Abstract
Introduction
Long-term, intermittent oral corticosteroid (OCS) use in children with asthma leads to significant decrements in bone mineral accretion (BMA). We aimed to identify genetic factors influencing OCS dose effects on BMA in children with asthma.
Methods
We first performed a gene by OCS interaction genome-wide association study (GWAS) of BMA in 489 white participants in the Childhood Asthma Management Program trial, who took short-term oral prednisone bursts when they experienced acute asthma exacerbations. We selected the top-ranked 2,000 single nucleotide polymorphisms (SNPs) in the GWAS and determined whether these SNPs also had cis-regulatory effects on dexamethasone-induced gene expression in osteoblast cells.
Results
We identified two SNPs (rs9896933 and rs2074439) associated with decreased BMA and related to the tubulin γ pathway. Rs9896933 met criteria for genome-wide significance (P = 3.15 ×10−8 in GWAS) and is located on the intron of tubulin folding cofactor D gene. Rs2074439 (P = 2.74 × 10−4 in GWAS) showed strong cis-regulatory effects on dexamethasone-induced tubulin γ gene expression in osteoblast cells (P = 8.64 × 10−4). Interestingly, we found that BMA worsened with increased dose of prednisone as the number of mutant alleles of the two SNPs increased.
Conclusions
We have identified two novel tubulin γ pathway SNPs, rs9896933 and rs2074439, showing independent interactive effects with cumulative corticosteroid dose on BMA in children with asthma receiving multiple OCS bursts.
Keywords: Asthma, Bone mineral density, Child, Corticosteroids
Introduction
Bone mineral accretion (BMA) is defined as the accrual in bone mineral density (BMD) over time. As adults normally lose BMD as they age, the achievement of BMD that occurs during childhood and adolescence is regarded as an important factor contributing to osteoporosis.1 Therefore, factors affecting BMA during this critical period may also increase the risk of developing osteoporosis later in life. The adverse effects of oral corticosteroid (OCS) on bone health have been well known for many years, and even intermittent OCS use may affect BMA in childhood. Our group previously reported that frequent OCS use in children with asthma led to significant decrements in BMA.2,3
Advances in genomic technology enables us to integrate gene expression data with genotype data. Through this integration, we significantly increase the possibility of identifying functional genetic variants underlying disease pathogenesis. For example, we observed dexamethasone specific cis-regulation of Tenascin C (TNC) expression in the primary osteoblast.4 We then performed an association study between six expression single nucleotide polymorphisms (SNPs) within TNC locus and increases in lung function after two months of inhaled corticosteroid (ICS) treatment in children with asthma. Finally, we demonstrated that this heritable cis-regulation of TNC expression explained the difference in response to ICSs by showing that four of six SNPs were significantly associated with lung function increases.4 Using a cellular expression quantitative trait loci (eQTL) approach, we have also recently identified several SNPs associated with ICS response at a genome-wide level.5
The purpose of the present study was to identify genetic factors with the potential to substantially modify the effects of OCS on BMA in children with asthma receiving multiple intermittent OCS courses over time. We initially performed a GWAS of OCS-induced BMA changes observed in children with asthma participating in the Childhood Asthma Management Program (CAMP) trial.5 They were followed for a mean of 4.3 years and took short-term oral prednisone bursts when they experienced acute exacerbations. To refine the potential for these SNPs to have a functional effect on bone, we determined whether the top-ranked SNPs had cis-regulatory effects on corticosteroid-induced gene expression in osteoblast cells. Finally, noting that two of the associated variants mapped to the same pathway, we tested the combined effects of the two SNPs on BMA.
MATERIALS AND METHODS
The CAMP trial was approved by the Institutional Review Board of the participating institutions and informed consent was obtained from the participating children and their parents.
