Abstract
Background and purpose
Edema formation, inflammation and increased blood-brain barrier permeability contribute to poor outcomes after intracerebral hemorrhage (ICH). This study examined the therapeutic effect of Dimethyl fumarate (DMF), a fumaric acid ester that activates nuclear factor erythroid-2 related factor 2 (Nrf2) and Nrf2 heterodimerization effector musculo-aponeurotic fibrosacoma-G (MAFG) in a murine ICH model.
Methods
Male CD-1 mice (n=176) were subjected to intrastriatal infusion of bacterial collagenase (n=120), autologous blood (n=18) or sham surgery (n=30). After ICH, animals either received vehicle, Dimethyl fumarate (10mg or 100mg/kg) or casein kinase 2 inhibitor (E)-3-(2,3,4,5-tetrabromophenyl)acrylic acid (TBCA). Thirty-eight mice also received scrambled siRNA or MAFG siRNA 24 hours before ICH. Brain water content and neurological function were evaluated.
Results
Dimethyl fumarate reduced Evans blue extravasation, decreased brain water content, and improved neurological deficits at 24 and 72 hours after ICH. Casein kinase 2 inhibitor TBCA and MAFG siRNA prevented the effect of Dimethyl fumarate on brain edema and neurological function. After ICH, ICAM-1 levels increased and Casein kinase 2 levels decreased. Dimethyl fumarate reduced ICAM-1 but enhanced Casein kinase 2 levels. Again, Casein kinase 2 inhibitor TBCA and MAFG siRNA abolished the effect of Dimethyl fumarate on ICAM-1 and Casein kinase 2. Dimethyl fumarate preserved pNrf2 and MAFG expression in the nuclear lysate after ICH and the effect of Dimethyl fumarate was abolished by Casein kinase 2 inhibitor TBCA and MAFG siRNA. Dimethyl fumarate reduced microglia activation in peri-hematoma areas after ICH. The protective effect of Dimethyl fumarate on brain edema and neurological function was repeated in a blood injection mouse model.
Conclusion
Dimethyl fumarate ameliorated inflammation, reduced blood barrier permeability, and improved neurological outcomes by Casein kinase 2 and Nrf2 signaling pathways after experimental ICH in mice.
Keywords: Dimethyl fumarate, intracerebral hemorrhage, inflammation, Evans Blue extravasation, casein kinase 2, phosphorylated nuclear factor erythroid- 2 related factor 2 (Nrf2), musculo-aponeurotic fibrosacoma-G
Introduction
Intracerebral hemorrhage (ICH) which accounts for about 15-20% of all deaths from stroke is the rupturing of small blood vessels in the brain parenchyma. Currently, no effective treatment options are available for this fatal stroke subtype, and, even if patients survive the initial injury, a series of secondary events may lead to cerebral edema, progression of neurobehavioral deficits, and possible death.1-4
Evidence from clinical and animal studies suggest that inflammation and oxidative stress which occur after hematoma formation are involved in ICH-induced secondary brain injury and neurological dysfunction.5,6 Vascular cell adhesion molecule-1 (VCAM-1) and intracellular adhesion molecule-1 (ICAM-1), adhesion molecules expressed in the endothelium that are important in inflammation after injury, are increased upon activation of the nuclear factor-κB (NF-κB)-mediated tumor necrosis factor α (TNFα) signaling pathway. TNFα increases early-onset endothelial adhesion by protein kinase C-dependent upregulation of ICAM-1 expression, which can worsen outcomes following ICH.7,8
Nuclear factor erythroid-2 related factor 2 (Nrf2), a major phase II gene regulator, is a broadly expressed transcription factor that binds to the antioxidant response element (ARE) consensus and regulates expression of phase II detoxifying enzymes.9 In addition to protecting against oxidative and electrophilic stress, recent studies demonstrated Nrf2 responds to pro-inflammatory stimuli and rescues cells/tissues from inflammatory injuries.10,11 As a transcription factor, it is essential for Nrf2 to translocate into the nucleus in order to stimulate the upregulation of cytoprotective genes.12 Translocation of Nrf2 from the cytoplasm to the nucleus and export of Nrf2 from the nucleus is regulated by nuclear localization signals, nuclear export sequences and phosphorylation.13 Nrf2 is the substrate of several protein kinases, including protein kinase C14, phosphatidylinositol 3-kinase15, glycogen synthase kinase-316, casein kinase 217, and Fyn.18 Casein Kinase 2, an ubiquitous eukaryotic kinase composed of catalytic (α or α1) and regulatory (β) subunits was elucidated as a major kinase for phosphorylating Nrf2 in in-vitro studies using neuroblastoma cells and human keratinocyte cell lines.17,19
Dimethyl fumarate (DMF), a fumaric acid ester that is effective in the treatment of relapsing/remitting multiple sclerosis, promotes Nrf2 activation and stabilization through direct modification of Keap1 at cysteine residue 151.20,21 Stabilization and phosphorylation of Nrf2 facilitates its nuclear import, forming heterodimers with MAFG, subsequently upregulating cytoprotective genes and inhibiting NF-κB nuclear translocation, thus decreasing expression of NF-κB-dependent genes, including inflammatory cytokines, chemokines, and adhesion molecules.13,22 Although Dimethyl fumarate stabilizes Nrf2, the role Casein Kinase 2 plays in phosphorylating Nrf2 and p-Nrf2 conferred neuroprotection after ICH has not been documented. In the present study, we aimed to test 2 hypotheses, (i) administration of Dimethyl fumarate will reduce brain edema and neurological dysfunction in mice after ICH (ii) Casein Kinase 2 phosphorylation of Nrf2 will promote Nrf2 nuclear translocation and antioxidant response element activation as well as ameliorate inflammation and blood brain barrier permeability after ICH. A schema of the study design is presented in appendix 1.
Materials and Methods
All procedures were conducted in accordance with the NIH guide for care and use of laboratory animals. Approval was obtained from the Institutional Animal Care and Use Committee of Loma Linda University. CD-1 mice weighing 29-38g (Charles River, Wilmington, MA) were housed in light and temperature controlled environment with access to food and water ad libitum.
Surgical Procedures
ICH
Intracerebral hemorrhage was induced in mice using either the collagenase injection model (cICH) or the autologous blood (bICH) double-injection model, as previously reported.23,24 Briefly, mice were treated with Atropine (0.22mg/kg) and anaesthetized with co-injection of Ketamine (100mg/kg) and Xylazine (10mg/kg) intraperiteoneally and positioned prone on a stereotactic head frame (Kopf Instruments, Tujunga CA), and eye ointment was applied to keep the eyes moist. A 1mm burr hole was drilled at 0.2mm rostral and 2.2mm right lateral of bregma, and a 27gauge needle (Microliter No. 701; Hamilton Company, NV) was inserted 3.5mm below the dura.
