Abstract
For tamoxifen-dependent Cre recombinase, also known as CreER recombinase, tamoxifen (TAM) is used to activate the Cre to generate time- and tissue-specific mouse mutants. TAM is a potent CreER system inducer; however, TAM is also an active selective estrogen receptor modulator (SERM) that can influence bone homeostasis. The purpose of this study was to optimize the TAM dose for Cre recombinase activation while minimizing the effects of TAM on bone turnover in young growing mice.
Methods
To evaluate the effects of TAM on bone turnover and bone mass, 1-month-old male wild-type mice were intraperitoneally injected with TAM at 0, 1, 10 or 100 mg/kg/day for four consecutive days. The distal femurs were analyzed one month after the last TAM injection by microCT, mechanical test, and surface-based bone histomorphometry. Similar doses of TAM were used in Col1 (2.3 kb)-CreERT2; mT/mG reporter mice to evaluate the dose-dependent efficacy of Cre-ER activation in bone tissue.
Results
A TAM dose of 100 mg/kg × 4 days significantly increased trabecular bone volume/total volume (BV/TV) of the distal femur, femur length, bone strength, and serum bone turnover markers compared to the 0 mg control group. In contrast, TAM doses ≤ 10 mg/kg did not significantly change any of these parameters compared to the 0 mg group, although a higher bone strength was observed in the 10 mg group. Surface-based histomorphometry revealed that the 100 mg/kg dose of TAM dose significantly increased trabecular bone formation and decreased periosteal bone formation at 1-week post-TAM treatment. Using the reporter mouse model Col1-CreERT2; mT/mG, we found that 10 mg/kg TAM induced Col1-CreERT2 activity in bone at a comparable level to the 100 mg/kg dose.
Conclusions
TAM treatment at 100 mg/kg/day × 4 days significantly affects bone homeostasis, resulting in an anabolic bone effect on trabecular bone in 1-month-old male mice. However, a lower dose of TAM at 10 mg/kg/day × 4 days can yield similar Col1-CreERT2 induction efficacy with minimum effects on bone turnover in young male mice.
Keywords: Tamoxifen, Col1a1 (2.3 kb), CreER, Estrogen, Bone turnover, Bone formation
1. Introduction
The Cre/Lox recombination-mediated conditional gene knockout technology is used to study gene functions in a specific tissue(s), especially when systemic inactivation of a particular gene causes embryonic lethality or profound developmental defects [1]. Furthermore, an inducible Cre recombinase can be used to inactivate a target gene at a certain time point, allowing the gene of interest to be functionally expressed prior to induction [1].
Tamoxifen (TAM)-inducible Cre-ER (or modified versions such as CreERT2) is one of the most widely used inducible genetic systems [2, 3]. It utilizes a mutated estrogen receptor (ER) fused to Cre as a transgene (Cre-ER), which only becomes activated and then translocates into the nucleus upon binding of the active tamoxifen (TAM) metabolite 4-hydroxytamoxifen (4-OHT), which mutates the ER. CreERT2 is currently the most successful Cre-ER version, which has very low background Cre activity and robust activation following TAM treatment. Control mice are treated with vehicle are usually compared to experimental animals treated with TAM (or 4-OHT) to study the phenotypes of CreER-driven gene knockout [1,4,5]. CreERT2 has been utilized to generate time-dependent and tissue-specific mutants in several tissues, including bone, endothelium, epithelium, fat, liver, and the nervous system [6]. CreER activation can be initiated by TAM administration in pregnant females, postnatal pups, or postnatal adults by oral gavage or intraperitoneal (I.P.) injection. The TAM dosage depends on the animal age, target tissue, and other factors. For example, oral gavage of 1–5 mg TAM was used for five consecutive days to achieve ubiquitous CreERT2 activity in 1-month- to 2-month-old mice, or 0.25–1 mg 4-OHT I.P. injection for five consecutive days for P12–P17 pups to activate CreER in bone [6–8]. In general, older Cre-ER animals require higher TAM doses and longer TAM treatment periods to achieve satisfactory Cre-ER induction. To induce CreERT2 activity in postnatal bone, previous reports by other investigators generally used a dose of 100 mg or higher per kilogram body weight (100 mg/kg/day) for three to five days [6,9].