CAMP BMA measurements
A total 1,041 children with mild-to-moderate asthma aged 5 to 12 years were randomized to budesonide, nedocromil, or placebo and followed for a mean of 4.3 years in the CAMP trial.6 For asthma exacerbations, oral prednisone bursts were prescribed per protocol as follows: 2 mg/kg per day (up to 60 mg) for 2 days followed by 1 mg/kg per day (up to 30 mg) for 2 days with an option to continue dosing if improvement was insufficient. BMD measurements (in grams per square centimeter) of the lumbar spine (L1-L4) were performed yearly during the study period by means of dual-energy x-ray absorptiometry with the Hologic (Waltham, MA, US) QDR-1500 (at 6 centers) or the Lunar (Madison, WS, US) DPX (at 2 centers). Hologic DEXA machines were further divided by the use of pencil-beam or fan-beam measurements. Detailed methods to convert Lunar measures to Hologic values and to adjust deviations between pencil- and fan-beam measurements were described in our previous report.3 BMA (in grams per square centimeter per year) represents the average gain of BMD over time and calculated as follows: (BMD at 4 years’ follow-up - BMD at baseline)/4 years. A 4-year follow-up was selected as this approximated the end of the trial. BMD z scores were calculated by using CAMP internal references.2
GWAS of bone mineral accretion
Genotyping was done by the HumanHap550v3 BeadChip or the Infinium HD 140 Human610-Quad BeadChip (Illumina Inc., San Diego, CA, US). SNPs showing significant deviation from Hardy-Weinberg equilibrium (P < 0.001), SNPs with low minor allele frequency (< 0.05) and SNPs with high rates of missing data (> 0. 01) were excluded. Using a linear regression gene-environment interaction model as implemented in the PLINK (http://pngu.mgh.harvard.edu/purcell/plink/),7 we performed GWAS to test the following model: BMA ~ genotype (additive genetic model) + cumulative dose of OCS + genotype*cumulative dose of OCS + covariates. Based on our previous report, age, sex, BMD, height, body mass index, serum concentration of vitamin D, and Tanner stage measured at baseline were included as covariates.3 Since we focused on the interactive effect between genotype and cumulative dose of OCS on BMA, we reported P values from the ‘genotype*cumulative dose of OCS’ term. Only the data from Caucasian children was used in the present study due to potential issues with population stratification.
Cis-regulation effect on corticosteroid-induced gene expression in osteoblast cells
We used data from our previous study.4 In brief, expression profiling of human primary bone cells derived from 113 donors (51 female and 62 male donors undergoing total hip or knee replacement at the Uppsala University Hospital, Uppsala, Sweden) and stimulated with dexamethasone (100nM) for 24 hours was measured using the Illumina HumRef-8v2 BeadChips (Illumina Inc. San Diego, CA, US). Genotyping was done by Illumina HapMap550K Duo chip (Illumina Inc., San Diego, CA, US) as previously described.4 We performed an association test between corticosteroid-induced gene expression and SNPs using a linear regression model implemented in the SNPTEST software (http://www.stats.ox.ac.uk/~marchini/software/gwas/gwas.html).8 An additive effect of SNP was assumed and two covariates (year of birth and sex) were included in a regression model. Cis-regulatory SNPs were defined when SNPs were located in a 1 Mb window flanking the target gene or within the target gene itself and their P values were less than 0.05.
Statistical analysis
Pearson's correlation and simple linear regression analysis were done to evaluate relations between cumulative dose of OCS and BMA according to the genotypes. Multiple linear regression models were constructed to test effects of cumulative dose of OCS on BMA adjusted by baseline covariates. All analyses were performed using the R (version 3.0.2) software (www.r-project.org).
RESULTS
A total of 489 Caucasian subjects were included in GWAS of OCS-induced BMA. Table 1 summarizes baseline characteristics. Median (range) number of OCS bursts was 3 (0-27). The cumulative dose of OCS showed a significantly negative association with BMA after adjustment for covariates (beta = −3.036 × 10−6 and P = 7.96 × 10−5, see Table E1 in this article's Online Repository). A total of 44,319 SNPs with P values less than 0.05 in GWAS were identified (see ‘BMA_Interaction_Pvalue0.05.xlsx’ in this article's Online Repository). Table 2 shows 9 SNPs which met the threshold for genome-wide significance (P < 1 × 10−7) for the interaction of genotype with corticosteroid dose on BMA. The QQ-plot and Manhattan plot for the interaction results were presented in this article's Online Repository (Figure E1). Power for the interaction analysis was calculated using Quanto software 1.2.4 (http://biostats.usc.edu/Quanto.html). Quanto is a program for computing either power or required sample size for association studies of genes, environmental factors, gene-environment interaction.9,10 An additive genetic model and alpha value (two-sided) of 0.05 were assumed. Minor allele frequency, mean and variance of BMA in CAMP subjects, genetic effect (beta of the ‘genotype’ term in our regression model), and environmental effect (beta of the ‘cumulative dose of OCS’ term in our regression model) were obtained from our results. For rs9896933, an estimated power was 0.68 for main (gene) effect and 0.58 (2 df test) for interaction (gene-environment interaction, that is, interaction between genotype and cumulative dose of OCS). In osteoblast cells, a total of 14,982 SNPs showed cis-regulatory effects on corticosteroid-induced gene expression (see ‘Osteoblast_cis_eQTL.xlsx’ in this article's Online Repository). We selected the top-ranked 2,000 SNPs in GWAS and found that 3 SNPs among them showed cis-regulatory effects on corticosteroid-induced gene expression in osteoblast cells (Table 3).
Table 1.
