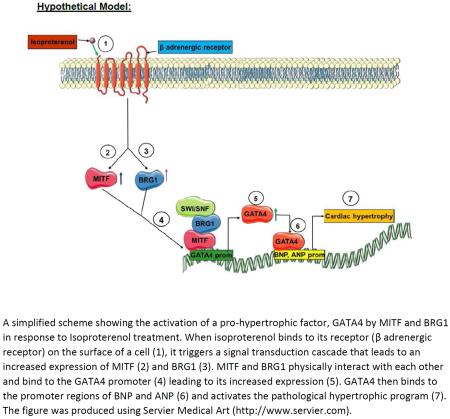
Abstract
The transcriptional regulation of pathological cardiac hypertrophy involves the interplay of transcription factors and chromatin remodeling enzymes. The Microphthalmia-associated transcription factor (MITF) is highly expressed in cardiomyocytes and is required for cardiac hypertrophy. However, the transcriptional mechanisms by which MITF promotes cardiac hypertrophy have not been elucidated. In this study, we tested the hypothesis that MITF promotes cardiac hypertrophy by activating transcription of pro-hypertrophy genes through interactions with the SWI/SNF chromatin remodeling complex. In an in vivo model of cardiac hypertrophy, expression of MITF and the BRG1 subunit of the SWI/SNF complex increased coordinately in response to pressure overload. Expression of MITF and BRG1 also increased in vitro when cardiomyocytes were stimulated with angiotensin II or a β-adrenergic agonist. Both MITF and BRG1 were required to increase cardiomyocyte size and activate expression of hypertrophy markers in response to β-adrenergic stimulation. We detected physical interactions between MITF and BRG1 in cardiomyocytes and found that they cooperate to regulate expression of a pro-hypertrophic transcription factor, GATA4. Our data show that MITF binds to the E box element in the GATA4 promoter and facilitates recruitment of BRG1. This is associated with enhanced expression of the GATA4 gene as evidenced by increased Histone3 lysine4 tri-methylation (H3K4me3) on the GATA4 promoter. Thus, in hypertrophic cardiomyoctes, MITF is a key transcriptional activator of a pro-hypertrophic gene, GATA4, and this regulation is dependent upon the BRG1 component of the SWI/SNF complex.
Keywords: Microphthalmia-Associated Transcription Factor (MITF), SWI/SNF Chromatin Remodeling Enzymes, BRG1, cardiomyocyte, transcription, GATA4, cardiac hypertrophy
Graphical Abstract
Introduction
Heart failure due to pathological cardiac hypertrophy is a leading cause of mortality worldwide [1, 2]. Pathological cardiac hypertrophy is an intricate process that involves transcriptional changes in the cardiomyocyte. It can be triggered in response to pressure overload or stimulation with neurohormonal factors such as endothelin-1 (ET-1), angiotensin II, α1and β-adrenergic agonists which activate signaling cascades that then promote a shift from the expression of adult proteins to expression of fetal genes. The fetal gene expression program is executed by the transcription factors GATA4, Nkx2.5, TBX5, and MEF2, whose roles in embryonic cardiac development are recapitulated in the hypertrophic cardiomyocyte [3, 4]. Recent studies show that the full spectrum of changes in the gene expression profile requires the activities of many additional transcription factors as well as chromatin remodeling enzymes [5–7]. Among these newly identified regulators of hypertrophy is the Microphthalmia-Associated Transcription Factor (MITF), which is required for the hypertrophic response to β-adrenergic signaling [8].
MITF is a basic helix-loop-helix leucine zipper transcription factor that regulates gene expression by binding to E box elements in the promoter region of its target genes [9]. The MITF gene is transcribed from alternative promoters that give rise to splice isoforms which differ at the amino terminus and have cell and tissue specific distribution [9]. MITF-M is the most well studied isoform, expressed abundantly in neural crest derived melanocytes and melanoma [10]. Other MITF isoforms are critical for the development and function of diverse cell lineages including mast cells and osteoclasts. Interestingly, the different MITF isoforms regulate unique and overlapping gene sets, demonstrating some degree of cell specificity as well as biological redundancy [11, 12]. Mice with mutations at the MITF locus have pigmentation defects, deafness, osteopetrosis, decreased heart to body weight ratio, and a dampened hypertrophic response to β-adrenergic signaling [8, 9].
The heart specific isoform, MITF-H, is highly expressed in cardiomyocytes and is essential for the hypertrophic response [8]. At present, only three genes that are directly regulated by MITF-H in cardiomyocytes have been identified [13–15]. Furthermore, little is known about how MITF-H activity is coordinated with the activities of other transcriptional regulators or with chromatin remodeling enzymes that regulate cardiomyocyte specific gene expression.
SWI/SNF enzymes are ATP dependent multi-subunit chromatin remodeling complexes that are required for heart development during mouse embryogenesis [16–18]. Heterogeneous complexes composed of a catalytic subunit, BRG1 or BRM, and 9–12 BRG1/BRM associated factors (BAFs) have distinct functions in mammalian cells [19–21]. BRG1 [16, 17], Baf60C [22, 23], Baf250A [24, 25], Baf180 [26], Baf45C/DPF3 [27, 28], and BAF200 [29] are all essential for cardiomyocyte differentiation and/or heart morphogenesis.
Components of the SWI/SNF complex have also been implicated in the regulation of cardiac hypertrophy [16, 30–32]. In mice, BRG1 is predominantly expressed during embryogenesis, then down-regulated in adult hearts, and re-activated in adult hearts that are subjected to stress. Conditional BRG1 deletion in adult mice prevents cardiac hypertrophy from developing after trans-aortic constriction (TAC) [16]. Furthermore, BRG1 and other SWI/SNF subunits are highly enriched on fetal cardiac gene promoters in hypertrophic hearts [30, 31].
Prior studies demonstrated independent roles for MITF and BRG1 in the regulation of cardiac hypertrophy [8, 13, 16]. However, the transcriptional mechanisms utilized by MITF to promote cardiac hypertrophy remain largely undefined. We previously showed that interactions between MITF and BRG1 promote MITF target gene expression in melanoma cells and drive aspects of melanoma tumorigenicity [21, 33]. These observations prompted us to test the hypothesis that MITF interacts with BRG1 in cardiomyocytes to activate pro-hypertrophy genes in response to hypertrophic stimulation.
We discovered that MITF and BRG1 cooperate to induce cardiomyocyte hypertrophy. In an in vivo model of cardiac hypertrophy, expression of MITF increased coordinately with that of BRG1 in response to pressure overload. MITF and BRG1 levels also increased in H9c2 immortalized cardiomyocytes that were stimulated with isoproterenol, a β-adrenergic agonist. Depletion of MITF or BRG1 by transfection with short interfering RNAs (siRNAs) attenuated the hypertrophic response as evidenced by decreased cell size and decreased expression of two cardiac hypertrophy genes, brain natriuretic peptide (BNP) and atrial natriuretic peptide (ANP). GATA4 is a transcriptional activator of both BNP and ANP. Importantly, the upstream region of the GATA4 gene has a conserved E box that is important for its expression and is potentially regulated by MITF [34]. We determined that depletion of MITF decreased GATA4 mRNA levels and that MITF can bind to the E box in the GATA4 promoter and trans-activate a GATA4 reporter containing an intact E box. Furthermore, MITF was detected on the endogenous GATA4 promoter, required to recruit BRG1, and to promote H3K4 tri-methylation. Thus, in stimulated cardiomyocytes, a key transcriptional activator of pro-hypertrophic gene expression, GATA4, is directly activated by MITF and this regulation is dependent upon the BRG1 component of the SWI/SNF complex.
