Abstract
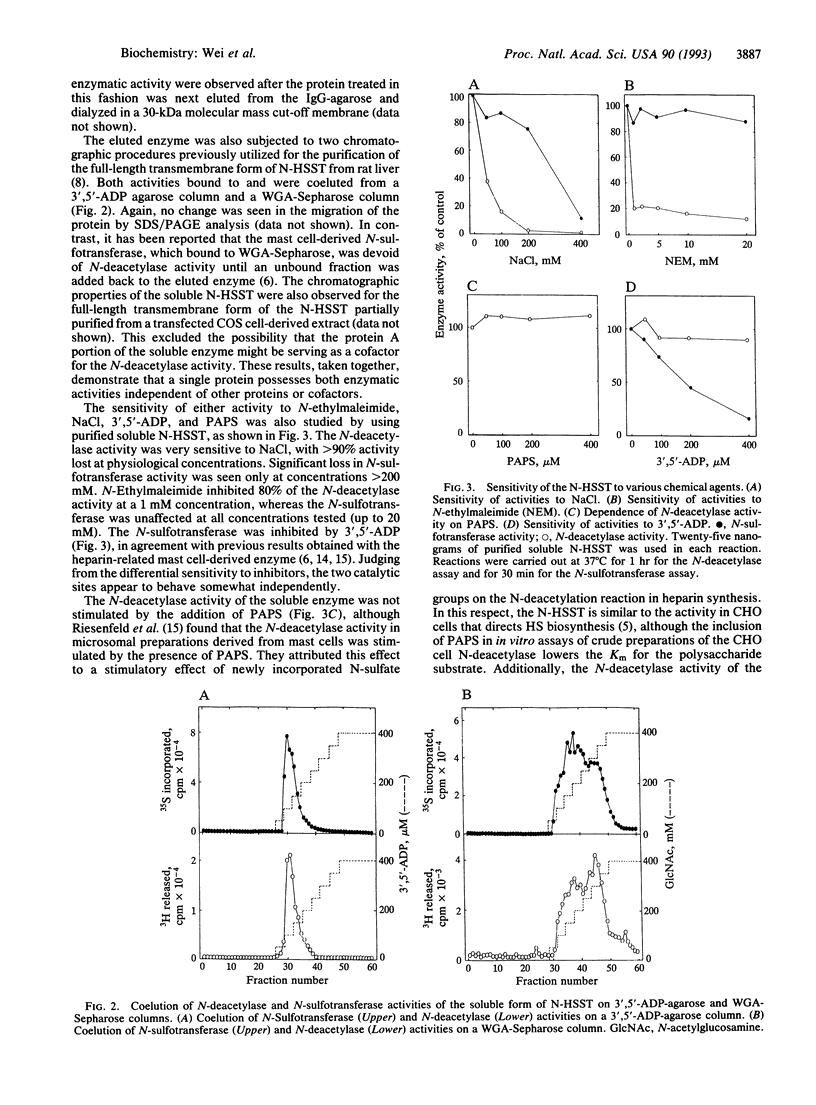
Heparan sulfate is a highly sulfated carbohydrate polymer that binds to and modulates the activities of numerous proteins. The formation of these protein-binding domains in heparan sulfate is dependent on a series of biosynthetic reactions that modify the polysaccharide backbone; the initiating and rate-limiting steps of this process are the N-deacetylation and N-sulfation of N-acetylglucosamine residues in the polymer. We now report that in the rat liver, biosynthesis of heparan sulfate utilizes a single protein that possesses both N-deacetylase and N-sulfotransferase activities. This was accomplished by demonstrating that both activities resided in a purified soluble fusion protein containing the Golgi-lumenal portion of the enzyme. We propose that this protein be renamed the rat liver Golgi heparan sulfate N-deacetylase/N-sulfotransferase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bame K. J., Lidholt K., Lindahl U., Esko J. D. Biosynthesis of heparan sulfate. Coordination of polymer-modification reactions in a Chinese hamster ovary cell mutant defective in N-sulfotransferase. J Biol Chem. 1991 Jun 5;266(16):10287–10293. [PubMed] [Google Scholar]
- Bame K. J., Reddy R. V., Esko J. D. Coupling of N-deacetylation and N-sulfation in a Chinese hamster ovary cell mutant defective in heparan sulfate N-sulfotransferase. J Biol Chem. 1991 Jul 5;266(19):12461–12468. [PubMed] [Google Scholar]
- Brandan E., Hirschberg C. B. Purification of rat liver N-heparan-sulfate sulfotransferase. J Biol Chem. 1988 Feb 15;263(5):2417–2422. [PubMed] [Google Scholar]
- Coleman P. F., Suttle D. P., Stark G. R. Purification from hamster cells of the multifunctional protein that initiates de novo synthesis of pyrimidine nucleotides. J Biol Chem. 1977 Sep 25;252(18):6379–6385. [PubMed] [Google Scholar]
- Daubner S. C., Schrimsher J. L., Schendel F. J., Young M., Henikoff S., Patterson D., Stubbe J., Benkovic S. J. A multifunctional protein possessing glycinamide ribonucleotide synthetase, glycinamide ribonucleotide transformylase, and aminoimidazole ribonucleotide synthetase activities in de novo purine biosynthesis. Biochemistry. 1985 Dec 3;24(25):7059–7062. doi: 10.1021/bi00346a006. [DOI] [PubMed] [Google Scholar]
- Floyd E. E., Jones M. E. Isolation and characterization of the orotidine 5'-monophosphate decarboxylase domain of the multifunctional protein uridine 5'-monophosphate synthase. J Biol Chem. 1985 Aug 5;260(16):9443–9451. [PubMed] [Google Scholar]
