Abstract
Data concerning the link between severity of abdominal aortic calcification (AAC) and fracture risk in postmenopausal women are discordant. This association may vary by skeletal site and duration of follow-up. Our aim was to assess the association between the AAC severity and fracture risk in older women over the short- and long-term. This is a case-cohort study nested in a large multicenter prospective cohort study. The association between AAC and fracture was assessed using Odds Ratios (OR) and 95% confidence intervals (95%CI) for vertebral fractures and using Hazard Risks (HR) and 95%CI for non-vertebral and hip fractures. AAC severity was evaluated from lateral spine radiographs using Kauppila’s semiquantitative score. Severe AAC (AAC score 5+) was associated with higher risk of vertebral fracture during 4 years of follow-up, after adjustment for confounders (age, BMI, walking, smoking, hip bone mineral density, prevalent vertebral fracture, systolic blood pressure, hormone replacement therapy) (OR=2.31, 95%CI: 1.24–4.30, p<0.01). In a similar model, severe AAC was associated with an increased in the hip fracture risk (HR=2.88, 95%CI: 1.00–8.36, p=0.05). AAC was not associated with the risk of any non-vertebral fracture. AAC was not associated with the fracture risk after 15-years of follow-up. In elderly women, severe AAC is associated with higher short-term risk of vertebral and hip fractures, but not with the long-term risk of these fractures. There is no association between AAC and risk of non-vertebral-non-hip fracture in older women. Our findings lend further support to the hypothesis that AAC and skeletal fragility are related.
Keywords: Abdominal aortic calcification, fragility fracture, bone mineral density, elderly women
Introduction
Calcification in the media of the abdominal aorta and osteoporosis share common pathways including genetic factors, hormones, cytokines, transdifferentiation of vascular smooth cells, abnormal mineral metabolism and other factors (1). Several studies have assessed the link between the severity of abdominal aortic calcification (AAC) and fracture risk in postmenopausal women, but their results are discordant. In some, but not all, cross-sectional studies, severe AAC was associated with lower bone mineral density (BMD) and higher odds of vertebral and hip fractures (2–8). In some (8–9), but not all (10–12), prospective studies, severe AAC was associated with higher risk of vertebral and hip fractures.
Several factors contribute to these discrepancies in results. Studies performed in small samples or in populations with few fracture events may lack statistical power (6–7). Fracture risk may be higher only in individuals with severe AAC (4,9) and a low threshold of AAC severity (e.g. present vs absent) may not be specific enough to observe an association (12). Severe AAC may be associated with specific fracture types such as major fragility fractures (e.g. vertebra, hip), but not with other non-vertebral fractures (5,11–12). Thus, the association may be non-significant in younger cohorts composed mainly of subjects with mild AAC and few fracture events. As aortic calcification is a dynamic process that may be related to bone metabolism, AAC may lose its predictive power in studies with long-term follow-up (10).
The strength of the association between AAC severity and risk of incident fracture may depend on the covariates included in the statistical model (5,8,12). Bone fragility and AAC share common risk factors e.g. smoking, low physical activity and diabetes mellitus (13–16). Severe AAC may be associated with lower bone mineral density (BMD) (2,8,17) and prevalent fractures (2–5). Additional studies are needed to examine potential confounders and mediators of the association. Severe AAC is associated with higher mortality and higher risk of cardiovascular events (18–19). Therefore, competing risks of mortality and higher rate of dropout in the subjects with severe AAC may falsely decrease the number of incident fractures ascertained in this group, resulting in null findings. Thus, it is uncertain whether an association between AAC and fracture risks exists in older adults, whether any association varies by skeletal site of fracture, AAC severity, and duration of follow-up.
Therefore, to determine whether severe AAC is independently associated with higher fracture risk of elderly women and to examine whether any association persists or wanes with long-term follow-up, we assessed baseline AAC in a sample of a large cohort of women aged ≥ 65 years who were followed prospectively for up to 20 years to ascertain incident fractures.
Subjects and Methods
Cohort
The Study of Osteoporotic Fractures is a multicenter study of risk factors for fracture in 9,704 non-black community-dwelling women aged 65 years and older recruited from population-based listings at four clinical centers (Portland, OR; Minneapolis, MN; Baltimore, MD; Monangehala Valley, PA) (20). Women unable to walk without the assistance of another person and women with bilateral hip prostheses were excluded. Written informed consent was obtained from all women and the institutional review boards at all participating centers approved the study protocol.
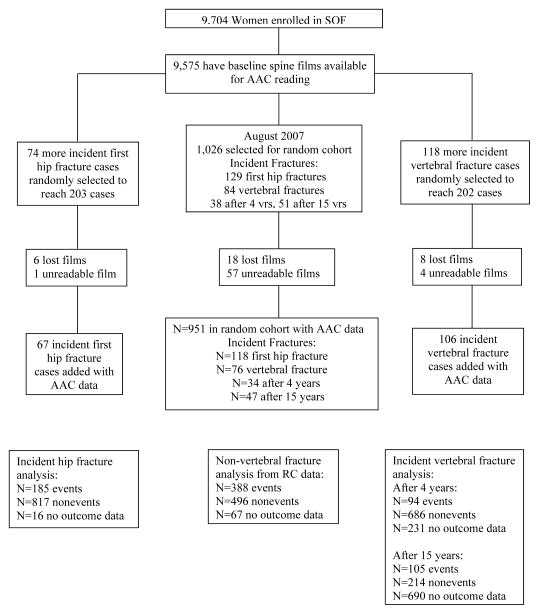
The AAC readings were performed in a subset selected using a nested case-cohort design. Of 9,704 women, 9,575 had baseline lateral spine X-rays available for AAC reading (Fig. 1). Among them, a random cohort of 1,026 women was selected. Within this randomly selected cohort there were 129 women with a first incident hip fracture during 14.2±5.5 years of follow up and 84 women with an incident vertebral fracture identified on a repeat spine film at an average of 3.7±0.4 years or 15.0±0.7 years of follow up. To reach a goal of approximately 200 cases of each type in the analyses, 74 more women with an incident first hip fracture and 118 more women with an incident vertebral fracture were randomly selected from the overall case groups of women with incident hip fracture and women with incident vertebral fracture, respectively. A small number of women in the randomly selected cohort or case groups had lost or unreadable films and were excluded from analyses examining the association of AAC with fracture risk (Fig. 1).
Fig. 1.
