Abstract
Alzheimer’s disease (AD) is currently being addressed by intensive investment in pre-clinical and clinical research on the amyloid hypothesis, but concern remains about the validity of the concept that soluble Aβ oligomers are principally responsible for initiating AD phenotypes. Here, we apply well-defined Aβ oligomers isolated from AD brains or made synthetically to document a systematic accrual of first subtle and then more profound changes in certain synaptic proteins in both primary neuronal cultures and behaving adult mice. Among the first (within hours) synaptic changes are selective decreases in surface levels of certain (e.g., GluA1) but not other (e.g., GluN2B) glutamate receptors and subtle microglial activation. After 4 days, numerous additional synaptic proteins are altered. Moreover, Aβ oligomers induce hyperphosphorylation of tau and subsequent neuritic dystrophy. All changes are prevented by scyllo-inositol in a dose-and stereoisomer-specific manner. Mechanistically, scyllo-inositol interferes quantitatively with the binding of Aβ oligomers to plasma membranes. These comprehensive analyses in culture and in vivo provide direct evidence that diffusible oligomers of human Aβ (without plaques) induce multiple phenotypic changes in healthy neurons, indicating their role as principal endogenous cytotoxins in AD. Our data recommend a re-examination of scyllo-inositol as an anti-oligomer therapeutic in humans with early AD.
Keywords: Soluble Aβ oligomers, synapses, microglia, scyllo-inositol, Alzheimer's disease
Introduction
Despite almost two decades of research on the apparent neurotoxicity of various assembly states of amyloid β-protein (Aβ), concerns about the hypothesis that soluble oligomeric forms are the principal initiating neurotoxins in Alzheimer’s disease (AD) remain prevalent (Benilova et al., 2012; Herrup, 2010; Pimplikar et al., 2010; Robakis, 2011; Small and Duff, 2008). The mechanistic uncertainty about whether soluble Aβ oligomers in the human brain are responsible for early and progressive synaptotoxicity has been augmented by the prominent failures of several experimental anti-Aβ therapeutics to achieve their pre-specified cognitive and behavioral endpoints in Phase 3 human trials. Nevertheless, a very large preclinical and clinical effort to validate anti-amyloid agents continues and represents by far the largest sector of AD therapeutic development at this writing. Accordingly, it becomes even more important to attempt to rigorously confirm or deny the effects of well-defined, diffusible Aβ species on neurons, particularly their synapses, and on innate immune cells in the brain.
Here, we have coupled extensive primary neuronal culture analyses of both synthetic and natural (AD brain-derived) soluble Aβ oligomers with the much less commonly used in vivo paradigm of intracerebroventicular (icv) microinjection of oligomers into behaving, wild-type adult mice, in order to examine the temporal evolution of the acute and subacute cellular effects. We have coupled these in vitro and in vivo analyses of oligomer bioactivity with the controlled application of an anti-Aβ small-molecule therapy, scyllo-inositol, in comparison to its stereoisomer, chiro-inositol, which has the same empirical formula but is far less active. We report a diverse array of time-dependent biological effects, including tau hyperphosphorylation and neuritic dystrophy, of soluble oligomers applied at pathophysiologically relevant concentrations. Then, we examine the mechanism of the highly consistent protection that scyllo-inositol provides, both in neuronal culture and in vivo . Our results directly support the Aβ oligomer hypothesis for the initiation of progressive neural injury and also provide a rationale for continuing the clinical development of scyllo-inositol, particularly in light of statistically significant benefits on certain cognitive and biomarker endpoints observed in mild AD patients during a ‘failed’ Phase 2 clinical trial of this natural product (Salloway et al., 2011).
Materials and methods
Human brain sample preparation
Frozen human cerebral cortices were provided by C. Lemere (BWH/HMS) or M. Frosch (MGH/HMS) under IRB-approved human studies protocols and by M. Farrell (Beaumont Hospital, Dublin) in accord with local Ethics Committee guidelines and ERC/IRB approval. Each subject’s clinical and neuropathological diagnoses are provided in Supplementary Table 1. Samples of temporal or frontal cortex containing white and grey matter were weighed. Freshly prepared, ice cold Tris-buffered saline (TBS) consisting of 20 mM Tris-HCl, 150 mM NaCl, pH 7.4, was added to the frozen cortex at 4:1 (TBS volume:brain wet wt) and homogenized with 25 strokes at a setting of 10 on a mechanical Dounce homogenizer. The homogenate was spun at 175,000 g in a TLA100.2 rotor on a Beckman TL 100. The supernate (called TBS extract) was aliquoted and stored at −80°C.
Immunoprecipitation/Western blot (IP/WB) analysis of Aβ
We used an IP/WB protocol described previously (Jin et al., 2011; Shankar et al., 2008; Walsh et al., 2002) to detect Aβ in TBS brain extracts or culture media. These were IP’ed with either Aβ antiserum AW7 (1:50) and Protein A sepharose (PAS; Sigma) or Aβ monoclonal antibody (mAb) 3D6 (3 μg/ml, gift of Elan, plc) and Protein G agarose (PGA; Roche) plus PAS. After bead washing, the immunoprecipitates were eluted with 10 μL 4 % LDS sample buffer, heated at 65 °C for 5 min and centrifuged at 14,000 rpm for 5 min. The supernatant was electrophoresed on a 26-well 4-12 % bis-Tris gel using MES running buffer (Invitrogen). Proteins were transferred to 0.2 μm nitrocellulose and Western blotted (WB) for Aβ with 1 μg/ml each of 6E10 (Covance) + 2G3 + 21F12 (mAb’s from Elan, plc) using the LiCor Odyssey Infrared Imaging System.
Immunoprecipitation (IP)-size exclusion chromatography (SEC)
TBS extracts of AD or control cortex were IP’ed with 3D6 (3 μg/ml) + 15 μL PAS and 15 μL PGA. After bead washing, the precipitates were eluted with 10 μL 4 % LDS sample buffer, heated at 65 °C for 5 min and centrifuged at 14,000 rpm for 5 min. The supernate was transferred to 500 μL TBS. IPed samples or culture medium (500 μL) was injected onto a Superdex 75 (10/30HR) column (Amersham Biosciences, Piscataway, NJ) and eluted at a flow rate of 0.8 ml/min into 1 ml SEC fractions using 50 mM ammonium acetate, pH 8.5. 250 μL were lyophilized, reconstituted in 15 μL of 2X LDS sample buffer, heated at 65 °C for 5 min and used for WB analysis. Soluble Aβ monomer-rich or dimer-rich SEC fractions from AD-TBS and the corresponding fractions from Cont-TBS were pooled separately and lyophilized prior to addition to the culture medium.
Production & characterization of cross-linked synthetic dimers
Aβ40 S26C was synthesized by the Biopolymer Laboratory at UCLA Medical School, and the correct sequence and purity confirmed by amino acid analysis, reverse-phase HPLC and mass spectrometry (Shankar et al., 2008). Disulfide-bonded Aβ dimers were generated by atmospheric oxidation of a 20 μM solution of Aβ40 S26C in 20 mM ammonium bicarbonate, pH 8.0, for 4 days at RT. To facilitate disassembly of aggregates formed during oxidation, the peptide solution was lyophilized and subsequently incubated in 5 M GuHCl, Tris-HCl, pH 8.0, for 4 hr. Disulfide crossed-linked Aβ dimers were then separated from unreacted monomer and higher aggregates by size exclusion chromatography. Briefly, two Superdex 75 10/30 HR columns were linked in series and eluted with 50 mM ammonium acetate, pH 8.5, at a flow rate of 0.5 ml/min. Fractions (0.5 ml) were collected, and an aliquot of each was electrophoresed on 16% tris-tricine polyacrylamide gels and detected by silver staining. SEC fractions found to contain exclusively dimeric Aβ were pooled and the peptide content determined by comparison to known standards. Samples were stored at -80 °C until used.
