Abstract
Objective
Although Huntington disease (HD) is caused by an autosomal dominant mutation, its phenotypic presentation differs widely. Variability in clinical phenotypes of HD may reflect the existence of disease subtypes. This hypothesis was tested in prodromal participants from the longitudinal Neurobiological Predictors of Huntington's Disease (PREDICT-HD) study.
Method
We performed clustering using longitudinal data assessing motor, cognitive, and depression symptoms. Using data from 521 participants with 2716 data points, we fit growth mixture models (GMM) that identify groups based on multivariate trajectories.
Results
In various GMM, different phases of disease progression were partitioned by progression trajectories of motor and cognitive signs, and by overall level of depression symptoms. More progressed motor signs were accompanied by more progressed cognitive signs, but not always by higher levels of depressive symptoms. In several models, there were at least two groups with similar trajectories for motor and cognitive signs that showed different levels for depression symptoms - one with a very low level of depression and the other with a higher level of depression.
Conclusions
Findings indicate that at least intermediate HD progression might be associated with different levels of depression. Depression is one of the few symptoms that is treatable in HD and has implications for clinical care. Identification of potential depression subtypes may also help to select appropriate patients for clinical trials.
Keywords: Depression, Huntington disease, genetics, cognition, movement disorders
Huntington disease (HD) is an autosomal dominant, progressive, and fatal neurodegenerative disease without any known cure. HD is accompanied by multidimensional signs and symptoms including movement disorder, cognitive decline, and behavioral disturbances caused by a cytosine-adenine-guanine (CAG) repeat in the 5’-translated region of the HTT gene on the short arm of chromosome 4 (The Huntington's Disease Collaborative Research Group, 1993).
Despite the single-gene etiology of HD, there is substantial variation in observed profiles among people with HD (Andrew et al., 1993; Claes et al., 1995; Biglan et al., 2013). For example, some individuals have motor dysfunction at clinical onset accompanied by no changes in mood or cognitive function for extended periods of time. Other individuals show milder motor dysfunction, but have relatively severe changes in mood and/or cognitive function. Still others have concomitant motor, mood, and cognitive signs and symptoms. The variability in clinical presentation is challenging to explain, as it is not accounted for by CAG length and age at diagnosis (Andrew et al., 1993; Claes et al., 1995; Rosenblatt et al., 2012). The identification of phenotypic subtypes within HD may hold promise for improved understanding of pathophysiology, shared mechanisms, genetic influence or environmental impact that may facilitate patient classification and therapeutic development.
One approach to subtype identification is to classify individuals according to their progression level at study entry using demographic (age) and genetic (CAG repeat length) information (Long et al., 2014; Zhang et al., 2011). Such efforts have resulted in many subgroups, most typically varying by what is considered disease progression during the prodromal period (i.e., before motor diagnosis). The Neurobiological Predictors of Huntington's Disease (PREDICT-HD) (Paulsen et al., 2006; Paulsen et al., 2008) and TRACK-HD (Tabrizi et al., 2009) studies have used this strategy to separate prodromal groups into subgroups according to their estimated proximity to motor diagnosis, labeling them either pre-A and pre-B, (Tabrizi et al., 2013), near-to or far-from motor diagnosis (Langbehn et al., 2004), or having a low, medium or high probability of motor diagnosis within the next few years (Zhang et al., 2011).
In contrast, the goal of this analysis is to find subgroups based on multivariate trajectories of signs and symptoms without regard to CAG expansion and age at entry. To our knowledge, no previous article has used longitudinal data to develop subtype models of HD in this manner. The goal of this study is to examine the trajectories of phenotypic variables to identify potential subgroups. One advantage of the exploratory clustering approach is that multiple phenotypic variables can simultaneously be examined, which might be more informative than a typical univariate outcome approach. Another advantage of clustering is that it is not based on a predefined criterion, so that disease subtypes might be discovered that do not necessarily conform to a priori conventional group definitions.
Materials and Methods
Participants
Participants were from the PREDICT-HD study, which is an observational longitudinal study designed to prospectively characterize and refine clinical, neurobiological, and neurobehavioral markers of HD prior to the point of traditional motor diagnosis in a population known to carry the HD CAG expansion (Paulsen et al., 2006; Paulsen et al., 2008; Paulsen et al., 2014). Individuals were recruited from 32 sites in North America, Australia, and Europe beginning in 2001. Participants had to be at least 18 years old and independently tested for the CAG gene mutation before study entry. Motor, cognitive, behavioral, and functional assessments were collected annually and key demographic characteristics were recorded at baseline. Study protocol was approved by each site's institutional review board, and informed consent was obtained from all participants. Baseline disease burden was indexed with the CAG-Age Product (CAP) score, computed as CAP = (Age at baseline) × (CAG–33.66) (Zhang et al., 2011). The CAP score was developed based on an accelerated failure time (AFT) model predicting motor diagnosis from age at entry, CAG length, and their interaction. CAP is similar to the “disease burden” score of Penny, Vonsattel, MacDonald, Gusella, & Myers (1997) and purports to index the cumulative toxicity of mutant huntingtin at the time of study entry.