Investigators who utilize CreER in musculoskeletal research have become increasingly aware of potential “off-target” effects of TAM on skeletal tissue, as TAM is a selective estrogen receptor modulator (SERM) and is well documented to inhibit bone resorption and influence bone homeostasis, particularly in young growing mice [10–12]. TAM is a selective estrogen receptor modulator with similar actions on bone cells to other SERMs, such as raloxifene, which increased bone mass and reduced vertebral fracture risk in elderly postmenopausal women with osteoporosis in clinical studies [13–15]. TAM reduces osteoclast activity and bone turnover in humans at a dose of 20 mg/day after 10 weeks of daily treatments [16,17]. However, a thorough evaluation of how TAM affects bone homeostasis when used as a CreER recombinase inducer has not been carefully evaluated.
This study utilized a well-characterized osteoblast-specific Col1a1 (2.3 kb)-CreERT2 driver to study the dose-dependent induction efficacy of TAM [7]. Unexpectedly, we found that 100 mg/kg/day × 4 days TAM treatment significantly affected bone mass independent of CreER status. Therefore, the purpose of this study was to optimize the TAM dose to activate CreER recombinase and reduce “off-target” effects on bone turnover.
2. Materials and methods
2.1. Mouse lines
The Col1a1 (2.3 kb)-CreERT2, Col1a1 (2.3 kb)-GFP, and mT/mG transgenic mice were purchased from Jackson Lab and maintained in the UC Davis vivarium. The mT/mG reporter mouse possesses loxp sites at both sides of a tdTomato (mT) cassette and expresses red fluorescence in all tissues, which is driven by a CMV enhancer/chicken beta-actin core promoter (pCA). When bred to Cre-expressing mice, the resulting offspring have the mT cassette deleted in Cre-expressing tissues, allowing expression of the enhanced green fluorescent protein (mG/GFP) cassette located just downstream of mT. Therefore, green fluorescence marks only those cells with Cre activity or those that have been exposed to Cre. All procedures were approved by the Institutional Animal Care and Use Committee of UC Davis.
2.2. MicroCT measurements
Distal femurs were scanned and measured using the VivaCT 40 (Scanco Medical, Bassersdorf, Switzerland) with a voxel resolution of 10 mm in all three spatial dimensions. A monoenergetic (70 keV) X-ray source was used, which has been reported to be safe for both structural bone endpoints and bone marrow cell viabilities in small animals. Approximately 200 slices were evaluated approximately 0.2 mm away from the distal end of the growth plate. The slices covered a total tissue volume of 2–3.5 mm3 for each scan and were used to obtain the cancellous bone volume/total volume (BV/TV) [18].
2.3. Tamoxifen treatment
To prepare the working tamoxifen solution, tamoxifen (T5648, Sigma-Aldrich, St. Louis, MO, USA) powder was weighed and dissolved ethanol, and vortexed for a few minutes. Corn oil (C8267, Sigma-Aldrich) was added to a 9:1 oil: ethanol mixture ratio with 20 mg/mL tamoxifen, and the solution was covered in aluminum foil and vigorously shaken in 37°C until it became a homogeneous gold color. The prepared TAM working solution was stored at +4 °C protected from light for up to one week.
2.4. Bone histomorphometry
All mice received intraperitoneal injections of calcein (10 mg/kg) and alizarin red (30 mg/kg) at seven-day intervals. Bone samples were dissected and fixed in 4% paraformaldehyde. Distal femurs were dehydrated in graded concentrations of ethanol and xylene and embedded un-decalcified in methyl methacrylate for sectioning with a Leica RM 2265 microtome (Leica Microsystems Nussloch GmbH, Germany) into 8-μm thick sections. The 8-μm sections were left unstained for measurement of surface-based histomorphometric indices using an Eclipse E400 fluorescent microscope (Nikon, Japan) linked to image analysis software (Bioquant Image Analysis Corporation, Nashville, TN, USA).
