Abstract
Background
Disrupted brain connectivity is implicated in the pathophysiology of late-life depression (LLD). There are few studies in this area using resting-state functional magnetic resonance imaging (rs-fMRI). In this pilot case-control study, we compare rs-fMRI data between age-matched depressed and non-depressed older adults.
Methods
Older participants (≥ 55 years) with current major depressive disorder (MDD) were recruited to participate in an ongoing study of LLD, and were compared to the age-matched, non-depressed controls. Rs-fMRI data were collected using a 3-Tesla MRI system. In this study, a data-driven approach was chosen and an independent component analysis (ICA) was performed.
Results
Seventeen subjects with MDD were compared to 31 controls. The depressed group showed increased connectivity in three main networks compared to the controls (p(corr) < 0.05), including connectivity between the default mode network (DMN) and the posterior superior temporal sulcus (pSTS). Increased connectivity was also observed within the visual network in the medial, lateral and ventral regions of the occipital lobes, and within the auditory network throughout the right superior temporal cortex.
Conclusion
This data-driven, pilot study finds patterns of increased connectivity that may be unique to LLD in the DMN, as well as visual and auditory networks. The functional implications of this aberrant connectivity remains to be determined. These findings should be further explored in larger samples.
Keywords: Late-life, geriatric depression, functional connectivity, neuroimaging, fMRI
Introduction
The most recent global burden of disease data identified depressive disorders as a leading contributor to health burden internationally, and these data suggested that major depressive disorder (MDD) was also a contributor to burden allocated to suicide and ischemic heart disease (Ferrari et al., 2013). As the global population ages this century to unprecedented levels (UN, 2013), the rates of late-life depression (LLD) are expected to increase in parallel (Ferrari et al., 2013). This will see major impacts on our communities given the effects of LLD on cognitive, mood and somatic symptoms, as well as general functioning. Given this major burden, a deeper understanding of the neurobiology of LLD is important for the development of novel diagnostic systems and therapies.
Important markers of both structural and functional neuroplasticity in depression come from neuroimaging studies. These markers allow for an in vivo understanding of dysfunctional neuroplasticity processes such as reduced neurogenesis, as well as impaired synaptic plasticity and long-term potentiation (Eyre and Baune, 2012). LLD studies of neuroimaging are suggested to differ from mid-life depression in a number of ways, hence making the LLD-specific research field essential. For example, the Default Mode Network (DMN) demonstrates less functional connectivity with age (Koch et al., 2010; Tomasi and Volkow, 2012). White matter hyperintensities (WMH) are common in LLD, but rare in midlife depression (Hopkins et al., 2006). A higher burden of WMHs are associated with greater limbic activation on emotional reactivity tasks (Aizenstein et al., 2011). The differences between LLD and midlife depression may be explained by mechanistic hypotheses. Taylor et al (Taylor et al., 2013) summarizes two key mechanistic constructs in LLD: the disconnection hypothesis and the hypoperfusion hypothesis. The disconnection hypothesis by Alexopoulos et al (Alexopoulos, 2002) suggests ischemia and white matter pathology may disrupt neural connections among regions modulating mood and cognition. In this model, widespread cerebral WMHs cause focal damage to tracts and circuits. Such focal damage could adversely affect the tract connectivity causing ‘disconnection’ of brain regions. This state is believed to adversely affect the function of connected regions at rest and during cognitive tasks, and may contribute to circuitry alterations mediating symptomatology. The hypoperfusion hypothesis (Taylor et al., 2013) is suggested given the commonly noted vascular dysfunction in LLD and the cerebral blood flow reductions which can alter brain function and contribute to symptomatology (Broadley et al., 2002; Chen et al., 2006; Greenstein et al., 2010; Paranthaman et al., 2010; Rajagopalan et al., 2001). Regional cerebral metabolic activity is tightly correlated with cerebral blood flow, which is regulated by complex interactions between neurons, glia and vasculature (Iadecola, 2004). In late-life, vascular disease disorders such as hypertension, diabetes and atherosclerosis often lead to vascular wall hypertrophy, reduced arterial lumen diameter, arterial stiffness and endothelial cell dysfunction (Dandona et al., 2004; Touyz, 2005).
Both WMH and hypoperfusion may have consequences on brain networks that can be investigated with neuroimaging research. The most research in neuroimaging of LLD surrounds the Default Mode Network (DMN), with the other commonly studied networks including the affective/frontolimbic network, the cognitive control network (CCN) and the corticostriatal network (Alexopoulos et al., 2012; Alexopoulos et al., 2013; Andreescu et al., 2013; Patel et al., 2015; Tadayonnejad and Ajilore, 2014; Tadayonnejad et al., 2014; Yuen et al., 2014). The DMN is a network of regions showing synchronized activity patterns when the brain is at rest, and connectivity is decreased when the mind is engaged on the external environment (Fox and Raichle, 2007; Raichle et al., 2001). The DMN includes areas in the medial prefrontal cortex (mPFC), the posterior cingulate cortex (PCC), the precuneus and the medial temporal lobe (MTL) (Buckner et al., 2008; Yeo et al., 2011). Evidence suggests involvement of the DMN in self-referential processing, including internal monitoring, autobiographical memory retrieval, future planning, and theory of mind (Buckner et al., 2008; Northoff and Bermpohl, 2004; Spreng et al., 2009). Dysfunction in the DMN may occur due to WMHs and hypoperfusion (Tadayonnejad et al., 2014) and may represent an imbalance between control systems involved in negative rumination and preferential internal over external attention, possibly reflecting depressive biases toward internal thoughts at the cost of engagement in the external environment (Andrews-Hanna et al., 2010; Kaiser et al., 2014). Self-referential processing dysfunction may lead to negativity bias pronounced with depression (Andrews-Hanna et al., 2010; Kaiser et al., 2014).
