical properties and density. J Orthop Res. 2002;20:764–71. doi: 10.1016/S0736-0266(01)00161-9. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Long F. β-catenin promotes bone formation and suppresses bone resorption in postnatal growing mice. J Bone Miner Res. 2013;28:1160–9. doi: 10.1002/jbmr.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Shi Y, Regan J, Karuppaiah K, Ornitz DM, Long F. Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PLoS One. 2014;9:e85161. doi: 10.1371/journal.pone.0085161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alam N, St-Arnaud R, Lauzier D, Rosen V, Hamdy RC. Are endogenous BMPs necessary for bone healing during distraction osteogenesis? Clin Orthop Relat Res. 2009;467:3190–8. doi: 10.1007/s11999-009-1065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bais MV, Wigner N, Young M, Toholka R, Graves DT, Morgan EF, et al. BMP2 is essential for post natal osteogenesis but not for recruitment of osteogenic stem cells. Bone. 2009;45:254–66. doi: 10.1016/j.bone.2009.04.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daluiski A, Engstrand T, Bahamonde ME, Gamer LW, Agius E, Stevenson SL, et al. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet. 2001;27:84–8. doi: 10.1038/83810. [DOI] [PubMed] [Google Scholar]
- 37.Wu AC, Kidd LJ, Cowling NR, Kelly WL, Forwood MR. Osteocyte expression of caspase-3, COX-2, IL-6 and sclerostin are spatially and temporally associated following stress fracture initiation. Bonekey Rep. 2014;3:571. doi: 10.1038/bonekey.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–86. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 39.Onishi T, Ishidou Y, Nagamine T, Yone K, Imamura T, Kato M, et al. Distinct and overlapping patterns of localization of bone morphogenetic protein (BMP) family members and a BMP type II receptor during fracture healing in rats. Bone. 1998;22:605–12. doi: 10.1016/s8756-3282(98)00056-8. [DOI] [PubMed] [Google Scholar]
- 40.Song J, McColl J, Camp E, Kennerley N, Mok GF, McCormick D, et al. Smad1 transcription factor integrates BMP2 and Wnt3a signals in migrating cardiac progenitor cells. Proc Natl Acad Sci U S A. 2014;111:7337–42. doi: 10.1073/pnas.1321764111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrée B, Duprez D, Vorbusch B, Arnold H-H, Brand T. BMP-2 induces ectopic expression of cardiac lineage markers and interferes with somite formation in chicken embryos. Mech Dev. 1998;70:119–31. doi: 10.1016/S0925-4773(97)00186-X. [DOI] [PubMed] [Google Scholar]
- 42.Umulis D, O’Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136:3715–28. doi: 10.1242/dev.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohkawara B, Iemura S, ten Dijke P, Ueno N. Action Range of BMP Is Defined by Its N-Terminal Basic Amino Acid Core. Curr Biol. 2002;12:205–9. doi: 10.1016/S0960-9822(01)00684-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.