2.5. Statistical analyses
Results are expressed as the mean ± standard deviation. Statistically significant differences between groups were measured by a one-way ANOVA followed by the Bonferroni test. A value of P < 0.05 was considered statistically significant. Data were analyzed using the GraphPad Prism software package (La Jolla, CA, USA).
3. Results
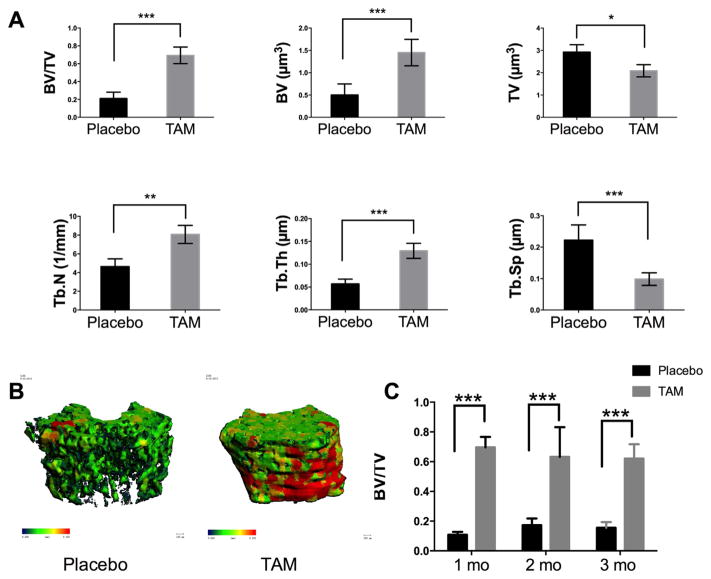
We first treated 1-month-old male mice on a C57/BL6-129 hybrid background with TAM in corn oil by intraperitoneal (IP) injection. The TAM dose was 100 mg/kg/day for four consecutive days, while the control animals received the same volume of corn oil. At one month after the last injection, we found that the distal femoral trabecular BV/TV in the TAM-treated mice was increased by over 300%, which was primarily due to an increase in bone volume, trabeculae number and trabeculae thickness [Fig. 1A, B]. We observed a similar increase in trabecular bone in both male or female mice when TAM was administered at 2 or 3 months of age at 100 mg/kg/day × 4 days [Fig. 1C]. We also observed a significant increase (~50%) in lumbar vertebrae BV/TV (Supplemental 1.D). The less dramatic response in lumbar vertebrae suggests that TAM affects rapidly growing bones or more mechanically loaded bones.
Fig. 1.
Tamoxifen (100 mg/kg/day) treatment for four days significantly increases trabecular bone mass. Male or female mice at 1 (A), 2, or 3 (C) months of age were treated with corn oil (placebo) or 100 mg/kg/day TAM for four consecutive days. Distal femurs were collected for microCT scanning one month after treatment. Three-dimensional images were reconstructed for trabeculae at the distal femurs at one month post-treatment (B). Differences between groups (corn oil (placebo) versus TAM in corn oil) were analyzed using an unpaired t-test. N = 6–8 mice per group; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We next tried to define a “non-anabolic” TAM dose and dosing interval to minimize TAM effects on long bones. We treated 1-month-old male mice with 0, 1, 10, or 100 mg/kg/day TAM × 4 days or a single 100 mg/kg and 300 mg/kg TAM injection. One month after TAM treatment, mice in the 1 or 10 mg × 4 dose group exhibited no significant changes in trabecular BV/TV in the distal femurs compared to the 0 mg (placebo-treated) group [Fig. 2A]. In contrast, 100 mg × 4 doses, 100 mg single dose or a 300 mg single dose significantly increased BV/TV in distal femurs [Fig. 2A and B]. Additionally, the 100 mg × 4 dose group had increased femur length in both male and female mice [Fig. 2C, Supplementary Fig. 1B], and maximal load was increased in both the 10 and 100 mg × 4 dose groups measured by femoral 3-point bending [Fig. 2D]. Serum osteocalcin, a bone formation marker, was not significantly higher in the 100 mg group [Fig. 2E], while the bone resorption marker serum CTX-1 was significantly inhibited in the 100 mg group [Fig. 2F]. Serum SOST levels were also significantly downregulated [Supplementary Fig. 1C].