Hippocampal neuronal cultures
Primary hippocampal cultures were generated from E18 Sprague-Dawley rat embryos (Jin et al., 2011). The hippocampus was dissected in Hank’s Balanced Salt Solution buffered with HEPES and dissociated with 0.125 % trypsin (Invitrogen) for 15 min at 37 °C followed by trituration. Dissociated cells were plated in 6-well plates or 24-well plates with coverslips pre-coated with poly-D lysine (100 μg/ml). After 4 days culturing in Neurobasal medium with B-27 supplement and glutamax (Invitrogen), Half the medium was exchanged every 4 days.
Intracerebroventricular (ICV) injection
Intracerebroventricular (ICV) injection was performed as described previously (Cirrito et al., 2003). Wild type mice (C57/Bl6, 5 mos old, female) were anesthetized using 1.5–2.5 % isoflurane. The head was shaved, and the skin was transected along the midline to expose the skull from several millimeters anterior and posterior to bregma and lambda. The animal was then placed in a small-animal stereotaxic device equipped with dual manipulator arms and an anesthetic mask (David Kopf Instruments, Tujunga, CA). To ensure the skull was level for each animal, measurements and adjustments were made so that bregma and lambda were at equal heights (with 0.1 mm tolerance), as well as 2 points equidistant from midline. Bore holes (0.75 mm) were made above the left cerebroventricle (coordinates: bregma −0.4 mm, 1.0 mm lateral; −2.5 mm relative to dura mater). Synthetic Aβ40 S26C (5 ng, 2.5 μl vol.) was injected at 0.5 μl/min via a Hamilton syringe into the ventricle of anesthetized mice. Mice were sacrificed 1 to 7 days post-injection for analysis.
[Detailed Materials and Methods are available in the Supplementary Materials.]
Results
Soluble A β oligomers isolated from AD cortex impair tau phosphorylation and neurite structure in hippocampal neurons: prevention by scyllo-inositol
To characterize in detail the potential neurotoxicity of soluble Aβ oligomers, we initially isolated endogenous Aβ species from the brains of clinically and neuropathologically typical AD cases using a method we refer to as IP-SEC (Shankar et al., 2008). Tris-buffered saline soluble extracts of AD cortex (AD-TBS) were prepared by ultracentrifugation, and these supernatants were immunoprecipitated (IP) with an Aβ-specific antiserum (AW7). Aβ monomers (AβM) and soluble oligomers, including SDS-stable dimers (AβD), in the resultant precipitates were separated by non-denaturing size-exclusion chromatography (SEC) (Fig. S1A). Identical IP-SEC fractions prepared from TBS extracts of age-matched normal cortex (Cont-TBS) contained no detectable Aβ species (Fig. S1A, B). Primary hippocampal neurons from E18 rat embryos maintained in culture for at least 18 days (≥18 DIV) were exposed to either the human monomers (SEC fractions 12-13 in Fig. S1A)) or dimers (SEC fractions 10-11) (final Aβ concentrations in the CM: ~0.5-2 nM) for 3 days and then fixed for immunocytochemistry (ICC). In accord with our previous work (Jin et al., 2011), application of the Aβ dimer-rich fraction (AβD) induced a marked disruption of neurite integrity after 3 days of exposure, seen as a fine “beaded” pattern of dystrophic neurites by both tubulin and tau ICC (Fig. 1A). In contrast, applications of the same amount of the adjacent monomer-rich SEC fraction (AβM) isolated simultaneously from the same AD cortical extract induced no detectable neurite alteration, serving as an important negative control throughout this study for the oligomer specificity of the effects (Fig.1A). We confirmed the continuous presence of the applied Aβ species by IPing the neuronal CM at the end of the 3-day exposure and recovering the respective monomer or dimer species (Fig. S1B).
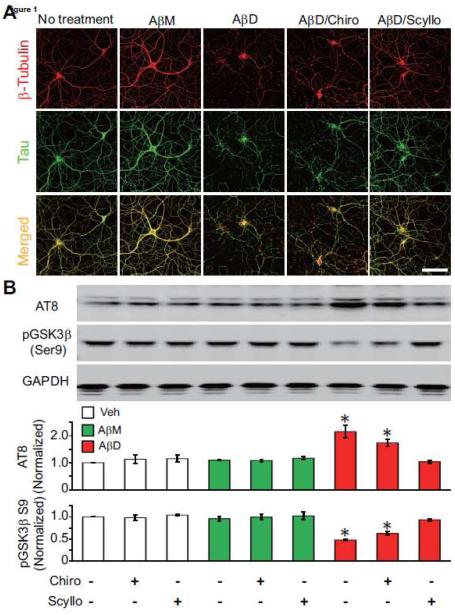
Figure 1. Soluble Aβ oligomers isolated from AD cortex induce neurite disruption and tau phosphorylation in primary hippocampal neurons: prevention by scyllo-inositol.
(A) Confocal micrographs showing the tau (green) and β-tubulin (red) immunoreactivities of the cytoskeleton of hippocampal neurons (DIV21) after 3-day treatment with Aβ monomers or Aβ dimers isolated from AD-TBS in the presence or absence of chiro- or scyllo-inositol (20 μM). Scale bar, 50 μm. (B) Representative Western blots showing the phosphorylation of endogenous rat Tau at the AT8 epitope (Ser202/205) and GSK3β at its auto-regulatory Ser9 epitope in primary hippocampal neurons (DIV19). Cells were treated under the indicated conditions (bottom) for just 1 day. GAPDH is a loading control. Bars: mean levels (N = 4) of phosphorylation of rat tau at the AT8 epitope and GSK3β at Ser9, normalized to the values in sister cultures without treatment. * significantly different from neurons without treatment (p < 0.05 by one-way ANOVA test). Error bars, s.e.m.
To learn whether this invariant effect of the natural AD dimers could be prevented pharmacologically, we co-administered scyllo-inositol (final concentration in the CM: 20 μM) with the Aβ dimer-rich SEC fraction to the mature hippocampal cultures for 3 days. We observed no significant alteration of neuritic architecture and tau and tubulin immunoreactivity compared to the negative controls of AβM treatment or no treatment (Fig. 1A). As a key control for this treatment, co-administering the stereoisomer chiro-inositol, which has the same empirical formula as scyllo-inositol, had no benefit on the neuritic dystrophy induced by the soluble Aβ oligomers (Fig. 1A).
