Abstract
Although gliosis is a normal response to brain injury, reports on the extent of astrogliosis in the degenerating substantia nigra in Parkinson's disease (PD) are conflicting. It has also been recently suggested that accumulation of nigral α-synuclein in this disorder might suppress astrocyte activation which in turn could exacerbate the degenerative process. This study examined brain protein levels (intact protein, fragments, and aggregates, if any) of astroglial markers and their relationship to α-synuclein in PD and in the positive control parkinson-plus conditions multiple system atrophy (MSA) and progressive supranuclear palsy (PSP). Autopsied brain homogenates of patients with PD (n=10), MSA (n=11), PSP (n=11) and matched controls (n=10) were examined for the astroglial markers glial fibrillary acidic protein (GFAP), vimentin, and heat shock protein-27 (Hsp27) by quantitative immunoblotting. As expected, both MSA (putamen > substantia nigra > caudate > frontal cortex) and PSP (substantia nigra > caudate > putamen, frontal cortex) showed widespread but regionally specific pattern of increased immunoreactivity of the markers, in particular for the partially proteolyzed fragments (all three) and aggregates (GFAP). In contrast, immunoreactivity of the three markers was largely normal in PD in brain regions examined with the exception of trends for variably increased levels of cleaved vimentin in substantia nigra and frontal cortex. In patients with PD, GFAP levels in the substantia nigra correlated inversely with α-synuclein accumulation whereas the opposite was true for MSA. Our biochemical findings of generally normal protein levels of astroglial markers in substantia nigra of PD, and negative correlation with α-synuclein concentration, are consistent with some recent neuropathology reports of mild astroglial response and with the speculation that astrogliosis might be suppressed in this disorder by excessive α-synuclein accumulation. Should astrogliosis protect, to some extent, the degenerating substantia nigra from damage, therapeutics aimed at normalization of astrocyte reaction in PD could be helpful.
Keywords: Parkinson's disease, α-synuclein, astrogliosis, glial fibrillary acidic protein, vimentin, heat shock protein-27, multiple system atrophy, progressive supranuclear palsy, substantia nigra, quantitative Western blot
Introduction
The pathological hallmarks of idiopathic Parkinson's disease (PD) are loss of dopaminergic neurones originating in the substantia nigra (SN) (Hassler, 1938; Greenfield and Bosanquet, 1953; Ehringer and Hornykiewicz, 1960) and accumulation of α-synuclein-immunoreactive aggregates in neuronal (Spillantini et al., 1997; Wakabayashi et al., 1997) and glial cells (Wakabayashi et al., 2000; Hishikawa et al., 2001; Braak et al., 2007) (see (Jellinger, 2012) for an extensive review).
As the cause of this “α-synucleinopathy” is unknown, there is a need to identify useful therapeutic targets in PD. Gliosis, a normal response to brain injury, has historically been described as a core feature in PD (Hassler, 1953; Forno, 1966; Earle, 1968; Bernheimer et al., 1973; Forno, 1990; Paulus and Jellinger, 1991; Forno et al., 1992) (but see below) and recently, a type of gliosis, astrogliosis, has been suggested as a novel drug target (Fellner et al., 2011; Halliday and Stevens, 2011; McGann et al., 2012; Colangelo et al., 2014). Although the functional role of astrogliosis is still debated, there has been some movement in the literature from the assumption that neurodegeneration-triggered astrogliosis, by contributing to neuroinflammation, is fundamentally harmful, to the possibility that astroglial support, at least in the early stage of neurodegeneration, could be neuroprotective by decreasing vulnerability to neurotoxic insults (Glass et al., 2010; Sofroniew and Vinters, 2010; Kraft et al., 2013; Marchetti et al., 2013; Verkhratsky et al., 2013a; Verkhratsky et al., 2013b). In keeping with this speculation, it has been argued that decreased integrity of astroglial cells, possibly an α-synuclein-related feature of PD, could actually contribute to the loss of nigral neurons (Halliday and Stevens, 2011).
The success of therapeutic strategies in PD targeted to astrocytes could depend to some extent on astrocyte integrity and number. Although not generally appreciated, the literature is still surprisingly unclear on the status of nigral astrogliosis in PD (Table 1) (Forno, 1966; Forno, 1990; Forno et al., 1992; Damier et al., 1993; Damier et al., 1996; Banati et al., 1998; Knott et al., 1999; Renkawek et al., 1999; Mirza et al., 2000; Choi et al., 2005; Miklossy et al., 2006; Thannickal et al., 2007; Song et al., 2009; Kurowska et al., 2011; Lastres-Becker et al., 2012; Sathe et al., 2012). As early as 1953 Hassler noted that when nigral neurones are lost in PD, “...bildet sich eine Gliawucherung an ihrer Stelle, die zunächst dicht ist und sich später auflockert...” [there develops in their place a proliferation of glia, which is at first dense, later becoming more scattered] (Hassler, 1953). Later studies (e.g., the classic 1973 Bernheimer investigation (Bernheimer et al., 1973)) reported “moderate” to “dense” nigral gliosis in almost all examined patients with PD. However, most of these early studies did not explicitly identify glial cell type and the literature has been somewhat confusing given the later discovery of marked microgliosis in SN in PD (McGeer et al., 1988; Hirsch et al., 2005; McGeer and McGeer, 2008; Hirsch and Hunot, 2009; McGeer and McGeer, 2011). Even employing a “new” procedure (immunostaining for the glial fibrillary acidic protein [GFAP] (Eng and Ghirnikar, 1994); see Table 1 and Discussion) now considered more specific for identification of astroglial cells, in particular reactive astrocytes and fibrous astrocytes in the white matter, the findings have been conflicting – with a recent study reporting minimal nigral astrogliosis in PD ((Song et al., 2009), see (Bruck et al., 2015) for a review) and speculating that astrogliosis might have been suppressed by α-synuclein accumulated in the astrocytes (Halliday and Stevens, 2011).
Table 1.
Review of literature on GFAP-astrogliosis in substantia nigra pars compacta (SN) of patients with Parkinson's disease (PD)
| Reference | No. of subjects | Methods | Marker employed | Main findings of astroglial changesb and comments |
|---|---|---|---|---|
| Negative findings: | ||||
| Banati et al. 1998 | 10 | IHC | GFAP | 0 (4/10), 1 (5/10), 2 (1/10). In contrast to prominent microglial activation, GFAP staining of astrocytes was at most moderate in one case. |
| Mirza et al. 2000 | 5 | aIHC | GFAP MTs (I/II) |
No change in either density or morphology of astrocytes. |
| Song et al. 2009 | 13 | IHC | GFAP | No morphological change, with at most only mild increase in GFAP immunoreactivity in SN. |
| Kurowska et al. 2011 | 2 | IHC | GFAP | No astrogliosis in host SN or in graft of one of the patients in putamen; grafts of another patient with better clinical outcome had marked astrogliosis. |
| Positive findings: | ||||
| Forno et al. 1966, 1990, 1992 | Unknown | HE or Holzer, IHC | GFAP | Glial scars in areas with severe neuronal loss in SN that were better viewed by HE/Holzer than by GFAP. |
| Damier et al. 1993, 1996 | 4-5 | aIHC | GFAP GPx MAO-B |
Increased astrocyte density in SN by GFAP [+68%] (+62-82%), GPx [+83%] (+9-166%), and MAO-B [+203%]. Morphology was not described but the increase was correlated with neuronal loss. |
| Knott et al. 1999 | 11 | IHC | GFAP Vimentin |
Reactive astrocytes positive for GFAP and vimentin are located in the immediate vicinity of degenerated neurons, forming “scars” where extensive neuronal loss occurred. |
| Renkawek et al. 1999 | 12 | IHC | GFAP Hsp27 |
Moderate GFAP-positive astrogliosis in SN. |
| Choi et al. 2005 | 7 | IHC, WB | GFAP MPO |
+500% in MPO WB immunoreactivity in SN that was co-localized with those of GFAP-astrocytes although morphology was not described. |
| Miklossy et al. 2006 | 14 | IHC | GFAP ICAM-1 |
1 (3/14), 2 (11/14) for GFAP; 2 (7/14), 3 (7/14) for ICAM-1; reactive astrocytes were identified by both markers. |
| Thannickal et al. 2007 | 10 | IHC | GFAP | GFAP-astrocytes increased with disease stage. |
| Lastres-Becker et al. 2012 | 3 | IHC, WB | GFAP | +100% in GFAP WB. Morphological changes showed astrogliosis. |
| Sathe et al. 2012 | 6 | IHC, WB | GFAP S100B |
+25% in S100B WB. S100B largely co-localizes with GFAP in astrocytes but the morphology was not described. |
IHC = immunohistochemistry; HE = haematoxylin-eosin; WB = Western blot; GFAP = glial fibrillary acidic protein; MTs = metallothioneins; GPx = glutathione peroxidase; MAO-B = monoamine oxidase subtype B; Hsp27 = heat shock protein-27; MPO = myeloperoxidase; ICAM-1 = intercellular adhesion molecule-1.
