Abstract
While melanoma is believed to be a highly immunogenic tumor and recent developments in immunotherapies are promising. Interferon-γ (IFN-γ) produced by immune cells plays a crucial role in tumor immune surveillance; however, it has also been reported to be pro-tumorigenic. In the current study, we found that IFN-γ enhances the expression of CD74, which interacts with its ligand, macrophage migration inhibitory factor (MIF), and thereby activates the PI3K/AKT pathway in melanoma, promoting tumor survival. IFN-γ increased phosphorylation of AKT Ser473 and upregulated total and cell surface expression of CD74 in human melanoma cell lines tested. CD74 was highly expressed in melanoma tissues. Moreover, the expression of CD74 on tumor cells correlated with plasma IFN-γ levels in melanoma patient samples. In our analysis of melanoma cell lines, all produced MIF constitutively. Blockade of CD74-MIF interaction reduced AKT phosphorylation and expression of pro-tumorigenic molecules, including interleukin-6, interleukin-8 and BCL-2. Inhibition of CD74-MIF interaction significantly suppressed tumor growth in the presence of IFN-γ in our xenograft mouse model. Thus, we conclude that IFN-γ promotes melanoma cell survival by regulating CD74-MIF signaling, suggesting that targeting the CD74-MIF interaction under IFN-γ–stimulatory conditions would be an effective therapeutic approach for melanoma.
Keywords: CD74, interferon-γ, AKT, MIF, inflammation
INTRODUCTION
Melanoma, the most aggressive skin cancer, is believed to be a highly immunogenic tumor. Therefore numerous immunotherapies such as cytokines, cancer vaccines and adoptive cell therapies have been developed (Gao et al., 2013; Zikich et al., 2013). Recent remarkable advances in immunotherapy have provided immune checkpoint inhibitors, which showed impressive clinical benefits, as treatment options for patients with metastatic melanoma (Pardoll, 2012; Robert et al., 2015). Although these immunotherapies have generated durable responses, strategies to improve response rate are still urgently required (Azijli et al., 2014; Zikich et al., 2013).
Interferon-γ (IFN-γ), a cytokine produced by immune cells, plays a crucial role in cancer immune surveillance, promoting antitumor responses (Dunn et al., 2006). IFN-γ also has a direct antitumor effect on tumor cells, including melanoma (Brown et al., 1987; Garbe et al., 1990; Gollob et al., 2005). On the other hand, several reports have suggested that IFN-γ may also have pro-tumorigenic effects in solid tumors under certain circumstances (Garbe et al., 1990; Porter et al., 2001; Taniguchi et al., 1987). A recent study in an ultraviolet B–irradiated mouse skin cancer model demonstrated that IFN-γ produced by macrophages promotes melanoma growth by inhibiting apoptosis (Zaidi et al., 2011). In fact, a Southwest Oncology Group randomized clinical trial showed that IFN-γ had an adverse effect on melanoma relapse and mortality rates (Meyskens et al., 1995). However, the molecular mechanisms of IFN-γ–mediated pro-tumor effects are not yet fully understood. Thus, elucidating the key signaling pathways related to melanoma cell survival, which are implicated in pro-tumor IFN-γ function, may provide new insight into potential therapeutic targets and strategies. CD74, also known as major histocompatibility complex (MHC) class II-associated invariant chain, is a type II transmembrane glycoprotein and has been reported to be upregulated by IFN-γ (Cao et al., 2000). Its role has been studied mainly in the immune system. While most newly synthesized CD74 protein functions as a chaperone of MHC class II Dα and Dβ chains (Roche et al., 1991), a small proportion of CD74 is found on the cell surface independent of the MHC class II complex (Henne et al., 1995; Wraight et al., 1990). Cell surface CD74 has been identified as the high-affinity receptor for the cytokine macrophage migration inhibitory factor (MIF), the binding of which induces signal transduction, including activation of ERK and PI3K/AKT signaling (Shi et al., 2006; Starlets et al., 2006). Moreover, CD74 contributes to the regulation of several pro-tumorigenic molecules, such as interleukin (IL)-8 and anti-apoptotic factor BCL-2 (Beswick et al., 2005; Binsky et al., 2007; Gore et al., 2008). Although one study reported CD74 expression in melanoma (Weeraratna et al., 2004), the role of CD74 in melanoma remains unclear.
In the current study, we tested our hypothesis that IFN-γ promotes melanoma cell survival by regulating CD74-MIF signaling. We found that IFN-γ enhances the expression of CD74, which interacts with MIF and thereby activates AKT signaling as well as other pro-tumorigenic molecules, suggesting that targeting the CD74-MIF interaction, especially under IFN-γ–stimulatory conditions, would be an effective therapeutic approach for melanoma.
RESULTS
IFN-γ upregulated phosphorylation of AKT Ser473
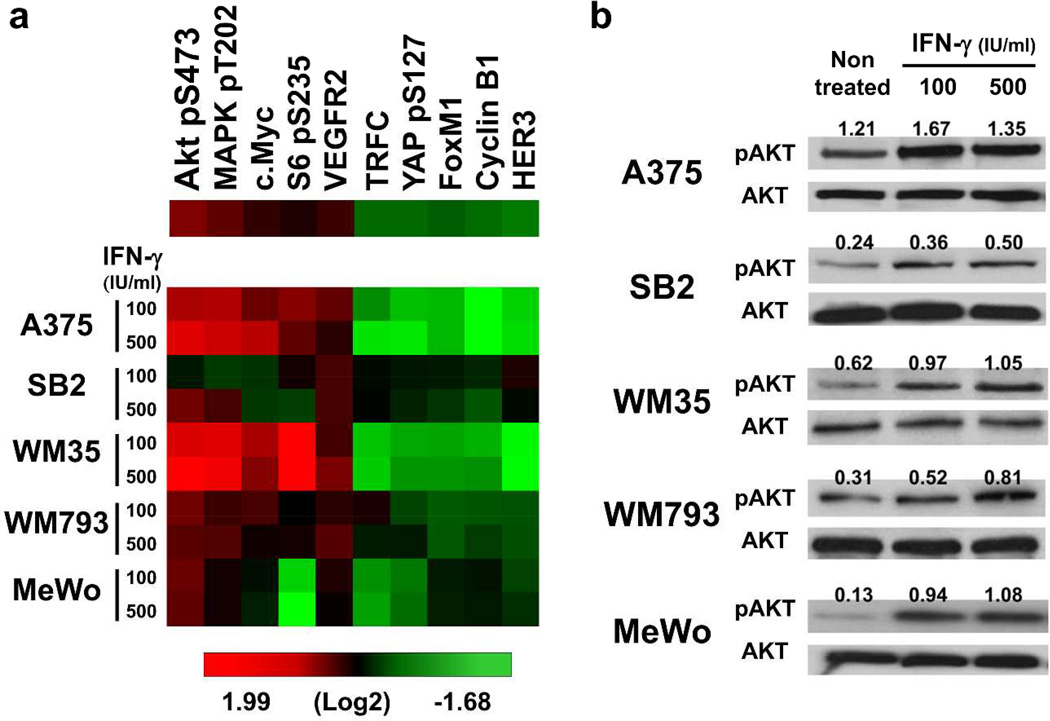
To assess the effect of IFN-γ on the major growth and survival signaling molecules in melanoma cells, we used a reverse phase protein array (RPPA). Cells of five melanoma lines were treated with 0, 100 or 500 IU/ml IFN-γ for 48 hours. Treatment with IFN-γ markedly upregulated the phosphorylation of AKT Ser473 in all cell lines tested (Figure 1a, Supplementary Figure S1). Upregulated phosphorylation of AKT Ser473 by IFN-γ stimulation was confirmed with Western blot analysis (Figure 1b). These results suggest that IFN-γ stimulation activates the AKT signaling pathways in human melanoma cells.
