Abstract
There are no effective treatments for millions of patients with intractable epilepsy. High-fat ketogenic diets may provide significant clinical benefit but are challenging to implement. Low carbohydrate levels appear to be essential for the ketogenic diet to work, but the active ingredients in dietary interventions remain elusive, and a role for ketogenesis has been challenged. A potential antiseizure role of dietary protein or of individual amino acids in the ketogenic diet is understudied. We investigated the two exclusively ketogenic amino acids, L-leucine and L-lysine, and found that only L-leucine potently protects mice when administered prior to the onset of seizures induced by kainic acid injection, but not by inducing ketosis. Unexpectedly, the D-enantiomer of leucine, which is found in trace amounts in the brain, worked as well or better than L-leucine against both kainic acid and 6 Hz electroshock-induced seizures. However, unlike L-leucine, D-leucine potently terminated seizures even after the onset of seizure activity. Furthermore, D-leucine, but not L-leucine, reduced long-term potentiation but had no effect on basal synaptic transmission in vitro. In a screen of candidate neuronal receptors, D-leucine failed to compete for binding by cognate ligands, potentially suggesting a novel target. Even at low doses, D-leucine suppressed ongoing seizures at least as effectively as diazepam but without sedative effects. These studies raise the possibility that D-leucine may represent a new class of anti-seizure agents, and that D-leucine may have a previously unknown function in eukaryotes.
Keywords: amino acids, epilepsy, kainic acid, preclinical testing
INTRODUCTION
Approximately one million people in the US have seizures that are not controlled by appropriately-chosen medications (Brodie et al., 2012; Kobau et al., 2008; Kwan and Brodie, 2000). However, the efficacy of epilepsy medications has not improved substantially in the past 50 years. One underutilized option for these patients is metabolism-based therapy (Hartman and Stafstrom, 2013), with the high-fat, low-carbohydrate ketogenic diet being the most widely used form (Hartman et al., 2007; Neal et al., 2008). However, the full benefit of the ketogenic diet is hampered by difficulties with implementation. Efforts to decipher the mechanisms of action have focused primarily on fatty acid metabolites and carbohydrate restriction (Masino and Rho, 2012). The other relevant ketogenic diet component is protein, which is minimized to induce systemic ketosis (Hartman and Vining, 2007; Laeger et al., 2014). Thus, the possibility that proteins or their amino acid components could actively contribute to efficacy of the ketogenic diet seems counterintuitive. Furthermore, free amino acids potently stimulate the nutrient-sensing kinase mTORC1 (mammalian target of rapamycin complex 1), which is already hyperactive in epilepsy patients with mutations in TSC1/2 (tuberous sclerosis complex) (Orlova and Crino, 2010). In addition, mTOR activity is elevated in rodent brains following kainic acid-induced seizures (Zeng et al., 2009). However, the role of mTOR in other epilepsy etiologies is not understood, and our studies of wild type mice indicate that the mTOR inhibitor rapamycin has minimal or no benefit in several acute seizure models (Hartman et al., 2012).
In addition to its role in nutrient signaling to mTORC1 (Bar-Peled and Sabatini, 2014; Cota et al., 2006; Sancak et al., 2008), L-leucine is a ketogenic amino acid, in that its degradation results in the production of ketone bodies, particularly acetoacetate and its metabolite β–hydroxybutyrate, both of which have been implicated as anticonvulsants (Bixel and Hamprecht, 1995; Masino and Rho, 2012). However, elevated L-leucine and other branched-chain amino acids have been associated with abnormal developmental outcomes and seizures (Mackenzie and Woolf, 1959; Menkes et al., 1954), dampening the exploration of potentially efficacious amino acids. We tested the ketogenic amino acid L-leucine in mice and found striking protection against seizure activity, but without evidence of elevated blood ketones. Remarkably, the enantiomer D-leucine was a more potent anti-seizure agent than L-leucine. The L-enantiomers of amino acids are generally assumed to account for most or all of their biological roles, including protein translation, signaling, transporter-mediated effects, and as metabolic substrates. However, D-leucine is found in food and is produced by bacteria (Ekborg-Ott and Armstrong, 1996; Mutaguchi et al., 2013), but has no known physiological function in eukaryotes. Our finding that D- but not L-leucine alters long-term potentiation (LTP) in acute brain slices is consistent with the possibility that D-leucine has previously unknown signaling activity in mammals. D-leucine also may offer a safer alternative over L-leucine.
METHODS
Seizure tests
Male mice were housed 3–4 per cage after being sorted on arrival to the facility by Animal Care staff. Kainic acid (Tocris Bioscience, Ellisville, MO, U.S.A. and Cayman Chemical, Ann Arbor, MI, U.S.A.) was injected intraperitoneally. At the time of the experiment, mice were randomized to different treatment groups by weight to ensure all cohorts had similar average weights (Supplemental Figs. 1B and 2B) and were all the same age (5 wks) at the time of treatment. Seizure tests were performed as described previously (Hartman et al., 2010). Briefly, kainic acid was injected i.p. and seizures were scored (on a 7-point scale) based on the maximum seizure severity during sequential 5 min epochs using a modified Racine scale (Morrison et al., 1996). Note that the seizure severity of vehicle-treated controls differs in different experiments primarily due to different vendor sources of kainic acid and inherent differences between different shipments of mice; however, control and experimental cohorts were closely paired. The 6 Hz test was administered exactly as described previously at 6 wks of age (2-wk leucine treatments were started at 4 wks of age), and, as in prior work, any change in behavior scored as a seizure after the electroshock was administered (Hartman et al., 2012). Results for all mice tested are presented here and all observations were made by personnel who were blind to the treatment condition during treatment administration and during seizure scoring in all experiments. Measurements of glucose and β-hydroxybutyrate were performed on one mouse from each cage (selected by a random number generator) exactly as described (Hartman et al., 2010). All animal protocols for this study were approved by the ACUC (JHU and UW) and were adhered to strictly.
Amino acid treatments
A single dose of each amino acid dissolved in H2O (Sigma-Aldrich, St. Louis, MO, U.S.A) was injected intraperitoneally (i.p.) into 5 week-old mice 3 h before kainic acid injection. The reported purity of the lots of L- and D-leucine used was 99% (based on optical rotation data); L-leucine had no more than 5% of the D-isomer, and D-leucine had 0.4% of the L-isomer (Sigma Aldrich, technical data). In separate experiments, L- and D-leucine was administered at the indicated time (15 or 20 min) after kainic acid injection. The 15–20 min time is midrange for the clinical operational definition of status epilepticus (defined as 5–30 min of seizure activity without a return to baseline, (Lowenstein, 1999)). In these experiments, all mice had experienced at least two seizures with a score ≥ 3 when D-leucine was administered, with seizures starting 10–15 min after kainic acid injection. Diazepam (5 mg/ml injectable solution, diluted 1:1.5 in phosphate-buffered saline; Hospira, Lake Forest, IL, U.S.A.) was injected i.p. at 20 min after kainic acid injection, when all mice had exhibited two or three stage ≥3 seizures.