Quantitative immunohistochemistry was used
Reactive astrocytes were rated as 0 (absence), 1 (mild), 2 (moderate), and 3 (severe/marked).
To address these uncertainties in the literature, we employed a simple quantitative immunoblotting approach to measure levels of several astrocytic marker proteins including GFAP, vimentin and heat shock protein-27 (Hsp27) (Tong et al., 2014) in homogenates of autopsied brain of patients with PD and, for comparison, in those with multiple system atrophy (MSA) (Adams et al., 1964; Gilman et al., 1999; Ozawa et al., 2004) and progressive supranuclear palsy (PSP) (Steele et al., 1964; Hauw et al., 1994; Togo and Dickson, 2002), with the latter disorders (as positive disease controls) considered to be characterized by marked and widespread pathological changes and gliosis (Togo and Dickson, 2002; Ozawa et al., 2004; Song et al., 2009). We paid special attention to the partially proteolyzed and aggregated species of the astroglial marker proteins as our recent data suggested that these species, probably formed as a result from increased protein turnover in activated or disturbed astrocytes, may be more sensitive markers than the intact and total proteins to detect brain pathological changes in the astrocyte (Tong et al., 2014). Our biochemical findings suggest that the astrocytic reaction in PD might be less than that in MSA and PSP and also support the speculation that α-synuclein could be toxic to astrocytes in this disorder.
Materials and Methods
Patients
Autopsied brains, collected at the Centre for Addiction and Mental Health in Toronto through collaboration with neurologists, were obtained from a total of 10 patients with PD, 11 patients with MSA, 11 patients with PSP, and 10 healthy control subjects. No significant difference (one-way ANOVA) was found in postmortem interval (PMI, in hours) (control: 12 ± 1; PD: 14 ± 2; MSA: 13 ± 2; PSP: 11 ± 1; mean ± SEM) or in age (years) (control: 70 ± 3; PD: 75 ± 2; MSA: 65 ± 3; PSP: 73 ± 3) among the four groups. One half-brain was used for neuropathological examination, whereas the other half was frozen for neurochemical analyses. The characteristics of the patients are summarized in Supplementary Table 1. No detailed information on the neuropsychological or mental function of the patients was available. The causes of death for the control subjects were cardiovascular illnesses (7), bronchopneumonia (2), and pulmonary edema (1).
Neuropathological assessment
Neuropathological findings (qualitative only) of neuronal loss by routine haematoxylineosin (HE) stain and assessments of the pathological hallmarks including Lewy bodies (LBs), glial cytoplasmic inclusions (GCIs) and neurofibrillary tangles (NFTs) in brains of patients with PD, MSA and PSP are summarized in Supplementary Table 1. HE stain of paraffin sections was assessed for neuronal loss qualitatively (0 = normal appearance; 1 = one focus of neuronal loss; 2 = more than one foci of neuronal loss; 3 = diffuse neuronal loss). LBs (HE) were confirmed in all of the PD patients (idiopathic) with significant LB pathology restricted to SN, locus coeruleus and other brain stem areas and with the anterior cingulate, hippocampus including transentorhinal cortex, and frontal and temporal neocortices showing no obvious LB pathology. In addition, biochemical analyses, previously conducted, confirmed accumulation of high molecular weight (HMW) α-synuclein species (using the monoclonal Syn-1, clone 42, Transduction Laboratories, Lexington, KY, USA) in SN in all of the PD cases, in putamen in three cases but not in the frontal cortex (Tong et al., 2010), confirming that the PD cases had brainstem-predominant α-synuclein accumulation. LBs were absent in all of the MSA cases. The presence of GCIs (by Bielschowsky silver impregnation and α-synuclein immunohistochemistry (1:100, mouse monoclonal LB509; Invitrogen, Frederick, MD, USA)) was confirmed in five MSA patients whereas the analysis of the remaining MSA patients was conducted prior to the consensus statement on the diagnosis of MSA (Gilman et al., 1999), which incorporated assessment of the neuropathology of GCIs. However, all MSA cases showed characteristic regional pattern of degenerative changes in the striatonigral and pontocerebellar brain regions. Further, characteristic heterogeneous accumulation of HMW α-synuclein species in widespread brain regions of all of the MSA cases was confirmed (Tong et al., 2010). For all of the PSP cases, pathological examination confirmed absence of LBs (although case #26 showed HMW α-synuclein accumulation in SN (Tong et al., 2010)) and the presence of neuronal loss in substantia nigra, globus pallidus, subthalamic nucleus, brainstem, and cerebellar dentate nucleus together with tau-positive neurofibrillary tangles (by Bielschowsky silver impregnation and tau immunohistochemistry (1;40,000, rabbit polyclonal; Dako, Carpinteria, CA, USA)) (Hauw et al., 1994). No systematic or quantitative assessment of gliosis (microglia and astrocytes) was performed during the neuropathological examination.
SDS-PAGE and western blotting
Brain dissection followed published procedures (Kish et al., 1988) using the atlas of Riley (Riley, 1943). Brain regions examined in this study included SN pars compacta, putamen, caudate, and frontal cortex (Brodmann area 9). SN samples (20-30 mg wet weight) were taken from the slice at the level of red nucleus, corresponding roughly to plates T4-1308 and T4-1443 of Riley (Riley, 1943); caudate and putamen samples were taken from the representative intermediate (along a dorsoventral gradient) subdivision of the middle (along a rostrocaudal gradient) portion of both nuclei, i.e. slices #5 or #6 for caudate and slices #7 or #8 for putamen as described in (Kish et al., 1988). Dissected tissue was homogenized (10×, vol/vol) by sonication in ice-cold 50 mM Tris-HCl, 2 mM EGTA, pH 7.4, containing 1% (vol/vol) protease inhibitors cocktail (cat# P8340, Sigma-Aldrich, St-Louis, MO). Aliquoted tissue homogenates were used for the quantitative immunoblotting assays of the glial marker proteins with a five-point tissue standards composed of a pooled human frontal cortical sample (see (Tong et al., 2014) for details and antibodies used). Levels of GFAP (in ng/μg protein) in the tissue standard were calibrated by sandwiched ELISA using purified porcine GFAP (cat# AG230, Chemicon International, Temecula, CA) as the standard (Tong et al., 2011b). Levels of the HMW aggregates of GFAP, the partially-digested low molecular weight (LMW) species of GFAP and vimentin, and the truncated Hsp27 were determined by interpolation from the same standards as for the respective intact protein. Glial marker findings for putamen only of MSA (as a positive control) and control subjects were previously reported (Tong et al., 2014). Levels of α-synuclein, either the full-length 17-kDa intact protein or the HMW species, in the SDS (10%)-solubilized membrane fraction (35,000 g × 15 min) of tissue homogenates were previously reported (Tong et al., 2010). Concentrations of the housekeeping “control” proteins neuron specific enolase (NSE) and α-tubulin were also determined by the same quantitative immunoblotting technique in the SDS-PAGE samples. For simplicity only, “immunoreactivity” of the proteins examined will be referred to as “levels”.
Statistical analyses
Statistical analyses on differences in levels of the glial markers among the control and the parkinsonian conditions were carried out using non-parametric Kruskal-Wallis ANOVA followed by Dunn's multiple comparison tests or uncorrected Mann-Whitney U tests because of non-normal distribution of some of the data, in particular for the proteolyzed species of the markers and markedly unequal variances in data distribution among the conditions (see Fig. 2 and Fig. 6 for examples). Differences in levels of the control proteins NSE and α-tubulin among the conditions were analyzed by ANOVA followed by post hoc Newman-Keuls multiple comparison tests. Correlations were examined by Pearson product-moment or Spearman rank order correlation analyses as indicated in the text. The criterion of statistical significance was P < 0.05.
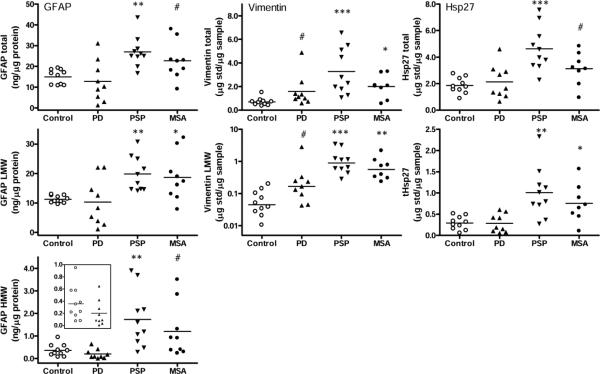
Fig. 2.
