Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) has emerged as a major nosocomial pathogen that is widespread in both health care facilities and the community at large as a result of direct host-to-host transmission. Several virulence factors are associated with pathogen transmission to naive hosts. Immunodominant surface antigen B (IsaB) is a virulence factor that helps Staphylococcus aureus to evade the host defense system. However, the mechanism of IsaB on host transmissibility remains unclear. We found that IsaB expression was elevated in transmissible MRSA. Wild-type isaB strains inhibited autophagic flux to promote bacterial survival and elicit inflammation in THP-1 cells and mouse skin. MRSA isolates with increased IsaB expression showed decreased autophagic flux, and the MRSA isolate with the lowest IsaB expression showed increased autophagic flux. In addition, recombinant IsaB rescued the virulence of the isaB deletion strain and increased the Group A streptococcus (GAS) virulence in vivo. Together, these results reveal that IsaB diminishes autophagic flux, thereby allowing MRSA to evade host degradation. These findings suggest that IsaB is a suitable target for preventing or treating MRSA infection.
Keywords: Methicillin-resistant Staphylococcus aureus, immunodominant surface antigen B, virulence factor, autophagy, host transmission
INTRODUCTION
For several decades, MRSA has been recognized as a leading cause of nosocomial infections worldwide (Chatterjee and Otto, 2013; Hogea et al., 2013). MRSA infections occur predominantly in hospitals, in which case they are called hospital acquired-MRSA (HA-MRSA); however, the incidence of community-acquired MRSA (CA-MRSA) infections has been increasing (Jappe et al., 2008; Otto, 2010; Xia et al., 2013). MRSA can spread to the bloodstream and cause sepsis, a leading cause of shock and circulatory collapse (Tarkowski et al., 2001). MRSA can also spread to other organs, including the lung, kidney, liver, spleen, heart and bones, which results in severe infections such as urethritis, pneumonia, endocarditis and osteomyelitis (Haim et al., 2010). MRSA is becoming increasingly difficult to treat because multidrug-resistant strains of MRSA are on the rise, leading to high mortality rates in humans (Westling, 2009). The increasing morbidity and mortality associated with MRSA infections have motivated researchers to make MRSA prevention and therapy a priority. Transmission between hosts is a vital aspect of the biology of any commensal or pathogenic microorganism (Massey et al., 2006). For example, pathogenic bacteria utilize virulence factors that attack or allow evasion of the host antimicrobial defense system, promoting bacterial survival and allowing infection of the host (Kassegne et al., 2014; Liu et al., 2009; Lo et al., 2011; Ogawa et al., 2011; Orvedahl and Levine, 2009; Otto, 2012). In addition, virulence factors may be required for the transmission of pathogens to naive hosts (Wickham et al., 2007).
Autophagy is a ‘self-eating’ pathway that generates autophagosomes and delivers abnormal organelles or misfolded proteins to lysosomes for degradation during stress or nutrient deprivation (Mizushima et al., 2008). Autophagy serves as an antimicrobial defense mechanism that eliminates invading bacteria (a process called xenophagy) and plays a crucial role in host cell resistance to infection (Knodler and Celli, 2011; Parihar et al., 2014). Although the precise mechanisms of autophagy on bacteria remain unclear, autophagosomes and phagosomes are involved in recruiting intracellular bacteria into lysosomes for bulk degradation (Levine et al., 2011; Petruccioli et al., 2012). In contrast, invading bacteria express virulence factors that attenuate host autophagic flux and allow escape from host attack: for example, IcsB of Shigella flexneri and the deubiquitinase SseL of Salmonella Typhimurium (Ogawa et al., 2005) (Mesquita et al., 2012). Furthermore, S. flexneri expresses the T3SS effector IcsB, which interferes with the binding between the surface protein VirG and the host protein ATG5 and thereby allows S. flexneri to avoid targeting by host autophagy (Ogawa et al., 2005). Ssel, a deubiquitinase of Salmonella, reduces ubiquitinated aggregates and eliminates recruitment of the autophagy adaptor SQSTM1, which in turn evades host autophagy degradation and promotes Salmonella replication in cells (Mesquita et al., 2012). Interestingly, S. aureus employees autophagosomes to replicate in human cervical cancer HeLa cells or fibroblast cells (Schnaith et al., 2007), indicating that autophagy is detrimental to these host cells during S. aureus infection. Nevertheless, autophagy is induced to degrade intracellular bacteria and serves as a safeguard for host cells during S. aureus infection (Amano et al., 2006; Mauthe et al., 2012), suggesting that the role of autophagy in S. aureus infections is not fully understood.
S. aureus immunodominant surface antigen B (IsaB) is classified as both a secreted and a cell-surface associated virulence factor, and it elicits an immune response during septicemia (Mackey-Lawrence et al., 2009). Analysis of global transcripts showed that isaB expression is induced in response to certain conditions and factors such as the presence of glucose, human serum, plasma (Mackey-Lawrence and Jefferson, 2013a), biofilms, neutrophil exposure, anaerobic conditions, bacteremia and infections, and following internalization by human epithelial cells, suggesting a role in immune evasion and virulence (Fuchs et al., 2007; Garzoni et al., 2007; Resch et al., 2005; Voyich et al., 2005). However, the role of IsaB in the autophagy of MRSA-infected cells and in the transmission of MRSA is not defined. In this study, we found that the expression of the IsaB virulence factor was elevated in transmissible MRSA. IsaB was involved in susceptibility to MRSA infection and inflammation via inhibition of autophagic flux in both human macrophage-like THP-1 cells and in vivo. Moreover, group A streptococcus (GAS), an invading pathogen that is easily degraded by host autophagy, became more virulent in cells treated with recombinant IsaB. Our study suggests that IsaB is a suitable target for preventing and treating MRSA infection.