For this analysis, observations from the first phase of PREDICT-HD (PREDICT-HD 1.0) were used from 2002–2009. To estimate multivariate trajectories for each participant more reliably, we restricted our analysis to participants who had at least four repeated measures for each of the chosen outcomes. This resulted in the exclusion of 300 participants with 661 observations. The final sample consisted of 521 participants and 2716 observations from six years of follow-up with an average of five motor, cognitive, and psychiatric measurements per participant. More details on the longitudinal structure of the sample are presented in Table 1.
Table 1.
Participant characteristics at baseline and the longitudinal structure of the sample
| Variables | N, Mean (SD), Median (Min-Max) or % |
|---|---|
| Number of participants | 521 |
| Age (yrs) | 42.26 (9.99) |
| Sex (% Male) | 38 |
| CAG repeat length | 42.27 (2.42) |
| Education (yrs) | 14.51 (2.67) |
| CAP score | 351.07 (80.78) |
| Antidepressant use (%) | 22 |
| History of depression (%) | 37 |
| Number of follow-ups | 5 (4-7) |
| Time from study entry to last follow-up (yrs) | 4.5 (2.76-6.58) |
| Average time between the repeated measurements (yrs) | 1.04 (0.21) |
Note. yrs = years; CAG = cytosine-adenine-guanine; CAP = CAG-Age Product.
Measures
To represent the triad of HD signs and symptoms, we chose variables to represent typical clinical outcomes in HD representing motor signs (detectable by an examiner), cognitive performance, and depression symptoms (self-report). The variables were selected based on our previous studywide analyses of longitudinal predictive outcomes in PREDICT-HD (Paulsen et al., 2008; Paulsen, Smith, Long, & The PREDICT-HD Investigators of the Huntington Study Group, 2013; Paulsen et al., 2014a, Paulsen et al., 2014b). The motor measure used was the total motor score (TMS) from the Unified Huntington's Disease Rating Scale (UHDRS) (The Huntington Study Group, 1996). The TMS is the sum of 31 items from various domains of motor impairment, each rated by a trained examiner during a standardized motor examination. The TMS is widely used in HD clinical trials and is considered the standard for indexing motor signs (Armstrong & Miyasaki, 2012). The cognitive measure used was the UHDRS Symbol Digit Modalities Test (SDMT), a timed psychomotor assessment requiring matching of symbols with digits using a simple substitution task, with higher scores indicating better cognitive function (Smith, 1982). SDMT was selected because it was the best cognitive performance measure in PREDICT-HD at discriminating between controls and prodromal HD individuals (Stout et al., 2012, Paulsen, Smith, Long, & The PREDICT-HD Investigators of the Huntington Study Group, 2013; Paulsen et al., 2014a). The depression measure used was the Beck Depression Inventory-II (BDI-II), a 21-question multiple-choice self-report inventory, with higher scores indicating more severe depressive symptoms (Beck, Steer, & Brown, 1996). The BDI-II was selected because it is arguably the most widely used self-report measure for assessing depression in HD (Ossig & Storch, 2015; De Souza, Jones & Rickards, 2010), and it has been reported that depression symptom severity is associated with poorer cognitive function in people with prodromal HD (Smith et al. 2012). In addition to the domains described above, we also examined daily functioning using the total functional capacity (TFC) from the UHDRS, which is an examiner-administered five-item checklist that assesses the ability of the participant to accomplish common daily tasks. Motor diagnosis was defined in the conventional form as a Diagnostic Confidence Level (DCL) rating of 4 (unequivocal motor signs of HD; ≥99% confidence) on the UHDRS motor exam.
Statistical Methods
To identify subgroups with distinct profiles, clustering was performed using growth mixture models (GMM) (Muthén & Muthén, 2000). GMM assumes the observed data constitute a mixture distribution that arises as a result of random sampling from several (i.e., more than one) population subgroups. Heterogeneity of the mixture distribution is indicative of the subgroups and can be characterized by a categorical latent variable for the group membership. GMM analysis is concerned with identifying a sufficient number of categories for the latent variables to account for the observed heterogeneity in the longitudinal profiles of the sample data. However, selection of the “correct” number of categories is a notoriously difficult task (Hardy, 1996). For this reason, we consider longitudinal data of the TMS, SDMT, and BDI-II, and examine a wide number of solutions (one to six groups) with associated maximum-likelihood-based fit indexes. To accelerate convergence, all continuous variables except for the time metric (years since study entry) were transformed to standard scores. After each GMM was fit, each individual was assigned to the most likely group, and a secondary analysis was performed to possibly characterize the groups beyond the longitudinal profiles. The following time-invariant baseline covariates were considered in the secondary analysis: age at study entry, CAG expansion, gender, years of education at study entry, TFC score at study entry, rate of diagnosis during follow-up, antidepressant use at study entry, and disease burden at study entry. A complete description of GMM and the detailed group comparison results are available in the appendix.
