To the Editor:
STAT3 mutated hyper-IgE (Job’s) syndrome (STAT3 HIES) is characterized by highly elevated serum IgE, recurrent pneumonias, eczema, skin abscesses, mucocutaneous candidiasis, and dental, vascular and skeletal abnormalities1. STAT3 also promotes CD4 Th17 differentiation and expression of the associated cytokines IL17 and IL222. Th17 cells are believed to enhance mucosal immunity through antimicrobial peptides, impairment of which may explain the typical epithelial infections in STAT3 HIES.
Cryptococcus, Histoplasma, and Coccidioides are endemic fungi that may cause disseminated infection involving the brain or gastrointestinal tract in patients with STAT3 HIES, a pattern distinct from other primary immunodeficiences3–5. We report five cases and review the literature on endemic fungal infections complicating HIES (Online Repository Table 1).
Case Reports
Histoplasmosis (H) 1
STAT3 HIES was diagnosed at age 7 in a boy with recurrent oto-sinopulmonary infections, skin abscesses, radial and skull fractures, eczema and oral aphthous ulcers.
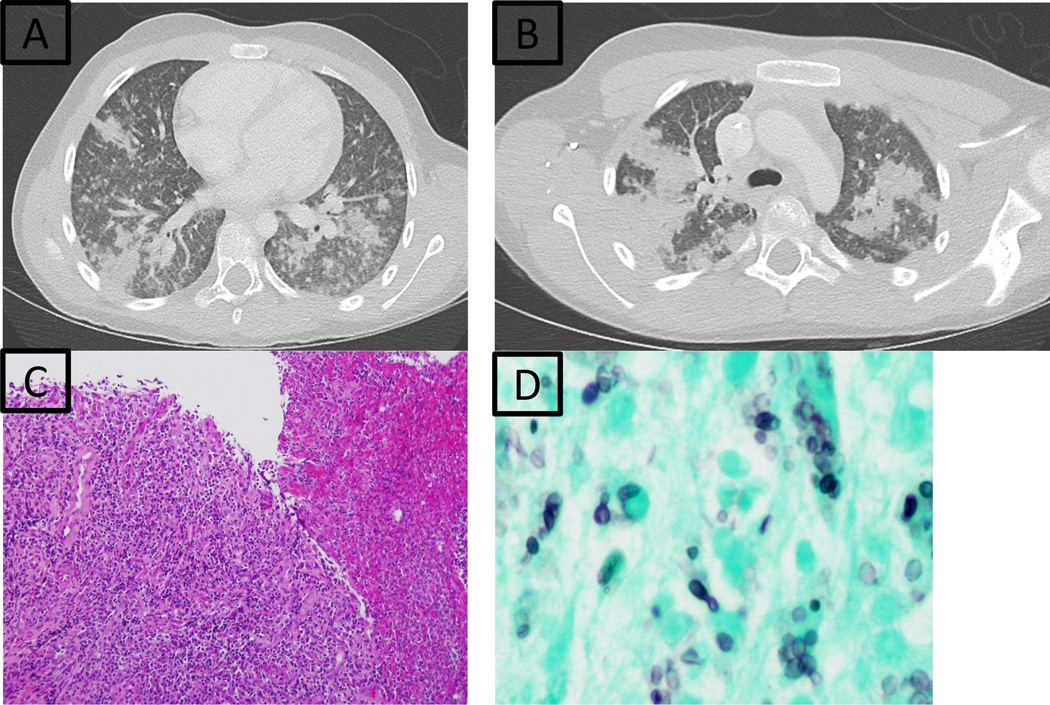
At 10 years, he had fever, abdominal pain and hives. Computerized tomography (CT) showed bilateral lung infiltrates (Fig. 1A,B) and hepatosplenomegaly. Histoplasma was diagnosed by urine and blood antigen; Gomori-methenamine silver (GMS) staining showed small yeasts in bronchial lavage. Secondary hemophagocytic lymphohistiocytosis was apparent with fever, elevated liver enzymes (5–10 times the upper limit of normal), ferritin (16.5 times the upper limit of normal) and thrombocytopenia to 63 K/uL (ref 206–369 K/uL). He received liposomal amphotericin B with defervescence in 3 days. CRP, hepatic and hematologic values normalized in 7 days. Infiltrates resolved by 3 months. Liposomal amphotericin B was replaced with itraconazole at 3.5 weeks due to nephrotoxicity. Itraconazole was poorly tolerated and was changed to posaconazole. He remains well on posaconazole 12 months later.
Figure 1.
Case H1: Chest CT with nodular infiltrates; bronchoscopy positive for Histoplasma (A and B); Case H2: Ulcerated duodenal mucosa with fibrinopurulent exudate (C) and budding Histoplasma yeast forms (D).