RESULTS
IsaB expression is induced in transmissible MRSA strain in vivo
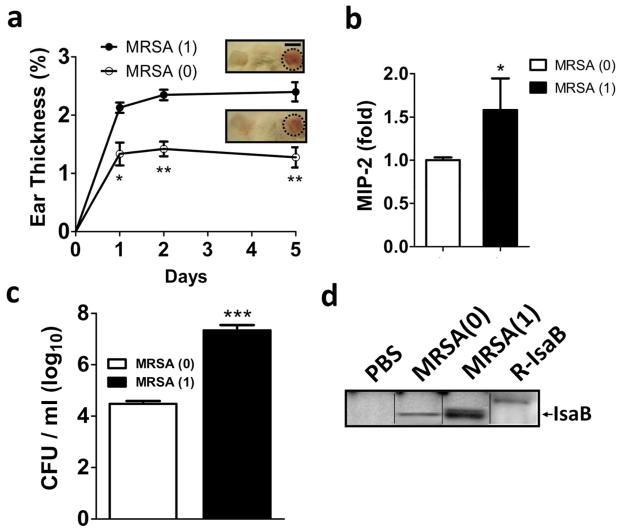
To compare the virulence of MRSA with and without host transmissibility, transmissible MRSA strain was isolated. After one host transmission of MRSA [MRSA(1)], severe ear inflammation (Figure 1a) and increased secretion of macrophage inflammatory peptide (MIP)-2 from skin tissue were observed in mice at day 5 post-infection (Figure 1b), in addition to a more than 100-fold increase in bacterial colonization of the ear skin (Figure 1c) over infection with parental MRSA [MRSA(0)]. These results suggest that after host transmission, MRSA became more virulent and caused severe skin inflammation and bacterial colonization. Because expression of IsaB is increased during S. aureus infection (Mackey-Lawrence and Jefferson, 2013a), we examined IsaB expression in transmissible MRSA by immunoblotting with the antibody generated against purified recombinant IsaB (Figure S1). The recombinant IsaB was validated by sequencing using nano-liquid chromatography linear trap quadrupole tandem mass spectrometry. Thirty-eight peptides were fully sequenced, and these matched well with the internal amino acids of MRSA IsaB (Table S1). We found that the expression level of IsaB was increased in transmissible MRSA (1) compared to that in parental MRSA (0) (Figure 1d). These results indicate that the virulence factor IsaB may be involved in MRSA host transmissibility.
Figure 1. Transmissible MRSA252 induces IsaB expression and inflammation in vivo.
(a) The right ears of mice were injected intradermally with MRSA(0) or MRSA(1) (1 × 107 CFU). Ear thickness was measured and calculated as a percentage, compared to the PBS-injected control, on days 1, 2, and 5 after MRSA injection. Representative images of skin inflammation are shown. Bar = 1 cm. (b) MIP-2 production in mice infected with MRSA(1) was measured by ELISA and normalized to that in mice infected with MRSA(0). (c) The infected ears on day 5 were homogenized for counting MRSA colonies. Data are expressed as the mean ± SEM (n=8). (d) The samples in (c) were subjected to immunoblotting. An arrow indicates IsaB protein, which has a molecular weight of 20 kDa.
IsaB diminishes autophagic flux to enhance bacterial survival in cells
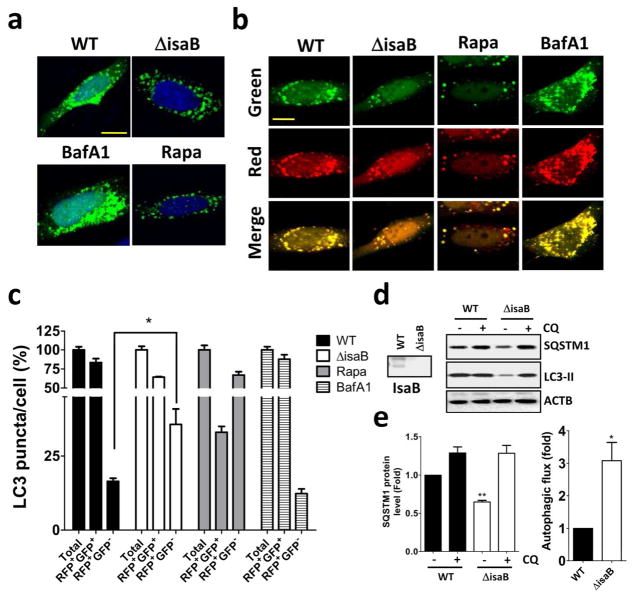
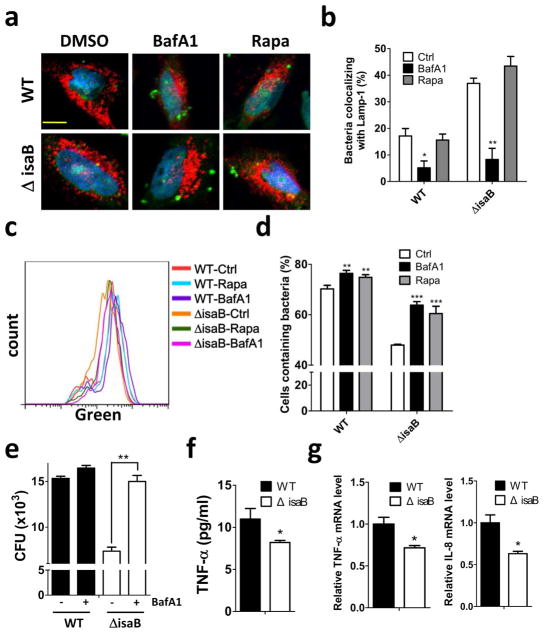
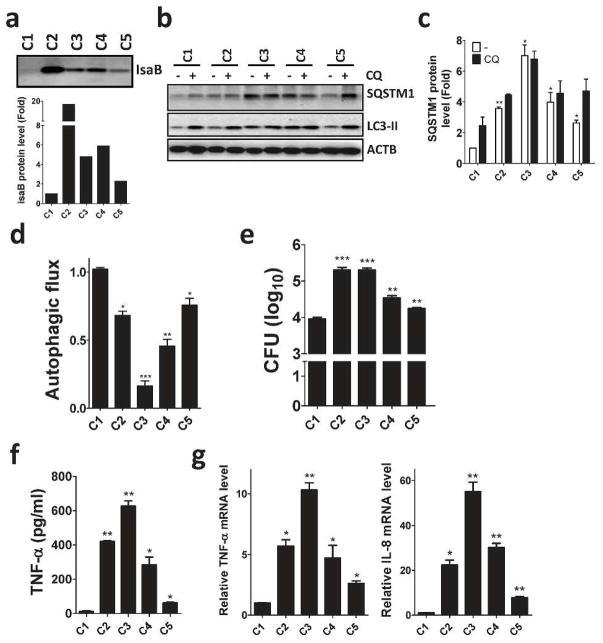
Autophagy plays a crucial role on the clearance of invading bacteria from host cells (Gong et al., 2012; Levine et al., 2011). To examine the potential mechanisms of virulence factor IsaB on MRSA transmissibility, wild-type and isogenic isaB deletion strains were used to infect human HeLa cells harboring GFP-MAP1LC3. Autophagy induction increases the number and intensity of small MAP1LC3-II puncta, whereas blocking the downstream steps of autophagosomes or lysosomes development not only increases the surface area and intensity of MAP1LC3-II puncta in the cytoplasm but also causes them to aggregate around the nuclear membrane (Zhang et al., 2007). We included rapamycin and bafilomycin A1 (BafA1) as controls for autophagic flux induction and inhibition, respectively (Figure 2a and 2b). In contrast, wild-type isaB strain apparently increased the surface area and intensity of GFP-MAP1LC3-II puncta in the cells (Figure 2a). HeLa cells harboring RFP-GFP-MAP1LC3 were further