Selectively increased phosphorylation of tau is well-established to contribute to neurodegeneration during the pathogenesis of AD and experimental models thereof [reviewed in (Mandelkow and Mandelkow, 2012)]. The degree of phosphorylation of certain residues within tau is significantly increased in AD cortex, in APP tg mouse brain, and in cultured neurons exposed to soluble Aβ oligomers. We therefore asked whether scyllo- or chiro-inositol can decrease or prevent the hyperphosphorylation of tau at an AD-relevant epitope known to be induced by soluble Aβ oligomers from AD cortex (Jin et al., 2011). After being cultured for 18-19 days, rat hippocampal neurons were exposed to the SEC-isolated human Aβ monomers or dimers for just one day, either without or with co-administration of chiro-or scyllo-inositol, and the phosphorylation state of tau at Ser202/Ser205 (mAb AT8 epitope) was assayed by quantitative western blotting. The AD dimer-rich SEC fraction, but not its matched AD monomer fraction from the same patient, induced a substantial increase in tau phosphorylation at the AT8 epitope (Fig. 1B). Importantly, the Aβ dimer fraction also activated glycogen synthase kinase 3β (GSK3β), the kinase reported to phosphorylate tau at the AT8 epitope (Joachim et al., 1987), as indicated by decreased phosphorylation of GSK3β at serine 9, an epitope known to negatively regulate its activity (Sutherland et al., 1993) (Fig. 1B). Aβ-free SEC fractions prepared identically from age-matched control human cortex (Cont-D; see Fig. S1B) did not induce altered phosphorylation of either protein (Fig. S2A). Treatment of hippocampal neurons with scyllo-or chiro-inositol alone did not change the phosphorylation states of tau or GSK3β at these respective epitopes, but the increased phosphorylation of tau at the AT8 epitope and decreased phosphorylation of GSK3β at serine 9 caused by the AβD fraction was almost fully prevented by co-administering scyllo-but not chiro-inositol (Fig. 1B).
To begin to examine the mechanism of the preventive effect of scyllo-inositol, we pre-treated mature (DIV 18) hippocampal neurons for 24 hr with chiro- or scyllo-inositol alone (20 μM for each), followed by replacement with fresh inositol-free medium and then applying the AβM or AβD SEC fractions alone. Such pre-treatment of the neurons with scyllo-inositol resulted in considerable but not full protection against the Aβ dimer-induced tau hyperphosphorylation and activation of GSK3β (Fig. S2A). This result suggests that the sites at which scyllo-inositol diminishes the Aβ dimer effects exist in part on the neurons themselves.
Prevention of soluble Aβ oligomer binding to the neuronal surface by scyllo-inositol
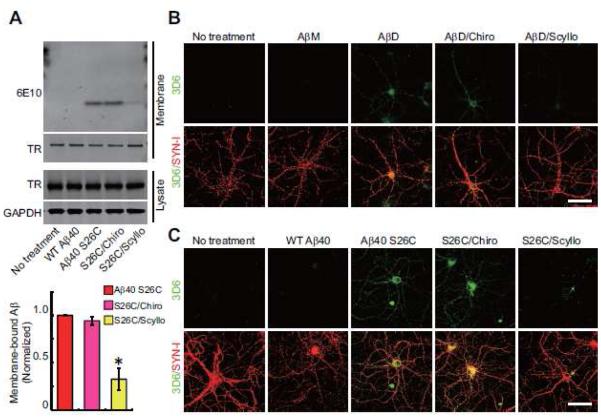
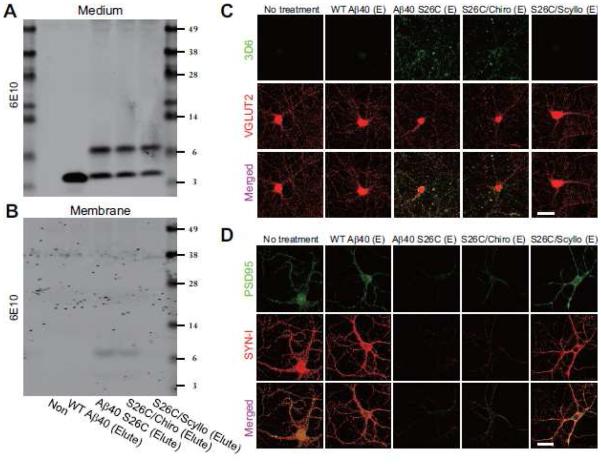
Soluble Aβ oligomers consist of amphipathic Aβ peptide assemblies, and they have been shown in some studies to insert into artificial lipid bilayers (Arispe et al., 1993). It is also reported that synthetic Aβ oligomers or soluble Aβ oligomers extracted from AD brain can bind to the plasma membranes of neurons and thus target synaptic sites in part (Lacor et al., 2007). Here, we examined a synthetic human Aβ40 peptide with serine 26 mutated to cysteine that forms stable, disulfide-bonded dimers [(Aβ40 S26C)2] under oxidizing conditions and can subsequently aggregate into a range of higher order (but still entirely soluble) oligomers and then confer consistent neurotoxic effects (Li et al., 2009; Li et al., 2011; O'Malley et al., 2014). Hippocampal neurons (DIV>18) were treated for 3 hr at 250 nM final concentration with either (Aβ40 S26C)2 or wild-type synthetic Aβ40 (wt Aβ40), which maintains its monomeric state (Fig. S1C, D). Surface biotinylation experiments on the intact neurons post-treatment showed the binding of the Aβ dimers but not the wt monomers to the neuronal plasma membrane by Western blotting (Fig. 2A). In addition, we used 3D6, a monoclonal antibody specific for the free N-terminus (Asp-1) of human Aβ, to immunostain fixed neurons after Aβ treatment under non-permeabilizing conditions. Consistently, punctate 3D6 immunoreactivity was only detected on the somata and neurites of cells treated with Aβ dimers, not monomers (Fig. 2B, C). To test whether the presence of scyllo-inositol can lessen the binding of Aβ dimers to the neuronal surface, scyllo-or chiro-inositol (final concentration 20 μM) was co-administered to hippocampal neurons with either the AβD-rich SEC fractions from human brain (~0.5-2 nM) or synthetic (Aβ40 S26C)2 oligomers (250 nM). The binding of the oligomers to the neuronal plasma membrane was significantly decreased by co-application of scyllo-but not chiro-inositol, as revealed both by surface biotinylation (Fig. 2A) and by surface immunocytochemistry with 3D6 (Fig. 2B, C). The inhibitory effect of scyllo-inositol on the binding capacity of Aβ dimers was dose-dependent, since co-administration of scyllo-inositol at lower concentrations (1 or 2 μM) with (Aβ40 S26C)2 did not significantly decrease the peptide’s binding to the neuronal surface, but doses of 5, 10 and 20 μM did so in that order (Fig. S3A). Interestingly, pre-treatment of the neurons with scyllo-inositol (20 μM) alone for 24 hr followed by wash-out (change to fresh medium) still substantially decreased the subsequent binding of oligomers to the neuronal surface (Fig. S2B).
Figure 2. Prevention of Aβ oligomer binding to the surface of hippocampal neurons by scyllo-inositol.
(A) Representative anti-Aβ Western blot showing streptavidin-precipitated biotinylated Aβ on primary hippocampal neurons (DIV18) after various peptide treatments. Western blotting of transferrin receptor (TR), and TR and GAPDH in the cell lysate served as controls. Bars: mean levels of membrane-bound Aβ; cultures treated with (Aβ40 S26C)2 dimer only were normalized to 1 (*, p<0.01 by one-way ANOVA test). N = 4 independent experiments; error bars, s.e.m. (B , C) Confocal micrographs of hippocampal neurons (DIV21) show surface-bound Aβ detected by 3D6 (green) alone or co-labeled for synapsin I (SYN-I, red) after 3 hr treatment with Aβ monomers or Aβ dimers from AD-TBS (B) or pure, synthetic wt Aβ40 or (Aβ40 S26C)2 dimer (C) in the presence or absence of chiro- or scyllo-inositol (20 μM). Scale bar, 50 μm.