Fig. 2.
Different doses of tamoxifen affect bone. One-month-old male mice were treated with TAM at 0 (placebo), 1, 10, or 100 mg/kg/day × 4 days, or a single 100 mg or 300 mg dose. Distal femoral BV/TV (A, B), femur length (C), maximal load (D), and serum bone turnover markers (E and F) were measured at one month post-treatment. N = 6–8 mice per group; *, P < 0.05; **, P < 0.01.
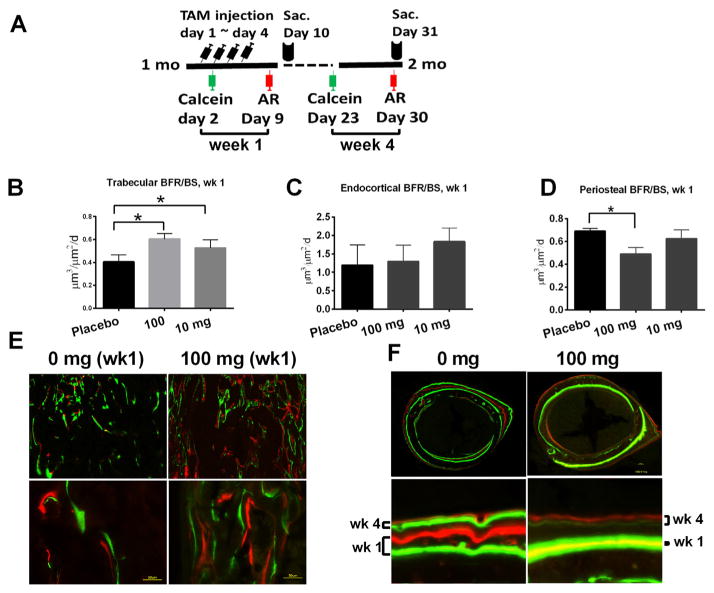
To better evaluate the short-term (1 week post-treatment) and longer-term (4 weeks post-treatment) effects of TAM on bone formation, we administered a calcein label one day after the initiation of TAM treatment followed by a alizarin red label on day 4 post-TAM treatment [Fig. 3A]. The first two labels allowed us to analyze changes in bone formation rate immediately following TAM treatment. We administered a second round of calcein/alizarin red labels -9 and -2 days before sacrifice, i.e., one month post treatment. These latter two labels allowed us to calculate changes in bone formation rate at the end of 1-month TAM treatment. We found that at 1-week post-treatment, 100 mg and 10 mg doses both significantly increased trabecular bone formation rate (BFR/BS) compared to the placebo-treated group [Fig. 3B and E]. We detected TAM did not affect the endocortical bone formation significantly [Fig. 3C] but observed periosteal bone formation was suppressed at the 100 mg dose [Fig. 3D and F]. There was no significant change in trabecular bone formation 4-week post-TAM treatment (data on file). However, periosteal bone formation was still decreased at the 100 mg dose with a low 3rd calcein label, but showed some recovery with the 4th alizarin label.
Fig. 3.