employed to inspect the role of IsaB on autophagic flux. Because GFP is quenched in acidic environments, such as that in an autolysosome, the RFP+GFP+ puncta and RFP+GFP− puncta were considered to be autophagosomes and autolysosomes, respectively (Zhou et al., 2012). The cells infected with wild-type strain had relatively fewer autolysosomes (RFP+GFP−) compared to the cells infected with isaB deletion strain (Figure 2b and 2c). Next, we examined MAP1LC3-II and SQSTM1 degradation in infected THP-1 cells cultured with or without chloroquine (CQ, an autophagic flux inhibitor) to determine the effect of IsaB on autophagic flux in host cells, as described previously (Liu et al., 2014). MAP1LC3-II and SQSTM1 proteins were accumulated in THP-1 cells when infected with wild-type strain (Figure 2d and 2e), indicating that IsaB may inhibit autophagic flux in host cells. To determine colocalization between bacteria and lysosomes, HeLa cells expressing the lysosomal marker Lamp1-RFP (Figure 3a) were infected with bacteria that were labeled with SYTO green fluorescent dye. The number of intracellular bacteria was increased in HeLa cells that were pretreated with BafA1, while the percentage of lysosomes colocalized with bacteria was reduced in these cells (Figure 3a and 3b). Furthermore, the survival of isaB deletion strain in THP-1 cells was significantly decreased (Figure 3c and 3d). The decreased bacterial survival of isaB deletion strain was recovered in both cells by pretreatment with rapamycin and BafA1 (Figure 3c, 3d, and 3e). However, silencing Atg5 and Ulk1, which are both essential genes for autophagosome formation, did not restore bacterial survival of isaB deletion strain in THP-1 cells (Figure S2), implying that IsaB may block autophagic flux and allow bacteria to survive in cells. We also found that TNF-α secretion was diminished in THP-1 cells infected with the isaB deletion strain compared with wild-type strain (Figure 3f). The mRNA levels of Tnf− α and Il-8 were reduced in the cells infected with isaB deletion mutant (Figure 3g), suggesting that IsaB may diminish autophagic flux, enhances the survival of S. aureus, and thereby elicit inflammation in host. More interestingly, clinical MRSA isolates expressing higher levels of IsaB accumulated more SQSTM1 protein and displayed less autophagic flux in THP-1 cells than MRSA (C1), which had the lowest IsaB expression level out of the 5 MRSA clinical isolates tested in this study (Figure 4). Moreover, production of the inflammatory cytokine TNF-α and mRNA levels of Tnf-α and Il-8 in the infected THP-1 cells were negatively correlated with autophagic activity (Figure 4), supporting the notion that IsaB may reduce autophagic flux and thereby allow MRSA to survive in the host cells. In addition to the role of bacterial virulence factors in autophagy, they are also involved in the induction of immune cell death, which may be important for bacterial survival and escape from host immune defense (Haim et al., 2010; Sumpter and Levine, 2010). Indeed, apoptosis was increased in macrophages infected with strains expressing high levels of IsaB (Figure S3a and S3b). By contrast, recombinant IsaB had no effect on apoptosis in macrophages (Figure S3c), suggesting that IsaB may have an additional role on cytotoxicity when bacteria are internalized into cells.
Figure 2. IsaB is involved in autophagic flux inhibition in cells.