Case H2
STAT3 HIES was diagnosed at age 5 in a girl with Staphylococcus aureus skin infections, sinusitis, asthma, minimal trauma fractures, and retained primary teeth.
At 13 years, she presented with abdominal pain, weight loss, severe iron deficient anemia, and elevated inflammatory markers. Endoscopy showed plaques in the distal two-thirds of her esophagus with diffuse exudative ulceration in her descending duodenum, terminal ileum, and transverse colon. Biopsies showed hyphae but were not cultured. Presumed candidiasis was treated with fluconazole. Despite initial improvement, abdominal pain recurred. Repeated endoscopy showed persistent ulceration (duodenum, transverse colon), patchy mucosal eosinophilia with granulomatous infiltrates (gastric antrum, duodenum, ascending, transverse, and descending colon); fungal elements were noted (esophagus, duodenum, terminal ileum, transverse colon, left colon). Histoplasma capsulatum grew on culture from the terminal ileum and colon (Fig. 1C,D). Urine histoplasma antigen was elevated at 14.42 ng/ml. Liposomal amphotericin B was replaced with itraconazole after one week due to nephrotoxicity. She improved rapidly and urine histoplasma antigen decreased after 2 months; 3 years later, she remains well and continues on itraconazole.
Case H3
STAT3 HIES was diagnosed at age 38 in a woman with recurrent otitis in childhood, retained primary teeth, scoliosis, cavitary lung lesions, and chronic pulmonary infections.
At age 21, she had months of fever, night sweats and dysphonia. ENT diagnosed laryngeal histoplasmosis (limited records available on means of diagnosis) and treated with amphotericin B followed by ketoconazole. She required reconstructive laryngoplasty. Thirty years later she has no recurrence of histoplasmosis.
Cryptococcus (Cr) 1
STAT3 HIES was diagnosed at age 3 in a boy with eczema, skin abscesses, and an affected father. At age 18, chest CT showed a lung lesion, and sputum culture grew Cryptococcus gattii. He was not adherent to voriconazole therapy. Five months later, Cryptococcus gattii meningitis was suspected because of headache, neck stiffness and vomiting and confirmed by CSF antigen and culture. Liposomal amphotericin B and flucytosine led to rapid improvement. Amphotericin was replaced with voriconazole after one week due to nephrotoxicity. Headache and vomiting returned, and he resumed liposomal amphotericin with aggressive hydration. Headache cleared rapidly but he had papilledema with high intracranial pressure (ICP) (peak opening pressure 45 mm Hg) without other neurologic findings. He received liposomal amphotericin for 7 weeks, followed by fluconazole. Serum creatinine fell below 1mg/dL over 4 months upon stopping liposomal amphotericin. Opening pressure remained elevated for about two years but without symptoms or vision loss. Three years later, he remains on fluconazole with negative CSF cryptococcal antigen, but positive serum cryptococcal antigen. He has a normal opening pressure and no other neurologic issues.
Coccidioides (Co) 1
STAT3 HIES was diagnosed at age 9 in a girl from the Midwest with recurrent pneumonia, thrush, eczema herpeticum, oral aphthous ulcers, retained primary teeth, and minimal trauma fractures.
At 4.5 years, following a visit to Arizona, she had fever, headache, vomiting and a seizure accompanied by eosinophilia (counts 690 – 1012 K/uL) and IgE levels of 5642 mg/dL. Pulmonary and meningeal Coccidioides immitis infection was diagnosed by cultures of bronchoscopic lavage and CSF. She received amphotericin B lipid complex for 6 days but developed renal insufficiency with peak creatinine of 1.0 mg/dL; fluconazole was continued with good response. Her course was complicated by an internal capsule cerebral vascular accident and hydrocephalus requiring ventriculoperitoneal shunt.
Seven years later, she has residual left hemiparesis, but no other coccidioidomycosis complications. She remains on fluconazole with low serum and urine Coccidioides antigen levels of 0.27 and 0.21 ng/mL, respectively (normal level 0 ng/mL).
Literature Review
We reviewed Pubmed, Web of Science, and Scopus using the search terms Job’s syndrome, hyperimmunoglobulin E syndrome, STAT3 HIES, combined with the terms fungi, mycosis, coccidioidomycosis, cryptococcus, histoplasmosis, and blastomycosis. We identified eleven cases of endemic fungal infection in clinically diagnosed Job’s syndrome (testing not performed for STAT3, or other causes of HIES phenotype such as PGM3 or DOCK8): five with histoplasmosisE1–5, one with histoplasmosis followed by cryptococcal meningitisE6, three with cryptococcosis aloneE7–9, and two with coccidioidomycosisE10–11. Histoplasmosis generally presented with abdominal symptoms, often without pulmonary disease; one case was mistaken for Crohn’s diseaseE4. Cryptococcus infection localized to the GI tract and meninges, and our patients closely mimicked two reported casesE7–8. Specifically, Patient Cr2 had C. neoformans causing a Crohn’s-like picture with a chronic perirectal abscess and a constricting lesion of the colon. Patient Cr3 had hematemesis from an esophageal ulcer, which grew C. neoformans. Meningeal Coccidioides occurred in our patient (Co1) and in two reported casesE10–11, and 2/3 of the patients had concomitant lung infection and/or coccidioidomycosis related stroke.