Substantia nigra. Scatter plots of levels (total, low molecular weight species [LMW] and high molecular weight aggregates [HMW]) of glial fibrillary acidic protein (GFAP), vimentin, and heat shock protein-27 (Hsp27) in controls and in patients with Parkinson's disease (PD), progressive supranuclear palsy (PSP) and multiple system atrophy (MSA). The inset shows the enlarged graph for GFAP aggregates in controls and PD. ***p<0.001, **p<0.01, and *p<0.05, PD, PSP, or MSA vs. controls (Kruskal-Wallis ANOVA followed by Dunn's multiple comparison tests; #p<0.05, PD, PSP, or MSA vs. controls (Mann-Whitney U-test in cases that failed Dunn's multiple comparison correction). tHsp27 = truncated Hsp27.
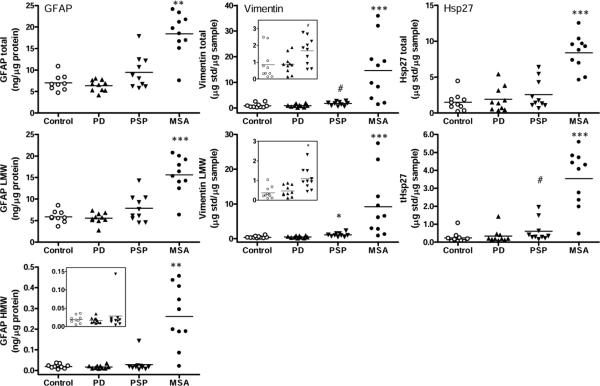
Fig. 6.
Putamen. Scatter plots of levels (total, low molecular weight species [LMW] and high molecular weight [HMW] aggregates) of glial fibrillary acidic protein (GFAP), vimentin, and heat shock protein-27 (Hsp27) in controls and in patients with Parkinson's disease (PD), progressive supranuclear palsy (PSP) and multiple system atrophy (MSA). The insets show enlarged graphs for vimentin and HMW GFAP in controls, PD and PSP. ***p<0.001, **p<0.01, and *p<0.05, PD, PSP, or MSA vs. controls (Kruskal-Wallis ANOVA followed by Dunn's multiple comparison tests; #p<0.05, PD, PSP, or MSA vs. controls (Mann-Whitney U-test in cases that failed Dunn's multiple comparison correction). tHsp27 = truncated Hsp27.
Results
Substantia Nigra Pars Compacta (SN)
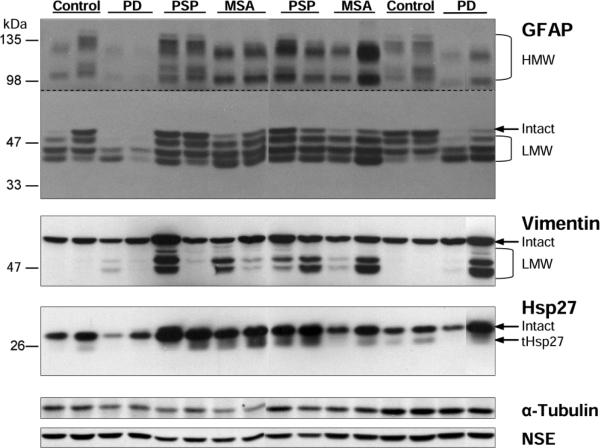
Similar to that reported in putamen of patients with MSA (Tong et al., 2011b; Tong et al., 2014), SN of both PSP and MSA patients showed the expected increased immunoreactivity for GFAP, vimentin and Hsp27. As shown in the representative immunoblots in Fig. 1, both total immunoreactivity (intact plus cleaved LMW species plus HMW aggregates, if any) and, in particular, that of the LMW species of the markers were increased in PSP and MSA. For GFAP, longer exposure also revealed the presence of two characteristic high molecular weight GFAP-immunoreactive protein bands at approximately 130 and 100 kDa, respectively, with levels correlated with those of the major GFAP bands at 43-50 kDa and markedly increased in PSP and MSA.
Fig. 1.
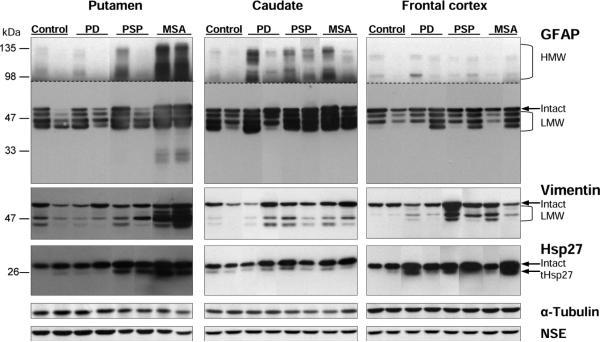
Substantia nigra: representative immunoblots of the astroglial markers including glial fibrillary acidic protein (GFAP), vimentin, and heat shock protein-27 (Hsp27) in controls and in patients with Parkinson's disease (PD), progressive supranuclear palsy (PSP) and multiple system atrophy (MSA). Protein bands for the intact markers and the partially proteolyzed low molecular weight (LMW) species and truncated Hsp27 (tHsp27) are identified. The top half of the blots for GFAP was over-exposed to show high molecular weight (HMW) aggregates of GFAP. Note that GFAP immunoreactivity was markedly decreased versus controls in some cases of PD whereas was significantly enhanced in both MSA and PSP, which also showed marked increase in immunoreactivity for vimentin and Hsp27, in particular the LMW species. Also shown are blots of the control proteins including α-tubulin and neuron specific enolase (NSE).
Quantitative data are presented in Table 2 (see scatter plots in Fig. 2 for distribution of individual values amongst the conditions). In general, the magnitude of difference between controls and the parkinsonian conditions was more marked for the LMW or HMW species than for the intact or total immunoreactivity of the markers. Patients with PSP had the most marked increase, with little overlap between individual patient and control values, in nigral levels of the astrocytic markers, expressed either in total (GFAP, +80%; vimentin, +373%; Hsp27, +149%), in only the LMW species (GFAP, +76%; vimentin, +1731%; Hsp27, +250%), or in the HMW species of GFAP (+384%). A milder, significant or non-significant trend for increased nigral levels of total (GFAP, +52%; vimentin, +187%; Hsp27, +68%), LMW (GFAP, +66%; vimentin, +982%; Hsp27, +162%) and HMW species (GFAP, +236%) of the markers was observed in MSA. In contrast, mean levels (total, LMW or HMW) of GFAP and Hsp27 in SN of PD were not significantly different from that of the controls. However, there was a trend for the variably increased levels of vimentin in SN of PD (total, +129%; LMW, +553%; or +70% and +131%, respectively, after exclusion of one outlier) that was significant in uncorrected Mann-Whitney U-tests (P=0.02 or 0.03 without the outlier). Levels of the control proteins NSE and α-tubulin were significantly decreased in SN of PSP (−47% and −27%, respectively) and MSA (−33% and −27%, respectively). In SN of PD, levels of NSE were normal whereas that of α-tubulin were decreased mildly (−14%; see also Fig. 1 for representative immunoblots of NSE and α-tubulin).
Table 2.