Electrophysiology
Male 7–8 week C57BL/6 mice littermates (Harlan Laboratories, Madison, WI) were randomly assigned to treatment groups and hippocampal slices were prepared as previously reported (Potter et al., 2010). Slices from 6 different animals were used for LTP studies, testing each slice three times for input/output and paired-pulse facilitation both before amino acid addition and after LTP measurements in the presence of amino acids. fEPSPs were recorded from CA1 stratum radiatum with ACSF-filled recording electrodes (2–5MΩ) (Stafstrom et al., 2009). A bipolar platinum-tungsten (92:8 Pt:Y) stimulating electrode was placed at the CA3/CA1 border along the Schaffer collateral pathway (Gerstein et al., 2012). Initial baseline synaptic transmission was assessed for each slice by applying increasing stimuli (0.5V – 20V, 25 nA – 1.5 µA, A-M Systems model 2200 stimulus isolator, Carlsborg, WA) in regular artificial cerebrospinal fluid (ACSF) without amino acids to determine the input:output (I/O) relationship. Subsequent LTP stimuli were set to evoke a fEPSP with half the maximum fEPSP slope. Prior to LTP induction, slices also were tested in regular ACSF without amino acids using a paired pulse facilitation (PPF) paradigm, which consisted of an initial single stimulus to the Schaffer collaterals followed by a second stimulus of equal magnitude at half maximum intensity as set by the I/O curve. This PPF paradigm was repeated with increasing inter-pulse intervals (10 msec to 300 msec). PPF at each time interval was recorded in triplicate and then averaged. fEPSP slopes from the second pulse were plotted as a percentage of initial slope.
After initial I/O and PPF data were recorded without amino acids, baseline pretreatment data were obtained for 30 min, followed by addition of the respective amino acids. After 60 min preincubation with amino acids, LTP was then induced by theta burst stimulation (10 bursts/train, 3 trains/stimulus, 20-sec inter-train interval). Each burst contained 4 stimuli at 100Hz with an interburst interval of 200 msec (Kumar, 2011). Synaptic efficacy was monitored continuously (0.05 Hz). Every 2 min, sweeps were averaged (pClamp, Molecular Devices). After 180 min, still in the presence of amino acids, I/O and PPF data were acquired by repeating the protocols described above to verify the integrity of the slice preparations.
For potassium bursting experiments, after 15 min of baseline recording in separate slices, epileptiform bursts were induced by increasing [K+]o to 7.5 mM (Traynelis and Dingledine, 1988). A stable baseline burst firing rate of 13.7 ± 1.0 was established over 30 min, after which ACSF with various added concentrations of D-leucine (100 µM, 1 mM, 10 mM) was perfused.
In vitro radioligand receptor binding studies
Detailed protocols are available for all assays on the NIMH-PDSP website (http://pdspdb.unc.edu/pdspWeb/?site=binding) (Besnard et al., 2012). Primary screening assays with D-Leu were performed at a final concentration of 10 µM in quadruplicate for 47 different receptors, and as described in Fig. 4D for kainic acid receptors. Results were normalized as relative percent inhibition, with specific binding in the absence of competing ligand set as 100% (no inhibition) and specific binding in the presence of reference compound as 0% (100% inhibition). The buffer for the secondary binding assay (Fig. 4D) was 50 mM Tris HCl, 2.5 mM CaCl2, pH 7.4.
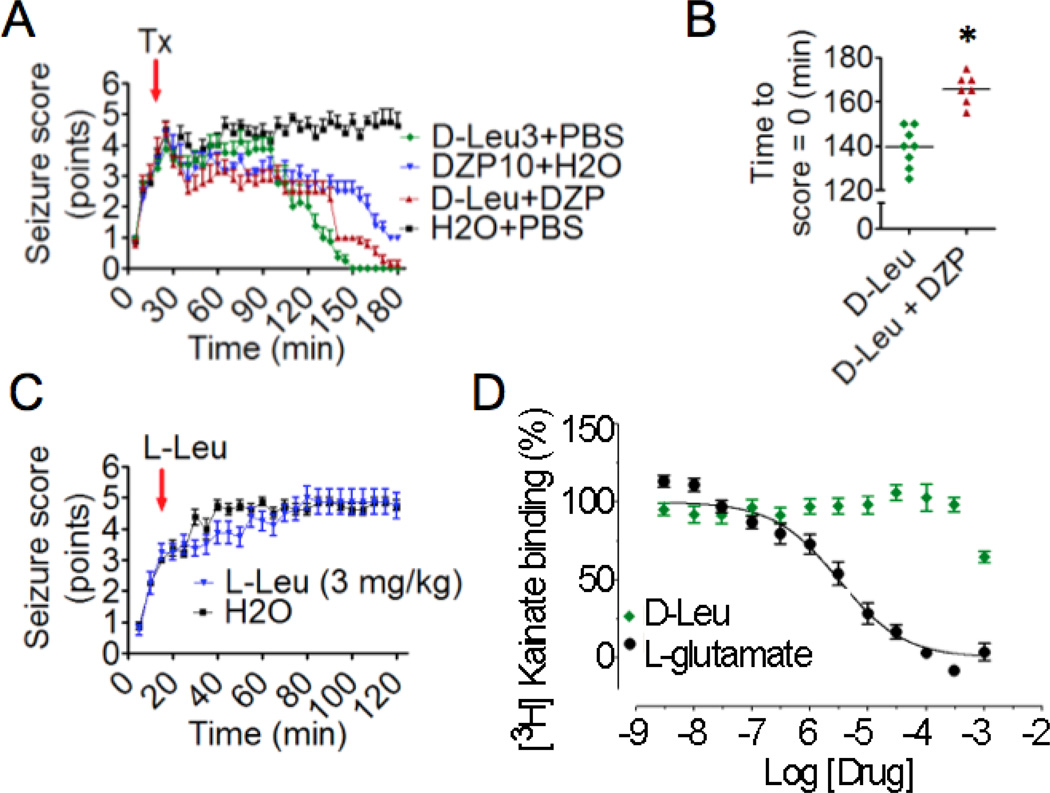
Figure 4. Comparison of D-leucine with diazepam treatments post seizure onset.
(A) Means of maximum seizure scores in consecutive 5-min intervals (±SEM) for 4 independent cohorts were determined using somewhat stronger seizure conditions (25 mg/kg kainic acid ip). There were no differences between treatment groups in mean seizure scores prior to treatment. At 20 min (red arrow) after kainic acid injection (25 mg/kg i.p.), mice were treated with a single dose of D-leucine (3 mg/kg), diazepam (10 mg/kg), a combination of D-leucine (3 mg/kg) and diazepam (10 mg/kg) or a combination of their respective vehicles (H2O and PBS), respectively (N = 8/treatment, P < 0.001 for comparisons between all treatment groups, multilevel mixed effects linear regression model). (B) Scatterplot showing time until seizure score = 0 (P= 2.7E-05, t-test). One mouse in the D-Leu + DZP never achieved a score = 0 and was therefore omitted from this analysis. Bars, mean score. (C) Mean seizure scores (±SEM) taken at 5-min intervals for 2 cohorts of mice treated with L-leucine (3 mg/kg) 15 min after injection with (25 mg/kg) kainic acid (red arrow). (N = 8/treatment, P ≤ 0.001, multilevel mixed effects linear regression model; see Methods). All animals tested are presented for panels A–C. (D) D-leucine and L-glutamate (positive control) were tested at the indicated concentrations for the ability to compete off bound 3H-kainic acid from its receptors in rat brain membrane preparations. Values indicate percent inhibition of kainic acid binding (mean ± SEM) in three independent assays, each in triplicate (Ki = >10,000 nM).