Results
MITF and BRG1 expression increases after transverse aortic constriction (TAC) induced cardiac hypertrophy and in cardiomyocytes treated with angiotensin II
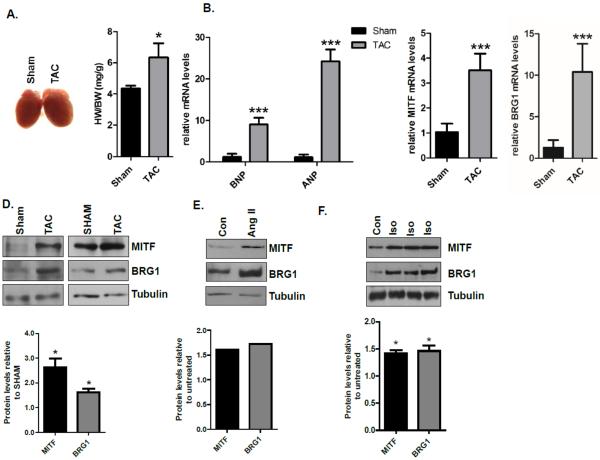
BRG1 expression is silenced in adult myocardium and re-activated in hearts subjected to pressure overload [16], but it is not known if MITF expression also increases under these conditions. To determine if MITF is activated coordinately with BRG1 when cardiac hypertrophy is induced by pressure overload, we subjected mice to trans-aortic constriction (TAC) and examined the expression of MITF and BRG1 in hypertrophic hearts. The development of cardiac hypertrophy was confirmed by an increase in heart weight (HW) to body weight (BW) ratio (Fig. 1A) and increased expression of two markers of hypertrophy, brain natriuretic peptide (BNP) and atrial natriuretic peptide (ANP) (Fig. 1B). Under these conditions, there was a significant increase in the expression of MITF and BRG1 at both the mRNA (Fig. 1C) and protein (Fig. 1D) levels in TAC mice compared to sham operated animals, two weeks after surgery.
Fig.1. MITF and BRG1 mRNA and protein levels increase after transverse aortic constriction (TAC).
(A) Representative images of hearts and heart weight/body weight (HW/BW) in sham and TAC mice 2 weeks after surgery. (B) Relative BNP and ANP expression levels were measured by quantitative RT- PCR (qRT – PCR) from left ventricles of sham and TAC mice described in (A). ANP and BNP levels were normalized to 18s rRNA. (C) MITF and BRG1 expression levels were assessed by qRT-PCR as in B. For experiments shown in (A), (B), and (C), the sample size was N=3 for sham, N=4 for TAC. Standard error bars are shown (* p<0.05, **p<0.01, and *** p<0.005). (D) Whole cell lysates from sham and TAC mice described in A were run on an SDS-polyacrylamide gel and immunoblotted with MITF and BRG1 antibodies. Tubulin was used as a loading control. Each blot is from an independent experiment in which cardiomyocytes were obtained from three to five mice. (E) Whole cell lysates from control and angiotensin II (200nM) treated primary adult mouse cardiomyocytes were prepared six hours after treatment, run on an SDS-polyacrylamide gel and immunoblotted with MITF and BRG1 antibodies. Tubulin was used as a loading control. The blot is from an experiment in which cardiomyocytes were obtained from four mice. (F) Whole cell lysates from control and isoproterenol (10μM) treated primary adult mouse cardiomyocytes were prepared six hours after treatment, run on an SDS-polyacrylamide gel, and immunoblotted with MITF and BRG1 antibodies. Tubulin was used as a loading control. Each lane on the gel contains whole cell lysate isolated from cardiomyocytes that were isolated from one mouse. Band densities were quantified using Image J software and shown in the graphs below each blot.
Pressure overload is associated with increased levels of angiotensin II [35]. To determine whether MITF and BRG1 expression increase coordinately in response to angiotensin II stimulation, we isolated adult mouse cardiomyocytes and cultured them in the presence and absence of angiotensin II. We detected an increase in MITF and BRG1 protein levels in angiotensin II treated cardiomyocytes compared to controls (Fig. 1E). We also detected increased MITF and BRG1 protein levels when adult mouse cardiomyocytes were stimulated with isoproterenol, a β-adrenergic receptor agonist (Fig. 1F).
MITF and BRG1 expression increases in H9c2 cardiomyocytes after β-adrenergic stimulated hypertrophy
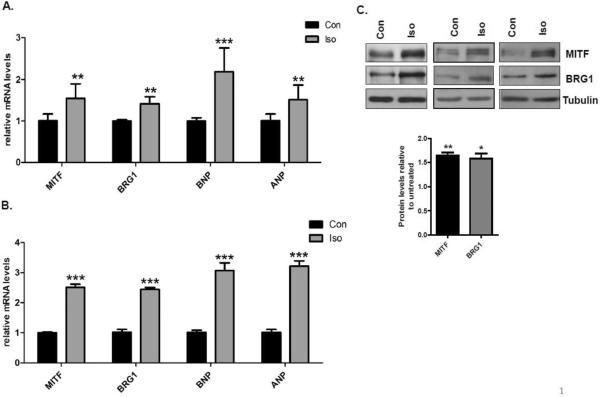
H9c2 cells are immortalized rat cardiomyocytes that respond similarly as primary rat cardiomyocytes to hypertrophy signals [36]. In order to determine if BRG1 levels increase coordinately with MITF, we treated H9c2 cells with 10μM isoproterenol to activate β-adrenergic receptors. The activation of hypertrophy was confirmed by significant increases in the mRNA levels of BNP and ANP four hours post β-adrenergic stimulation (Fig. 2A) and further increases after 24 hours (Fig. 2B). There was a coordinate increase in MITF and BRG1 mRNA levels at both these time points (Fig. 2A, 2B) and also at the protein level (Fig. 2C). Thus, MITF and BRG1 are coordinately activated by multiple hypertrophic signals. Expression of these two proteins increases in vitro by β-adrenergic stimulation of immortalized cardiomyocytes (Fig. 2A, B, C). MITF and BRG1 are also coordinately activated in primary cardiomyocytes from adult mice by β-adrenergic stimulation (Fig. 1F) and by treatment with angiotensin II (Fig. 1E) as well as in vivo by pressure overload (Fig. 1A, B, C, D).
Fig.2. Isoproterenol induced expression of MITF and BRG1 in H9c2 cells.
(A) RNA was harvested from untreated H9c2 cells (con) and H9c2 cells that were treated with 10μM isoproterenol (Iso) for 4 hours. Relative MITF, BRG1, BNP, ANP expression levels were measured by quantitative RT- PCR (qRT – PCR) and normalized to 18S rRNA. (B) Cells treated as in A were harvested 24 hours after isoproterenol stimulation. RT-qPCR was performed as in A. Standard error bars and statistical significance are shown (*p<0.05, ** p<0.01 and *** p<0.005). (C) Whole cell extracts were prepared from H9C2 cells treated as in (A), run on an SDS-PAGE gel, and immunoblotted with anitbodies to MITF and BRG1. Tubulin is a loading control. The blots shown are from three independent experiments. Band densities were quantified using Image J software and shown in the graphs below each blot.
