Abstract
Cryolipolysis is a non-invasive, skin cooling treatment for local fat reduction that causes prolonged hypoesthesia over the treated area. We tested the hypothesis that cryolipolysis can attenuate nociception of a range of sensory stimuli, including stimuli that evoke itch. The effects of cryolipolysis on sensory phenomena were evaluated by quantitative sensory testing (QST) in 11 healthy subjects over a period of 56 days. Mechanical and thermal pain thresholds were measured on treated and contralateral untreated (control) flanks. Itch duration was evaluated following histamine iontophoresis. Unmyelinated epidermal nerve fiber and myelinated dermal nerve fiber densities were quantified in skin biopsies from six subjects. Cryolipolysis produced a marked decrease in mechanical and thermal pain sensitivity. Hyposensitivity started between two to seven days after cryolipolysis and persisted for at least thirty-five days post-treatment. Skin biopsies revealed that cryolipolysis decreased epidermal nerve fiber density as well as dermal myelinated nerve fiber density, which persisted throughout the study. In conclusion, cryolipolysis causes significant and prolonged decreases in cutaneous sensitivity. Our data suggest that controlled skin cooling to specifically target cutaneous nerve fibers has the potential to be useful for prolonged relief of cutaneous pain and might have a use as a research tool to isolate and study cutaneous itch-sensing nerves in human skin.
Introduction
Cryolipolyis is controlled deep cooling of the skin and subcutaneous fat, used clinically for non-invasive and selective reduction of subcutaneous fat (Manstein et al., 2008; Nelson et al., 2009). Crystallization of cytoplasmic lipids occurs in adipocytes at temperatures well above the freezing point of water. This crystallization is hypothesized to produce selective injury, stress and apoptosis of adipocytes, followed by panniculitis and gradual fat loss (Zelickson et al., 2009). During treatment, metal plates cooled to a temperature below 0°C are applied to the skin for 60 minutes. Prolonged hypoesthesia in the treated area is a common side effect, resolving 1-2 months after treatment. The mechanism for this unexpected, prolonged hypoesthesia is unknown. In a case study report, histology of biopsies taken from a single patient after cryolipolysis treatment did not show inflammation or necrosis of nerves (Coleman et al., 2009). A small sample-sized human study using non-quantitative neurologic evaluation, noted modest, reversible changes sensory function, occurring in 66% of the patients after treatment (Coleman et al., 2009). These findings suggest that skin cooling alters cutaneous sensory nerve function, but does not address the neurosensory pathways and mechanisms involved.
The aim of this study was to characterize the long-term effects of a single cryolipolysis treatment to the flank of healthy subjects on cutaneous nerve fiber density and sensory functions, including pain and itch, over a 56-day follow up period. Quantitative sensory tests (QSTs) assessed cutaneous sensitivity to mechanical and thermal stimuli. Itch duration after local histamine iontophoresis was also determined. Skin biopsies were obtained from six subjects for quantitative histopathologic analysis of myelinated and unmyelinated cutaneous nerve fibers. We hypothesized that myelinated nerves may undergo preferential injury during cryolipolysis due to their lipid-rich myelin sheath, followed by decreased peripheral nerve function and gradual regeneration.
Results
Participants
Figure 1 shows the experimental timeline. Six females (55%) and 5 males (45%) participated in the study (N=11). Mean age was 37.6 ± 8.4 years (range 27-53 years). Mean BMI was 27.1 ± 2.0 kg/m2 (range 22.5-29.1 kg/m2). Four subjects (36%) were randomized for right flank treatment, and 7 subjects (64%) for left flank treatment. All subjects tolerated cryolipolysis treatment, sensory testing, and skin biopsy without significant adverse effects. Six subjects (55%) reported mild to moderate pain in the treated area 10 minutes after treatment. At 48-72 hours post treatment only two subjects (18%) 2/11 reported mild discomfort in the treated area. At Day 7 post treatment no subjects reported pain.
Figure 1. Experimental time line.
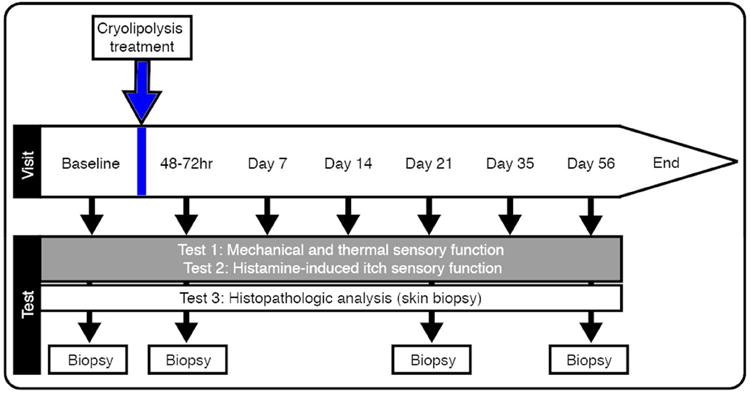
Experiments were performed in the test-order listed. Arrows indicate time-point each experiment was performed.
Effects of cryolipolysis on pain and sensory thresholds
Table 1 summarizes mechanical and thermal pain thresholds for the Treated and Control sides at each time point. Mechanical pain threshold (MPT) was significantly higher (i.e. cutaneous hyposensitivity occurred) when compared to baseline threshold at 48-72hrs after cryolipolysis treatment [Baseline: 13g (7-44g) vs. 42-72hrs: 56 g (14-127g), Z = -2.58, p=0.01). Mechanical pain hyposensitivity was observed at each time point until Day 56 post-treatment (Table 1). There were no significant changes from baseline threshold at the Control site at any time point during the follow-up period (Table 1).
Table 1.