We are aware of 3 studies in LLD exploring the DMN specifically, 2 via seed region analysis (Alexopoulos et al., 2012; Kenny et al., 2010) and 1 by independent components analysis (ICA) (Sexton et al., 2012). ICA is a useful methodology as it provides a data-driven approach to defining resting-state networks. The only ICA study in LLD of which we are aware comes from Sexton et al (Sexton et al., 2012), who explored a cross-sectional, multimodal neuroimaging approach to a mixture of patients with current LLD or past history of LLD. No significant differences in functional connectivity were detected between the current (or past) LLD and age-matched healthy control groups in the DMN, anterior DMN, posterior DMN, cognitive control network (CCN), or affective/frontolimbic network.
Our study is the first to apply the ICA methodology in older adults with a current major depressive episode. We used a cross-sectional analysis of high-resolution rs-fMRI data. We hypothesized that LLD would be associated with aberrant connectivity within the DMN; therefore, this network was targeted in our primary analysis. We also performed exploratory analyses of LLD-related differences in functional connectivity in other resting-state networks to determine whether LLD is associated with broader dysfunction.
Methods
Participants
From November 2013 to December 2014, we recruited 17 older adults (≥ 55 years) to participate in the ongoing study of geriatric depression (NCT01902004), and 31 non-depressed age-matched controls. After describing the details of the study to interested and eligible subjects, written informed consent was obtained in accordance with the procedures set by the UCLA Institutional Review Board (IRB).
Depressed subjects
Inclusion criteria were: 1) current episode of unipolar MDD according to DSM-5 criteria; 2) Hamilton Depression Rating Scale (HDRS-24) score ≥ 16; 3) Mini-Mental State Exam (MMSE) score ≥ 24; and 4) subjective memory complaints. Exclusion criteria were: 1) history of any other psychiatric disorders (other than unipolar MDD); 2) severe or acute unstable medical illness; 3) acute suicidal, violent behavior or history of suicide attempt within the last year; or 4) any other central nervous system diseases. Subjects were free of psychotropic medications for at least two weeks before participating in the study.
Non-depressed subjects
Inclusion criteria were: 1) Mini-Mental State Exam (MMSE) score ≥ 24; 2) subjective memory complaints; 3) no current, or history of, depression. Exclusion criteria were: 1) history of any psychiatric disorders or dementia; 2) severe or acute unstable medical illness; 3) any other central nervous system diseases; 4) no psychotropic medications use.
Clinical Measures
Mood evaluation included the Hamilton Rating Scale of Depression (HDRS-24; (Hamilton, 1960)), the Hamilton Anxiety Scale (HAS; (Hamilton, 1959)). Health functioning, medical and vascular comorbidity were collected using the Stroke Risk Factor Prediction Chart (AHA, 1990); and the Cumulative Illness Rating Scale-Geriatric (CIRS-G; (Miller et al., 1992)). Stress coping/resilience was measured by the Connor-Davidson Resilience scale (CD-RISC) (Connor and Davidson, 2003).
Image acquisition
Functional resting imaging data were collected with a 3T TIM Trio scanner (Siemens AG, Munich & Berlin, Germany). Participants’ heads were positioned comfortably within a 32-channel head coil, and head motion was minimized with firm cushions. We instructed participants to close eyes and stay awake during image acquisition. Resting-state functional images were acquired for 5 minutes and 41 seconds with a multi-band gradient-echo echo-planar imaging (EPI) sequence sensitive to BOLD contrast effects. We acquired 275 contiguous EPI resting-state volumes, and the parameters for functional imaging were repetition time 1.24 seconds, echo time 38.2 ms, flip angle 65°, field of view 21.2 × 21.2 cm2, acquisition matrix 118 × 118, 1.8 mm3 iso-voxel size (no gap), 78 slices, and 6 bands. We also acquired anatomic images with 3D MPRAGE sequence (acquisition matrix 256 × 256 with 1 mm thick contiguous slices) for co-registration with the functional data.
