Abstract
There is no clear consensus among state newborn screening programs on whether routine second screening of newborns identifies clinically relevant cases of congenital adrenal hyperplasia. This retrospective study evaluated laboratory practices, along with biochemical and medical characteristics of congenital adrenal hyperplasia (CAH) cases (1) detected on the first newborn screen in one-screen compared to two-screen states, and (2) detected on the first versus the second screen in the two-screen states, to determine the effectiveness of a second screen. A total of 374 confirmed cases of CAH from 2 one-screen states and 5 two-screen states were included in this study. Demographic data and diagnostic information on each reported case were collected and analyzed. Additionally, laboratory data, including screening methodologies and algorithms, were evaluated. The one-screen states reported 99 cases of CAH out of 1,740,586 (1 in 17,500) newborns screened: 88 (89%) identified on first screen and 5 (5%) identified on targeted second screen. The two-screen states reported 275 cases of CAH out of 2,629,627 (1 in 9,500) newborns screened: 165 (60%) identified on first screen and 99 (36%) identified on second screen. Using a multivariate model, the only significant predictor of whether a case was identified on the first or second screen in the two-screen states was the type of CAH. Compared with classical salt-wasting CAH, classical simple virilizing and non-classical CAH cases were less likely to be detected on the first versus the second screen. The routine second newborn screen is important for identifying children with CAH, particularly simple virilizing and non-classical forms, which might otherwise not be captured through a single screen.
Keywords: Congenital adrenal hyperplasia, Newborn screening, Genetic testing, 17-Hydroxyprogesterone, Routine second screen
1. Introduction
Nearly all newborns in the United States receive a state-mandated newborn screen, which enables early identification and treatment of disorders that can cause intellectual disability, morbidity, and mortality. In the 1960s when newborn screening (NBS) began, it was typical that the heel stick blood specimens used for screening were collected at 48-96 hours after birth. This practice enabled adequate nutritional intake for detection of metabolic disorders and allowed for the natural rise and fall of analytic marker concentrations that occur during the first day of life. In more recent years, the practice of early hospital discharge of newborns has significantly impacted NBS; many newborns have specimens collected at or before 24 hours of life, and some are even collected before 12 hours of life, leading to an increased chance of missing clinically significant cases [1, 2]. As a response to this concern, the Maternal and Child Health Bureau recommended that (1) the NBS specimen be collected from all newborns as close as possible to the time of discharge from the nursery, and in no case later than 7 days of age; and (2) if the initial specimen is collected before 24 hours of age, a second specimen should be collected before 2 weeks of age [3].
Congenital adrenal hyperplasia (CAH), caused by steroid 21-hydroxylase deficiency, was first proposed for NBS in 1977 because infants with the classical salt-wasting form have impairment of mineralocorticoid and glucocorticoid synthesis, leading to hyponatremic dehydration, shock, and eventually death, if untreated. Early identification and treatment was shown to prevent life-threatening adrenal crisis [4]. There are 2 other milder forms of CAH: classical simple virilizing and non-classical. Patients with classical simple virilizing CAH have ambiguous genitalia due to the exposure to androgens, but do not experience salt-wasting crisis. Patients with the non-classical form of CAH can be largely asymptomatic [4]. The estimated prevalence of the 2 classical forms of CAH in the United States is 1 in 16,000 to 1 in 20,000 [5].
The analytic marker used to screen for CAH cases, 17-hydroxyprogesterone (17-OHP), is typically elevated at birth and declines to stable concentrations by 1 to 3 weeks of age in healthy newborns. In contrast, 17-OHP concentrations in newborns with CAH increase over time after birth [5, 6]. Milder forms of CAH may be missed on an initial screen (false-negative) because of insufficient 17-OHP elevations at the time of collection, which is typically within the first 24 to 48 hours after birth [7-9]. Additionally, low birth weight (BW) and prematurity can contribute to an increased 17-OHP concentration in unaffected newborns, leading to false-positive cases; adjustments to screening cutoff values based on BW and gestational age have been used by NBS laboratories to minimize false-positive rates [10-13]. Given the disease spectrum and the fluctuations of the 17-OHP hormone concentration, especially within the first few days of life, diagnostic accuracy can be challenging.