Levels (total, LMW and HMW species) of the astroglial markers GFAP, vimentin, and Hsp27 and the control proteins NSE and α-Tubulin in brain of patients with PD, PSP, and MSA
| Control (n=10) | PD (n=9-10) | PSP (n=10-11) | MSA (n=8-10) | P | |
|---|---|---|---|---|---|
| Substantia Nigra pars compacta | |||||
| GFAP-total | 15.0 ± 1.12 | 12.7 ± 3.30 | 27.0 ± 2.32** (+80%) |
22.71 ± 3.03# (+52%) |
0.001 |
| -LMW | 11.2 ± 0.40 | 10.3 ± 2.67 | 19.8 ± 1.85** (+76%) |
18.7 ± 2.69* (+66%) |
0.002 |
| -HMW | 0.36 ± 0.09 | 0.20 ± 0.07 | 1.73 ± 0.40** (+384%) |
1.20 ± 0.40# (+236%) |
0.0003 |
| Vimentin-total | 0.70 ± 0.10 | 1.59 ± 0.45# (+129%) |
3.29 ± 0.62*** (+373%) |
1.99 ± 0.35* (187%) | 0.0005 |
| -LMW | 0.07 ± 0.02 | 0.46 ± 0.30# (+553%) |
1.29 ± 0.36*** (+1731%) |
0.76 ± 0.23** (+982%) |
<0.0001 |
| Hsp27-total | 1.86 ± 0.19 | 2.14 ± 0.42 | 4.64 ± 0.53*** (+149%) |
3.13 ± 0.44# (+68%) |
0.0005 |
| -tHsp27 | 0.29 ± 0.05 | 0.28 ± 0.07 | 1.01 ± 0.19** (+250%) |
0.75 ± 0.16* (+162%) |
0.0007 |
| NSE | 0.94 ± 0.06 | 0.90 ± 0.04 | 0.50 ± 0.04*** (−47%) |
0.63 ± 0.07** (−33%) |
<0.0001 |
| α-Tubulin | 1.36 ± 0.05 | 1.17 ± 0.07* (−14%) |
0.99 ± 0.06** (−27%) |
0.99 ± 0.07** (−27%) |
0.0006 |
| Putamen | |||||
| GFAP-total | 7.01 ± 0.62 | 6.39 ± 0.41 | 9.49 ± 1.22 | 18.46 ± 1.53** (+163%) |
0.0001 |
| -LMW | 5.87 ± 0.47 | 5.58 ± 0.40 | 7.86 ± 0.99 | 15.63 ± 1.35*** (+166%) |
0.0002 |
| -HMW | 0.019 ± 0.003 | 0.017 ± 0.003 | 0.028 ± 0.013 | 0.261 ± 0.47** (+1290%) |
0.0004 |
| Vimentin-total | 0.85 ± 0.28 | 0.88 ± 0.18 | 1.68 ± 0.25# (+97%) |
14.61 ± 3.82*** (+1614%) |
<0.0001 |
| -LMW | 0.37 ± 0.09 | 0.47 ± 0.09 | 1.10 ± 0.18* (+200%) |
9.18 ± 2.90*** (+2412%) |
<0.0001 |
| Hsp27-total | 1.51 ± 0.39 | 1.92 ± 0.54 | 2.58 ± 0.65 | 8.40 ± 0.77*** (+455%) |
<0.0001 |
| -tHsp27 | 0.23 ± 0.10 | 0.34 ± 0.13 | 0.60 ± 0.19# (+158%) |
3.54 ± 0.50*** (+1408%) |
<0.0001 |
| NSE | 1.09 ± 0.06 | 1.17 ± 0.06 | 1.14 ± 0.10 | 0.49 ± 0.11*** (−56%) |
<0.0001 |
| α-Tubulin | 1.01 ± 0.06 | 1.07 ± 0.07 | 1.02 ± 0.06 | 0.67 ± 0.08** (−33%) |
0.001 |
| Caudate | |||||
| GFAP-total | 6.27 ± 1.20 | 8.91 ± 1.15 | 11.22 ± 0.55* (+79%) |
10.41 ± 1.16# (+66%) |
0.03 |
| -LMW | 4.73 ± 0.91 | 6.66 ± 0.93 | 8.28 ± 0.59* (+75%) |
8.37 ± 0.89* (+77%) |
0.02 |
| -HMW | 0.020 ± 0.007 | 0.039 ± 0.009 | 0.039 ± 0.006# (+98%) |
0.038 ± 0.007 | 0.14 |
| Vimentin-total | 2.79 ± 0.41 | 4.45 ± 0.73 | 5.73 ± 0.52** (+105%) |
4.07 ± 0.46 | 0.007 |
| -LMW | 1.02 ± 0.23 | 2.10 ± 0.44 | 2.77 ± 0.35** (+171%) |
2.16 ± 0.29# (+112%) |
0.007 |
| Hsp27-total | 2.11 ± 0.16 | 2.39 ± 0.18 | 3.32 ± 0.34** (+57%) |
2.89 ± 0.24# (+37%) |
0.006 |
| -tHsp27 | 0.24 ± 0.04 | 0.32 ± 0.04 | 0.50 ± 0.10# (+109%) |
0.62 ± 0.10** (+158%) |
0.007 |
| NSE | 1.30 ± 0.05 | 1.30 ± 0.04 | 1.36 ± 0.04 | 1.11 ± 0.07* (−15%) |
0.007 |
| α-Tubulin | 1.36 ± 0.08 | 1.28 ± 0.05 | 1.21 ± 0.06 | 1.11 ± 0.06* (−19%) |
0.049 |
| Frontal cortex | |||||
| GFAP-total | 3.11 ± 0.70 | 4.24 ± 0.63 | 4.83 ± 0.49 | 4.46 ± 0.70 | 0.19 |
| -LMW | 1.88 ± 0.54 | 2.68 ± 0.53 | 3.09 ± 0.33 | 3.16 ± 0.57 | 0.09 |
| -HMW | 0.014 ± 0.004 | 0.022 ± 0.003 | 0.024 ± 0.006 | 0.019 ± 0.004 | 0.20 |
| Vimentin-total | 1.14 ± 0.20 | 1.65 ± 0.23 | 2.82 ± 0.68# (+148%) |
1.86 ± 0.40 | 0.07 |
| -LMW | 0.21 ± 0.05 | 0.47 ± 0.06# (+128%) |
1.10 ± 0.39*** (+430%) |
0.69 ± 0.20** (+233%) |
0.0006 |
| Hsp27-total | 1.80 ± 0.14 | 2.49 ± 0.19# (+39%) |
2.55 ± 0.15# (+42%) |
2.05 ± 0.28 | 0.02 |
| -tHsp27 | 0.19 ± 0.03 | 0.30 ± 0.05 | 0.32 ± 0.04 | 0.31 ± 0.09 | 0.15 |
| NSE | 1.20 ± 0.05 | 1.24 ± 0.06 | 1.26 ± 0.08 | 1.31 ± 0.08 | 0.72 |
| α-Tubulin | 1.07 ± 0.05 | 1.08 ± 0.06 | 1.07 ± 0.06 | 1.14 ± 0.09 | 0.86 |
Data, expressed in mean ± SEM (%change versus control in cases with significant changes), are in μg std/μg sample protein except those for GFAP that are in ng/μg protein. GFAP = glial fibrillary acidic protein; Hsp27 = heat shock protein-27; tHsp27 = truncated Hsp27; NSE = neuron specific enolase; LMW = low molecular weight; HMW = high molecular weight; PD = Parkinson's disease; PSP = progressive supranuclear palsy; MSA = multiple system atrophy.
p<0.001
p<0.01
p<0.05, PD, PSP, or MSA vs. controls (Kruskal-Wallis ANOVA followed by Dunn's multiple comparison tests for GFAP, vimentin, and Hsp27; one-way ANOVA followed by post hoc Newman-Keuls multiple comparison tests for NSE and α-Tubulin)
p<0.05, PD, PSP, or MSA vs. controls (Mann-Whitney U-test in cases that failed Dunn's multiple comparison correction).
Amongst the patients with PD, a wide scatter of GFAP immunoreactivity was observed in SN. Five of the nine patients had GFAP levels (total, LMW) below the lowest value of the controls (Fig. 2; see Fig. 1 for example blots).
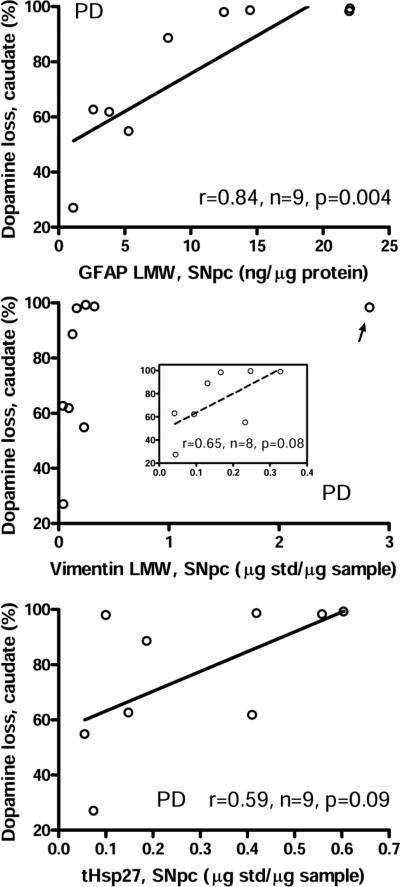
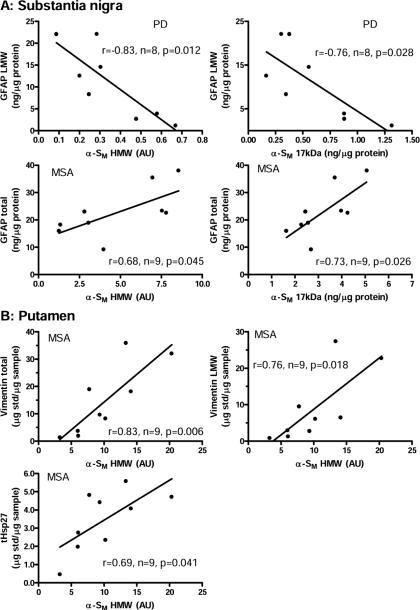
Levels of astroglial markers in SN were positively correlated (Pearson) with the extent of dopamine loss previously reported (Tong et al., 2010) in the caudate (Fig. 3) and putamen (data not shown) in PD. Significant negative correlations (Pearson) were observed between LMW GFAP immunoreactivity and previously reported (Tong et al., 2010) levels of α-synuclein in the membrane fraction (either intact 17 kDa α-synuclein or HMW species) in SN in PD (r=−0.76 and −0.83, P = 0.028 and 0.012, respectively; Fig. 4A) (supplementary Table 2 provides all correlations examined between levels of the glial markers and α-synuclein accumulation).