Statistics
In the kainic acid test, the slope of the curve generated by seizure score points was analyzed using a multilevel mixed effects linear regression model with individual measurements over time for each individual mouse (random effect), individual mice within a cohort (random effect), and cohorts within a treatment group (fixed effect) (Stata 13.1, Stata Corporation, College Station, TX, U.S.A.). Additional comparisons of the other parameters between groups were made using a t-test or one-way ANOVAs with post-hoc Tukey tests, as appropriate (GraphPad Prism 4, LaJolla, CA, U.S.A.). The level of significance was P ≤ 0.05 for two comparisons, and with Bonferroni corrections for multiple comparisons (i.e., when six comparisons were made, P ≤ 0.008 was used). In the 6 Hz test, probit analyses were used to determine the primary outcome, the convulsive current 50 (CC50), the current where 50% of mice experienced any seizure behavior (Minitab 16, State College, PA, U.S.A.). Electrophysiology data were analyzed by 2-way ANOVA (treatment and time) with repeated measures (mixed model) and Bonferroni post-hoc tests (GraphPad Prism 6, LaJolla, CA, U.S.A.).
RESULTS
L-leucine pretreatment protects against induced seizures
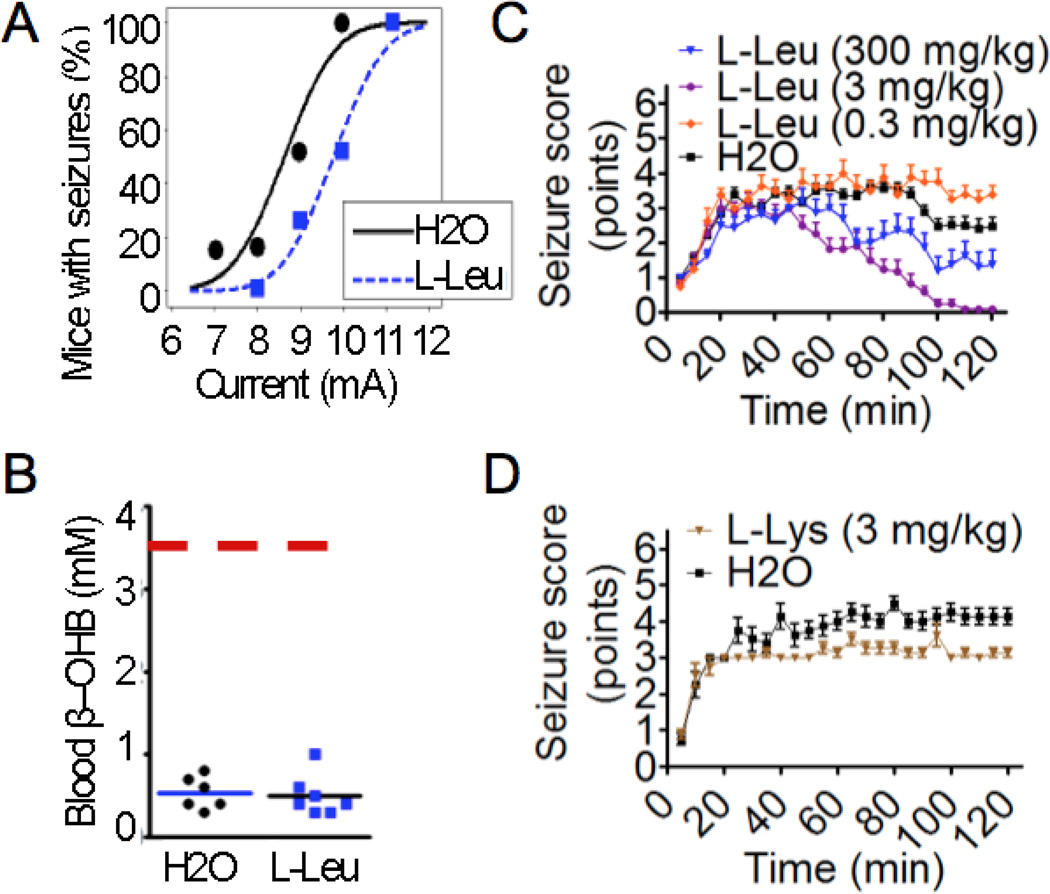
Early studies in rats found that branched chain amino acids, including L-leucine, delay the onset of seizures induced by picrotoxin or pentylenetetrazol (PTZ) but without affecting seizure duration (Dufour et al., 1999; Skeie et al., 1994). We tested L-leucine in a seizure model that we developed previously to demonstrate efficacy of the ketogenic diet, which includes a prolonged pre-treatment period more typical of clinical use (Hartman et al., 2008; Hartman et al., 2010). Mice exposed to L-leucine in the drinking water for 12–14 days were protected from 6 Hz-induced seizures, with a margin of protection similar to the ketogenic diet in this test (Fig. 1A, Supplemental Fig. 1A). However, L-leucine did not induce systemic ketosis based on normal blood levels of β-hydroxybutyrate and glucose (Fig. 1B, Supplemental Fig. 1B).
Figure 1. L-leucine pretreatment protects against seizures.
(A) Probability of mice having a seizure at the indicated 6 Hz stimulus currents for mice pretreated without or with L-leucine (1.5% w/v) in drinking water for 12–14 days. Data are from 3 independent cohorts. Data points represent the percentage of mice from all cohorts that had seizures at a given current. Probit analysis curves represent a probability function, therefore not all points lie on the curve (N = 24–28/treatment, P=0.02, probit analysis). (B) Blood β–hydroxybutyrate levels on the day of seizure testing from randomly preselected mice (using a random number generator) from the same cohorts in Fig. 1A (H2O N= 6 mice, L-leucine N = 7 mice, P = 0.79, t-test). Solid bars indicate the mean; dashed line indicates the level of β–hydroxybutyrate seen in mice fed a ketogenic diet (Hartman et al., 2010). (C) Mean maximum seizure scores (±SEM) for each consecutive 5-min interval for 2 h after injection with kainic acid (23.5 mg/kg) for 3–4 independent cohorts of mice pretreated with L-leucine or water (vehicle) for 3 h (N = 8–36/treatment, P < 0.001, multilevel mixed effects linear regression model). (D) Mean maximum seizure scores (±SEM) at consecutive 5-min intervals in the kainic acid (23.5 mg/kg) seizure test for 2 independent cohorts of mice pretreated with L-lysine (3 mg/kg) or water (vehicle) for 3 h. (N = 8/treatment, P <0.001, multilevel mixed effects linear regression model). (A–D) All animals tested are presented.