Newborn screening programs adopt cutoff values for analytic markers that maximize detection of all true cases and minimize the number of false-positive results. The number of false-positive screening results for CAH and other disorders on the NBS panel is a concern to programs because it causes unnecessary testing of newborns, undue parental anxiety, and added costs and strain to the follow-up programs and the medical system [14, 15]. However, a larger concern, and one that is much more difficult to quantify, is false-negative screen results, which can lead to missed cases or delays in identification of newborns with treatable conditions. Most states perform a single screen on term newborns that have a satisfactory specimen collected between 24-48 hours after birth. For newborns that do not meet these criteria, additional screening (targeted second screen) might be recommended, based on the state-specific screening algorithm. To minimize the chance of missing clinically significant disorders on a single screen, 9 states have mandated that a routine second screen be performed on all newborns at 8-14 days of age, and 3 states have a recommended routine second screen that is obtained on ≥85% of newborns in those states. Taken together, approximately 22.5% of all U.S. newborns receive a routine second screen.
It is not uniformly agreed upon that NBS programs should detect all forms of CAH, as opposed to only the severe salt-wasting form. Additionally, evidence has been inconsistent as to the effectiveness of the routine second newborn screen to detect cases of CAH missed by the first screen. In Washington state from 1978-1992, an initial newborn screen failed to detect 21 newborns that were subsequently identified on the second screen, including 2 with CAH (5% of all identified CAH cases) [16]. In Wisconsin, where only 1 newborn screen is performed, data on newborns with false-negative results for CAH from 2000-2003 were analyzed [17]. Eight newborns during this time period were not identified by the newborn screen, and subsequently received a diagnosis of 21-hydroxylase deficiency, although none had the salt-wasting form of CAH; these results suggested that the initial screen successfully identified all newborns with the more severe form of the disorder. A study in Texas (a two-screen state) also reported that the first screen detected newborns with the salt-wasting form of CAH, while the second screen detected primarily newborns with the simple virilizing or non-classical forms of CAH [18]. In a study from Colorado, also a two-screen state, the sensitivity of the first screen was determined to be 71.8% (false-negative rate of 28.2%) for detecting classical CAH, defined as both salt-wasting and simple virilizing forms [19]. Minnesota, a single screen state, reported 4 classical CAH cases (3 simple virilizing and 1 salt-wasting) missed over a 5 year period when using a first tier NBS protocol measuring only 17-OHP concentration in specimens (false-negative rate of 15.4%) [8, 9].
To address the ongoing debate among state NBS programs regarding the utility of the routine second screen to identify clinically relevant cases of CAH missed by the first screen alone, plans for the retrospective study reported here were initiated with support from the Health and Human Services Secretary's Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC). The specific objectives of this study were to examine the effectiveness of a routine second screen for CAH by evaluating laboratory practices along with biochemical and medical characteristics of CAH cases (1) detected in one-screen compared to two-screen states, and (2) detected on the first versus the second screen in the two-screen states.
2. Methods
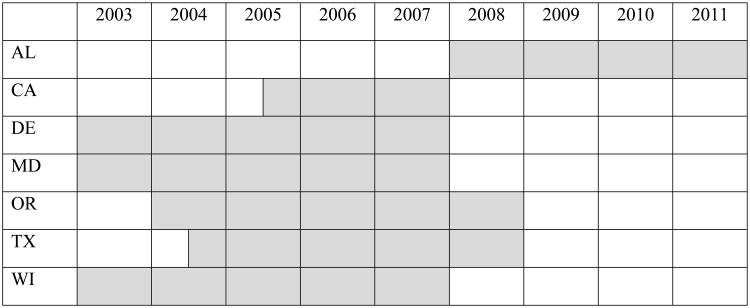
A 5-year retrospective study was planned by representatives from 14 state NBS programs, endocrinologists, and representatives from the Health Resources and Services Administration, the National Newborn Screening and Genetics Resources Center, the Centers for Disease Control and Prevention, the Food and Drug Administration, the Association of Public Health Laboratories (APHL), the Health and Human Service's SACHDNC, Pediatrix Screening, and the CAH Research Education & Support Foundation. Upon execution of the study, data from confirmed cases of CAH were obtained from 2 one-screen states (CA, WI) and 5 two-screen states (AL, DE, MD, OR, TX). Data submitted to the study spanned a 3-5 year period of time (Fig 1). Two-screen states were defined as states with a legally-mandated requirement to routinely collect a second blood specimen from all newborns, or states with a recommended second screen that results in greater than 85% of all newborns receiving a second screen at 8-14 days after birth. All participating states received Institutional Review Board approval for the study.
Fig 1.
Source of congenital adrenal hyperplasia cases by year.
2.1. Screening methodologies and algorithms
All participating states quantified 17‐OHP as the analytic marker for CAH using a dissociation-enhanced lanthanide fluoroimmunoassay. A fixed cutoff based on BW was used to identify newborns at risk for CAH in both of the one-screen states and in 4 of the two-screen states. One of the two-screen states used a floating cutoff that was determined daily based on a percent from the mean 17-OHP value obtained on the normal population and on low BW newborns. All states had a unique algorithm for repeat screening and reporting of abnormal results, although in general, depending upon the 17-OHP concentration, states either recommended repeating the newborn screen (by collecting a second specimen) or performing confirmatory testing and a clinical assessment. The screening algorithm for 1 of the one-screen states included collection of targeted second screens at specific intervals after birth for newborns that had extended hospital stays due to low BW or illness.
