Abstract
Alcoholism afflicts 1 in 13 US adults, and comorbidity with depression is common. Levels of serotonin (5-HT) metabolites in alcoholic or depressed humans and rat strains are lower compared to healthy counterparts. Rats bred for ethanol (EtOH) preference are common in EtOH studies, however out-bred strains better model the range of EtOH consumption in humans. We examined voluntary EtOH consumption in out-bred Sprague-Dawley (SD) rats placed in the 20% EtOH intermittent access drinking paradigm (IA). Acquisition of 20% EtOH consumption (g EtOH/kg/24h) was assessed during the first 6-8 weeks of IA. Rats naturally separated into two groups (Drinkers or Non-drinkers) based on EtOH intake above or below 0.5g/kg/24hr prior to treatment intervention. We examined the effect of central 5-HT depletion on EtOH consumption by infusing 5,7-dihyroxytryptamine (5,7-DHT; i.c.v., 200-300 μg) or vehicle and measured EtOH consumption for 4 weeks post-operatively in IA. Compared to baseline, there was no effect of vehicle or 5,7-DHT on EtOH consumption during the postoperative period. Quantification of 5-HT depletion in the dorsal raphe nucleus (DRN) using tryptophan hydroxylase-2 (TPH2) immunohistochemistry resulted in a 76% decrease in staining with 5,7-DHT treatment. Interestingly, preservation of the ventromedial (VM) sub-regions was evident in all animals treated with 5,7-DHT, regardless of drinking behavior. In addition, Drinkers treated with 5,7-DHT had significantly more TPH2 depletion in the DRN compared to Non-drinkers. Our findings indicate that out-bred SD rats exhibit a natural EtOH consumption behavior (Drinker or Non-drinker) that is stable across time and independent of 5-HT depletion in the CNS. In addition, rats that regularly consumed > 0.5g EtOH/kg had greater sensitivity to 5,7-DHT in the DRN, indicating an interaction between EtOH and sensitivity of DRN 5-HT cells to neurotoxic substances. This may contribute to the dysfunctionality of the 5-HT system in alcoholic humans and lead to a better understanding of current pharmacological treatments for this addiction.
Keywords: Sprague-Dawley; ethanol; dorsal raphe nucleus; serotonin; 5,7-dihydroxytryptamine; intermittent access
1. Introduction
Alcoholism afflicts 1 in every 13 US adults, and comorbidity with depression is common (National Institute on Drug Abuse, 2010). Much like depressed patients, alcoholics have been shown consistently to have reduced indicators of central nervous system (CNS) serotonin (5-hydroxytryptamine; 5-HT), including decreased levels of the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA), compared to healthy counterparts (LeMarquand, et al., 1994a,b; Nevo and Hamon, 1995). These findings have been recapitulated in both rat models of depression and high alcohol-preferring rat strains (Badawy, 2002; Charney, 1998; Charney et al., 1990; McBride, 2010; Overstreet et al., 2007), suggesting a link between CNS 5-HT neurotransmission and the consumption of alcohol.
Animal models that can successfully mimic human alcohol consumption are of great value given the numerous factors that contribute to ethanol (EtOH) intake in people (Carnicella et al., 2014; Novier et al, 2015). The intermittent-access two-bottle choice drinking paradigm (IA) is one such model, and has seen a resurgence in the literature in the last 5 – 7 years since its conception in the 1970s (Wayner et al., 1972; Wise, 1973). This methodology gives rodents the option to consume EtOH or water in their home-cages and induces EtOH intake, with animals reaching stable baseline levels from days to weeks of exposure. This model is successful in inducing voluntary EtOH consumption across both alcohol-preferring and out-bred strains of rats (see Carnicella et al., 2014 for a review), adding to its validity.