Tamoxifen treatment regulates bone formation. One-month-old male mice were given TAM, calcein, and alizarin red labeling at indicated times (A). A group of mice were sacrificed at day 10 for histomorphometric evaluation of trabecular bone (B). Another group of mice were sacrificed at day 31, and bone formation was measured at the endocortical bone surface (C) and the periosteal surface (D) at one week post-TAM treatment. Representative images for the distal femurs (E) and middle-femurs (F) from 0 and 100 mg/kg groups.
We next examined the Cre activation efficacy for each TAM dose. To achieve this goal, we crossed Col1a1 (2.3 kb)-CreERT2 mice with the mT/mG reporter mice. The mT/mG line allowed us to detect CreER activation by tracing green fluorescent protein (GFP) expression [Fig. 4A] [19]. The Col1a1 promoter (2.3 kb) is a mature osteoblast-specific promoter that is widely used in bone research [7]. We used a transgenic Col1a1-GFP mouse with constitutive GFP expression driven by a 2.3 kb rat type 1, alpha 1 (Col1a1) promoter as a positive control [20]. We then treated 1-month-old male double transgenic Col1-CreERT2; mT/mG mice with 0, 1, 10, or 100 mg/kg/day TAM for four consecutive days. Three days after the last TAM injection, distal femurs were removed and then were cryosectioned. We observed CreER activation in the 100 mg and 10 mg groups, as indicated by robust GFP expression in trabecular bone regions underneath the growth plate and at the endocortical bone surface. In contrast, there was substantially reduced GFP expression in the 1 mg group, corresponding to lower Cre activation [Fig. 4B]. We performed quantitative calculation of the induction rate by counting the GFP-positive bone surface in reference to the total endosteal surface using the Bioquant Image Analysis System. We found that the 10 mg group had a similar induction rate to the 100 mg group, which was approximately 51% and 58%, respectively [Fig. 4C]. The GFP-positive surface in endosteal bones from age- and sex-matched Col1a1-GFP mice was approximately 70% [Fig. 4B and C]. These data indicate that the 10 mg dose was comparable to the 100 mg dose in activating Col1a1-CreERT2 in osteoblasts.
Fig. 4.
Different doses of tamoxifen induce Col1a1-CreERT2 activity in trabecular and cortical bones. In Col1-CreERT2; mT/mG system, cells with inactive Cre and their descendants would exclusively express tdTomato (red fluorescence) in the absence of TAM; upon 4-OHT (active metabolite of TAM) engaging the ER in the cytoplasm, Cre-ER would translocate into the nucleus and activate GFP expression (green fluorescence) (A). One-month-old male Col1-CreERT2; mT/mG mice were treated with 0, 1, 10, or 100 mg/kg/day TAM for 4 days. Femurs were collected for cryosection, and fluorescent images were acquired at the distal femur region and middle shaft region (B). Col1a1-GFP was used as a positive control (only green fluorescence present). The induction rate (GFP-positive surface/total endosteal bone surface) was quantitated (C).
4. Discussion
The goal of this technical report was not to examine the effects of Tamoxifen on adult skeleton or bone turnover used as a “SERM” but to raise investigators’ awareness about the side effects of TAM on bone when used as a Cre inducer. TAM is most often used to induce Cre activity soon after birth or during gestation. Like estrogen, TAM’s effect on bone is complex and varies by bone location, timing, genetic background, and many other contributing factors. Therefore, this report can only serve as a technical protocol for optimizing TAM dose for its use as a Cre inducer in different Cre strains and experimental settings in a case-by-case manner.