Image analysis
The rs-fMRI images were pre-processed in FSL (FMRIB Software Library (FSL, www.fmrib.ox.ac.uk/fsl) for motion correction, high-pass filter (0.01 Hz), image normalization and 5 mm3 Gaussian spatial smoothing. MELODIC (Multivariate Exploratory Linear Decomposition into Independent Components, a tool of FSL) was used to remove significant head motion, scanner, and physiological artifacts using ICA. The processed functional data from all participants were temporally concatenated to form a 4D data set, which was decomposed into group-level independent components (ICs) using ICA. The MELODIC automated dimensionality estimate was used to determine the number and order of the ICs (Beckmann and Smith, 2004). Each component includes brain structures that share the same temporal pattern of signal after mixture modeling was applied. The dual regression approach was subsequently used to back-reconstruct individual-specific connectivity maps associated with each group-level component, which been shown to be an effective and reliable approach to analyses of rsfMRI data (FMRIB, 2015). This approach yielded 36 ICs; 26 of these overlapped grey matter and were considered biologically plausible. For each of these 26 ICs, we compared functional connectivity between the depression and control groups in analyses restricted (i.e., masked) to voxels having 0.5 or higher probability of inclusion in each group-level IC image (Beckmann and Smith, 2004). One-tailed tests compared depression and control groups in each IC at a single-voxel threshold of z > 1.64, p < 0.05, with age and sex serving as nuisance covariates. Although Bonferroni correction is sometimes suggested to address the inclusion of two one-tailed tests (i.e., depression > controls, depression < controls) as executed per standard procedures in FSL, we chose a more lenient single-voxel threshold (p < 0.05) for these exploratory analyses a priori, with correction for cluster extent to address the multiple comparisons problem using Random Field Theory at p(corr) < 0.05 (Worsley et al., 2004).
Statistical analysis of demographic and clinical data
In addition to the abovementioned imaging analyses, a statistical analysis was performed on the demographic and clinical data obtained from the depressed and non-depressed groups. Data were checked for outliers and normality assumptions. The 2 study groups were compared on demographic characteristics using t tests for continuous variables and χ2 tests for categorical variables. Significance levels were set at 0.05 for demographic, medical and neuropsychological data. Correction for multiple comparisons were not performed as this was an exploratory study (Bender and Lange, 2001).
Results
Baseline characteristics
Seventeen depressed older adult and 31 non-depressed older adult participants were included in this analysis. Clinical and demographic characteristics of the sample are presented in table 1. Compared to non-depressed comparators, the depressed group had greater depressive and lower resilience scores.
Table 1.
Demographic and clinic characteristics for depression and healthy control subjects
| Variables | Non-depressed Group (n=31) | Depressed Group (n=17) | Analysis | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | T test | ||
| Age (yrs) | 67.48 (8.87) | 67.28 (6.64) | T(46) = −.09, p = 0.93 | |
| Education (yrs) | 16.23 (1.56) | 16.33 (2.20) | T(46) =.20, p = 0.84 | |
| HAMD | 3.32 (3.51) | 17.89 (2.89) | T(46) =14.91, p < 0.01* | |
| CVRF | 7.71 (4.55) | 7.50 (3.60) | T(46) =−0.17, p = 0.87 | |
| CIRS total | 2.03 (2.07) | 2.78 (2.42) | T(46) =1.14, p = 0.26 | |
| MMSE | 28.84 (1.13) | 28.28 (1.64) | T(46) =−1.42, p = 0.16 | |
| CD-RISC | 75.26 (11.44) | 59.11 (16.27) | T(46) =−4.07, p < 0.01* | |
|
| ||||
| N (%) | N (%) | Chi-Square (p) | ||
|
| ||||
| Sex | Male | 14 (45) | 5 (29) | |
| Female | 17 (55) | 12 (71) | χ (1) = .66, p = 0.55 | |
|
| ||||
| Race | White | 23 (74) | 13 (76) | |
| Other | 8 (26) | 4 (24) | χ (4) = 2.22, p = 0.70 | |
|
| ||||
| Handedness | Right | 25 (81) | 14 (82) | |
| Left | 6 (19) | 3 (18) | χ (1) = .002, p = 1.00 | |
CD-RISC = The Connor-Davison Resilience Scale 25; HAMD = The Hamilton Depression Rating Scale; CVRF = Cardiovascular Risk Factors; CIRS = Cumulative Illness Rating Scale; MMSE = Mini-Mental State Examination.
p≤0.05
MRI Results
Primary analysis
Default Mode Network
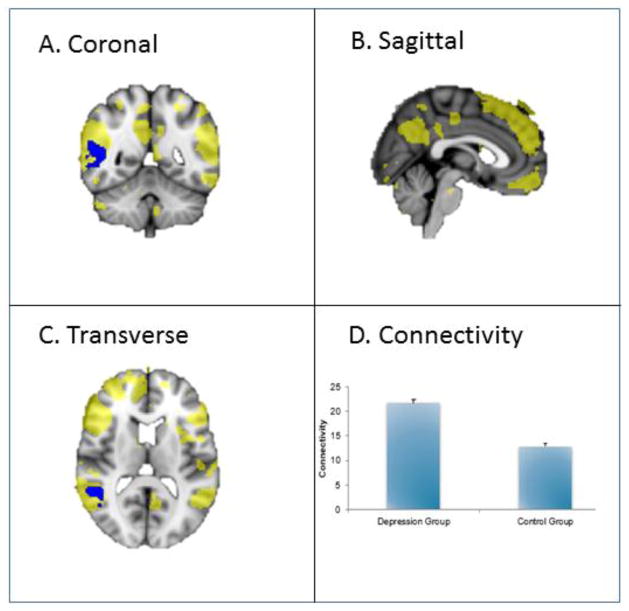
The right posterior superior temporal sulcus (pSTS) showed increased connectivity with the DMN in depressed vs. non-depressed. The DMN recruited the following brain regions: medial and superior frontal gyrus, extending to middle and inferior frontal gyrus, angular gyrus spreading to middle temporal gyrus, and precuneus cortex, right angular gyrus and middle temporal gyrus. See figure 1 for a graphical illustration of these findings.