We reported previously that the ketogenic diet does not protect against seizures induced by kainic acid, an agonist of two types of glutamate receptors (kainic acid and AMPA) (Hartman et al., 2010; Stawski et al., 2010). In contrast, we found that a single low dose of 3 mg/kg L-leucine injected 3 h prior to kainic acid-induced seizures potently suppressed seizure activity, while the lowest dose tested (0.3 mg/kg) was statistically different from vehicle, but without a substantial overall benefit (Fig. 1C & Supplemental Figs. 1C & D). Efficacy at such a low dose suggests L-leucine may act by a receptor signaling mechanism rather than as a metabolic substrate (Skeie et al., 1994; Yudkoff et al., 2005). Interestingly, the highest dose of 300 mg/kg L-leucine, which was selected based on the earlier rat studies (Dufour et al., 1999; Skeie et al., 1994), had only intermediate protection, possibly reflecting branched-chain toxicity (Fig. 1C & Supplemental Figs. 1C & D). The ketogenic diet may fail to protect in this assay (Hartman et al., 2010; Stawski et al., 2010) because its mechanism differs from L-leucine or because the mouse-adapted ketogenic diet was not optimized to detect the beneficial effects of amino acids.
To further evaluate whether L-leucine-mediated protection is related to ketogenic effects not detected by our assays, L-lysine, the other amino acid that is only ketogenic (i.e., not also glucogenic) was tested against kainic acid-induced seizures. L-lysine (3 mg/kg) did not provide substantial clinical protection (although it was statistically significant for seizure duration) and was not further investigated (Fig. 1D & Supplemental Fig. 1E).
Pretreatment with the enantiomer D-leucine potently protects against induced seizures
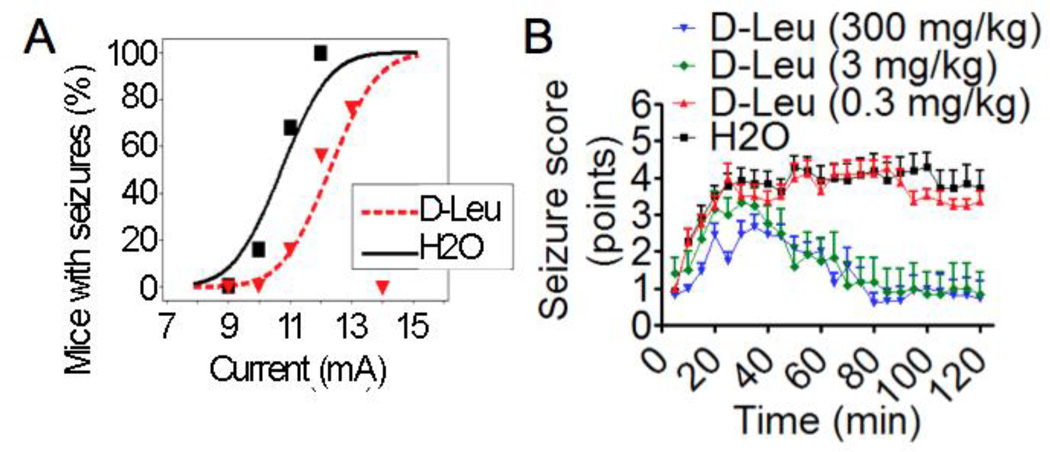
We considered the possibility that a contaminant present in commercial sources of L-leucine (prepared from a bacterial source, Sigma Aldrich technical data) was responsible for the observed anti-seizure activity. The most abundant contaminant is the enantiomer D-leucine. D-leucine is not synthesized by animals, but is ingested from dietary sources and is present in brain tissue, including the hippocampus (rats and mice), pineal gland (rats), cerebrum (mice), and other brain regions at lower concentrations (Hamase et al., 1997; Hamase et al., 2001). We found that two weeks of D-leucine in drinking water (1.5% w/v) protected in the 6 Hz test (Fig. 2A, Supplemental Figs. 2A). Similar to L-leucine, but unlike the ketogenic diet (Hartman et al., 2010), D-leucine had no apparent effect on systemic ketosis as blood levels of β–hydroxybutyrate and glucose were unchanged, and there was no difference in food or water consumption, or on body weights (Supplemental Figs. 1B and 2B). Thus, it appears unlikely that seizure protection by L- and D-leucine is mediated via effects on systemic metabolic changes.
Figure 2. D-leucine pretreatment protects against seizures.
(A) Probability of mice having 6 Hz-induced seizures as described in Fig. 1A except treating with D-leucine (1.5% w/v). Data are from 3 independent cohorts; all mice tested are presented. (N = 21–22/treatment, P = 0.02, probit analysis). (B) Means of maximum seizure scores for consecutive 5-min intervals (±SEM) in the kainic acid (23.5 mg/kg) seizure test for 3–4 independent cohorts of mice pretreated with D-leucine or water (vehicle) for 3 h and then observed for 2 h following kainic acid administration (N = 8–15/treatment, P < 0.001, multilevel mixed effects linear regression model). (A–B) All animals tested are presented.
The high dose (300 mg/kg) of D-leucine potently suppressed seizure activity induced by kainic acid, similar to the 3 mg/kg dose (Fig. 2B, Supplemental Fig. 2C & D). D-leucine appears to be a more potent anticonvulsant than L-leucine because the vehicle-treated mice had seizure intensities were somewhat more intense than in experiments testing L-leucine (compare H2O controls in Figs. 1C & 2B, Supplemental Figs. 1D & 2D). D-leucine may also reduce seizure activity somewhat earlier than L-leucine. Cross contamination of either D-leucine or L-leucine with their enantiomers cannot explain the observed protection by either enantiomer, as the level of contamination in the 3 mg/kg dose is less than the ineffective 0.3 mg/kg dose (see Methods). However, these data do not exclude the possibility that L- and/or D-leucine is racemized following injection, although the short time frame to onset of effect seems inconsistent with this hypothesis.