Baseline characteristics of subjects enrolled in CAMP
| Total numbers | 489 |
| Male sex, no. (%) | 302 (61.7) |
| Age (y), mean (SD) | 8.8 (2.1) |
| Height (cm), mean (SD) | 132.0 (19.8) |
| BMI (kg/m2), median (IQR) | 17.1 (15.8- 19.7) |
| BMD (g/cm2), median (IQR) | 0.782 (0.683 - 0.935) |
| Vitamin D (ng/mL), median (IQR) | 36.6 (28.8 - 47.9) |
| Tanner stage, no. (%) | |
| I/II/III/IV/V | 353 (72.2)/100 (20.4)/24 (4.9)/11 (2.2)/1 (0.3) |
| Cumulative dose of prednisolone (mg), median (IQR) | 360 (120 - 900) |
| Bone mineral accretion (g/cm2/y), median (IQR) | 0.038 (0.024 - 0.063) |
SD; Standard deviation, IQR; Interquartile range, BMI; Body mass index, BMD; Bone mineral density, BMA; Bone mineral accretion
Table 2.
Nine SNPs with genome-wide significance identified in GWAS of OCS-induced BMA
| Rank | SNP | Chromosome | Gene | Beta | P value |
|---|---|---|---|---|---|
| 1 | rs7003550 | 8 | intergenic | −8.15 × 10−6 | 1.46 × 10−8 |
| 2 | rs9896933 | 17 | TBCD | −8.56 × 10−6 | 3.15 × 10−8 |
| 3 | rs7599706 | 2 | intergenic | −1.49 × 10−5 | 3.98 × 10−8 |
| 4 | rs12447718 | 16 | ZFPM1 | −1.31 × 10−5 | 4.26 × 10−8 |
| 5 | rs4368243 | 18 | intergenic | −5.99 × 10−6 | 6.35 × 10−8 |
| 6 | rs2316527 | 7 | DPP6 | −1.13 × 10−5 | 7.63 × 10−8 |
| 7 | rs4484658 | 8 | intergenic | −8.90 × 10−6 | 8.09 × 10−8 |
| 8 | rs7506840 | 18 | LOC100130480 | −1.11 × 10−5 | 8.95 × 10−8 |
| 9 | rs10485681 | 20 | intergenic | −8.63 × 10−6 | 9.91 × 10−8 |
Beta; Beta coefficient, TBCD; Tubulin folding cofactor D, ZFPM1; zinc finger protein, FOG family member 1, DPP6; Dipeptidyl-peptidase 6
Table 3.
Three SNPs showing cis-regulatory effects on corticosteroid-induced gene expressions in osteoblast among the top-ranked 2,000 SNPs in GWAS
| SNP | GWAS |
Cis-regulation effect in osteoblast |
|
|---|---|---|---|
| P value | Gene induced by dexamethasone | P value | |
| rs949637 | 0.0008123 | FLJ16171 | 0.0008897 |
| rs2074439 | 0.0002743 | TUBG1 | 0.0008635 |
| rs2030737 | 0.0002953 | KY | 0.0001267 |
FLJ16171; FLJ16171 protein (on the chromosome 5q35), TUBG1; Tubulin, gamma 1 (on the chromosome 17q21), KY; Kyphoscoliosis peptidase (on the chromosome 3q22.2)
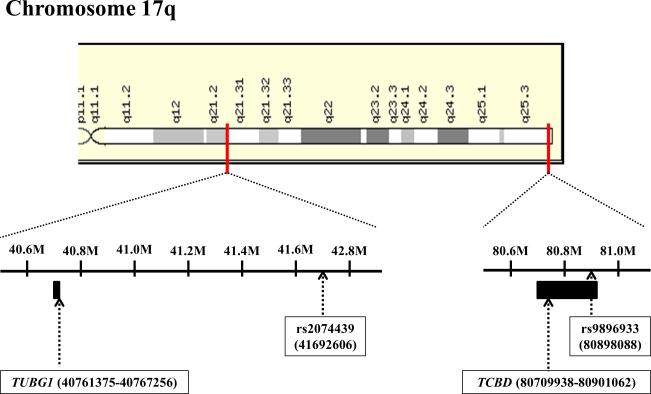
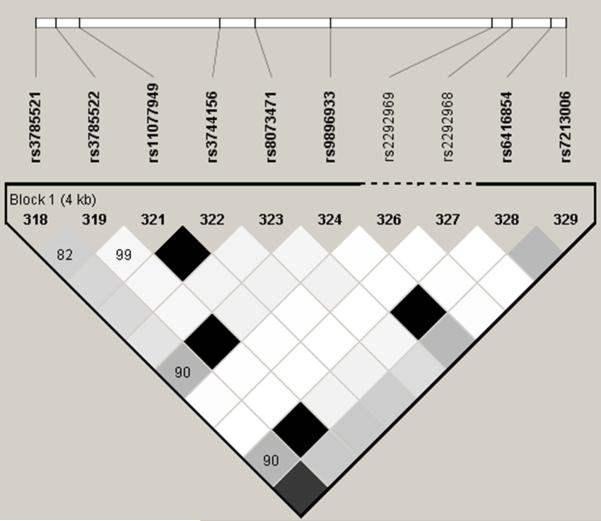
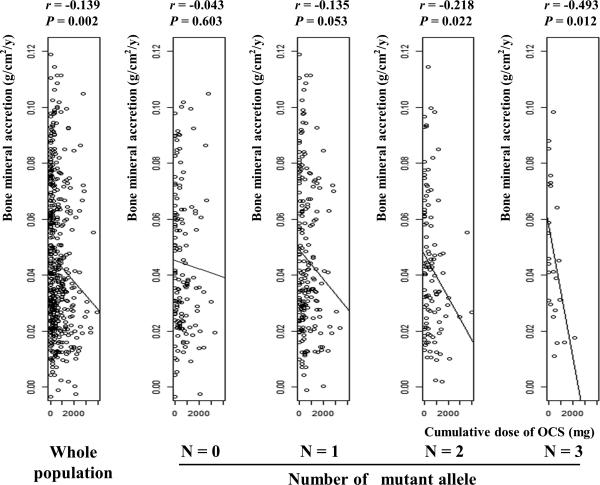