For cICH, 0.075units of bacterial collagenase (Type VII-S, Sigma-Aldrich, St. Louis, MO) dissolved in 0.5μl saline was infused into the right basal ganglia at a rate of 0.1667μl/min using a Namonite Syringe Pump (Harvard Apparatus, Holliston, MA). The needle was left for an additional 5 minutes (to prevent the backflow of bacterial collagenase along the needle tract) and withdrawn slowly at a rate of 1mm/minute. The cranial burr hole was sealed with bone wax, the scalp was sutured, and 0.4ml of normal saline was injected subcutaneously to avoid postsurgical dehydration. Animals were allowed to recover fully under observation. Mice received DMF (10 or 100 mg/kg) intraperitoneally 1 h after ICH.
For bICH, blood was collected from the tail artery and transferred quickly to a glass syringe. The 27 gauge needle of the syringe was then inserted 3.0 mm below the dura through the burr hole and 5μL of autologous blood was infused at a rate of 2μL/min. Next, the needle was advanced an additional 0.5 mm and, after a waiting period of 5 minutes; 25μL of blood was infused into the right striatum. The needle was left in place for an additional 10 minutes after completing the infusion and withdrawn at a rate of 1 mm/min; the burr hole was sealed with bone wax and mice were allowed to recover under observation. Mice received DMF (100 mg/kg) intraperitoneally 1 h after ICH.
Sham operated animals were subjected to needle insertion only.
ICV
Anesthetized mice were fixed prone onto a stereotactic head apparatus (Kopf Instruments, Tujunga CA), and a 1mm burr hole was drilled at 0.3mm posterior and 1 mm left lateral of bregma. A 27 gauge needle of a 10μL Hamilton syringe (Microliter No. 701; Hamilton Company, NV) was inserted through a burr hole 2.5mm below the dura, as previously described.25,26 Following the manufacturer's instructions, and methods described by Ma et al with slight modifications, MAF-G siRNA or control siRNA, 1.2nm each in 3μL siRNA diluted in sterile RNAse free water (Santa Cruz Biotechnology, CA), was injected by a micro infusion pump (Harvard Apparatus, Holliston, MA) at a rate of 0.3750μl/min 24 hours before ICH-induction. The needle was removed over a 5 minute period after waiting for 7 minutes; the burr hole was sealed with bone wax.26 In order to enhance the gene silence efficiency, MAF-G siRNAs from two different sources were mixed:
-
1)
sense 5’ GGAAGAGAUCAUCCAGCUGtt 3’
antisense, 5’ CAGCUGGAUGAUCUCUUCCtt 3’;
-
2)
sense 5’CAGCGUCAUCACAAUAGUAtt 3’;
5’CCUUGAUCAUCUUCGUUGUtt 3’;
5’CUGUGGCUGUUGGAGUUUAtt 3’;
Antisense, 5’UACUAUUGUGAUGACGCUGtt3’;
5’ACAACGAAGAUGAUCAAGGtt 3’;
5’UAAACUCCAACAGCCACAGtt 3’
The sequence for control siRNA is
Sense: 5’UUCUCCGAACGUGUCACGUtt 3’
Antisense: 5’ACGUGACACGUUCGGAGAAtt 3’.
Mice received DMF (100 mg/kg) intraperitoneally 1 h after ICH.
Experimental Groups and Pharmacological Intervention
One hundred and seventy six CD-1 mice were used in this study. Mice were randomly divided into the following groups: Sham (n=36); ICH + Vehicle (n=52); ICH + 10mg/kg DMF (n=6); ICH + 100mg/kg DMF (n=32); ICH + TBCA (100μm/kg) + 100mg/kg DMF (n=14); Control siRNA + ICH (n=4); Control siRNA + ICH + 100mg/kg DMF (n=14); MAFG siRNA + ICH (n=4); MAFG siRNA + ICH + 100mg/kg DMF (n=14).
Assessment of Neurological Function
Behavioral outcomes were assessed by observers blinded to treatment at 24 and 72 hours after ICH. The sensorimotor Garcia test evaluating spontaneous activity, axial sensation, vibrissae proprioception, limb symmetry, lateral turning, forelimb outstretching, and climbing was used. 27 Each test received a score between zero (worst performance) and 3 (best performance).
In the forelimb placement test, reflexive motor ability of contralateral forelimb to vibrissae elicited excitation was assessed; score was expressed as the number of successful left paw placements out of a total of 10 consecutive vibrissae stimulations.28 For the corner turn test mice were allowed to advance into a 30° corner and exit by turning either left or right. Choice of turning was recorded for a total of 10 trials and a score was calculated as number of left turns/all trials ×100.28,29
Brain water content (BWC) measurement
Brain water content was measured at 24 and 72 hours post ICH, as previously described.26,30 Mice were decapitated under lethal isoflurane anesthesia and the brains quickly removed. A 4mm section, 2mm anterior and posterior of the needle tract, was separated and divided into ipsilateral and contralateral cortices and basal ganglia. The cerebellum was additionally collected as an internal control. All brain samples were weighed using an analytical microbalance (APX-60 Denver Instrument, Bohemia, NY) to obtain wet weight (WW). Samples were dried at 100° C for 24 hours and dry weight (DW) determined. BWC was calculated as (WW-DW)/WW × 100
Evans Blue extravasation
Evans Blue extravasation assays were conducted at 24 hours after ICH surgery as previously reported.31 Briefly, mice received an intraperitoneal injection of 4ml/kg Evans blue dye (4%), 3h prior to euthanasia and transcardiac perfusion with PBS. Ipsilateral and contralateral hemispheres were homogenized separately in 1200 μl of PBS, sonicated and centrifuged. Trichloroacetic acid (500 μl) was added to 500 μl of the supernatant layer and incubated over night at 4°C. This solution was re-centrifuged and extravasated Evans blue dye was quantified using a spectrophotometer at 610nm (Thermo Fisher Scientific Inc. Waltham, MA). Results were presented as μg of Evans Blue dye per g of brain tissue.
Hemoglobin assay
The hemoglobin assay was performed as previously described.26,32 Ipsilateral and contralateral hemispheres were homogenized separately, sonicated and centrifuged. Drabkin's reagent (800μl) was added to 200μl of supernatant and left to react for 15 minutes. Absorbance of hemoglobin was measured at 540nm using a spectrophotometer and quantified using a standard curve.
Evaluation of the role of Nrf2 phosphorylation in DMF-mediated neuroprotection
In order to confirm the role of MAFG and Casein Kinase 2-dependent phosphorylation of Nrf2 in DMF conferred neuroprotection, 1.2nm of MAFG siRNA was administered via ICV route 24 hours prior to ICH. Efficiency of MAFG gene knockdown was confirmed by western blotting. Two groups of mice treated with control siRNA or MAFG siRNA respectively 24h before ICH and received DMF 1h after ICH. A separate group of mice not treated with MAFG siRNA received 100μm/kg of Casein Kinase 2 inhibitor, TBCA then DMF 1h after ICH. Neurological deficits and brain water content were measured at 24 hours after ICH insult.