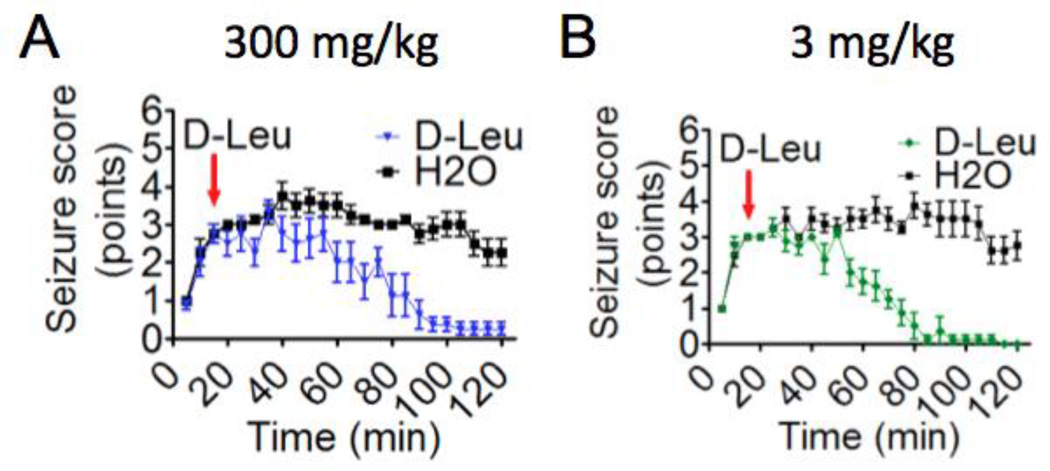
D-leucine terminates ongoing seizures
To more closely mimic clinical settings, D-leucine was administered 15 min after animals were injected with kainic acid (approximately 5–10 min after the onset of seizure activity). Strikingly, both the high (300 mg) and low (3 mg) dose of D-leucine abolished behavioral seizure activity in all mice tested (Fig. 3, Supplemental Fig. 3). D-leucine was also compared to diazepam, the most commonly used medicine to stop status epilepticus in rodents (and a commonly used medicine in the clinic) that also is a strong sedative. To provide an even greater challenge for treatment, mice received a higher dose of kainic acid, and treatment with D-leucine and/or diazepam was delayed until 20 min after kainic acid. All treatments led to improved seizure scores (Fig. 4A, Supplemental Fig. 4A & B). However, none of the mice treated with diazepam alone returned to baseline by 3 h (i.e., seizure score = 0, and easy arousability with gentle cage movement). Furthermore, mice treated with D-leucine alone returned to baseline in less time than the drug combination (Fig. 4B). In contrast to D-leucine, L-leucine provided no substantial benefit against a similar seizure stimulus although L-leucine was statistically significant (Fig. 4C, Supplemental Fig. 4C). Thus, D-leucine stops ongoing seizures more effectively than diazepam, while L-leucine was ineffective after seizure onset.
Figure 3. D-leucine treatment post seizure onset protects against seizures induced by kainic acid (i.p.).
(A) Mean seizure scores (±SEM) for 2 cohorts of mice treated with D-leucine (300 mg/kg) 15 min after the onset of kainic acid-induced seizures (23.5 mg/kg i.p.) (red arrow). (N = 8/treatment, P <0.001, multilevel mixed effects linear regression model). (B) Mean seizure scores (±SEM) taken at 5-min intervals for 2 cohorts of mice treated with D-leucine (3 mg/kg) 15 min after seizure induction (red arrow). (N = 8/treatment, P < 0.001, multilevel mixed effects linear regression model). (A–B) All animals tested are presented.
No D-leucine receptor candidates
To investigate potential anti-seizure mechanisms, we determined if D-leucine might compete with kainic acid binding to endogenous receptors. However, D-leucine failed to compete off radiolabeled kainic acid from its receptors in tissue preparations, in contrast to L-glutamate (Fig. 4D). Therefore, protection in the kainic acid test is not likely due to competition of D-leucine for kainic acid binding sites on glutamatergic receptors. In pursuit of a D-leucine receptor, a panel of candidate neuronal receptors was screened for potential binding to D-leucine by the National Institute of Mental Health Psychoactive Drug Screening Program (NIMH-PDSP) (Besnard et al., 2012). In these assays, D-leucine was tested for the ability to compete with radiolabeled ligands specific for a panel of 48 common neuronal receptors and transporters, including G protein-coupled receptors, ion channels, and transporters as described (Besnard et al., 2012). As expected, D-leucine failed to compete for binding to kainic acid receptors, but also did not reduce the binding of other radioligands to the remaining 47 receptors tested (glutamatergic receptors, GABAA, serotonergic, adrenergic, dopaminergic, histaminergic, muscarinic, Sigma 1/2, and opiate receptors) and transporters (serotonin, dopamine, and norepinephrine) (Supplemental Fig. 5). These results indicate that D-leucine does not competitively bind the same sites or allosterically reduce affinity of ligands for their cognate receptors. On a positive note, D-leucine also appears to lack non-specific interactions with this panel of central nervous system receptors, ruling out off-site binding targets commonly observed with other small molecules.
A candidate D-leucine receptor was not identified in this screen. However, because only one binding site was tested for each receptor or transporter (e.g., the MK-801 site on the NMDA receptor), it is formally possible that D-leucine binds to other sites on one or more of these proteins. The lack of detectable effects on substrate binding, combined with the effective dosing range in animal seizure tests, suggest a safety margin that may be of clinical utility.
D-Leucine attenuates long-term potentiation but not basal synaptic activity
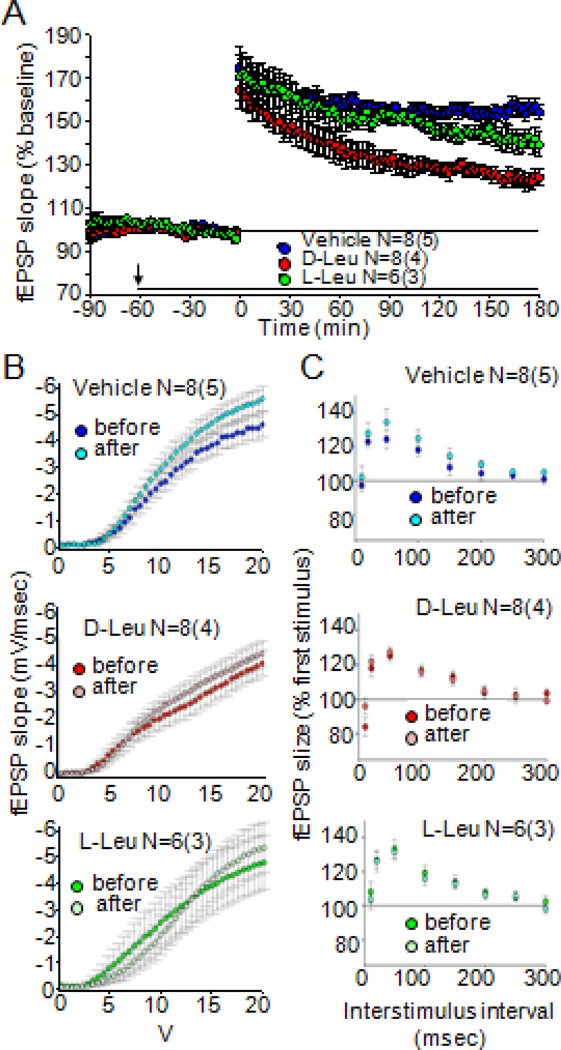
LTP represents the enduring enhancement of signal transmission between two neurons that results from their synchronous stimulation and thus, represents a form of synaptic plasticity. LTP measurement is one of the most common methods used to measure synaptic function, which (chronically) underlies not only epileptic seizures but also routine tasks, such as memory formation. We therefore tested D-leucine in an LTP paradigm (theta burst stimulation, TBS) to mimic the physiological firing of hippocampal neurons (Vertes and Kocsis, 1997). Field excitatory postsynaptic potentials (fEPSP) slopes were significantly attenuated after 2 mM D-leucine application during the early protein synthesis-independent maintenance phase, whereas 2 mM L-leucine had no effect (LTP induction was not affected by application of D- or L-leucine compared to controls) (Fig. 5A). Notably, the effect of D-leucine is milder than that reported for diazepam, which completely blocks LTP (del Cerro et al., 1992). Furthermore, neither amino acid had a significant effect on basal synaptic function based input/output relations before amino acid addition compared with after LTP plateau in the presence of amino acids (Fig. 5B).