We focused on two SNPs, rs9896933 (minor allele frequency = 0.173, ranked at the 2nd in GWAS; P = 3.15 ×10−8) and rs2074439 (minor allele frequency = 0.34, ranked at the 1,256th in GWAS; P = 2.74 ×10−4), given that (1) rs9896933 is located on the intron of the tubulin folding cofactor D (TBCD) gene and is in tight LD linkage disequilibrium (i.e. highly correlated) with rs3785522, a non-synonymous coding SNP on TBCD (r2 = 0.945) (Figure 1A and 1B), (2) rs2074439 is located on the chromosome 17q21 (Figure 1B) which was previously identified as a QTL associated with bone mineral density in European pedigrees,11 (3) tubulin γ 1 (TUBG1) gene regulated by rs2074439 showed significantly reduced expressions in osteoblast after incubation with dexamethasone for 24hr,4 and (4) a biologic interaction between TUBG1 and TBCD has been reported.12 Negative correlations between cumulative dose of OCS and BMA stratified by genotype of rs9896933 and rs2074439 were shown (Figure E2 and E3). We failed to demonstrate a significant epistasis between rs9896933 and rs2074439. However, we found that an interaction between rs9896933 and the cumulative dose of OCS and an interaction between rs2074439 and the cumulative dose of OCS were still significant, when both terms were put into a regression model together [BMA ~ cumulative dose of OCS + rs9896933 genotype (additive model) + rs2074439 genotype (additive model) + cumulative dose of OCS*rs9896933 genotype + cumulative dose of OCS*rs2074439 genotype + covariates] (Table E2). This meant that both interactions were independently significant when adjusting for each other. Figure 2 shows correlations between cumulative dose of OCS and BMA stratified by the number of mutant allele of rs9896933 and rs2074439. A dosage effect of mutant alleles on a slope obtained from simple linear regression analysis was observed (Figure E4). Table E3 shows beta and P values of associations between cumulative dose of OCS and BMA in each group obtained from multiple linear regression analysis adjusted by baseline covariates. We also evaluated whether rs9896933 showed the same interactive effect with ICSs on BMA in asthmatic children without OCS bursts. For this, we compared BMA stratified by rs9896933 of subjects who were randomized to ICS to that of subjects who were randomized to placebo. We found that rs9896933 genotype was not significantly associated with BMA in both groups (Figure E5).
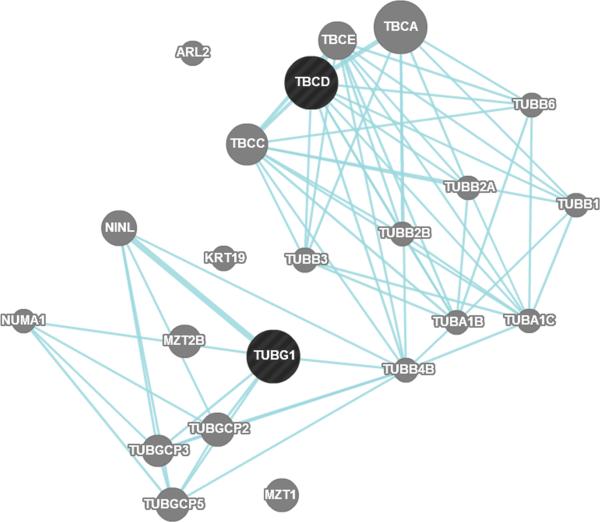
Figure 1. Position of identified SNPs and genes on the chromosome 17q.