Results
Growth Mixture Modeling
Participant baseline characteristics and the longitudinal structure of the sample are presented in Table 1. Table 2 shows the fit statistics for the one- to six-group solutions. Goodness-of-fit statistics improved as the number of groups increased. A popular method of evaluating GMM fit is the Bayesian Information Criterion (BIC) (Magidson & Vermunt 2004). As Table 2 shows, the BIC decreased quickly moving from the one-group to two-group model (10.5% BIC decrease) and continued to decrease as the number of groups increased. However, the rate of decrease slowed as the number of groups increased, and the five- and six-group models had very similar BIC values (0.1% BIC decrease moving from the former to the latter).
Table 2.
Model comparison of growth mixture models with different numbers of groups
| No. of Class | No. of Parameters | logL | AIC | BIC | BICadj | BIC Difference (k-1 class vs. k class) | BIC % Change |
|---|---|---|---|---|---|---|---|
| 1 | 30 | −6950 | 14554 | 14681 | 14586 | NA | NA |
| 2 | 61 | −6383 | 12888 | 13147 | 12954 | 1534 | 10.5 % |
| 3 | 92 | −5998 | 12181 | 12572 | 12280 | 575 | 4.4 % |
| 4 | 123 | −5742 | 11729 | 12253 | 11862 | 319 | 2.6 % |
| 5 | 154 | −5575 | 11459 | 12114 | 11625 | 139 | 1.2 % |
| 6 | 185 | −5471 | 11312 | 12099 | 11512 | 15 | 0.1 % |
Note. logL = log likelihood; AIC = Akaike's information criteria; BIC = Bayesian information criteria; BICadj = sample-size adjusted BIC; BIC Difference = BIC difference between k-1 class model and k class model (k≥2); BIC % change = BIC Difference /BIC of k-1 class model (k≥2)
Longitudinal Trajectories
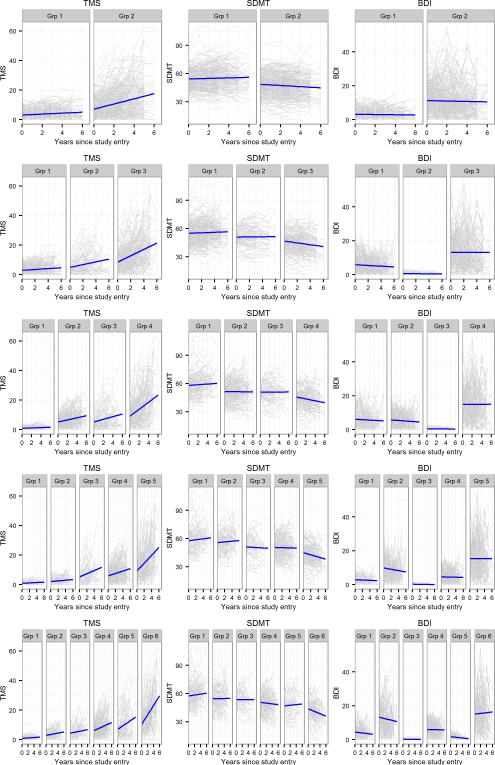
Figure 1 shows the trajectories for TMS, SDMT, and BDI-II by GMM groups (two-group in first row and six-group in last row). Each panel shows individual empirical trajectories (grey thin lines) with the fitted group mean trajectories (blue thick lines) separately for the three outcome variables. In general, as more groups were added, the groups were distinguished by progression trajectories for TMS and SDMT, and by depression levels for the BDI-II. That is, the slope of the longitudinal trajectories for TMS and SDMT indicated increasing progression going from left to right. In contrast, the slope of the depression trajectories did not show systematic worsening. In addition, the overall depression levels varied as the number of groups increased. For all models, the last group showed the worst longitudinal trajectories for motor and cognitive, and the highest depression symptoms. However, the other progression groups had combinations of different levels of depression, and the depression level within a group remained relatively constant over time in many cases.
Figure 1.
Motor, cognitive, and depressive signs and symptoms in people with prodromal HD measured by total motor score (TMS), symbol digit (SDMT), and Beck Depression Inventory (BDI). Each row shows two to six group trajectories for each outcome. Each panel shows variability of individual trajectories (grey) around the group mean trajectory (blue) by group for each outcome. Grp = group.
Variability of Individual Trajectories
As displayed in Figure 1, individual trajectories were distributed around the group mean trajectories and the variability differed by group. Individuals were assigned to a group based on their most likely membership probability (highest posterior probability). For SDMT, the individual trajectory variability was relatively similar across all groups, whereas for TMS, the variability increased from Group 1 to the last group. For the BDI-II, the individual trajectory variation was greatest for the last group and smallest for the group with the lowest level of depression. As an example, in the four-group solution (row 3 of Figure 1), some individuals in the last group had much worse BDI-II scores than the group mean profile, and on the high end were within the range of moderate (20 ≤ BDI-II ≤ 28) or severe depression (29 ≤ BDI-II ≤ 63). Few individuals in groups 1 to 3 had BDI-II scores within the ranges of moderate or severe depression.