- Gallagher J. T., Lyon M., Steward W. P. Structure and function of heparan sulphate proteoglycans. Biochem J. 1986 Jun 1;236(2):313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habuchi H., Suzuki S., Saito T., Tamura T., Harada T., Yoshida K., Kimata K. Structure of a heparan sulphate oligosaccharide that binds to basic fibroblast growth factor. Biochem J. 1992 Aug 1;285(Pt 3):805–813. doi: 10.1042/bj2850805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Orellana A., Gil G., Hirschberg C. B. Molecular cloning and expression of rat liver N-heparan sulfate sulfotransferase. J Biol Chem. 1992 Aug 5;267(22):15744–15750. [PubMed] [Google Scholar]
- Ishihara M., Guo Y., Swiedler S. J. Selective impairment of the synthesis of basic fibroblast growth factor binding domains of heparan sulphate in a COS cell mutant defective in N-sulphotransferase. Glycobiology. 1993 Feb;3(1):83–88. doi: 10.1093/glycob/3.1.83. [DOI] [PubMed] [Google Scholar]
- Ishihara M., Kiefer M. C., Barr P. J., Guo Y., Swiedler S. J. Selection of COS cell mutants defective in the biosynthesis of heparan sulfate proteoglycan. Anal Biochem. 1992 Nov 1;206(2):400–407. doi: 10.1016/0003-2697(92)90385-k. [DOI] [PubMed] [Google Scholar]
- Ishihara M., Tyrrell D. J., Kiefer M. C., Barr P. J., Swiedler S. J. A cell-based assay for evaluating the interaction of heparin-like molecules and basic fibroblast growth factor. Anal Biochem. 1992 May 1;202(2):310–315. doi: 10.1016/0003-2697(92)90111-j. [DOI] [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Kjellén L., Pettersson I., Unger E., Lindahl U. Two enzymes in one: N-deacetylation and N-sulfation in heparin biosynthesis are catalyzed by the same protein. Adv Exp Med Biol. 1992;313:107–111. doi: 10.1007/978-1-4899-2444-5_11. [DOI] [PubMed] [Google Scholar]
- Lee L., Kelly R. E., Pastra-Landis S. C., Evans D. R. Oligomeric structure of the multifunctional protein CAD that initiates pyrimidine biosynthesis in mammalian cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6802–6806. doi: 10.1073/pnas.82.20.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson I., Kusche M., Unger E., Wlad H., Nylund L., Lindahl U., Kjellén L. Biosynthesis of heparin. Purification of a 110-kDa mouse mastocytoma protein required for both glucosaminyl N-deacetylation and N-sulfation. J Biol Chem. 1991 May 5;266(13):8044–8049. [PubMed] [Google Scholar]
- Riesenfeld J., Hök M., Lindahl U. Biosynthesis of heparin. Assay and properties of the microsomal N-acetyl-D-glucosaminyl N-deacetylase. J Biol Chem. 1980 Feb 10;255(3):922–928. [PubMed] [Google Scholar]
- Riesenfeld J., Hök M., Lindahl U. Biosynthesis of heparin. Concerted action of early polymer-modification reactions. J Biol Chem. 1982 Jan 10;257(1):421–425. [PubMed] [Google Scholar]
- Sanchez-Lopez R., Nicholson R., Gesnel M. C., Matrisian L. M., Breathnach R. Structure-function relationships in the collagenase family member transin. J Biol Chem. 1988 Aug 25;263(24):11892–11899. [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]