Western Blotting
Mice were euthanized after surgery, and ipsilateral hemispheres were isolated and processed as previously described.26 Cytoplasmic and nuclear fraction proteins were isolated and equal amounts of protein (20-100μg) were separated by SDS-PAGE, then transferred onto nitrocellulose membranes and incubated with the respective primary and secondary antibodies. The following primary antibodies were obtained from Santa Cruz Biotechnology: anti-ICAM-1; Casein Kinase 2α and β-actin. Antibodies to MAFG and p-Nrf2 were purchased from Abcam and Enzo Life Sciences respectively. All secondary antibodies were purchased from Santa Cruz Biotechnology. Immunoblots were visualized with the ECL Plus chemiluminescence reagent kit (Amersham Bioscience, Arlington Heights, IL) and densitometrically quantified using ImageJ software (National Institute of Health). Results are expressed as relative density ratio, normalized to the mean value of β-actin.
Immunofluorescence Staining
Mice were euthanized at 24 hours after surgery, and brain specimens were processed as previously described with minor modifications.33 Immunofluorescence was performed using the microglia marker anti-OX42 antibody (1:100, Abcam) and DAPI. Microphotographs were taken with the aid of a fluorescent microscope and Magna Fire SP system (Olympus).
Statistical Analysis
Data were expressed as mean ± SEM and statistically analyzed by one-way ANOVA followed by the Tukey test. All behavior data were expressed as mean ± SEM and analyzed by one-way ANOVA on ranks followed by the Tukey test. A probability value of <0.05 was considered statistically significant. All statistical analyses were performed using Sigma Plot version 11.0 for Windows.
Results
DMF attenuated neurological deficits and brain edema at 24 and 72 hours after ICH
Neurological deficits and brain edema were evaluated at 24 and 72 hours after ICH in mice. Mice subjected to ICH showed significant neurological deficits in the Garcia neuroscore, forelimb placement, and corner turn tests compared to sham operated animals (p < 0.05; Figures 1A and B). Mice treated with low dose dimethyl fumarate (10mg/kg) after ICH did not show a significant improvement in Garcia neuroscore and the corner turn test compared to vehicle (p>0.05). Treatment with high dose dimethyl fumarate improved neurological deficits at 24 and 72 hours after ICH.
Figure 1.
Statistical analysis of behavioral outcomes (A & B) and brain water content (C & D) at 24 and 72h after intracerebral hemorrhage induction or sham surgery. Dimethyl fumarate (DMF) (100mg) improved neurological deficits at 24 and 72h after ICH (A & B). Brain water content in the ipsilateral basal ganglia and cortices were reduced by DMF 100mg but not by 10mg DMF (C & D). Data are expressed as mean ± SEM. *p<0.05 compared to sham, #p<0.05 compared to Vehicle, & p<0.05 compared to DMF 10mg, n=6 per group. cICH indicates collagenase induced intracerebral hemorrhage, Garcia, Garcia test, CTT, corner turn test, FPT forelimb placement test.
Treatment with high dose dimethyl fumarate (100mg/kg) also significantly reduced brain water content in the ipsilateral basal ganglia and cortex compared to vehicle treated groups (p<0.05) at 24 and 72 hours after ICH (Figures 1C and 1D). Low dose dimethyl fumarate did not produce a significant reduction in brain water content at 24 hours post-injury compared to vehicle treated groups.
DMF reduced Evans blue dye extravasation and had no effect on Hematoma volume after ICH
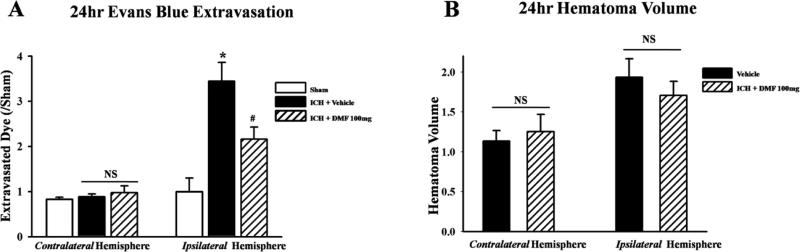
Treatment with dimethyl fumarate reduced amount of extravasated Evans blue dye measured in the ipsilateral hemisphere compared to vehicle treated groups (p<0.05); there was no significant difference between sham operated and dimethyl fumarate treated animals (Figure 2A). Dimethyl fumarate treatment did not reduce hematoma volume, there was no significant difference between vehicle and dimethyl fumarate treated animals (2B).
Figure 2.
Statistical analysis of Evans blue dye extravasation and hematoma volume after 24h after ICH. Dimethyl fumarate (DMF) reduced amount of extravasated dye in the ipsilateralhemisphere (A) but did not reduce hematoma volume after ICH (B). Data are expressed as mean ± SEM. *p<0.05 compared to sham, #p<0.05 compared to Vehicle. NS means not significant, n=6 per group.
Knockdown of MAFG protein and CK2 inhibition reversed the protective effects of Dimethyl fumarate after ICH
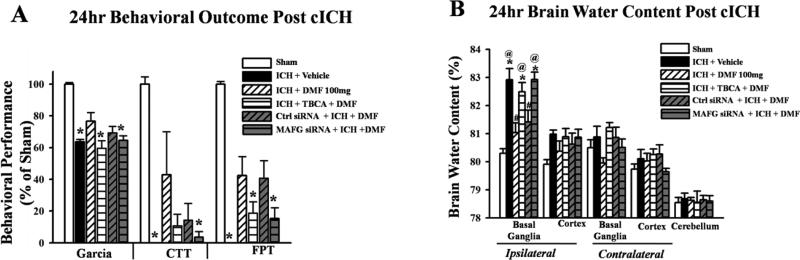
A significant improvement in neurological deficits and reduction in brain water content and were observed in the Dimethyl fumarate and control (scrambled) siRNA + DMF treated groups, compared to vehicle after ICH. The knockdown of MAFG using siRNA and inhibition of Casein Kinase 2 by TBCA reversed the effects of Dimethyl fumarate, producing worse neurological deficits (Figures 3A and B) and a significantly increasing brain water after ICH content compared to sham operated animals (p<0.05).
Figure 3.
Statistical analysis of behavioral outcomes (A) and brain water content (B) at 24 hrs after intracerebral hemorrhage induction or sham surgery. Treatment with Dimethyl fumarate (DMF) and control siRNA (+ DMF) improved neurological deficits after ICH while TBCA (+ DMF) and MAFG siRNA (+ DMF) worsened these indices (A). DMF and control siRNA improved brain water content in the ipsilateral hemisphere while these were worsened by TBCA and MAFG siRNA. Data are expressed as mean ± SEM. *p<0.05 compared to sham, #p<0.05 compared to Vehicle, @ p<0.05 compared to DMF 100mg, n=6 per group. cICH collagenase induces intracerebral hemorrhage, Garcia, Garcia test, CTT, corner turn test, FPT forelimb test.