Flowchart for the sampling for abdominal aortic calcification readings in the Study of Osteoporotic Fractures
The hip fracture analyses included 185 incident hip fracture cases and 817 women without incident hip fracture. The vertebral fracture analysis had 2 stages. In the first stage we examined the association between AAC identified on baseline spine film and incident vertebral fracture identified on repeat spine film an average of 3.7±0.4 years later (94 incident vertebral fracture cases and 686 women without incident vertebral fracture). In the second stage we examined the association between AAC identified on baseline spine film and incident vertebral fracture identified on repeat spine film and average of 15.0±0.7 years later (105 incident vertebral fracture cases and 214 women without incident vertebral facture). Finally, in an analysis limited to the randomly selected cohort, we examined the association of baseline AAC with risk of incident non-vertebral fracture (388 women with at least one event and 496 women without incident non-vertebral fracture during an average follow-up of 14.2±5.5 years).
Assessment of the abdominal aortic calcification (AAC) score
AAC was assessed using Kauppila’s visual semiquantitative score from the digitized baseline lateral radiographs of the lumbar spine centered at L3 (21–22). Films were read by one reader (JN) blinded to fracture status. Severity of calcific deposits in the anterior and posterior walls of the abdominal aorta adjacent to the first four lumbar vertebrae were assessed in the 8 segments defined using the midpoint of the intervertebral space above and below the vertebrae as boundaries. Severity scores for these segments (0–3) were added to yield an AAC score (0–24). AAC score was categorized into three groups: 0, 1–4, 5+, corresponding to 49, 26 and 25% of the investigated group, respectively. The most common problems causing films to be coded as unreadable included bad quality, insufficient visualization of the area anterior to the spine, or artifacts in the image.
A set of 38 randomly selected films were read by the reader a second time throughout the reading process to assess intra-reader reproducibility. A second expert (DPK) read 241 randomly selected films to assess inter-reader reliability. The reproducibility for AAC score was analyzed as a log-transformed continuous variable using the intraclass correlation coefficient (ICC). The intra-reader ICC was 0.97 (95% confidence interval, 95%CI: 0.95–0.99). The inter-reader ICC was 0.86 (95%CI: 0.83–0.89). The acceptability of the film for AAC readability agreed for 87% of the films for intra-reader reliability and 94% of the films for inter-reader reliability. The weighted kappa score for the selected categorization (0/1–4/5+) for intra-reader reproducibility was 0.89 (95%CI: 0.77–1.00). The weighted kappa score for inter-reader reproducibility was 0.75 (95%CI: 0.69–0.82).
Assessment of the incident fractures
SOF participants were contacted by mail or telephone and asked about incident fractures every four months; follow-up was >95% complete (23). All nonspine fractures were self-reported and confirmed by review of radiological reports; hip fractures were also validated by reviewing preoperative radiographs (23). The participant was also interviewed to determine the circumstances of incident fractures. Participant deaths were identified during tri-annual contacts (next-of-kin interview) and death certificates were obtained. We excluded fractures that occurred because of excessive trauma. For the outcome of first incident hip fracture, those who had a prior hip fracture were not included in the analyses. Follow-up time was 14.2±5.5 years. In a sensitivity analysis, follow-up time was truncated to 5 years to examine associations at a similar follow-up time as the vertebral fracture outcome.
For the assessment of the incident vertebral fractures, lateral radiographs of the thoracic and lumbar spine were obtained at the first, third and eighth visit (24). The average time interval was 3.7 years between the first and the third visits, and 15.0 years between the first and the eighth visits. Incident vertebral fracture was defined as a 20% and 4 mm decrease in the anterior, middle, or posterior heights of any vertebra on the follow-up radiographs compared with the respective heights on the baseline radiograph.
Measurement of bone mineral density
Bone mineral density (g/cm2) of the total hip and its subregions was measured using dual X-ray absorptiometry with Hologic QDR-1000 scanners (Hologic, Inc., Waltham, MA) (25–26). Femoral neck phantom scan results were assessed for quality control. The intra-clinic coefficient of variation (CV) for circulating femoral neck phantom ranged from 0.62% to 1.86%. The inter-clinic CV was 1.2%.
Other covariates
All participants responded to an interviewer-assisted questionnaire. Smoking habits were classified at baseline as current, previous and never. Information was obtained on whether participants walked as a form of exercise and history of falls in past year. Participants were asked about a physician diagnosis of selected medical conditions, e.g. diabetes mellitus. Hormone replacement therapy (HRT) use was categorized as current, previous or never using baseline as reference point (27). Vertebral morphometry was performed on the baseline films by trained technicians (24). Prevalent vertebral fractures were diagnosed using Black’s criterion of 3SD<mean height ratio (28). At baseline blood pressure was measured with a mercury sphygmomanometer with subjects in a supine position after at least a 5-minute rest (29). Body weight and height were measured using a balance beam scale and a Harpenden stadiometer (27). Gait speed was measured in m/s on a standard 6-m walking course.
Statistical analyses
Characteristics for those women selected for the random cohort were compared to those eligible but were not selected were compared using a t-test for continuous normally distributed variables, a Wilcoxon rank-sum test for skewed continuous variables, and a chi-square test for categorical variables. Among the 951 women with technically adequate baseline spine films in the randomly selected cohort, characteristics at the baseline exam were summarized by category of AAC as mean±SD or n (%). Tests for linear trend for these variables across the AAC levels were performed using the Cochran-Armitage trend test for 2-category variables, a Jonckheere-Terpstra test for 3 category variables, and linear regression for continuous variables. Summaries were preformed comparing characteristics of those with incident hip fractures and those with incident vertebral fracture to women in the random cohort without incident fracture of the specific type using analysis of variance for continuous variables and chi-square test for categorical variables.
The association between AAC severity and risk of incident vertebral fracture was examined using logistic regression, and presented as odds ratios with their 95% confidence interval (OR, 95%CI). The dependent variable was modeled as ≥ 1 incident vertebral fracture vs. no incident fracture. The time to first incident hip or non-vertebral fracture was evaluated using Cox proportional hazard models, with results presented as hazard ratio (HR) and 95%CI. The association of AAC and incident nonspine fracture was analyzed among those women in the randomly selected cohort. The association of AAC and incident hip fracture was analyzed among the women in the case-cohort group selected for this outcome, with weighting to accommodate the case-cohort design (30–31).