A. Linkage disequilibrium plot between rs9896933 and rs3785522 (HapMap CEPH population) B. Rs9896933, rs2074439, TCBD and TUBG1 C. Analysis of the potential pathway interactions between TBCD and TUBG1 via GeneMania (www.genemania.org)
Figure 2. Correlations between cumulative dose of OCS and BMA stratified by the number of mutant alleles of rs9896933 and rs2074439.
Regression lines of whole population, subjects without any mutant alleles, subjects with one mutant allele, subjects with two mutant alleles, and subjects with three mutant alleles are shown. The slope obtained from simple linear regression analysis becomes steeper as the number of mutant alleles of two SNPs increases. P values were obtained from Pearson's correlation analysis, r; correlation coefficient
DISCUSSION
For their unrivaled anti-inflammatory and immunomodulatory effects, OCSs are recommended in the current treatment guidelines for pediatric asthma.13-16 However, OCSs frequently cause many adverse outcomes including osteoporosis. Multiple OCS bursts over time have been associated with lower bone mineral density in children with asthma.2,3 Various factors including gender, age, height, and pubertal stage are known to affect BMA associated with OCS use in children.17 However, there has been no study evaluating a genetic influence on BMA induced by OCS bursts in children with asthma. In the present study, we identified two SNPs of potential functional significance (rs9896933 and rs2074439) that were involved in the γ tubulin pathway and had significant interactive effects with a cumulative dose of OCS on BMA in children with asthma. For asthmatic children without OCS bursts, effects of those two SNPs on BMA were not different between those assigned to ICS and those assigned to placebo. However, for the proper interpretation, we should make it clear that medium dose of ICS (total daily dose of budesonide was 400 μg) was administered in the CAMP study.6 Notably, the association between cumulative dose of OCS and lower BMA increased as the number of mutant alleles of two SNPs increased.
Rs9896933 is located on the intron of TBCD and is in tight LD with rs3785522, a non-synonymous coding SNP in TBCD gene. We evaluated the functional implication of rs3785522 using ‘The Functional Single Nucleotide Polymorphism (F-SNP)’, a web-based tool (http://compbio.cs.queensu.ca/F-SNP/) integrating information obtained from 16 bioinformatics tools and databases about the functional effects of SNPs.18 F-SNP indicated 0.912 for the FS score of rs3785522. The FS score is a functional SNP scoring system provided by F-SNP to distinguish features of disease-related SNPs versus neutral SNPs.19 The median FS score for neutral SNPs is 0.1764, whereas for disease-related SNPs, the median rises to 0.5.19 Thus, rs3785522 may be functionally important. Similarly, through its association with corticosteroid-induced changes in expression of TUBG1, rs2074439 (or a variant in LD with it) represents a regulatory variant in the γ tubulin pathway.
Osteoblast cells that synthesize new bone matrix on bone-forming surface play an important role in bone homeostasis.20 Decreased bone formation is an important mechanism for corticosteroid-induced BMD loss.21 Therefore, corticosteroid effects on the osteoblast have been extensively evaluated. Corticosteroids prevent terminal osteoblast differentiation,22 induces osteoblast apoptosis,23 and inhibits osteoblast production of bone matrix.24 While these effects are known, the role of microtubules on corticosteroid-induced BMD loss in the osteoblast cells has been not previously elucidated. Microtubules are major components of the cytoskeleton and provide scaffolding, sequestering, and delivery functions. It is known that microtubules are involved in various signaling pathways, such as sonic hedgehog/Gli, Wnt/β-catenin, G-protein, and mitogen-activated protein kinase pathways which are related to skeletal development and homeostasis.25,26 Interestingly, recent reports noted that inhibition of microtubule assembly increases osteoblast differentiation in vitro, and murine bone mass in vivo.27,28 Formation of new microtubule polymers from heterodimers of α and β tubulin is termed ‘nucleation’ and requires another member of the tubulin family, γ tubulin.29 γ tubulin forms γ tubulin ring complexes with additional proteins and these γ tubulin complexes may also be involved in regulating microtubule dynamics.30 Tubulin folding cofactor D coded by TBCD is one of the five tubulin-specific cochaperones A-E that mediate the nucleation of tubulin.31 Tubulin folding cofactor D plays a critical role in recruiting γ tubulin ring complexes and initiating microtubule growth and excess of tubulin folding cofactor D inhibits microtubule assembly.12 Taken together, it is possible that a disruption in the γ tubulin pathway in the osteoblast cells may result in BMD changes and these changes may be modified via genetic factors. Interestingly, androgen was reported to promote γ tubulin recruitment and microtubule assembly in human fibroblast32 and pregnenolone was reported to bind to microtubule-associated protein 2 and to stimulate microtubule assembly.33 As mentioned before, inhibition of microtubule assembly in osteoblast is possibly linked to BMD decrease. Therefore, it is also possible that corticosteroid-induced BMD loss is associated with the γ tubulin pathway.