2.2 Data elements
Individual-level anonymous data were submitted to the study coordinating center at the APHL on all confirmed cases of CAH. These data elements included newborn demographics (e.g., sex, race/ethnicity) and factors that might affect the screening result (e.g., BW, gestational age, dietary intake, exposure to medications, and whether the newborn received a blood transfusion or was in the neonatal intensive care unit (NICU) prior to collection of the NBS specimen). Laboratory factors were obtained on each case, such as assay specific information (e.g., type of assay used to measure the 17-OHP concentration, the measured 17-OHP concentration, and screening cutoff values) and timing (age of newborn at specimen collection and time from collection to analysis). Clinical characteristics pertaining to case diagnosis were also collected (prenatal treatment with steroids, how newborn sex was determined, clinical manifestations at presentation, degree of virilization, and what treatment, if any, was initiated and when). Newborn screening laboratories provided information on the type of CAH that was diagnosed and whether the newborn was identified on the first screen, routine second screen, targeted second screen (for the one-screen states), or detected clinically. Together, these data enabled a secondary assessment by a metabolic specialist (S.K.S.) to determine if cases were correctly characterized by type of CAH and by identification on the first or second screen. To ensure anonymity of the source for reported case data, APHL hosted the repository for submitted data and entered it into a secure web-based portal.
Due to this being a retrospective study, some data elements were missing or not provided. Therefore, only the following variables could be included in analyses: race/ethnicity (non-Hispanic white (NHW), Hispanic, non-Hispanic black (NHB), Asian/Pacific Islander (A/PI), other), sex (male, female), feeding status at the time of first screen (breastfeeding only, formula only, breastfeeding and formula, other), BW (<2500 g, 2500-3999 g, ≥4000 g), NICU admission at time of first screen (no, yes), blood transfusion prior to first screen (no, yes), age of newborn at first screen specimen collection (0-1 days, ≥2 days), time from first screen specimen collection to laboratory assay completion (0-3 days, 4-5 days, 6-7 days, ≥8 days), initial abnormal screen identifying the potential case (first screen, second screen (for the two-screen states), targeted second screen (for the one-screen states), detected clinically (i.e., no abnormal screening result(s)), and type of CAH (classical salt-wasting, classical simple virilizing, non-classical, not specified).
In addition to the above data on confirmed CAH cases, NBS laboratory programs submitted aggregate data on all newborns screened during the time periods shown in Fig. 1 for the following variables: race/ethnicity, sex, feeding status at the time of first screen, BW, NICU admission at time of first screen, blood transfusion prior to first screen, age of newborn at first screen specimen collection, and time from first screen specimen collection to laboratory assay completion. Additionally, the two-screen states submitted aggregate data for race/ethnicity for all newborns receiving a second screen.
2.3. Data analysis
Univariate logistic regressions were conducted to determine which characteristics were predictive of a case of CAH being detected on the first versus the second screen in the two-screen states. Predictive modeling was performed with statistically significant variables using multivariate logistic regression. Analyses were conducted using SAS 9.3 (SAS Institute, Inc., Cary, NC).
The total number of newborns screened and the number screened with each characteristic were used to calculate estimated frequencies among cases in the one-screen and the two-screen states. Frequencies overall and for each type of CAH were compared between the one- and two-screen states by Z-test for 2 proportions.
3. Results
A total of 374 out of 4,370,213 newborns screened (1 in 11,685) in participating states (AL, CA, DE, MD, OR, TX, WI) were identified with CAH during 3 to 5 year intervals (Fig 1) from 2003-2011. The one-screen states (CA, WI) reported 99 cases of CAH out of 1,740,586 (1 in 17,500) newborns screened; 88 (89%) were identified on the first screen and 5 (5%) were identified on a targeted second screen. The two-screen states (AL, DE, MD, OR, TX) reported 275 cases of CAH out of 2,629,627 (1 in 9,500) newborns screened; 165 (60%) were identified on the first screen and 99 (36%) were identified on the second screen. Only 2 out of the 5 two-screen states reported cases of CAH identified on the second screen, so only cases from those 2 states were included in the first screen versus second screen analyses described in the next section. Although the study did not request comprehensive data on cases not identified by NBS, 10 CAH cases not detected by NBS (delayed diagnosis) were submitted to the study. Of these 10 cases, 6 were from the one-screen states and 4 were from the two-screen states. In the two-screen states, there were 7 cases labeled as “unknown” because an initial specimen submitted for NBS was deemed unsatisfactory by the laboratory; the case was detected on a subsequent screening specimen, but because of the initial unsatisfactory specimen, it is unknown whether the case would have been detected on the first screen. The data summarizing the screen that identified each case are shown in Table 1.