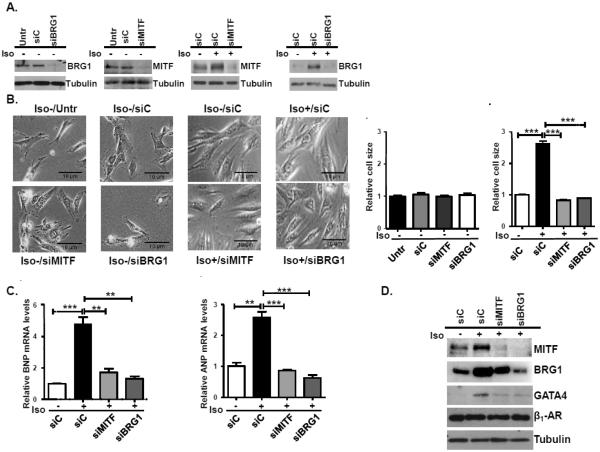
Depletion of MITF and BRG1 attenuates the β-adrenergic induced increase in cell size and expression of cardiac hypertrophy markers but not the expression of the β-adrenergic 1 receptor
To determine if MITF and BRG1 contribute to the activation of hypertrophy by β-adrenergic signaling, we transfected H9c2 cells with scrambled siRNA (siC) or siRNA that targets MITF or BRG1 and then induced hypertrophy with isoproterenol (Fig. 3A). Depletion of MITF or BRG1 did not affect cell size in untreated H9C2 cells. Consistent with previous findings, there was a significant increase in cell size after treatment with isoproterenol for 96 hours in cells that were transfected with a control siRNA [37]. However, isoproterenol treated cells that were depleted of MITF or BRG1 did not increase in size (Fig. 3B).
Fig.3. Isoproterenol induced hypertrophy of H9C2 cells is abrogated by down-regulation of MITF and BRG1.
(A) H9c2 cells were transfected with control siRNA (siC), siMITF, or siBRG1 for 72 hours and then were cultured in the presence or absence of isoproterenol (Iso). Whole cell extracts were prepared from cells that were harvested 4 hours after isoproterenol treatment, run on an SDS-PAGE gel, and immunoblotted with anitbodies to MITF or BRG1. Tubulin is a loading control. (B) (Left): Representative microscopic images (20×magnification) of control and isoproterenol treated cells transfected with a control siRNA (siC), siMITF, or siBRG1 96 hours after treatment (Right): Cell size was quantified using Image J software. The results are from three independent experiments. Standard errors and statistical significance are shown (*p<0.05, ** p<0.01 and *** p<0.005). (C) RNA was harvested from H9c2 cells treated as in (B). BNP and ANP mRNA levels were determined by qRT-PCR with18S rRNA as the normalization control. Standard error and statistical significance are shown. D. Protein was isolated from H9c2 cells treated as in (B) and probed with antibodies to MITF, BRG1, GATA4, β1-adrenergic receptor (β1-AR), or tubulin.
We further tested if MITF and BRG1 are both required for activation of BNP and ANP upon induction of hypertrophy. We observed a significant increase in BNP and ANP mRNA levels in control cells (siC) that were treated with isoproterenol compared to untreated cells. However, BNP and ANP mRNA levels were significantly reduced in cells depleted of MITF or BRG1 (Fig. 3C).
In order to identify which steps of the hypertrophic response to β-adrenergic stimulation require MITF and BRG1, we investigated the effects of MITF and BRG1 knockdown on the expression of two key regulators of the pathway, the β1-adrenergic receptor 1 (β1-AR) and GATA4, a critical pro-hypertrophic transcription factor that activates ANP and BNP expression. Knockdown of either MITF or BRG1 did not affect β1-AR expression but abrogated the expression of GATA4, a transcriptional regulator of both ANP and BNP (3D). Interestingly, BRG1 knockdown also severely inhibited MITF expression while MITF knockdown had only a small effect on BRG1 expression. These data suggest a degree of cross-regulation of MITF and BRG1 levels, both of which are required for activation of GATA4 expression.
MITF activates expression of the pro-hypertophic transcription factor GATA4 by binding to the E box in its promoter
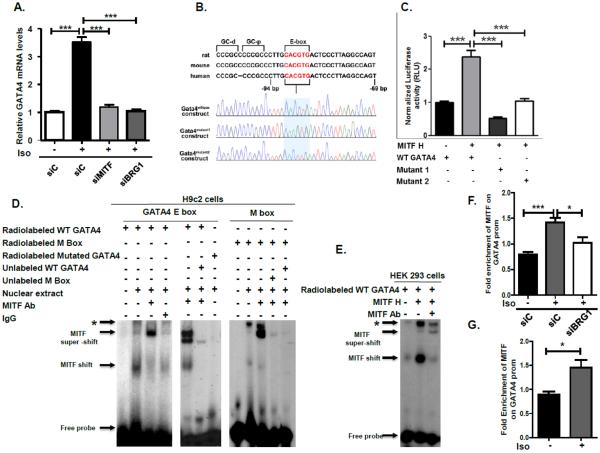
We investigated the possibility that MITF directly regulates the expression of GATA4, which has a conserved E box that was shown to be important for its expression [34, 38, 39]. Previous studies showed that GATA4 mRNA levels are up-regulated in isoproterenol-treated hearts [40, 41]. We found that GATA4 mRNA was induced by isoproterenol treatment of H9c2 cardiomyocytes that were transfected with control siRNA and that depletion of either MITF or BRG1 inhibited activation of GATA4 expression by isoproterenol treatment (Fig. 4A).
Fig. 4. Regulation of the GATA4 promoter by MITF.
A) H9c2 cells were transfected with either scrambled siRNA or siRNA that targets MITF or BRG1, then cultured in the presence or absence of isoproterenol for 96 hours. GATA4 mRNA levels were determined by qRT-PCR with 18S rRNA as the normalization control. Standard error and statistical significance are shown. (*p<0.05, ** p<0.01 and *** p<0.005).
B) Top: Schematic showing an E box element and a GC box motif that are conserved across rat, mouse and human GATA4 promoters. Bottom: The sequence of the cloned GATA 4 wild type and mutant constructs used in the luciferase assays. C) HEK 293 cells were co-transfected with MITF-H or an empty vector and either wild-type GATA4 or GATA4 mutant1 (C to A) or mutant 2 (G to T) luc-reporter constructs. A Beta-Gal expression vector was co-transfected as a control. Luciferase readings of three separate transfections were normalized to the respective beta-galactosidase readings and to protein concentration, then plotted. Standard error and statistical significance are shown. D) Left: A 32P – labeled oligonucleotide corresponding to the −96 to −79 bp region (5'-cgggatCCCTTGCACGTGACTCCC-3') of the GATA4 promoter (containing E box but lacking GC box motif) was incubated with nuclear extract from isoproterenol stimulated H9c2 cells. Supershifts were conducted by incubating with MITF antibody or with an IgG control. 30X unlabeled wild-type GATA4 oligonucleotide was used in competition experiments (middle). As a control, a A 32P- oligonucleotide containing a mutant GATA4 E box (5cgggatCCCTTGCACGAAACTCCC-3') was incubated with nuclear extract and MITF antibody. Right: A 32P – labeled oligonucleotide corresponding to a region of the TYRP1 promoter containing the conserved M box (5'CAGTGGGGAGGGAGTCATGTGCTGCCTAGT-3') was incubated with nuclear extract from isoproterenol stimulated H9c2 cells. 30X unlabeled M box or GATA4 E box was used in competition experiments. E) A 32P – labeled olignucleotide containing the GATA4 E box was incubated with nuclear extract from HEK 293 cells that were transfected with either an empty vector (−) or MITF-H. Super shifts were carried out with MITF antibody. * indicates mobility shifts that were not affected by MITF antibody. F) Chromatin immunoprecipitations (ChIPs) were performed using an antibody to MITF or control IgG on H9C2 cells that were transfected with control siRNA or RNA that targets BRG1 and G) primary cardiomyocytes from adult mice that were untreated or treated with isoproterenol. MITF enrichment was normalized to enrichment of control IgG. Fold enrichment of MITF on the GATA4 promoter is shown relative to a control PNOC region. The data are representative of three independent experiments. Standard error bars and statistical significance are shown.