Mechanical and thermal pain thresholds over the control and cryolipolysis treated areas, and pairwise comparisons for (1) side tested and (2) post-treatment time A.
| Pairwise Comparisons | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Side | Time | |||||
| QST | Time | Control | Treatment | Tx vs. Control | Control: vs BL | Tx: vs. BL |
|
| ||||||
| MPT (g)B | ||||||
| Baseline | 36 (11-57) | 13 (7-44) | 0.11 (Z:-1.60) | - | - | |
| 48-72h | 26 (9-73) | 56 (14-127) | 0.03* (Z:-2.23) | 0.68 (Z:-0.42) | 0.01* (Z=-2.58) | |
| Day 7 | 30 (9-46) | 32 (9-103) | 0.09 (Z:-1.68) | 0.59 (Z:-0.53) | 0.05* (Z:-1.89) | |
| Day 14 | 22 (7-73) | 26 (11-180) | 0.05* (Z:-1.99) | 0.88 (Z:-0.15) | 0.02* (Z:-2.29) | |
| Day 21 | 34 (10-73) | 45 (8-180) | 0.06 (Z:-1.89) | 0.51 (Z:-0.66) | 0.03* (Z:-2.23) | |
| Day 35 | 37 (12-100) | 49 (17-127) | 0.31 (Z:-1.00) | 0.21 (Z:-1.25) | 0.01* (Z: -2.58) | |
| Day 56 | 30 (13-87) | 45 (17-60) | 0.80 (Z:-0.26) | 0.37 (Z:-0.89) | 0.02* (Z:-2.29) | |
|
| ||||||
| CPT (°C)C | ||||||
| Baseline | 22.9 (20.1-25.8) | 24.1 (21.3-26.9) | 0.27 | - | - | |
| 48-72h | 22.1 (19.3-25.0) | 18.3 (15.5-21.1) | <0.001* | 0.46 | <0.001* | |
| Day 7 | 23 (20.1-25.8) | 20.5 (17.6-23.3) | 0.026* | 0.97 | 0.001* | |
| Day 14 | 21.8 (19.0-24.6) | 18.2 (15.3-21.0) | <0.001* | 0.30 | <0.001* | |
| Day 21 | 21.8 (19.0-24.6) | 17.4 (14.6-20.3) | <0.001* | 0.30 | <0.001* | |
| Day 35 | 22 (19.1-24.8) | 21.5 (18.7-24.3) | 0.68 | 0.37 | 0.016* | |
| Day 56 | 22.1(19.2-24.9) | 22.9 (20.1-25.8) | 0.43 | 0.43 | 0.27 | |
|
| ||||||
| HPT (°C)C | ||||||
| Baseline | 40.9 (39.4-42.4) | 42.1 (40.6-43.6) | 0.035* | - | - | |
| 48-72h | 42.1 (40.6-43.5) | 43 (41.5-44.5) | 0.10 | 0.047* | 0.12 | |
| Day 7 | 41.6 (40.1-43.1) | 43 (41.5-44.5) | 0.014* | 0.26 | 0.12 | |
| Day 14 | 42 (40.5-43.4) | 43.5 (42.0-45.0) | 0.007* | 0.07 | 0.017* | |
| Day 21 | 42.8 (41.3-44.3) | 44 (42.5-45.5) | 0.035* | 0.001* | 0.001* | |
| Day 35 | 41.9 (40.4-43.4) | 42.1(40.6-43.6) | 0.71 | 0.08 | 0.98 | |
| Day 56 | 42.3 (40.8-43.8) | 42.8 (41.3-44.3) | 0.46 | 0.013* | 0.23 | |
Abbreviations: BL – Baseline; CPT- Cold Pain Threshold; HPT- Heat Pain Threshold; MPT- Mechanical Pain Threshold; QST – Quantitative Sensory Testing; Tx – Treatment.
Mechanical, cold and heat pain are transmitted by myelinated Aδ and unmyelinated C fibers (Backonja et al., 2013)
Median (25th to 75th percentile); p-values determined from Wilcoxon Signed Ranks Test
Mean (95% Confidence Interval); p values determined from mixed model repeated measures ANOVA with Bonferroni correction (assuming significance at p<0.05). We observed that for some QST measures, the differences were very small (i.e. <2°C) despite showing statistical significance.
Indicates statistically significant differences
Cold pain threshold (CPT) significantly increased after cryolipolysis treatment (Treated side: F6, 430=11.38, p<0.01; Figure 2). Onset of cold pain hyposensitivity began at 48-72hrs after treatment [Baseline: 24.1°C (21.3-26.9°C) vs. 42-72hrs: 18.3°C (15.5-21.1°C); p<0.001]. Cold pain hyposensitivity persisted until Day 35 and returned to baseline sensitivity by the end of the study (Table 1). There were no significant changes in cold pain threshold at the Control site at any time point during the follow-up period (Control side: F6, 430 = 0.41, p=0.87; Figure 2). Heat pain threshold (HPT) was less affected by cryolipolysis treatment than MPT and CPT. There were significant increases in HPT after cryolipolysis treatment at both Treated and Control sides (Treated side: F6, 430=2.86, p=0.01; Control side: F6, 430=2.14, p=0.05). However, the magnitude of the change from baseline was very small (<2°C) for both Treated and Control sides, and warrant caution in their clinical interpretation.
Figure 2. Cryolipolysis reduces cutaneous sensitivity to mechanical and thermal stimuli following cryolipolysis.
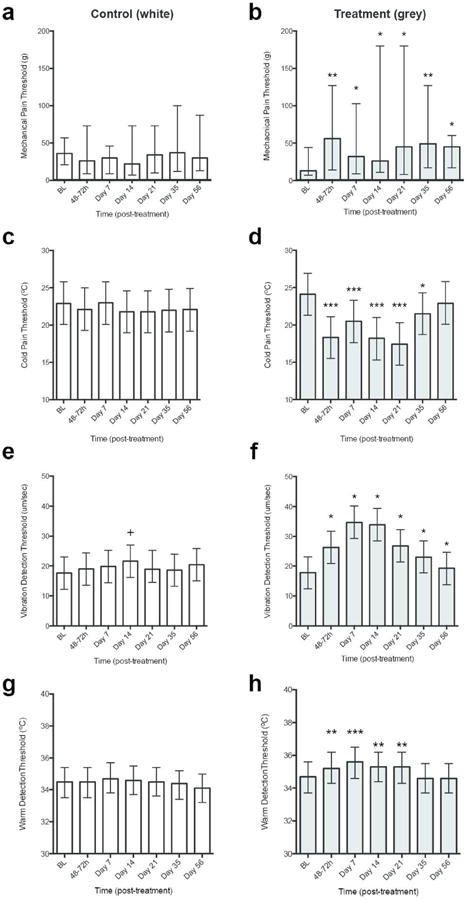
Sensory threshold for Control side shown in white bars and Treatment side shown in grey bars. (a and b) Mechanical pain threshold (MPT); (c and d) Cold detection threshold (CDT); (e and f) Vibration detection threshold (VDT); (g and h) Warmth detection threshold (WDT). Data for each side is compared to baseline sensory threshold. MPT data: bars represent median; whiskers represent LQ to UQ; p-values determined from Wilcoxon Signed Ranks Test. VDT, WDT, and CDT data: bars represent mean; whiskers represent 95% CI; p values determined from Mixed Model Repeated Measures ANOVA with Bonferroni correction (assuming significance at p<0.05). Asterisks indicate level of significance compared to Treatment baseline: *, p<0.05; **, p<0.01, ***, p<0.005. Crosses (+) indicate level of significance compared to Control baseline (+ p=<0.05, +++p=<0.001). BL, Baseline.