Discussion
To our knowledge, this is the first study examining subtypes of multidimensional longitudinal signs and symptoms in people with prodromal HD. Our research is an extension of previous cross-sectional studies which found different phenotypic profiles among people with prodromal HD at the time of diagnosis (Biglan et al., 2013). Using longitudinal data, we employed a clustering approach (GMM) that identifies subgroups based on multivariate trajectories. The results suggest clustering was characterized by increasing rates of longitudinal progression for motor signs and cognitive performance, but by differing and unsystematic levels of depression symptoms. For the cluster solutions with two or more groups, the first group always showed minimal deterioration and the last group always showed the fastest change in motor and cognitive signs, as well as the highest level of depression (see Figure 1). The intermediate groups showed increasing deterioration predominantly in motor signs, but depression did not show a corresponding progression correlation. In fact, the level of depression varied by progression groups, with some similar motor and cognitive progression groups having BDI-II levels reflecting virtually no depression, and others showing higher depression.
The pattern of results reflects characteristics of the PREDICT-HD sample. Participants in the study had a variety of progression levels at study entry. Though the longitudinal profiles spanned several years, progression levels did not change drastically among the participants because HD is a relatively slowly evolving neurodegenerative disease (Ross et al., 2014). Thus, the progression subgroups with different rates of motor and cognitive change should not be considered as fixed types. Rather, each panel offers a snapshot of change for a particular epoch of the disease, with each cohort having a relatively homogeneous level of progression. Based on other analysis from our group (e.g., Paulsen et al., 2014a), there is evidence that individuals with HD might progress along the lines of the pattern suggested by the motor signs panels in Figure 1. The left panels depict no or little change early in the disease, and the right panels depict accelerated deterioration later in the disease.
The novelty of this study is the result concerning depression symptoms. Depression slope and overall level did not track with the other two clustering variables, especially for the groups that might be considered as representing “moderate” or “intermediate” progression in the multiple group solutions (e.g., the six-group solution). The inconsistent correlation of psychiatric symptoms compared with motor and cognitive signs is similar to a previous cross-sectional analysis of depressive symptoms in PREDICT-HD (Epping et al., 2013). Our findings support the idea that individuals with the HD mutation can develop depressive symptoms at any time during the HD prodrome, regardless of their level of motor or cognitive impairment. This is of interest because depression is one of the few symptoms/ signs in HD that is amenable to treatment (Ossig & Storch, 2015; Tyagi, Tyagi, Shekhar, Singh & Kori, 2010). The depression pattern is also interesting regarding the course of HD, as depression symptoms may not index progression in the same sense as motor signs or cognitive performance. Analysis of the BDI-II in isolation shows that it does not discriminate among healthy controls and CAG expanded individuals, and is not good at distinguishing among different levels of HD progression (Paulsen et al., 2014a). The importance of depression may be that it can occur in conjunction with other HD signs/symptoms. In this sense, the findings of this study may help clinicians better understand and implement treatment regimens for persons with HD. Considering the trajectories, several groups showed depression symptoms that may benefit from intervention or may worsen to a severity level requiring treatment. Knowledge of such profiles may assist clinicians to better detect and follow HD patients with depression who may show worsening over time.
The advanced progression represented by the highest cluster group warrants additional study. In all models, the last group showed the most elevated level of depression symptoms, along with the highest levels of motor and cognitive impairments. The last group also had the largest depression variability (see Figure 1), with individuals ranging from severe depression (29 ≤BDI ≤ 63) to minimal depression (BDI ≤ 13). Variability indicates HD progression may or may not be accompanied by clinical depression. One possibility is that the intermediate progression groups may or may not have depression. Because all cohorts end up in the last panel in terms of motor signs and cognitive performance, it could be that constant depression levels could lead to the high observed variability. This finding is consistent with earlier reports of cross-sectional data suggesting the highest levels of depression are found in the phase of disease just prior to diagnosis and in stages II–III where independence in daily living skills decline (Paulsen et al., 2005; Nehl, Ready, Hamilton, & Paulsen, 2001). Additional variables – perhaps genotypic variables – might be needed to account for the inflated variation observed in the most progressed group.
An important consideration is whether the results were influenced by existing depression treatments. For the groups that showed consistently lower levels of depression over time, there is little or no evidence this was due to antidepressant medication. In fact, there was a positive correlation between depression and medication. In the low-level groups, participants reported 5% to 10% of antidepressant use at baseline, which were lower rates than for the groups with higher depression levels. For example, in the 6-group model, for Group 3 and 5, only 6% and 10% of participants respectively, reported taking an antidepressant at baseline. This was in comparison to the other groups that reported antidepressant use of 13% for Group 1, 34% for Group 2, 21% for Group 4, and 41% for Group 6.
The subgroups discovered in this study can be related to groups from previous analysis (Zhang et al., 2011). When the subgroups were compared to baseline CAP groups (Table S1 in the Supplemental Materials), all cluster solutions showed that the first group tended to be comprised mainly of individuals in the Low and Medium groups, whereas the last group tended to include more individuals in the High group. The fact that the cluster analysis produced groups that tended to be stratified by CAP score provides evidence for the validity of CAP as an index for progression. Clustering was based solely on phenotypes, but the groups were roughly characterized by genotype and exposure (age) as computed in CAP. Of interest is that depression does not appear to systematically vary by CAP, except perhaps very early or very late in the disease. There may be contexts in which psychiatric phenotypes, such as depression, may account for variability separately or in addition to CAP. Additional psychiatric domains might show similar results as depression and should be considered in future research.