When we used a commonly utilized tamoxifen dose (100 mg/kg/day × 4 days, IP) to activate the Cre recombinase in an inducible Col1a1 (2.3 kb)-ERT mouse line, we found a 3-fold and 50% increases in trabecular bone volume at the distal femurs and the lumbar vertebral bodies in the Col1a1 (2.3 kb)-ERT mice of both sexes and in their wild-type (WT) littermates. We observed a similar bone gain in two- or three-month-old WT mice treated with 100 mg/kg/day tamoxifen × 4 days or single 100 mg/kg or 300 mg/kg tamoxifen dose in 1-month-old mice. We detected a similar but less dramatic anabolic response in C57/CBA mice (Supplementary Fig. 1A). Mechanically, 100 mg/kg/day TAM × 4 days significantly increased femoral trabecular bone formation, but inhibited periosteal apposition, decreased bone resorption and resulted in net bone gain at the distal femurs. Alternatively, we found that 10 mg/kg/day TAM × 4 days did not significantly affect bone turnover or bone mass, but still activated Cre to a comparable level to the 100 mg/kg/day dose.
The magnitude of trabecular bone gain following a short-term and relatively high-dose tamoxifen administration was unexpected. One previous study found that a bi-weekly I.P. injection of 0.1 mg 4-OHT (an active TAM metabolite) per mouse (2 weeks of age) for 12 weeks significantly increased trabecular bone mass and cortical bone thickness in female mice [11]. However, our current study was not designed to address the differences between tamoxifen and 4-OHT, and TAM/4-OHT treatments may have skeletal effects on younger mice or embryos. Our observation that 100 mg TAM treatment increased femur length is consistent with a previous study. Perry and colleagues found that tamoxifen treatment increased femur length was associated with an increase in width of the growth plate and proliferating zone, which suggests that tamoxifen inhibits the tendency of endogenous estrogen on bone growth [10]. Our observation on SOST is also consistent with previous reports that estrogen and tamoxifen decrease SOST expression through ER beta, which can modulate the response to mechanical strain or BMP2 treatment in vitro [21,22].
Apart from bone, tamoxifen can also affect other systems. TAM IP injections given at ~120 mg/kg/day × 5 days for CreER induction changed serum testosterone (~500% increase), follicle-stimulating hormone (~30% decrease) and luteinizing hormone (~60% decrease) levels at five days post-TAM treatment [23]. The fact that tamoxifen treatment has such a significant effect on bone homeostasis, bone marrow microenvironment, and the endocrine system raises significant concerns.
Tamoxifen-inducible CreER is an excellent system for genetic mouse studies. One concern is that the Cre may be leaky because CreER could accidentally enter nuclei to knock out “floxed” genes in the absence of tamoxifen binding [24]. In this case, it is necessary to control for the non-tamoxifen-treated effect. Thus, TAM-treated mice should be compared to vehicle-treated mice with the same genotype, but this comparison would be problematic due to the bone effects of tamoxifen. Fortunately, we observed very low Cre-mediated recombination using the mT/mG model system in the Col1a1 (2.3) ERT2 mouse line and observed very low to no background Cre activation prior to tamoxifen administration (Fig. 4C).
Additionally, we have performed a dose response study and determined an “optimal” tamoxifen dose of 10 mg/kg/day × 4 days with minimal effects on bone turnover and bone mass but with retention of CreER induction efficacy in Col1a1 (2.3)-CreERT2 mice. The optimal tamoxifen dosage we identified here for the Col1a1 (2.3)-CreERT2 line may not apply to other mouse strains with different genetic backgrounds or other tissue-specific promoters. Based on these data, we recommend the inclusion of a tamoxifen-treated WT control in addition to placebo-treated controls for the WT and Cre-ERT mice for all studies using CreER system, especially those examining bone phenotypic changes as primary endpoints. Studies using CreERT2 system shall report findings on both sexes. A tamoxifen titration study may be necessary to define an optimal tamoxifen dose or dose range to activate Cre without significantly affecting bone or other tissues of interest.