Treatment with DMF reduced expression of ICAM-1 and increased expression of p-Nrf2 and MAFG after ICH
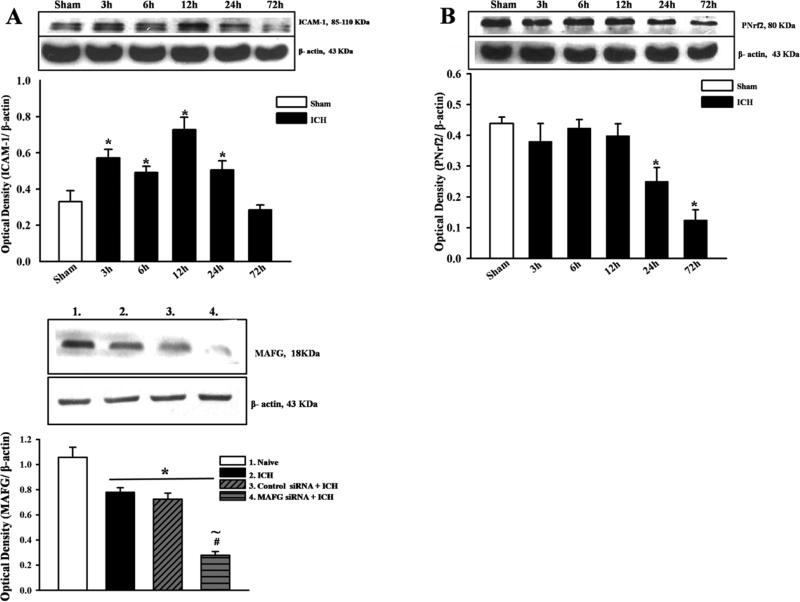
Western blot analysis of ICAM-1 in the ipsilateral hemisphere after ICH showed an increase in as early as 3 hours and up to 24 hours after ICH while decrease in p-Nrf2 expression was noticeable by 24h after ICH (Figures 4A and B). Expression of MAFG was significantly reduced after MAFG siRNA was administered (Figure 4C).
Figure 4.
Representative Western blots and densitometric quantification of for expression profile of ICAM-1 (A) p-Nrf2 (B) and MAFG siRNA (C) after ICH. Expression of ICAM-1 increased from 3hrs and peaked at 12-24hrs after ICH (A); expression of p-Nrf2 was significantly different from sham operated animals at 24hrs after ICH (B); expression of MAFG was significantly reduced after injection of MAFG siRNA. Data are expressed as mean ± SEM. *p<0.05 compared to sham, n=4 per group. #p<0.05 compared to Vehicle, ~ p<0.05 compared to control siRNA + ICH. cICH collagenase induces intracerebral hemorrhage, NS indicates no significant difference.
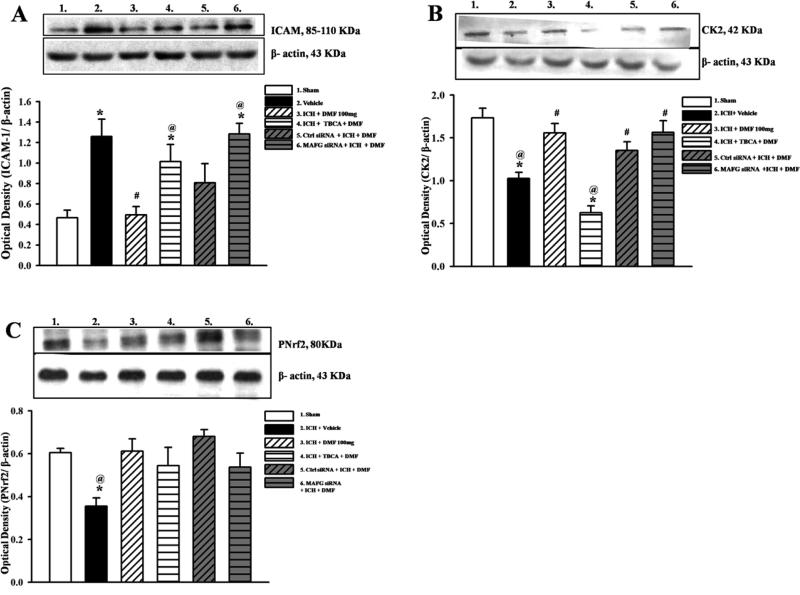
TBCA+DMF treated and MAFG siRNA knockdown + DMF treated groups showed increased ICAM-1 expression levels after ICH compared to sham (p<0.05). ICAM-1 expression was significantly reduced in Dimethyl fumarate treated and control siRNA groups (p<0.05; Figure 5A). Casein Kinase 2 expression at 24 hours was significantly reduced in the vehicle and inhibitor treated groups (p<0.05) compared to sham, but there was no difference between sham, Dimethyl fumarate treated, control siRNA + DMF and MAFG siRNA + DMF treated groups after ICH (Figure 5B).
Figure 5.
Representative Western blots and densitometric quantification of ICAM-1/β-actin (A), CK2/β-actin (B), and p-Nrf2/β-actin (C) in cytoplasmic cell lysate at 24 hours after intracerebral hemorrhage or sham surgery. Dimethyl fumarate (DMF) and control siRNA reduced ICAM-1 expression; TBCA and MAFG siRNA increased ICAM-1 expression (A). CK2 expression was increased in DMF treated, control and MAFG siRNA groups but reduced in vehicle and TBCA treated groups (B). p-Nrf2 expression was significantly reduced in vehicle treated group.(C). Data are expressed as mean ± SEM. N=6 per group. *p<0.05 compared to sham, #p<0.05 compared to Vehicle, @ p<0.05 compared to DMF 100mg, NS indicates no significant difference.
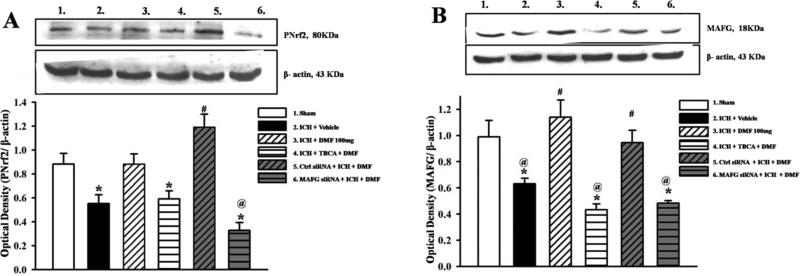
In the cytosol, p-Nrf2 expression was reduced in vehicle treated group (Figure 5C), while p-Nrf2 expression was significantly increased in the nuclear fraction of the cell lysate in Dimethyl fumarate treated and control siRNA groups compared to vehicle (p<0.05) but not compared to sham (Figure 6A). Treatment with Dimethyl fumarate increased the expression of MAFG significantly compared to vehicle (p<0.05). However, Casein Kinase 2 inhibitor groups and siRNA MAFG treated groups reversed this effect (Figure 6B).