An eQTL is a locus that explains a fraction of the genetic variance of a gene expression phenotype and standard eQTL analysis is particularly useful for identifying pharmacogenomic loci.34 However, eQTL may differ according to the cell type35 and thus functional validation using eQTL is optimally conducted in cell lines that are relevant to the disease. We demonstrated an epistasis between cis-eQTL SNP of TUBG1 and the second-most-significant SNP in GWAS which is in tight LD with a possibly functional non-synonymous coding SNP in TBCD. A biologic interaction between γ tubulin and tubulin folding cofactor D was reported,11 and we found that TBCD and TUBG1 shared a common pathway using the program GeneMania (http://genemania.org/) (Figure 1C). Combined, these findings suggest that two SNPs identified in the present study may have a causative role in OCS-induced BMA in children with asthma. We believed that children with asthma were a relevant population to reveal genetic factors associated BMA caused by corticosteroids, because they carry little risk for BMD loss compared to the elderly or to patients with other diseases. However, we could not obtain BMA data from a similar group of asthmatic children for the replication of our results, which is an important limitation in generalizing our results. Confirmation in an independent cohort is warranted. In addition, considering that only 3 SNPs out of 2,000 identified in the GWAS showed cis-regulatory effects, different pharmacologic profiles between dexamethasone and prednisone need to be mentioned. Dexamethasone is more potent and has longer half-life compared to prednisone36 and thus the involved genes and pathways perhaps differ somewhat, although clinical responses to both drugs in reducing acute asthma exacerbations were not different.37,38
Besides the rs9896933 relationship with the γ tubulin pathway, 8 other SNPs with genome-wide significance identified in GWAS of BMA were noted. So far, there have been no reports showing direct associations between these SNPs and bone mineral density. However, rs7003550, the top-ranked SNP, is of particular interest. According to the SCAN database, rs7003550 significantly affects on the expression of the p21 protein activated kinase 2 (PAK2) gene (P = 5 × 10−5) and adaptor protein, phosphotyrosine interaction, PH domain and leucine zipper containing 1 (APPL1) gene (P = 0.0001) in Caucasians.39 The SCAN database provides summary information from eQTL mapping of HapMap SNPs to gene expression (evaluated by the Affymetrix exon array) in the full set of HapMap Caucasians.40 The PAK are critical effectors that link Rho GTPases to cytoskeleton reorganization and have been reported to take a role in methylglyoxal-induced41 and citrinin-induced42 osteoblast apoptosis. The protein encoded by APPL1 gene has been shown to be involved in the regulation of cell proliferation, and in the crosstalk between the adiponectin and insulin signaling pathways.43 Interestingly, the effect of adiponectin on bone metabolism is mediated by APPL1.44 Taken together, rs7003550 may be involved in OCS-induced decrease in BMA by modulating PAK2 and APPL1 gene expressions.
In conclusion, we have identified novel variants that influence the magnitude of the effect of OCSs on BMA based on an integrative genomic approach. Two of these variants of potential functional significance map to the γ tubulin pathway and may provide novel functional insights into the mechanisms by which systemic OCSs affect bone health. More studies testing other diseases, such as ulcerative colitis, juvenile idiopathic arthritis, and other autoimmune diseases that feature corticosteroids are needed to get a complete picture of the mechanisms involved and risk estimates in susceptible children.
Supplementary Material
Clinical implication.
This suggests that genetic factors may contribute to decreased BMA in asthmatics children from OCS by instituting alternative long-term control measures.
Capsule summary.
We have identified two novel SNPs, rs9896933 and rs2074439, showing interactive effects with OCS on BMA in children with asthma receiving multiple OCS bursts when adjusting for each other. Our results indicate that a tubulin γ pathway may be involved in OCS-induced decreases in BMA.
Acknowledgement
This work was supported by U01 HL65899, R01 HL092197, and R01 NR013391. The Childhood Asthma Management Program is supported by contracts NO1-HR-16044, 16045, 16046, 16047, 16048, 16049, 16050, 16051, and 16052 with the National Heart, Lung, and Blood Institute and General Clinical Research Center grants M01RR00051, M01RR0099718-24, M01RR02719-14, and RR00036 from the National Center for Research Resources.