To determine if MITF regulates transcription of GATA4 through the conserved E box, GATA4 promoter-luciferase reporter constructs with a wild type E box and constructs with mutations in the E box were generated (Fig. 4B). The wild type and mutant GATA4 promoter constructs were co-transfected into HEK 293T cells with an empty vector or an expression vector containing the heart specific MITF isoform (MITF-H). We found that MITF-H could drive the expression of a wild-type GATA4 promoter-luciferase reporter but that mutations in the E box element severely inhibited activation (Fig. 4C).
The ability of MITF to bind to the GATA4 promoter was assessed by electrophoretic mobility shift assays (EMSAs) using a radiolabeled probe containing the GATA4 upstream region with the E box element. A DNA-protein complex was detected upon incubation of a wild-type GATA4 E box with nuclear extract from isoproterenol-induced H9c2 cells. This band was super-shifted by incubation with MITF antibody but not with an IgG control antibody (Fig. 4D, left). The observed binding was specific as competition with the excess unlabeled wild type GATA4 E Box abolished the mobility shifts and a radiolabeled probe with mutated GATA4 was not shifted (Fig. 4D, middle). These results suggest that MITF can bind to the E box element of the GATA4 promoter in isoproterenol induced H9c2 cells.
The GATA4 promoter contains an E box element comprised of 5'-CACGTG-3', This E box motif is different from the M box, an 11 bp specialized E box with a hexamer core of 5'-CATGTG-3', found in the promoters of many melanocyte specific genes including tyrosinase and tyrosinase related protein 1 (TYRP1) [42]. To determine if the MITF isoform present in cardiomyocytes can also bind the M box, we performed EMSAs using a radiolabeled oligonucleotide containing the TYRP1 upstream region with the M box element. The same mobility shifts were detected with the TYRP1 M box probe as with the GATA4 probe (Fig. 4D, right). Importantly, MITF antibody promoted the formation of a super-shift while both the unlabeled TYRP1 and GATA4 oligonucleotides competed with the M box probe for binding to MITF. Thus, MITF binds to the GATA4 upstream region as well as it does to the TYRP1 upstream region.
Our results suggested that the MITF-H isoform binds the GATA4 E box because the MITF-H isoform is thought to be the only MITF isoform that is expressed in cardiomyocytes [14]. To confirm that the MITF-H isoforms binds to the GATA4 E box, we transfected HEK 293T cells with an empty vector or with a vector expressing MITF-H and performed EMSAs using radio-labeled GATA4 E box. A prominent band that was super-shifted with MITF antibody was detected when the probe was incubated with nuclear extract prepared from MITF-H transfected cells (Fig. 4E). ChIP analysis confirmed that MITF binds to the GATA4 promoter in isoproterenol induced H9C2 cardiomyocytes and that knockdown of BRG1 partially abrogates MITF associateion with this promoter (Fig. 4F). Furthermore, we also detected MITF binding to the GATA4 promoter in primary cardiomyocytes that were isolated from adult mice and treated with isoproterenol (Fig. 4G). Thus, our data show that MITF transcriptionally activates GATA4 expression by binding to the E box in the GATA4 promoter and that the level of MITF association with this promoter is partially dependent on BRG1.
MITF and BRG1 physically interact in cardiomyocytes and in HEK 293T cells
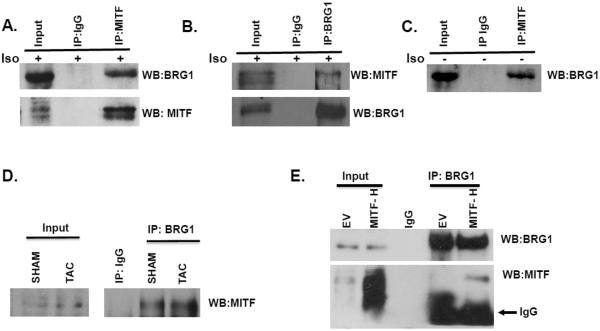
Our experiments indicated that depletion of either MITF or BRG1 was sufficient to block the increase in cell size and abrogate β-adrenergic stimulation of BNP and ANP expression. To determine if MITF and BRG1 act together in a concerted manner in the context of cardiomyocytes, we carried out co-immunoprecipitation (co-IP) experiments in H9c2 cells that were treated with isoproterenol. We found that an antibody to BRG1 pulled down MITF (Fig. 5A) and in a reciprocal co- IP, an MITF antibody pulled down BRG1 (Fig. 5B). Interestingly an MITF-BRG1 interaction was also detected in untreated H9C2 cells (Fig. 5C). Furthermore, MITF and BRG1 interact in the left ventricles of mice that were either sham treated or TAC treated (Fig. 5D). Thus, endogenous MITF and BRG1 proteins likely interact in both normal and hypertrophic cardiomyocytes.
Fig. 5. The heart specific isoform of MITF (MITF-H) interacts with BRG1.
(A) H9C2 cells were treated with isoproterenol (10μM). Cells were harvested 4 hours later and whole cell extracts were used for immunoprecipitation with an antibody to MITF or a control IgG. The immunoprecipitated material was run on an SDS – polyacrylamide gel and immunoblotted for BRG1 and MITF. 8–10% of the input was loaded onto the gel. (B) The reciprocal experiment was performed with an antibody to BRG1 or control IgG on H9C2 cells treated as in (A). (C) Untreated H9C2 cells were used for immunoprecipitation with an antibody to MITF or a control IgG as in A. (D) Whole cell extracts were prepared from the left ventricles of sham or TAC treated mice, two weeks after treatment, and used for immunoprecipitation with a BRG1 antibody or IgG. The immunoprecipitated material was run on an SDS – polyacrylamide gel and immunoblotted for MITF. 8–10% of the input was loaded onto the gel. (D) An empty vector (EV) or an MITF-H expression plasmid was transfected into HEK293 cells. Cells were harvested 48 hours later and the whole cell extract was used for immunoprecipitation with a BRG1 antibody. The immunoprecipitated material was run on an SDS-polyacrylamide gel and immunoblotted for BRG1 and MITF. 8–10% of the input was loaded onto the gel.
The physical interaction between the MITF-H isoform and BRG1 was confirmed by transfecting HEK 293T cells with a plasmid expressing MITF-H and carrying out immunoprecipitation experiments with a BRG1 antibody. MITF-H was coimmunoprecipated with BRG1 under these conditions (Fig 5C). These results show that both endogenous MITF in cardiomyocytes as well as ectopically expressed MITF-H physically interact with BRG1.