Scyllo-inositol inhibits soluble Aβ oligomer-induced synaptic loss in hippocampal neurons
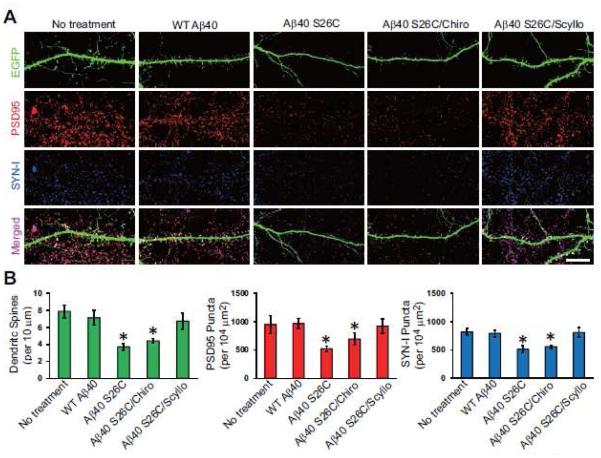
Previous studies suggest that soluble Aβ assemblies, including dimers and oligomers thereof, may serve as a synaptotoxic factor during AD pathogenesis. Next, we asked whether scyllo-inositol also protects neurons from synaptotoxicity induced by the soluble Aβ dimer/oligomer-rich fractions. We treated cultured wt hippocampal neurons (≥18 DIV) which had been transfected with EGFP at DIV 14 with either wt Aβ40 monomers or Aβ40 S26C dimers (each at 250 nM) for 24 hr. After treatment, neurons were fixed and immunolabeled with the pre-synaptic marker synapsin I and the post-synaptic marker PSD95. High-resolution confocal microscopy of the dendrites of mature EGFP-transfected neurons clearly showed the structures of dendritic spines, which are intimately, associated with the synaptic markers PSD95 and synapsin I (Fig. 3A). On the dendrites of the (Aβ40 S26C)2-treated neurons, the density of dendritic spines and synaptic clusters (i.e., PSD95+synapsin I positive puncta) decreased markedly (Fig. 3A, B). However, on the dendrites of neurons treated with the same concentration of wt Aβ40 monomer, there was no significant synapse loss. Co-administration of scyllo-but not chiro-inositol (20 μM) almost completely prevented the alterations of synapses by (Aβ40 S26C)2, as indicated by the densities of dendritic spines and synaptic clusters (Fig. 3). The inhibitory effect of scyllo-inositol on Aβ dimer-induced synaptic loss was also dose-dependent: it could only be observed clearly at concentrations of 5 μM and higher (Fig. S3C-E).
Figure 3. Scyllo-inositol inhibits.
A β oligomer-induced synaptic loss in primary hippocampal neurons. (A) Confocal images showing EGFP-labeled dendritic spines (green), endogenous PSD95 (red) and synapsin I (SYN-I, blue) of hippocampal neurons (DIV21) transfected with EGFP and exposed for 1 d to synthetic wt Aβ40 or (Aβ40 S26C)2 dimer with or without co-administration of chiro-inositol or scyllo-inositol (20 μM). Scale bar, 10 μm. (B) Bars: mean density of dendritic spines and PSD95/SYN-I puncta under different conditions of treatment. * significantly different from neurons without treatment (p < 0.05 by one-way ANOVA test). Fifteen cells per condition were analyzed. Error bars, s.e.m.
To evaluate in more depth the protective effect of scyllo-inositol against the synaptotoxicity induced by soluble Aβ oligomers, we performed surface biotinylation experiments on intact neurons under different treatment conditions. Exposure of neurons for 24 hr to (Aβ40 S26C)2 oligomers (at 250 nM) significantly decreased the levels of the synaptic proteins GluN2A, PSD95, synapsin I and synaptophysin on the neuronal surface, as established by quantitative western blotting after streptavidin pull-down (Fig. S4A, B). In contrast, the protein levels of GluN2B, β2-integrin and transferrin receptor on the neuronal surface were not significantly changed (Fig. S4). This selective decrease of certain synaptic proteins on the neuronal membrane by soluble dimers was completely prevented by co-treatment with scyllo-inositol but only modestly and not significantly ameliorated by chiro-inositol (Fig. S4B).
Soluble Aβ oligomers induce glutamate receptor internalization in hippocampal neurons: prevention by scyllo-inositol
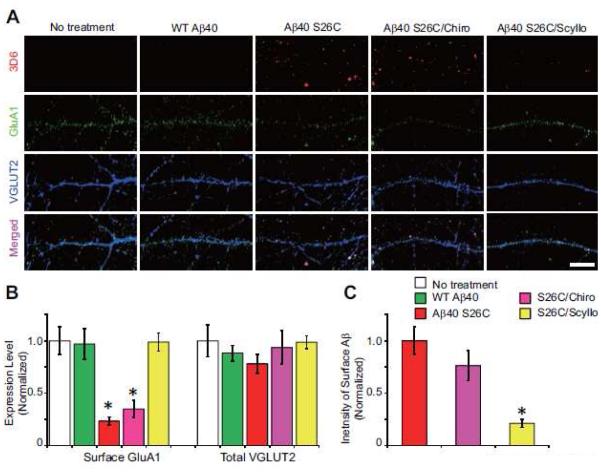
Treatment of hippocampal neurons with soluble Aβ oligomers induced synaptic loss, as indicated by the above decreases in dendritic spines and certain synaptic membrane proteins (e.g. GluN2A). To extend these results, we used surface labeling to detect the distributions of Aβ oligomers and the AMPA receptor subunit 1 (GluA1) on hippocampal neurons under different treatment conditions. Primary neurons (≥18 DIV) were treated with wt Aβ40 monomers or (Aβ40 S26C)2 oligomers (each at 250 nM) for just 3 to 12 hr; then, we simultaneously surface-labeled the treated cells for 10 min with the human-specific Aβ antibody 3D6 and an anti-GluA1 antibody which recognizes its N-terminus. After fixation under non-permeabilizing conditions, surface-labeled Aβ oligomers and GluA1 were detected by fluorophore-conjugated secondary IgGs, as observed by high-resolution confocal microscopy. After treatment for 3 hr, (Aβ40 S26C)2 dimers (but not wt Aβ40 monomers) were immunodetected on the neuronal plasma membrane in a punctate manner and were co-localized in part with puncta of GluA1 and VGLUT2 [Fig. S5A-C]. It should be noted that during this 3 hr treatment, neither Aβ monomers nor dimers significantly decreased the overall levels of GluA1 and VGLUT2 on the neuronal surface (Fig. S5B). However, (Aβ40 S26C)2 oligomers, but not wt Aβ40 monomers, significantly decreased the phosphorylation of GluA1 at serine 845 (Fig. S6). This decrease after just 3 hr is important mechanistically, because phosphorylation at Ser845 was previously reported to be critical for surface stabilization of the GluA1 receptor at synaptic sites (He et al., 2009; Roche et al., 1996). After treatment for 12 hr, (Aβ40 S26C)2 oligomers, but not wt Aβ40 monomers, markedly decreased the surface expression level of GluA1(Fig. 4A, B; Fig. S5D), with a much smaller but still significant decrease of VGLUT2 (Fig. S6A). When the hippocampal neurons were treated with (Aβ40 S26C)2 in the presence of scyllo-inositol for 12 hr, the oligomers no longer bound significantly to the synaptic puncta (Fig. 4A, C), and surface GluA1 clusters were preserved (Fig 4B).