Figure 1. Default Mode Network engagement in a cross-sectional study of late-life depression as compared to control.
This figure shows increased connectivity in the DMN in depression versus control groups (p < 0.05, corrected). The blue regions indicate higher connectivity in depression versus control group. The yellow region demonstrates the recruited areas of the DMN. See A, B and C. The bar graph (D) indicates mean connectivity for depression and control groups.
Exploratory Analyses of Resting-State Networks
Of the 25 remaining resting-state networks analyzed, 4 networks demonstrated significant differences between groups, including 3 visual networks overlapping occipital cortex and one auditory network overlapping superior temporal cortex. These findings are discussed below.
Visual Networks
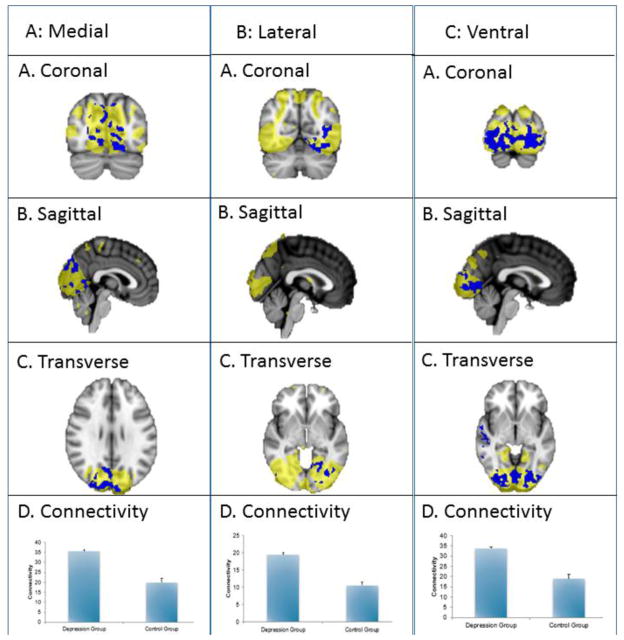
Three visual resting-state networks showed significant differences in functional connectivity between depressed and non-depressed subjects, including medial, lateral, and ventral visual networks. Within the medial visual network, which overlapped with early visual/occipital cortex, diffuse clusters of increased connectivity were found (see figure 2, column A). The second lateral network primarily overlapped posterior and ventral occipital cortex and inferior temporal cortex, and a cluster in the left occipital fusiform gyrus showed increased connectivity with this network (see figure 2, column B). The third network showed increased connectivity diffusely throughout the bilateral occipital cortex and bilateral lingual gyrus. Other areas recruited were in the intracalcarine cortex (see figure 2, column C).
Figure 2. Visual network engagement in a cross-sectional study of late-life depression as compared to control.
This figure shows increased connectivity in the visual networks in depression versus control groups (p < 0.05, corrected). The blue regions indicate higher connectivity in depression versus control group. The yellow region demonstrates the recruited areas. See components A, B and C in each column. The bar graph (D) indicates mean connectivity for depression and control groups.
Auditory Network
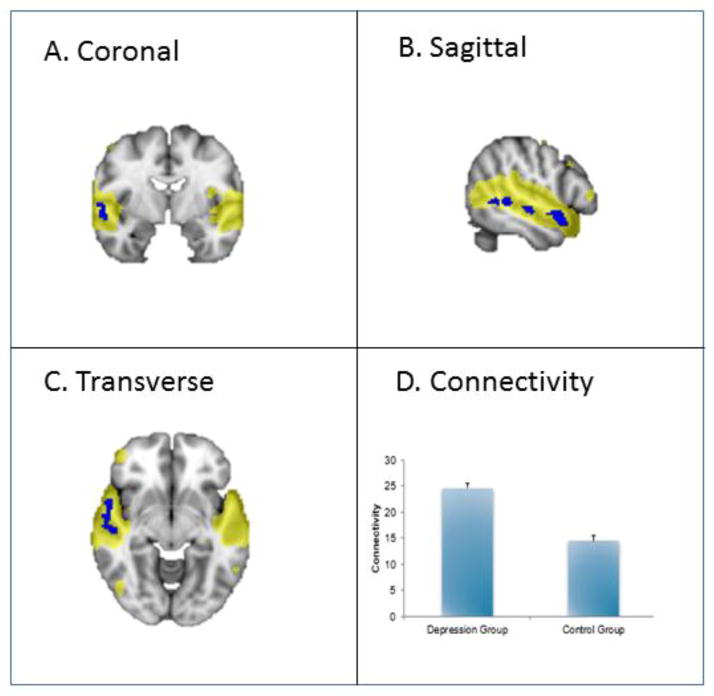
An auditory resting-state network was found engaging bilateral superior temporal cortex. Additionally, multiple clusters of increased connectivity in depressed vs non-depressed groups were found throughout the right middle and superior temporal gyrus, extending to the right temple pole.