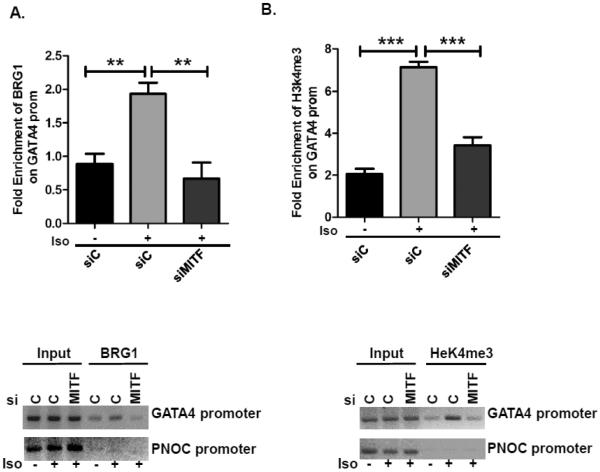
MITF promotes recruitment of BRG1 to the GATA4 promoter and histone H3K4 trimethylation (H3K4me3)
We tested the hypothesis that MITF-H binds the GATA4 promoter and activates gene expression by recruiting the BRG1 component of the SWI/SNF complex to the GATA4 promoter, thereby promoting chromatin changes. To test this hypothesis, we carried out chromatin immunoprecipitations (ChIP) after depleting MITF in H9c2 cells and treating the cells with isoproterenol to induce hypertrophy. Significant enrichment of BRG1 was observed on the GATA4 promoter in cells that were treated with isoproterenol. However, enrichment was significantly abrogated in cells that were depleted of MITF (Fig. 6A) as was enrichment of H3K4me3, a histone mark associated with active transcription (Fig. 6B). In combination, our results indicate that GATA4 is an MITF target gene that is directly activated by both MITF and BRG1.
Fig. 6. MITF promotes BRG1 recruitment to the GATA4 promoter after Isoproterenol treatment.
(A) H9c2 cells were transfected with either scrambled siRNA or siRNA that targets MITF or BRG1 then treated with isoproterenol for 4 hours as in Fig. 3A. Cells were processed for the chromatin immunoprecipitaions (ChIP) assay with an antibody to BRG1 or IgG (A) or with an antibody to H3K4me3 (B). BRG1 and H3K4me3 enrichment was normalized to enrichment of control IgG. And is shown relative to a control PNOC region. (TOP): ChIPs were evaluated by qPCR. (Bottom): ChIPs were run on 2% agarose gels and stained with ethidium bromide.
Discussion
The Microphthalmia-Associated Transcription Factor (MITF) is a pleiotropic regulator of gene expression in diverse cell types. Several MITF isoforms are transcribed from alternative promoters that give rise to distinct 5′ first exons which are spliced on to common 3'exons, generating proteins that are expressed in a cell specific manner. Although the mechanisms that regulate the expression of the melanocyte specific isoform, MITF-M, have been well characterized, less is known about how the other MITF isoforms are regulated. The results of this study indicate that expression of MITF in the heart, in cultured primary cardiomyocytes, and in H9c2 immortalized cardiomyocytes, can be induced by multiple hypertrophic stimuli, including pressure overload, angiotensin II, and β-adrenergic agonists. Interestingly, a previous study determined that the MITF-H promoter and not the MITF-M promoter can be induced by β-adrenergic stimulation in H9c2 cardiomyocytes, and other studies suggested that distinct transcriptional mechanisms regulate usage of the different MITF promoters [8, 43]. Interestingly, depletion of BRG1 was previously reported to abrogate MITF expression in melanoma cells[44]. Our data indicate that depletion of BRG1 also abrogates MITF-H expression in cardiomyocytes and reduces MITF-H occupancy on a newly identified MITF-H target, the GATA4 promoter. Additional experiments are required to identify the precise signaling cascades and transcription factors that promote MITF-H expression in cardiac hypertrophy and to understand how BRG1 contributes to this regulation.
Several studies have demonstrated a critical role for MITF-H in the regulation of pathological cardiac hypertrophy [8, 13]. However, the precise transcriptional mechanisms by which this MITF isoform promotes gene expression in the heart are not well understood. Only, three genes, myosin light-chain 1a (MLC-1a), and erbin have been shown to be directly regulated by MITF in cardiomyocytes [13–15]. Of these three MITF targets, only mir-541 and erbin have a role in cardiac hypertrophy. In this study, we determined that MITF-H binds to a conserved E box in the promoter of the GATA4 gene and activates GATA4 expression in cardiomyocytes in response to β-adrenergic stimulation. Thus, our data indicate that GATA4 is a novel MITF-H target gene.
Interestingly, we found that in addition to the E box present in the GATA4 promoter, MITF-H can bind to the canonical M box present in melanocyte specific genes. It has been demonstrated that some MITF isoforms can bind but cannot trans-activate melanocyte specific promoters [12]. Genome-wide studies conducted in melanocytes and melanoma cells indicate that the MITF isoforms present in these cells regulate expression of melanocyte specific genes, pro-proliferative, and survival genes [45–47]. A unique set of MITF targets were identified in mast cells, including genes that are important for mast cell function, signaling, and adhesion [11]. Hence, distinct isoforms differentially regulate gene expression in a context specific manner, potentially through interactions with cell specific factors. The requirement for MITF-H for the activation of GATA4, a transcription factor that binds to the promoters of multiple hypertrophy genes, suggests that MITF-H has an extensive role in the hypertrophic response to β-adrenergic stimulation and potentially in response to other stimuli of hypertrophy. Future experiments involving chromatin immunoprecipitation coupled to high throughput sequencing (ChIP-seq) and RNA sequencing in cardiomyocytes will allow us to identify direct MITF-H gene targets and illuminate the mechanisms by which MITF, a pro-proliferative transcription factor in melanoma, can instead promote hypertrophy in a terminally differentiated cell such as the cardiomyocyte.
Although existing evidence suggests that different MITF isoforms interact with cell specific factors to regulate distinct transcriptional profiles and biological responses, BRG1 is a common co-activator utilized by multiple MITF isoforms in different cell types. MITF isoforms present in melanoma cells and osteoclasts interact with BRG1 and recruit BRG1 to target promoters [21, 48]. We now show that MITF-H interacts with BRG1 in cardiomyocytes and recruits BRG1 to the promoter of a hypertrophy gene. Consistent with our hypothesis that MITF-H cooperates with BRG1 to promote hypertrophy, multiple stimuli trigger expression of BRG1 coordinately with expression of MITF while depletion of either MITF or BRG1 abrogates expression of GATA4 and the hypertrophic response. Interestingly, we detected physical interactions between MITF and BRG1 in unstimulated cardiomyocytes as well as in hypertrophic cardiomyocytes. However, knockdown of MITF and BRG1 in unstimulated cardiomyocytes did not affect cell size. Furthermore, MITF and BRG1 recruitment to the GATA4 promoter increased with isoproterenol stimulation. These data suggest that MITF and BRG1 cooperate to regulate distinct genes in unstimulated and hypertrophic cardiomyocytes. In melanocytes and melanoma cells, MITF extensively regulates BRG1 genomic occupancy by promoting BRG1recruitment to loci that encode transcriptional regulators and genes that promote pigmentation, and proliferation. Presumably, distinct MITF isoforms in other cell types recruit BRG1 to unique genomic loci to obtain the appropriate gene expression profile. Identifying the genomic loci to which MITF directs BRG1 in cardiomyocytes will give insight into the mechanisms by which the MITF-H isoform regulates gene expression in a cardiomyocyte specific manner.
In conclusion, our work shows that MITF and BRG1 are activated by hypertrophic stimulation of cardiomyocytes and cooperate to regulate expression of GATA4, a key transcriptional regulator of BNP and ANP as well as expression of other fetal cardiac genes [49]. Despite, GATA4 being a vital transcription factor in cardiac hypertrophy, the mechanisms that regulate GATA4 expression in response to hypertrophic stimulation have not been well characterized. The E box present in the GATA4 proximal promoter is required for transcriptional activation of GATA4 during differentiation of P19.CL6 embryonic carcinoma cells into beating cardiomyocytes [39] and for GATA4 expression in gonadal and other cell types [34]. Ubiquitously expressed Upstream Stimulatory Factors (USFs) bind to the E box and trans-activate the Gata4 promoter [34]. However, the requirement for USFs in cardiac hypertrophy has not been determined. Our data indicate that MITF-H binds the E box and activates transcription and we confirm the presence of other E box binding factors present in both H9c2 and 293T cells, which are potentially USF factors. Interestingly, in melanocytes and melanoma cells, USF-1 plays an important role in the tanning response by binding and trans-activating E boxes present in several MITF target promoters. In future studies, we will determine if USF proteins contribute to GATA4 regulation in cardiac hypertrophy and elucidate the mechanisms that regulate transcription factor occupancy of the E box.