(a) HeLa cells harboring GFP-MAP1LC3 or (b) RFP-GFP- MAP1LC3 were infected with wild-type (WT) S. aureus or an isogenic deletion mutant (ΔisaB) (MOI of 200) for 6 h to observe GFP-MAP1LC3 puncta and autophagic flux using confocal microscopy. Rapamycin (0.5 μM) or BafA1 (100 nM) are used as a control for autophagy induction and inhibition, respectively. Bar = 10 μm. (c) The percentage of autophagosomes (RFP+GFP+) and autolysosomes (RFP+GFP−) in each cell as (b) were quantitated from a pool of at least 10 images. (d) Human macrophage-like THP-1 cells were infected with wild-type (WT) S. aureus or an isogenic deletion mutant (ΔisaB) (MOI of 200) for 6 hrs in the presence or absence of 20 μM CQ. The accumulation of SQSTM1 and MAP1LC3-II in infected THP-1 cells was examined by immunoblotting. (e) The quantification of SQSTM1 protein levels and autophagic flux is shown. The results are expressed as the mean±SEM from 3 independent experiments.
Figure 3. Autophagic flux inhibition increases the viability of S. aureus in host cells.
HeLa cells harboring Lamp1-RFP (red) were infected with SYTO (green)-labeled wild-type (WT) S. aureus or an isogenic deletion mutant (ΔisaB) (MOI of 200) for 5 hrs in the absence or presence of BafA1 (100 nM) or rapamycin (0.5 μM). The cells were then fixed and observed under confocal microscopy. Bar = 10 μm. (b) Colocalization of bacteria and Lamp1 in cells as (a) was quantitated. (c) Human macrophage-like THP-1 cells were infected with SYTO (green)-labeled bacteria (MOI of 200) for 6 hrs with or without BafA1 (100 nM) or Rapamycin (0.5 μM). The infected cells were harvested to determine the amount of bacteria in the host cells using flow cytometer. (d) The percentage of bacteria in cells was quantitated by FlowJo software and analyzed by Prism 5.0. (e) Colony formation by the cells infected with bacteria (MOI of 200) for 6 hrs in the presence or absence of BafA1 was quantified. (f) The secretion of the inflammatory cytokine TNF-α and (g) the mRNA levels of Tnf-α and Il-8 in infected THP-1 cells were determined by ELISA and real-time PCR, respectively. The results are expressed as the mean ± SEM from 3 independent experiments.
Figure 4. IsaB expression in clinical MRSA isolates is associated with autophagy and inflammation in THP-1 cells.
(a) Clinical MRSA isolates were lysed to examine IsaB protein levels by immunoblotting. (b) Human macrophage-like THP-1 cells were infected with clinical MRSA isolates (MOI of 10) for 6 h in the presence (+) or absence (-) of 20 μM CQ for 2 h prior to harvesting. Cells were then lysed to examine SQSTM1, MAP1LC3-II protein levels by immunoblotting. Because the clinical MRSA isolate C1 shows the lowest IsaB expression of the 5 tested clinical MRSA isolates, the protein levels of (c) SQSTM1 and (d) MAP1LC3-II in the infected cells were quantified using the C1-infected cells as a control. (e) Colony formation, (f) secretion of TNF-α and (g) the mRNA levels of Tnf-α and Il-8 in infected THP-1 cells were determined by ELISA and real-time PCR, respectively. The results are expressed as the mean ± SEM from 3 independent experiments.
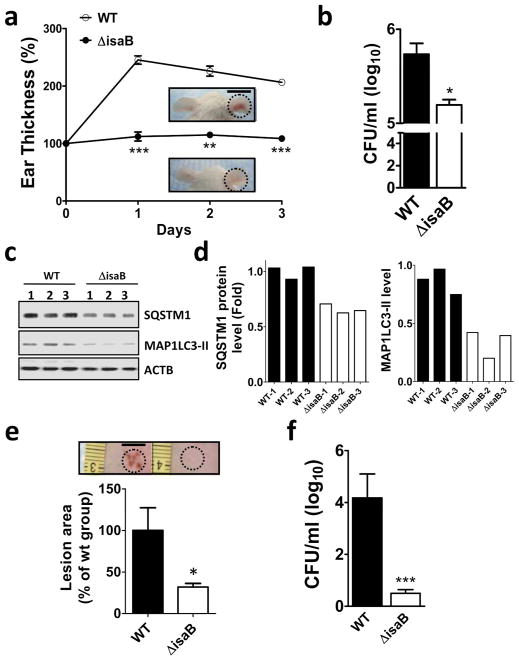
The wild-type isaB strain inhibits autophagic flux and increases survival in vivo
To examine the involvement of IsaB in MRSA virulence in vivo, mice ears were injected with a wild-type isaB strain or deletion strain and skin lesions were used as an indication of inflammation. At day 3, the ears injected with isaB deletion strain displayed significantly less inflammation than mice whose ears were injected with the wild-type strain (Figure 5a, WT). Next, the mouse ears injected with either the wild-type strain or isaB-deletion strain were excised and homogenized to assess bacterial colonization (Figure 5b), and immunoblotting of MAP1LC3 and SQSTM1 was conducted to assess host autophagic flux (Figure 5c and 5d). The survival of isaB-deletion strain was much lower than that of the wild-type strain in vivo (Figure 5b). In contrast to mouse ears infected with the isaB-deletion mutant, those infected with the wild-type strain showed increased accumulation of SQSTM1 and lapidated MAP1LC3-II, further confirming that IsaB inhibits host autophagic flux in vivo (Figure 5c and 5d). The lesion size and bacterial survival in mouse dorsal skin was also significantly reduced in the mice injected with the isaB deletion strain (Figure 5e and 5f). These results further support the notion that IsaB is involved in modulating autophagic flux to promote bacterial survival and elicit the host inflammatory response.
Figure 5. IsaB inhibits autophagic flux and triggers inflammation in vivo.