Figure 1. IFN-γ upregulates AKT Ser473 phosphorylation.
Five melanoma cell lines were treated with 0, 100 or 500 IU/ml IFN-γ for 48 hours and then analyzed for protein expression and phosphorylation. (a) Heat map of RPPA analysis. The top five upregulated and top five downregulated proteins by IFN-γ were selected from 171 proteins and phosphorylation of key signaling molecules. Red, high expression; green, low expression. (b) Western blot analysis. Numbers above each band indicate the relative expression level of phosphorylated AKT Ser473 (pAKT) to total AKT (AKT), calculated by subtracting the intensity of the background before dividing the band pixel density of the target protein by the band pixel density of loading controls.
Human melanoma expressed CD74
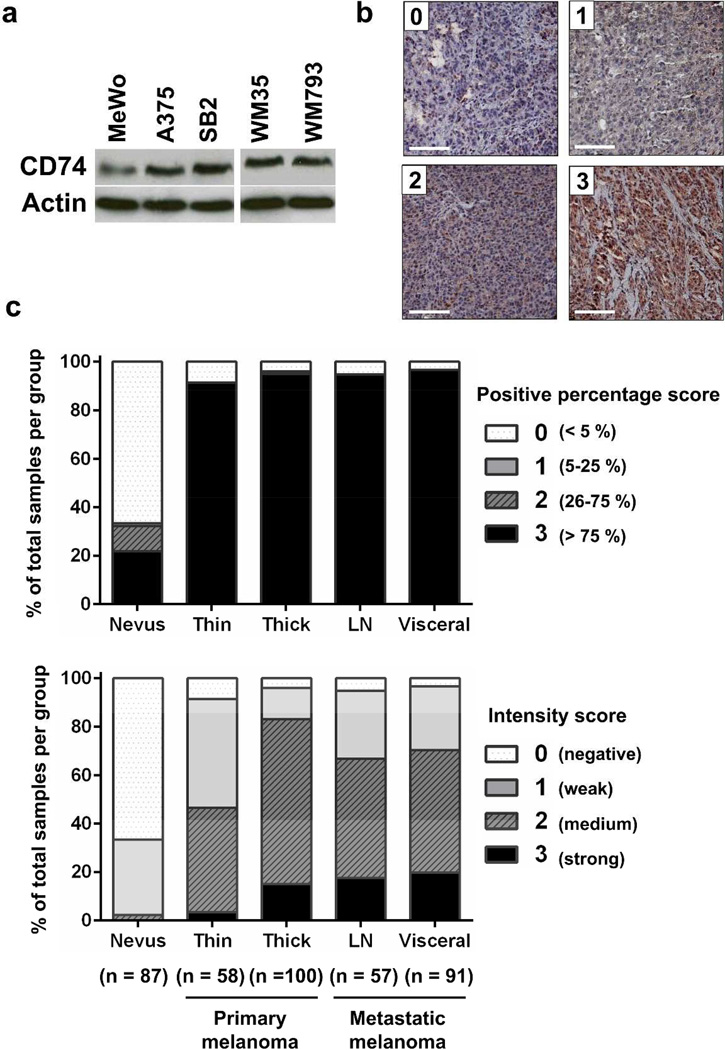
To explore which molecules functionally mediate the effect of IFN-γ, we first profiled the expression of inflammation-related genes in A375 melanoma cells and normal human epidermal melanocytes (NHEMs). Our PCR array result showed 10 genes, CD70, IL-1β, MGLL, SERPINA3, MDK, IL13RA2, CD74, VEGFA, CXCL1 and IL-8, were increased 50-fold or more in A375 cells compared with NHEMs (Supplementary Figure S2a). On the basis of an earlier report that CD74 expression is upregulated by IFN-γ (Cao et al., 2000), our interests focused on CD74 among these genes. The mRNA and protein expression of CD74 were verified in various melanoma cell lines (Figure 2a, Supplementary Figure S2b). To determine whether CD74 is expressed in melanoma tissues, we next performed immunohistochemical staining for CD74 on a melanoma progression tissue microarray (TMA) containing tissue cores from benign nevi, primary cutaneous melanomas, melanoma metastases to lymph nodes and melanoma metastases to visceral organs (Nazarian et al., 2010). CD74 protein was expressed (percentage score 1–3) in 33.3% of benign nevi, 94.3% of primary melanomas, and 95.9% of metastatic melanomas. The CD74 staining intensity in primary and metastatic melanomas was also significantly higher than that in nevi (P < 0.0001, Figure 2b,c). These results suggest that melanoma cells express CD74 and that expression of CD74 is associated with the progression from melanocytes and benign nevi to clinically evident melanoma.
Figure 2. CD74 expression in melanoma cell lines and tissues.
(a) Five melanoma cell lines were analyzed by Western blot analysis for CD74 expression. (b) Representative immunohistochemical staining intensities: score 0 (negative), 1 (weak), 2 (medium) and 3 (strong). Scale bar = 100 µm. (c) TMA analysis for CD74 expression during melanoma progression. CD74 staining was scored for the percentage (upper) and intensity (lower) of positive tumor cells. n indicates the number of tissue cores in each group. LN, lymph node. Both percentage and intensity score of each melanoma group were significantly higher than those for nevus group (P < 0.0001).