Superior Parietal Network
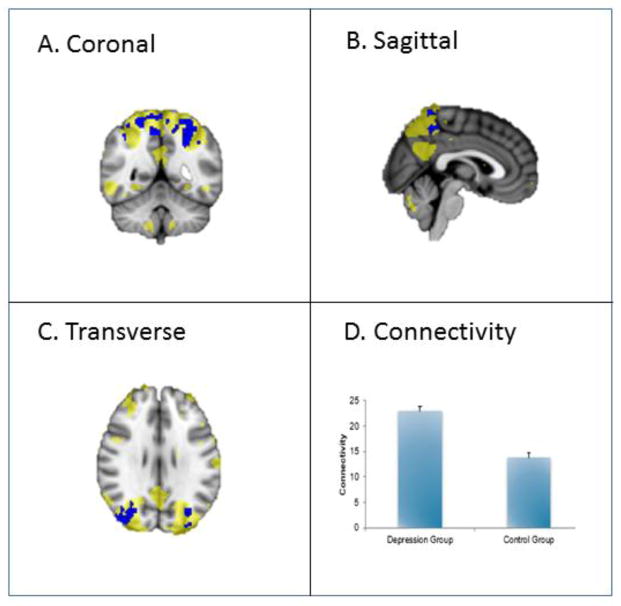
Increased connectivity in this network was found bilaterally throughout the superior divisions of the precuneus cortex in the depressed vs. non-depressed groups. Recruitment of the network was also found in the superior divisions of the bilateral occipital cortex.
Posthoc analyses
No significant differences were found between HAM-D scores and functional connectivity in the above-mentioned brain regions. Further, resilience was not found to significantly correlate with functional connectivity in these brain regions either. Finally, correlations between network connectivity and illness duration in the depressed subjects were not significant.
Discussion
Few studies explore resting-state functional connectivity in LLD populations. Understanding this area is important given the rising burden of disease, the need for more precise diagnostic systems and poor treatment response in LLD. In this study we found increased connectivity in multiple resting-state networks in subjects with LLD as compared with non-depressed older adults – the DMN, as well as visual and auditory networks.
Our study is unique in utilizing an ICA analysis of rs-fMRI data and finding aberrant DMN connectivity in an LLD population. Findings in the DMN show increased connectivity within the right pSTS. Previous research associated the pSTS with an array of tasks relevant to social perception including facial processing, motion processing, the integration of audio and visual information, as well as theory of mind (i.e. deciphering the beliefs and perspectives of others) (Lahnakoski et al., 2012). These types of socially-related functions are characteristically impaired in depression (Hirschfeld et al., 2000). To our knowledge, there are no structural or functional neuroimaging studies exploring the pSTS in either midlife or LLD, making our results novel. Our results showing hyperconnectivity in the pSTS most likely represent dysfunctional social functioning, however this remains to be tested empirically. The rs-fMRI component of the multimodal Sexton et al. (Sexton et al., 2012) study, the only study with which we can truly compare our data, utilized data-driven ICA analysis in 36 mixed recovered or current LLD participants with low severity of depressive symptoms compared to 25 age-matched controls. They did not find any functional connectivity differences between groups. The lack of findings of connectivity from this study may be due to relatively low depression severity. The mean HAMD score in the mixed recovered vs. current LLD group was 4.19 (SD 4.77). Our depressed group, by comparison had mean HAM-D scores of 17.89 (2.89). Another study explored the DMN using seed-based approaches to rs-fMRI analysis. Although seed-based vs. ICA rs-fMRI analyses are difficult to compare directly, some similarities between these studies emerge. The other study by Alexopoulos et al (Alexopoulos et al., 2012) explored rs-fMRI DMN data in LLD. In this study they engaged 26 non-MCI older adults, 16 with MDD (mean age 69 +/− 5.5) and 10 with no MDD (mean age 68.6 +/− 7.0). DMN activity was assessed from a seed placed in the PCC. Interestingly, hyperconnectivity in the DMN, specifically the left precuneus/medial parietal region, subgenual anterior cingulate cortex and lateral parietal regions, distinguished depressed from non-depressed subjects. This again has significant overlap with our study results, suggesting compatible results. In our exploratory analyses, we demonstrated increased connectivity between in the medial and lateral parietal cortices and the superior parietal regions. In the Alexopoulos et al. (Alexopoulos et al., 2012) study, hyperconnectivity within the DMN was positively associated with pessimism. This suggests the DMN may be a useful target which underlies characteristics, like pessimism, which can perpetuate depression and reduce treatment response.