Fig. 3.
Correlations (Pearson) between the extent of dopamine loss (expressed as percentage loss) reported previously (Tong et al., 2010) in the caudate nucleus and levels of the low molecular weight species (LMW) of glial fibrillary acidic protein (GFAP), vimentin, and truncated heat shock protein-27 (tHsp27) in the substantia nigra of patients with Parkinson's disease (PD). The inset shows enlarged portion and linear correlation for vimentin without the outlier (arrow).
Fig. 4.
Correlations (Pearson) between levels (total or low molecular weight species [LMW]) of glial fibrillary acidic protein (GFAP), vimentin, and truncated heat shock protein-27 (tHsp27) and that of α-synuclein (17 kDa intact or high molecular weight species [HMW]) accumulation in the membrane fraction (α-SM) reported previously (Tong et al., 2010) in substantia nigra (A) of patients with Parkinson's disease (PD) and multiple system atrophy (MSA) and in putamen (B) of MSA. AU = arbitrary unit.
Unlike the situation in PD, α-synuclein accumulation (17 kDa or HMW) in SN of MSA correlated (Pearson) positively with GFAP levels (total) (r=0.73 and 0.68, P = 0.026 and 0.045, respectively; Fig. 4A; see also supplementary Table 2). No significant correlation was observed between levels of the astroglial markers (total, LMW, or HMW) and those of 17 kDa α-synuclein in SN of PSP. Among the PSP cases examined, only one subject had a marked above-normal accumulation of HMW α-synuclein in SN (case #5 of (Tong et al., 2010)).
Putamen, Caudate, and Frontal Cortex
In the striatum (caudate and putamen), astroglial marker levels generally paralleled the extent of neuronal loss reported in the literature (Togo and Dickson, 2002; Ozawa et al., 2004; Song et al., 2009), with PD striatum showing no statistically significant change, and with putamen more affected than caudate in MSA, and the reverse for PSP (Table 2; see Fig. 5 for representative immunoblots and Fig. 6 and supplementary Fig. 1 for the scatter plots for putamen and caudate, respectively). In general, again, the LMW or HMW species of the markers had higher magnitude of change than the intact or total immunoreactivity. Despite the lack of significant change in PD striatum, caudate but not the more dopamine depleted putamen showed noticeably high levels of both aggregated GFAP and cleaved vimentin (with Pearson correlation r=0.87, n=10, P=0.001 between the two measures) in four (out of 10) PD cases (see Fig. 5 for examples of blots and supplementary Fig. 1 for scatter plots). No significant difference from controls was observed in levels of NSE and α-tubulin in the striatum of PD and PSP whereas levels of the control proteins were decreased mildly (caudate, −15% and −19%, respectively) to markedly (putamen, −56% and −33%, respectively) in MSA striatum.
Fig. 5.
Putamen, caudate and frontal cortex: representative immunoblots of the astroglial markers including glial fibrillary acidic protein (GFAP), vimentin, and heat shock protein-27 (Hsp27) in controls and in patients with Parkinson's disease (PD), progressive supranuclear palsy (PSP) and multiple system atrophy (MSA). Protein bands for the intact markers and the partially proteolyzed low molecular weight (LMW) species and truncated Hsp27 (tHsp27) are identified. The top half of the blots for GFAP was over-exposed to show high molecular weight (HMW) aggregates of GFAP. Note markedly increased immunoreactivity (especially of the HMW and LMW species) in MSA putamen and in PSP/MSA caudate. Also shown are blots of the control proteins including α-tubulin and neuron specific enolase (NSE).
In the striatum, statistically significant positive correlations (Pearson) were observed between HMW α-synuclein accumulation and levels of vimentin (total, r=0.83, P=0.006; LMW; r=0.76, P=0.018) or truncated Hsp27 (tHsp27, r=0.69, P=0.041) in MSA putamen (Fig. 4B; see also supplementary Table 2).
In the frontal cortex (Table 2), no significant difference from controls was observed in GFAP levels in the parkinsonian conditions. Significant or trends for increase in levels of vimentin and Hsp27 were observed in PSP (vimentin-LMW, +430%; vimentin total, +148%; Hsp27 total, +42%), MSA (vimentin-LMW, +233%), and PD (vimentin-LMW, +128%; Hsp27 total, +39%) (see Fig. 5 for examples of blots and supplementary Fig. 2 for scatter plots). Levels of NSE and α-tubulin were all normal in the parkinsonian frontal cortices.
No statistically significant correlation was observed between regional levels of the glial markers and PMI, age, disease duration, levodopa treatment, or clinical subtypes of the subjects including MSA with predominant cerebellar features (MSA-C) versus MSA with predominant parkinsonian features (MSA-P). There was also no significant correlation between nigral or striatal levels of the astroglial markers and dopamine loss in the striatum in either MSA or PSP, consistent with the widespread pathological changes in the Parkinson-plus conditions.
Discussion
We found that changes, on average, in levels of astrocyte markers in SN of patients with PD were much milder than those in MSA and PSP and were negatively correlated with α-synuclein levels. This provides support for the speculation that α-synuclein accumulation in PD might suppress astrocyte activation (Halliday and Stevens, 2011) – in principle, a potentially deleterious event.
Astroglial markers in PD: the literature vs. present findings
It is surprising that after more than 60 years of neuropathological investigation, the status of astrogliosis in SN of patients with PD is uncertain (Bruck et al., 2015). Historically, marked nigral ”gliosis” has been reported (Hassler, 1953; Forno, 1966; Earle, 1968; Bernheimeret al., 1973; Paulus and Jellinger, 1991) in PD. With general stains, such as HE (Forno, 1966; Earle, 1968; Forno, 1990; Forno et al., 1992) or Kluever-Barrera (Paulus and Jellinger, 1991), it may be difficult to formally identify astrocytes. However, more specific stains such as Holzer (Forno, 1966; Forno et al., 1992) and Arendt-Kanzler (Bernheimer et al., 1973), revealing fibrous astrocytes, were also used. Later discovery of marked microgliosis in SN of PD, similar to that in MSA and PSP, may have also made the situation somewhat confusing as activation of microglia and astrocytes often coexists in many neurodegenerative conditions. The advent of GFAP immunohistochemistry, now the standard tool for assessment of astrogliosis, has been claimed as a more sensitive and specific method to detect astroglial activation (Kamo et al., 1987; Eng and Ghirnikar, 1994). However, even when employing the now routine GFAP antibody, the question of nigral astrogliosis in PD is unresolved, with contradictory results obtained for the two quantitative investigations (Damier et al., 1993; Mirza et al., 2000) (Table 1). Thus, some reviews suggest that reactive astrogliosis in SN of PD is “abundant” (McGeer and McGeer, 2008), “massive” (Hirsch et al., 2005), or “at most mild” (Vila et al., 2001). We addressed some of the above issues by employing antibodies to three proteins, namely GFAP, vimentin, and Hsp27, considered being different components of astroglial filaments that are important to the structure, propagation, hypertrophy, and scar formation of astroglial cells (see (Tong et al., 2014) for discussion on limitations of the markers). For ease of quantitation and also for assessment of turnover of the marker proteins upon gliosis, we employed a simple Western blot assay in brain homogenates.
Consistent with results of the qualitative assessment of GFAP immunohistochemistry reported by Song (Song et al., 2009), differences (vs. control values) in brain glial marker (especially vimentin and its truncated forms and aggregates of GFAP) levels were generally more marked in brain in MSA (e.g., 17 fold increase in putamen) and PSP (five fold increase in SN) than in PD. Findings in the striatum and frontal cortex of PD were largely negative although there were indications (e.g., GFAP aggregation and vimentin truncation) of astroglial activation in the caudate and frontal cortex at least in some of the patients, which is consistent with some literature ((Mythri et al., 2011; Charron et al., 2014), but see (Banati et al., 1998; Mirza et al., 2000)). In the SN, in which nigral neuronal damage was roughly comparable amongst the three disorders by qualitative neuropathological assessment (Supplementary Table 1) and by similar extent of loss of the vesicular monoamine transporter 2 (VMAT2) in SN (Tong et al., 2011a) and dopamine in the striatum (Tong et al., 2010), there was only a trend for increased levels of one of the markers (vimentin) in PD, whereas concentrations of one or more glial markers were significantly above-normal in the other conditions. Interestingly, for GFAP, presently the most commonly used astroglial marker, levels of this marker (total, LMW, and HMW) in about half of the PD cases were actually below the lowest value of the controls. In this respect, our quantitative results are consistent with some previous “negative” reports of astrogliosis in PD substantia nigra (Banati et al., 1998; Mirza et al., 2000; Song et al., 2009; Kurowska et al., 2011) (Table 1) and the notion that astroglial changes in PD are relatively mild as compared to those in PSP and MSA (Song et al., 2009). Nevertheless, our biochemical data do show high variability among subjects (see Fig. 2) and suggest some astrocytic disturbance in PD. In this regard, although nigral levels of intact glial markers were not significantly above-normal, we did observe increased (+553%; +131% if excluding an outlier) nigral concentrations of truncated vimentin and higher percentage ratio of truncated versus intact vimentin in PD (35%) than in controls (11%). [Note however that this “turnover” ratio in PD was still less than those in MSA (78%) and PSP (67%)]. As we have previously suggested (Tong et al., 2014), increased levels of glial marker fragments in the absence of changes in intact protein might represent an early stage of astrocytic pathology associated with increased astrocytic remodeling or filament breakdown that might not be easily detected by immunohistochemical procedures.
