To the Editor
Efficient expansion of tissue-specific stem cells and progenitor cells is critical for therapeutic strategies in regenerative medicine. 3T3-J2 cells have been used clinically for expansion of human primary epidermal keratinocytes (HPEKs) (Green, 2008). While 3T3-J2 cells were originally derived from mouse embryonic fibroblasts (MEFs), they are vastly superior in their ability to support long-term keratinocyte proliferation (Supplementary Figure S1 online). Determining the mechanistic basis for this effect may identify the strategies to improve the efficacy of therapeutic expansion of epidermal keratinocytes.
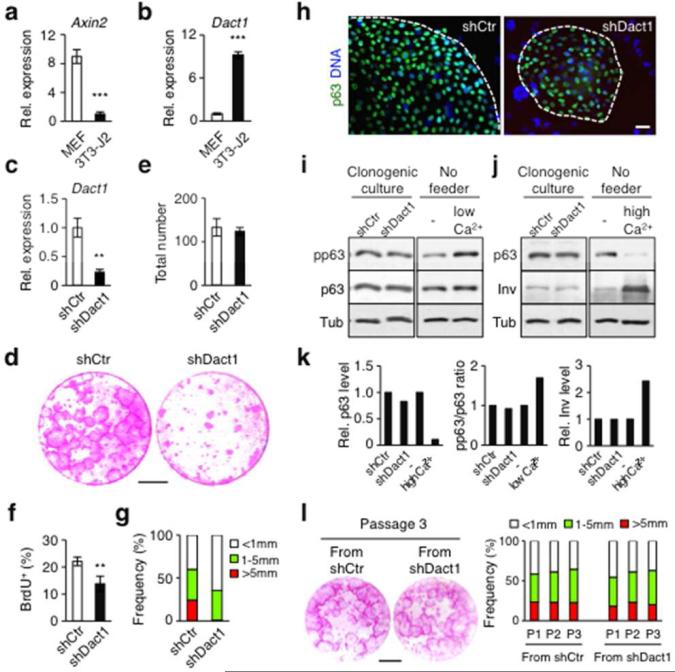
As Wnt/β-catenin signaling stimulates keratinocyte proliferation (Lim et al., 2013), we first asked if increased Wnt expression by 3T3-J2 cells could contribute to long-term keratinocyte proliferation. However, gene expression analysis showed that 3T3-J2 cells and MEFs express Wnt ligands at similar levels (data not shown), suggesting that enhanced Wnt production by 3T3-J2 cells is unlikely to account for their superior ability to support keratinocyte proliferation. Unexpectedly, despite similar levels of Wnt receptors and inhibitors (data not shown), expression of Axin2, a well-characterized Wnt/β-catenin target was significantly lower in 3T3-J2 cells than in MEFs (Figure 1a), suggesting that Wnt/β-catenin signaling is reduced in 3T3-J2 cells. In support, we found that 3T3-J2 cells expressed a significantly higher level of the Wnt/β-catenin antagonist Dact1 (Cheyette et al., 2002) than did MEFs (Figure 1b). As Dact1 can influence both Wnt-dependent and Wnt-independent signaling (Kivimäe et al., 2011), we next set out to determine if Dact1 expression influenced the ability of 3T3-J2 cells to support keratinocyte proliferation.
Figure 1. Suppression of Dact1 in 3T3-J2 cells reduces clonogenic expansion of HPEKs.
(a) Relative expression of Axin2. (b) Expression of Dact1 in 3T3-J2 cells. (c) Suppression of Dact1 by shRNA (shDact1) in 3T3-J2 cells. shCtr, control shRNA. (d) Rhodamine staining of epidermal clones grown for 14 days. Bar=10 mm. (e) Total number of epidermal clones at day 14. (f) Proliferation of keratinocytes in clonogenic culture at day 7. (g) Size distribution of epidermal clones at day 14. (h) Expression of p63 in representative epidermal clones. Bar=20 μm. (i and j) Western blot of keratinocytes from clonogenic cultures. HPEKs stimulated with low (i: 0.3 mM) or high (j: 1.3 mM) calcium, as positive controls. (k) Quantification of i and j. (l) Serial culture of equal plated numbers of keratinocytes, grown for 14 days with γ-irradiated fresh 3T3-J2 cells. Left: rhodamine staining at passage (P) 3. Right: size distribution of epidermal clones over multiple passages. Bar=10 mm. Data shown are mean±SEM (n=3). **P<0.01; ***P<0.001.
Notably, we found that silencing of Dact1 in 3T3-J2 cells reduced keratinocyte proliferation with a corresponding reduction in epidermal clone size in clonogenic culture (Barrandon and Green, 1987) (Figure 1c-g and Supplementary Figure S2a-c online). As enforced Wnt/β-catenin signaling in 3T3-J2 cells by ectopically expressed Wnt/β-catenin ligands had similar effects on keratinocyte proliferation (Supplementary Figure S3 online), these results indicate that Dact1 plays a critical role in the ability of 3T3-J2 cells to support keratinocyte proliferation, potentially by attenuating Wnt/β-catenin signaling in 3T3-J2 cells.