Our results provide evidence of a novel mechanism by which GATA4 and hence, pathological cardiac hypertrophy is regulated. Detailed understanding and modulation of this molecular pathway may provide an alternative approach for tackling this medical condition.
Materials and Methods
Cell culture
H9c2 cells were purchased from the American Tissue Culture Collection (Manassas, VA, USA). Cells were sub-cultured weekly in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 1% glutamine and 1% penicillin/streptomycin. Cells grown to 60–70% confluency were serum deprived for 24 h and treated with isoproterenol (10 μM).
MITF-H Plasmid and transient transfections
The MITF-H plasmid was described in [8] and transfected into HEK 293T cells using liptofectamine LTX (Invitrogen).
Cell extracts and immunoblot analysis
Cells were lysed as described [50]. Antiserum to BRG1 was previously described [50]. Antibody against MITF (cat# ab12039) was from Abcam (Cambridge, MA, USA). Tubulin antibody (cat#21485) was from Cell Signaling Technology (Boston, MA, USA).
RNA isolation and Quantitative Real Time PCR
Total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA) and cDNA was prepared using the QuantiTect Reverse Transcription kit (Qiagen, Germantown, MD). Quantitative PCR (qPCR) was performed and analyzed as described [21]. Primers used for mouse and rat MITF: 5'-CAGACCCACCTGGAAAACC-3' and 5'-ATGCTGAGCTCAGGACTTGG-3', mouse BRG1: 5'-TCTGAGGTGGACGCCCGACACATTA-3' and 5'-TAAGGACCTGCGTCAACTTGCAGTG -3', mouse BNP: 5'-CACCGCTGGGAGGTCACT-3' and 5'-GTGAGGCCTTGGTCCTTCAA-3', mouse ANP: 5'-GCTCCTTCTCCATCACCCTG-3' and 5'-ACCGGCATCTTCTCCTCCA-3', mouse 18srRNA: 5'-AGGTTCTGGCCAACGGTCTAG-3' and 5'-CCCTCTATGGGCTCGAATTTT-3', rat BRG1: 5'-GAGGTGCGTGTGCTTCGCCT-3' and 5'-GCGAGAACCACGGCCGAACA-3', rat BNP: 5'-ACCGGATCGGCGCAGTCAGT-3' and 5'-GCCGCAGGCAGAGTCAGAAGC-3', rat ANP: 5'-ACCTGGAGGAGAAGATGCCG-3' and 5'-TGTTGCAGCCTAGTCCGCTC-3', rat GATA4: 5'-CCGTCTACGTGCCCACTC-3' and 5'-GTGTAAGCGGCTCCCTCAG-3', rat 18srRNA: 5'-AGTCCCTGCCCTTTGTACACA -3' and 5'-GATCCGAGGGCCTCACTAAAC-3'.
siRNA Knockdown
The siRNA sequences for control and MITF were described previously: siControl, 5′-(UUCUCCGAACGUGUCACGU)-3′; siMITF, 5′-(AGCAGUACCUUUCUACCAC)-3′ [33]. A pool of siRNAs targeting BRG1 was transfected according to manufacturer's instructions (Dharmacon, Lafayette, CO). After 72 hours in transfection media, cells were serum deprived for 24 h and treated with isoproterenol (10 μM) either for 4 or 96 hours.
Co-immunoprecipitations
The mouse MITF-H plasmid used in transfections was described in [8]. HEK 293T cells were obtained from ATCC and transfected with empty pcDNA 3.1 or pcDNA3.1 MITF-H using Lipofectamine LTX (Invitrogen, Carlsbad, CA). Cells were harvested 48 hours post-transfection. Co-immunoprecipitations were performed as previously described (43) using MITF (mouse) or BRG1 (rabbit) antibody. Species matched IgG (cat# sc2025 and sc2017, Santa Cruz Biotechnology, CA) was used as a control.
Cell area measurement
The surface area of H9c2 cells was measured using phase-contrast microscopy. Cells were serum starved for about 24 h and then stimulated with isoproterenol (10 μM) treatment for 96 hours. Images were obtained using an Olympus IX70 microscope equipped with a digital camera and SPOT imaging software (Diagnostics Instruments, Inc. Sterling Heights, MI). Cell surface area was measured using Image J software (National Institutes of Health).
Transverse aortic constriction (TAC)
Animal studies were performed in accordance with protocols that were approved by the Institutional Animal Care Committee. Two month-old male C57BL/6 mice were used. The TAC procedure was conducted as described in [51]. Briefly, 2-month old male C57BL/6 mice were anesthetized with 2–5% isoflurane. Endotracheal intubation was performed with a rodent intubation system (Biotex, Inc) and connected to a mini-ventilator (Harvard Apparatus). Mice were given 0.05 mg/kg buprenorphine (s.c.) before the operation and were maintained unconscious with 0.8–1.5% isoflurane. A partial thoracotomy was performed and the thymus was separated to expose the transverse aorta. A 6-0 silk suture was used for tying the aorta to a 27-G blunt needle, which was removed immediately after ligation. The lungs were re-inflated before closing the chest. The pectorales muscles were sutured with a 5–0 absorbable chromic gut. The skin was sutured with a 6-0 nylon suture. The entire procedure was identical in sham mice, except for the aorta ligation. Mice were sacrificed 2 weeks after surgery, and body and organ weights were recorded. Left ventricles were snap-frozen in liquid nitrogen and stored at −80°C.
Primary cardiomyocyte isolation and culture
Cardiomyocytes were isolated from hearts obtained from 3 month old male mice as described in [52]. 10μM isoproterenol or 20nM angiotensin II was used to stimulate hypertrophy.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared using the NE-PER™ Nuclear and Cytoplasmic Extraction Reagents kit (Thermo Fisher Scientific™) according to the manufacturer's protocol. EMSA was performed using as described in [14]. Briefly, double-stranded synthetic wild-type GATA4 E box (5'- cgggatCCCTTGCACGTGACTCCC-3'), mutated E box (5'-cgggatCCCTTGCACGAAACTCCC-3'), and TYRP1 M box (5'CAGTGGGGAGGGAGTCATGTGCTGCCTAGT-3') probes were prepared by end-labeling with [γ-32P] ATP (MP Biomedicals) and T4 polynucleotide kinase. A typical binding assay contained 0.8 – 1.0 × 105 cpm double stranded probe and 7–8 μg of nuclear extract protein in binding buffer (10% glycerol, 2.5 mM MgCl2, 1 mM DTT, 50 mM KCl, 10 mM Hepes pH 7.9). In competition experiments, nuclear extracts were pre-incubated with 30 X molar excess non-radioactive double stranded probe for 90 min. 32P-labeled probes were added to the reactions and incubation continued for 30 min at room temperature. For super-shift experiments, non-specific IgG (Santa Cruz) or MITF (C5, Abcam) antibody was added and incubation continued for 30 min. The reactions were subjected to electrophoresis in a 6% non-denaturing polyacrylamide gel using 0.5 X TBE buffer.