IFN-γ stimulation increased transcription and cell surface expression of CD74
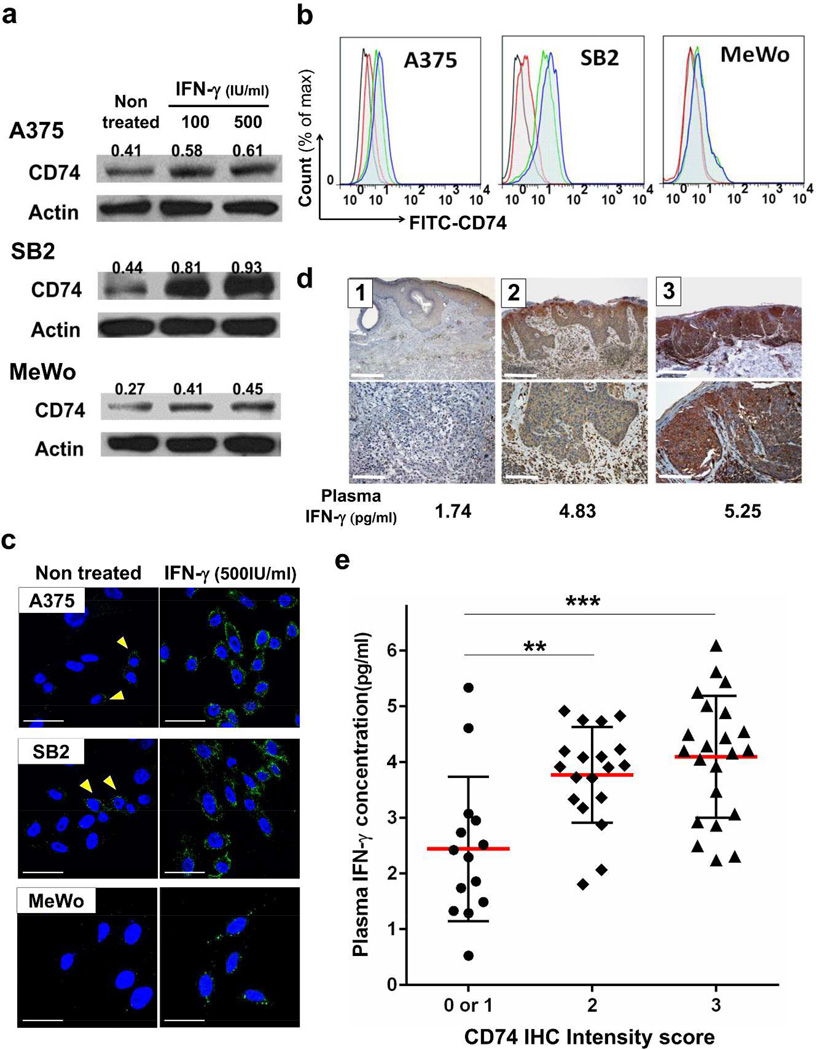
On the basis of the above results, we hypothesized that CD74 is one of the molecules implicated in the pro-tumor function of IFN-γ in melanoma cells. To ascertain whether IFN-γ stimulation augments the expression of CD74 in melanoma cells, cells treated with IFN-γ for 48 hours were assessed for mRNA and protein expressions. IFN-γ stimulation transcriptionally upregulated the expression of CD74 in all cell lines tested (Figure 3a, Supplementary Figure S3a,b). Cell surface CD74 has been reported to have a role as a signaling molecule (Starlets et al., 2006). We therefore evaluated the presence of CD74 on the cell surface using flow cytometry and immunofluorescence confocal microscopic analysis. We observed modest constitutive CD74 expression on A375, SB2, WM35 and WM793 cells; however MeWo cells had no detectable surface expression without IFN-γ treatment. IFN-γ increased cell surface expression of CD74 in all cell lines examined (Figure 3b,c, Supplementary Figure S3c). These results confirm that IFN-γ upregulates not only total but also cell surface expression of CD74 in melanoma cells.
Figure 3. CD74 expression is upregulated by IFN-γ and correlates with plasma IFN-γ levels.
(a, b and c) Melanoma cell lines were treated with 0, 100 or 500 IU/ml IFN-γ for 48 hours and then assessed for CD74 expression. (a) Western blot analysis for total CD74 protein. Numbers above each band indicate relative CD74 expression levels. (b) Flow cytometric analysis for cell surface CD74. Histogram: black, isotype control; red, untreated cells stained with FITC-CD74 antibody; green, 100 IU/ml IFN-γ–treated cells with FITC-CD74 antibody; blue, 500 IU/ml IFN-γ–treated cells with FITC-CD74 antibody. (c) Immunofluorescence confocal microscopic analysis of cell surface CD74. Green (Alexa-488), CD74. Blue (DAPI), nucleus. A375 and SB2 cells showed slight expression under normal conditions (arrows). Scale bar = 30 µm. (d) Representative images of weak (left panels, score 1), medium (middle panels, score 2) and strong CD74 staining (right panels, score 3). Upper panel, Scale bar = 1 mm.; lower panel, Scale bar = 250 µm. Corresponding plasma IFN-γ levels are listed. (e) The association between CD74 staining at the tumor site and plasma IFN-γ levels in 55 patients with melanoma. Red bars, means. Error bars, standard deviations. **P < 0.01, ***P < 0.001.
Expression of CD74 in melanoma tissues correlated with plasma IFN-γ levels
Our in vitro results indicated that IFN-γ, which is available in the tumor microenvironment, can induce cell surface CD74 expression in melanoma cells. We next analyzed the correlation between plasma IFN-γ levels and CD74 immunohistochemical staining intensity in tumors from 55 melanoma patients. Patients with high CD74 staining intensities (score 2 or 3) in tumor cells had significantly higher plasma IFN-γ levels than patients with low CD74 staining intensity (score 0 or 1) (Figure 3d,e). Our analysis suggests that the expression of CD74 in tumor cells is associated with IFN-γ levels in patients with melanoma.
Autocrine MIF-CD74 signaling regulated phosphorylation of AKT Ser473 and expression of BCL-2, IL-6 and IL-8
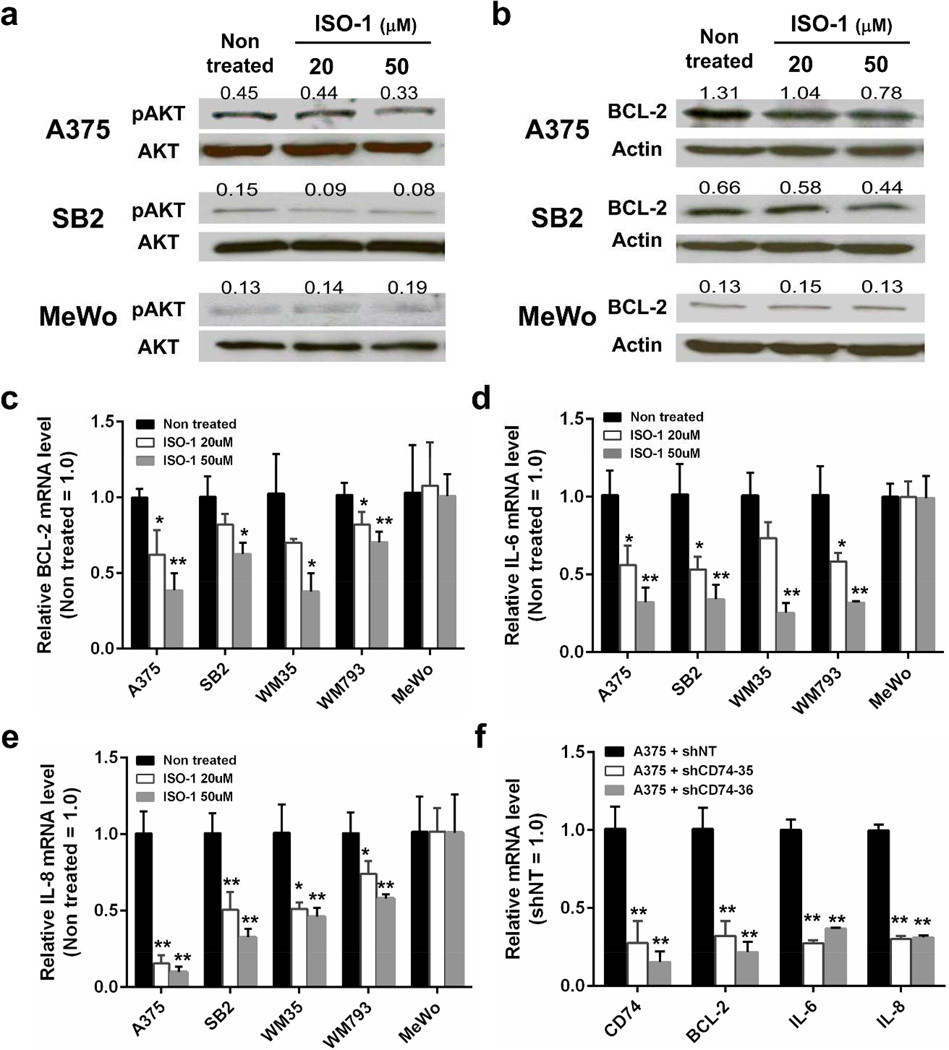
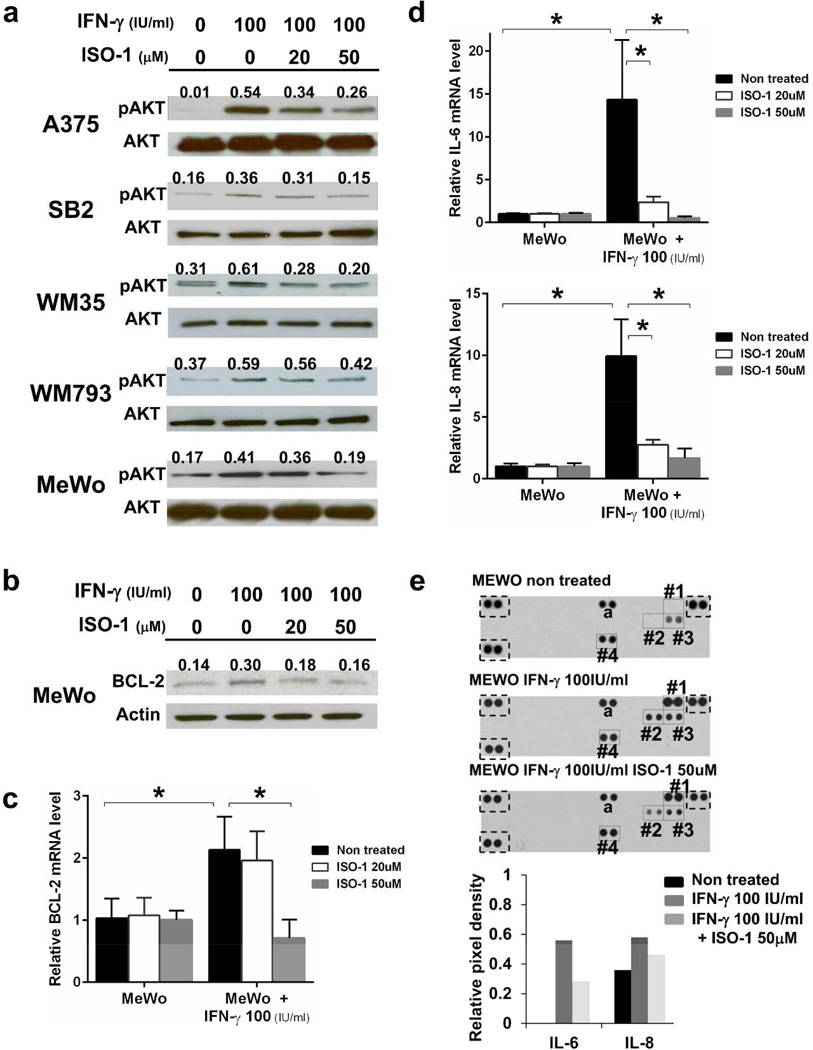