For all fracture outcomes, AAC was analyzed as a continuous variable (1 unit increase) or categorized as AAC score =0 (reference), 1–4 (mild AAC) and 5+ (at least moderate AAC). Tests for linear trend across the AAC categories were performed by including an ordinal variable (0,1,2) as an independent variable in the models. All models were first adjusted for age, because fracture risk and AAC severity increase with age. Multivariable models included potential confounders of the association between AAC and fracture risk (i.e. age, BMI, prevalent vertebral fracture, smoking, walking for exercise, systolic blood pressure and HRT use as well as, additionally for total hip BMD). Models were further adjusted by prior falls, gait speed, and history of diabetes mellitus. The Fine and Gray model was integrated into multivariable models to calculate HR (95%CI) allowing for competing mortality risks (32). All associations were also examined using a secondary classification of AAC (0, 1–6, 7+) to determine if the results were robust to this more severe definition of AAC. All analyses were performed using the SAS 9.2 software (SAS Institute, Cary, NC, USA) and Stata version 12.1 (StataCorp, College Station, TX, USA).
Results
Randomly selected cohort
Randomly selected women self-reported diabetes slightly more often than those women who were not selected for inclusion in the random selected cohort (9 vs 7%, p=0.02). The two groups did not differ in terms of age, BMI, lifestyle, blood pressure, BMD, gait speed, prior falls or prevalent vertebral fractures. In the randomly selected cohort of 951 women, 25% (n=238) had severe AAC (AAC score 5+) (Table 1). As severity of AAC increased, on average so did age, systolic blood pressure, lumbar spine BMD, prevalence of smoking and existing radiographic vertebral fractures. As the AAC severity increased, the average femoral neck and total hip BMD decreased as did the proportion of women reporting walking for exercise, a history of diabetes mellitus and HRT use.
Table 1.
Characteristics of 951 women in random sample by category of AAC score
| Characteristic | AAC = 0 (49%, n=469) | AAC = 1–4 (26%, n=244) | AAC = 5+ (25%, n=238) | p for trend |
|---|---|---|---|---|
| Age (yrs) | 70.6 ± 4.4 | 72.2 ± 5.3 | 73.6 ± 5.4 | <0.001 |
| Body mass index (kg/m2) | 26.2 ± 4.5 | 27.0 ± 4.4 | 26.4 ± 4.8 | 0.11 |
| Smoking status, n (%) | <0.001 | |||
| Never | 316 (68) | 125 (51) | 118 (50) | |
| Past | 123 (26) | 94 (39) | 95 (40) | |
| Current | 28 (6) | 25 (10) | 25 (10) | |
| Walks for exercise, n (%) | 256 (55) | 125 (51) | 110 (46) | 0.04 |
| Diabetes mellitus, n (%) | 27 (5.78) | 23 (9.47) | 29 (12.18) | 0.003 |
| Current HRT use, n (%) | 0.004 | |||
| Never | 247 (53) | 150 (63) | 151 (64) | |
| Past | 141 (30) | 64 (27) | 62 (26) | |
| Current | 77 (17) | 23 (10) | 22 (9) | |
| Prevalent vertebral fracture, n (%) | 81 (17) | 51 (21) | 46 (19) | 0.41 |
| Systolic blood pressure (mm Hg) | 139 ± 18 | 144 ± 20 | 146 ± 21 | <0.001 |
| Lumbar spine BMD (g/cm2) | 0.84 ± 0.17 | 0.87 ± 0.17 | 0.87 ± 0.15 | 0.05 |
| Total hip BMD (g/cm2) | 0.77 ± 0.12 | 0.77 ± 0.13 | 0.74 ± 0.12 | 0.03 |
| Femoral neck BMD (g/cm2) | 0.66 ± 0.10 | 0.66 ± 0.11 | 0.63 ± 0.09 | 0.02 |
| Gait speed, m/s | 1.05 ± 0.20 | 1.01 ± 0.24 | 0.96 ± 0.22 | <0.001 |
HRT – hormone replacement therapy, BMD – bone mineral density
Incident radiographic vertebral fracture
Women with an incident radiographic vertebral fracture at 4 years of follow-up (n=94) were on average older (74 vs. 71 years), had lower hip BMD (0.68 vs. 0.77 g/cm2) and had more prevalent vertebral fractures (51 vs. 18%) (p<0.001 for all) vs. women who did not sustain vertebral fracture during an average of 4 years of follow-up. After adjustment for age, the odds of incident vertebral fracture at 4 years increased with AAC severity and were twofold higher in women with AAC 5+ vs. women without AAC (Table 2). The association was not altered by adjustment for confounders. Further adjustment for total hip BMD reduced the analytical sample size to 725 women (80 cases). Vertebral fracture risk increased with AAC severity and was higher in women with severe AAC (AAC score 5+ vs. 0: OR=2.31, 95%CI: 1.24–4.30, p<0.01). The relationship between severe AAC (5+ vs. 0) and odds of incident vertebral fracture remained after adjustment for gait speed (OR=2.29, 95%CI: 1.23–4.28, p<0.01), prior falls (OR = 2.23, 95%CI: 1.19–4.17, p<0.05) and diabetes mellitus (OR = 2.23, 95%CI: 1.19–4.17, p<0.05). Comparisons of 149 women having AAC score 7+ with women without AAC provided similar results.
Table 2.
Association between severity of abdominal aortic calcification (AAC) and the risk of vertebral fracture in elderly women from the SOF study
| Adjusted for age | Adjusted for age, BMI, regular walking, smoking habits, HRT prevalent vertebral fracture, systolic blood pressure | Adjusted for age, BMI, regular walking, smoking habits, HRT, prevalent vertebral fracture, systolic blood pressure and total hip BMD | Adjusted for age, BMI, regular walking, smoking habits, HRT, prevalent vertebral fracture, systolic blood pressure, hip BMD and prior falls in past year | Adjusted for age, BMI, regular walking, smoking habits, HRT, prevalent vertebral fracture, systolic blood pressure, hip BMD prior falls in past year, and diabetes mellitus | |
|---|---|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| 4-year follow-up | n/N = 94/780 | n/N = 92/768 | n/N = 80/725 | n/N = 79/722 | n/N = 79/641 |
| AAC score# | 1.06 (1.01 – 1.11)a | 1.05 (0.99 – 1.11) | 1.07 (1.01 – 1.14)a | 1.07 (1.01 – 1.14)a | 1.07 (1.01 – 1.14)a |
| AAC score = 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| AAC score = 1–4 | 1.65 (0.95 – 2.87) | 1.41 (0.78 – 2.56) | 1.49 (0.77 – 2.87) | 1.39 (0.71 – 2.69) | 1.38 (0.71 – 2.69) |
| AAC score = 5+ | 1.95 (1.13 – 3.35)a | 1.94 (1.09 – 3.44)a | 2.31 (1.24 – 4.30)b | 2.23 (1.19 – 4.17)a | 2.23 (1.19 – 4.17)a |
| p for trend | <0.05 | 0.02 | <0.01 | 0.01 | 0.01 |
|
| |||||
| 15-year follow-up | n/N = 105/319 | n/N = 104/310 | n/N = 98/295 | n/N = 98/294 | n/N = 98/196 |
| AAC score# | 0.98 (0.91 – 1.06) | 0.98 (0.90– 1.06) | 0.98 (0.90– 1.06) | 0.98 (0.90– 1.06) | 0.98 (0.90– 1.06) |
| AAC score = 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| AAC score =1–4 | 1.07 (0.60 – 1.89) | 1.13 (0.60 – 2.13) | 1.10 (0.57 – 2.12) | 1.12 (0.58 – 2.17) | 1.10 (0.57 – 2.14) |
| AAC score = 5+ | 0.91 (0.46 – 1.77) | 0.93 (0.45 – 1.90) | 0.93 (0.44 – 1.94) | 0.94 (0.45 – 1.97) | 0.93 (0.45 – 1.96) |
| p for trend | 0.86 | 0.93 | 0.91 | 0.95 | 0.93 |
per 1 unit increase;
p<0.05,
p<0.01;
n - number of women with fractures in the analysis, N – total number of women in the analysis BMI – body mass index, BMD – bone mineral density, HRT – hormone replacement therapy
The 15-year odds of incident radiographic vertebral fracture were assessed in 319 women, including 105 women with incident vertebral fracture. There was no evidence of any association between AAC severity and odds of incident vertebral fracture over the long-term regardless of the model used (age-adjusted, multivariable, multivariable with further adjustment for hip BMD or gait speed) and regardless of how AAC score was expressed in the model (continuous or categories, highest cutoff for AAC score 5 or 7). For example, the odds of vertebral fracture in women with AAC score 5+ was not different from that in women without AAC (OR after adjustment for multiple confounders =0.93, 95%CI: 0.44–1.94).