Cloning and Luciferase reporter assay
The reporter construct rGata4-pGL3/Luc was synthesized by PCR amplification of the rat Gata4 gene segment from −238 to +40 using gtatCTCGAGAGAGCGGAGCGCTGGTACTGA as the forward primer with the XhoI restriction site and gataAAGCTTCTGCAGCAACAGCGGAGCCT as the reverse primer with the HindIII restriction site. The PCR-amplified product was cloned in to a pGL3 basic vector that lacks eukaryotic promoter and enhancer sequences (Promega). The mutant plasmid prGata4-88lucmut and prGata4-87lucmut were obtained by site-specific mutagenesis using the Stratagene site-directed mutagenesis kit. The rGata4-pGL3/Luc plasmid was used as a template to mutate the −88C allele to −88A allele and −87G allele to −87T and designated as rGata4-pGL3/Luc −88 (Mutant 1) and rGata4-pGL3/Luc −87 (Mutant 2) respectively. The nucleotide sequence of oligonucleotides used for mutation of the CACGTG/E box site were C88A Primer1: CTAAGGGAGTCACTTGCAAGGGCGGGG, C88A Primer2: CCCCGCCCTTGCAAGTGACTCCCTTAG and G87T Primer1 CCTAAGGGAGTCAAGTGCAAGGGCGGG, G87T Primer2 CCCGCCCTTGCACTTGACTCCCTTAGG. All clones were verified by sequencing. For the luciferase assays, HEK293T cells were transfected with 300 ng of either wild-type GATA4 or mutated GATA4 reporter construct, 50 ng of an internal control - pCMV beta-galactosidase expressing construct and 3 μg of either empty vector or MITF-H expression constructs. The total amount of DNA (5 μg) transfected was kept constant by the addition of control pGL3 basic vector. Cells were transfected using Lipofectamine LTX (Invitrogen) according to manufacturer's protocols. After 48 hours post transfection, luciferase and beta-galactosidase assays (Promega) were performed. Luciferase activity was normalized to the internal control beta-galactosidase.
Chromatin immunoprecipitations (ChIPs)
ChIPs were performed as described [21]. The BRG1 antibody was previously described [53]. The antibody against MITF was from Abcam (C5) and the antibodies against Histone 3 lysine 4 trimethylation (H3K4me3) was obtained from Active Motif (Carlsbad, CA). Primers used were as follows: rat GATA4 promoter: 5'-GCATGGACTTTGCCTGCT-3' and 5'-CCTGCTCTGACTGGCCTAAG-3' and rat PNOC promoter: 5'-CAGACAGGGAGGACATGGAT-3' and 5'-GGACTGCAAAGTGCAGACAA-3'. ChIPs were analyzed by quantitative PCR (qPCR) using SYBR green or were run on a 2% agarose gel and stained with ethidium bromide.
Statistical Analysis
Statistical significance was calculated by the Student's t test when comparing two sets of data and a one way ANOVA followed by the Bonferroni multiple comparison tests for comparing more than two sets of data using Graphpad Prism, version 5.03.
Highlights.
MITF and BRG1 levels are elevated in hypertrophic hearts and cardiomyocytes.
MITF and BRG1 regulate cardiomyocyte size in response to β-adrenergic stimulation.
MITF and BRG1physically interact in hypertrophic cardiomyocytes.
MITF binds to the GATA4 E box and activates expression of a GATA4 reporter.
MITF facilitates BRG1 recruitment to the GATA4 promoter and chromatin modifications.
Acknowledgments
This work was supported by National Institute of Health grants ARO59379, HL036573, HL020176, and HL112641.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interests The authors declare that they have no conflict of interest.
References
- 1.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7(8):589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB. Vital epidemiologic clues in heart failure. J Clin Epidemiol. 2000;53(3):229–35. doi: 10.1016/s0895-4356(99)00135-3. [DOI] [PubMed] [Google Scholar]
- 3.Oka T, et al. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res. 2006;98(6):837–45. doi: 10.1161/01.RES.0000215985.18538.c4. [DOI] [PubMed] [Google Scholar]
- 4.Okazaki H, et al. Angiotensin II type 1 receptor blocker prevents atrial structural remodeling in rats with hypertension induced by chronic nitric oxide inhibition. Hypertens Res. 2006;29(4):277–84. doi: 10.1291/hypres.29.277. [DOI] [PubMed] [Google Scholar]
- 5.Gaspar-Pereira S, et al. The NF-kappaB subunit c-Rel stimulates cardiac hypertrophy and fibrosis. Am J Pathol. 2012;180(3):929–39. doi: 10.1016/j.ajpath.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Q, et al. Interaction between NFkappaB and NFAT coordinates cardiac hypertrophy and pathological remodeling. Circ Res. 2012;110(8):1077–86. doi: 10.1161/CIRCRESAHA.111.260729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou J, et al. C/EBPbeta knockdown protects cardiomyocytes from hypertrophy via inhibition of p65-NFkappaB. Mol Cell Endocrinol. 2014;390(1–2):18–25. doi: 10.1016/j.mce.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Tshori S, et al. Transcription factor MITF regulates cardiac growth and hypertrophy. J Clin Invest. 2006;116(10):2673–81. doi: 10.1172/JCI27643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steingrimsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet. 2004;38:365–411. doi: 10.1146/annurev.genet.38.072902.092717. [DOI] [PubMed] [Google Scholar]
- 10.Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 2006;12(9):406–14. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Shahlaee AH, et al. Distinct and shared transcriptomes are regulated by microphthalmia-associated transcription factor isoforms in mast cells. J Immunol. 2007;178(1):378–88. doi: 10.4049/jimmunol.178.1.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takemoto CM, Yoon YJ, Fisher DE. The identification and functional characterization of a novel mast cell isoform of the microphthalmia-associated transcription factor. J Biol Chem. 2002;277(33):30244–52. doi: 10.1074/jbc.M201441200. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, et al. Cardiac hypertrophy is negatively regulated by miR-541. Cell Death Dis. 2014;5:e1171. doi: 10.1038/cddis.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tshori S, et al. Microphthalmia transcription factor isoforms in mast cells and the heart. Mol Cell Biol. 2007;27(11):3911–9. doi: 10.1128/MCB.01455-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rachmin I, et al. FHL2 switches MITF from activator to repressor of Erbin expression during cardiac hypertrophy. Int J Cardiol. 2015;195:85–94. doi: 10.1016/j.ijcard.2015.05.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hang CT, et al. Chromatin regulation by Brg1 underlies heart muscle development and disease. Nature. 2010;466(7302):62–7. doi: 10.1038/nature09130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stankunas K, et al. Endocardial Brg1 represses ADAMTS1 to maintain the microenvironment for myocardial morphogenesis. Dev Cell. 2008;14(2):298–311. doi: 10.1016/j.devcel.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willis MS, et al. Functional redundancy of SWI/SNF catalytic subunits in maintaining vascular endothelial cells in the adult heart. Circ Res. 2012;111(5):e111–22. doi: 10.1161/CIRCRESAHA.112.265587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang L, Nogales E, Ciferri C. Structure and function of SWI/SNF chromatin remodeling complexes and mechanistic implications for transcription. Prog Biophys Mol Biol. 2010;102(2–3):122–8. doi: 10.1016/j.pbiomolbio.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vignali M, et al. ATP-dependent chromatin-remodeling complexes. Mol Cell Biol. 2000;20(6):1899–910. doi: 10.1128/mcb.20.6.1899-1910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keenen B, et al. Heterogeneous SWI/SNF chromatin remodeling complexes promote expression of microphthalmia-associated transcription factor target genes in melanoma. Oncogene. 