Our results have potential implications for the planning of HD intervention studies. The success of clinical trials depends on the sensitivity of endpoints for detecting treatment effects.
An important issue in the recruitment of HD participants is whether those recruited are displaying sufficient levels of symptom severity to demonstrate alleviation (Long et al., 2014; Paulsen et al., 2013; Paulsen & Long, 2012). Once a clinical endpoint has been identified (e.g., motor signs), natural history deterioration in untreated patients is necessary for an intervention to show a treatment effect by varying with the natural disease decline. Subgroup analysis might be used to help identify appropriate HD candidates for clinical trials. For example, patients with a profile consistent with the last group in Figure 1 (for all solutions) might be best for studying whether an effective treatment can improve depression, cognition, and motor function simultaneously. Motor and cognitive function show significant longitudinal deterioration and depression shows an elevated overall level. Subgroups of HD without depression may be less useful since it has been reported that depressive symptoms are associated with poorer cognitive performance (Smith et al., 2012) and treatments may improve both depressive symptoms and motor signs (Barone et al., 2010).
There are some limitations of the current study. Our sample might not be representative of all people with prodromal HD since only a limited number of people go through genetic testing and participate voluntarily in the PREDICT-HD study. It has been reported that those who agree to genetic testing expect fewer problems handling positive gene test results (Codori, Hanson, & Brandt, 1994). This suggests depression prevalence in people with prodromal HD might be underestimated by our sample (Epping et al., 2013). As BDI-II is a self-report measure of depression symptoms, it might underestimate depression severity, especially for more progressed people who may have decreased insight or awareness of symptoms (Epping et al., 2013; Duff et al., 2010; Downing et al., 2013; Kim et al., in press).
Another caveat is that no attempt was made to ascertain the “correct” number of groups, though the fit indexes in Table 2 offer an indication of relative importance. There appears to be relatively strong evidence against a one-group solution, but identification of a best multiple-group model is difficult because the fit indexes decreased as the number of groups increased (see Table 2). Solutions beyond six groups were not considered because they did not converge. Though seven-group and higher solutions might be of interest, it is argued that the current results are still useful. The change in the fit statistics for the last two solutions was relatively slight. We might expect even less change for higher-group solutions indicating that the added insight might be limited. Moreover, since the clusters appear to be generated by progression status at study entry, we expect additional groups to represent even greater gradations of initial progression. Given the similarity in conclusions based on our four-, five-, and six-group solutions, it is reasonable that the seven-group and higher solutions would show similar patterns and lead to the same conclusions about the combination of motor signs, cognitive performance, and depression symptoms.
The identification of distinct subgroups was based on the selected outcome measures. The variables we used to cluster participants were not exhaustive, as signs and symptoms can also be influenced by other factors such as comorbid conditions and lifestyle behaviors. Using different outcome measures might yield different subgroups. GMM can also be performed using both the outcome measures and other patient characteristics. This unified model accounts for classification uncertainty and provides unbiased parameter estimates and standard errors for the secondary analyses (McIntosh, 2013). This research can be extended to examine the association between different multidimensional trajectories and a health-related outcome such as a diagnosis of HD.
In summary, we found that people with prodromal HD represent a heterogeneous group with regards to depression symptoms. Identifying progression cohorts with different levels of depression might lead to better treatment regimens and elucidation of the underlying pathophysiology of HD.
Supplementary Material
Acknowledgments
This research is supported by the National Institutes for Health, National Institute of Neurological Disorders and Stroke (5R01NS040068), CHDI Foundation, Inc (A3917) and (6266), Cognitive and Functional Brain Changes in Preclinical Huntington's Disease (HD) (5R01NS054893).
Jane S. Paulsen has received grant funding from the National Institutes of Health, CHDI Foundation, Inc., served on an advisory board for Lundbeck, LLC and has a consulting agreement with ProPhase, LLC. Ji-in Kim, Jeffrey D. Long, James A. Mills, and Elizabeth McCusker have no competing interests to declare.
We thank the PREDICT-HD sites, the study participants, the National Research Roster for Huntington Disease Patients and Families, the Huntington's Disease Society of America and the Huntington Study Group. This publication was supported by the National Center for Advancing Translational Sciences, and the National Institutes of Health (NIH), through Grant 2 UL1 TR000442-06.