Cell surface CD74 is a receptor of MIF that is a cytokine produced by a variety of cell types, including melanoma cells (Oliveira et al., 2014). We confirmed MIF expression in all cell lines tested (Supplementary Figure S4a,b), and MIF was secreted into the culture supernatant (Supplementary Figure S5b). Hence, we postulated that autocrine MIF-cell surface CD74 signaling play a certain role in melanoma cells. To identity the function of the CD74-MIF interaction, we employed (S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid (ISO-1), which inhibits MIF binding to CD74 (Leng et al., 2011). Treatment with ISO-1 decreased the phosphorylation of AKT Ser473 in A375 and SB2 cells, which constitutively have modest CD74 on their cell surfaces (Figure 4a). Because the PI3K/AKT pathway is a key signaling pathway in cell survival, we also analyzed the expression of the anti-apoptotic protein BCL-2. mRNA and protein expressions of BCL-2 were suppressed by ISO-1 in A375, SB2, WM35 and WM793 cells (Figure 4b,c, Supplementary Figure S5a). Because CD74 has been reported to be involved in IL-6 and IL-8 production, we next examined IL-6 and IL-8 expression. Treatment with ISO-1 significantly reduced IL-6 and IL-8 mRNA expression in four melanoma cell lines that have cell surface CD74 (Figure 4d,e). Analysis of culture supernatants using an antibody-based cytokine and chemokine array showed that treatment with ISO-1 suppressed IL-8 secretion in A375, SB2 and WM793 cells and suppressed IL-6 secretion in WM793 cells (Supplementary Figure S5b). Interestingly, ISO-1 had no effect on AKT phosphorylation or BCL-2, IL-6 and IL-8 expression in cell surface CD74-negative MeWo cells. Furthermore, similar downregulation of BCL-2, IL-6 and IL-8 was observed in CD74-knocked-down cells (Figure 4f, Supplementary Figure S5c). These results suggest that MIF secreted from melanoma cells binds to cell surface CD74 in an autocrine manner and plays a role in melanoma cell survival by regulating the phosphorylation of AKT Ser473 and expression of BCL-2, IL-6 and IL-8.
Figure 4. Autocrine MIF-CD74 interaction regulates AKT Ser473 phosphorylation and BCL-2, IL-6 and IL-8 expression.
(a and b) Cells were treated with ISO-1 for 48 hours and then analyzed by Western blot analysis for AKT Ser473 phosphorylation (a) and BCL-2 expression (b). Numbers above each band indicate the relative level of phosphorylated AKT Ser473 (pAKT) to total AKT (AKT) or BCL-2 to actin. (c, d and e) mRNA expression levels of BCL-2 (c), IL-6 (d) and IL-8 (e) in melanoma cells treated with ISO-1 for 24 hours were measured using qRT-PCR. (f) CD74 knockdown A375 cells were analyzed for mRNA expression of the indicated genes by qRT-PCR. shNT, non-target shRNA. *P < 0.05, **P < 0.01.
IFN-γ stimulation augmented CD74-MIF signaling
On the basis of our finding that IFN-γ upregulates cell surface expression of CD74, we hypothesized that autocrine MIF-CD74 signaling would mediate melanoma cell survival under IFN-γ stimulation. Increased phosphorylation of AKT Ser473 by IFN-γ stimulation was suppressed by ISO-1 in a dose-dependent manner in all cell lines tested (Figure 5a). In further analysis of the cell surface CD74-negative MeWo cells, ISO-1 suppressed BCL-2 expression upregulattion by IFN-γ (Figure 5b,c). Additionally, IFN-γ stimulation markedly increased IL-6 and IL-8 expression and secretion, which were strikingly decreased by ISO-1 treatment (Figure 5d,e). Interestingly, neither treatment with IFN-γ nor treatment with ISO-1 affected the expression of MIF (Figure 5e). Taken together, these results demonstrated that IFN-γ–induced cell surface expression of CD74 promotes the activation of AKT signaling, expression of BCL-2 and secretion of IL-6 and IL-8 through autocrine MIF-CD74 signaling in melanoma cells.
Figure 5. IFN-γ stimulation augments MIF-CD74 signaling.