Table 1. Congenital adrenal hyperplasia cases detected on first or second screens.
| Initial Abnormal Screen | One-Screen States N (%) | Two-Screen States N (%) | Total N (%) |
|---|---|---|---|
| First Screen | 88 (89%) | 165 (60%) | 253 (67.6%) |
| Second Screen | NA | 99 (36%) | 99 (26.5%) |
| Targeted Second | 5 (5%) | NA | 5 (1.3%) |
| Unknowna | 0 | 7 (2.5%) | 7 (1.9%) |
| Not Detected by NBS | 6 (6%) | 4 (1.5%) | 10 (2.7%) |
| Total Infants Screened | 1,740,586 | 2,629,627 | 4,370,213 |
| TOTAL | 99 (1/17,500) | 275 (1/9,500) | 374 (1/11,685) |
Unknown cases had an initial specimen submitted for NBS that was deemed unsatisfactory by the laboratory; the case was detected on a subsequent screening specimen, but because of the initial unsatisfactory specimen, it is unknown whether the case would have been detected on the first screen.
N=number; NA=not-applicable; NBS=newborn screening
3.1. First screen versus second screen: two-screen states
In unadjusted univariate analyses, race/ethnicity, type of CAH, NICU admission at time of first screen, and age of newborn at first screen specimen collection were identified as being predictive of cases identified on the first versus the second screen in the two-screen states. Cases identified on the first versus second screen in the two-screen states were less likely to be Hispanic than NHW (odds ratio (OR)=0.44; 95% confidence interval (CI)=0.24-0.80); less likely to have simple virilizing or non-classical CAH than salt-wasting CAH (OR=0.05; 95% CI=0.02-0.14 and OR=0.02; 95% CI=0.01-0.06, respectively); more likely to have been admitted to the NICU at the time of the first screen (OR=10.98; 95% CI=4.11-29.32); and more likely to have had the first specimen collected at ≥48 hours than <48 hours (OR=1.94; 95% CI=1.13-3.33). There was no significant difference in the sex, BW, or feeding status at the time of the first screen for the newborns detected on the first versus the second screen in the two-screen states.
Using a multivariate model, the only significant predictor of whether a case was identified on the first or second screen in the two-screen states was the type of CAH. Compared with salt-wasting CAH, simple virilizing and non-classical CAH cases were less likely to be detected on the first versus the second screen (OR=0.08; 95% CI=0.03-0.22 and OR=0.03; 95% CI=0.01-0.08, respectively). Of all the salt-wasting cases identified in the two-screen states, 6.5% (9/139) were detected on the second screen, while 51% (23/45) and 74% (60/81) of all the simple virilizing and non-classical cases, respectively, were identified on the second screen (Table 2). Among the non-salt-wasting CAH cases detected on the second screen, 19 of the 23 simple virilizing CAH cases (83%) and 20 of the 60 non-classical CAH cases (33%) were reported to have been treated with glucocorticoids and/or mineralocorticoids.
Table 2. Number and detection rate of congenital adrenal hyperplasia cases by disease type.
| CAH Type | One-Screen States | Two-Screen States | ||||
|---|---|---|---|---|---|---|
| Total Cases (Detection rate) | Total Cases (Detection rate) | |||||
| First Screen N (%)c | Targeted 2nd Screen N (%)c | Delayed Dxa or Unknownb N (%)c | First Screen N (%)c | 2nd Screen N (%)c | Delayed Dxa or Unknownb N (%)c | |
| Salt-Wasting | 75 (1/23,208) | 139 (1/18,918) | ||||
| 69 (70%) | 2 (2%) | 4 (4%) | 125 (45%) | 9 (3%) | 5 (2%) | |
| Simple Virilizing | 14 (1/124,328) | 45 (1/58,436) | ||||
| 12 (12%) | 0 (0%) | 2 (2%) | 20 (7%) | 23 (8%) | 2 (0.5%) | |
| Non-classical | 8 (1/217,573) | 81 (1/32,465) | ||||
| 5 (5%) | 3 (3%) | 0 (0%) | 17 (7%) | 60 (22%) | 4 (1.5%) | |
| Not Specified | 2 (2%) | 0 (0%) | 0 (0%) | 3 (1%) | 7 (3%) | 0 (0%) |
| Total CAH Cases | 99 (1/17,500) | 275 (1/9,500) | ||||
| 88 (89%) | 5 (5%) | 6 (6%) | 165 (60%) | 99 (36%) | 11 (4%) | |
Delayed diagnosis were cases not detected by newborn screening
An unknown case had an initial specimen submitted for newborn screening that was deemed unsatisfactory by the laboratory; the case was detected on a subsequent screening specimen, but because of the initial unsatisfactory specimen, it is unknown whether the case would have been detected on the first screen.