Figure 4. Scyllo-inositol inhibits soluble.
A β oligomer-induced glutamate receptor internalization in hippocampal neurons. (A) Confocal images of surface-labeled Aβ (detected by 3D6, red), surface-labeled GluA1 (green) and total VGLUT2 (blue) in hippocampal neurons (DIV18) after 12 hr treatment with synthetic wt Aβ40 or (Aβ40 S26C)2 dimer with or without co-administration of chiro- or scyllo-inositol (20 μM). Scale bar, 10 μm. (B) and (C) Bars: mean intensity of surface GluA1, total VGLUT2 (B) and surface-labeled Aβ (C) under indicated conditions of treatment. * significantly different from neurons without treatment (p < 0.05 by one-way ANOVA test). Fifteen cells per condition were analyzed. Error bars, s.e.m.
Pre-incubation of scyllo-inositol with soluble Aβ oligomers prevents their binding to neurons and synaptotoxic effects
As described above, scyllo- but not chiro-inositol co-administered with natural (AD) or synthetic Aβ dimers/oligomers in the culture medium consistently decreased their binding to the surface of healthy neurons and their subsequent neurotoxic effects. To further investigate the mechanisms by which scyllo-inositol prevented Aβ oligomer-induced toxicity, we incubated Aβ40 S26C dimers or else wt Aβ40 monomers (each at 500 nM) with scyllo-or chiro-inositol (20 μM) at 4°C for 6 hr in vitro, followed by immunoprecipitation with an anti-Aβ antiserum (R1282) and Protein A-sepharose beads overnight. The Aβ peptides bound to the beads were eluted by glycine (20 mM, pH 3.0), followed by adjusting the pH to 7.4 and exposing primary hippocampal neurons (≥DIV18) to these eluates. After the treatment of neurons, the Aβ species remaining in the CM were detected by Western blotting (Fig. 5A). Consistent with our previous observations, only the (Aβ40 S26C)2 oligomers, not the wt Aβ40 monomers, bound detectably to the surface of intact neurons and induced synaptic loss, as indicated by western blotting the membrane-bound Aβ (Fig. 5B) or by surface immunolabeling of Aβ on the intact cells (using 3D6 and VGLUT2, Fig. 5C). We found that the pre-incubation of Aβ40 S26C oligomers with scyllo-inositol followed by elution and neuronal application significantly decreased the binding ability and the neurotoxic effects of the Aβ dimers on the neurons (Fig. 5B-D). Pre-incubation with chiro-inositol did not have these benefits. Thus, pre-treating soluble Aβ dimers/oligomers with scyllo-inositol alone may change the conformation, residue exposure or other properties of the dimers, thereby decreasing their subsequent binding to the neuronal surface.
Figure 5. Pre-incubation of scyllo-inositol with soluble Aβ oligomers prevents their binding to neurons and synaptotoxic effects.
Representative blots showing eluted A β in culture medium (A) and bound on membrane (B) of hippocampal neurons (DIV18) under different treatments. (C) Confocal images show surface-labeled Aβ (detected by 3D6, green), and total VGLUT2 (red) of hippocampal neurons (DIV18) after 12 hr treatment with eluted wt Aβ40 or (Aβ40 S26C)2 dimer, with or without co-administration of chiro-or scyllo-inositol (20 μM). Scale bar, 20 μm. (D) Confocal images showing endogenous PSD95 (green) and synapsin I (SYN-I, red) in hippocampal neurons (DIV18) after 1 d treatment with eluted synthetic wt Aβ40 or (Aβ40 S26C)2 dimer, with or without co-administration of chiro-or scyllo-inositol (20 μM). Scale bar, 20 μm.
In vivo effects of soluble A β oligomers on synapses and microglia: prevention by scyllo-inositol
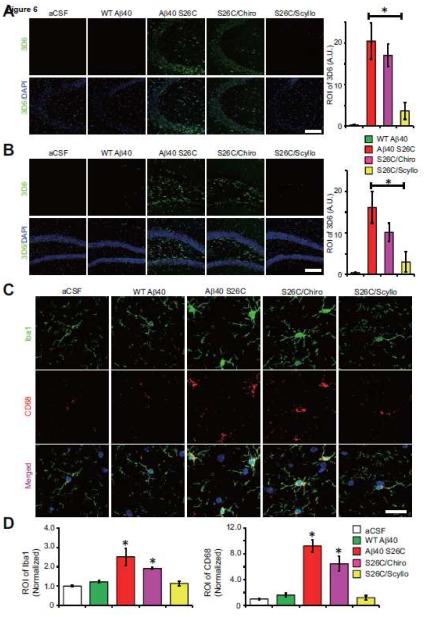
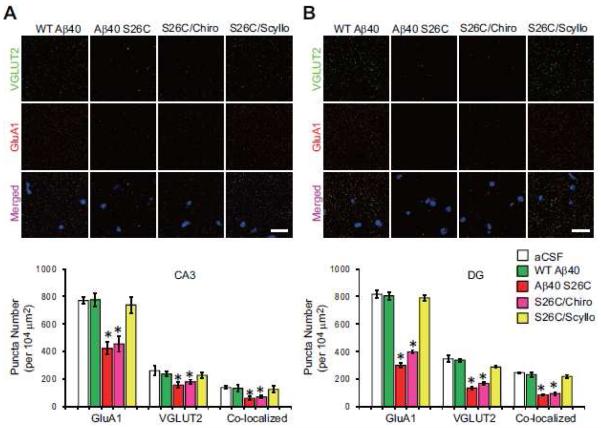
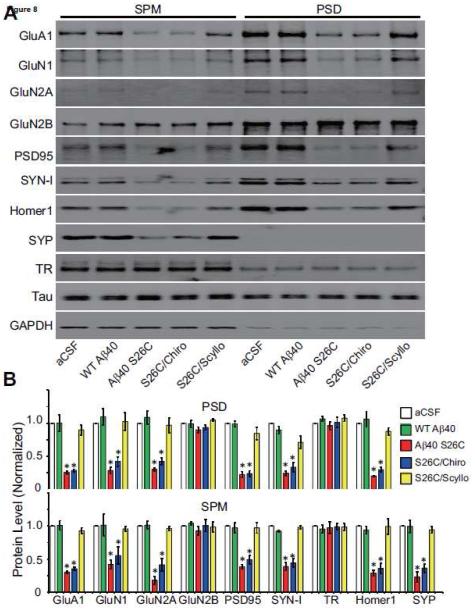
Although the above characterization of the biological effects of Aβ oligomers on healthy primary neurons produced highly consistent and well-controlled results, it was important to obtain in vivo confirmation of such reductionist experiments in the intact living brain. To this end, wt Aβ40 monomers or (Aβ40 S26C)2 dimers (5 ng of each) or vehicle alone (artificial cerebrospinal fluid (aCSF)) were introduced into the cerebrospinal fluid of healthy, wt C57/Bl6 adult mice (age 5-6 mos) by intracerebroventricular (ICV) microinjection. The injected mice were euthanized either 1 or 4 days after treatment. One day post-injection (1 dpi), (Aβ40 S26C)2 oligomers but not Aβ40 monomers were detectable in the hippocampus of the ICV-injected mice by immunohistochemistry (IHC) with the human-Aβ-specific antibody 3D6 (Fig. 6; Fig. S7A). Many of the Aβ-immunoreactive cells were neurons in CA3 and DG of hippocampus, as shown by co-localization of the 3D6 signal with GluA1 (Fig. S7B). ICV injection of (Aβ40 S26C)2 also led after 1 day to modest activation of microglia (compared to the aCSF and wt Aβ40 monomer injections), as revealed by IHC with the microglia marker Iba1 and the general monocyte/macrophage marker CD68 (Fig. S8A, B), but did not yet induce detectable synapse loss in CA3 and DG (Fig. S9A), and there was minimal binding of Aβ40 S26C oligomers to the activated microglia (Fig. S8C). When the (Aβ40 S26C)2 dimers were co-injected with scyllo-inositol, their in vivo binding to hippocampal cells was markedly diminished, while chiro-inositol produced a smaller and non-significant lowering (Fig. 6A, B). At four days post-injection (4 dpi), Aβ40 S26C oligomers induced a more significant activation of microglia and monocytes (Fig. 6C, D), and Aβ40 S26C oligomers were immunodetected as bound to activated microglia (Fig. S8C). Importantly, there was now significant synaptic loss in the molecular layer of CA3/DG, as shown by quantification of puncta by IHC using the pre-synaptic markers VGLUT2 or SYN-I and the post-synaptic markers GluA1 or PSD95 (Fig. 7; Fig. S9D, E). Furthermore, by biochemical fractionation of the hippocampi 4 dpi, we also observed significant decreases of numerous synaptic proteins in the synaptic plasma membrane (SPM) fraction and post-synaptic density (PSD) fraction of the S26C oligomer-injected brains (Fig. 8; Fig. S9B, C). Co-injection of the oligomers with scyllo-but not chiro-inositol largely prevented the microglial activation (Fig. 6C, D; Fig. S8A, B) and synaptic loss (Figs. 7, 8) observed 4 days after injection.