(a) The isaB wild-type (WT) or the isaB deletion (ΔisaB) S. aureus strain (1 × 107 CFU) was subcutaneously injected into the ears of mice (3 mice for each group). The ear thickness of each infected mouse was measured every day for 3 days. Representative images of ear skin inflammation are shown. Bar = 1 cm. (b) Infected right ears were homogenized on day 3 after injection to determine the level of bacterial colonization. (c) The homogenized ears were analyzed for SQSTM1 and MAP1LC3-II protein levels. (d) Quantified SQSTM1 and MAP1LC3-II protein levels in infected ears are shown. (e) The dorsal skin of mice was injected intradermally with wild type (WT) or isaB-deletion mutant (ΔisaB) strain (4 mice for each group). Representative images of dorsal skin lesions after 3 days of infection are shown and were quantified with ImageJ software. Bar = 0.5 cm. (f) Colonization by wild-type (WT) or isaB-deletion (ΔisaB) strain in the skin was counted on day 3 after injection. The results are expressed as the mean ± SEM.
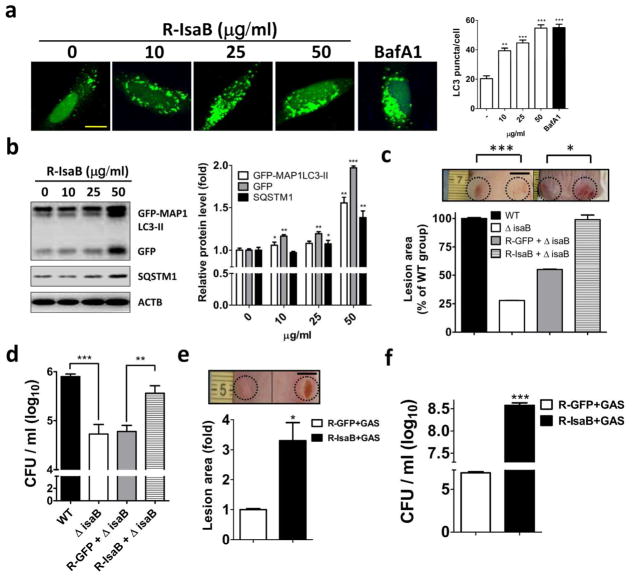
Recombinant IsaB inhibits autophagic flux in vitro and enhances bacterial colonization in vivo
To examine the role of IsaB on autophagic flux, cells were treated with recombinant MRSA IsaB and then assessed for MAP1LC3-II puncta accumulation, with BafA1 used as the positive control. We found that treatment with recombinant IsaB led to the accumulation of MAP1LC3-II puncta in a dose-dependent manner (Figure 6a). Likewise, recombinant IsaB resulted in significant accumulation of autophagic markers, including GFP-MAP1LC3-II, GFP and SQSTM1, in infected MEF stably expressing GFP-MAP1LC3 (Mizushima et al., 2010) (Figure 6b). Interestingly, the decreased lesion size in the mice skin that were infected with the isaB deletion strain was significantly restored when the mice were injected with recombinant IsaB, whereas recombinant GFP had little effect on virulence reconstitution (Figure 6c). Similar results were observed for bacterial colony formation (Figure 6d). Because intracellular GAS are degraded by host autophagy within 5 h post-infection (Yamaguchi et al., 2009), we hypothesized that exogenous MRSA IsaB might promote GAS virulence. Indeed, the lesion size and number of GAS colonies were significantly increased when mice were injected with recombinant IsaB compared to injections with recombinant GFP (Figure 6e and 6f), revealing that the virulence factor IsaB may inhibit autophagic flux and thereby eradicate bacterial degradation in vivo. Furthermore, the number of MRSA(1) colonies and lesion size in the ears and dorsal skin of IsaB-immunized mice were significantly lower than in GFP-immunized mice (Figure S4 and S5). These results show that IsaB is a potential vaccine for the MRSA.
Figure 6. Recombinant IsaB diminishes autophagic flux and reconstitutes the virulence of the isaB-deficient strain and GAS.
(a) HeLa cells harboring GFP-MAP1LC3 were treated with recombinant IsaB for 6 h and fixed to determine the effect of IsaB on GFP-MAP1LC3-II puncta, using florescence microscopy. BafA1 (100 nM) is used as a control for autophagic flux inhibition. Bar = 10 μm. (b) MEF cells stably harboring GFP-MAP1LC3 were treated with recombinant IsaB for 6 h. Protein levels of GFP-MAP1LC3-II, GFP, and SQSTM1 in treated cells were determined by immunoblotting. Protein levels were normalized with ACTB and are shown as quantified in the right panel. (c) Mice were injected with the isaB deletion strain (1 × 107 CFU) and 50 μg recombinant MRSA IsaB (R-IsaB ± ΔisaB) or GFP (R-GFP ±V isaB). Lesion sizes were quantified on day 3 after injection using ImageJ software (n = 4). Bar = 0.5 cm. (d) Bacterial survival on day 3 after injection was quantified in agar plates. (e) Live GAS NZ131 (1 × 107 CFU) mixed with 50 μg recombinant IsaB (R-IsaB+GAS) or GFP (R-GFP+GAS) was subcutaneously injected into the dorsal skin of mice. Lesion sizes and (f) bacterial survival on day 3 after injection were quantified using ImageJ software. Representative images of dorsal skin lesion are shown. Bar = 0.5 cm. The results are expressed as the mean ± SEM.