Table 2 summarizes mechanical and thermal detection thresholds for the Treated and Control sides at each time point. Vibration detection (VDT), warmth detection (WDT) and cool detection (CDT) thresholds significantly increased after cryolipolysis treatment (Treated side: VDT: F6, 882=31.14, p<0.001; WDT: F6, 581=6.89, p<0.001; CDT: F6, 581=10.89, p<0.001). There were no grossly significant changes in detection threshold at the Control site (Control side: VDT: F6, 882=1.23, p=0.29; WDT: F6, 581=1.74, p=0.11; CDT: F6, 581=0.63, p=0.70; Figure 2). Vibration and warmth detection modalities were affected by cryolipolysis treatment to the greatest degree as shown by the rapid onset and prolonged duration of hyposensitivity. Onset of vibration, warmth and cool detection hyposensitivity began at 48-72hrs after treatment [VDT: Baseline: 17.8μm/sec (12.4-23.2μm/sec) vs. 42-72hrs: 26.3μm/sec (20.9-31.7μm/sec), p<0.001; WDT: Baseline: 34.7°C (33.7-35.6°C) vs. 42-72hrs: 35.2°C (33.7-35.6°C), p=0.01; CDT: Baseline: 29.5°C (28.1-30.8°C) vs. 42-72hrs: 26.2°C (24.8-27.5°C), p<0.001]. Vibration detection hyposensitivity was maintained until Day 35 and returned to baseline sensitivity by Day 56 (Figure 2). Warmth detection hyposensitivity persisted until Day 21 and returned to baseline sensitivity by Day 35 (Figure 2). Cold detection hyposensitivity returned to baseline levels at Day 7. There were no significant changes from baseline for mechanical detection thresholds at the Treated or Control Side during the study (Table 2).
Table 2.
Mechanical and thermal sensory detection thresholds over the control and cryolipolyis treated areas, with pairwise comparisons for (1) side tested and (2) post-treatment timeA.
| Pairwise Comparisons | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Side | Time | |||||
| QST | Time | Control | Treatment | Tx vs. Control | Control: vs BL | Tx: vs. BL |
|
| ||||||
| MDT (g)B: | ||||||
| Baseline | 0.43 (0.26-0.52) | 0.40 (0.24-0.66) | 0.35 (Z:-0.94) | - | - | |
| 48-72h | 0.40 (0.21-0.93) | 0.87 (0.40-1.27) | 0.18 (Z:-1.33) | 0.65 (Z:-0.46) | 0.07 (Z:-1.84) | |
| Day 7 | 0.35 (0.20-0.67) | 0.83 (0.45-1.30) | 0.04* (Z:-2.07) | 0.36 (Z:-0.92) | 0.1 (Z:-1.63) | |
| Day 14 | 0.33 (0.21-0.53) | 0.47 (0.40-1.60) | 0.15 (Z:-1.43) | 0.42 (Z:-0.80) | 0.33 (Z:-0.98) | |
| Day 21 | 0.39 (0.24-0.60) | 1.00 (0.40-1.13) | 0.05* (Z:-1.99) | 0.53 (Z:-0.62) | 0.06 (Z:-1.91) | |
| Day 35 | 0.32 (0.24-0.47) | 0.47 (0.47-0.80) | 0.18 (Z:-1.33) | 0.36 (Z:-0.92) | 0.24 (Z:-1.17) | |
| Day 56 | 0.40 (0.24-0.47) | 0.47 (0.32-0.47) | 0.44 (Z:-0.77) | 0.26 (Z:-1.13) | 0.93 (Z:-0.09) | |
|
| ||||||
| VDT (μm/sec)C | ||||||
| Baseline | 17.6 (12.2-23.1) | 17.8 (12.4-23.2) | 0.93 | - | - | |
| 48-72h | 19.0 (13.6-24.4) | 26.3 (20.9-31.7) | <0.001* | 0.42 | <0.001* | |
| Day 7 | 19.8 (14.4-25.3) | 34.7 (29.3-40.2) | <0.001* | 0.2 | <0.001* | |
| Day 14 | 21.6 (16.2-27.0) | 33.9 (28.5-39.4) | <0.001* | 0.02 | <0.001* | |
| Day 21 | 18.9 (14.5-25.3) | 26.8 (21.4-32.3) | <0.001* | 0.18 | <0.001* | |
| Day 35 | 18.6 (13.2-24.0) | 23.1 (17.7-28.5) | <0.001* | 0.57 | 0.01 | |
| Day 56 | 20.4 (15.0-25.9) | 19.3 (13.8-24.7) | 0.48 | 0.09 | 0.37 | |
|
| ||||||
| CDT (°C)C | ||||||
| Baseline | 29.0 (27.6-30.3) | 29.5 (28.1-30.8) | 0.36 | - | - | |
| 48-72h | 29.6 (28.2-31.0) | 26.2 (24.8-27.5) | <0.001* | 0.249 | <0.001* | |
| Day 7 | 29.2 (27.8-30.6) | 28.6 (27.2-30.0) | 0.23 | 0.63 | 0.1 | |
| Day 14 | 29.5 (28.1-30.1) | 29.1 (27.7-30.5) | 0.44 | 0.302 | 0.51 | |
| Day 21 | 29.8 (28.4-31.1) | 28.7 (27.3-30.1) | 0.05 | 0.137 | 0.16 | |
| Day 35 | 29.8 (28.4-31.2) | 29.4 (28.0-30.8) | 0.46 | 0.119 | 0.93 | |
| Day 56 | 29.7 (28.3-31.0) | 29.9 (28.5-31.2) | 0.69 | 0.193 | 0.44 | |
|
| ||||||
| WDT (°C)C | ||||||
| Baseline | 34.5 (33.5-35.4) | 34.7 (33.7-35.6) | 0.365 | - | - | |
| 48-72h | 34.5 (33.5-35.4) | 35.2 (34.3-36.2) | <0.001* | 0.98 | 0.01* | |
| Day 7 | 34.7 (33.8-35.7) | 35.6 (34.6-36.5) | <0.001* | 0.24 | <0.001* | |
| Day 14 | 34.6 (33.7-35.5) | 35.3 (34.4-36.2) | 0.001* | 0.62 | 0.004* | |
| Day 21 | 34.5 (33.6-35.4) | 35.3 (34.3-36.2) | <0.001* | 0.92 | 0.006* | |
| Day 35 | 34.4 (33.4-35.2) | 34.6 (33.7-35.5) | 0.22 | 0.45 | 0.66 | |
| Day 56 | 34.1 (33.2-35.0) | 34.6 (33.7-35.5) | 0.02 | 0.08 | 0.67 | |
Abbreviations: BL – Baseline; CDT- Cool Detection Threshold; MDT- Mechanical Detection Threshold; QST – Quantitative Sensory Testing; Tx – Treatment; VDT- Vibration Detection Threshold; WDT- Warmth Detection Threshold.