Abbreviations
- BMA
Bone mineral accretion
- BMD
Bone mineral density
- CAMP
Childhood asthma management program
- eQTL
Expression quantitative trait loci
- GWAS
Genome wide association study
- ICS
Inhaled corticosteroid
- OCS
Oral corticosteroid
- SNP
Single nucleotide polymorphism
- TBCD
Tubulin folding cofactor D
- TUBG1
Tubulin gamma 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–95. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 2.Kelly HW, Van Natta ML, Covar RA, Tonascia J, Green RP, Strunk RC. Effect of long-term corticosteroid use on bone mineral density in children: a prospective longitudinal assessment in the childhood Asthma Management Program (CAMP) study. Pediatrics. 2008;122:e53–61. doi: 10.1542/peds.2007-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tse SM, Kelly HW, Litonjua AA, Van Natta ML, Weiss ST, Tantisira KG. Corticosteroid use and bone mineral accretion in children with asthma: effect modification by vitamin D. J Allergy Clin Immunol. 2012;130:53–60. doi: 10.1016/j.jaci.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundberg E, Adoue V, Kwan T, Ge B, Duan QL, Lam KC, et al. Global analysis of the impact of environmental perturbation on cis-regulation of gene expression. PLoS Genet. 2011;7:e1001279. doi: 10.1371/journal.pgen.1001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu W, Rogers AJ, Damask A, Raby BA, Klanderman BJ, Duan QL, et al. Pharmacogenomics: Novel Loci Identification via Integrating Gene Differential Analysis and eQTL Analysis. Hum Mol Genet. 2014;23:5017–24. doi: 10.1093/hmg/ddu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Childhood Asthma Management Program Research Group Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med. 2000;343:1054–63. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 7.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchini J, Howie B. Genotype imputation for genome-wide association studies. Nat Rev Genet. 2010;11:499–511. doi: 10.1038/nrg2796. [DOI] [PubMed] [Google Scholar]
- 9.Gauderman WJ, Morrison JM. [December 26th, 2014];Quanto 1.2.4: A computer program for power and sample size calculations for genetic-epidemiology studies. Available at http://biostats.usc.edu/Quanto.html.
- 10.Kraft P, Yen YC, Stram DO, Morrison J, Gauderman WJ. Exploiting gene-environment interaction to detect genetic associations. Hum. Hered. 2007;63:111–9. doi: 10.1159/000099183. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman JM, Ostertag A, Saint-Pierre A, Cohen-Solal M, Boland A, Van Pottelbergh I, et al. Genome-wide linkage screen of bone mineral density (BMD) in European pedigrees ascertained through a male relative with low BMD values: evidence for quantitative trait loci on 17q21-23, 11q12-13, 13q12-14, and 22q11. J Clin Endocrinol Metab. 2008;93:3755–62. doi: 10.1210/jc.2008-0678. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham LA, Kahn RA. Cofactor D functions as a centrosomal protein and is required for the recruitment of the gamma-tubulin ring complex at centrosomes and organization of the mitotic spindle. J Biol Chem. 2008;283:7155–65. doi: 10.1074/jbc.M706753200. [DOI] [PubMed] [Google Scholar]
- 13.The Global Initiative for Asthma (GINA) [May 18th, 2014];The guideline for asthma management and prevention. 2010 Available at http://www.ginasthma.org/guidelines-pocket-guide-for-asthma-management.html.
- 14.The National Asthma Education and Prevention Program (NAEPP) [May 18th, 2014];National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. 2007 Available at http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf.
- 15.British Thoracic Society, Scottish Intercollegiate Guidelines Network [May 18th, 2014];British guideline on the management of asthma. Available at http://www.sign.ac.uk/pdf/sign101.pdf.
- 16.Potter Paul C. Current guidelines for the management of asthma in young children. Allergy Asthma Immunol Res. 2010;2:1–13. doi: 10.4168/aair.2010.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown JJ, Zacharin MR. Proposals for prevention and management of steroid induced osteoporosis in children and adolescents. J Paediatr Child Health. 2005;41:553–7. doi: 10.1111/j.1440-1754.2005.00718.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee PH, Shatkay H. F-SNP: computationally predicted functional SNPs for disease association studies. Nucleic Acids Res. 2008;36:D820–4. doi: 10.1093/nar/gkm904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee PH, Shatkay H. An integrative scoring system for ranking SNPs by their potential deleterious effects. Bioinformatics. 2009;25:1048–55. doi: 10.1093/bioinformatics/btp103. [DOI] [PubMed] [Google Scholar]
- 20.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–9. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canalis E, Bilezikian JP, Angeli A, Giustina A. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004;34:593–8. doi: 10.1016/j.bone.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Pereira RC, Delany AM, Canalis E. Effects of cortisol and bone morphogenetic protein-2 on stromal cell differentiation: correlation with CCAAT-enhancer binding protein expression. Bone. 2002;30:685–91. doi: 10.1016/s8756-3282(02)00687-7. [DOI] [PubMed] [Google Scholar]