DISCUSSION
MRSA infection remains a significant threat to human health in the second decade of the 21st century. It is widely accepted that increased virulence of MRSA is associated with increased transmissibility (Massey et al., 2006). Virulence factors of MRSA strains have been reported to attenuate antimicrobial systems in host cells (Kraus and Peschel, 2008). Although several antibiotics are used to treat MRSA infection, multidrug-resistant strains of MRSA are a growing threat to public health (Han et al., 2007). Therefore, increasing our understanding of how MRSA virulence factors counteract host antimicrobial mechanisms can provide critical insight into the pathogenesis of MRSA and may advance preventive and therapeutic strategies. In this study, we report the following findings. First, the level of IsaB is elevated in transmissible MRSA(1), which is more virulent than parental MRSA(0). Second, elevated IsaB inhibits host autophagic flux and increase MRSA survival, thereby eliciting inflammation in both cell culture and animal models. Third, recombinant IsaB inhibits autophagic flux and increases host susceptibility to infection with GAS. These findings shed light on the virulence mechanisms of IsaB in skin pathogenesis during MRSA infection.
Autophagy is an innate antimicrobial defense mechanism that eliminates intracellular bacteria and reduces excessive inflammation in host cells (Petruccioli et al., 2012). Some bacteria have developed strategies to avoid degradation by host autophagic responses. These bacteria express virulence factors that block autophagy by inhibiting essential molecules or steps in autophagy, such as the ATG protein, autophagic recognition (Sumpter and Levine, 2010; Yoshikawa et al., 2009), and membrane (autophagosome and lysosome) fusion (Gong et al., 2012). However, the role of autophagy can be either beneficial or detrimental to host cells during S. aureus infection. S. aureus becomes trapped and is degraded in autophagosomes in human osteosarcoma U2OS cells (Mauthe et al., 2012), but accessory gene regulator (agr), a virulence factor, is involved in inducing autophagosomes to form a niche for S. aureus replication in HeLa cells (Schnaith et al., 2007). Our current data show that the level of IsaB is elevated in transmissible MRSA and that IsaB may negatively regulate autophagic flux in human macrophage-like THP-1 cells. Clinical MRSA isolates expressing high levels of IsaB also display reduced autophagic flux, increased survival, and elevated inflammatory cytokine production, in line with previous findings showing that autophagic flux is beneficial for host degradation of MRSA (Amano et al., 2006; Mauthe et al., 2012). Regarding the role of IsaB in autophagic flux, our study found that BafA1 largely rescued the survival of isaB deletion strain in THP-1 cells, whereas autophagy inhibition by RNAi had minimal effects on bacterial survival in the cells. BafA1, an inhibitor of vacuolar H+-ATPase, has been found to inhibit the acidification of lysosomes and further block the fusion of autophagosomes with lysosomes (Klionsky et al., 2008). IsaB expression in S. aureus is induced in acidic environments (pH 4-5) (Garzoni et al., 2007; Mackey-Lawrence and Jefferson, 2013b), implying that elevated IsaB in MRSA may prevent lysosomal acidification and indirectly impair autophagic flux. However, further study is required to determine the detailed mechanisms of IsaB on autophagy regulation in host cells.
Autophagy modulates pro-inflammatory transcription factors and IL-1β activation and secretion to suppress inflammation (Dupont et al., 2011; Harris et al., 2011; Yuk and Jo, 2013). Our results show that IsaB induces the production of the inflammatory cytokines TNF-α and IL-8, but not the activation or secretion of IL-1β (data not shown), suggesting that IsaB-inhibited autophagic flux may enhance the ability of pro-inflammatory transcription factors to augment inflammation. In addition to autophagy, immune cell death is also involved in pathogenic factor-induced inflammation. Our results also show that strains expressing high levels of IsaB induce apoptosis in macrophages. IsaB expression is elevated when S. aureus is internalized into human cells or exposed to neutrophils (Garzoni et al., 2007; Voyich et al., 2005). Neutrophils are the immune cells that immediately migrate to infected sites to kill S. aureus, mainly through opsonophagocytosis (van Kessel et al., 2014), implying that IsaB may play a role in opsonophagocytosis of neutrophils. Nevertheless, understanding the role of IsaB in neutrophil-mediated opsonophagocytosis will require further study aimed at clarifying these missing links.
In this study, we observed elevated levels of IsaB in transmissible MRSA. Elevated IsaB inhibits host autophagic flux and promotes MRSA virulence. Immunization with IsaB effectively protects mice from skin lesions caused by MRSA(1). Of note, IsaB is a secreted or cell surface virulence factor widely expressed in most strains of S. aureus, including MRSA and MSSA (Mackey-Lawrence and Jefferson, 2013a; Mackey-Lawrence et al., 2009). Thus, our study suggests that IsaB is a potential target for preventing or treating S. aureus infection, which could greatly benefit human health.
MATERIALS AND METHODS
Isolation of the transmissible MRSA252 strain
Female ICR mice (3 to 6 weeks old; Harlan) were maintained at the University of California, San Diego (UCSD), in accordance with institutional guidelines. MRSA252 (25 μl in PBS; 1 × 107 CFU), or 25 μl of PBS, used as a negative control, was subcutaneously injected into the central portion of the left ear of mice. Infected ear skin was harvested and homogenized on day 3 after infection. The homogenate was plated on tryptic soy agar to pick bacterial colonies for further experiments. The MRSA252 strain before host transmission was named MRSA(0) and MRSA252 after one generation of host transmission was named MRSA(1). All animal experiments were approved by UCSD Institutional Animal Care and Use committee.