Hip fracture
On average, the 185 women with a first incident hip fracture were older (73 vs. 72 yr), weighed less, had lower BMI (25 vs. 27 kg/m2) and hip BMD (0.69 vs. 0.77 g/cm2), and were more likely to have an existing radiographic vertebral fractures (30 vs. 16%) (p<0.001 for all) vs. women without an incident hip fracture during the entire follow-up period of 15 years.
Of the 185 hip fracture cases during the long-term follow-up, a total of 32 women experienced a hip fracture during the first 5 years (Table 3). After adjustment for age, 5-year hip fracture risk increased with AAC severity and was more than twofold higher in women with AAC score 5+ vs. 0: HR=2.41, 95%CI: 1.04–5.52, p<0.05). The association persisted despite adjustment for confounders. Further adjustment for total hip BMD reduced the analytical sample to 793 women (22 cases). In this model, severe AAC was associated with higher hip fracture risk, but the association was of marginal significance due to the lower number of cases (p= 0.05). The association remained after adjustment for gait speed (AAC score 5+ vs. 0, HR=2.88, 95%CI: 0.99–8.39, p=0.056), prior fall (OR=2.76, 95%CI: 0.996–7.63, p=0.0509) and diabetes mellitus (OR=2.65, 95%CI: 0.98–7.18, p=0.0559).
Table 3.
Association between severity of abdominal aortic calcification (AAC) and the risk of hip fracture in elderly women from the SOF study
| Adjusted for age | Adjusted for age, BMI, regular walking, smoking habits, HRT, prevalent vertebral fracture, systolic blood pressure | Adjusted for age, BMI, regular walking, smoking habits, HRT, prevalent vertebral fracture, systolic blood pressure and total hip BMD | Adjusted for age, BMI, regular walking, smoking habits, HRT, prevalent vertebral fracture, systolic blood pressure, hip BMD and prior falls in past year | Adjusted for age, BMI, regular walking, smoking habits, HRT, prevalent vertebral fracture, systolic blood pressure, hip BMD prior falls in past year, and diabetes mellitus | |
|---|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | |
| 5-year follow-up | n/N = 32/952 | n/N = 30/935 | n/N = 22/793 | n/N = 22/791 | n/N = 22/769 |
| AAC score# | 1.08 (1.01 – 1.15)a | 1.08 (1.01 – 1.16)a | 1.07 (0.97 – 1.18) | 1.06 (0.96 – 1.17) | 1.06 (0.96 – 1.17) |
| AAC score = 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| AAC score = 1–4 | 1.45 (0.57 – 3.71) | 1.15 (0.40 – 3.37) | 1.41 (0.38 – 5.25) | 1.36 (0.38 – 4.95) | 1.41 (0.38 – 5.18) |
| AAC score = 5+ | 2.41 (1.04 – 5.52)a | 2.42 (0.98 – 5.99)* | 2.88 (1.00 – 8.36)§ | 2.76 (0.996 – 7.63)£ | 2.65 (0.98 – 7.18)**** |
| p for trend | <0.05 | 0.053 | 0.05 | 0.057 | 0.06 |
|
| |||||
| 15-year follow-up | n/N = 185/1002 | n/N = 183/985 | n/N = 155/835 | n/N = 155/833 | n/N = 155/678 |
| AAC score# | 1.02 (0.98 – 1.06) | 1.02 (0.98 – 1.06) | 1.01 (0.96 – 1.05) | 1.01 (0.96 – 1.05) | 1.01 (0.96 – 1.05) |
| AAC score = 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| AAC score = 1–4 | 1.08 (0.74 – 1.58) | 1.04 (0.68 – 1.58) | 0.91 (0.56 – 1.48) | 0.91 (0.56 – 1.47) | 0.93 (0.57 – 1.51) |
| AAC score = 5+ | 1.23 (0.83 – 1.81) | 1.27 (0.86 – 1.88) | 1.22 (0.78 – 1.91) | 1.22 (0.78 – 1.91) | 1.22 (0.78 – 1.91) |
| p for trend | 0.31 | 0.25 | 0.45 | 0.46 | 0.46 |
per 1 unit increase;
p<0.05,
p = 0.055,
p = 0.05,
p = 0.0509,
p=0.0559
n - number of women with fractures in the analysis, N – total number of women in the analysis BMI – body mass index, BMD – bone mineral density, HRT – hormone replacement therapy
When a more stringent threshold of severe AAC was used (7+, n=149), the magnitude of the association was somewhat attenuated and the confidence interval was wider (multivariable model HR= 2.14, 95%CI: 0.63–7.24, p=0.22).
During the 15-year follow-up, 185 women experienced a hip fracture. There was no evidence of a long-term association between AAC severity and hip fracture risk regardless of the model examined (age-adjusted, multivariable, multivariable with adjustment for hip BMD or gait speed) or how AAC score was expressed in the model. For example, the long-term risk of hip fracture in women with AAC=5+ did not differ from that in women without AAC (HR after adjustment for multiple confounders =1.22, 95%CI: 0.78–1.91). In models accounting for the competing risk of mortality, findings regarding shorter term and long-term associations of AAC with hip fracture risk were similar to those of the primary analysis.