Figure 5. D-leucine inhibits long-term potentiation in hippocampal area CA1.
(A) Field EPSP slope as a function of time before and after theta burst stimulation (TBS). Over the 3 h following TBS, there is significant reduction of the percent change in fEPSP slope over time (i.e., a lower post-TBS fEPSP slope) with 2 mM D-leucine [(main effect of treatment F(1,15)=26.83, P = 0.0001)] but not 2 mM L-leucine treatment compared to control (main effect of treatment F(1,13)=1.584, P = 0.23); 2 mM D-leucine compared to 2 mM L-leucine also was significant (main effect of treatment, F(1,13)= 8.431, P = 0.01). Pooled data from N slices (number of animals indicated in parentheses). Arrow, application of amino acids for duration of experiment (black bar). (B) Input/output curves show no effect on fEPSP slope as a function of stimulation voltage before and after D-leucine and L-leucine compared to untreated controls (main effect of treatment, F=(4,44)=0.2898, P = 0.83). (C) Paired-pulse facilitation (PPF) protocol showing no effect of amino acids on fEPSP amplitude (percent first stimulus) as a function of interstimulus interval (main effect of treatment, F=(4,44) =1.387, P =0.26).
To assess any possible presynaptic contribution to synaptic transmission, paired-pulse facilitation (PPF), a form of short-term plasticity, was also examined. There was a similar degree of PPF after treatment with either vehicle or the leucine isomers at all inter-stimulus intervals tested (Fig. 5C). These results verify the viability of the slice preparations.
To characterize the effects of D-leucine on neuronal hyperexcitability (i.e., one measure of the propensity to develop seizures), we elicited epileptiform discharges using elevated extracellular potassium ([K+]o) (Traynelis and Dingledine, 1988). There was no difference in burst firing rate with any D-leucine concentration compared to pre-leucine baseline or wash, indicating a lack of obvious effects on induced epileptiform activity (Supplemental Fig. 6).
DISCUSSION
We found that very low concentrations of D-leucine potently terminate ongoing seizures or raise seizure threshold in mice. This finding is surprising because a function for endogenous D-leucine in epilepsy or any other process in eukaryotic biology is not known. D-leucine is not incorporated into mammalian proteins (Payne et al., 2012) and does not stimulate mTOR activity appreciably (Fox et al., 1998).
The distinction between L- and D-enantiomers may be important clinically as branched chain L-amino acids are under investigation for therapeutic use in patients with low levels due to branched-chain ketoacid dehydrogenase kinase deficiency (OMIM 614923) (Novarino et al., 2012). Except for this rare disorder, treatment of idiopathic epilepsy with high doses of L-leucine has potential safety issues because of the neurotoxicity reported in patients with mutations leading to elevated branched chain amino acids including L-leucine (maple syrup urine disease) (Mackenzie and Woolf, 1959; Menkes et al., 1954). The potential clinical utility of L-leucine for epilepsy is further challenged by the finding that acute L-leucine exposure induces hypoglycemia via insulin secretion in mice (Freinkel et al., 1976; Reaven and Greenberg, 1965).
Although a leucine racemase has not been identified in mammals, we cannot rule out the possibility that there may be a low level of conversion of D- to L-leucine via oxidative deamination (D-amino acid oxidase), followed by transaminase-mediated reamination (D'Aniello et al., 1993; Ercal et al., 1996). However, this is not supported by our finding that D-leucine was more effective at the highest dose tested (300 mg/kg) than L-leucine in the kainic acid test. We also cannot rule out the possibility that a product of D-leucine degradation accounts for its effects, although our hippocampal slice data (Fig. 5) suggest that renal and hepatic-derived metabolites are not the underlying mechanisms.
Comparison to other treatments
Kainic acid is a widely-used model of focal-onset seizures, the most common cause of epilepsy in adults (Hauser et al., 1993). The pretreatment of mice with clinically approved drugs diazepam, valproate, ethosuximide, and topiramate decreases the severity of seizures after an intravenous infusion of kainic acid, although results for phenobarbital are mixed (Cramer et al., 1994; Kaminski et al., 2004; Steppuhn and Turski, 1993). In contrast, phenytoin, carbamazepine, and lamotrigine are inactive in this test (Cramer et al., 1994; Hartman et al., 2010; Noh et al., 2003; Samala et al., 2008). However, diazepam administered after onset of seizures (given after 5 min of repetitive seizure activity) stops the progression of i.p. kainic acid-induced status epilepticus (Fritsch et al., 2010), similar to our findings. Thus, D-leucine appears to compare favorably to common clinical antiseizure treatments over the duration of the observation period in the kainic acid test.
Potential mechanisms of action
We found that D-leucine inhibits LTP in the hippocampal CA1 region, with the deviation of fEPSP slopes occurring roughly 30–60 min after stimulation, earlier than reported changes in protein synthesis (Malenka and Bear, 2004). Others reported that induction of LTP is completely blocked in the dentate gyrus in hippocampal slices from rats that had received intrahippocampal L-leucine injections 24 h prior, intended to mimic the high intracerebral levels in patients with maple syrup urine disease (Glaser et al., 2010). In contrast, we found that D-leucine only reduced (but did not block) LTP, and D-leucine did not affect high-K+ bursting frequency, in contrast to GABAergic allosteric potentiators (pentobarbital, benzodiazepines) (Clifford et al., 1982; Korn et al., 1987). The sodium channel blocker phenytoin also does not affect bursting but is ineffective in the 6 Hz test, differentiating it from D-leucine (Barton et al., 2001). These data suggest that D-leucine has a different mechanism of action from other agents that reduce bursting.
Some effective anti-seizure medications (e.g., diazepam) completely inhibit LTP (del Cerro et al., 1992), restricting interpretations of potential physiological mechanisms for seizure prevention (West et al., 2014). One general concern is that a medicine that inhibits LTP may also have an adverse effect on learning and memory. However, discordant effects between LTP and behavior have been noted for another antiseizure medicine, felbamate (Pugliese and Corradetti, 1996), which lowers LTP but does not lead to deficits in learning in rodents (Smith et al., 1994). Thus, the relationship between the LTP effect of D-leucine and learning require further study.
The only other reports showing an in vivo effect of D-leucine suggest an opioid-related mechanism based on evidence that D-leucine inhibits transport of enkephalins across the blood-brain barrier (Banks and Kastin, 1991), and that naloxone substantially reverses these analgesic effects (Ninomiya et al., 1990). However, the role of specific opioid receptors in epilepsy is unclear (Yajima et al., 2000). D-leucine is unlikely to block seizures by this mechanism because the lowest effective dose of D-leucine in our seizure tests is orders of magnitude lower than required for analgesia (Cheng and Pomeranz, 1980). Furthermore, our binding assays failed to detect binding of D-leucine to mu, delta, or kappa opioid receptors. However, because D-leucine is not incorporated through biosynthetic processes into proteins in eukaryotes, we anticipate that D-leucine has a specific receptor. Although not tested here, D-amino acids (including D-leucine) have been reported to bind Tas1R2/R3 G-protein-coupled receptors but a role for these receptors in epilepsy has not been reported (Bassoli et al., 2014; Yarmolinsky et al., 2009).