Figure 6.
Representative Western blots and densitometric quantification of p-Nrf2/β-actin (A), MAFG/β-actin (B) in nuclear cell lysate at 24 hours after intracerebral hemorrhage or sham surgery. Treatment with DMF and control siRNA significantly increased p-Nrf2 expression while TBCA and MAFG siRNA reduced p-Nrf2 expression (A). MAFG expression was also significantly increased by DMF and control siRNA treated groups and reduced in vehicle, inhibitor and MAFG siRNA treated groups. Data are expressed as mean ± SEM. N=6 per group. *p<0.05 compared to sham, #p<0.05 compared to Vehicle, @ p<0.05 compared to DMF 100mg.
Administration of DMF reduced microglia activation
Immunoflorescence staining with activated microglia marker OX42 showed a reduction in number of activated microglia in the peri-hematomal region in Dimethyl fumarate treated animals. There was a significant difference between DMF treated and vehicle treated groups after ICH (Figures 7A&B).
Figure 7.
Representative microphotographs of immunofluorescence staining showing microglia activation (FITC/green) and DAPI (blue) 24h hours after ICH (A) and quantification of activated microglia (B). DMF treatment decreased perihematomal microglia activation. DMF treated animals had fewer activated microglia cells in the perihematomal regions compared to vehicle groups. Arrows represent activated microglia. Data are expressed as mean ± SEM. *p<0.05 compared to sham, #p<0.05 compared to Vehicle
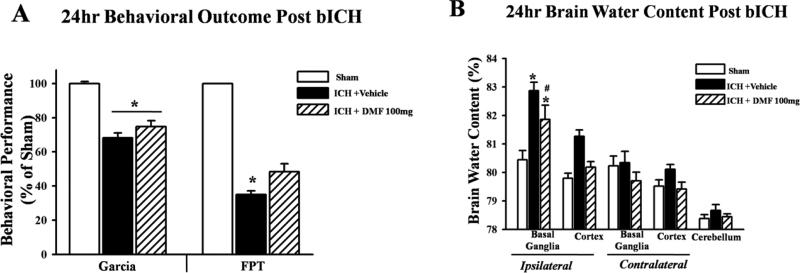
Brain water content and neurological deficits were also evaluated in a collagenase independent mouse model of ICH via a double injection of autologous blood into the right striatum. DMF treatment significantly improved neurological deficits in the forelimb placement test compared to vehicle (p<0.05) and reduced brain water content at 24 hours post-ICH injury (Figures 8A and B).
DMF treatment reduced brain water content and neurological deficits in autologous blood injection ICH model
Neurological deficits and brain edema were evaluated at 24 hours after bICH in mice. Mice subjected to bICH showed significant neurological deficits in the Garcia neuroscore and forelimb placement, compared to sham operated animals (p < 0.05; Figures 8A). Treatment with high dose Dimethyl fumarate improved neurological deficits at 24 hours after bICH.
Figure 8.
Statistical analysis of behavioral outcomes (A) and brain water content (B) at 24h after intracerebral hemorrhage induction or sham surgery. Treatment with DMF improved the forelimb placement test (A) and reduced brain water content (B). Data are expressed as mean ± SEM. *p<0.05 compared to sham, #p<0.05 compared to Vehicle, n=6 per group. bICH indicates blood induced intracerebral hemorrhage, Garcia, Garcia test, CTT, corner turn test, FPT forelimb placement test.
Treatment with high dose Dimethyl fumarate (100mg/kg) also significantly reduced brain water content in the ipsilateral basal ganglia compared to vehicle treated groups (p<0.05) at 24 hours after bICH (Figures 8B).
Discussion
In this study, treatment with Dimethyl fumarate (i) improved neurological deficits and reduced brain water content after collagenase induced ICH; (ii) reduced Evans Blue dye extravasation after ICH; (iii) decreased expression of ICAM-1 and increased expression of Casein Kinase 2 in the cytoplasm while nuclear expression of p-Nrf2 and MAFG were elevated, (iv) decreased activation of microglia in the peri hematomal region and (v) improved neurological deficits and reduced brain edema in the blood induced ICH.
Brain edema typified by increased brain water content is an important complication after ICH and occurs in the acute and delayed phases of ICH. Dimethyl fumarate, a fumaric acid ester (FAE) and an α,β-unsaturated electrophilic compound activates the Nrf2 transcriptional pathway, reducing oxidative stress, inflammation and neuronal demyelination.21,34 High dose Dimethyl fumarate (100mg/kg) reduced brain water content at 24 and 72 hours after collagenase induced ICH (compared to vehicle treated animals). The forepaw placement tests and corner turn tests which are used to assess patterns of turning and sensorimotor impairments in pre-clinical studies, and the composite Garcia test detects neurological deficits of unilateral ischemia or hemorrhagic brain injuries were utilized in this study. Prior studies demonstrated a correlation between increased brain edema and poor outcomes in patients; while in rodent models of ICH improvement in forepaw placement test correlates with decreased brain water content after ICH .28 Thus, interventions reducing brain edema are expected to improve neurological deficits in ICH patients. Accordingly, we observed that high dose Dimethyl fumarate (100mg/kg) improved neurological deficits in the Garcia test, forelimb placement test, and corner turn test at 24 hours as well as in the Garcia test and forelimb placement test at 72 hours after ICH and was used for subsequent experiments. These findings support our initial hypothesis that administration of Dimethyl fumarate will ameliorate brain edema and improve neurological outcomes after ICH.
The reduced brain water content by Dimethyl fumarate could be partly due to preservation of blood brain barrier integrity after ICH. As reduced brain water measurement technique used in this study does not distinguish between vasogenic and cytotoxic edema,32 Evans Blue extravasation assay was used to assess the functional integrity of the blood brain barrier. The blood brain vasculature consists of tight junction and adherens junction proteins which regulate the movement of substances between the brain and the blood and maintains ionic homeostasis in the microenvironment of the brain. Injury to the closely knit structure results in increased cell permeability and edema formation.35 In vasogenic edema, characterized by increase in extracellular volume, the loss of these tight endothelial junctions of the blood brain barrier, allows intravascular proteins and fluid to penetrate the parenchymal extracellular space, while in cytotoxic edema, the blood brain barrier remains intact but impaired functioning of the sodium and potassium pump in the results in intracellular retention of sodium and water.36 The Evans Blue dye has a high affinity to serum albumin, and because albumin cannot cross the blood-brain barrier, the dye can be used to assess the permeability of the blood brain barrier.37 Treatment with Dimethyl fumarate improved the blood brain barrier permeability as shown by less albumin-bound dye extravasation in to the ipsilateral hemisphere compared to vehicle treated groups indicating an improvement in the integrity of the blood brain barrier with treatment.