Supplementary Material
Supplemental 1 (A) Male or female mice (on the C57/CD-1 genetic background) at 1 month of age were treated with corn oil (placebo) or 100 or 10 mg/kg/day TAM for four consecutive days, or 100 mg/kg for one time. Distal femurs were collected for microCT analysis one month after treatment; (B) one-month-old female mice were treated with corn oil (placebo) or 100 mg/kg/day TAM for four consecutive days, and femurs were collected and measured with a beam caliper one month after treatment; (C) one-month-old male mice were treated with corn oil (placebo) or 100 mg/kg/day TAM for four consecutive days, and serum was collected for SOST ELISA assay one month after treatment; N = 6–8 per group. (D) Male mice at 1 month of age were treated with corn oil (placebo) or 100 mg/kg/day TAM for four consecutive days, and lumbar vertebrae 3 were collected for microCT analysis one month after treatment.
Acknowledgments
This work was funded by the NIH grants P50 AR063043 (to WY) and R01 AR061366 (to WY). We thank the SCOR External Advisory Board member Dr. Mark Johnson and the Internal Advisory Board members Dr. Robert Nissenson and Dr. Edward Hsiao for technical support.
Abbreviations
- BV
bone volume
- Cre
Cre recombinase
- Col1
type I collagen
- mutant ER
mutant estrogen receptor
- GFP
green fluorescent protein
- I.P
intraperitoneal
- microCT
micro-computed tomography
- WT
wild-type
- TAM
tamoxifen
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bone.2015.07.034.
References
- 1.Gierut JJ, Jacks TE, Haigis KM. Strategies to achieve conditional gene mutation in mice. Cold Spring Harb Protoc. 2014;2014:339–349. doi: 10.1101/pdb.top069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metzger D, Clifford J, Chiba H, Chambon P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc Natl Acad Sci U S A. 1995;92:6991–6995. doi: 10.1073/pnas.92.15.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwenk F, Kuhn R, Angrand PO, Rajewsky K, Stewart AF. Temporally and spatially regulated somatic mutagenesis in mice. Nucleic Acids Res. 1998;26:1427–1432. doi: 10.1093/nar/26.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oury F, Khrimian L, Denny CA, Gardin A, Chamouni A, Goeden N, Huang YY, Lee H, Srinivas P, Gao XB, Suyama S, Langer T, Mann JJ, Horvath TL, Bonnin A, Karsenty G. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013;155:228–241. doi: 10.1016/j.cell.2013.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitamura H, Cambier S, Somanath S, Barker T, Minagawa S, Markovics J, Goodsell A, Publicover J, Reichardt L, Jablons D, Wolters P, Hill A, Marks JD, Lou J, Pittet JF, Gauldie J, Baron JL, Nishimura SL. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin alphavbeta8-mediated activation of TGF-beta. J Clin Invest. 2011;121:2863–2875. doi: 10.1172/JCI45589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feil S, Valtcheva N, Feil R. Inducible Cre mice. Methods Mol Biol. 2009;530:343–363. doi: 10.1007/978-1-59745-471-1_18. [DOI] [PubMed] [Google Scholar]
- 7.Kim JE, Nakashima K, de Crombrugghe B. Transgenic mice expressing a ligand-inducible Cre recombinase in osteoblasts and odontoblasts: a new tool to examine physiology and disease of postnatal bone and tooth. Am J Pathol. 2004;165:1875–1882. doi: 10.1016/S0002-9440(10)63240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leone DP, Genoud S, Atanasoski S, Grausenburger R, Berger P, Metzger D, Macklin WB, Chambon P, Suter U. Tamoxifen-inducible glia-specific Cre mice for somatic mutagenesis in oligodendrocytes and Schwann cells. Mol Cell Neurosci. 2003;22:430–440. doi: 10.1016/s1044-7431(03)00029-0. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 10.Perry MJ, Gujra S, Whitworth T, Tobias JH. Tamoxifen stimulates cancellous bone formation in long bones of female mice. Endocrinology. 2005;146:1060–1065. doi: 10.1210/en.2004-1114. [DOI] [PubMed] [Google Scholar]