Bacterial and cell culture
The MRSA252 strain, wild-type S. aureus 10833 strain, isaB deletion 10833ΔisaB::erm strain (with erythromycin antibiotic), and clinical MRSA isolates were cultured in 3% (w/v) trypticase soy broth (Sigma-Aldrich) under aerobic conditions at 37°C overnight with shaking (250 rpm). Human macrophage-like THP-1 cells were cultured in RPMI-1640 Medium (Invitrogen) and infected with bacteria for assessment of bacterial survival, inflammatory cytokine production and immunoblotting. MEF cells that stably expressing GFP-MAP1LC3 and human cervical cancer HeLa cells transfected with RFP-GFP-MAP1LC3, LAMP1-RFP or GFP-MAP1LC3 were cultured in DMEM (Invitrogen) and either infected with bacteria or treated with recombinant IsaB; autophagic flux and localization were then assessed with confocal microscopy. Detailed information about bacteria, antibodies, cytokines, plasmids and recombinant IsaB are described in the Supplementary Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants (R01-AI067395-01, R21-R022754-01, 1R41AR056169, and R21-I58002-01 to C-MH), the Ministry of Science and Technology (102-2311-B-075B-001 to C-WS) and Kaohsiung Veterans General Hospital (VGHKS103-110 to C-WS). We thank Dr. Kimberly K Jefferson and Dr. Noboru Mizushima for kindly providing the wild-type/isogenic isaB deletion strains and the MEF cells that stably expressing GFP-MAP1LC3, respectively. We also thank Dr. Tamotsu Yoshimori and Dr. Walther Mothes for sharing the plasmids pEGFP-MAP1LC3, RFP-GFP-MAP1LC3 and Lamp1-RFP.
ABBREVIATIONS
- MRSA
Methicillin-resistant Staphylococcus aureus
- IsaB
Immunodominant surface antigen B
- TNF-α
Tumor necrosis factor alpha
- IL-8
Interleukin 8
- SQSTM1
Sequestosome 1
- MAP1LC3
Microtubule-associated proteins 1A/1B light chain 3
- CFU
Colony-forming unit
- GAS
Group A Streptococcus
- MIP-2
Macrophage inflammatory protein-2
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Amano A, Nakagawa I, Yoshimori T. Autophagy in innate immunity against intracellular bacteria. Journal of biochemistry. 2006;140:161–6. doi: 10.1093/jb/mvj162. [DOI] [PubMed] [Google Scholar]
- Chatterjee SS, Otto M. Improved understanding of factors driving methicillin-resistant Staphylococcus aureus epidemic waves. Clin Epidemiol. 2013;5:205–17. doi: 10.2147/CLEP.S37071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont N, Jiang S, Pilli M, et al. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1beta. The EMBO journal. 2011;30:4701–11. doi: 10.1038/emboj.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S, Pane-Farre J, Kohler C, et al. Anaerobic gene expression in Staphylococcus aureus. J Bacteriol. 2007;189:4275–89. doi: 10.1128/JB.00081-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzoni C, Francois P, Huyghe A, et al. A global view of Staphylococcus aureus whole genome expression upon internalization in human epithelial cells. BMC Genomics. 2007;8:171. doi: 10.1186/1471-2164-8-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L, Devenish RJ, Prescott M. Autophagy as a macrophage response to bacterial infection. IUBMB life. 2012;64:740–7. doi: 10.1002/iub.1070. [DOI] [PubMed] [Google Scholar]
- Haim M, Trost A, Maier CJ, et al. Cytokeratin 8 interacts with clumping factor B: a new possible virulence factor target. Microbiology. 2010;156:3710–21. doi: 10.1099/mic.0.034413-0. [DOI] [PubMed] [Google Scholar]
- Han LL, McDougal LK, Gorwitz RJ, et al. High frequencies of clindamycin and tetracycline resistance in methicillin-resistant Staphylococcus aureus pulsed-field type USA300 isolates collected at a Boston ambulatory health center. J Clin Microbiol. 2007;45:1350–2. doi: 10.1128/JCM.02274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, Hartman M, Roche C, et al. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. The Journal of biological chemistry. 2011;286:9587–97. doi: 10.1074/jbc.M110.202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogea C, VANE T, Acosta CJ. A basic dynamic transmission model of Staphylococcus aureus in the US population. Epidemiol Infect. 2013:1–11. doi: 10.1017/S0950268813001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jappe U, Heuck D, Strommenger B, et al. Staphylococcus aureus in dermatology outpatients with special emphasis on community-associated methicillin-resistant strains. J Invest Dermatol. 2008;128:2655–64. doi: 10.1038/jid.2008.133. [DOI] [PubMed] [Google Scholar]
- Kassegne K, Hu W, Ojcius DM, et al. Identification of collagenase as a critical virulence factor for invasiveness and transmission of pathogenic Leptospira species. J Infect Dis. 2014;209:1105–15. doi: 10.1093/infdis/jit659. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Elazar Z, Seglen PO, et al. Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy. 2008;4:849–50. doi: 10.4161/auto.6845. [DOI] [PubMed] [Google Scholar]
- Knodler LA, Celli J. Eating the strangers within: host control of intracellular bacteria via xenophagy. Cell Microbiol. 2011;13:1319–27. doi: 10.1111/j.1462-5822.2011.01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus D, Peschel A. Staphylococcus aureus evasion of innate antimicrobial defense. Future microbiology. 2008;3:437–51. doi: 10.2217/17460913.3.4.437. [DOI] [PubMed] [Google Scholar]
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–35. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PF, Leung CM, Chang YH, et al. ATG4B promotes colorectal cancer growth independent of autophagic flux. Autophagy. 2014;10:1454–65. doi: 10.4161/auto.29556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PF, Lo CW, Chen CH, et al. Use of nanoparticles as therapy for methicillin-resistant Staphylococcus aureus infections. Curr Drug Metab. 2009;10:875–84. doi: 10.2174/138920009790274522. [DOI] [PubMed] [Google Scholar]