Non-vertebral fracture
The group with non-vertebral fractures includes women with hip fracture. AAC severity was not associated with risk of any non-vertebral fracture irrespective of the follow-up duration (5 or 15 years), how AAC was expressed (continuous or categorical variable), what cutoff was used to define the high score category (5+ or 7+) and which variables were used for adjustment (age, multivariable, multivariable adjusted for hip BMD or gait speed) (Table 4). Accounting for the competing risk of mortality did not change these results.
Table 4.
Association between severity of abdominal aortic calcification (AAC) and the risk of non-vertebral fracture (including hip fracture) in elderly women from the SOF study
| Adjusted for age | Adjusted for age, BMI, regular walking, smoking habits, HRT, prevalent vertebral fracture, systolic blood pressure | Adjusted for age, BMI, regular walking, smoking habits, HRT, prevalent vertebral fracture, systolic blood pressure and total hip BMD | Adjusted for age, BMI, regular walking, smoking habits, HRT, prevalent vertebral fracture, systolic blood pressure, hip BMD and prior falls in past year | Adjusted for age, BMI, regular walking, smoking habits, HRT, prevalent vertebral fracture, systolic blood pressure, hip BMD prior falls in past year, and diabetes mellitus | |
|---|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | |
| 5-year follow-up | n/N = 147/884 | n/N = 144/869 | n/N = 122/736 | n/N = 121/734 | n/N = 121/610 |
| AAC score# | 1.01 (0.97 – 1.05) | 1.00 (0.96 – 1.04) | 0.99 (0.95 – 1.04) | 0.99 (0.94 – 1.04) | 0.99 (0.94 – 1.04) |
| AAC score = 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| AAC score = 1–4 | 1.44 (0.98 – 2.12) | 1.32 (0.88 – 1.98) | 1.23 (0.79 – 1.93) | 1.24 (0.79 – 1.94) | 1.25 (0.80 – 1.95) |
| AAC score = 5+ | 1.22 (0.81 – 1.84) | 1.12 (0.73 – 1.72) | 1.00 (0.62 – 1.60) | 0.97 (0.61 – 1.56) | 0.95 (0.59 – 1.52) |
| p for trend | 0.26 | 0.54 | 0.96 | 0.97 | 0.89 |
|
| |||||
| 15-year follow-up | n/N = 388/884 | n/N = 384/869 | n/N = 338/736 | n/N = 337/734 | n/N = 336/731 |
| AAC score# | 0.99 (0.97 – 1.02) | 0.99 (0.96 – 1.02) | 0.98 (0.95 – 1.01) | 0.98 (0.95 – 1.01) | 0.98 (0.95 – 1.01) |
| AAC score = 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| AAC score = 1–4 | 1.16 (0.92 – 1.48) | 1.13 (0.88 – 1.45) | 1.05 (0.80 – 1.37) | 1.07 (0.82 – 1.41) | 1.09 (0.83 – 1.43) |
| AAC score = 5+ | 1.07 (0.83 – 1.38) | 1.03 (0.79 – 1.34) | 0.98 (0.74 – 1.30) | 0.99 (0.74 – 1.31) | 0.98 (0.74 – 1.30) |
| p for trend | 0.50 | 0.73 | 0.94 | 0.99 | 0.96 |
per 1 unit increase;
BMI – body mass index, BMD – bone mineral density, HRT – hormone replacement therapy n - number of women with fractures in the analysis, N – total number of women in the analysis
Discussion
In this case-cohort analysis nested in a large cohort of elderly women, severe AAC was associated with higher risks of incident radiographic vertebral fracture and hip fracture (but not any non-vertebral fracture) during a follow-up period of 4 to 5 years. However, associations of AAC with risks of vertebral and hip fractures waned with increasing duration of follow-up and the waning of the association at 15 years of follow-up was not accounted for by the higher competing risk of death among women with severe AAC.
Our finding that severe AAC is associated with higher risk of hip fracture, but not with the risk of other non-vertebral fracture confirms previous results. In 1471 postmenopausal women, AAC was associated with higher incidence of hip fracture, but not with the incidence of “total” fracture (12). In 327 postmenopausal women consulted for osteoporosis, presence of AAC was associated with “vertebral or hip fracture” (adjusted for multiple confounders), but not with the wrist fracture or “other fractures” (5). In 5400 elderly men, severe AAC was associated with higher risk of hip fracture, but not with the risk of nonspine nonhip fracture (33). In a cross-sectional study performed in 624 men and women aged 50–89, severe AAC was associated with higher odds of vertebral fracture, but not with the nonspine fracture (34). Few studies found significant link between severe AAC and overall fracture risk (9,35). These associations were significant only for individuals with severe AAC (>6) and hip fractures comprised a major proportion of the total fracture groups (e.g. in the study of Zhou et al., 50 out of 94 women with nonspine fractures sustained hip fracture) (9).
Vertebral and hip fractures are fragility fractures and poor bone strength is their major determinant. Other non-spine fractures are associated with other risk factors, e.g. fall, trauma (36–37). The association of severe AAC with hip and vertebral fractures was significant after adjustment for BMD similarly to previous data (8–9,33,35) indicating that the link between AAC and fracture risk is not mediated by BMD. Of note, in older subjects, severe AAC was associated with lower trabecular, but not cortical, density at the spine and proximal femur (3,38). As vertebral bodies consist mainly of trabecular bone and long bones consist mainly of cortical bone, different links of AAC with density in both compartments may contribute to the differences which we found. However, the associations of severe AAC with trabecular density are not consistent (17,39–40). Fracture risk is higher in women with higher levels of bone turnover markers (BTM) (41). Women with AAC had low BTM levels (3), but data on this topic are scarce. Severe AAC may obstruct lumbar arteries originating from the aortic wall and supplying lumbar vertebrae with nutrients and oxygen (42). However, the adjustment for poor blood flow has no impact on the link between AAC and fracture (33). Subjects with severe AAC may have more and more dangerous falls (e.g. due to lower muscle strength and poor protective reflextes). However, this association remained significant after adjustment for prior falls and gait speed (9,33,35). In addition, severe AAC did not predict other fractures of the lower limbs (33). Of note, adjustment for diabetes mellitus had no impact on the link between severe AAC and fracture risk, although diabetic patients may have poor bone microarchitecture and higher risk of fall (43–44). Thus, the mechanism of the preferential association of severe AAC with major fragility fractures remains to be elucidated.