Aberrant connectivity within sensory networks was a prominent feature of this depressed cohort in exploratory analyses, and this finding is novel given few studies examine sensory networks in depression. Three separate components were found in the visual network region, whereby increased connectivity was seen diffusely throughout the occipital lobes. We believe this visual network hyperconnectivity should be considered in terms of functional and neuropathological underpinnings. From a neuropathological perspective, there are a number of hypotheses for this connectivity dysfunction. The occipital lobes have been implicated on a physiological level whereby those with MDD have reduced muscarinic acetylcholine receptor (M)2 receptor binding in this region (Nikolaus et al., 2012), reduced fractional anisotropy (marker of white matter integrity) (Huang et al., 2011) and reduced magnetization transfer ratios (Kumar et al., 2004). A recent study by Maller et al (Maller et al., 2014) explored the phenomenon of occipital bending in adults with MDD (51 depressed, 48 health controls). This is the first study in adult depression and hasn’t been explored in LLD. Occipital bending is a phenomenon of occipital lobe asymmetry within psychiatric populations. This study found the prevalence of occipital bending is three times higher among depressed adults than non-depressed, though its effects on brain function are unclear and may not be restricted to occipital cortex. It is believed incomplete neural pruning may lead to restricted cranial space for brain growth, or ventricular enlargement may exacerbate natural occipital curvature (Maller et al., 2014). From a functional perspective, visual processing deficits may be related to our hyperconnectivity findings and are recognized as a component of LLD (Potter et al., 2013). Visual search may be impaired in LLD. It is an established model for studying how manipulation of task attributes affects speed of performance (Eckstein, 2011). In a study by Potter et al (Potter et al., 2013), visual search performance was compared in 32 LLD and 32 control participants. Data in this study showed specific slowing in the comparison stage of visual search in LLD, rather than in the encoding/response stages. They also found greater overall slowing in LLD during inefficient versus efficient search. There were no group differences on traditional neuropsychological measures of processing speed. This study therefore highlights the importance of specific analysis of processing speed, and that of the visual network. No study has explored fMRI or rs-fMRI correlations to visual search performance in an LLD sample. Our study also found hyperconnectivity in the auditory networks. There are very few studies conducted on the auditory network or auditory processing in LLD, none using neuroimaging. One study found deficits in pre-attentive auditory processing, specifically mismatch negativity, in LLD, measured using event-related potentials, were associated with poorer semantic fluency and high levels of functional disability (Naismith et al., 2012). This impairment was localized to the temporal and not frontocentral area. This is likely relevant to the findings of our study, whereby hyperconnectivity may be related to impaired auditory processing.
It may be possible to incorporating our findings together from DMN, auditory and visual networks. Our speculation is auditory and visual processing dysfunction noted in LLD, and via functional hyperconnectivity, is related to increased connectivity between DMN and the pSTS, perhaps causing impaired facial processing, motion processing, and integration of audio and visual information.
Our study didn’t find any involvement of other major resting state networks such as the salience, cognitive control, and corticostriatal networks. This is likely due to differences in samples and resting state analytical techniques between studies. As mentioned previously, ours is the first study exploring LLD vs. non-depressed subjects with the ICA technique, therefore there are no comparisons to our study to date. When considering the salience network, Yuen et al investigated resting-state connectivity in LLD with right anterior insula seed region (Yuen et al., 2014), and found that LLD subjects (n=16) exhibited increased connectivity in the bilateral precuenus compared with normal controls (n=10). In our data, depressed subjects also showed higher connectivity relative to the healthy controls in the bilateral precuneus for salience network, but this effect did not survive cluster-level correction.
This study has a number of limitations. Our study involved a cross-sectional comparison of two relatively small groups that prevents identification of causation, hence future longitudinal assessments are key. Our use of ICA analysis may be seen as a limitation. The data-driven approach using ICA analysis is useful to understanding patterns in the data without a preexisting model hypothesized. This is as opposed to the model-driven and/or seed-region approach which can also be used whereby the analysis is constrained to brain regions of interest or hypothesis. Our study didn’t correct for multiple comparisons which is therefore an area to improve on in future studies.
Conclusion
This pilot study finds increased connectivity in the DMN, visual and auditory networks in LLD versus non-depressed older adults. Our findings of hyperconnectivity in the DMN, pSTS, visual and auditory networks may suggest dysfunction. Aberrant connectivity within the DMN and pSTS may suggest social functioning and self-referential processing impairment. We speculate pSTS hyperconnectivity may represent dysfunction of integration of visual and auditory inputs, leading to visual and auditory impairments. We recommend further large-scale research to understand the neuropsychological correlation to these findings, the relevance of WMHs and occipital bending.
Figure 3. Auditory network engagement in a cross-sectional study of late-life depression as compared to control.
This figure shows increased connectivity in the auditory network in depression versus control groups (p < 0.05, corrected). The blue regions indicate higher connectivity in depression versus control group. The yellow region demonstrates the recruited areas. See A, B and C. The bar graph (D) indicates mean connectivity for depression and control groups.