Mechanical detection using von Frey hairs and vibration is transmitted by myelinated Aβ afferent fibers; cool detection is transmitted by myelinated Aδ and unmyelinated C-fibers; warmth detection is primarily transmitted by unmyelinated C-fibers. (Backonja et al., 2013)
Median (25th to 75th percentile); p-values determined from Wilcoxon Signed Ranks Test
Mean (95% Confidence Interval); p values determined from Mixed Model Repeated Measures ANOVA with Bonferroni correction (assuming significance at p<0.05). We observed that for some QST measures, the differences were very small (i.e. <1°C) despite showing statistical significance
Indicates statistically significant differences
Itch
Histamine iontophoresis evoked pruritus at the Treated and Control sides in all subjects. In the Treated side the mean itch duration at baseline was 9.7 min (CI: 6.8-12.7 min). Itch duration on the Treated side vs baseline, reduced by 48-72 hours post-treatment (p = 0.008), and remained attenuated until the end of the study (Day 14 p = 0.003, Days 21, 35 and 56 p < 0.001), but not compared to the control side (Figure S1 and Table S1). No significant changes were detected in mean or peak itch intensity or duration between control and treated side for any time point following histamine iontophoresis (Table S2 and S3).
Confocal imaging of cutaneous nerves
Six subjects underwent skin biopsy at baseline, 48-72hrs, Days 21 and 56 post-treatment for microscopic quantitative analysis of unmyelinated, epidermal nerve fiber density (ENF) and dermal myelinated nerve fiber density (Table 3). Cryolipolysis significantly reduced the density of ENFs, starting 48-72 hours and becoming more prominent at Days 21 and 56 post-treatment (Figure 3A and Table 3). Density of dermal myelinated fibers was unchanged at 48-72hrs but was significantly reduced starting from Day 21 (a 56% decrease, p<0.001) compared to baseline (Figure 3B). By Day 56 post-treatment, some recovery was seen but there were still significantly lower dermal myelinated nerve fiber density than baseline (Figure 3B and Table 3).
Table 3.
Histopathologic analysis of the cryolipolysis treated area over a 56 day period A.
| Time | Myelinated Fibers/mm2 | p-value | ENF/mm2 | p-value |
|---|---|---|---|---|
|
| ||||
| Baseline | 5.57 (4.12-7.02) | - | 8.96 (7.44-10.47) | - |
| 48-72h | 5.33 (3.88-6.78) | 1 | 7.43 (5.91-8.94) | 0.024* |
| Day 21 | 2.43 (0.98-3.88) | <0.001* | 6.45 (4.93-7.97) | <0.001* |
| Day 56 | 2.74 (1.29-4.19) | <0.001* | 6.1 (4.59-7.62) | <0.001* |
Abbreviations: ENF-number of Epidermal Nerve Fibers.
Mean (95% Confidence Interval). Pairwise comparison of post-treatment time-point vs. baseline values determined from Repeated Measures ANOVA.
Figure 3. Cryolipolysis reduces epidermal nerve fiber density and myelinated dermal fiber density.
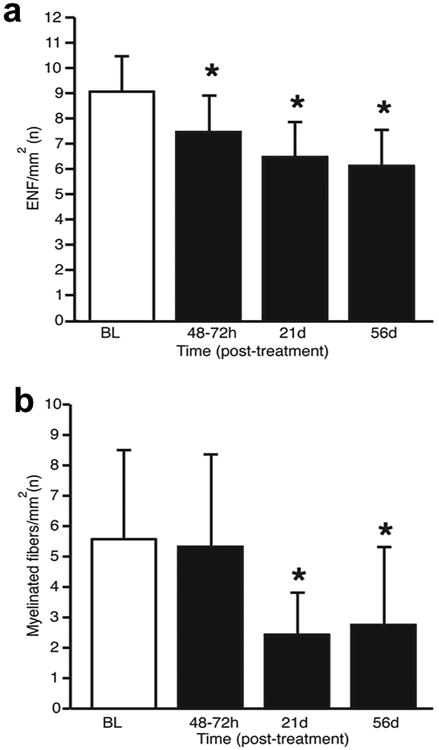
(a) Density of epidermal nerve fibers (ENF), (b) Density of myelinated dermal nerve fibers. Data shown as mean (SD). P-values determined using mixed model repeated measures ANOVA with Bonferroni correction. Asterisks indicate level of significant compared to baseline. *, p<0.05.
Discussion
It has been reported in the past that cryolipolysis induces prolonged hypoesthesia in the treated area (Coleman et al., 2009), but this study specified and quantified the modality, magnitude and duration of sensory losses caused by cryolipolysis treatment. We also discovered that cryolipolysis causes prolonged reduction in the density of both myelinated and unmyelinated cutaneous nerves, with myelinated nerve fibers in the dermis being the most greatly affected.