Several potential mechanisms may link AAC and fracture risk. Osteoprotegerin (OPG) knockout is characterized by osteoporosis and vascular calcification (45). Inflammatory status is associated with higher risk of fracture and severe AAC (46–47). These results are consistent with our findings; however, it is not clear how to account for them in the statistical analyses, e.g. serum OPG levels may not necessarily reflect its tissular levels.
The association between severe AAC and fracture risk was stronger in short-term follow-up (4–5 years) than in the 15-year follow-up, even after accounting for the competing risk of death and despite higher number of incident fractures during the longer follow-up. Previously, severe AAC predicted fracture in the follow-ups of less than 8 years in women (8–9) and of less than 11 years in men (33,35), but not in a longer follow-up (10).
Vascular calcification is a dynamic process. Its progression has its own variability and its own determinants (2,8,34,48–50). In postmenopausal women, more rapid AAC progression is associated with greater bone loss (2,8,34,49), whereas greater bone loss is associated with higher fracture risk (51). However, the associations between AAC progression and bone loss were weak with high residual variability and their strength of varied according to skeletal site (8,34). Thus, a single assessment of AAC severity may not capture its long-term progression over the subsequent 15 years. This progression may depend on the factors which appear after baseline such as diseases (diabetes, kidney disease) or treatment (statins). In parallel, loss of bone is shaped by its specific determinants. Thus, AAC may predict fracture during the first years of follow-up, when bone status is still determined by the known factors (8–9). Later on, predictive value of AAC wanes with increasing duration of the follow-up because bone and AAC are influenced by their specific, new and unknown determinants or by the unpredictable changes in the previously existing factors. Consequently, AAC are not predictive of fracture in very long follow-ups, even after accounting for the competing risk of death (10).
Our data suggest that only severe and not mild AAC are associated with higher risk of hip and vertebral fracture. These data are consistent with the previous studies. In the cross-sectional studies, significantly higher odds of vertebral fractures were associated with severe AAC, but not with mild AAC (34,52). In the prospective studies higher risk of fracture was found only in individuals with severe AAC regardless of the sex, population and site of fracture (9,33,35). As mentioned above, severe AAC was more strongly associated with more severe types of fracture, e.g. with hip fracture and not with other non-vertebral non-hip fracture. Similarly, there was a positive association of AAC with the number and the severity of vertebral fractures categorized using Genant’s semi-quantitative score (53–54).
As severe AAC is associated with higher morbidity and mortality (18–19), severe AAC may reflect poor health status and frailty accompanied by deterioration in bone strength. AAC severity and fracture risk increase with age and share common risk factors (13–16). Subjects with severe AAC may have lower BMD (2,8–9,17,33,35). However, in this and previous studies, the association between severe AAC and fracture risk remained significant after adjustment for age, BMD, co-morbidities and other risk factors (3,8–9,33,52,54).
The strengths of our study include the prospective design, a high number of fully adjudicated incident fractures and adjustment for multiple confounders. There are also limitations. The SOF cohort consists of home-dwelling elderly women and its results may not be extrapolated on other populations. Assessment of AAC using the semi-quantitative score may be less accurate than computed tomography. Self-reported incident fractures were confirmed, but false negatives are possible. We considered the effects of multiple potential confounders on the associations between AAC and fracture risk, but the residual confounding remains possible in this observational study. We cannot adjust the models for creatinine, bone turnover markers or vitamin D because few women selected for this study had measurements. Of note, we have previously shown that the impact of adjustment for kidney function on the strength of the association between AAC and fracture risk is small in magnitude (33).
In summary, more severe AAC in elderly women is associated with more than twofold higher short-term risk of vertebral and hip fractures and the association is not explained by lower BMD among those with greater AAC. However, the associations between AAC and risk of these fragility fractures wane with increasing follow-up and the lack of long term associations is not explained by a higher competing risk of death among women with more severe AAC. There is no evidence of a short-term or long-term association between AAC and risk of any non-vertebral fracture in older women. The higher short-term risk of fragility fracture in women with severe AAC strengthens the hypothesis of the link between vascular calcification and fracture risk. Further studies are needed to elucidate the mechanisms underlying the associations between AAC and risk of fracture.
Highlights.
In older women severe abdominal aortic calcification (AAC) is associated with higher risk of hip and spine fracture during 4–5 years of follow-up.
Severe AAC was not associated with the risk of non-vertebral-non-hip fracture in women.
In elderly women, severe AAC is associated with higher risk of major osteoporotic fractures during a short-term follow-up (4–5 years), but not during a long-term follow-up (15 years).
Acknowledgments
The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01AR35584, R01 AG005394, R01 AG027574, R01 AG027576, and R01 AR41398.
The Authors thank Dr Jesse Nandhavan for the assessment of aortic calcification.
Footnotes
Conflict of interest: All the authors declare that they have no conflict of interest as concerns this manuscript.