Figure 4. Superior parietal and occipital network engagement in a cross-sectional study of late-life depression as compared to control.
This figure shows increased connectivity in the superior temporal and occipital network in depression versus control groups (p < 0.05, corrected). The blue regions indicate higher connectivity in depression versus control group. The yellow region demonstrates the recruited areas. See A, B and C. The bar graph (D) indicates the connectivity for depression and control groups.
Highlights.
Disrupted brain connectivity is implicated in the pathophysiology of late-life depression.
We compare resting state-fMRI data between depressed and non-depressed older adults.
In this study the data-driven approach was chosen and an independent component analysis (ICA) was performed.
This pilot study finds patterns of increased connectivity in the DMN, as well as visual and auditory networks.
Footnotes
Conflict of interest
The authors report no conflict of interest.
Financial disclosures
This work was supported by the NIH grants MH077650, MH86481 and AT003480, and grants from the Forest Research Institute and Actavis Pharmaceutical, and Alzheimer’s Research and Prevention Foundation to Dr. Lavretsky
All other co-authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AHA; Association, A.H, editor. Stroke Risk Factor Predictor Chart. Dallas, TX: 1990. [Google Scholar]
- Aizenstein HJ, Andreescu C, Edelman KL, Cochran JL, Price J, Butters MA, Karp J, Patel M, Reynolds CF., 3rd fMRI correlates of white matter hyperintensities in late-life depression. The American journal of psychiatry. 2011;168:1075–1082. doi: 10.1176/appi.ajp.2011.10060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS. Frontostriatal and limbic dysfunction in late-life depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2002;10:687–695. [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. Journal of affective disorders. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Yuen G, Kanellopoulos D, Seirup JK, Lim KO, Gunning FM. Functional connectivity in apathy of late-life depression: a preliminary study. Journal of affective disorders. 2013;149:398–405. doi: 10.1016/j.jad.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C, Tudorascu DL, Butters MA, Tamburo E, Patel M, Price J, Karp JF, Reynolds CF, 3rd, Aizenstein H. Resting state functional connectivity and treatment response in late-life depression. Psychiatry research. 2013;214:313–321. doi: 10.1016/j.pscychresns.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE transactions on medical imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Bender R, Lange S. Adjusting for multiple testing--when and how? Journal of clinical epidemiology. 2001;54:343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Broadley AJ, Korszun A, Jones CJ, Frenneaux MP. Arterial endothelial function is impaired in treated depression. Heart. 2002;88:521–523. doi: 10.1136/heart.88.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Chen CS, Chen CC, Kuo YT, Chiang IC, Ko CH, Lin HF. Carotid intima-media thickness in late-onset major depressive disorder. International journal of geriatric psychiatry. 2006;21:36–42. doi: 10.1002/gps.1420. [DOI] [PubMed] [Google Scholar]
- Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC) Depression and anxiety. 2003;18:76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- Dandona P, Chaudhuri A, Aljada A. Endothelial dysfunction and hypertension in diabetes mellitus. The Medical clinics of North America. 2004;88:911–931. x–xi. doi: 10.1016/j.mcna.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Eckstein MP. Visual search: a retrospective. Journal of vision. 2011:11. doi: 10.1167/11.5.14. [DOI] [PubMed] [Google Scholar]
- Eyre H, Baune BT. Neuroplastic changes in depression: a role for the immune system. Psychoneuroendocrinology. 2012;37:1397–1416. doi: 10.1016/j.psyneuen.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Vos T, Whiteford HA. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS medicine. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FMRIB. Dual Regression. Analysis Group, F; Oxford, UK: 2015. [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Greenstein AS, Paranthaman R, Burns A, Jackson A, Malik RA, Baldwin RC, Heagerty AM. Cerebrovascular damage in late-life depression is associated with structural and functional abnormalities of subcutaneous small arteries. Hypertension. 2010;56:734–740. doi: 10.1161/HYPERTENSIONAHA.110.152801. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. The British journal of medical psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld RM, Montgomery SA, Keller MB, Kasper S, Schatzberg AF, Moller HJ, Healy D, Baldwin D, Humble M, Versiani M, Montenegro R, Bourgeois M. Social functioning in depression: a review. The Journal of clinical psychiatry. 2000;61:268–275. doi: 10.4088/jcp.v61n0405. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Beck CJ, Burnett DL, Weaver LK, Victoroff J, Bigler ED. Prevalence of white matter hyperintensities in a young healthy population. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2006;16:243–251. doi: 10.1111/j.1552-6569.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- Huang H, Fan X, Williamson DE, Rao U. White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:684–691. doi: 10.1038/npp.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nature reviews Neuroscience. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Spielberg JM, Warren SL, Sutton BP, Miller GA, Heller W, Banich MT. Distracted and down: neural mechanisms of affective interference in subclinical depression. Social cognitive and affective neuroscience. 2014 doi: 10.1093/scan/nsu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny ER, O’Brien JT, Cousins DA, Richardson J, Thomas AJ, Firbank MJ, Blamire AM. Functional connectivity in late-life depression using resting-state functional magnetic resonance imaging. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2010;18:643–651. doi: 10.1097/JGP.0b013e3181cabd0e. [DOI] [PubMed] [Google Scholar]