Percent indicates the proportion of the total (i.e., percent of 99 cases for the one-screen states and percent of 275 cases for the two-screen states)
CAH=congenital adrenal hyperplasia; N=number; Dx=diagnosis
3.2. One-screen versus two-screen states
The overall detection rate of salt-wasting CAH was statistically similar between the one- and two-screen states (1-in-23,208 vs. 1-in-18,918; p=0.153); however, the detection rates of simple virilizing CAH (1-in-124,328 vs. 1-in-58,436; p=0.012) and non-classical CAH (1-in-217,573 vs. 1-in-32,465; p<0.001) were significantly higher in the two-screen states (Tables 2 and 3).
Table 3. Detection rate of congenital adrenal hyperplasia cases.
| Cases Identified on First Screen and Detection Rates | |||
|---|---|---|---|
| CAH Type | One-Screen States | Two-Screen States | P-valuea |
| Salt-Wasting | 69 1/25,226 |
125 1/21,037 |
0.225 |
| Simple Virilizing | 12 1/145,049 |
20 1/131,481 |
0.788 |
| Non-classical | 5 1/348,117 |
17 1/154,684 |
0.101 |
| Total Number of Cases Identified and Detection Rates | |||
| CAH Type | One-Screen States | Two-Screen States | P-valuea |
| Salt-Wasting | 75 1/23,208 |
139 1/18,918 |
0.153 |
| Simple Virilizing | 14 1/124,328 |
45 1/58,436 |
0.012 |
| Non-classical | 8 1/217,573 |
81 1/32,465 |
<0.001 |
Based on Z-test for 2 proportions; significant p-values are shown in bold font
Comparing the detection rate of CAH cases identified on just the first screen in the one-screen versus the two-screen states (Table 3), there was no statistically significant difference for salt-wasting CAH (1-in-25,226 vs. 1-in-21,037; p=0.225), simple virilizing CAH (1-in-145,049 vs. 1-in-131,481; p=0.788), or non-classical CAH (1-in-348,117 vs. 1-in-154,684; p=0.101). A known factor that can influence the number of cases identified on the first screen, timing of initial specimen collection, was also statistically similar between the one-screen and two-screen states (1.94 and 1.99 days, respectively).
Because there was no significant difference in the total number of newborns with salt-wasting CAH identified in the one- and two-screen states, the data on all salt-wasting CAH cases were combined to evaluate characteristics of the disorder. With respect to race/ethnicity, the highest detection rates of classical salt-wasting CAH were among the NHW (1/17,963) and Hispanic (1/19,231) newborns, while there were lower detection rates among NHB (1/32,039) and A/PI (1/70,402) newborns. The prevalence of certain characteristics among salt-wasting CAH cases was compared to the prevalence among all screened newborns (Table 4). A common characteristic of salt-wasting cases was admission to the NICU before collection of the first newborn screen. Thirty seven percent of all newborns with CAH were in the NICU at the timing of the first screen, compared with only 7.6% of all screened newborns (p<0.001). In addition, newborns in the NICU with salt-wasting CAH were nearly 3 times more likely to be female than male (OR=2.90; 95% CI=1.22-6.92). There was also a statistically smaller percentage of low BW newborns (<2500 g) with salt-wasting CAH (8.6%) compared to the percentage of low BW newborns in the screened population (14.6%). No statistical difference was found in the distributions by sex, BW (greater than 2500 g), or blood transfusion prior to first screen between newborns identified with salt-wasting CAH and the screened population of newborns.