Based on seizure protection in both chemically-induced and electroshock tests, the data presented here suggest that D-leucine may be a novel treatment for seizures. Surprisingly, D-leucine was effective after the onset of seizures, whereas L-leucine was not. In addition, D-leucine was more potent as an antiseizure drug than in prior studies as an analgesic agent. These findings may open a new area of investigation for the therapeutic use of D-amino acids in neurological disorders.
Supplementary Material
HIGHLIGHTS.
The ketogenic amino acid L-leucine potently suppresses seizures in mouse models
D-leucine is more effective than L-leucine but neither induces ketosis
Only D-leucine works after seizure onset and lowers long-term potentiation
Putative D-leucine receptor is not among panel of 48 neuronal receptors tested
Acknowledgments
We thank Drs. Bryan L. Roth and Xi-Ping Huang at the National Institute of Mental Health Psychoactive Drug Screening Program (NIMH-PDSP) for D-leucine receptor binding studies. We thank Dr. Michael V.L. Bennett for thoughtful comments on the manuscript. This study was supported by K08NS070931 and the Pakula Family (ALH), R01NS083373 and R01NS037402 (JMH) and a generous gift from the Mathias Koch Memorial Fund (CES). For assistance with linear regression analyses, we thank Carol B. Thompson (Johns Hopkins Bloomberg School of Public Health Biostatistics Center), supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health grant 1UL1TR001079). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders And Stroke, National Center for Research Resources, National Center for Advancing Translational Sciences, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
ALH, JMH, and CES conceived and designed experiments; ALH, PS, KO, and CES performed the research; ALH, PS, KO, CES, and JMH analyzed the data; JMH, ALH, and CES prepared the manuscript.
The authors declare no conflict of interest.
REFERENCES
- Banks WA, Kastin AJ. Leucine modulates peptide transport system-1 across the blood-brain barrier at the stereospecific site within the central nervous system. J Pharm Pharmacol. 1991;43:252–254. doi: 10.1111/j.2042-7158.1991.tb06678.x. [DOI] [PubMed] [Google Scholar]
- Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014;24:400–406. doi: 10.1016/j.tcb.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton ME, et al. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–227. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- Bassoli A, et al. The taste of D- and L-amino acids: In vitro binding assays with cloned human bitter (TAS2Rs) and sweet (TAS1R2/TAS1R3) receptors. Food Chem. 2014;150:27–33. doi: 10.1016/j.foodchem.2013.10.106. [DOI] [PubMed] [Google Scholar]
- Besnard J, et al. Automated design of ligands to polypharmacological profiles. Nature. 2012;492:215–220. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixel MG, Hamprecht B. Generation of ketone bodies from leucine by cultured astroglial cells. J Neurochem. 1995;65:2450–2461. doi: 10.1046/j.1471-4159.1995.65062450.x. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, et al. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78:1548–1554. doi: 10.1212/WNL.0b013e3182563b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng RS, Pomeranz B. A combined treatment with D-amino acids and electroacupuncture produces a greater analgesia than either treatment alone; naloxone reverses these effects. Pain. 1980;8:231–236. doi: 10.1016/0304-3959(88)90010-3. [DOI] [PubMed] [Google Scholar]
- Clifford DB, et al. Effect of anticonvulsant drugs on kainic acid-induced epileptiform activity. Exp Neurol. 1982;76:156–167. doi: 10.1016/0014-4886(82)90109-1. [DOI] [PubMed] [Google Scholar]
- Cota D, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Cramer CL, et al. Kainic acid and 4-aminopyridine seizure models in mice: evaluation of efficacy of anti-epileptic agents and calcium antagonists. Life Sci. 1994;54:PL271–PL275. doi: 10.1016/0024-3205(94)00845-0. [DOI] [PubMed] [Google Scholar]
- D'Aniello A, et al. Biological role of D-amino acid oxidase and D-aspartate oxidase. Effects of D-amino acids. J Biol Chem. 1993;268:26941–26949. [PubMed] [Google Scholar]
- del Cerro S, et al. Benzodiazepines block long-term potentiation in slices of hippocampus and piriform cortex. Neuroscience. 1992;49:1–6. doi: 10.1016/0306-4522(92)90071-9. [DOI] [PubMed] [Google Scholar]
- Dufour F, et al. Modulation of pentylenetetrazol-induced seizure activity by branched-chain amino acids and alpha-ketoisocaproate. Brain Res. 1999;815:400–404. doi: 10.1016/s0006-8993(98)01188-3. [DOI] [PubMed] [Google Scholar]
- Ekborg-Ott KH, Armstrong DW. Evaluation of the concentration and enantiomeric purity of selected free amino acids in fermented malt beverages (beers) Chirality. 1996;8:49–57. doi: 10.1002/(SICI)1520-636X(1996)8:1<49::AID-CHIR10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Ercal N, et al. In vitro study of the metabolic effects of D-amino acids. Chirality. 1996;8:24–29. doi: 10.1002/(SICI)1520-636X(1996)8:1<24::AID-CHIR6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Fox HL, et al. Amino acid effects on translational repressor 4E-BP1 are mediated primarily by L-leucine in isolated adipocytes. Am J Physiol. 1998;275:C1232–C1238. doi: 10.1152/ajpcell.1998.275.5.C1232. [DOI] [PubMed] [Google Scholar]
- Freinkel N, et al. Insulin release and phosphate ion efflux from rat pancreatic islets induced by L-leucine and its nonmetabolizable analogue, 2-aminobicyclo[2.2.1]heptane-2-carboxylic acid. Proc Natl Acad Sci U S A. 1976;73:3403–3407. doi: 10.1073/pnas.73.10.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch B, et al. Treatment of early and late kainic acid-induced status epilepticus with the noncompetitive AMPA receptor antagonist GYKI 52466. Epilepsia. 2010;51:108–117. doi: 10.1111/j.1528-1167.2009.02205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein H, et al. Rescue of synaptic plasticity and spatial learning deficits in the hippocampus of Homer1 knockout mice by recombinant Adeno-associated viral gene delivery of Homer1c. Neurobiol Learn Mem. 2012;97:17–29. doi: 10.1016/j.nlm.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser V, et al. The intra-hippocampal leucine administration impairs memory consolidation and LTP generation in rats. Cell Mol Neurobiol. 2010;30:1067–1075. doi: 10.1007/s10571-010-9538-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamase K, et al. Regional distribution and postnatal changes of D-amino acids in rat brain. Biochim Biophys Acta. 1997;1334:214–222. doi: 10.1016/s0304-4165(96)00095-5. [DOI] [PubMed] [Google Scholar]
- Hamase K, et al. Determination of free D-proline and D-leucine in the brains of mutant mice lacking D-amino acid oxidase activity. Anal Biochem. 2001;298:253–258. doi: 10.1006/abio.2001.5382. [DOI] [PubMed] [Google Scholar]