- 23.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–82. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delany AM, Gabbitas BY, Canalis E. Cortisol downregulates osteoblast alpha 1 (I) procollagen mRNA by transcriptional and posttranscriptional mechanisms. J Cell Biochem. 1995;57:488–94. doi: 10.1002/jcb.240570314. [DOI] [PubMed] [Google Scholar]
- 25.Gundersen GG, Cook TA. Microtubules and signal transduction. Curr Opin Cell Biol. 1999;11:81–94. doi: 10.1016/s0955-0674(99)80010-6. [DOI] [PubMed] [Google Scholar]
- 26.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–65. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 27.Zhao M, Ko SY, Liu JH, Chen D, Zhang J, Wang B, et al. Inhibition of microtubule assembly in osteoblasts stimulates bone morphogenetic protein 2 expression and bone formation through transcription factor Gli2. Mol Cell Biol. 2009;29:1291–305. doi: 10.1128/MCB.01566-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou W, Greenblatt MB, Brady N, Lotinun S, Zhai B, de Rivera H, et al. The microtubule-associated protein DCAMKL1 regulates osteoblast function via repression of Runx2. J Exp Med. 2013;210:1793–806. doi: 10.1084/jem.20111790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moudjou M, Bordes N, Paintrand M, Bornens M. gamma-Tubulin in mammalian cells: the centrosomal and the cytosolic forms. J Cell Sci. 1996;109:875–87. doi: 10.1242/jcs.109.4.875. [DOI] [PubMed] [Google Scholar]
- 30.Raynaud-Messina B, Merdes A. Gamma-tubulin complexes and microtubule organization. Curr Opin Cell Biol. 2007;19:24–30. doi: 10.1016/j.ceb.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Tian G, Huang Y, Rommelaere H, Vandekerckhove J, Ampe C, Cowan NJ. Pathway leading to correctly folded beta-tubulin. Cell. 1996;86:287–96. doi: 10.1016/s0092-8674(00)80100-2. [DOI] [PubMed] [Google Scholar]
- 32.Colello D, Reverte CG, Ward R, Jones CW, Magidson V, Khodjakov A, et al. Androgen and Src signaling regulate centrosome activity. J Cell Sci. 2010;123:2094–102. doi: 10.1242/jcs.057505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murakami K, Fellous A, Baulieu EE, Robel P. Pregnenolone binds to microtubule-associated protein 2 and stimulates microtubule assembly. Proc Natl Acad Sci U S A. 2000;97:3579–84. doi: 10.1073/pnas.97.7.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dahlin A, Tantisira KG. Integrative systems biology approaches in asthma pharmacogenomics. Pharmacogenomics. 2012;13:1387–404. doi: 10.2217/pgs.12.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fairfax BP, Makino S, Radhakrishnan J, Plant K, Leslie S, Dilthey A, et al. Genetics of gene expression in primary immune cells identifies cell type-specific master regulators and roles of HLA alleles. Nat Genet. 2012;44:502–10. doi: 10.1038/ng.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zoorob RJ, Cender D. A different look at corticosteroids. Am Fam Physician. 1998;58:443–50. [PubMed] [Google Scholar]
- 37.Greenberg RA, Kerby G, Roosevelt GE. A comparison of oral dexamethasone with oral prednisone in pediatric asthma exacerbations treated in the emergency department. Clin Pediatr. 2008;47:817–23. doi: 10.1177/0009922808316988. [DOI] [PubMed] [Google Scholar]
- 38.Shefrin AE, Goldman RD. Use of dexamethasone and prednisone in acute asthma exacerbations in pediatric patients. Can Fam Physician. 2009;55:704–6. [PMC free article] [PubMed] [Google Scholar]
- 39. [May 18th, 2014];The SCAN database. Available at http://www.scandb.org/newinterface/about.html.
- 40.Gamazon ER, Zhang W, Konkashbaev A, Duan S, Kistner EO, Nicolae DL, et al. SCAN: SNP and copy number annotation. Bioinformatics. 2010;26:259–62. doi: 10.1093/bioinformatics/btp644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan WH1, Wu HJ, Shiao NH. Apoptotic signaling in methylglyoxal-treated human osteoblasts involves oxidative stress, c-Jun N-terminal kinase, caspase-3, and p21-activated kinase 2. J Cell Biochem. 2007;100:1056–69. doi: 10.1002/jcb.21114. [DOI] [PubMed] [Google Scholar]
- 42.Huang YT, Lai CY, Lou SL, Yeh JM, Chan WH. Activation of JNK and PAK2 is essential for citrinin-induced apoptosis in a human osteoblast cell line. Environ Toxicol. 2009;24:343–56. doi: 10.1002/tox.20434. [DOI] [PubMed] [Google Scholar]
- 43.Deepa SS, Dong LQ. APPL1: role in adiponectin signaling and beyond. Am J Physiol Endocrinol Metab. 2009;296:E22–36. doi: 10.1152/ajpendo.90731.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tu Q, Zhang J, Dong LQ, Saunders E, Luo E, Tang J, et al. Adiponectin inhibits osteoclastogenesis and bone resorption via APPL1-mediated suppression of Akt1. J Biol Chem. 2011;286:12542–53. doi: 10.1074/jbc.M110.152405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.