Discussion
These sixteen cases, seven with confirmed STAT3 mutations, highlight the susceptibility of HIES patients to endemic fungi. Impaired Th17 cell differentiation is a consistent immune defect in STAT3 HIES, which may mediate protection against Cryptococcus and the other endemic mycoses6,7. The GI predominant dissemination, frequently without pulmonary disease, in STAT3 HIES suggests that Th17 cells may be important in upregulation of GI epithelial antimicrobial peptides for host control of Histoplasma and Cryptococcus.
The three STAT3 HIES patients with Coccidioides presented with meningeal disease, while five documented patients with coccidoidomycosis and IL-12/IFN-γ/STAT1 defects experienced lung, bone, (4/5) and lymph node disease (3/5)E12–14. Thus, the specific immune pathway affected may determine the site of fungal dissemination.
These findings underscore the importance of considering endemic fungal infections in STAT3 HIES patients with gastrointestinal complaints, especially since Crohn’s disease is uncommon in these patients8 and delay of antifungal therapy prolongs disease. Although disseminated endemic fungal infection remains uncommon in STAT3 HIES, recognition of this susceptibility is important for those living in or traveling to endemic regions. Due to the severity of some of the infections, such as the risk of meningitis with Coccidioides exposure, strong consideration should be given for antifungal prophylaxis for those at heightened risk. Itraconazole (5 mg/kg per day; maximum 200 mg daily) can be given to patients residing in areas of increased Histoplasma risk, and fluconazole (3–6 mg/kg per day for a maximum of 200 mg daily) can be considered for patients traveling to or residing in Coccidioides endemic regions.
Nephrotoxicity occurred in 40% of STAT3-HIES patients within a week of starting amphotericin therapy, despite having normal kidney function at initiation. This compares to a nephrotoxicity rate of approximately 14% in the general population9. Although there are insufficient data to suggest heightened toxicity, increased vigilance to nephrotoxicity in this population is prudent.
Supplementary Material
Acknowledgments
CDO was funded through the National Institutes of Health (NIH) Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc, The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Inc. and Mr. and Mrs. Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at: http://fnih.org/work/education-training-0/medical-research-scholars-program
Publication Disclaimer: The views expressed in this article are those of the authors and do not reflect the official policy of the U.S. Government. This research was supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.
Funding: This study was funded in part by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases and National Cancer Institute, National Institutes of Health, USA. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics approval: The patients described in these clinical cases were enrolled in protocols approved by the institutional review board of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. The patients and/or their families provided written informed consent.
References
- 1.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 Mutations in the Hyper-IgE Syndrome. N Engl J Med. 2007;357:1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 2.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sowerwine KJ, Holland SM, Freeman AF. Hyper-IgE syndrome update. Ann N Y Acad Sci. 2012;1250:25–32. doi: 10.1111/j.1749-6632.2011.06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antachopoulos C, Walsh TJ, Roilides E. Fungal infections in primary immunodeficiencies. Eur J Pediatr. 2007;166:1099–1117. doi: 10.1007/s00431-007-0527-7. [DOI] [PubMed] [Google Scholar]
- 5.Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Johnson RH, Stevens DA, et al. Coccidioidomycosis. Clin Infect Dis. 2005;41:1217–1223. doi: 10.1086/496991. [DOI] [PubMed] [Google Scholar]
- 6.Valdez PA, Vithayathil PJ, Janelsins BM, Shaffer AL, Williamson PR, Datta SK. Prostaglandin E2 suppresses antifungal immunity by inhibiting interferon regulatory factor 4 function and interleukin-17 expression in T cells. Immunity. 2012;36:668–679. doi: 10.1016/j.immuni.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wozniak KL, Hardison SE, Kolls JK, Wormley FL Jr. Role of IL-17A on Resolution of Pulmonary C. neoformans Infection. PLoS ONE. 2011;6:e17204. doi: 10.1371/journal.pone.0017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberti-Flor JJ, Granda A. Ileocecal histoplasmosis mimicking Crohn’s disease in a patient with Job’s syndrome. Digestion. 1986;33:176–180. doi: 10.1159/000199290. [DOI] [PubMed] [Google Scholar]
- 9.Mistro S, Maciel I de M, de Menezes RG, Maia ZP, Schooley RT, Badaró R. Does lipid emulsion reduce amphotericin B nephrotoxicity? A systematic review and meta-analysis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2012;54:1774–1777. doi: 10.1093/cid/cis290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.