Footnotes
PREDICT-HD Investigators, Coordinators, Motor Raters, Cognitive Raters
Isabella De Soriano, Courtney Shadrick, and Amanda Miller (University of Iowa, Iowa City, Iowa, USA);
Edmond Chiu, Joy Preston, Anita Goh, Stephanie Antonopoulos, and Samantha Loi (St. Vincent's Hospital, The University of Melbourne, Kew, Victoria, Australia);
Phyllis Chua, and Angela Komiti (The University of Melbourne, Royal Melbourne Hospital, Melbourne, Victoria, Australia);
Lynn Raymond, Joji Decolongon, Mannie Fan, and Allison Coleman (University of British Columbia, Vancouver, British Columbia, Canada);
Christopher A. Ross, Mark Varvaris, Maryjane Ong, and Nadine Yoritomo (Johns Hopkins University, Baltimore, Maryland, USA);
William M. Mallonee and Greg Suter (Hereditary Neurological Disease Centre, Wichita, Kansas, USA);
Ali Samii, Emily P. Freney, and Alma Macaraeg (University of Washington and VA Puget Sound Health Care System, Seattle, Washington, USA);
Randi Jones, Cathy Wood-Siverio, and Stewart A. Factor (Emory University School of Medicine, Atlanta, Georgia, USA);
Roger A. Barker, Sarah Mason, and Natalie Valle Guzman (John van Geest Centre for Brain Repair, Cambridge, UK);
Elizabeth McCusker, Jane Griffith, Clement Loy, Jillian McMillan, and David Gunn (Westmead Hospital, Sydney, New South Wales, Australia);
Michael Orth, Sigurd Süβmuth, Katrin Barth, Sonja Trautmann, Daniela Schwenk, and Carolin Eschenbach (University of Ulm, Ulm, Germany);
Kimberly Quaid, Melissa Wesson, and Joanne Wojcieszek (Indiana University School of Medicine, Indianapolis, IN, USA);
Mark Guttman, Alanna Sheinberg, Albie Law, and Irita Karmalkar (Centre for Addiction and Mental Health, University of Toronto, Markham, Ontario, Canada);
Susan Perlman and Brian Clemente (UCLA Medical Center, Los Angeles, California, USA);
Michael D. Geschwind, Sharon Sha, Joseph Winer, and Gabriela Satris (University of California, San Francisco, California, USA);
Tom Warner and Maggie Burrows (National Hospital for Neurology and Neurosurgery, London, UK);
Anne Rosser, Kathy Price, and Sarah Hunt (Cardiff University, Cardiff, Wales, UK);
Frederick Marshall, Amy Chesire, Mary Wodarski, and Charlyne Hickey (University of Rochester, Rochester, New York, USA);
Peter Panegyres, Joseph Lee, Maria Tedesco, and Brenton Maxwell (Neurosciences Unit, Graylands, Selby-Lemnos & Special Care Health Services, Perth, Western Australia, Australia);
Joel Perlmutter, Stacey Barton, and Shineeka Smith (Washington University, St. Louis, Missouri, USA);
Zosia Miedzybrodzka, Daniela Rae, Vivien Vaughan, and Mariella D'Alessandro (Clinical Genetics Centre, Aberdeen, Scotland, UK);
David Craufurd, Judith Bek, and Elizabeth Howard (University of Manchester, Manchester, UK);
Pietro Mazzoni, Karen Marder, and Paula Wasserman (Columbia University Medical Center, New York, New York, USA);
Rajeev Kumar, Diane Erickson, Christina Reeves, and Breanna Nickels (Colorado Neurological Institute, Englewood, Colorado, USA);
Vicki Wheelock, Lisa Kjer, Amanda Martin, and Sarah Farias (University of California, Davis, Sacramento, California, USA);
Wayne Martin, Oksana Suchowersky, Pamela King, Marguerite Wieler, and Satwinder Sran (University of Alberta, Edmonton, Alberta, Canada);
Anwar Ahmed, Stephen Rao, Christine Reece, Alex Bura, and Lyla Mourany (Cleveland Clinic Foundation, Cleveland, Ohio, USA);
Executive Committee
Principal Investigator Jane S. Paulsen, Jeffrey D. Long, Hans J. Johnson, Thomas Brashers-Krug, Phil Danzer, Amanda Miller, H. Jeremy Bockholt, and Kelsey Montross.
Scientific Consultants
Deborah Harrington (University of California, San Diego); Holly Westervelt (Rhode Island Hospital/Alpert Medical School of Brown University); Elizabeth Aylward (Seattle Children's Research Institute); Stephen Rao (Cleveland Clinic); David J. Moser, Janet Williams, Nancy Downing, Vincent A. Magnotta, Hans J. Johnson, Thomas Brashers-Krug, Jatin Vaidya, Daniel O'Leary, and Eun Young Kim (University of Iowa).
Core Sections
Biostatistics: Jeffrey D. Long, Ji-In Kim, Spencer Lourens (University of Iowa); Ying Zhang and Wenjing Lu (University of Indiana).
Ethics: Cheryl Erwin (Texas Tech University Health Sciences Center); Thomas Brashers-Krug, Janet Williams (University of Iowa); and Martha Nance (University of Minnesota).
Biomedical Informatics: H. Jeremy Bockholt, Jason Evans, and Roland Zschiegner (University of Iowa).
Contributor Information
Ji-in Kim, Department of Psychiatry, Carver College of Medicine, University of Iowa.
Jeffrey D. Long, Department of Psychiatry, Carver College of Medicine, and Department of Biostatistics, College of Public Health, University of Iowa
James A. Mills, Department of Psychiatry, Carver College of Medicine, University of Iowa
Elizabeth McCusker, Department of Neurology, Westmead Hospital, University of Sydney.
Jane S. Paulsen, Departments of Psychiatry and Neurology, Carver College of Medicine, and Department of Psychology, University of Iowa.