Table 4. Characteristics of classical salt-wasting congenital adrenal hyperplasia cases.
| Characteristic | % Among Salt-Wasting CAH Cases (N) | % Among All Screened Newborns | P-Value |
|---|---|---|---|
| Sex | |||
| Male | 52.3% (112) | 51.1% | 0.725a |
| Female | 47.7% (102) | 48.9% | |
| Birth Weight | |||
| <2500 g | 8.6% (17) | 14.6% | 0.017b |
| 2500-3999 g | 80.7% (161) | 78.3% | 0.239b |
| ≥4000 g | 9.6% (19) | 7.1% | 0.164b |
| NICU Admission Prior to Screening | |||
| Yes | 36.8% (67) | 7.6% | <0.001a |
| No | 63.2% (115) | 92.4% | |
| Blood Transfusion Prior to Screening | |||
| Yes | 1.0 % (2) | 0.3% | 0.067a |
| No | 99.0% (206) | 99.7% |
Based on chi-squared test
Based on Z-test for 2 proportions
Significant p-values are shown in bold font
CAH=congenital adrenal hyperplasia; N=number; NICU=neonatal intensive care unit
4. Discussion
The purpose of this study was to determine the effectiveness of a routine second screen to identify additional, clinically relevant cases of CAH that might be missed on the first screen. It was found that the detection rate for all classical salt-wasting CAH cases was comparable between the one- and two-screen states. However, for simple virilizing and non-classical CAH, the detection rate was significantly higher in the two-screen states, as compared to the one-screen states. Comparing cases of simple virilizing and non-classical CAH detected on the first screen, the detection rates were statistically similar between the one- and two-screen states. Thus, this study showed that a single screen has a comparable detection rate of CAH cases for each type in the one- and two-screen states, but that the second screen has the potential to detect a substantial number of classical simple virilizing and non-classical CAH cases (51% and 74% of cases, respectively, identified on the second screen in the two-screen states). It is important to note that if the two-screen states performed only a single screen using their current algorithms, 36% of all CAH cases would not have been identified, the majority being simple virilizing and non-classical cases, but also including 6.5% of the classical salt-wasting cases. Of the CAH cases identified on the second screen in the two-screen states, all of the salt-wasting cases, 83% of the simple virilizing cases, and 33% of the non-classical cases required medical treatment, suggesting that they were clinically significant.
The overall detection rate of CAH cases (all types) identified in the two-screen states (1 in 9,500) was significantly higher than the rate for all CAH cases identified in the one-screen states (1 in 17,500). This difference is largely attributed to the detection of a significant number of simple virilizing and non-classical CAH cases on the second screen in the two-screen states, which is consistent with previously published papers [8, 17]. However, differences in screening algorithms may have also impacted the overall detection rates. All 7 states used a similar assay to measure 17-OHP and 6 of the 7 states used a fixed cutoff. However, 1 state (a two-screen state) used a floating cutoff algorithm to identify specimens with elevated 17-OHP. The use of a floating cutoff, as opposed to a fixed cutoff, can increase the number of cases referred for additional testing, whether it be a repeat newborn screen or confirmatory testing and a clinical evaluation. Determination of cutoffs and when to refer patients for confirmatory testing and clinical evaluation is unique to each NBS program and largely depends on the willingness of a state to accept a high false-positive rate in order to minimize the chance of a false-negative (missed) case. Neither the false-positive rates nor the positive predictive values were assessed as part of the study. Due to the retrospective nature of this study, differences in screening algorithms and cutoffs could not be controlled. Consequently, the data may be biased by the unique screening practices of each state, especially those states that contributed the largest number of cases. Furthermore, the results and conclusions of this study are not generalizable to all one-screen and two-screen states, but are limited to those that participated in the study.
A total of 10 cases (6 from the one-screen states and 4 from the two-screen states) submitted to our study were not identified by routine screening, suggesting that delayed diagnoses are not unique to either one-screen or two-screen states. Sensitivity of the NBS assay to detect all cases of CAH was not addressed in this analysis, as we did not ascertain all delayed diagnosis cases. However, given these reported cases, as well as literature reports of CAH cases not being detected by NBS [8, 17], healthcare providers in both one- and two-screen states should be cautioned that a newborn with a normal NBS result could still have a CAH diagnosis.
Similar to what has been reported in the literature, this study showed differences in the detection rate of salt-wasting CAH by race/ethnicity [20]. NHW and Hispanic newborns had the highest rates of salt-wasting CAH, while NHB and A/PI newborns had significantly lower rates. Another common characteristic among salt-wasting CAH cases was the higher prevalence of newborns, particularly females, in the NICU at the time of screening, as compared to all screened newborns. This finding is most likely due to female salt-wasters generally presenting with ambiguous genitalia and being admitted to the NICU for medical workup and potentially earlier identification of CAH.
Another challenge for this study was a lack of consistent case classifications. Individual case classifications were determined by the medical providers within each state based on clinical evaluation and confirmatory test results. Although traditionally, CAH has been divided into 3 types – classical salt-wasting, classical simple virilizing, non-classical -- an argument could be made that CAH is a disease continuum reflecting the severity of the enzyme deficiency without clear distinctions. Given the lack of uniformity in case definitions, there may have been variation in the classification of CAH type, leading to a misrepresentation within our dataset. Review of clinical data variables (by author S.K.S.) was intended to minimize misclassification. However, a need exists for consistent use of case definitions within the NBS community.