Although less proliferative, epidermal clones in Dact1-silenced 3T3-J2 cell culture expressed p63, a transcription factor essential for the proliferative potential of epidermal stem cells (Senoo et al., 2007), at a similar level as those in control culture (Figure 1h). Further, p63 phosphorylation, an early epidermal differentiation marker (Suzuki and Senoo, 2012), and expression of involucrin, a marker of terminal differentiation (Banks-Schlegel and Green, 1981), were similar in both cultures (Figure 1i-k). Moreover, keratinocytes from both cultures showed similar clonogenic potential in subsequent passages (Figure 1l and Supplementary Figure S2d online). These results indicate that loss of Dact1 in 3T3-J2 cells reduces proliferation of keratinocytes without promoting their differentiation.
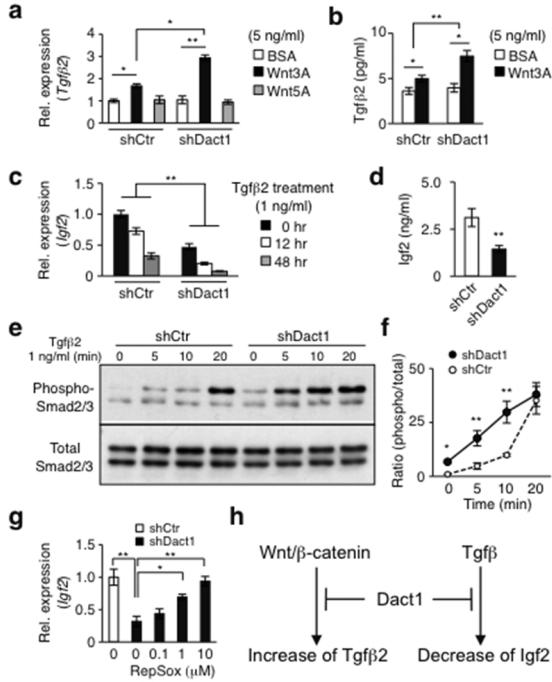
As transforming growth factor-β (Tgfβ) also inhibits proliferation of keratinocytes without influencing their differentiation (Shipley et al., 1986), we next determined if the Dact1- dependent regulation of keratinocyte proliferation by 3T3-J2 cells involved Tgfβ. As HPEKs express Wnt/β-catenin ligands (Lim et al., 2013), we first asked if Dact1-silencing influenced Tgfβ expression in response to exogenous Wnt stimulation. Notably, silencing of Dact1 in 3T3-J2 cells enhanced expression of Tgfβ2, but not Tgfβ1 or Tgfβ3, in response to Wnt3A stimulation compared to control cells at both the mRNA and protein levels (Figure 2a and b). However, the basal Tgfβ2 expression was unchanged in Dact1-silenced 3T3-J2 cells (Figure 2a and b), suggesting that increased Tgfβ2 expression by 3T3-J2 cells required additional Wnt/β-catenin stimuli provided by HPEKs. In support, treatment of 3T3-J2 cells with HPEK-conditioned media stimulated Tgβ2 expression while this effect was significantly suppressed when HPEK-conditioned media were prepared in the presence of LGK974, an inhibitor of Porcupine essential for Wnt secretion (Liu et al., 2013) (Figure S4). These results suggest that in our co-culture system, Dact1 counteracts Tgfβ2 upregulation by 3T3-J2 cells in response to HPEK-derived Wnt ligands, thereby preventing Tgfβ2-mediated inhibition of keratinocyte proliferation.
Figure 2. Dact1 attenuates Wnt and Tgfβ signaling in 3T3-J2 cells.
(a and b) Enhanced Tgfβ2 mRNA (a) and protein (b) levels in Dact1-silenced 3T3-J2 cells at day 4 by Wnt3A. (c) Reduced Igf2 expression by loss of Dact1 and further suppression by Tgfβ2 treatment in Dact1-silenced 3T3-J2 cells. (d) Decreased basal Igf2 protein levels in Dact1-silenced 3T3-J2 cells. (e and f) Enhanced Smad2/3 phosphorylation in Dact1-silenced 3T3-J2 cells. (g) Recovery of Igf2 expression by RepSox in Dact1-silenced 3T3-J2 cells. (h) Model for the role of Dact1. Data shown are mean±SEM (n=3). *P<0.05; **P<0.01.
Among known growth-promoting factors, insulin-like growth factors (Igfs) have been implicated in the ability of 3T3-J2 cells to promote keratinocyte proliferation (Barreca et al., 1992). Consistent with these findings, silencing of Dact1 in 3T3-J2 cells reduced Igf2 at both the mRNA and protein levels while Igf1 was undetectable (Figure 2c and d), suggesting that Dact1 plays an important role in the transcriptional control of Igf2 in 3T3-J2 cells. Moreover, Tgfβ signaling can suppress Igf expression in human mesenchymal stem cells and other murine fibroblasts (Ochiai et al., 2012). Notably, we found that Tgfβ2 treatment further reduced Igf2 expression in Dact1-silenced 3T3-J2 cells, but less so in control 3T3-J2 cells (Figure 2c). These results suggest that Dact1 also attenuates Tgfβ signaling, thereby maintaining the relatively high levels of Igf2 critical for 3T3-J2 cell-mediated keratinocyte proliferation.