References
- 1.Persy V, D'Haese P. Vascular calcification and bone disease: the calcification paradox. Trends Mol Med. 2009;15:405–16. doi: 10.1016/j.molmed.2009.07.001. doi:0.1016/j.molmed.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89:4246–53. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 3.Kim KJ, Kim KM, Park KH, Choi HS, Rhee Y, Lee YH, Cha BS, Kim MJ, Oh SM, Brown JK, Lim SK. Aortic calcification and bone metabolism: the relationship between aortic calcification, BMD, vertebral fracture, 25-hydroxyvitamin D, and osteocalcin. Calcif Tissue Int. 2012;91:370–8. doi: 10.1007/s00223-012-9642-1. [DOI] [PubMed] [Google Scholar]
- 4.El Maghraoui A, Rezqi A, Mounach A, Achemlal L, Bezza A, Dehhaoui M, Ghozlani I. Vertebral fractures and abdominal aortic calcification in postmenopausal women. A cohort study. Bone. 2013;56:213–9. doi: 10.1016/j.bone.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Rajzbaum G, Roger VL, Bézie Y, Chauffert M, Bréville P, Roux F, Safar ME, Blacher J. French women, fractures and aortic calcifications. J Intern Med. 2005;257:117–9. doi: 10.1111/j.1365-2796.2004.01430.x. [DOI] [PubMed] [Google Scholar]
- 6.Frye MA, Melton LJ, 3rd, Bryant SC, Fitzpatrick LA, Wahner HW, Schwartz RS, Riggs BL. Osteoporosis and calcification of the aorta. Bone Miner. 1992;19:185–94. doi: 10.1016/0169-6009(92)90925-4. [DOI] [PubMed] [Google Scholar]
- 7.Simon SP, Fodor D, Muntean L, Poanta L, Cristea P, Rednic S. Bone mineral density, vertebral fractures and body mass index in postmenopausal women with abdominal aortic calcification. Endocr Res. 2014;39:1–6. doi: 10.3109/07435800.2013.794425. [DOI] [PubMed] [Google Scholar]
- 8.Bagger YZ, Tankó LB, Alexandersen P, Qin G, Christiansen C. Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. J Intern Med. 2006;259:598–605. doi: 10.1111/j.1365-2796.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhou R, Zhou H, Cui M, Chen L, Xu J. The association between aortic calcification and fracture risk in postmenopausal women in China: the prospective Chongqing osteoporosis study. PLoS One. 2014;9(5):e93882. doi: 10.1371/journal.pone.0093882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samelson EJ, Cupples LA, Broe KE, Hannan MT, O'Donnell CJ, Kiel DP. Vascular calcification in middle age and long-term risk of hip fracture: the Framingham Study. J Bone Miner Res. 2007;22:1449–54. doi: 10.1359/jbmr.070519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flipon E, Liabeuf S, Fardellone P, Mentaverri R, Ryckelynck T, Grados F, Kamel S, Massy ZA, Dargent-Molina P, Brazier M. Is vascular calcification associated with bone mineral density and osteoporotic fractures in ambulatory, elderly women? Osteoporos Int. 2012;23:1533–9. doi: 10.1007/s00198-011-1762-3. [DOI] [PubMed] [Google Scholar]
- 12.Wang TK, Bolland MJ, van Pelt NC, Horne AM, Mason BH, Ames RW, Grey AB, Ruygrok PN, Gamble GD, Reid IR. Relationships between vascular calcification, calcium metabolism, bone density, and fractures. J Bone Miner Res. 2010;25:2777–85. doi: 10.1002/jbmr.183. [DOI] [PubMed] [Google Scholar]
- 13.Kim ED, Kim JS, Kim SS, Jung JG, Yun SJ, Kim JY, Ryu JS. Association of abdominal aortic calcification with lifestyle and risk factors of cardiovascular disease. Korean J Fam Med. 2013;34:213–20. doi: 10.4082/kjfm.2013.34.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sennerby U, Farahmand B, Ahlbom A, Ljunghall S, Michaëlsson K. Cardiovascular diseases and future risk of hip fracture in women. Osteoporos Int. 2007;18:1355–62. doi: 10.1007/s00198-007-0386-0. [DOI] [PubMed] [Google Scholar]
- 15.Feskanich D, Willett W, Colditz G. Walking and leisure-time activity and risk of hip fracture in postmenopausal women. JAMA. 2002;288:2300–6. doi: 10.1001/jama.288.18.2300. [DOI] [PubMed] [Google Scholar]
- 16.Jutberger H, Lorentzon M, Barrett-Connor E, Johansson H, Kanis JA, Ljunggren O, Karlsson MK, Rosengren BE, Redlund-Johnell I, Orwoll E, Ohlsson C, Mellström D. Smoking predicts incident fractures in elderly men: Mr OS Sweden. J Bone Miner Res. 2010;25:1010–6. doi: 10.1359/jbmr.091112. [DOI] [PubMed] [Google Scholar]
- 17.Farhat GN, Cauley JA, Matthews KA, Newman AB, Johnston J, Mackey R, Edmundowicz D, Sutton-Tyrrell K. Volumetric BMD and vascular calcification in middle-aged women: the Study of Women's Health Across the Nation. J Bone Miner Res. 2006;21:1839–46. doi: 10.1359/jbmr.060903. [DOI] [PubMed] [Google Scholar]
- 18.Bastos Gonçalves F, Voûte MT, Hoeks SE, Chonchol MB, Boersma EE, Stolker RJ, Verhagen HJ. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta-analysis. Heart. 2012;98:988–94. doi: 10.1136/heartjnl-2011-301464. [DOI] [PubMed] [Google Scholar]
- 19.Wilson PW, Kauppila LI, O'Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–34. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 20.Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, Mascioli SR, Scott JC, Seeley DG, Steiger P, Vogt TM. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990;263:665–8. [PubMed] [Google Scholar]
- 21.Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132:245–50. doi: 10.1016/s0021-9150(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 22.Ettinger B, Black DM, Nevitt MC, Rundle AC, Cauley JA, Cummings SR, Genant HK. Contribution of vertebral deformities to chronic back pain and disability. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1992;7:449–56. doi: 10.1002/jbmr.5650070413. [DOI] [PubMed] [Google Scholar]
- 23.Nevitt MC, Cummings SR, Browner WS, et al. The accuracy of self-report of fractures in elderly women: evidence from a prospective study. Am J Epidemiol. 1992;135:490–499. doi: 10.1093/oxfordjournals.aje.a116315. [DOI] [PubMed] [Google Scholar]
- 24.Schousboe JT, Fink HA, Taylor BC, Stone KL, Hillier TA, Nevitt MC, Ensrud KE. Association between self-reported prior wrist fractures and risk of subsequent hip and radiographic vertebral fractures in older women: a prospective study. J Bone Miner Res. 2005;20:100–6. doi: 10.1359/JBMR.041025. [DOI] [PubMed] [Google Scholar]
- 25.Steiger P, Cummings SR, Black DM, Spencer NE, Genant HK. Age-related decrements in bone mineral density in women over 65. J Bone Miner Res. 1992;7:625–32. doi: 10.1002/jbmr.5650070606. [DOI] [PubMed] [Google Scholar]
- 26.Ensrud KE, Palermo L, Black DM, Cauley J, Jergas M, Orwoll ES, Nevitt MC, Fox KM, Cummings SR. Hip and calcaneal bone loss increase with advancing age: longitudinal results from the study of osteoporotic fractures. J Bone Miner Res. 1995;10:1778–87. doi: 10.1002/jbmr.5650101122. [DOI] [PubMed] [Google Scholar]