A single cryolipolysis treatment significantly reduced pain sensitivity to mechanical and thermal modalities within 48-72hrs post-treatment. Mechanical and thermal hyposensitivity persisted for at least 21 days. There was significant loss of unmyelinated epidermal and myelinated dermal nerve fibers starting several days after cryolipolysis, which then recovered gradually, but did not return to baseline during this study. Dermal myelinated nerve fibers were particularly affected, which is consistent with our hypothesis that cryolipolysis preferentially affects lipid-rich structures, including the myelin sheath (Morell and Quarles, 1999). Myelinated nerve fibers in the dermis are composed of the larger, thickly myelinated A-beta fibers that transmit innocuous tactile and vibrational stimuli, and smaller, thinly myelinated A-delta fibers that transmit, cold perception, rapid heat and noxious mechanical stimuli (Provitera et al., 2007). The profoundly reduced mechanosensation to both tactile and vibratory stimuli following cryolipolysis is consistent with loss of myelinated A-beta fibers. Although acute pain is transmitted primarily by C and A-delta fibers, many types of chronic pain are characterized by allodynia, pain to light touch, and there is an ongoing controversy about the degree to which different types of allodynia involve large myelinated fibers versus small unmyelinated fiber terminals in the dorsal horn of the spinal cord (Janig, 2011; Nagi et al., 2011). Myelinated A-delta fibers also play a role in itch sensation (Ringkamp et al., 2011). Studies have shown that large myelinated fibers are most vulnerable to cold injury, which is supported by our study findings (Irwin, 1996; Jia and Pollock, 1997, 1998). An unexpected finding in our study is the decrease in unmyelinated epidermal nerve fiber density after cryolipolysis treatment, which can also contribute to reduction in pain sensation. Based on only one patient, a previous report found no changes in epidermal nerve fiber density at 6 weeks after cryolipolysis treatment (Coleman et al., 2009). In contrast we analyzed multiple biopsies from six subjects, using a rigorous approach with thick sections (70μm) for double immunostaining, and high resolution confocal imaging every 2μm for the entire thickness, to allow 3D visualization of individual nerve fibers for quantification (Figures 3 and 4). In animals, low temperatures for long exposure times can damage unmyelinated nerve fibers (Jia and Pollock, 1998). The mechanisms by which cryolipolysis affects unmyelinated nerve fibers may be multifactorial, such as cold-induced lipid crystallization, ischemia reperfusion injury, metabolic stress or stress signaling pathways. We could not further analyze the specific nerve fiber loss, due to limited biopsy tissue in this human study.
Figure 4. Confocal microscopy imaging of biopsy samples.
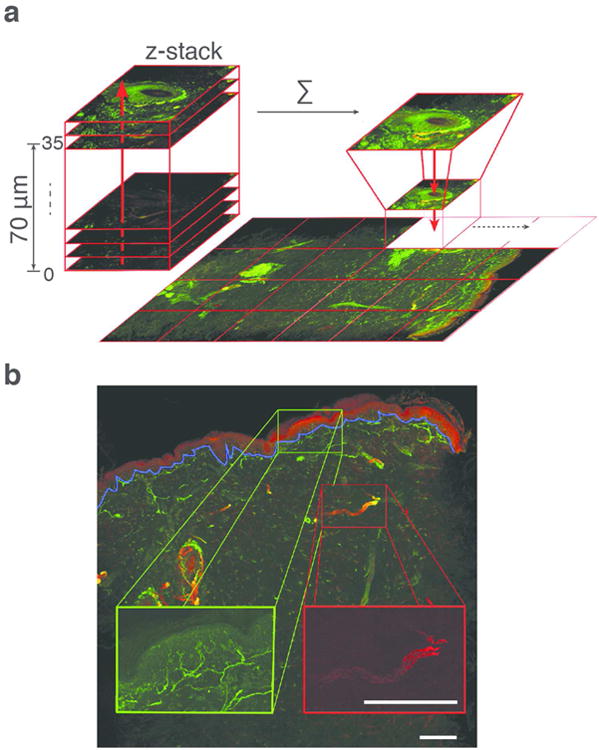
(a) A single biopsy sample demonstrating the confocal imaging technique. The entire 3 mm skin biopsy specimen with 70 μm thickness was imaged every 2 μm. Frames were stacked to create a 3D projection of each confocal image. Thirty-five frames per biopsy were generated and then stitched together using custom-built routines in ImageJ/Fiji. (b) 3D projection of confocal image. The green channel representing fibers stained with anti-PGP9.5 was used to quantify epidermal nerve fibers, and the red channel representing fibers stained with anti-MBP antibody was used to quantify the dermal myelinated fibers. Scale bar = 250 μm
There was gradual but excellent recovery of all cutaneous sensation by 56 days after cryolipolysis, with the exception of histamine-induced itch duration and MPT. However, cutaneous nerve fiber density had not fully recovered. Similarly, ENF density has been reported to lag behind the complete recovery of sensory function after high-dose capsaicin treatment (Kennedy et al., 2010).
Histamine iontophoresis is widely used to study experimental itch (Ikoma et al., 2005; Ishiuji et al., 2008; Magerl et al., 1990; Ward et al., 1996; Yosipovitch et al., 2007). When compared to the baseline value, there was significant, sustained reduction in histamine-induced itch duration at 48-72hrs, and days 14, 21, 35 and 56 after treatment but not compared to the control side (Figure S1 and Table S1). There was no significant change in mean or peak itch intensity after cryolipolysis (Table S2 and S3). The possible reduction of histamine induced itch duration with cryolipolysis could be an experimental artifact in light of the fact that peak itch and mean itch intensity did not change and further studies are needed to substantiate the claim that cryolypolysis is able to alter itch duration.
Itch and pain are distinct sensations but they are closely related with largely overlapping receptors and neuronal subtypes. Unmyelinated C fibers and thinly myelinated A delta fibers are the primary sensory nerves that mediate pain and itch sensation (Basbaum et al., 2009; Ikoma et al., 2006). Histamine-sensitive itch nerves also respond to noxious heat and/or to painful chemicals such as capsaicin, making isolation of the nerves that transmit pain from those that transmit histamine-induced itch experimentally impossible in humans (Schmelz et al., 1997; Schmelz et al., 2003). In mice, Han et al recently identified the existence of itch-specific cutaneous nerves that express the MrgprA3 receptor (Han et al., 2013). This distinct subset of sensory neurons that they identified in mouse skin is crucial for itch induced by various pruritogens but dispensable for acute and chronic pain (Han et al., 2013). Itch-specific fibers have yet to be identified in human skin, although our data argue that such a population may exist or at least that cryolipolysis selectively affects nociceptors that respond only to pain, as opposed to those more widely tuned to itch and pain. In our patients we noted that cryolipolysis induced specific loss of mechanical and thermal pain sensation without altering the peak or mean itch sensation. Because cryolipolysis did not affect itch but reduced mechanical and thermal pain sensation, this technique could potentially be used to study the subpopulation of cutaneous C and A delta fibers that selectively responds to itch in human skin. This would open new avenues for itch research in understanding this population of neurons in humans and developing targeted therapies.