This large, multi-state study has a number of strengths over previous studies conducted by individual states. Data on a total of 374 CAH cases out of 4,370,213 newborns screened in both the one- and two-screen states were reported and submitted to this study. Evaluation of retrospective data collected on the laboratory practices, along with biochemical and medical characteristics of the CAH cases, allowed for multivariate analysis of factors associated with whether a case is detected on the first or the second screen in the two-screen states. Finally, this is the only comparative study between one-screen and two-screen states.
5. Conclusion
The collected data demonstrates that a single initial screen has a comparable detection rate of each CAH type in both the one-screen and two-screen states that participated in this study. However, the study also provides evidence that the second screen is instrumental in identifying simple virilizing and non-classical cases of CAH, which might otherwise not be identified through the first screen and could result in delayed diagnosis and treatment. Controversy remains, however, as to whether NBS programs should identify only the most serious forms or the full spectrum of CAH. Additionally, both the one- and two-screen states reported cases of CAH not identified through screening, suggesting that delayed diagnoses are not unique to either screening algorithm.
Acknowledgments
We gratefully acknowledge the study data contributors: Gail J. Mick, MD, Danita Rollin, BS, MT(ASCP), and Cindy Ashley, RN, BSN, Alabama Department of Public Health; Fred Lorey, PhD and Hao Tang, PhD, California Department of Public Health; Louis E. Bartoshesky MD, MPH, Delaware Division of Public Health; Fizza Gulamali-Majid, PhD and Hiten Dholakia, Maryland Department of Health and Mental Hygiene; Judi Tuerck, RN, MS and Cheryl Hermerath, MBA, DLM (ASCP), RM (NRM), Northwest Regional Newborn Screening Program; Susan M. Tanksley, PhD, Paula Guerin, RN, BSN, and Art Cowes, BS, Texas Department of State Health Services; and Gary Hoffman, BS and Karen Kennedy-Parker, MT (ASCP), Wisconsin State Laboratory of Hygiene. We thank Joshua Hernandez, MCTS from the Association of Public Health Laboratories for database development and support. We thank Bradford L. Therrell, PhD from the National Newborn Screening and Genetics Resource Center for funding support of the Work Group meeting of stakeholders and his helpful discussions in the planning stage for this study.
Funding Sources: This study was supported by Cooperative Agreement #1U50HK000105 from the Centers for Disease Control and Prevention.
Abbreviations
- NBS
newborn screening
- CAH
congenital adrenal hyperplasia
- 17-OHP
17-hydroxyprogesterone
- BW
birth weight
- SACHDNC
Secretary's Advisory Committee on Heritable Disorders in Newborns and Children
- APHL
Association of Public Health Laboratories
- NICU
neonatal intensive care unit
- NHW
non-Hispanic white
- NHB
non-Hispanic black
- A/PI
Asian/Pacific Islander
- OR
odds ratio
- CI
confidence interval
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Association of Public Health Laboratories.
References
- 1.Lee KS, Perlman M. The impact of early obstetric discharge on newborn health care. Current opinion in pediatrics. 1996;8(2):96–101. doi: 10.1097/00008480-199604000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Therrell BL, Jr, Pass KA, Levy HL. Early hospital discharge: impact on newborn screening. In: Pass KA, Levy HL, editors. Early Hospital Discharge: Impact on Newborn Screening: Proceedings of a Conference Held in Washington, D.C.; March 31-April 1, 1995; Atlanta, GA: Council of Regional Networks for Genetics Services, Emory University School of Medicine; 1996. pp. 75–86. [Google Scholar]
- 3.Newborn screening: A blueprint for the future executive summary: newborn screening task force report. Pediatrics. 2000;106(2 Pt 2):386–388. [PubMed] [Google Scholar]
- 4.White PC. Neonatal screening for congenital adrenal hyperplasia. Nature reviews Endocrinology. 2009;5(9):490–498. doi: 10.1038/nrendo.2009.148. [DOI] [PubMed] [Google Scholar]