Does α-synuclein suppress astrogliosis?
Halliday and colleagues (Halliday and Stevens, 2011) speculate that accumulation in astrocytes of α-synuclein, the presumed key etio-pathological factor in PD and other synucleinopathies, might damage astrocytes in the SN and in so doing promote degeneration of dopamine neurones. The first part of this speculation is now supported by our observation of an inverse correlation between nigral α-synuclein and GFAP levels in PD (non-significant trends for other astroglial markers). The specificity of our finding is suggested by a positive correlation between levels of astrocytic markers and α-synuclein in both SN and putamen of MSA. Although it is possible that the stage of disease progression in PD might have influenced astroglial status and α-synuclein accumulation differently, the relationship between disease stage and α-synuclein accumulation is still not clear. Previously (Tong et al., 2010), we did not observe any correlation between levels of α-synuclein and pathological rating of LBs or disease duration/stage in PD. Greffard has reported no change in nigral α-synuclein burden with disease duration (Greffard et al., 2010) whereas a positive correlation between nigral α-synuclein burden and dopamine neuronal damage has been observed in one investigation (Kovacs et al., 2008).
The difference between PD and MSA could be explained by different magnitude of α-synuclein accumulation (Tong et al., 2010) and more specifically, localization of some α-synuclein to astrocytes in PD (Wakabayashi et al., 2000; Hishikawa et al., 2001; Braak et al., 2007), which is correlated with Lewy pathology (Hishikawa et al., 2001), whereas in MSA there is little accumulation of the protein in astrocytes ((Song et al., 2009), but see (Wenning and Jellinger, 2005)), with massive α-synuclein accumulation predominantly localized to oligodendrocytes in this disorder (Tu et al., 1998; Wakabayashi et al., 1998). One Investigation suggests that astrocytes that accumulate α-synuclein in PD are mainly of the protoplasmic type whereas reactive astrocytosis in MSA might affect mainly fibrous astrocytes (Song et al., 2009); however, a majority of the previous studies did not distinguish the two astrocyte subtypes. The mechanism by which α-synuclein might inhibit nigral astrogliosis in PD is conjectural but could involve suppression of proteasomal (Middeldorp et al., 2009) and/or mitochondrial (Schmidt et al., 2011; Braidy et al., 2013) functions (see also (Halliday and Stevens, 2011)). Astrocytes, which normally express low levels of α-synuclein (Maroteaux et al., 1988; Shibayama-Imazu et al., 1993) (but see (Mori et al., 2002)), can take up α-synuclein originally expressed by neurons in a phenotype (e.g., GFAP vs S100B) and brain region specific manner. In a study of α-synuclein-overexpressed mice (Marxreiter et al., 2013), exogenous α-synuclein was co-localized with S100B positive but not GFAP-positive astrocytes in the hippocampus whereas astrocytes in the olfactory bulb did not accumulate α-synuclein. It is also possible that uptake of α-synuclein might have changed the functionality of hippocampal astrocytes in these mice, e.g., suppression of GFAP while promotion of S100B expression. In in vitro studies, overexpression of α-synuclein in culture of astrocytes caused death of the glial cells (Stefanova et al., 2001) although exogenous recombinant α-synuclein activated cultured astrocytes (Klegeris et al., 2006; Koob et al., 2010; Fellner et al., 2013). Interestingly, in two patients with PD who received intra-putamen fetal dopamine neuron grafts, the one patient who had, at autopsy, marked GFAP-astrogliosis in the graft had more surviving grafted dopamine neurons than the other patient (note: neither patient showed astrogliosis in SN) (Kurowska et al., 2011). It should be emphasized that our study focused on ‘classic’ brainstem-predominant idiopathic PD, i.e., at Braak stage III/IV (Braak et al., 2003), with low incidence of α-synuclein accumulation and glial changes in the striatum and frontal cortex. In a recent study (Kovacs et al., 2014) of subjects with predominantly more advanced Lewy-related pathology (Braak V/VI in 35 out of 57 subjects) and accompanying neurofibrillary degeneration, a positive correlation was reported between GFAP immunopositivity and glial or ‚dots’ α-synuclein immunoreactivity in cortical areas. This “discrepancy” could be explained by different brain regions and pathological conditions involved, and/or different antibodies for α-synuclein employed. Further quantitative studies are required to examine the details of endogenous α-synuclein interaction with astrocytes in vivo, including the potentially different behaviors of astrocyte subtypes (Marxreiter et al., 2013) and conformationally different “strains” of α-synuclein “prions” (Bousset et al., 2013).
Limitations and therapeutic considerations
The many limitations of our investigation include a limited sample size, large and unequal data variance among the conditions, which preclude the use of more vigorous statistical tools (e.g., ANCOVA following by Bonferroni adjustments). The correlations reported were also not corrected for multiple comparisons and thus should be interpreted with caution. However, we emphasize that our primary focus is on the possibility of differences between PD and controls, with MSA and PSP groups only as positive disease controls. There was also the generic uncertainty that differences in levels of protein markers in brain homogenates might not correlate with meaningful differences in astrocyte “pathology” (see (Tong et al., 2014) for discussion). We addressed these concerns by selecting multiple measures of astrocyte proteins and by including two different positive controls (MSA, PSP) in which marked astrogliosis in multiple brain regions has long been established and accepted. We also provided detailed analyses of different immunoreactive species (total, intact, LMW proteolyzed/truncated products and HMW aggregates) of the marker proteins, with the altered species apparently being more sensitive biochemical indices of astrocytic activation/disturbance. There is further the possibility that difference in the extent of nigral astrogliosis between PD versus PSP/MSA is explained by different magnitude of neuronal loss in this midbrain region. Although no detailed quantitative cytoarchitectural data are available in our parkinsonian cases, qualitative neuropathological assessment of neuronal loss showed that the conditions were similar in mean ratings (see supplementary Table 1) of nigral neuronal damage (PD, 2.3; MSA, 2.2; PSP, 2.6; p>0.05, Kruskal-Wallis ANOVA). Nevertheless, it must be emphasized that our biochemical data in brain homogenates can only be suggestive of actual changes or relative lack thereof in astrocyte activity.
Could impaired astrocytic response in PD, possibly due to α-synuclein suppression, promote degeneration of nigral neurones? The answer depends in part on the nature of the still uncertain functional role(s), probably biphasic, of astrocytes in neurodegenerative disease. Halliday and colleagues (Song et al., 2009; Halliday and Stevens, 2011) have championed the possibility, supported by some animal data (Solano et al., 2008; Chen et al., 2009; Mullett and Hinkle, 2009; Gu et al., 2010; Schmidt et al., 2011), that astrocyte dysfunction, possibly α-synuclein-related, might be involved in both disease initiation and progression in PD – and suggest that normalized astrocyte activity could protect nigral neurones. In this scenario impaired glial response could lead, for example, to loss of neuroprotective trophic factors and overproduction of inflammatory molecules (Fellner et al., 2011; Halliday and Stevens, 2011). Our findings are at least consistent with the possibility that astrocyte response in PD nigra might be “subnormal” in a neurodegenerative condition and complement speculations in the literature that the astrocyte (e.g. promotion of astrocytic signaling molecules) could represent a therapeutic target in this disorder, particularly at an early stage of the disease.
Supplementary Material
Highlights.
➢ Astroglial status in PD substantia nigra has been controversial
➢ PD nigra, unlike PSP and MSA, is shown to have normal levels of astrocyte markers
➢ Astrocyte marker levels correlate inversely with α-synuclein accumulation in PD
➢ MSA instead has a positive correlation between changes in astrocyte and α-synuclein
➢ Cleaved GFAP, vimentin and Hsp27 and aggregates of GFAP are more sensitive markers
Acknowledgment
This study was supported in part by the US NIDA/NIH DA07182, the New Zealand Institute of Environmental Science and Research, Ltd., and the Centre for Addiction and Mental Health Foundation.