Cells were treated with ISO-1 for 24 hours (c and d) or 48 hours (a, b and e) under IFN-γ–stimulatory conditions. AKT Ser473 phosphorylation (a) and BCL-2 protein expression (b) were detected by Western blot analysis. qRT-PCR was performed to measure mRNA of BCL-2 (c), IL-6 and IL-8 (d). *P < 0.05. (e) Cell culture supernates were subjected to focused cytokine array. Dots square, positive control; #1, IFN-γ; #2, IL-6; #3, IL-8; #4, MIF; a, CXCL1. Lower bar graph indicates relative pixel densities of IL-6 and IL-8.
ISO-1 suppressed tumor growth in the presence of IFN-γ in a xenograft mouse model
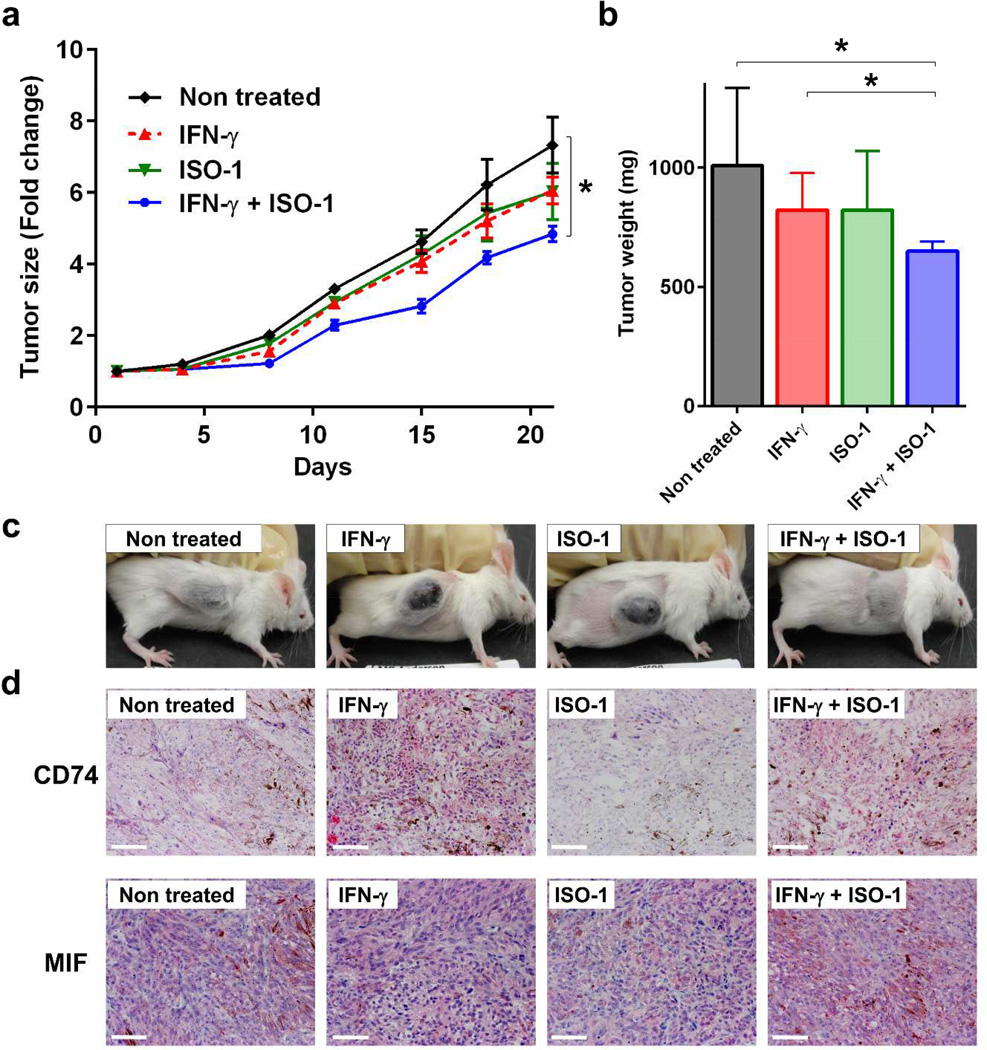
To evaluate the function of the CD74-MIF interaction in vivo, we used a xenograft mouse model. Cell surface CD74-negative MeWo cells were injected into the flank of SCID Beige mice, and then mice were treated with ISO-1 by daily intraperitoneal injection. As IFN-γ is species-specific and its production could be insufficient in immune-deficient mice, IFN-γ was administered with or without ISO-1 to induce CD74 expression in MeWo cells (Supplementary Figure S6). Although no significant difference in tumor growth between untreated controls and mice treated with IFN-γ alone or ISO-1 alone was observed, ISO-1 treatment significantly suppressed tumor growth in the presence of IFN-γ compared with untreated control or IFN-γ alone (Figure 6a). Tumor weights of mice treated with IFN-γ and ISO-1 on day 21 were significantly lower than those of untreated control mice (Figure 6b). We also observed elevated CD74 expression in tumors of IFN-γ–administered mice, and MIF was expressed in MeWo xenografts (Figure 6d). These results suggest that inhibition of CD74-MIF interaction represses tumor growth under IFN-γ–stimulatory conditions.
Figure 6. MIF-CD74 inhibition suppresses tumor growth under IFN-γ–stimulatory conditions in a xenograft melanoma model.
Cell surface CD74-negative MeWo cells were subcutaneously injected into the flank of SCID Beige mice. Six days after cell injection, mice were randomly assigned to treatment groups (6 mice per group) and started on daily intraperitoneal treatment with ISO-1 (500 µg/mouse) and/or IFN-γ (1000 IU/mouse). ISO-1 and IFN-γ were administered for 21 days, and tumor growth was monitored. (a) Fold changes of tumor size. (b) Tumor weight on day 21. Error bars, standard deviations. *P < 0.05. (c) Representative mouse from each group (day 21). (d) Immunohistochemical staining for CD74 (upper) and MIF (lower) on day 21. Scale bar = 100 µm. ; AEC chromogen counterstained with hematoxylin.
DISCUSSION
In this study, we found that the cell surface CD74 responding to IFN-γ enhances activation of AKT signaling and upregulation of pro-tumorigenic molecules by binding to MIF, thereby resulting in melanoma cell survival.
Unlike type I IFNs (IFN-α and IFN-β), which are approved for use in adjuvant therapies for melanoma, IFN-γ (the only known type II IFN) has been reported to be both anti-tumorigenic and pro-tumorigenic (Zaidi and Merlino, 2011). Regarding the anti-tumorigenic properties of IFN-γ, treatment with high concentrations (600–1200 IU/ml) of recombinant IFN-γ resulted in 50% growth inhibition in some human melanoma cell lines (Garbe et al., 1990). In contrast, our previous study showed that treatment with 1000 IU/ml IFN-γ for 24 hours did not suppress the viability of A375 or MeWo cells (Ekmekcioglu et al., 2008). Additionally, IFN-γ enhanced lung colonization of intravenously inoculated B16 melanoma cells (Taniguchi et al., 1987). Although the mechanisms underlying pro-tumorigenic IFN-γ have recently been actively investigated in terms of immunosuppressive pathways (Spranger et al., 2013; Zaidi and Merlino, 2011), the molecular mechanisms by which IFN-γ becomes pro-tumorigenic in tumor cells remain poorly understood. We now speculate that upregulation of cell surface CD74 plays a certain role in this process by promoting the survival of melanoma cells. Upregulation of cell surface CD74 expression by IFN-γ stimulation has been reported in several cancer cell lines, including one melanoma cell line (Beswick et al., 2005; Ong et al., 1999). In the current study, we demonstrated that IFN-γ upregulated both transcription and cell surface expression of CD74 in all five melanoma cell lines tested. Moreover, tumor CD74 expression had a positive association with plasma IFN-γ levels in patients with melanoma, suggesting that increased CD74 expression on melanoma cells reflects stimulation by IFN-γ in the tumor microenvironment.