Hematoma formation is another important outcome after ICH. Hematoma volume can be an independent predictor of outcome, and as such, can greatly affect other consequences of ICH, including blood brain barrier integrity and brain water content.38,39 To test whether Dimethyl fumarate was improving other indices after ICH by reducing hematoma volume, we measured hemorrhage volumes in Dimethyl fumarate and vehicle treated groups using the hemoglobin assay. We found no significant difference in hemorrhage volumes between the two groups. This result indicates that Dimethyl fumarate has no effect on reducing the hematoma formation and expansion that occurs after ICH. Hence, we conclude that Dimethyl fumarate directly reduces brain water content and improves the integrity of the blood brain barrier. This finding also suggests that our model of ICH was consistent, having similar hemorrhage volumes; thus making our findings more conclusive.
Both in vitro and in vivo studies have indicated that fumaric acid esters, the family to which Dimethyl fumarate belongs, may lead to a shift in cytokine production from a Th1 pattern with production of IFNγ and TNFα to a Th2 pattern characterized by the production of interleukin (IL)-4 and IL-5. 40,41 Activation of the Nrf2/ARE system by Nrf2 signaling activators down-regulate the overproduction of pro-inflammatory cytokines, such as IL-1, IL-6, and TNFα expression of these cytokines was significantly higher in Nrf2-deficient mice than in wild-type animals.42-44 We therefore examined if Dimethyl fumarate treatment had any anti-inflammatory effects. After confirmation of the up-regulation of ICAM-1 after ICH, we evaluated the expression of ICAM-1 in our intervention groups. Dimethyl fumarate treatment significantly reduced ICAM-1 levels, compared to vehicle, while TBCA and MAFG knockdown groups had significantly higher levels of ICAM-1 (compared to sham). To the best of our knowledge, this is the first study to elucidate the role of MAFG and phosphorylated Nrf2 in the down-regulation of ICAM-1 production. ICAM-1, which is upregulated after ischemic and hemorrhagic injury, plays an important role in inducing neuro-inflammation and mediating the progression of injury after acute stroke.5,45 Our results are in line with the work of Thimmulappa et al and Zhao et al who found that activators of the Nrf2/ARE pathway reduced myeloperoxidase, an inflammatory mediator in experimentally induced sepsis, and ICH. 46,47
Immunofluoresecnce staining for microglia activation was also evaluated in this study and number of activated microglia were quantified. Microglial activation has been demonstrated to be an important contributor to inflammation-related brain injury after ICH.48,49 Dimethyl fumarate treated animals showed fewer activated microglia cells in the peri-hematomal region compared to vehicle treated animals. Consistent with our results discussed above, which demonstrate the anti-inflammatory effects of Dimethyl fumarate, Dimethyl fumarate-treated animals showed fewer activated microglial cells in the perihematoma, compared to vehicle-treated animals
We then explored the anti-inflammatory mechanism of Dimethyl fumarate treatment after ICH. We proposed that Dimethyl fumarate acts by the Casein Kinase 2-mediated phosphorylation of Nrf2. Nrf2, a major phase II gene regulator, is a broadly expressed transcription factor that binds to the antioxidant response element (ARE) consensus and regulates expression of phase II detoxifying enzymes.9 The key mediators regulating nuclear import and export of transcription factors, such as Nrf2, are nuclear localization signals and nuclear export sequences. The phosphorylation of Nrf2 may also contribute to its nuclear transportation.13 In unstressed conditions, Nrf2 is sequestered in the cytoplasm by Kelch-like ECH associated protein 1 (Keap1), a cytoskeleton associated protein. Keap1 functions as a negative regulator of Nrf2 by promoting Nrf2 ubiquitination and proteasomal degradation.50 When liberated from Keap1 repression, Nrf2 becomes stabilized and translocates into the nucleus to form a heterodimer with small MAF (sMAF) proteins.51 The Nrf2–sMaf dimer then binds to the ARE, which is a cis-acting DNA regulatory element localized in the promoter region of many cellular defense genes.52
To confirm the activity of our proposed signaling pathway, we tested if Casein Kinase 2 inhibition by (E)-3-(2,3,4,5-tetrabromophenylacrylic acid (TBCA), and MAFG knockdown by siRNA, could reverse the protective effects of Dimethyl fumarate in reducing brain edema and neurological deficits. We investigated: (i) the role of Casein Kinase 2 in stabilizing Nrf2 following its dissociation from Keap1 via administration of the Casein Kinase 2 inhibitor, TBCA, 30 minutes prior to Dimethyl fumarate treatment and (ii) the occurrence of the MAFG/p-Nrf2 heterodimerization in the nucleus and the downregulation of inflammatory cytokine activators by using MAFG siRNA. TBCA is a cell-permeable tetrabrominated cinnamic acid compound that acts as a potent and ATP-competitive inhibitor of Casein Kinase 2 (IC50 = 110 nM, Ki = 77 nM); it has a 5-fold selectivity over other Casein Kinase 2 inhibitors.53 MAFG is one of the small MAF proteins that form heterodimers with activated Nrf2 in the nucleus. MAFG/Nrf2 heterodimers bind to the ARE, thus inducing transcription of target genes, resulting in reduced production of ROS and inflammatory cytokines.17 Consistent with what we expected, our results showed that mice treated with TBCA and MAFG siRNA showed a significant increase in brain water content (compared to sham) and had greater deficits in the Garcia and forelimb placement tests, reversing the effects of Dimethyl fumarate.
Western blot analysis for the expression of p-Nrf2 after ICH showed significant reduction compared to sham, by 24 hours, and up to 72 hours after ICH. Moreover, analysis of the cytoplasmic expression of p-Nrf2 at 24 hours after ICH was reduced in vehicle treated groups, indicating a possible role for other kinase-mediated phosphorylation of stabilized Nrf2, since Casein Kinase 2 inhibition did not change its expression. In the nuclear cell lysate, however, the expression of p-Nrf2 was significantly increased in Dimethyl fumarate-treated and control siRNA groups, when compared to vehicle-treated animals. Levels of p-Nrf2 in TBCA-treated also animals were also significantly different compared to sham operated and vehicle-treated animals. We speculate that Dimethyl fumarate might also act by affecting the nuclear shuttling of p-Nrf2, but further investigation is needed to verify this theory.
Quantification of Casein Kinase 2 in the cytoplasmic cell lysate yielded lower values in the TBCA-treated group, as expected. However, an increased expression was observed in Dimethyl fumarate-treated and control siRNA groups, indicating that Dimethyl fumarate might be activating Casein Kinase 2. MAFG expression levels were significantly lower in vehicle-treated, Casein Kinase 2 inhibitor, and MAFG knockdown groups. Further studies are needed to establish a link, if there is any, between Dimethyl fumarate and Casein Kinase 2 activation.