- 5.White PC. Optimizing newborn screening for congenital adrenal hyperplasia. The Journal of pediatrics. 2013;163(1):10–12. doi: 10.1016/j.jpeds.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Honour J. Biochemical aspects of congenital adrenal hyperplasia. Journal of inherited metabolic disease. 1986;9(Suppl 1):124–134. doi: 10.1007/BF01800866. [DOI] [PubMed] [Google Scholar]
- 7.Therrell BL, Jr, Berenbaum SA, Manter-Kapanke V, Simmank J, Korman K, Prentice L, Gonzalez J, Gunn S. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics. 1998;101(4 Pt 1):583–590. doi: 10.1542/peds.101.4.583. [DOI] [PubMed] [Google Scholar]
- 8.Sarafoglou K, Banks K, Kyllo J, Pittock S, Thomas W. Cases of congenital adrenal hyperplasia missed by newborn screening in Minnesota. JAMA : the journal of the American Medical Association. 2012;307(22):2371–2374. doi: 10.1001/jama.2012.5281. [DOI] [PubMed] [Google Scholar]
- 9.Sarafoglou K, Banks K, Gaviglio A, Hietala A, McCann M, Thomas W. Comparison of one-tier and two-tier newborn screening metrics for congenital adrenal hyperplasia. Pediatrics. 2012;130(5):e1261–1268. doi: 10.1542/peds.2012-1219. [DOI] [PubMed] [Google Scholar]
- 10.Allen DB, Hoffman GL, Fitzpatrick P, Laessig R, Maby S, Slyper A. Improved precision of newborn screening for congenital adrenal hyperplasia using weight-adjusted criteria for 17-hydroxyprogesterone levels. The Journal of pediatrics. 1997;130(1):128–133. doi: 10.1016/s0022-3476(97)70321-4. [DOI] [PubMed] [Google Scholar]
- 11.Nordenstrom A, Wedell A, Hagenfeldt L, Marcus C, Larsson A. Neonatal screening for congenital adrenal hyperplasia: 17-hydroxyprogesterone levels and CYP21 genotypes in preterm infants. Pediatrics. 2001;108(4):E68. doi: 10.1542/peds.108.4.e68. [DOI] [PubMed] [Google Scholar]
- 12.Gidlof S, Wedell A, Guthenberg C, von Dobeln U, Nordenstrom A. Nationwide Neonatal Screening for Congenital Adrenal Hyperplasia in Sweden: A 26-Year Longitudinal Prospective Population-Based Study. JAMA pediatrics. 2014:1–8. doi: 10.1001/jamapediatrics.2013.5321. [DOI] [PubMed] [Google Scholar]
- 13.van der Kamp HJ, Oudshoorn CG, Elvers BH, van Baarle M, Otten BJ, Wit JM, Verkerk PH. Cutoff levels of 17-alpha-hydroxyprogesterone in neonatal screening for congenital adrenal hyperplasia should be based on gestational age rather than on birth weight. The Journal of clinical endocrinology and metabolism. 2005;90(7):3904–3907. doi: 10.1210/jc.2004-2136. [DOI] [PubMed] [Google Scholar]
- 14.Bodegard G, Fyro K, Larsson A. Psychological reactions in 102 families with a newborn who has a falsely positive screening test for congenital hypothyroidism. Acta paediatrica Scandinavica. 1983;(304):1–21. doi: 10.1111/j.1651-2227.1983.tb09850.x. [DOI] [PubMed] [Google Scholar]
- 15.Kwon C, Farrell PM. The magnitude and challenge of false-positive newborn screening test results. Archives of pediatrics & adolescent medicine. 2000;154(7):714–718. doi: 10.1001/archpedi.154.7.714. [DOI] [PubMed] [Google Scholar]
- 16.Doyle DL, Sanderson M, Bentvelzen J, Fineman RM. Factors which influence the rate of receiving a routine second newborn screening test in Washington State. American journal of medical genetics. 1995;59(4):417–420. doi: 10.1002/ajmg.1320590404. [DOI] [PubMed] [Google Scholar]
- 17.Varness TS, Allen DB, Hoffman GL. Newborn screening for congenital adrenal hyperplasia has reduced sensitivity in girls. The Journal of pediatrics. 2005;147(4):493–498. doi: 10.1016/j.jpeds.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 18.Brosnan CA, Brosnan P, Therrell BL, Slater CH, Swint JM, Annegers JF, Riley WJ. A comparative cost analysis of newborn screening for classic congenital adrenal hyperplasia in Texas. Public Health Rep. 1998;113(2):170–178. [PMC free article] [PubMed] [Google Scholar]
- 19.Chan CL, McFann K, Taylor L, Wright D, Zeitler PS, Barker JM. Congenital adrenal hyperplasia and the second newborn screen. The Journal of pediatrics. 2013;163(1):109–113 e101. doi: 10.1016/j.jpeds.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Pass KA, Neto EC. Update: newborn screening for endocrinopathies. Endocrinology and metabolism clinics of North America. 2009;38(4):827–837. doi: 10.1016/j.ecl.2009.08.005. [DOI] [PubMed] [Google Scholar]