Although genetically modified alcohol-preferring rats show robust EtOH intake across a number of drinking schedules (see Rodd et al., 2004), these models do not accurately reflect EtOH consumption across the genetically diverse human population (Carnicella et al., 2014; Moorman and Aston-Jones, 2009; Novier et al., 2015). Many studies using out-bred rat strains in the IA drinking paradigm establish minimum EtOH consumption criteria for inclusion in experiments, excluding rats who do not drink robustly (Carnicella et al., 2014; Li et al., 2011; Wayner et al., 1972), thus artificially creating a group of animals that might respond to experimental manipulations but do not truly represent the population in question. A greater proportion of Sprague-Dawley (SD) rats are slow to drink to stable baseline levels, and the current state of the literature suggests that this population has been excluded from earlier experiments (Carnicella et al., 2014; Moorman and Aston-Jones, 2009), removing a vital control group in the process. Given these apparent individual differences in EtOH preference and consumption, SD rats may better represent the human population than alcohol-preferring strains and yield valuable information regarding the effects of EtOH exposure across a more diverse genetic background (Novier et al, 2015).
Intermittent access has been used successfully in a number of recent studies to examine CNS pharmacology that may influence EtOH consumption in the rat. Many investigations have focused on the mesolimbic dopamine system (Bito-Onon et al., 2011; Carnicella et al, 2009; Li et al., 2010; Moorman and Aston-Jones, 2009), and even include the use of acupuncture (Li et al., 2011). Earlier, several studies showed that depletion of 5-HT with synthesis inhibitors or neurotoxins can modify alcohol intake in several strains of rat (Ellison et al., 1979; Geller, 1973; Hill and Goldstein, 1974; Ho et al., 1974; Melchior and Myers, 1976; Myers and Melchior, 1975; Richardson and Novakovski, 1978), however the effects across studies have been less consistent than data from stimulation of 5-HT neurotransmission, which consistently produces a reduction in EtOH consumption (Nevo and Hamon, 1995; for review see McBride, 2010). In addition, many studies of 5-HT depletion have employed high alcohol-preferring rat strains (Contreras et la., 1990; Murphy et al., 1985; for review see LeMarquand, et al., 1994b), thus their drinking may already be at ceiling levels, with no ability of pharmacological manipulations to further increase EtOH consumption. With regard to out-bred strains, the lack of consistency may be due to differences in methodology (Badawy, 2002), to the exclusion of animals that did not reach EtOH consumption criteria or, importantly, the inclusion of these animals, negating any effects that may have been seen only in higher consuming animals.
Few of these studies included the SD rat, yet many 5-HT and depression studies in our laboratory and others have used this out-bred strain (Connor et al., 1999; Kirby et al., 1995, 1997, 2007; Kirby and Lucki, 1997; Robertson et al., 2005; Roche et al., 2003). While there has been a resurgence in the use of IA in recent years (Ahmadiantehrani et al, 2013; Bito-Onon et al., 2010; Li et al., 2010, 2011; Simms et al., 2008), studies utilizing this methodology in SD rats have not investigated the role of 5-HT in EtOH consumption. Although this relationship has been studied extensively in the past, variations in methodology ranging from induction of EtOH consumption to quantification of 5-HT depletion challenge the integration of findings across experiments (Adell and Myers, 1995; Moorman and Aston-Jones, 2009; Myers and Melchior, 1975; Nevo and Hamon, 1995; Wang et al., 1996).
The purpose of our investigation is to use the IA paradigm in the SD rat to investigate individual differences in voluntary EtOH consumption and the potential effect of 5-HT depletion on this behavior. We predict that SD rats will show a natural preference or aversion for EtOH (Carnicella et al., 2014; Li et al., 2011; Moorman and Aston-Jones, 2009). In addition, given the link between EtOH consumption and 5-HT levels in both laboratory animals and humans (Badawy, 2002; LeMarquand, et al., 1994a,b; McBride, 2010; Nevo and Hamon, 1995), we predict central 5-HT depletion will result in an increase in EtOH consumption (Wang et al., 1996). To measure 5-HT depletion we employed immunohistochemical quantification of TPH2-labeled cells across the anatomically and functionally diverse subdivisions of the serotonergic dorsal raphe nucleus (DRN).
2. Methods
2.1. Animals and Housing
Naïve male Sprague-Dawley rats (150 – 200 g upon arrival, Taconic Farms, Cranbury, NJ) were allowed to acclimate to the animal facility and handling for one week before being singly housed in Plexiglas cages with wire tops and ventilated lids. Animals had ad-libitum access to standard rat chow and water and were on a 12 hour light cycle (lights on at 7:00 a.m.). All procedures were approved by the Temple University School of Medicine Institutional Animal Care and Use Committee and followed all guidelines in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals.