- Koch W, Teipel S, Mueller S, Buerger K, Bokde AL, Hampel H, Coates U, Reiser M, Meindl T. Effects of aging on default mode network activity in resting state fMRI: does the method of analysis matter? NeuroImage. 2010;51:280–287. doi: 10.1016/j.neuroimage.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Kumar A, Gupta RC, Albert Thomas M, Alger J, Wyckoff N, Hwang S. Biophysical changes in normal-appearing white matter and subcortical nuclei in late-life major depression detected using magnetization transfer. Psychiatry research. 2004;130:131–140. doi: 10.1016/j.pscychresns.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Lahnakoski JM, Glerean E, Salmi J, Jaaskelainen IP, Sams M, Hari R, Nummenmaa L. Naturalistic FMRI mapping reveals superior temporal sulcus as the hub for the distributed brain network for social perception. Frontiers in human neuroscience. 2012;6:233. doi: 10.3389/fnhum.2012.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller JJ, Thomson RH, Rosenfeld JV, Anderson R, Daskalakis ZJ, Fitzgerald PB. Occipital bending in depression. Brain : a journal of neurology. 2014;137:1830–1837. doi: 10.1093/brain/awu072. [DOI] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF., 3rd Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry research. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- Naismith SL, Mowszowski L, Ward PB, Diamond K, Paradise M, Kaur M, Lewis SJ, Hickie IB, Hermens DF. Reduced temporal mismatch negativity in late-life depression: an event-related potential index of cognitive deficit and functional disability? Journal of affective disorders. 2012;138:71–78. doi: 10.1016/j.jad.2011.12.028. [DOI] [PubMed] [Google Scholar]
- Nikolaus S, Hautzel H, Heinzel A, Muller HW. Key players in major and bipolar depression--a retrospective analysis of in vivo imaging studies. Behavioural brain research. 2012;232:358–390. doi: 10.1016/j.bbr.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in cognitive sciences. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Paranthaman R, Greenstein AS, Burns AS, Cruickshank JK, Heagerty AM, Jackson A, Malik RA, Scott ML, Baldwin RC. Vascular function in older adults with depressive disorder. Biological psychiatry. 2010;68:133–139. doi: 10.1016/j.biopsych.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Patel MJ, Andreescu C, Price JC, Edelman KL, Reynolds CF, 3rd, Aizenstein HJ. Machine learning approaches for integrating clinical and imaging features in late-life depression classification and response prediction. International journal of geriatric psychiatry. 2015 doi: 10.1002/gps.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter GG, Madden DJ, Costello MC, Steffens DC. Reduced comparison speed during visual search in late life depression. Journal of clinical and experimental neuropsychology. 2013;35:1060–1070. doi: 10.1080/13803395.2013.856381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E, Pitt B. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. The American journal of cardiology. 2001;88:196–198. A197. doi: 10.1016/s0002-9149(01)01623-x. [DOI] [PubMed] [Google Scholar]
- Sexton CE, Allan CL, Le Masurier M, McDermott LM, Kalu UG, Herrmann LL, Maurer M, Bradley KM, Mackay CE, Ebmeier KP. Magnetic resonance imaging in late-life depression: multimodal examination of network disruption. Archives of general psychiatry. 2012;69:680–689. doi: 10.1001/archgenpsychiatry.2011.1862. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of cognitive neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Tadayonnejad R, Ajilore O. Brain network dysfunction in late-life depression: a literature review. Journal of geriatric psychiatry and neurology. 2014;27:5–12. doi: 10.1177/0891988713516539. [DOI] [PubMed] [Google Scholar]
- Tadayonnejad R, Yang S, Kumar A, Ajilore O. Clinical, cognitive, and functional connectivity correlations of resting-state intrinsic brain activity alterations in unmedicated depression. Journal of affective disorders. 2014;172C:241–250. doi: 10.1016/j.jad.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Molecular psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Aging and functional brain networks. Molecular psychiatry. 2012;17:471–549. 458. doi: 10.1038/mp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touyz RM. Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: role of angiotensin II. Experimental physiology. 2005;90:449–455. doi: 10.1113/expphysiol.2005.030080. [DOI] [PubMed] [Google Scholar]
- UN; Population Division, D.o.E.a.S.A, editor World Population Ageing 2013. 2013. [Google Scholar]
- Worsley KJ, Taylor JE, Tomaiuolo F, Lerch J. Unified univariate and multivariate random field theory. NeuroImage. 2004;23(Suppl 1):S189–195. doi: 10.1016/j.neuroimage.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen GS, Gunning-Dixon FM, Hoptman MJ, AbdelMalak B, McGovern AR, Seirup JK, Alexopoulos GS. The salience network in the apathy of late-life depression. International journal of geriatric psychiatry. 2014;29:1116–1124. doi: 10.1002/gps.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]