Although CD74 expression and overexpression of MIF (a ligand of cell surface CD74) in melanoma have been reported (Oliveira et al., 2014; Weeraratna et al., 2004), the functional roles of CD74 and the CD74-MIF interaction in melanoma have not been analyzed to date. In our study, all examined melanoma cell lines expressed MIF, and modest cell surface CD74 expression was detected in four of five cell lines. These results provide circumstantial evidence of autocrine MIF-CD74 signaling. Indeed, inhibition of MIF binding to CD74 using ISO-1 suppressed the phosphorylation of AKT Ser473, as well as the expression of BCL-2, IL-6 and IL-8 in cell surface CD74-positive cell lines. Similarly, downregulation of BCL-2, IL-6 and IL-8 by CD74-knockdown was observed, indicating the importance of CD74 in this context. We also attempted knockdown of MIF; however, there was no significant difference in BCL-2, IL-6 and IL-8 expression, perhaps because melanoma cells could secrete enough MIF to bind CD74 on the cell membrane even though siRNA efficiently reduced mRNA expression of MIF (Supplementary Figure S4c,d). In melanoma, BCL-2 contributes to apoptosis resistance and chemotherapy resistance (Bedikian et al., 2006), and IL-6 and IL-8 are associated with invasiveness and metastatic potential (Bar-Eli, 1999; Kushiro et al., 2012). Additionally, secretion of both IL-6 and IL-8 from tumor cells has been reported to enrich the Foxp3+ CD4-regulatory T-cell subset among T cells migrating toward melanoma, thereby create an immunosuppressive microenvironment (Eikawa et al., 2010). Taken together, these findings suggest that CD74-MIF autocrine signaling is active in melanoma and may play roles in tumor cell survival, invasion and immunosuppression.
We also demonstrated that the cell surface CD74-MIF signaling is augmented in response to IFN-γ. Blockade of the CD74-MIF interaction by ISO-1 reduced AKT phosphorylation, promoting cell survival. Importantly, these findings were observed regardless of the BRAF or NRAS mutation status, implying that the augmentation of CD74-MIF signaling by IFN-γ is a universal phenomenon in melanoma cells. Despite high rates of clinical response, resistance to BRAF inhibitors emerges in most patients with metastatic melanoma (Salama and Flaherty, 2013). Given that the AKT pathway is one of the mediators of BRAF inhibitor resistance (Perna et al., 2015), the MIF-CD74 axis in melanoma cells may maintain AKT phosphorylation and interrupt the benefits of BRAF inhibitors, perhaps by IFN-γ from the melanoma microenvironment.
In our mouse study using MeWo cells, which have no cell surface CD74 without IFN-γ stimulation, inhibition of CD74-MIF interaction significantly suppressed tumor growth in the presence of IFN-γ. This result suggested that CD74-MIF signaling promotes melanoma cell survival in response to IFN-γ in vivo. Although the current study was limited to immune-deficient conditions, our findings provide evidence that CD74-MIF inhibition may have therapeutic potential for melanoma when combined with immune stimulatory therapies.
In conclusion, IFN-γ enhances the expression of CD74, which then is able to interact with and autocrine, or extracellular MIF, or both and thereby activate the PI3K/AKT pathway in melanoma, promoting tumor survival. Targeting the CD74-MIF interaction, especially under IFN-γ–stimulatory conditions, would be an effective therapeutic approach for melanoma.
MATERIALS AND METHODS
Patient samples
This research project was approved by both the Surveillance Committee for Human Subjects Research and the Vice President for Research at The University of Texas MD Anderson Cancer Center. Fifty-five melanoma patients presenting with clinically localized disease in the Melanoma Surgery Clinic at MD Anderson between September 2004 and April 2007 were included, and written informed consent was obtained from all patients according to the Declaration of Helsinki Principles. All patients underwent standard definitive surgery for their primary tumors. Plasma samples (obtained from each patient before wide local tumor excision and sentinel lymph node biopsy) were stored at −80°C until analysis.
Cell lines and reagents
The human melanoma cell lines A375 and MeWo were obtained from the American Type Culture Collection. WM35 and WM793 cells and SB2 cells were provided by Dr. Robert Kerbel (Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada) and Dr. Jeffrey Gershenwald (MD Anderson), respectively. All melanoma cells were grown under conditions as described elsewhere (Ekmekcioglu et al., 2008). Normal human melanocytes (NHEMs) were obtained from Lonza (Walkersville, MD) and cultured in Melanocyte Cell Basal Medium-4 supplemented with the MGM-4 Bullet Kit (Lonza). All cells were grown at 37°C in a 5% CO2 atmosphere and cultured to 50–60% confluence a day before the start of the experiment. The cell lines were validated via STR DNA fingerprinting using the AmpF/STR identifier kit according to the manufacturer’s instructions (Applied Biosystems, Carlsbad, CA).
Cells were treated with the MIF inhibitor ISO-1 (Calbiochem, La Jolla, CA) and human recombinant IFN-γ protein (eBioscience, San Diego, CA). The primers corresponding to shRNA targeting CD74 sequences were synthesized (Sigma-Aldrich, St Louis, MO), annealed and inserted between BamHI and EcoRI restriction sites in the multiple cloning cassette of the pSIREN-Shuttle vector (Clontech, Mountain View, CA). Sequences used are listed in Supplementary Table S1. The U6 promoter-shRNA cassettes were then excised and recloned to KpnI and ApaI sites of the pEPI-1 vector containing GFP expression marker, kindly provided by Dr. A. C. Jenke (HELIOS Children's Hospital, Wuppertal, Germany). The construct was transfected into melanoma cells using Fugene6 (Promega, Madison, WI), and transfected cells were treated with puromycin (Invitrogen, Carlsbad, CA) for 2 weeks. Puromycin-resistant colonies were collected by trypsinization and used without cloning.
Reverse phase protein array
The RPPA method used was described elsewhere (Tibes et al., 2006). Briefly, cells were lysed in lysis buffer and then SDS/2-ME buffer was added and the solution incubated for 5 minutes at 95°C. The lysate material was printed onto nitrocellulose-coated glass slides using the GeneTac arrayer (Genomic Solutions, Ann Arbor, MI). Slides were blocked for endogenous peroxidase, avidin and biotin activity, and primary antibody was added. Next, a biotinylated secondary antibody and a streptavidin-biotin complex were added. The DAKO signal amplification system (Copenhagen, Denmark) was used to detect and amplify antibody-binding intensity. The intensity of each spot was calculated using MicroVigene software (VigeneTech, North Billerica, MA).