- 27.Cauley JA, Seeley DG, Ensrud K, Ettinger B, Black D, Cummings SR. Estrogen replacement therapy and fractures in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1995;122:9–16. doi: 10.7326/0003-4819-122-1-199501010-00002. [DOI] [PubMed] [Google Scholar]
- 28.Eastell R, Cedel SL, Wahner HW, Riggs BL, Melton LJ., 3rd Classification of vertebral fractures. J Bone Miner Res. 1991;6:207–15. doi: 10.1002/jbmr.5650060302. [DOI] [PubMed] [Google Scholar]
- 29.Elkins JS, Yaffe K, Cauley JA, Fink HA, Hillier TA, Johnston SC. Pre-existing hypertension and the impact of stroke on cognitive function. Ann Neurol. 2005;58:68–74. doi: 10.1002/ana.20522. [DOI] [PubMed] [Google Scholar]
- 30.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 31.Barlow WE, Ichikawa L, Rosner D, et al. Analysis of Case-Cohort Designs. J Clin Epidemiol. 1999;52:1165–1172. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 32.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. JASA. 1999;94:496–509. [Google Scholar]
- 33.Szulc P, Blackwell T, Schousboe JT, Bauer DC, Cawthon P, Lane NE, Cummings SR, Orwoll ES, Black DM, Ensrud KE. High hip fracture risk in men with severe aortic calcification: MrOS study. J Bone Miner Res. 2014;29:968–75. doi: 10.1002/jbmr.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naves M, Rodríguez-García M, Díaz-López JB, Gómez-Alonso C, Cannata-Andía JB. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int. 2008;19:1161–6. doi: 10.1007/s00198-007-0539-1. [DOI] [PubMed] [Google Scholar]
- 35.Szulc P, Kiel DP, Delmas PD. Calcifications in the abdominal aorta predict fractures in men: MINOS study. J Bone Miner Res. 2008;23:95–102. doi: 10.1359/jbmr.070903. [DOI] [PubMed] [Google Scholar]
- 36.Kelsey JL, Samelson EJ. Variation in risk factors for fractures at different sites. Curr Osteoporos Rep. 2009;7:127–33. doi: 10.1007/s11914-009-0022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.FitzGerald G, Boonen S, Compston JE, Pfeilschifter J, LaCroix AZ, Hosmer DW, Jr, Hooven FH, Gehlbach SH. Differing risk profiles for individual fracture sites: evidence from the Global Longitudinal Study of Osteoporosis in Women (GLOW) J Bone Miner Res. 2012;27:1907–15. doi: 10.1002/jbmr.1652. [DOI] [PubMed] [Google Scholar]
- 38.Chan JJ, Cupples LA, Kiel DP, O'Donnell CJ, Hoffmann U, Samelson EJ. QCT Volumetric bone mineral density and vascular and valvular calcification: The Framingham Study. J Bone Miner Res. 2015 Apr 13; doi: 10.1002/jbmr.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hyder JA, Allison MA, Wong N, Papa A, Lang TF, Sirlin C, Gapstur SM, Ouyang P, Carr JJ, Criqui MH. Association of coronary artery and aortic calcium with lumbar bone density: the MESA Abdominal Aortic Calcium Study. Am J Epidemiol. 2009;169:186–94. doi: 10.1093/aje/kwn303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chow JT, Khosla S, Melton LJ, 3rd, Atkinson EJ, Camp JJ, Kearns AE. Abdominal aortic calcification, BMD, and bone microstructure: a population-based study. J Bone Miner Res. 2008;23:1601–12. doi: 10.1359/jbmr.080504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szulc P, Delmas PD. Biochemical markers of bone turnover: potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos Int. 2008;19:1683–704. doi: 10.1007/s00198-008-0660-9. [DOI] [PubMed] [Google Scholar]
- 42.Ratcliffe JF. The arterial anatomy of the adult human lumbar vertebral body: a microarteriographic study. J Anat. 1980;131(Pt 1):57–79. [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz AV, Hillier TA, Sellmeyer DE, Resnick HE, Gregg E, Ensrud KE, Schreiner PJ, Margolis KL, Cauley JA, Nevitt MC, Black DM, Cummings SR. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care. 2002;25:1749–54. doi: 10.2337/diacare.25.10.1749. [DOI] [PubMed] [Google Scholar]
- 44.Burghardt AJ, Issever AS, Schwartz AV, Davis KA, Masharani U, Majumdar S, Link TM. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95:5045–55. doi: 10.1210/jc.2010-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–8. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cauley JA, Danielson ME, Boudreau RM, Forrest KY, Zmuda JM, Pahor M, Tylavsky FA, Cummings SR, Harris TB, Newman AB. Inflammatory markers and incident fracture risk in older men and women: the Health Aging and Body Composition Study. J Bone Miner Res. 2007;22:1088–95. doi: 10.1359/jbmr.070409. [DOI] [PubMed] [Google Scholar]
- 47.Takasu J, Katz R, Shavelle DM, O'Brien K, Mao S, Carr JJ, Cushman M, Budoff MJ. Inflammation and descending thoracic aortic calcification as detected by computed tomography: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2008;199:201–6. doi: 10.1016/j.atherosclerosis.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Confavreux CB, Szulc P, Casey R, Boutroy S, Varennes A, Vilayphiou N, Goudable J, Chapurlat RD. Higher serum osteocalcin is associated with lower abdominal aortic calcification progression and longer 10-year survival in elderly men of the MINOS cohort. J Clin Endocrinol Metab. 2013;98:1084–92. doi: 10.1210/jc.2012-3426. [DOI] [PubMed] [Google Scholar]
- 49.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68:271–6. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- 50.Naves-Díaz M, Cabezas-Rodríguez I, Barrio-Vázquez S, Fernández E, Díaz-López JB, Cannata-Andía JB. Low calcidiol levels and risk of progression of aortic calcification. Osteoporos Int. 2012;23:1177–82. doi: 10.1007/s00198-011-1550-0. [DOI] [PubMed] [Google Scholar]
- 51.Sornay-Rendu E, Munoz F, Duboeuf F, Delmas PD. Rate of forearm bone loss is associated with an increased risk of fracture independently of bone mass in postmenopausal women: the OFELY study. J Bone Miner Res. 2005;20:1929–35. doi: 10.1359/JBMR.050704. [DOI] [PubMed] [Google Scholar]
- 52.Szulc P, Samelson EJ, Sornay-Rendu E, Chapurlat R, Kiel DP. Severity of aortic calcification is positively associated with vertebral fracture in older men--a densitometry study in the STRAMBO cohort. Osteoporos Int. 2013;24:1177–84. doi: 10.1007/s00198-012-2101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iwamoto J, Matsumoto H, Takeda T, Sato Y, Uzawa M. A radiographic study on the associations of age and prevalence of vertebral fractures with abdominal aortic calcification in Japanese postmenopausal women and men. J Osteoporos. 2010;2010:748380. doi: 10.4061/2010/748380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El Maghraoui A, Rezqi A, Mounach A, Achemlal L, Bezza A, Ghozlani I. Relationship between vertebral fracture prevalence and abdominal aortic calcification in men. Rheumatology (Oxford) 2012;51:1714–20. doi: 10.1093/rheumatology/kes126. [DOI] [PubMed] [Google Scholar]