References
- Anderson MJ, Miyasaki JM. Evidence-based guideline: Pharmacologic treatment of chorea in Huntington disease: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2012;79:597–603. doi: 10.1212/WNL.0b013e318263c443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew SE, Goldberg YP, Kremer B, Telenius H, Theilmann J, Adam S, Hayden MR. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nature Genetics. 1993;4:398–403. doi: 10.1038/ng0893-398. [DOI] [PubMed] [Google Scholar]
- Barone P, Poewe W, Albrecht S, Debieuvre C, Massey D, Rascol O, Weintraub D. Pramipexole for the treatment of depressive signs in patients with Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurology. 2010;9:573–580. doi: 10.1016/S1474-4422(10)70106-X. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GW. Beck Depression Inventory-II Manual. The Psychological Corporation; NY: 1996. [Google Scholar]
- Biglan KM, Zhang Y, Long JD, Geschwind M, Kang GA, Killoran A, The PREDICT-HD Investigators of the Huntington Study Group Refining the diagnosis of Huntington disease: the PREDICT-HD study. Frontiers in Aging Neuroscience. 2013;5:12. doi: 10.3389/fnagi.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes S, Van Zand K, Legius E, Dom R, Malfroid M, Baro F, Cassiman JJ. Correlations between triplet repeat expansion and clinical features in Huntington's disease. Archives of Neurology. 1995;52:749–753. doi: 10.1001/archneur.1995.00540320021009. [DOI] [PubMed] [Google Scholar]
- Codori AM, Hanson R, Brandt J. Self-selection in predictive testing for Huntington's disease. American Journal of Medical Genetics. 1994;54:167–173. doi: 10.1002/ajmg.1320540303. [DOI] [PubMed] [Google Scholar]
- De Souza J, Jones LA, Rickards H. Validation of self-report depression rating scales in Huntington's disease. Movement Disorders. 2010;25:91–96. doi: 10.1002/mds.22837. [DOI] [PubMed] [Google Scholar]
- Downing NR, Kim J-I, Williams JK, Long JD, Mills JA, Paulsen JS, PREDICT-HD Investigators and Coordinators of the Huntington Study Group WHODAS 2.0 in prodromal Huntington disease: measures of functioning in neuropsychiatric disease. European Journal of Human Genetics. 2014;22:958–963. doi: 10.1038/ejhg.2013.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Wang C, Stout JC, The PREDICT-HD Investigators of the Huntington Study Group “Frontal” behaviors before the diagnosis of Huntington's disease and their relationship to markers of disease progression: evidence of early lack of awareness. Journal of Neuropsychiatry and Clinical Neurosciences. 2010;22:196–207. doi: 10.1176/appi.neuropsych.22.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping EA, Mills JA, Beglinger LJ, Fiedorowicz LJ, Craufurd D, Smith MM, The PREDICT-HD Investigators and Coordinators of the Huntington Study Group Characterization of depression in prodromal Huntington disease in the neurobiological predictors of HD (PREDICT-HD) study. Journal of Psychiatric Research. 2013;47:1423–1431. doi: 10.1016/j.jpsychires.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy A. On the number of clusters. Computational Statistics & Data Analysis. 1996;23:83–96. [Google Scholar]
- The Huntington's Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- The Huntington Study Group Unified Huntington's Disease Rating Scale: reliability and consistency. Movement Disorders. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, National Comorbidity Survey Replication The epidemiology of major depressive disorder: Results from the national comorbidity survey replication (ncs-r). The Journal of the American Medical Association. 2003;28:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kim J-I, Long JD, Mills JA, Downing N, Williams JK, Paulsen JS, PREDICT-HD Investigators and Coordinators of the Huntington Study Group Performance of the 12-item WHODAS 2.0 in prodromal Huntington disease. European Journal of Human Genetics. doi: 10.1038/ejhg.2015.11. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR, The International Huntington's Disease Collaborative Group A new model for prediction of the age of onset and penetrance for Huntington's disease based on CAG length. Clinical Genetics. 2004;65:267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- Long JD, Paulsen JS, Marder K, Zhang Y, Kim JI, Mills JA, The Researchers of the PREDICT-HD Huntington's Study Group Tracking motor impairments in the progression of Huntington Disease. Movement Disorders. 2014;29:311–319. doi: 10.1002/mds.25657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magidson J, Vermunt J. Latent class models. In: Kaplan D, editor. Handbook of quantitative methodology for the social sciences. Sage; Newbury Park, CA: 2004. pp. 175–198. [Google Scholar]
- McIntosh CN. Pitfalls in subgroup analysis based on growth mixture models: a commentary on Van Leeuwen et al. (2012). Quality of Life Research. 2013;22:2625–2629. doi: 10.1007/s11136-013-0385-x. [DOI] [PubMed] [Google Scholar]
- Muthén B, Muthén LK. Integrating Person-Centered and Variable-Centered Analyses: Growth Mixture Modeling With Latent Trajectory Classes. Alcoholism: Clinical and Experimental Research. 2000;24:882–891. [PubMed] [Google Scholar]