Antibodies and western blotting
Goat polyclonal anti-human actin (I-19; Santa Cruz, Carlsbad, CA), mouse monoclonal anti-human CD74 (PIN.1; Novus Biologicals, Littleton, CO), mouse monoclonal anti-human BCL-2 (124; GeneTex, San Antonio, TX), goat polyclonal anti-human MIF (R&D Systems, Minneapolis, MN) and rabbit monoclonal anti-human total AKT, phosphorylated AKT Ser473 (Cell Signaling Technology, Danvers, MA) antibodies were used as primary antibodies; horseradish peroxidase-labeled anti-mouse, anti-rabbit and anti-goat antibodies (1:1000; DAKO) were used as secondary antibodies. Amersham ECL Western blot detection reagent (GE Healthcare, Piscataway, NJ) was used to detect protein expression. Protein expression bands were captured on Kodak BioMax film and recorded using a computer scanner. Band pixel density was quantified with Photoshop CS5.1 software (Adobe, San Jose, CA).
Melanoma tissue microarray and immunohistochemistry
A melanocytic tumor progression TMA composed of 480 cores from clinically stratified melanocytic samples obtained from 170 patients served as a template for investigating IL-1 in human melanocytic tumors (Nazarian et al., 2010; Qin et al., 2011). The immunohistochemistry protocol used was described previously (Ekmekcioglu et al., 2006). Mouse monoclonal anti-human CD74 (PIN.1) was used as the primary antibody. Immunolabeling was scored separately for two variables: percentage of melanoma cells with positive staining (<5%, 0; 5–25%, 1; 26–75%, 2; >75%, 3) and overall intensity of the immunoreactivity in positive cells (negative, 0; weak, 1; medium, 2; strong, 3). Specimens were considered to be positive if either the percentage or intensity score was 1 to 3. All specimens were manually scored by two researchers (SE, and KT) independently, without prior knowledge of other clinical information.
Measurement of plasma IFN-γ levels
Plasma IFN-γ levels were determined using a commercially available enzyme-linked immunosorbent assay kit (BioSource International, Camarillo, CA) according to the manufacturer’s recommendations.
Extraction of RNA and quantitative real-time PCR
Total cellular RNA was isolated using the RNeasy mini kit (Qiagen, Valencia, CA). RNA samples were treated with TURBO DNase (Ambion, Foster City, CA) to remove genomic DNA and converted to first-strand cDNA using the GeneAmp RNAPCR kit (Applied Biosystems). Quantitative real-time PCR (qRT-PCR) analysis was performed using a Mastercycler ep realplex real-time PCR system (Eppendorf, Westbury, NY) with RT2 SYBR Green qPCR Master Mix (Qiagen). Primer sequences used are listed in Supplementary Table S1. Human GAPDH was used as an internal reference. Fold-induction values were calculated using the 2−ΔΔCt method.
Focused protein array
Secreted proteins in each culture supernatant were measured using a human cytokine array panel A kit (Proteome Profiler; R&D Systems). Protein expression dots were scanned using a computer scanner, and dot pixel density was quantified using Photoshop CS5.1 software. Relative dot density was calculated by subtracting the intensity of the background before dividing the average dot pixel density of duplicate target proteins by the average dot pixel density of three positive controls.
CD74 cell surface expression analysis
Melanoma cells were cultured with or without IFN-γ (100 IU/ml) for 48 hours. Cells were subsequently treated with chondroitinase ABC (0.1 U/ml; Sigma-Aldrich) for 4 hours at 37°C, washed with ice-cold phosphate-buffered saline, detached by gentle pipetting, and subsequently fixed with 1% paraformaldehyde in phosphate-buffered saline. Cells were stained with FITC-conjugated mouse monoclonal anti-human CD74 antibody (M-B741) or its isotype control antibody (IgG2a, κ; BD Biosciences, San Jose, CA) and analyzed using a FACSCalibur flow cytometer (BD Biosciences). For immunofluorescence staining, cells were incubated with mouse anti-CD74 antibody (M-B741) or isotype control antibody (BD Biosciences) for 1 hour and then incubated with Alexa Fluor 488-labeled goat anti-mouse IgG antibody (Life Technologies) for 1 hour. Slides were photographed using a confocal fluorescence microscope (FV1000; Olympus).
Mouse xenograft model study
The mouse study was conducted at TD2 (Scottsdale, AZ). MeWo cells (1.5 × 107) were subcutaneously inoculated into the flanks of SCID Beige mice. Six days after cell injection, mice were randomly assigned to receive intraperitoneal treatment with ISO-1 (500 µg/day) and/or IFN-γ (1000 IU/day) daily for 21 days. Tumor growth was monitored for 21 days. Tumor weights were recorded when mice were euthanized on day 21 (Supplementary Figure S6).
Statistical analysis
All statistical analyses were performed using SAS software (SAS Institute) and S-PLUS software (TIBCO Software) with significance determined using Student’s t test or Mann-Whitney U test. P < 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the members of the MD Anderson Characterized Cell Line Core for their critical fingerprinting analysis of melanoma cell lines and the members of our Melanoma Informatics, Tissue Resource, and Pathology Core, who provided the de-identified tissue samples for this research project and maintained accurate patient information. The progression TMA was prepared as a SPORE collaborative effort with gracious support provided by Dr. Lyn Duncan of MGH Dermatopathology Unit, Boston, MA. We also thank Wendy D. Schober and Nalini Patel for assistance with the flow cytometric analysis, Dr. Junna Oba and Ms. Ping Liu for helping with the statistical analysis, Dr. Hirohito Yamaguchi for assistance with anti-apoptotic protein expression analysis, Ms. Sandra A. Kinney for excellent technical help and Mr. Donald Norwood for editorial assistance.
Financial Support
This work was supported by grant P50 CA09345 from the National Institutes of Health (SE, EAG), the Melanoma Specialized Programs of Research Excellence grant (EAG), the National Institutes of Health through MD Anderson’s Cancer Center Support Grant P30-CA016672 (EAG), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (EAG), the Japanese Strategic Young Researcher Overseas Visits Program for Accelerating Brain Circulation (KT), JSPS KAKENHI grant number 26860895 (KT) and the American Cancer Society Mentored Research Scholar Grant 118447-MSRG-10-052-01-LIB (ZB).