Figure 6. Prevention of Aβ oligomer binding to the neuronal surface and Aβ oligomer-induced activation of microglia in vivo by scyllo-inositol.
Confocal micrographs showing surface membrane-bound Aβ detected by 3D6 (green), and DAPI (blue) in CA3 (A) and DG (B) of hippocampus 1 day after ICV injection with synthetic wt Aβ40 or (Aβ40 S26C)2 dimer in the presence or absence of chiro- or scyllo-inositol (20 μM). Scale bar, 100 μm. Bars: mean intensity of 3D6 labeling under indicated conditions. *significantly different from mice injected with wt Aβ40 (p < 0.05, one-way ANOVA test). Eighteen slices per condition were analyzed. Error bars, s.e.m. (C) Confocal images showing the pattern of microglia labeled by Iba1 (green) and CD68 (red) in CA3 of hippocampus 4 day after ICV injection with synthetic wt Aβ40 or (Aβ40 S26C)2 dimer in the presence or absence of chiro- or Scyllo-inositol (20 μM). Scale bar, 20 μm. (D) Bars: mean intensity of Iba1 and CD68 under different conditions. * significantly different mice injected with aCSF (p < 0.05 by one-way ANOVA test). Fifteen slices per condition were analyzed. Error bars, s.e.m.
Figure 7. Scyllo-inositol inhibits Aβ oligomer-induced synaptic loss in vivo.
(A) and (B) Confocal micrographs showing the pattern of pre-synaptic puncta labeled by VGLUT2 (green), post-synaptic puncta labeled by GluA1 (red), and DAPI (blue) in the molecular layer of CA3 (A) or DG (B) of hippocampus 4 day after ICV injection with synthetic WT Aβ40 or (Aβ40 S26C)2 dimer in the presence or absence of chiro- or scyllo-inositol (20 μM). Scale bar, 20 μm. Bars: mean density of VGLUT2, GluA1 and co-localized synaptic puncta under different conditions. * significantly different from those of mice injected with aCSF (p < 0.05 by one-way ANOVA test). Fifteen slices per condition were analyzed. Error bars, s.e.m.
Figure 8. Scyllo-inositol inhibits Aβ oligomer-induced internalization of synaptic proteins in vivo.
(A) Representative Western blots showing certain membrane proteins in fractions of synaptic plasma membrane (SPM) or post-synaptic density (PSD) isolated from the hippocampus 4 day after ICV injection with synthetic wt Aβ40 or (Aβ40 S26C)2 dimer in the presence or absence of chiro-or scyllo-inositol (20 μM). GAPDH is a control. (B) Bar, average level of synaptic or membrane protein in different conditions normalized to GAPDH. Asterisks indicate data significantly different from those of mice injected with aCSF alone (p < 0.01 by one-way ANOVA test). Data are from 4 independent experiments.
Scyllo-inositol shifts Aβ oligomers to smaller species
Our results above suggest that scyllo-inositol protects neurons from the neurotoxicity induced by soluble Aβ oligomers by preventing the binding of the oligomers to the neuronal surface. Early reports on the activity of scyllo-inositol relevant to AD showed that it can inhibit Aβ fibril assembly and accelerate fibril disassembly in vitro (McLaurin et al., 1998; McLaurin et al., 2000); hence its protective role may also involve prevention of further aggregation of Aβ oligomers. To address this possibility, we directly assessed the aggregation state of wt Aβ40 monomers and (Aβ40 S26C)2 oligomers in the conditioned medium after treatment of primary hippocampal neurons, using SEC and Western blotting (Fig. S10). WB of each SEC fraction of the CMs showed that the co-administration with scyllo-inositol decreased the aggregation state of the S26C oligomers in the CM, as indicated by lowering of the amount of high MW Aβ soluble aggregates in the void volume fraction (fx’ 7 in Fig. S10). In accord, scyllo-inositol co-treatment was also associated with a decrease in the level of soluble dimers in SEC fraction 11 (~ 8 kDa) of the CM and an increase in monomer levels in the late SEC fractions (fx’ 14-16). All three of these quantitative changes were statistically significant (Fig. S10B). In contrast, identical co-treatment with the control stereoisomer, chiro-inositol, produced an SEC signal distribution closely similar to that of untreated oligomers (Figs. S10A, B).
Discussion
The central issue facing the >40 million Alzheimer sufferers worldwide and the many more pre-symptomatic humans currently harboring the disease process is the design and validation of disease-slowing therapeutics. To achieve this urgent goal, scientists must accurately decipher the fundamental biochemical mechanism of the disease. Great effort is now being expended on amyloid-lowering strategies, but how can we be certain that this is an effective approach? An ‘Achilles’ heel’ of the amyloid or Aβ hypothesis of AD is the widely-cited occurrence of some or many β-amyloid deposits in humans dying with little or no evidence of clinically meaningful cognitive impairment. A recent discovery about this apparent paradox is the evidence that such ‘high pathology controls’ can have amyloid plaques as abundant as those in end-stage AD patients but have much lower levels of soluble Aβ oligomers per plaque in the cortex (Esparza et al., 2013). In that work, the advent of selective ELISAs that detect a broad range of soluble Aβ oligomers but not monomers allowed the authors to establish a ratio of soluble Aβ oligomers to amyloid plaque number in postmortem brain tissue. This ratio was found to completely distinguish high pathology control brains from AD brains with no overlap [see Figs. 2J and N in (Esparza et al., 2013)]. Other groups are in the process of obtaining similar results. If this discriminatory difference in the soluble oligomer-to-plaque ratio is confirmed, then the ability to quantitatively sequester soluble Aβ oligomers into fibrillar plaques may help explain why some humans can handle AD-type amyloid plaque pathology with little symptomatic consequence, at least for a time, whereas others develop progressive dementia.