We also validated the protective effects of Dimethyl fumarate treatment in the collagenase independent mouse model of ICH via a double injection of autologous blood into the right striatum to avoid possible interference of bacterial collagenase with the inflammatory response. Intrastriatal injection of bacterial collagenase causes the disruption of cerebral capillaries and mimics spontaneous bleed occurring over several hours as seen in about 30% of all ICH patients.4,54 However, bacterial collagenase is thought to produce an exaggerated inflammatory response in the brain; although in vivo studies have not confirmed this hypothesis.55,56 High dose Dimethyl fumarate (100mg/kg) reduced brain water content and neurological deficits in the autologous blood injection model at 24 hours post-ictus confirming the neuroprotective effect of Dimethyl fumarate after experimental ICH.
In summary, the Dimethyl fumarate-induced dissociation of Nrf2 from Keap1, and the consequent Casein Kinase 2-mediated phosphorylation of Nrf2, resulted in neuroprotection after ICH by reducing brain edema and improving functional outcomes.
Supplementary Material
The highlights of this study.
Treatment with Dimethyl fumarate after intracerebral hemorrhage in mice:
-
(i)
activated Casein kinase 2 and Nrf2 signaling pathway
-
(ii)
ameliorated inflammation
-
(iii)
improved neurological function and protected blood brain barrier integrity
Acknowledgments
Sources of Funding
This study was supported by National Institute of Health grant NS082184 to JHZ.
Abbreviations
- DMF
dimethyl fumarate
- MAF-G
musculo-aponeurotic fibrosacoma-G
- Nrf2
nuclear factor erythroid-2 related factor 2
- p-Nrf2
phosphorylated nuclear factor erythroid-2 related factor 2
- TBCA
(E)-3-(2,3,4,5 tetrabromophenyl)acrylic acid
- CK2
casein kinase 2
- bICH
blood induced intracerebral hemorrhage
- cICH
collagenase induced intracerebral hemorrhage
- ICV
intracerebroventricular injection
- ICAM-1
Intracellular adhesion molecule-1
- DAPI
4',6-diamidino-2-phenylindole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Ikram MA, Wieberdink RG, Koudstaal PJ. International Epidemiology of Intracerebral Hemorrhage. Curr Atheroscler Rep. 2012;14:300–306. doi: 10.1007/s11883-012-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11(8):720–731. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandey AS, Xi G. Intracerebral hemorrhage: a multimodality approach to improving outcome. Transl Stroke Res. 2014;5:313–315. doi: 10.1007/s12975-014-0344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Q, Zhang J, Guo J, Tang J, Tao Y, Li L, et al. Chronic hydrocephalus and perihematomal tissue injury developed in a rat model of intracerebral hemorrhage with ventricular extension. Transl Stroke Res. 2014 doi: 10.1007/s12975-014-0367-5. doi 10.1007/s12975-014-0367-5. [DOI] [PubMed] [Google Scholar]
- 5.Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27:268–278. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Yang Q, Chen G, Zhang JH. An update on inflammation in the acute phase of intracerebral hemorrhage Transl. Stroke Res. 2015;6:4–8. doi: 10.1007/s12975-014-0384-4. [DOI] [PubMed] [Google Scholar]
- 7.Javaid K, Rahman A, Anwar NK, Frey RS, Minshall RD, Malik AB. Tumor necrosis factor-α induces early-onset endothelial adhesivity by protein kinase C-ζ dependent activation of intercellular adhesion molecule-1. Circ Res. 2003;92:1089–1097. doi: 10.1161/01.RES.0000072971.88704.CB. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Doré S. Inflammation after intracerebral hemorrhage. J. Cereb Blood Flow Metab. 2007;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- 9.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem and Biophysical Res Comm. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 10.Chen XL, Dodd G, Thomas S, Zhang X, Wasserman MA, Rovin BH, Kunsch C. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am. J. Physiol. Heart Circ. Physiol. 2006;290:1862–1870. doi: 10.1152/ajpheart.00651.2005. [DOI] [PubMed] [Google Scholar]
- 11.Zhao X, Aronowski J. Nrf2 to pre-condition the brain against injury caused by products of hemolysis after ICH. Transl Stroke Res. 2013;4:71–75. doi: 10.1007/s12975-012-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog in Neurobiol. 2013;100:30–47. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain AK, Bloom DA, Jaiswal AK. Nuclear import and export signals in control of Nrf2. J Bio Chem. 2005;280:29158–29168. doi: 10.1074/jbc.M502083200. [DOI] [PubMed] [Google Scholar]
- 14.Numazawa S, Ishikawa M, Yoshida A, Tanaka S, Yoshida T. Atypical protein kinase C mediates activation of NF-E2-related factor 2 in response to oxidative stress. Am J Physiol Cell Physiol. 2003;285:C334–C342. doi: 10.1152/ajpcell.00043.2003. [DOI] [PubMed] [Google Scholar]
- 15.Lee JM, Hanson JM, Chu WA, Johnson JA. Phosphatidylinositol 3-kinase, not extracellular signal-regulated kinase, regulates activation of the antioxidant-responsive element in IMR-32 human neuroblastoma cells. J Biol Chem. 2001;276:20011–20016. doi: 10.1074/jbc.M100734200. [DOI] [PubMed] [Google Scholar]
- 16.Rada P, Rojo AI, Evrard-Todeschi N, Innamorato NG, Cotte A, Jaworski T, et al. Structural and functional characterization of Nrf2 degradation by the GSK-3/β-TrCP axis. Mol Cell Biol. 2012;32:3486–99. doi: 10.1128/MCB.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apopa PL, He X, Qiang Ma Q. Phosphorylation of Nrf2 in the transcription activation domain by casein kinase 2 (CK2) is critical for the nuclear translocation and transcription activation function of Nrf2 in IMR-32 neuroblastoma cells. J Biochem and Mol Tox. 2008;22:63–76. doi: 10.1002/jbt.20212. [DOI] [PubMed] [Google Scholar]
- 18.Jain AK, Jaiswal A. Phosphorylation of tyrosine 568 controls nuclear export of Nrf2. J Biol Chem. 2006;281:12132–12142. doi: 10.1074/jbc.M511198200. [DOI] [PubMed] [Google Scholar]
- 19.Pi J, Bai Y, Reece JM, Williams J, Liu D, Freeman ML, et al. Molecular mechanism of human Nrf2 activation and degradation: Role of sequential phosphorylation by protein kinase CK2. Free Rad Biol Med. 2007;42:1797–1806. doi: 10.1016/j.freeradbiomed.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kappos L, Gold R, Miller DH, Macmanus DG, Havrdova E, Limmroth V, et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet. 2008;372:1463–1472. doi: 10.1016/S0140-6736(08)61619-0. [DOI] [PubMed] [Google Scholar]