Abbreviations
- IFN-γ
interferon-γ
- MIF
macrophage migration inhibitory factor
- IL
interleukin
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Azijli K, Stelloo E, Peters GJ, et al. New developments in the treatment of metastatic melanoma: immune checkpoint inhibitors and targeted therapies. Anticancer Res. 2014;34:1493–1505. [PubMed] [Google Scholar]
- Bar-Eli M. Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology. 1999;67:12–18. doi: 10.1159/000028045. [DOI] [PubMed] [Google Scholar]
- Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738–4745. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- Beswick EJ, Bland DA, Suarez G, et al. Helicobacter pylori binds to CD74 on gastric epithelial cells and stimulates interleukin-8 production. Infect Immun. 2005;73:2736–2743. doi: 10.1128/IAI.73.5.2736-2743.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binsky I, Haran M, Starlets D, et al. IL-8 secreted in a macrophage migration-inhibitory factor- and CD74-dependent manner regulates B cell chronic lymphocytic leukemia survival. Proc Natl Acad Sci USA. 2007;104:13408–13413. doi: 10.1073/pnas.0701553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TJ, Lioubin MN, Marquardt H. Purification and characterization of cytostatic lymphokines produced by activated human T lymphocytes. Synergistic antiproliferative activity of transforming growth factor beta 1, interferon-gamma, and oncostatin M for human melanoma cells. J Immunol. 1987;139:2977–2983. [PubMed] [Google Scholar]
- Cao ZA, Moore BB, Quezada D, et al. Identification of an IFN-gamma responsive region in an intron of the invariant chain gene. Eur J Immunol. 2000;30:2604–2611. doi: 10.1002/1521-4141(200009)30:9<2604::AID-IMMU2604>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- Eikawa S, Ohue Y, Kitaoka K, et al. Enrichment of Foxp3+ CD4 regulatory T cells in migrated T cells to IL-6– and IL-8–expressing tumors through predominant induction of CXCR1 by IL-6. J Immunol. 2010;185:6734–6740. doi: 10.4049/jimmunol.1000225. [DOI] [PubMed] [Google Scholar]
- Ekmekcioglu S, Ellerhorst JA, Prieto VG, et al. Tumor iNOS predicts poor survival for stage III melanoma patients. Int J Cancer. 2006;119:861–866. doi: 10.1002/ijc.21767. [DOI] [PubMed] [Google Scholar]
- Ekmekcioglu S, Mumm JB, Udtha M, et al. Killing of human melanoma cells induced by activation of class I interferon-regulated signaling pathways via MDA-7/IL-24. Cytokine. 2008;43:34–44. doi: 10.1016/j.cyto.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Bernatchez C, Sharma P, et al. Advances in the development of cancer immunotherapies. Trends Immunol. 2013;34:90–98. doi: 10.1016/j.it.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe C, Krasagakis K, Zouboulis CC, et al. Antitumor activities of interferon alpha, beta, and gamma and their combinations on human melanoma cells in vitro: changes of proliferation, melanin synthesis, and immunophenotype. J Invest Dermatol. 1990;95:231S–237S. doi: 10.1111/1523-1747.ep12875837. [DOI] [PubMed] [Google Scholar]
- Gollob JA, Sciambi CJ, Huang Z, et al. Gene expression changes and signaling events associated with the direct antimelanoma effect of IFN-γ. Cancer Res. 2005;65:8869–8877. doi: 10.1158/0008-5472.CAN-05-1387. [DOI] [PubMed] [Google Scholar]
- Gore Y, Starlets D, Maharshak N, et al. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex. J Biol Chem. 2008;283:2784–2792. doi: 10.1074/jbc.M703265200. [DOI] [PubMed] [Google Scholar]
- Henne C, Schwenk F, Koch N, et al. Surface expression of the invariant chain (CD74) is independent of concomitant expression of major histocompatibility complex class II antigens. Immunology. 1995;84:177–182. [PMC free article] [PubMed] [Google Scholar]
- Kushiro K, Chu RA, Verma A, et al. Adipocytes promote B16BL6 melanoma cell invasion and the epithelial-to-mesenchymal transition. Cancer microenviron. 2012;5:73–82. doi: 10.1007/s12307-011-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng L, Chen L, Fan J, et al. A small-molecule macrophage migration inhibitory factor antagonist protects against glomerulonephritis in lupus-prone NZB/NZW F1 and MRL/lpr mice. J Immunol. 2011;186:527–538. doi: 10.4049/jimmunol.1001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyskens FL, Jr, Kopecky KJ, Taylor CW, et al. Randomized trial of adjuvant human interferon gamma versus observation in high-risk cutaneous melanoma: a Southwest Oncology Group study. J Natl Cancer Inst. 1995;87:1710–1713. doi: 10.1093/jnci/87.22.1710. [DOI] [PubMed] [Google Scholar]
- Nazarian RM, Prieto VG, Elder DE, et al. Melanoma biomarker expression in melanocytic tumor progression: a tissue microarray study. J Cutan Pathol. 2010;37(Suppl 1):41–47. doi: 10.1111/j.1600-0560.2010.01505.x. [DOI] [PubMed] [Google Scholar]
- Oliveira CS, de Bock CE, Molloy TJ, et al. Macrophage migration inhibitory factor engages PI3K/Akt signalling and is a prognostic factor in metastatic melanoma. BMC cancer. 2014;14:630. doi: 10.1186/1471-2407-14-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong GL, Goldenberg DM, Hansen HJ, et al. Cell surface expression and metabolism of major histocompatibility complex class II invariant chain (CD74) by diverse cell lines. Immunology. 1999;98:296–302. doi: 10.1046/j.1365-2567.1999.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna D, Karreth FA, Rust AG, et al. BRAF inhibitor resistance mediated by the AKT pathway in an oncogenic BRAF mouse melanoma model. Proc Natl Acad Sci USA. 2015;112:E536–E545. doi: 10.1073/pnas.1418163112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter GA, Abdalla J, Lu M, et al. Significance of plasma cytokine levels in melanoma patients with histologically negative sentinel lymph nodes. Ann Surg Oncol. 2001;8:116–122. doi: 10.1007/s10434-001-0116-3. [DOI] [PubMed] [Google Scholar]
- Qin Y, Ekmekcioglu S, Liu P, et al. Constitutive aberrant endogenous interleukin-1 facilitates inflammation and growth in human melanoma. Mol Cancer Res. 2011;9:1537–1550. doi: 10.1158/1541-7786.MCR-11-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- Roche PA, Marks MS, Cresswell P. Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature. 1991;354:392–394. doi: 10.1038/354392a0. [DOI] [PubMed] [Google Scholar]
- Salama AK, Flaherty KT. BRAF in melanoma: current strategies and future directions. Clin Cancer Res. 2013;19:4326–4334. doi: 10.1158/1078-0432.CCR-13-0779. [DOI] [PubMed] [Google Scholar]
- Shi X, Leng L, Wang T, et al. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity. 2006;25:595–606. doi: 10.1016/j.immuni.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starlets D, Gore Y, Binsky I, et al. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood. 2006;107:4807–4816. doi: 10.1182/blood-2005-11-4334. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Petersson M, Hoglund P, et al. Interferon γ induces lung colonization by intravenously inoculated B16 melanoma cells in parallel with enhanced expression of class I major histocompatibility complex antigens. Proc Natl Acad Sci USA. 1987;84:3405–3409. doi: 10.1073/pnas.84.10.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibes R, Qiu Y, Lu Y, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5:2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- Weeraratna AT, Becker D, Carr KM, et al. Generation and analysis of melanoma SAGE libraries: SAGE advice on the melanoma transcriptome. Oncogene. 2004;23:2264–2274. doi: 10.1038/sj.onc.1207337. [DOI] [PubMed] [Google Scholar]
- Wraight CJ, van Endert P, Moller P, et al. Human major histocompatibility complex class II invariant chain is expressed on the cell surface. J Biol Chem. 1990;265:5787–5792. [PubMed] [Google Scholar]
- Zaidi MR, Davis S, Noonan FP, et al. Interferon-γ links ultraviolet radiation to melanomagenesis in mice. Nature. 2011;469:548–553. doi: 10.1038/nature09666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi MR, Merlino G. The two faces of interferon-γ in cancer. Clin Cancer Res. 2011;17:6118–6124. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikich D, Schachter J, Besser MJ. Immunotherapy for the management of advanced melanoma: the next steps. Am J Clin Dermatol. 2013;14:261–272. doi: 10.1007/s40257-013-0013-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.