Here, we examine the bioactivity of biochemically defined soluble Aβ oligomers on a range of cellular and biochemical outcomes directly relevant to Alzheimer’s disease. We believe this study has several specific advantages. First, there is no doubt about the identity of the Aβ oligomers: specific immunoprecipitation and SEC of soluble extracts of typical AD brains yields abundant such oligomers, including dimers that exit the SEC column at ~8 kDa as expected, and their bioactivities are closely matched by pure, synthetic Aβ dimers that aggregate into a range of higher MW oligomers (although the synthetic oligomers are far less potent than the natural oligomers in conferring toxicity). Second, the reductionist approach used here allows a temporal analysis of early and direct effects of diffusible Aβ oligomers per se on healthy primary neurons from normal rodents, without the confounding effects of pre-existing biochemical pathology (amyloid deposits, complex cellular changes) observed in adult genetically manipulated rodents. Third, the highly consistent in vitro effects observed in culture can be reproduced in vivo in behaving adult animals via the useful but infrequently used icv microinjection approach, again allowing a dissection of early events prior to frank neurodegeneration and cell death. Fourth, rigorous controls for specificity are provided by observing the lack of effect of equal amounts of soluble Aβ monomers isolated simultaneously in the same SEC run from the same brain extract of the same AD patient (or the ‘dimer-equivalent’ SEC fractions from age-matched normal human brains), and this is again mimicked by pure synthetic Aβ peptide that remains monomeric, compared to synthetic dimers. Fifth, a simple, highly stereospecific natural compound (scyllo-inositol), whose anti-Aβ protective activity has previously been established by several labs in preclinical studies and then shown to confer some symptomatic and biomarker benefits in mild AD patients in a clinical trial (discussed below), invariably prevents the negative effects of the natural and synthetic soluble oligomers, whereas a stereoisomer with the same empirical formula does not. Sixth, the mechanism of this agent is shown here to involve both binding to soluble Aβ oligomers themselves and interference with their binding to neuronal plasma membranes, thereby preventing multiple downstream consequences, including abnormal tau phosphorylation, synaptic protein deficits, decreased receptor endocytosis, neuritic dystrophy and microglial activation. Together, these various findings support the concept that diffusible oligomers of Aβ that bind to the hydrophobic surfaces of neuronal and other plasma membranes can serve as a key initiator of multiple aspects of the AD cellular and biochemical phenotype.
The enhancement by soluble Aβ oligomers of tau phosphorylation at an AD-relevant epitope after 1 day exposure and subsequent neuritic alteration at days 2 and 3 confirms that soluble oligomers present in AD cortex can directly induce the other cardinal abnormality of AD: tau hyperphosphorylation and neuritic dystrophy. But beyond this mechanistic connection between the two key AD lesions, the heterogeneous, time-dependent effects of soluble oligomers on some but not other neuronal receptors and signaling molecules indicate that Aβ oligomers are selective in their negative effects on synaptic protein pathways, at least during the early phase of Aβ neurotoxicity. Our data suggest a temporal hierarchy of response, with the likelihood that over time, many additional neuronal proteins become dysfunctional or lost. Among the first changes we saw in healthy neurons were decreases in the surface levels of synapsin I and synaptophysin pre-synaptically and GluN2A and PSD95 post-synaptically after 24 hours of oligomer binding to the plasma membrane. This was accompanied by a decrease in synapsin I/PSD95 co-labeled synaptic clusters, signifying that synapse loss can begin within 24 hr of previously healthy neurons being bound in a punctate manner on both soma and neurites by diffusible oligomers. At this early stage of fresh oligomer exposure, surface levels of numerous proteins such as GluN2B receptors, β2-integrins and transferrin receptors are not altered. The dynamic, time-dependent effects of this very early oligomer bioactivity are shown by our observing internalization of GluA1 AMPA receptor subunits after 12 hr of surface oligomer binding, but not after just 3 hr, even though the oligomers already co-localize with GluA1 and VGLUT2 at this earlier time point. And even when the surface AMPA receptor level was decreased at 12 hr, VGLUT2 remained unchanged at the same surface loci. When we turned to in vivo exposures, we saw oligomer binding on neuronal surfaces 1 day post-icv injection but no noticeable synapse change, although local microglia already showed modest activation. By day 4 post-injection, the diffusible oligomers had now activated microglia much more strongly, and there was evidence of statistically significant synapse loss, as marked by decreased synapsin I, GluA1, VGLUT2 and PSD95 at the neuronal surface. Now, numerous synaptic and signaling proteins in purified synaptic plasma membrane (SPM) preparations were significantly decreased. These data demonstrate that one could use this paradigm to perform many other timed analyses for a large range of neuronal and microglial proteins of interest in culture or in vivo, but the numerous examples we present here make the point of an evolving attack on the integrity of some but not all (e.g., GluN2B, transferrin) receptors on the neuronal plasma membrane during the first hours to days after the surface binding of entirely soluble Aβ oligomers.
The oligomer specificity of these results is further supported by our use in all the above-summarized analyses of co-treatments with scyllo- vs. chiro-inositol. Co-exposure of neurons to soluble Aβ oligomers plus the former compound, a natural sugar present at lower levels in the brain, invariably protected neurons and microglia from the negative effects of the oligomers. The benefit for preservation of synaptic proteins was tightly dose-dependent, beginning as low as 5 μM (Fig. S3). Thus, partial neutralization of Aβ oligomer effects requires at least 10-20 fold molar excess of scyllo-inositol over soluble oligomers. Mechanistically, we show that pretreatment of healthy neurons with scyllo-inositol followed by washing off excess compound protected the cells from the adverse biochemical effects of subsequent exposure to diffusible oligomers, although not quite as effectively as co-administration. In this context, we find that scyllo-inositol directly interferes with the binding of the oligomers to the neuronal plasma membrane, as shown both by biotinylation and immunocytochemistry of surface-bound Aβ (Fig. 2). Further, we show that pre-incubation of pure Aβ oligomers with scyllo-inositol followed by IP of the peptides and washing can also prevent their cellular binding and synaptotoxicity. Thus, scyllo-inositol decreases the cytotoxicity of soluble Aβ oligomers by quantitatively interfering with their binding to plasma membranes. As a potentially additional mechanism, SEC fractionation of CM from neurons after treatment with Aβ plus scyllo-inositol revealed that the amounts of large soluble oligomers in the void volume and also the ~8 kDa dimers decreased, while free monomer levels increased (Fig. S10), suggesting that scyllo-inositol, by binding to oligomers, may enhance disassembly of Aβ aggregates, in concert with earlier in vitro work (McLaurin et al., 1998; McLaurin et al., 2000). We confirmed that the anti-oligomer protection by scyllo-inositol is as readily observed in living animals (Figs. 6-8) as it is in cell culture (Figs. 1, 3-4).