- 21.Linker RA, Lee D, Ryan S, van Dam AM, Conrad R, Bista P. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134:678–692. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 22.Stoof TJ, Flier J, Sampat S, Nieboer C, Tensen CP, Boorsma DM. The antipsoriatic drug dimethylfumarate strongly suppresses chemokine production in human keratinocytes and peripheral blood mononuclear cells. Br J Dermatol. 2001;144:1114–11120. doi: 10.1046/j.1365-2133.2001.04220.x. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg GA, Mun-Bryce S, Wesley, Mario Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801–807. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Fields J, Dore S. The development of an improved preclinical mouse model of intracerebral hemorrhage using double infusion of autologous whole blood. Brain Res. 2008;1222:214–221. doi: 10.1016/j.brainres.2008.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullier M, Bouret SG, Vincent Prevot V, Dehouck BJ. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol. 2010;518:943–962. doi: 10.1002/cne.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma Q, Manaenko A, Khatibi NH, Chen w, Zhang JH, Tang J. Vascular adhesion protein-1 inhibition provides antiinflammatory protection after an intracerebral hemorrhagic stroke in mice. J. Cereb. Blood Flow Metab. 2011a;31:881–893. doi: 10.1038/jcbfm.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats: statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 28.Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- 29.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 30.Tang J, Liu J, Zhou C, Alexander JS, Nanda A, Granger DN, et al. MMP-9 deficiency enhances collagenase-induced intracerebral hemorrhage and brain injury in mutant mice. J Cereb Blood Flow Metab. 2004;24:1133–1145. doi: 10.1097/01.WCB.0000135593.05952.DE. [DOI] [PubMed] [Google Scholar]
- 31.Qingyi Ma, Huang B, Khatibi N, Rolland W, II, Suzuki H, Zhang JH, Jiping T. PDGFR-α inhibition preserves blood brain barrier after intracerebral hemorrhage. Ann Neurol. 2011b;70:920–931. doi: 10.1002/ana.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manaenko A, Chen H, Kammerd J, Zhang JH, Tang J. Comparison Evans Blue injection routes: intravenous versus intraperitoneal, for measurement of blood–brain barrier in a mice hemorrhage model. J. Neurosci Meth. 2011;195:206–210. doi: 10.1016/j.jneumeth.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ostrowski RP, Colohan AR, Zhang JH. Mechanisms of hyperbaricoxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2005;25:554–571. doi: 10.1038/sj.jcbfm.9600048. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt TJ, Muharrem AK, Ulrich M. Reactivity of dimethyl fumarate and methylhydrogen fumarate towards glutathione and N-acetyl-l-cystein -preparation of S-substituted thiosuccinic acid esters. Bioorganic & Med Chem. 2007;15:333–342. doi: 10.1016/j.bmc.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 35.Stamatovic SM, Keep RF, Andjelkovic AV. Brain endothelial cell-cell junctions: how to “open” the blood brain barrier. Current Neuropharmacology. 2008;6:179–192. doi: 10.2174/157015908785777210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain edema in focal ischemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawkins BT, Egleton RD. Fluorescence imaging of blood–brain barrier disruption. Journal of Neuroscience Meth. 2006;151:262–267. doi: 10.1016/j.jneumeth.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Schlunk F, Schulz E, Lauer A, Yigitkanli K, Pfeilschifter W, Steinmetz, et al. Warfarin pretreatment reduces cell death and MMP-9 activity in experimental intracerebral hemorrhage. Transl Stroke Res. 2014 doi: 10.1007/s12975-014-0377-3. doi:10.1007/s12975-014-0377-3. [DOI] [PubMed] [Google Scholar]
- 39.Zhao X, Sun G, Zhang H, Ting SM, Song S, Gonzales N, et al. Polymorphonuclear neutrophil in brain parenchyma after experimental intracerebral hemorrhage. Transl Stroke Res. 2014;5:554–561. doi: 10.1007/s12975-014-0341-2. [DOI] [PubMed] [Google Scholar]
- 40.de Jong R, Bezemer AC, Zomerdijk TP, van de Pouw-Kraan T, Ottenhoff TH, Nibbering PH. Selective stimulation of T helper 2 cytokine responses by the anti-psoriasis agent monomethylfumarate. Eur J Immunol. 1996;26:2067–2074. doi: 10.1002/eji.1830260916. [DOI] [PubMed] [Google Scholar]
- 41.Ockenfels HM, Schultewolter T, Ockenfels G, Funk R, Goos M. The antipsoriatic agent dimethylfumarate immunomodulates T-cell cytokine secretion and inhibits cytokines of the psoriatic cytokine network. Br J Dermatol. 1998;139:390–395. doi: 10.1046/j.1365-2133.1998.02400.x. [DOI] [PubMed] [Google Scholar]
- 42.Bondeson J, Sundle R. Auranofin inhibits the induction of interleukin 1 beta and tumor necrosis factor alpha mRNA in macrophages. Biochem. Pharmacol. 1995;50:1753–1759. doi: 10.1016/0006-2952(95)02030-6. [DOI] [PubMed] [Google Scholar]
- 43.Lee JM, Johnson JA. An important role of Nrf2–ARE pathway in the cellular defense mechanism. J Biochem. Mol Biol. 2004;37:139–143. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- 44.Kim J, Cha Y, Surh Y. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mut Res. 2010;690:12–23. doi: 10.1016/j.mrfmmm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Supanc V, Biloglav Z, Kes VB, Demarin V. Role of cell adhesion molecules in acute ischemic stroke. Ann Saudi Med. 2011;31:365–370. doi: 10.4103/0256-4947.83217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, et al. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis J. Clin. Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, et al. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007;38:3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- 48.Gong C, Hoff JT, Keep RF. Acute inflammatory reaction following experimental intracerebral hemorrhage in rat. Brain Res. 2000;871:57–65. doi: 10.1016/s0006-8993(00)02427-6. [DOI] [PubMed] [Google Scholar]
- 49.Chen S, Yang Q, Chen G, Zhang JH. An update on inflammation in the acute phase of intracerebral hemorrhage. Transl Stroke Res. 2015;6:4–8. doi: 10.1007/s12975-014-0384-4. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kensler TW, Wakabayashi N, Biswa S. Cell survival responses to environmental stresses via the Keap1–Nrf2–ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 52.Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol. 2011;85:241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 53.Pagano M, Poletto G, Di Maira G, Cozza G, Ruzzene M, Sarno S, et al. Tetrabromocinnamic acid (TBCA) and related compounds represent a new class of specific protein kinase CK2 inhibitors. Chem Bio Chem. 2007;8:129–139. doi: 10.1002/cbic.200600293. [DOI] [PubMed] [Google Scholar]
- 54.MacLellan CL, Silasi G, Poon CC, Edmundson CL, Buist R, Peeling J, et al. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb.Blood Flow Metab. 2008;28:516–525. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- 55.Matsushita K, Meng W, Wang X, Asahi M, Asahi K, Moskowitz MA, et al. Evidence for apoptosis after intercerebral hemorrhage in rat striatum. J Cereb Blood Flow Metab. 2000;20:396–404. doi: 10.1097/00004647-200002000-00022. [DOI] [PubMed] [Google Scholar]
- 56.Xiong XY, Wang J, Qian ZM, Yang QW. Iron and intracerebral hemorrhage: from mechanism to translation. Transl Stroke Res. 2014;5:429–411. doi: 10.1007/s12975-013-0317-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.