2010;29(1):81–92. doi: 10.1038/onc.2009.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lickert H, et al. Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature. 2004;432(7013):107–12. doi: 10.1038/nature03071. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi JK, et al. Baf60c is a nuclear Notch signaling component required for the establishment of left-right asymmetry. Proc Natl Acad Sci U S A. 2007;104(3):846–51. doi: 10.1073/pnas.0608118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei I, et al. SWI/SNF protein component BAF250a regulates cardiac progenitor cell differentiation by modulating chromatin accessibility during second heart field development. J Biol Chem. 2012;287(29):24255–62. doi: 10.1074/jbc.M112.365080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh AP, Archer TK. Analysis of the SWI/SNF chromatin-remodeling complex during early heart development and BAF250a repression cardiac gene transcription during P19 cell differentiation. Nucleic Acids Res. 2014;42(5):2958–75. doi: 10.1093/nar/gkt1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, et al. Polybromo protein BAF180 functions in mammalian cardiac chamber maturation. Genes Dev. 2004;18(24):3106–16. doi: 10.1101/gad.1238104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lange M, et al. Regulation of muscle development by DPF3, a novel histone acetylation and methylation reader of the BAF chromatin remodeling complex. Genes Dev. 2008;22(17):2370–84. doi: 10.1101/gad.471408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaynak B, et al. Genome-wide array analysis of normal and malformed human hearts. Circulation. 2003;107(19):2467–74. doi: 10.1161/01.CIR.0000066694.21510.E2. [DOI] [PubMed] [Google Scholar]
- 29.He L, et al. BAF200 is required for heart morphogenesis and coronary artery development. PLoS One. 2014;9(10):e109493. doi: 10.1371/journal.pone.0109493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang L, et al. Cardiac genes show contextual SWI/SNF interactions with distinguishable gene activities. Epigenetics. 2011;6(6):760–8. doi: 10.4161/epi.6.6.16007. [DOI] [PubMed] [Google Scholar]
- 31.Mehrotra A, Joe B, de la Serna IL. SWI/SNF chromatin remodeling enzymes are associated with cardiac hypertrophy in a genetic rat model of hypertension. J Cell Physiol. 2013;228(12):2337–42. doi: 10.1002/jcp.24404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weng X, et al. A crosstalk between chromatin remodeling and histone H3K4 methyltransferase complexes in endothelial cells regulates angiotensin II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2015;82:48–58. doi: 10.1016/j.yjmcc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Saladi SV, et al. BRG1 promotes survival of UV-irradiated melanoma cells by cooperating with MITF to activate the melanoma inhibitor of apoptosis gene. Pigment Cell Melanoma Res. 2013;26(3):377–91. doi: 10.1111/pcmr.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazaud Guittot S, et al. The proximal Gata4 promoter directs reporter gene expression to sertoli cells during mouse gonadal development. Biol Reprod. 2007;76(1):85–95. doi: 10.1095/biolreprod.106.055137. [DOI] [PubMed] [Google Scholar]
- 35.Schunkert H, et al. Increased rat cardiac angiotensin converting enzyme activity and mRNA expression in pressure overload left ventricular hypertrophy. Effects on coronary resistance, contractility, and relaxation. J Clin Invest. 1990;86(6):1913–20. doi: 10.1172/JCI114924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watkins SJ, Borthwick GM, Arthur HM. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell Dev Biol Anim. 2011;47(2):125–31. doi: 10.1007/s11626-010-9368-1. [DOI] [PubMed] [Google Scholar]
- 37.Somvanshi RK, Qiu X, Kumar U. Isoproterenol induced hypertrophy and associated signaling pathways are modulated by somatostatin in H9c2 cells. Int J Cardiol. 2013;167(3):1012–22. doi: 10.1016/j.ijcard.2012.03.077. [DOI] [PubMed] [Google Scholar]
- 38.Ishibashi T, et al. Conserved GC-boxes, E-box and GATA motif are essential for GATA-4 gene expression in P19CL6 cells. Biochem Biophys Res Commun. 2011;413(2):171–5. doi: 10.1016/j.bbrc.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 39.Ohara Y, et al. GATA-4 gene organization and analysis of its promoter. Biol Pharm Bull. 2006;29(3):410–9. doi: 10.1248/bpb.29.410. [DOI] [PubMed] [Google Scholar]
- 40.Gotic I, et al. Lamina-associated polypeptide 2alpha loss impairs heart function and stress response in mice. Circ Res. 2010;106(2):346–53. doi: 10.1161/CIRCRESAHA.109.205724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saadane N, Alpert L, Chalifour LE. Expression of immediate early genes, GATA-4, and Nkx-2.5 in adrenergic-induced cardiac hypertrophy and during regression in adult mice. Br J Pharmacol. 1999;127(5):1165–76. doi: 10.1038/sj.bjp.0702676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowings P, Yavuzer U, Goding CR. Positive and negative elements regulate a melanocyte-specific promoter. Mol Cell Biol. 1992;12(8):3653–62. doi: 10.1128/mcb.12.8.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Udono T, et al. Structural organization of the human microphthalmia-associated transcription factor gene containing four alternative promoters. Biochim Biophys Acta. 2000;1491(1–3):205–19. doi: 10.1016/s0167-4781(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 44.Vachtenheim J, Ondrusova L, Borovansky J. SWI/SNF chromatin remodeling complex is critical for the expression of microphthalmia-associated transcription factor in melanoma cells. Biochem Biophys Res Commun. 2010;392(3):454–9. doi: 10.1016/j.bbrc.2010.01.048. [DOI] [PubMed] [Google Scholar]
- 45.Hoek KS, et al. Novel MITF targets identified using a two-step DNA microarray strategy. Pigment Cell Melanoma Res. 2008;21(6):665–76. doi: 10.1111/j.1755-148X.2008.00505.x. [DOI] [PubMed] [Google Scholar]
- 46.Laurette P, et al. Transcription factor MITF and remodeller BRG1 define chromatin organisation at regulatory elements in melanoma cells. Elife. 2015;4 doi: 10.7554/eLife.06857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strub T, et al. Essential role of microphthalmia transcription factor for DNA replication, mitosis and genomic stability in melanoma. Oncogene. 2011;30(20):2319–32. doi: 10.1038/onc.2010.612. [DOI] [PubMed] [Google Scholar]
- 48.Sharma SM, et al. MITF and PU.1 recruit p38 MAPK and NFATc1 to target genes during osteoclast differentiation. J Biol Chem. 2007;282(21):15921–9. doi: 10.1074/jbc.M609723200. [DOI] [PubMed] [Google Scholar]
- 49.McBride K, Nemer M. Regulation of the ANF and BNP promoters by GATA factors: lessons learned for cardiac transcription. Can J Physiol Pharmacol. 2001;79(8):673–81. [PubMed] [Google Scholar]
- 50.de la Serna IL, et al. The microphthalmia-associated transcription factor requires SWI/SNF enzymes to activate melanocyte-specific genes. J Biol Chem. 2006;281(29):20233–41. doi: 10.1074/jbc.M512052200. [DOI] [PubMed] [Google Scholar]
- 51.deAlmeida AC, van Oort RJ, Wehrens XH. Transverse aortic constriction in mice. J Vis Exp. 2010;(38) doi: 10.3791/1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li D, et al. Isolation and culture of adult mouse cardiomyocytes for cell signaling and in vitro cardiac hypertrophy. J Vis Exp. 2014;(87) doi: 10.3791/51357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de La Serna IL, et al. Mammalian SWI-SNF complexes contribute to activation of the hsp70 gene. Mol Cell Biol. 2000;20(8):2839–51. doi: 10.1128/mcb.20.8.2839-2851.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]