- 11.Starnes LM, Downey CM, Boyd SK, Jirik FR. Increased bone mass in male and female mice following tamoxifen administration. Genesis. 2007;45:229–235. doi: 10.1002/dvg.20294. [DOI] [PubMed] [Google Scholar]
- 12.Manolagas SC, O’Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol. 2013;9:699–712. doi: 10.1038/nrendo.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 14.Allen MR, Iwata K, Sato M, Burr DB. Raloxifene enhances vertebral mechanical properties independent of bone density. Bone. 2006;39:1130–1135. doi: 10.1016/j.bone.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Amugongo SK, Yao W, Jia J, Dai W, Lay YA, Jiang L, Harvey D, Zimmermann EA, Schaible E, Dave N, Ritchie RO, Kimmel DB, Lane NE. Effect of sequential treatments with alendronate, parathyroid hormone (1–34) and raloxifene on cortical bone mass and strength in ovariectomized rats. Bone. 2014;67:257–268. doi: 10.1016/j.bone.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward RL, Morgan G, Dalley D, Kelly PJ. Tamoxifen reduces bone turnover and prevents lumbar spine and proximal femoral bone loss in early postmenopausal women. Bone Miner. 1993;22:87–94. doi: 10.1016/s0169-6009(08)80220-6. [DOI] [PubMed] [Google Scholar]
- 17.Kenny AM, Prestwood KM, Pilbeam CC, Raisz LG. The short term effects of tamoxifen on bone turnover in older women. J Clin Endocrinol Metab. 1995;80:3287–3291. doi: 10.1210/jcem.80.11.7593440. [DOI] [PubMed] [Google Scholar]
- 18.Yao W, Dai W, Shahnazari M, Pham A, Chen Z, Chen H, Guan M, Lane NE. Inhibition of the progesterone nuclear receptor during the bone linear growth phase increases peak bone mass in female mice. PLoS One. 2010;5:e11410. doi: 10.1371/journal.pone.0011410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 20.Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC, Rowe D. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J Bone Miner Res. 2002;17:15–25. doi: 10.1359/jbmr.2002.17.1.15. [DOI] [PubMed] [Google Scholar]
- 21.Galea GL, Meakin LB, Sugiyama T, Zebda N, Sunters A, Taipaleenmaki H, Stein GS, van Wijnen AJ, Lanyon LE, Price JS. Estrogen receptor alpha mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor beta. J Biol Chem. 2013;288:9035–9048. doi: 10.1074/jbc.M112.405456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim RY, Yang HJ, Song YM, Kim IS, Hwang SJ. Estrogen modulates bone morphogenetic protein-induced sclerostin expression through the Wnt signaling pathway. Tissue Eng A. 2015 doi: 10.1089/ten.TEA.2014.0585. [DOI] [PubMed] [Google Scholar]
- 23.Willems A, De Gendt K, Deboel L, Swinnen JV, Verhoeven G. The development of an inducible androgen receptor knockout model in mouse to study the postmeiotic effects of androgens on germ cell development. Spermatogenesis. 2011;1:341–353. doi: 10.4161/spmg.1.4.18740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manolagas SC, Kronenberg HM. Reproducibility of results in preclinical studies: a perspective from the bone field. J Bone Miner Res. 2014;29:2131–2140. doi: 10.1002/jbmr.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental 1 (A) Male or female mice (on the C57/CD-1 genetic background) at 1 month of age were treated with corn oil (placebo) or 100 or 10 mg/kg/day TAM for four consecutive days, or 100 mg/kg for one time. Distal femurs were collected for microCT analysis one month after treatment; (B) one-month-old female mice were treated with corn oil (placebo) or 100 mg/kg/day TAM for four consecutive days, and femurs were collected and measured with a beam caliper one month after treatment; (C) one-month-old male mice were treated with corn oil (placebo) or 100 mg/kg/day TAM for four consecutive days, and serum was collected for SOST ELISA assay one month after treatment; N = 6–8 per group. (D) Male mice at 1 month of age were treated with corn oil (placebo) or 100 mg/kg/day TAM for four consecutive days, and lumbar vertebrae 3 were collected for microCT analysis one month after treatment.