- Lo CW, Lai YK, Liu YT, et al. Staphylococcus aureus hijacks a skin commensal to intensify its virulence: immunization targeting beta-hemolysin and CAMP factor. J Invest Dermatol. 2011;131:401–9. doi: 10.1038/jid.2010.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey-Lawrence NM, Jefferson KK. Regulation of Staphylococcus aureus immunodominant antigen B (IsaB) Microbiol Res. 2013a;168:113–8. doi: 10.1016/j.micres.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey-Lawrence NM, Jefferson KK. Regulation of Staphylococcus aureus immunodominant antigen B (IsaB) Microbiol Res. 2013b;168:113–8. doi: 10.1016/j.micres.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey-Lawrence NM, Potter DE, Cerca N, et al. Staphylococcus aureus immunodominant surface antigen B is a cell-surface associated nucleic acid binding protein. BMC Microbiol. 2009;9:61. doi: 10.1186/1471-2180-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey RC, Horsburgh MJ, Lina G, et al. The evolution and maintenance of virulence in Staphylococcus aureus: a role for host-to-host transmission? Nat Rev Microbiol. 2006;4:953–8. doi: 10.1038/nrmicro1551. [DOI] [PubMed] [Google Scholar]
- Mauthe M, Yu W, Krut O, et al. WIPI-1 Positive Autophagosome-Like Vesicles Entrap Pathogenic Staphylococcus aureus for Lysosomal Degradation. International journal of cell biology. 2012;2012:179207. doi: 10.1155/2012/179207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita FS, Thomas M, Sachse M, et al. The Salmonella deubiquitinase SseL inhibits selective autophagy of cytosolic aggregates. PLoS Pathog. 2012;8:e1002743. doi: 10.1371/journal.ppat.1002743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, et al. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–26. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Yoshikawa Y, Mimuro H, et al. Autophagy targeting of Listeria monocytogenes and the bacterial countermeasure. Autophagy. 2011;7:310–4. doi: 10.4161/auto.7.3.14581. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Yoshimori T, Suzuki T, et al. Escape of intracellular Shigella from autophagy. Science. 2005;307:727–31. doi: 10.1126/science.1106036. [DOI] [PubMed] [Google Scholar]
- Orvedahl A, Levine B. Eating the enemy within: autophagy in infectious diseases. Cell death and differentiation. 2009;16:57–69. doi: 10.1038/cdd.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. Looking toward basic science for potential drug discovery targets against community-associated MRSA. Med Res Rev. 2010;30:1–22. doi: 10.1002/med.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. MRSA virulence and spread. Cell Microbiol. 2012;14:1513–21. doi: 10.1111/j.1462-5822.2012.01832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar SP, Guler R, Khutlang R, et al. Statin therapy reduces the mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J Infect Dis. 2014;209:754–63. doi: 10.1093/infdis/jit550. [DOI] [PubMed] [Google Scholar]
- Petruccioli E, Romagnoli A, Corazzari M, et al. Specific T cells restore the autophagic flux inhibited by Mycobacterium tuberculosis in human primary macrophages. J Infect Dis. 2012;205:1425–35. doi: 10.1093/infdis/jis226. [DOI] [PubMed] [Google Scholar]
- Resch A, Rosenstein R, Nerz C, et al. Differential gene expression profiling of Staphylococcus aureus cultivated under biofilm and planktonic conditions. Appl Environ Microb. 2005;71:2663–76. doi: 10.1128/AEM.71.5.2663-2676.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaith A, Kashkar H, Leggio SA, et al. Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. The Journal of biological chemistry. 2007;282:2695–706. doi: 10.1074/jbc.M609784200. [DOI] [PubMed] [Google Scholar]
- Sumpter R, Jr, Levine B. Autophagy and innate immunity: triggering, targeting and tuning. Seminars in cell & developmental biology. 2010;21:699–711. doi: 10.1016/j.semcdb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski A, Collins LV, Gjertsson I, et al. Model systems: modeling human staphylococcal arthritis and sepsis in the mouse. Trends Microbiol. 2001;9:321–6. doi: 10.1016/s0966-842x(01)02078-9. [DOI] [PubMed] [Google Scholar]
- van Kessel KP, Bestebroer J, van Strijp JA. Neutrophil-Mediated Phagocytosis of Staphylococcus aureus. Frontiers in immunology. 2014;5:467. doi: 10.3389/fimmu.2014.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyich JM, Braughton KR, Sturdevant DE, et al. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005;175:3907–19. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- Westling K. Cost-effectiveness analysis of treatment of methicillin-resistant Staphylococcus aureus bacteremia and endocarditis is a difficult issue. Clin Infect Dis. 2009;49:699–701. doi: 10.1086/604711. [DOI] [PubMed] [Google Scholar]
- Wickham ME, Brown NF, Boyle EC, et al. Virulence is positively selected by transmission success between mammalian hosts. Curr Biol. 2007;17:783–8. doi: 10.1016/j.cub.2007.03.067. [DOI] [PubMed] [Google Scholar]
- Xia JF, Gao JJ, Kokudo N, et al. Methicillin-resistant Staphylococcus aureus antibiotic resistance and virulence. Biosci Trends. 2013;7:113–21. [PubMed] [Google Scholar]
- Yamaguchi H, Nakagawa I, Yamamoto A, et al. An initial step of GAS-containing autophagosome-like vacuoles formation requires Rab7. PLoS Pathog. 2009;5:e1000670. doi: 10.1371/journal.ppat.1000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa Y, Ogawa M, Hain T, et al. Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nature cell biology. 2009;11:1233–40. doi: 10.1038/ncb1967. [DOI] [PubMed] [Google Scholar]
- Yuk JM, Jo EK. Crosstalk between autophagy and inflammasomes. Molecules and cells. 2013;36:393–9. doi: 10.1007/s10059-013-0298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yu J, Pan H, et al. Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc Natl Acad Sci U S A. 2007;104:19023–8. doi: 10.1073/pnas.0709695104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Zhong W, Zhou J, et al. Monitoring autophagic flux by an improved tandem fluorescent-tagged LC3 (mTagRFP-mWasabi-LC3) reveals that high-dose rapamycin impairs autophagic flux in cancer cells. Autophagy. 2012;8:1215–26. doi: 10.4161/auto.20284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.