In further support of Dact1-mediated attenuation of Tgfβ signaling, we found that Smad2/3 phosphorylation, an indicator of activated Tgfβ signaling (Kamato et al., 2013), was higher in Dact1-silenced 3T3-J2 cells than in control cells in response to Tgfβ signaling (Figure 2e and f). Further, treatment with RepSox, a Tgfβ receptor type I kinase inhibitor (Ichida et al., 2009), restored Igf2 levels in Dact1-silenced 3T3-J2 cells in a dose-dependent manner (Figure 2g). These results indicate that Dact1 counteracts Tgfβ signaling, which otherwise reduces Igf2 levels in 3T3-J2 cells.
Although 3T3-J2 cells have been utilized extensively in the last ~50 years in both stem cell biology and regenerative medicine, their extraordinary capacity to support keratinocyte proliferation is not yet understood. By dissecting signaling pathways, we have revealed the existence of a uniquely configured mechanism in 3T3-J2 cells that optimizes support for keratinocyte proliferation. Our present study shows that Dact1 plays a critical role in this process, likely through attenuation of both Wnt-induced Tgfβ2 expression and Tgfβ-mediated downregulation of Igf2 expression in 3T3-J2 cells (Figure 2h). Although the involvement of other signaling pathways and molecules need to be fully investigated, our current findings hold important implications for dissecting gene programs controlling keratinocyte proliferation and improving the efficacy of therapeutic expansion of epidermal keratinocytes.
Supplementary Material
Acknowledgements
We thank Dr. L. King for critical comments and proof reading of the manuscript, and R. Rengert for editorial assistance. The authors also thank Drs. M. Bhattacharya and J. Tobias for help in the initial development of this study. This work was supported by grants from the Pennsylvania Department of Health, the Skin Disease Research Center (P30AR057217) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR066755) to M.S.
Abbreviations
- Dact1
dapper antagonist of β-catenin 1
- HPEK
human primary epidermal keratinocyte
- Igf
insulin-like growth factor
- MEF
mouse embryonic fibroblast
- Tgfβ
transforming growth factor-β
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Banks-Schlegel S, Green H. Involucrin synthesis and tissue assembly by keratinocytes in natural and cultured human epithelia. J Cell Biol. 1981;90:732–7. doi: 10.1083/jcb.90.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84:2302–6. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreca A, De Luca M, Del Monte P, et al. In vitro paracrine regulation of human keratinocyte growth by fibroblast-derived insulin-like growth factors. J Cell Physiol. 1992;151:262–8. doi: 10.1002/jcp.1041510207. [DOI] [PubMed] [Google Scholar]
- Cheyette BN, Waxman JS, Miller JR, et al. Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev Cell. 2002;2:449–61. doi: 10.1016/s1534-5807(02)00140-5. [DOI] [PubMed] [Google Scholar]
- Green H. The birth of therapy with cultured cells. Bioessays. 2008;30:897–903. doi: 10.1002/bies.20797. [DOI] [PubMed] [Google Scholar]
- Ichida JK, Blanchard J, Lam K, et al. A small-molecule inhibitor of Tgf-beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamato D, Burch ML, Piva TJ, et al. Transforming growth factor-β signalling: role and consequences of Smad linker region phosphorylation. Cell Signal. 2013;25:2017–24. doi: 10.1016/j.cellsig.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Kivimäe S, Yang XY, Cheyette BNR. All Dact (Dapper/Frodo) scaffold proteins dimerize and exhibit conserved interactions with Vangl, Dvl, and serine/threonine kinases. BMC Biochem. 2011;12:33. doi: 10.1186/1471-2091-12-33. doi: 10.1186/1471-2091-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim X, Tan SH, Koh WL, et al. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science. 2013;342:1226–30. doi: 10.1126/science.1239730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Pan S, Hsieh MH, et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci USA. 2013;110:20224–9. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai H, Okada S, Saito A, et al. Inhibition of insulin-like growth factor-1 (IGF-1) expression by prolonged transforming growth factor-β1 (TGF-β1) administration suppresses osteoblast differentiation. J Biol Chem. 2012;287:22654–61. doi: 10.1074/jbc.M111.279091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, et al. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–36. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- Shipley GD, Pittelkow MR, Wille JJ, Jr, et al. Reversible inhibition of normal human prokeratinocyte proliferation by type beta transforming growth factor-growth inhibitor in serum-free medium. Cancer Res. 1986;46:2068–71. [PubMed] [Google Scholar]
- Suzuki D, Senoo M. Increased p63 phosphorylation marks early transition of epidermal stem cells to progenitors. J Invest Dermatol. 2012;132:2461–4. doi: 10.1038/jid.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.