Study limitations
QST was performed according to standardized instructions and by the same experimenter (L.G) to ensure inter-experimental reliability. Nevertheless, QST measures are subjective and can be influenced by a range of physical factors including experimental anxiety, attention, learning, and environmental conditions. Subjects knew which flank was treated and this knowledge may have affected their sensory ratings. Thermal probes with a small surface area are more sensitive at detecting sensory neuropathies and it is possible we may be underestimating the changes in thermal sensory threshold. With a large thermode, the stimulus may inadvertently spread to neighboring receptive fields; smaller thermodes are more sensitive for testing C-fiber mediated thresholds than larger ones due to the effects of spatial summation (Hilz et al., 1999; Khalili et al., 2001). Nevertheless, it is remarkable how changes in cutaneous sensitivity seen over a 56-day period following cryolipolysis treatment persist when compared to the baseline.
Clinical implications
Cryolipolysis is designed to target subcutaneous fat. Our study shows that a single cycle of controlled sub-0°C cold exposure (cryolipolysis treatment) leads to prolonged hyposensitivity to multiple sensory modalities. Based on these findings, if a controlled cooling device could be optimized to selectively target cutaneous nerves, it has the potential to provide long-lasting relief of cutaneous pain, although further experiments are required to clarify it's ability to modulate itch. The optimal cooling parameters, treatment interval, and potential clinical applications in dermatology remain to be established.
Materials and methods
Study design (Figure 1)
This open-label, prospective, IRB-approved study was conducted at the Massachusetts General Hospital (Boston, USA) between December 2012 and July 2013, registered at ClinicalTrials.gov (NCT01673113), and conforms to Declaration of Helsinki and Good Clinical Practice Guidelines. Written, informed consent was obtained from 11 healthy, adult subjects prior to participation, who were randomized to receive a single cryolipolysis treatment on either the left or right flank. Sensory functions were evaluated using QST, followed by histamine iontophoresis to evaluate itch duration on the treated and control (untreated) flank of each subject, before and at 48-72 hours, Days 7, 14, 21, 35 and 56 post-treatment. In 6 subjects, skin biopsies were taken of the treated flank, for analysis of nerve fiber density at baseline, 48-72 hours, Days 21 and 56. Subjects were 18-65 years old, with visible adiposity on both flanks. Subjects with neurologic disorders, known cold sensitivity, allergy or hypersensitivity to histamine, or dermatologic condition in the area to be treated were excluded.
Cryolipolysis procedure
Cryolipolysis treatment was performed approximately 150 cm2 above the iliac crest, on one side of the body. Cutaneous landmarks, and photographs were used to ensure that the same area was tested on follow-up visits. Cryolipolysis was performed with EzApp6.3 applicator (Zeltiq Aesthetics, Pleasanton CA, USA) at cooling intensity factor 41.6, corresponding to heat transfer of -73mW/cm2, for 1 hour. Immediately after treatment all subjects had edema and erythema and 6/11 subjects reported having pain in the treated area. At 48-72 hours, 5/11 subjects still had mild erythema and only 2/11 subjects reported mild discomfort in the treated area. All subjects (100%) were free of pain at Day 7 post treatment.
Quantitative sensory testing (QST)
QST evaluated mechanical (MDT), vibration (VDT), cool (CDT) and warmth (WDT) detection threshold, and mechanical (MPT), cold (CPT) and heat pain thresholds (HPT). Method of Limits approach with stimuli of increasing intensity, starting at sub-threshold levels was used. The QST protocol was performed in the following order:
Mechanical stimuli
MDT and MPT were evaluated first, using von Frey filaments (North Coast Medical, Gilroy, CA, USA). The up-down method, which evaluates the threshold force for appearance and disappearance of a touch sensation reported by the subject, was used until 3 values were obtained. MPT was determined by the minimum force to elicit sharp “prick-like” pain sensation. VDT was evaluated using a computerized vibrometer with 1cm2 contact probe placed perpendicularly on the skin (TSA-II, Medoc Inc., Ramat Yishai, Israel). This device gradually increased the vibration magnitude until the subject pressed a “stop” button to indicate when they first felt vibration. This test was repeated 8 times.
Thermal stimuli
Cool and warmth detection thresholds (CDT, WDT) and cold and hot pain thresholds (CPT, HPT) were determined using a 3cm2 Peltier thermal contact probe (TSA II, Medoc Inc., Ramat Yishai, Israel). Starting from 32°C, a stimulus ramp of 1.0°C/s was used for CDT and WDT, and 1.5°C/s for CPT and HPT assessment. The subject was asked to identify the sensations of warmth and cool, and hot and cold by pressing a “stop” button when each sensation was felt. CDT and WDT were measured 4 times, and HPT and CPT measured 3 times.
Histamine iontophoresis
A 20mm2 iontophoresis electrode (Perimed, Stockholm, Sweden) was used to deliver histamine base 1 mg/ml (Histatrol ALK Abello, Port Washington, NY) via a cathode current of 200μA for 1 minute. All subjects reported itch sensation at the end of iontophoresis. Before iontophoresis each subject was asked to rate their itch intensity on a standardized visual analogue scale (VAS) of 0 (no itch) to 10 (worst-itch). After iontophoresis each subject was asked to continue rating the itch intensity at 30-second intervals for total of 10 minutes. The duration of itch was calculated from the end of iontophoresis to the complete resolution of itch. Erythema and wheal diameters were measured at 10 min after iontophoresis.