- Hartman AL, et al. The neuropharmacology of the ketogenic diet. Pediatr Neurol. 2007;36:281–292. doi: 10.1016/j.pediatrneurol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, et al. Efficacy of the ketogenic diet in the 6-Hz seizure test. Epilepsia. 2008;49:334–339. doi: 10.1111/j.1528-1167.2007.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, et al. The mTOR Inhibitor Rapamycin Has Limited Acute Anticonvulsant Effects in Mice. Plos One. 2012;7 doi: 10.1371/journal.pone.0045156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, Stafstrom CE. Harnessing the power of metabolism for seizure prevention: focus on dietary treatments. Epilepsy Behav. 2013;26:266–272. doi: 10.1016/j.yebeh.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, Vining EP. Clinical aspects of the ketogenic diet. Epilepsia. 2007;48:31–42. doi: 10.1111/j.1528-1167.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- Hartman AL, et al. Seizure tests distinguish intermittent fasting from the ketogenic diet. Epilepsia. 2010;51:1395–1402. doi: 10.1111/j.1528-1167.2010.02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser WA, et al. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, et al. Topiramate selectively protects against seizures induced by ATPA, a GluR5 kainate receptor agonist. Neuropharmacology. 2004;46:1097–1104. doi: 10.1016/j.neuropharm.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Kobau R, et al. Epilepsy surveillance among adults-19 States, Behavioral Risk Factor Surveillance System, 2005. MMWR Surveill Summ. 2008;57:1–20. [PubMed] [Google Scholar]
- Korn SJ, et al. Epileptiform burst activity induced by potassium in the hippocampus and its regulation by GABA-mediated inhibition. J Neurophysiol. 1987;57:325–340. doi: 10.1152/jn.1987.57.1.325. [DOI] [PubMed] [Google Scholar]
- Kumar A. Long-Term Potentiation at CA3-CA1 Hippocampal Synapses with Special Emphasis on Aging, Disease, and Stress. Front Aging Neurosci. 2011;3:7. doi: 10.3389/fnagi.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Laeger T, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124:3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein DH. Status epilepticus: an overview of the clinical problem. Epilepsia. 1999;40(Suppl 1):S3–S8. doi: 10.1111/j.1528-1157.1999.tb00872.x. discussion S21-2. [DOI] [PubMed] [Google Scholar]
- Mackenzie DY, Woolf LI. Maple syrup urine disease; an inborn error of the metabolism of valine, leucine, and isoleucine associated with gross mental deficiency. Br Med J. 1959;1:90–91. doi: 10.1136/bmj.1.5114.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Masino SA, Rho JM. Mechanisms of Ketogenic Diet Action. In: Noebels JL, et al., editors. Jasper's Basic Mechanisms of the Epilepsies. Bethesda: National Center for Biotechnology Information (US); 2012. [PubMed] [Google Scholar]
- Menkes JH, et al. A new syndrome: progressive familial infantile cerebral dysfunction associated with an unusual urinary substance. Pediatrics. 1954;14:462–467. [PubMed] [Google Scholar]
- Morrison RS, et al. Loss of the p53 tumor suppressor gene protects neurons from kainate-induced cell death. J Neurosci. 1996;16:1337–1345. doi: 10.1523/JNEUROSCI.16-04-01337.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutaguchi Y, et al. Identification, purification, and characterization of a novel amino acid racemase, isoleucine 2-epimerase, from Lactobacillus species. J Bacteriol. 2013;195:5207–5215. doi: 10.1128/JB.00709-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal EG, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, et al. Analgesic effects of D-amino acids in four inbred strains of mice. Comp Biochem Physiol C. 1990;97:341–343. doi: 10.1016/0742-8413(90)90151-x. [DOI] [PubMed] [Google Scholar]
- Noh HS, et al. The protective effect of a ketogenic diet on kainic acid-induced hippocampal cell death in the male ICR mice. Epilepsy Res. 2003;53:119–128. doi: 10.1016/s0920-1211(02)00262-0. [DOI] [PubMed] [Google Scholar]
- Novarino G, et al. Mutations in BCKD-kinase lead to a potentially treatable form of autism with epilepsy. Science. 2012;338:394–397. doi: 10.1126/science.1224631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova KA, Crino PB. The tuberous sclerosis complex. Ann N Y Acad Sci. 2010;1184:87–105. doi: 10.1111/j.1749-6632.2009.05117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne EM, et al. L-Leucine improves the anemia and developmental defects associated with Diamond-Blackfan anemia and del(5q) MDS by activating the mTOR pathway. Blood. 2012;120:2214–2224. doi: 10.1182/blood-2011-10-382986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter WB, et al. Metabolic regulation of neuronal plasticity by the energy sensor AMPK. PLoS One. 2010;5:e8996. doi: 10.1371/journal.pone.0008996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese AM, Corradetti R. Effects of the antiepileptic drug felbamate on long-term potentiation in the CA1 region of rat hippocampal slices. Neurosci Lett. 1996;215:21–24. doi: 10.1016/s0304-3940(96)12948-7. [DOI] [PubMed] [Google Scholar]
- Reaven G, Greenberg RE. Experimental Leucine-Induced Hypoglycemia in Mice. Metabolism. 1965;14:625–631. doi: 10.1016/s0026-0495(65)80025-7. [DOI] [PubMed] [Google Scholar]
- Samala R, et al. Anticonvulsant profile of a balanced ketogenic diet in acute mouse seizure models. Epilepsy Res. 2008;81:119–127. doi: 10.1016/j.eplepsyres.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Sancak Y, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeie B, et al. Effects of valine, leucine, isoleucine, and a balanced amino acid solution on the seizure threshold to picrotoxin in rats. Pharmacol Biochem Behav. 1994;48:101–103. doi: 10.1016/0091-3057(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Smith RD, et al. Felbamate, a novel antiepileptic agent, does not affect cognition in rodents. Behav Pharmacol. 1994;5:365–368. doi: 10.1097/00008877-199406000-00016. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, et al. Anticonvulsant and antiepileptic actions of 2-deoxy-D-glucose in epilepsy models. Ann Neurol. 2009;65:435–447. doi: 10.1002/ana.21603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawski P, et al. Pharmacology of ionotropic glutamate receptors: A structural perspective. Bioorg Med Chem. 2010;18:7759–7772. doi: 10.1016/j.bmc.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Steppuhn KG, Turski L. Modulation of the seizure threshold for excitatory amino acids in mice by antiepileptic drugs and chemoconvulsants. J Pharmacol Exp Ther. 1993;265:1063–1070. [PubMed] [Google Scholar]
- Traynelis SF, Dingledine R. Potassium-induced spontaneous electrographic seizures in the rat hippocampal slice. J Neurophysiol. 1988;59:259–276. doi: 10.1152/jn.1988.59.1.259. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- West PJ, et al. Antiseizure drugs differentially modulate theta-burst induced long-term potentiation in C57BL/6 mice. Epilepsia. 2014;55:214–223. doi: 10.1111/epi.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima Y, et al. Effects of differential modulation of mu-, delta- and kappa-opioid systems on bicuculline-induced convulsions in the mouse. Brain Res. 2000;862:120–126. doi: 10.1016/s0006-8993(00)02096-5. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky DA, et al. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkoff M, et al. Response of brain amino acid metabolism to ketosis. Neurochem Int. 2005;47:119–128. doi: 10.1016/j.neuint.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Zeng LH, et al. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.