Abbreviations
- GCI
glial cytoplasmic inclusion
- GFAP
glial fibrillary acidic protein
- HMW
high molecular weight
- Hsp27
heat shock protein-27
- LB
Lewy body
- LMW
low molecular weight
- MSA
multiple system atrophy
- NFT
neurofibrillary tangle
- NSE
neuron-specific enolase
- PD
Parkinson's disease
- PSP
progressive supranuclear palsy
- SN
substantia nigra pars compacta
- tHsp27
truncated heat shock protein-27
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data:
Supplementary data to this article can be found online at xxx.
References
- Adams RD, Vanbogaert L, Vandereecken H. Striato-Nigral Degeneration. J Neuropathol Exp Neurol. 1964;23:584–608. [PubMed] [Google Scholar]
- Banati RB, Daniel SE, Blunt SB. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson's disease. Mov Disord. 1998;13:221–7. doi: 10.1002/mds.870130205. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–55. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Bousset L, Pieri L, Ruiz-Arlandis G, Gath J, Jensen PH, Habenstein B, et al. Structural and functional characterization of two alpha-synuclein strains. Nat Commun. 2013;4:2575. doi: 10.1038/ncomms3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Sastre M, Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson's disease. Acta Neuropathol. 2007;114:231–41. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- Braidy N, Gai WP, Xu YH, Sachdev P, Guillemin GJ, Jiang XM, et al. Uptake and mitochondrial dysfunction of alpha-synuclein in human astrocytes, cortical neurons and fibroblasts. Transl Neurodegener. 2013;2:20. doi: 10.1186/2047-9158-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck D, Wenning GK, Stefanova N, Fellner L. Glia and alpha-synuclein in neurodegeneration: A complex interaction. Neurobiol Dis. 2015 doi: 10.1016/j.nbd.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron G, Doudnikoff E, Canron MH, Li Q, Vega C, Marais S, et al. Astrocytosis in parkinsonism: considering tripartite striatal synapses in physiopathology? Front Aging Neurosci. 2014;6:258. doi: 10.3389/fnagi.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, et al. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson's disease: Critical role for the astrocyte. Proc Natl Acad Sci U S A. 2009;106:2933–8. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DK, Pennathur S, Perier C, Tieu K, Teismann P, Wu DC, et al. Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson's disease in mice. J Neurosci. 2005;25:6594–600. doi: 10.1523/JNEUROSCI.0970-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangelo AM, Alberghina L, Papa M. Astrogliosis as a therapeutic target for neurodegenerative diseases. Neurosci Lett. 2014;565:59–64. doi: 10.1016/j.neulet.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F. Glutathione peroxidase, glial cells and Parkinson's disease. Neuroscience. 1993;52:1–6. doi: 10.1016/0306-4522(93)90175-f. [DOI] [PubMed] [Google Scholar]
- Damier P, Kastner A, Agid Y, Hirsch EC. Does monoamine oxidase type B play a role in dopaminergic nerve cell death in Parkinson's disease? Neurology. 1996;46:1262–9. doi: 10.1212/wnl.46.5.1262. [DOI] [PubMed] [Google Scholar]
- Earle KM. Studies on Parkinson's disease including x-ray fluorescent spectroscopy of formalin fixed brain tissue. J Neuropathol Exp Neurol. 1968;27:1–14. doi: 10.1097/00005072-196801000-00001. [DOI] [PubMed] [Google Scholar]
- Ehringer H, Hornykiewicz O. Verteilung von Noradrenalin und Dopamin (3-Hydroxytyramin) im Gehirn des menches und ihr Verhalten bei Erkrankungen des extrapyramiden Systems. Klin Wochenschr. 1960;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol. 1994;4:229–37. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, et al. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia. 2013;61:349–60. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner L, Jellinger KA, Wenning GK, Stefanova N. Glial dysfunction in the pathogenesis of alpha-synucleinopathies: emerging concepts. Acta Neuropathol. 2011;121:675–93. doi: 10.1007/s00401-011-0833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forno LS. Pathology of parkinsonism. A preliminary report of 24 cases. J. Neurosurg. 1966;24(suppl.):266–279. [Google Scholar]
- Forno LS. Pathology of Parkinson's disease: the importance of the substantia nigra and Lewy bodies. In: Stern Gerald M., editor. Parkinson's disease. Chapman and Hall Medical; London: 1990. [Google Scholar]
- Forno LS, DeLanney LE, Irwin I, Di Monte D, Langston JW. Astrocytes and Parkinson's disease. Prog Brain Res. 1992;94:429–36. doi: 10.1016/s0079-6123(08)61770-7. [DOI] [PubMed] [Google Scholar]
- Gilman S, Low PA, Quinn N, Albanese A, Ben-Shlomo Y, Fowler CJ, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163:94–8. doi: 10.1016/s0022-510x(98)00304-9. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–34. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield JG, Bosanquet FD. The brain-stem lesions in Parkinsonism. J Neurol Neurosurg Psychiatry. 1953;16:213–26. doi: 10.1136/jnnp.16.4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greffard S, Verny M, Bonnet AM, Seilhean D, Hauw JJ, Duyckaerts C. A stable proportion of Lewy body bearing neurons in the substantia nigra suggests a model in which the Lewy body causes neuronal death. Neurobiol Aging. 2010;31:99–103. doi: 10.1016/j.neurobiolaging.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Gu XL, Long CX, Sun L, Xie C, Lin X, Cai H. Astrocytic expression of Parkinson's disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol Brain. 2010;3:12. doi: 10.1186/1756-6606-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, Stevens CH. Glia: initiators and progressors of pathology in Parkinson's disease. Mov Disord. 2011;26:6–17. doi: 10.1002/mds.23455. [DOI] [PubMed] [Google Scholar]
- Hassler R. Zur Pathologie der Paralysis agitans und des postenzephalitischen Parkinsonismus. J für Psychologie und Neurologie. 1938;48:387–476. [Google Scholar]
- Hassler R. Extrapyramidal-motorische Syndrome und Erkrankungen. In: Bergmann GV, Frey W, Schwiegh H, Jung R, editors. Hb.Inn.Med. vol. V, part III. Springer; Berlin, Göttingen, Heidelberg: 1953. p. 822. [Google Scholar]
- Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology. 1994;44:2015–9. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–97. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S, Hartmann A. Neuroinflammatory processes in Parkinson's disease. Parkinsonism Relat Disord. 2005;11(Suppl 1):S9–S15. doi: 10.1016/j.parkreldis.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Hishikawa N, Hashizume Y, Yoshida M, Sobue G. Widespread occurrence of argyrophilic glial inclusions in Parkinson's disease. Neuropathol Appl Neurobiol. 2001;27:362–72. doi: 10.1046/j.1365-2990.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Neuropathology of sporadic Parkinson's disease: evaluation and changes of concepts. Mov Disord. 2012;27:8–30. doi: 10.1002/mds.23795. [DOI] [PubMed] [Google Scholar]
- Kamo H, Haebara H, Akiguchi I, Kameyama M, Kimura H, McGeer PL. A distinctive distribution of reactive astroglia in the precentral cortex in amyotrophic lateral sclerosis. Acta Neuropathol. 1987;74:33–8. doi: 10.1007/BF00688335. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–80. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Klegeris A, Giasson BI, Zhang H, Maguire J, Pelech S, McGeer PL. Alpha-synuclein and its disease-causing mutants induce ICAM-1 and IL-6 in human astrocytes and astrocytoma cells. Faseb J. 2006;20:2000–8. doi: 10.1096/fj.06-6183com. [DOI] [PubMed] [Google Scholar]
- Knott C, Wilkin GP, Stern G. Astrocytes and microglia in the substantia nigra and caudate-putamen in Parkinson's disease. Parkinsonism Relat Disord. 1999;5:115–22. doi: 10.1016/s1353-8020(99)00022-x. [DOI] [PubMed] [Google Scholar]
- Koob AO, Paulino AD, Masliah E. GFAP reactivity, apolipoprotein E redistribution and cholesterol reduction in human astrocytes treated with alpha-synuclein. Neurosci Lett. 2010;469:11–4. doi: 10.1016/j.neulet.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Breydo L, Green R, Kis V, Puska G, Lorincz P, et al. Intracellular processing of disease-associated alpha-synuclein in the human brain suggests prion-like cell-to-cell spread. Neurobiol Dis. 2014;69:76–92. doi: 10.1016/j.nbd.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Milenkovic IJ, Preusser M, Budka H. Nigral burden of alpha-synuclein correlates with striatal dopamine deficit. Mov Disord. 2008;23:1608–12. doi: 10.1002/mds.22207. [DOI] [PubMed] [Google Scholar]