Skin biopsy and immunohistochemistry
Skin biopsies (3 mm) were obtained, processed and analyzed according to consensus standards (Lauria et al., 2010). Tissue was placed in Zamboni's fixative (NewcommerSupply, Middleton, WI, USA) for 24hrs at room temperature, rinsed with 0.01M phosphate buffered saline (PBS) and placed in cryoprotectant (20% glycerol in 0.1M Sorrenson's phosphate buffer) for a minimum of 24hrs at 4°C. Serial frozen sections 70μm thick were made. Sections were permeabilized in 0.5% Triton X-100 for 30 minutes at room temperature, incubated in TNB (0.1M Tris HCl, 0.15M NaCl and 0.5% Boehringer milk powder) with 1% Triton X-100 for 2hrs at room temperature, then treated overnight at 4°C with primary mouse antibodies against myelin basic protein (Calbiochem Cat#NE1019-100ul, 1:1000) and anti-human PGP9.5 from rabbits (ABDserotec Cat#7863-0504, 1:1000) diluted in TNB with 0.5 % triton X-100. Sections were washed in 0.01M PBS with 0.5% triton X-100 and fluorescent secondary antibodies FITC-conjugated donkey anti-rabbit and Cy3-conjugated donkey anti-mouse (Jackson ImmunoResearch; 1:500) applied for 2hrs at room temperature. Sections were washed in 0.01M PBS with 0.5% Triton X-100, then 1mM CuSO4 for 10 minutes, and mounted on glass slides with VECTASHIELD Mounting Medium (Vector Labs, Burlingame, CA).
Confocal microscopy and imaging
An Olympus Fluoview FV1000 laser scanning confocal microscope with IX81 inverted microscope base was used, with 20× 0.75NA (UPLSAPO) objective lens for imaging. An automated translation stage with sub-micrometer accuracy was used for high resolution, large area mosaic collection. Skin biopsy specimen sections were imaged every 2μm in depth at 512 × 512 pixels per frame. Frames were stacked to create a 3D projection of each confocal image. Thirty-five frames per biopsy were generated and then stitched together using custom-built routines developed in ImageJ/Fiji (Schindelin et al., 2012). Multichannel z-stacks were then split and every channel was projected to a single plane using a maximum projection method. To analyze the whole captured mosaic, single frames were stitched to form a single large image using a plugin implemented previously (Preibisch et al., 2009) (Figure 4).
Epidermal Nerve Fiber Density (ENF) and dermal myelinated nerve fiber density quantification
The number of ENFs, and of dermal myelinated fibers, was counted using ImageJ and NeuronJ software. The entire 3 mm skin biopsy confocal image was quantified and two sections per biopsy were analyzed per sample. ENF density was defined as the number of individual fibers per mm of basement membrane, as they crossed the dermal-epidermal junction; secondary branching within the epidermis was excluded from the quantification. Quantification of dermal myelinated fibers was performed as previously described (Doppler et al., 2012). Briefly, the total number of individual dermal myelinated fibers per mm2 were counted by two independent observers, one blinded and one unblinded to sample identification.
Statistical analysis
Sample size calculation
Calculations indicated over 80% power for capturing treatment effects on QST, using F-Test ANOVA (version 7.0, nQuery Advisor, Statistical Solutions, Saugus, MA). For biopsy analysis, the sample size of six patients provided 80% power to detect a minimum change of 30% at each time point using the F-test in repeated-measures ANOVA, with one standard deviation changes from baseline considered clinically significant (Vittinghoff E, 2005).
Statistics
Demographic data reported as mean, standard deviation; QST, histamine-iontophoresis and histology reported as mean, 95% confidence interval limits unless otherwise stated. All data were tested for normality using the Kolmogorov-Smirnow goodness of fit test. All outcome measures except mechanical QST (MDT and MPT) followed a normal distribution. To test for differences in sensitivity following cryolipolysis treatment, QST thresholds were compared between: (1) post-treatment versus baseline, and (2) treated area versus control, at all time points. A Mixed Model Repeated Measures ANOVA was used for QST data with a normal distribution; to correct for multiple comparisons two-tailed p<0.05 with Bonferroni correction were considered significant. A Wilcoxon signed-rank test was used for non-parametric data (MDT and MPT). Histamine-induced itch intensity and duration were analyzed using two-way repeated measures ANOVA. Histological results from skin punch biopsies, including myelinated fibers and ENF were analyzed using a linear mixed model repeated measures ANOVA to account for four samples from the same patient, assessed at the four different time points by two independent observers, to account for the within-subject variability of the paired data (treated and control flanks) using a compound symmetry covariance structure and the F-test to assess changes from baseline with a Bonferroni adjusted P-value (Sahai and Ageel, 2000). To test inter-observer agreement, the two observers were evaluated on each variable and found to be comparable (Pearson r >0.80 for each variable). All statistical analyses were performed with GraphPad Prism 5.04 (GraphPad Software Inc., CA), SPSS (IBM Corp., NY) or SAS v9.4 (SAS Institute, USA).
Supplementary Material
Figure S1: Histamine-induced itch duration following cryolipolysis. Data shown as mean (SD). P-values determined using mixed model repeated measures ANOVA with Bonferroni correction. Asterisks indicate level of significance compared to baseline. *, p<0.05.
Table S1: Histamine-induced itch duration (min) in the control and cryolipolysis treated areasA.
Table S2: Histamine-induced mean itch intensity in the control and cryolipolysis treated areasA.
Table S3: Histamine-induced peak itch intensity in the control and cryolipolysis treated areasA.
Table S4: Histamine-induced erythema diameter (cm) in the control and cryolipolysis treated areasA.
Acknowledgments
We thank Drs. Anne Louise Oaklander and Max Klein for useful discussions. L.G. was supported by a National Institutes of Health (NIH) T32 training grant.
Funding: This was an investigator-initiated study supported by discretionary funds.
Abbreviations
- QST
quantitative sensory testing
- BMI
body mass index
- MPT
mechanical pain threshold
- CPT
cold pain threshold
- HPT
heat pain threshold
- MDT
mechanical detection threshold
- VDT
vibration detection threshold
- CDT
cool detection threshold
- WDT
warmth detection thresholds
Footnotes
Conflict of interest statement: RRA receives a portion of royalties paid to Massachusetts General Hospital by Zeltiq, according to institutional policies. The remaining authors state no conflict of interest.