- Nehl C, Ready RE, Hamilton J, Paulsen JS. Effects of Depression on Working Memory in Presymptomatic Huntington's Disease. Journal of Neuropsychiatry and Clinical Neurosciences. 2001;13:342–346. doi: 10.1176/jnp.13.3.342. [DOI] [PubMed] [Google Scholar]
- Ossig C, Storch A. Depression in Huntington's Disease. In: Reichmann H, editor. Neuropsychiatric Symptoms of Movement Disorders. Springer; Switzerland: 2015. pp. 201–209. [Google Scholar]
- Paulsen JS, Hayden M, Stout JC, Langbehn DR, Aylward E, Ross CA, The PREDICT-HD Investigators of the Huntington Study Group Preparing for preventive clinical trials: the PREDICT-HD Study. Archives of Neurology. 2006;63:883–890. doi: 10.1001/archneur.63.6.883. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, The PREDICT-HD Investigators and Coordinators of the Huntington Study Group Detection of Huntington's disease decades before diagnosis: the PREDICT-HD Study. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79:874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Long JD. Neurodegenerative disease: Establishing a clinical trial battery for Huntington disease. Nature Reviews Neurology. 2012;8:250–251. doi: 10.1038/nrneurol.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Long JD, Johnson HJ, Aylward EH, Ross CA, Williams JK, The PREDICT-HD Investigators and Coordinators of the Huntington Study Group Clinical and Biomarker Changes in Premanifest Huntington Disease Show Trial Feasibility: A Decade of the PREDICT-HD Study. Frontiers in Aging Neuroscience. 2014a;6:78. doi: 10.3389/fnagi.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Long JD, Ross CA, Harrington DL, Erwin CJ, Williams JK, the PREDICT-HD Investigators and Coordinators of the Huntington Study Group Prediction of manifest Huntington's disease with clinical and imaging measures: a prospective observational study. Lancet Neurology. 2014b;13:1193–1201. doi: 10.1016/S1474-4422(14)70238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Nehl C, Hoth KF, Kanz JE, Benjamin M, Conybeare R, Turner B. Depression and Stages of Huntington's Disease. Journal of Neuropsychiatry and Clinical Neurosciences. 2005;17:496–502. doi: 10.1176/jnp.17.4.496. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Smith MM, Long JD, The PREDICT-HD Investigators and Coordinators of the Huntington Study Group Cognitive decline in prodromal Huntington Disease: implications for clinical trials. Journal of Neurology, Neurosurgery, and Psychiatry. 2013;84:1233–1239. doi: 10.1136/jnnp-2013-305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney JB, Jr., Vonsattel JP, MacDonald ME, Gusella JF, Myers RH. CAG repeat number governs the development rate of pathology in Huntington's disease. Annals of Neurology. 1997;41:689–692. doi: 10.1002/ana.410410521. [DOI] [PubMed] [Google Scholar]
- Rosenblatt AB, Kumar BV, Mo A, Welsh CS, Margolis RL, Ross CA. Age, CAG repeat length, and clinical progression in Huntington's disease. Movement Disorders. 2012;27:272–276. doi: 10.1002/mds.24024. [DOI] [PubMed] [Google Scholar]
- Ross CA, Aylward EH, Wild EJ, Langbehn DR, Long JD, Warner JH, Tabrizi SJ. Huntington disease: natural history, biomarkers and prospects for therapeutics. Nature Reviews: Neurology. 2014;10:204–216. doi: 10.1038/nrneurol.2014.24. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test: Manual. Western Psychological Services; Torrance, CA: 1982. [Google Scholar]
- Smith MM, Mills JA, Epping EA, Westervelt HJ, Paulsen JS, The PREDICT-HD Investigators and Coordinators of the Huntington Study Group Depressive sign severity is related to poorer cognitive performance in prodromal Huntington disease. Neuropsychology. 2012;26:664–669. doi: 10.1037/a0029218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Jones R, Labuschagne I, O'Regan AM, Say MJ, Dumas EM, Frost C. Evaluation of longitudinal 12 and 24 month cognitive outcomes in premanifest and early Huntington's disease. Journal of Neurology, Neurosurgery and Psychiatry. 2012;83:687–694. doi: 10.1136/jnnp-2011-301940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, The TRACK-HD Investigators Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurology. 2009;8:791–801. doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi SJ, Scahill RI, Owen G, Durr A, Leavitt BR, Roos RA, The TRACK-HD Investigators Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington's disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurology. 2013;12:637–649. doi: 10.1016/S1474-4422(13)70088-7. [DOI] [PubMed] [Google Scholar]
- Tyagi SN, Tyagi LK, Shekhar R, Singh M, Kori ML. Symptomatic Treatment and Management of Huntington's Disease: An Overview. Global Journal of Pharmacology. 2010;4:6–12. [Google Scholar]
- Zhang Y, Long JD, Mills JA, Warner JH, Lu W, Paulsen JS, The PREDICT-HD Investigators and Coordinators of the Huntington Study Group Indexing disease progression at study entry with individuals at-risk for Huntington disease. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2011;156:751–763. doi: 10.1002/ajmg.b.31232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.