- Kraft AW, Hu X, Yoon H, Yan P, Xiao Q, Wang Y, et al. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. Faseb J. 2013;27:187–98. doi: 10.1096/fj.12-208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowska Z, Englund E, Widner H, Lindvall O, Li JY, Brundin P. Signs of degeneration in 12-22-year old grafts of mesencephalic dopamine neurons in patients with Parkinson's disease. J Parkinsons Dis. 2011;1:83–92. doi: 10.3233/JPD-2011-11004. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, Ulusoy A, Innamorato NG, Sahin G, Rabano A, Kirik D, et al. alpha-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson's disease. Hum Mol Genet. 2012;21:3173–92. doi: 10.1093/hmg/dds143. [DOI] [PubMed] [Google Scholar]
- Marchetti B, L'Episcopo F, Morale MC, Tirolo C, Testa N, Caniglia S, et al. Uncovering novel actors in astrocyte-neuron crosstalk in Parkinson's disease: the Wnt/beta-catenin signaling cascade as the common final pathway for neuroprotection and self-repair. Eur J Neurosci. 2013;37:1550–63. doi: 10.1111/ejn.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–15. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marxreiter F, Ettle B, May VE, Esmer H, Patrick C, Kragh CL, et al. Glial A30P alpha-synuclein pathology segregates neurogenesis from anxiety-related behavior in conditional transgenic mice. Neurobiol Dis. 2013;59:38–51. doi: 10.1016/j.nbd.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann JC, Lioy DT, Mandel G. Astrocytes conspire with neurons during progression of neurological disease. Curr Opin Neurobiol. 2012;22:850–8. doi: 10.1016/j.conb.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLADR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–91. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Glial reactions in Parkinson's disease. Mov Disord. 2008;23:474–83. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. History of innate immunity in neurodegenerative disorders. Front Pharmacol. 2011;2:77. doi: 10.3389/fphar.2011.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp J, Kamphuis W, Sluijs JA, Achoui D, Leenaars CH, Feenstra MG, et al. Intermediate filament transcription in astrocytes is repressed by proteasome inhibition. Faseb J. 2009;23:2710–26. doi: 10.1096/fj.08-127696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklossy J, Doudet DD, Schwab C, Yu S, McGeer EG, McGeer PL. Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp Neurol. 2006;197:275–83. doi: 10.1016/j.expneurol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Mirza B, Hadberg H, Thomsen P, Moos T. The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson's disease. Neuroscience. 2000;95:425–32. doi: 10.1016/s0306-4522(99)00455-8. [DOI] [PubMed] [Google Scholar]
- Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K. Demonstration of alpha-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinase K and formic acid pretreatment. Exp Neurol. 2002;176:98–104. doi: 10.1006/exnr.2002.7929. [DOI] [PubMed] [Google Scholar]
- Mullett SJ, Hinkle DA. DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiol Dis. 2009;33:28–36. doi: 10.1016/j.nbd.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mythri RB, Venkateshappa C, Harish G, Mahadevan A, Muthane UB, Yasha TC, et al. Evaluation of markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson's disease brains. Neurochem Res. 2011;36:1452–63. doi: 10.1007/s11064-011-0471-9. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Paviour D, Quinn NP, Josephs KA, Sangha H, Kilford L, et al. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain. 2004;127:2657–71. doi: 10.1093/brain/awh303. [DOI] [PubMed] [Google Scholar]
- Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of Parkinson's disease. J Neuropathol Exp Neurol. 1991;50:743–55. doi: 10.1097/00005072-199111000-00006. [DOI] [PubMed] [Google Scholar]
- Renkawek K, Stege GJ, Bosman GJ. Dementia, gliosis and expression of the small heat shock proteins hsp27 and alpha B-crystallin in Parkinson's disease. Neuroreport. 1999;10:2273–6. doi: 10.1097/00001756-199908020-00009. [DOI] [PubMed] [Google Scholar]
- Riley RA. An atlas of the basal ganglia, brain stem and spinal cord. The Williams & Wilkins Company; Baltimore, MD.: 1943. [Google Scholar]
- Sathe K, Maetzler W, Lang JD, Mounsey RB, Fleckenstein C, Martin HL, et al. S100B is increased in Parkinson's disease and ablation protects against MPTP-induced toxicity through the RAGE and TNF-alpha pathway. Brain. 2012;135:3336–47. doi: 10.1093/brain/aws250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Linnartz B, Mendritzki S, Sczepan T, Lubbert M, Stichel CC, et al. Genetic mouse models for Parkinson's disease display severe pathology in glial cell mitochondria. Hum Mol Genet. 2011;20:1197–211. doi: 10.1093/hmg/ddq564. [DOI] [PubMed] [Google Scholar]
- Shibayama-Imazu T, Okahashi I, Omata K, Nakajo S, Ochiai H, Nakai Y, et al. Cell and tissue distribution and developmental change of neuron specific 14 kDa protein (phosphoneuroprotein 14). Brain Res. 1993;622:17–25. doi: 10.1016/0006-8993(93)90796-p. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano RM, Casarejos MJ, Menendez-Cuervo J, Rodriguez-Navarro JA, Garcia de Yebenes J, Mena MA. Glial dysfunction in parkin null mice: effects of aging. J Neurosci. 2008;28:598–611. doi: 10.1523/JNEUROSCI.4609-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YJ, Halliday GM, Holton JL, Lashley T, O'Sullivan SS, McCann H, et al. Degeneration in different parkinsonian syndromes relates to astrocyte type and astrocyte protein expression. J Neuropathol Exp Neurol. 2009;68:1073–83. doi: 10.1097/NEN.0b013e3181b66f1b. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Steele JC, Richardson JC, Olszewski J. Progressive Supranuclear Palsy. A Heterogeneous Degeneration Involving The Brain Stem, Basal Ganglia And Cerebellum With Vertical Gaze And Pseudobulbar Palsy, Nuchal Dystonia And Dementia. Arch Neurol. 1964;10:333–59. doi: 10.1001/archneur.1964.00460160003001. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Klimaschewski L, Poewe W, Wenning GK, Reindl M. Glial cell death induced by overexpression of alpha-synuclein. J Neurosci Res. 2001;65:432–8. doi: 10.1002/jnr.1171. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson's disease. Brain. 2007;130:1586–95. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo T, Dickson DW. Tau accumulation in astrocytes in progressive supranuclear palsy is a degenerative rather than a reactive process. Acta Neuropathol. 2002;104:398–402. doi: 10.1007/s00401-002-0569-x. [DOI] [PubMed] [Google Scholar]
- Tong J, Boileau I, Furukawa Y, Chang LJ, Wilson AA, Houle S, et al. Distribution of vesicular monoamine transporter 2 protein in human brain: implications for brain imaging studies. J Cereb Blood Flow Metab. 2011a;31:2065–75. doi: 10.1038/jcbfm.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Fitzmaurice P, Furukawa Y, Schmunk GA, Wickham DJ, Ang LC, et al. Is brain gliosis a characteristic of chronic methamphetamine use in the human? Neurobiol Dis. 2014;67:107–18. doi: 10.1016/j.nbd.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Tong J, Furukawa Y, Sherwin A, Hornykiewicz O, Kish SJ. Heterogeneous intrastriatal pattern of proteins regulating axon growth in normal adult human brain. Neurobiol Dis. 2011b;41:458–68. doi: 10.1016/j.nbd.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Wong H, Guttman M, Ang LC, Forno LS, Shimadzu M, et al. Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson's disease and progressive supranuclear palsy: a comparative investigation. Brain. 2010;133:172–88. doi: 10.1093/brain/awp282. [DOI] [PubMed] [Google Scholar]
- Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, et al. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–22. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Rodriguez JJ, Parpura V. Astroglia in neurological diseases. Future Neurol. 2013a;8:149–158. doi: 10.2217/fnl.12.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Rodriguez JJ, Steardo L. Astrogliopathology: A Central Element of Neuropsychiatric Diseases? Neuroscientist. 2013b doi: 10.1177/1073858413510208. [DOI] [PubMed] [Google Scholar]
- Vila M, Jackson-Lewis V, Guegan C, Wu DC, Teismann P, Choi DK, et al. The role of glial cells in Parkinson's disease. Curr Opin Neurol. 2001;14:483–9. doi: 10.1097/00019052-200108000-00009. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, Takahashi H. NACP/alpha-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson's disease brains. Acta Neuropathol. 2000;99:14–20. doi: 10.1007/pl00007400. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Matsumoto K, Takayama K, Yoshimoto M, Takahashi H. NACP, a presynaptic protein, immunoreactivity in Lewy bodies in Parkinson's disease. Neurosci Lett. 1997;239:45–8. doi: 10.1016/s0304-3940(97)00891-4. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H. Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett. 1998;249:180–2. doi: 10.1016/s0304-3940(98)00407-8. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Jellinger KA. The role of alpha-synuclein in the pathogenesis of multiple system atrophy. Acta Neuropathol. 2005;109:129–40. doi: 10.1007/s00401-004-0935-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.