References
- Backonja MM, Attal N, Baron R, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain. 2013;154:1807–19. doi: 10.1016/j.pain.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, et al. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman SR, Sachdeva K, Egbert BM, et al. Clinical efficacy of noninvasive cryolipolysis and its effects on peripheral nerves. Aesthetic Plast Surg. 2009;33:482–8. doi: 10.1007/s00266-008-9286-8. [DOI] [PubMed] [Google Scholar]
- Doppler K, Werner C, Henneges C, et al. Analysis of myelinated fibers in human skin biopsies of patients with neuropathies. J Neurol. 2012;259:1879–87. doi: 10.1007/s00415-012-6432-7. [DOI] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16:174–82. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilz MJ, Stemper B, Axelrod FB, et al. Quantitative thermal perception testing in adults. J Clin Neurophysiol. 1999;16:462–71. doi: 10.1097/00004691-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Handwerker H, Miyachi Y, et al. Electrically evoked itch in humans. Pain. 2005;113:148–54. doi: 10.1016/j.pain.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Stander S, et al. The neurobiology of itch. Nature Reviews Neuroscience. 2006;7:535–47. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Irwin MS. Nature and mechanism of peripheral nerve damage in an experimental model of non-freezing cold injury. Annals of the Royal College of Surgeons of England. 1996;78:372–9. [PMC free article] [PubMed] [Google Scholar]
- Ishiuji Y, Coghill RC, Patel TS, et al. Repetitive scratching and noxious heat do not inhibit histamine-induced itch in atopic dermatitis. Br J Dermatol. 2008;158:78–83. doi: 10.1111/j.1365-2133.2007.08281.x. [DOI] [PubMed] [Google Scholar]
- Janig W. Mechanical allodynia generated by stimulation of unmyelinated afferent nerve fibres. J Physiol. 2011;589:4407–8. doi: 10.1113/jphysiol.2011.217083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Pollock M. The pathogenesis of non-freezing cold nerve injury. Observations in the rat. Brain: A Journal of Neurology. 1997;120(Pt 4):631–46. doi: 10.1093/brain/120.4.631. [DOI] [PubMed] [Google Scholar]
- Jia J, Pollock M. Cold injury to nerves is not due to ischaemia alone. Brain: A Journal of Neurology. 1998;121(Pt 5):989–1001. doi: 10.1093/brain/121.5.989. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Vanhove GF, Lu SP, et al. A randomized, controlled, open-label study of the long-term effects of NGX-4010, a high-concentration capsaicin patch, on epidermal nerve fiber density and sensory function in healthy volunteers. J Pain. 2010;11:579–87. doi: 10.1016/j.jpain.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Khalili N, Wendelschafer-Crabb G, Kennedy WR, et al. Influence of thermode size for detecting heat pain dysfunction in a capsaicin model of epidermal nerve fiber loss. Pain. 2001;91:241–50. doi: 10.1016/S0304-3959(00)00444-9. [DOI] [PubMed] [Google Scholar]
- Lauria G, Hsieh ST, Johansson O, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Societ y. Eur J Neurol. 2010;17:903–12. e44–9. doi: 10.1111/j.1468-1331.2010.03023.x. [DOI] [PubMed] [Google Scholar]
- Magerl W, Westerman RA, Mohner B, et al. Properties of transdermal histamine iontophoresis: differential effects of season, gender, and body region. J Invest Dermatol. 1990;94:347–52. doi: 10.1111/1523-1747.ep12874474. [DOI] [PubMed] [Google Scholar]
- Manstein D, Laubach H, Watanabe K, et al. Selective cryolysis: a novel method of non-invasive fat removal. Lasers Surg Med. 2008;40:595–604. doi: 10.1002/lsm.20719. [DOI] [PubMed] [Google Scholar]
- Morell P, Quarles RH. Characteristic Composition of Myelin 1999 [Google Scholar]
- Nagi SS, Rubin TK, Chelvanayagam DK, et al. Allodynia mediated by C-tactile afferents in human hairy skin. J Physiol. 2011;589:4065–75. doi: 10.1113/jphysiol.2011.211326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AA, Wasserman D, Avram MM. Cryolipolysis for reduction of excess adipose tissue. Semin Cutan Med Surg. 2009;28:244–9. doi: 10.1016/j.sder.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Preibisch S, Saalfeld S, Tomancak P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics. 2009;25:1463–5. doi: 10.1093/bioinformatics/btp184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provitera V, Nolano M, Pagano A, et al. Myelinated nerve endings in human skin. Muscle Nerve. 2007;35:767–75. doi: 10.1002/mus.20771. [DOI] [PubMed] [Google Scholar]
- Ringkamp M, Schepers RJ, Shimada SG, et al. A role for nociceptive, myelinated nerve fibers in itch sensation. J Neurosci. 2011;31:14841–9. doi: 10.1523/JNEUROSCI.3005-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai H, Ageel MI. The Analysis of Variance. Birkh√ § user Boston; Boston, MA: 2000. [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, et al. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Weidner C, et al. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–8. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- Vittinghoff E GD, Shiboski SC, McCulloch CE. Regression methods in biostatistics Linear, logistic, survival, and repeated measures models. New York: Springer; 2005. pp. 253–89. [Google Scholar]
- Ward L, Wright E, McMahon SB. A comparison of the effects of noxious and innocuous counterstimuli on experimentally induced itch and pain. Pain. 1996;64:129–38. doi: 10.1016/0304-3959(95)00080-1. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Duque MI, Fast K, et al. Scratching and noxious heat stimuli inhibit itch in humans: a psychophysical study. Br J Dermatol. 2007;156:629–34. doi: 10.1111/j.1365-2133.2006.07711.x. [DOI] [PubMed] [Google Scholar]
- Zelickson B, Egbert BM, Preciado J, et al. Cryolipolysis for noninvasive fat cell destruction: initial results from a pig model. Dermatol Surg. 2009;35:1462–70. doi: 10.1111/j.1524-4725.2009.01259.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Histamine-induced itch duration following cryolipolysis. Data shown as mean (SD). P-values determined using mixed model repeated measures ANOVA with Bonferroni correction. Asterisks indicate level of significance compared to baseline. *, p<0.05.
Table S1: Histamine-induced itch duration (min) in the control and cryolipolysis treated areasA.
Table S2: Histamine-induced mean itch intensity in the control and cryolipolysis treated areasA.
Table S3: Histamine-induced peak itch intensity in the control and cryolipolysis treated areasA.
Table S4: Histamine-induced erythema diameter (cm) in the control and cryolipolysis treated areasA.