These new data add to a small body of preclinical and clinical work on scyllo-inositol for AD. Oral treatment of APP transgenic mice with scyllo-inositol decreased the abundance of high MW oligomers of Aβ in the brain, lowered amyloid plaque amounts, decreased microgliosis and astrocytosis, improved synaptic physiology and cognitive impairment, and even delayed premature mortality (McLaurin et al., 2006). In another study, scyllo- but not chiro-inositol dose-dependently prevented hippocampal LTP inhibition in wt mice, and feeding it to wt rats in the drinking water prevented the effects of icv-injected Aβ oligomers on a lever-pressing memory task (Townsend et al., 2006). Most importantly, scyllo-inositol was used to treat humans with mild-moderate AD for 18 months in a double blind, placebo controlled Phase 2 trial (Salloway et al., 2011). The primary endpoints on the Neuropsychological Test Battery (NTB) and the ADCS Activities of Daily Living scale were not met. However, prospectively defined subgroup analyses of the mild AD patients revealed a significant and clinically relevant treatment benefit on the NTB (p=0.007) in the per protocol study (PPS) group, and an exploratory responder analysis likewise favored treatment in this mild subset (p=0.026). Among biomarkers, CSF Aβ42 levels showed a small but significant (p=0.009) decrease in the PPS group.
Conclusion
In summary, we report systematic and detailed analyses of time-dependent changes in select pre- and post-synaptic proteins in healthy wt neurons caused by the surface binding of soluble Aβ oligomer-rich fractions obtained from typical sporadic AD cortices as well as pure, synthetic Aβ dimers and larger but still soluble oligomers thereof. The principal results were confirmed in vivo in wake, behaving adult rats injected icv with the diffusible oligomers. In mature cultured neurons, tau underwent hyperphosphorylation at an AD-relevant epitope followed by widespread neuritic dystrophy. There was also a time-dependent activation of local microglia in culture and in vivo that occurred pari pasu with the synaptic membrane protein changes. All of these in vitro and in vivo effects were prevented by co-administration of scyllo-inositol in a dose- and stereoisomer-specific manner. These well-controlled results provide direct evidence that soluble, diffusible oligomers of human Aβ, in the absence of amyloid plaques, can induce multiple AD-like changes in healthy neurons, thus strongly supporting such species as principal endogenous cytotoxins in AD brain. Further, our data recommend a re-examination of scyllo-inositol as a potential anti-oligomer therapeutic in humans with very mild or pre-symptomatic Alzheimer’s disease.
Supplementary Material
Highlights.
Aβ oligomers induce changes in specific synaptic receptors and signaling proteins.
Aβ oligomers thus have the properties of principal endogenous synaptotoxins of AD.
All synaptotoxic changes by Aβ oligomers are prevented by scyllo-inositol.
Mechanistically, scyllo-inositol decreases Aβ oligomer binding to plasma membranes.
Acknowledgements
We thank D. Walsh (ARCND, BWH) for generously providing the Aβ40 S26C peptides, D. Schenk (formerly of Elan, plc) and F. Bard (Janssen Pharmaceuticals) for the gift of 3D6 and 21F12 antibodies, Ting Yang for technical assistance, and other members of the Selkoe lab for helpful discussions. Supported by NIH grant AG027443 and AG006173 (DJS).
Abbreviations
- Aβ
amyloid β-protein
- AD
Alzheimer’s disease
- IP
immunoprecipitation
- SEC
size exclusion chromatography
- AβM
Aβ monomers
- AβD
LDS-stable Aβ dimers
- Chiro
chiro-inositol
- Scyllo
scyllo-inositol
- ICC
immunocytochemistry
- IHC
immunohistochemistry
- WB
western blotting
- PSD95
postsynaptic density protein 95
- GluN1/2A/2B
NMDA-type glutamate receptor 1/2A/2B
- GluA1
AMPA-type glutamate receptor 1
- SYP
synaptophysin
- SYN-1
synapsin-1
- VGLUT2
vesicular glutamate transporter 2
- wt
wild-type
- icv
intracerebroventicular
- dpi
day post-injection
- DG
dentate gyrus of hippocampus
- CA3
Cornu Ammonis Area 3 of hippocampus
- SPM
synaptic plasma membrane
- PSD
post-synaptic density
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
The authors declare no conflict of interest.
REFERENCES
- Arispe N, et al. Alzheimer disease amyloid β protein forms calcium channels in bilayer membranes: Blockade by trimethamine and aluminum. Proc. Natl. Acad. Sci. USA. 1993;90:567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benilova I, et al. The toxic Abeta oligomer and Alzheimer's disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–57. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003;23:8844–53. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza TJ, et al. Amyloid-beta oligomerization in Alzheimer dementia versus high-pathology controls. Ann Neurol. 2013;73:104–19. doi: 10.1002/ana.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, et al. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci U S A. 2009;106:20033–8. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K. Reimagining Alzheimer's disease--an age-based hypothesis. J Neurosci. 2010;30:16755–62. doi: 10.1523/JNEUROSCI.4521-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, et al. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci U S A. 2011;108:5819–24. doi: 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachim C, et al. Tau epitopes are incorporated into a range of lesions in Alzheimer's disease. J Neuropath Exp Neurol. 1987;46:611–622. doi: 10.1097/00005072-198711000-00001. [DOI] [PubMed] [Google Scholar]
- Lacor PN, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, et al. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, et al. Soluble Abeta oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J Neurosci. 2011;31:6627–38. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med. 2012;2:a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin J, et al. Phosphatidylinositol and inositol involvement in Alzheimer amyloid-beta fibril growth and arrest. J Mol Biol. 1998;278:183–94. doi: 10.1006/jmbi.1998.1677. [DOI] [PubMed] [Google Scholar]
- McLaurin J, et al. Inositol stereoisomers stabilize an oligomeric aggregate of Alzheimer amyloid beta peptide and inhibit abeta-induced toxicity. J Biol Chem. 2000;275:18495–502. doi: 10.1074/jbc.M906994199. [DOI] [PubMed] [Google Scholar]
- McLaurin J, et al. Cyclohexanehexol inhibitors of Abeta aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat Med. 2006;12:801–8. doi: 10.1038/nm1423. [DOI] [PubMed] [Google Scholar]
- O'Malley TT, et al. Abeta dimers differ from monomers in structural propensity, aggregation paths and population of synaptotoxic assemblies. Biochem J. 2014;461:413–26. doi: 10.1042/BJ20140219. [DOI] [PubMed] [Google Scholar]
- Pimplikar SW, et al. Amyloid-independent mechanisms in Alzheimer's disease pathogenesis. J Neurosci. 2010;30:14946–54. doi: 10.1523/JNEUROSCI.4305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robakis NK. Mechanisms of AD neurodegeneration may be independent of Abeta and its derivatives. Neurobiol Aging. 2011;32:372–9. doi: 10.1016/j.neurobiolaging.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, et al. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–88. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Salloway S, et al. A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurology. 2011;77:1253–62. doi: 10.1212/WNL.0b013e3182309fa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Duff K. Linking Abeta and tau in late-onset Alzheimer's disease: a dual pathway hypothesis. Neuron. 2008;60:534–42. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland C, et al. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J. 1993;296:15–9. doi: 10.1042/bj2960015. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M, et al. Orally available compound prevents deficits in memory caused by the Alzheimer amyloid-beta oligomers. Ann Neurol. 2006;60:668–76. doi: 10.1002/ana.21051. [DOI] [PubMed] [Google Scholar]
- Walsh D, et al. Naturally secreted oligomers of the Alzheimer amyloid ?protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.