2.2. Two-Bottle Choice Intermittent Access to 20% EtOH
After being individually housed, all animals (six cohorts, n = 56) were given home cage access to two - 4 ounce plastic bottles fitted with spring loaded ball bearing sipper tubes (Critter Cages, www.critter-cages.com; Wayner et al., 1972) secured to the wire top of the cage. Each bottle contained tap water for a period of one week to acclimate animals to the bottles. Following, all rats were started on the IA two-bottle choice paradigm in their home cages (Simms et al., 2008; Wayner et al., 1972; Wise, 1973). This model involves access to 20% v/v (EtOH/tap water) for a 24 hr period three times a week with ad libitum water. The EtOH bottle was presented alongside a water bottle in the home cage for 24 hr on Monday, Wednesday and Friday and replaced with a second water bottle between EtOH sessions. Bottles were changed between 9:30 a.m. and 11:30 a.m., during the light cycle. Ethanol bottle placement was alternated with water for each session to minimize the effect of potential side preferences. Each bottle was weighed 24 hr after presentation and animals were weighed daily to calculate EtOH intake (g EtOH/kg body weight/24 hr). All animals were run in the IA paradigm for 6-8 weeks prior to treatment. Multiple cohorts of animals were run sequentially, such that no two groups were on the same timeline of IA at any given time-point. This methodology was intended to minimize effects of events within the animal facility or changes in personnel executing the experiments on EtOH consumption. One group of animals (n = 8) was designated as control animals and did not receive any pharmacological treatment or surgical procedures. This group remained in the IA paradigm for a total of 12 weeks. A second group of animals (n = 8) was allocated to a separate study after completing 8 weeks of IA acquisition.
2.3. Pharmacological modulation of 5-HT levels
2.3.1. CNS inhibition of 5-HT via 5,7-dihydroxytryptamine administration
Following 6-8 weeks of IA, rats were administered 200-300 μg (100-150 μg per side) free base 5,7-DHT (5,7-dihydroxytryptophan-creatine salt; Sigma-Aldrich, USA) dissolved in saline + 0.1% ascorbic acid vehicle via bilateral intracerebroventricular (i.c.v.) injections. Bilateral infusions were done to maximize delivery of the solution into the ventricles and to ensure symmetrical distribution of the neurotoxin to all portions of the 5-HT system. Treatment control animals received vehicle via the same procedure. Anesthesia was induced with 8 mg/kg Xylazine (AnaSed; Lloyd, Inc., Shenandoah, IA) and 40 mg/kg Ketamine (Hospira, Lake Forrest, IL) and maintained with isoflurane gas anesthesia (0.5-2.0%) as needed (Jankowska et al., 1994; Melchior and Myers, 1976). Animals were pre-treated with desipramine (25 mg/kg; Sigma-Aldrich, USA) i.p. 30 minutes prior to 5,7-DHT infusion in order to prevent catacholaminergic cell loss (Baumgarten et al., 1982; Myers and Quarfordt, 1991). After being placed in a stereotaxic frame (Narshige, Japan), bilateral craniotomies were drilled above the lateral ventricles (-0.8 mm from Bregma, 2.1 mm bilateral; Paxinos and Watson, 2005) and a 32G 10 μL Hamilton Syringe (Hamilton Company, Reno, NV) was lowered 3.3 mm from the surface of the brain into each lateral ventricle. A volume of 7.5 μL 5,7-DHT (14-20 μg free base/μL) was infused over 5 minutes per side (200-300 μg total) with a 5 minute interval in between. Bone wax was used to seal the craniotomies and the incision was closed with skin staples. Following perfusion, Nissl staining was used to confirm the location of each infusion track (data not shown).
2.3.2 Post-operative care
All animals received 2 mL/100 g body weight Lactated Ringers Solution and 2.5 mg/kg enrofloxacin antibiotic (Baytril; Bayer Corporation, USA) subcutaneously as well as 5 mg/kg ketoprofen (Ketofen; Pfizer Inc., NY, USA) intramuscularly for pain management. Lactated Ringer Solution (1 mL/100 g) and enrofloxacin (2.5 mg/kg) were administered subcutaneously for 1-3 days post-operatively.
After the first eight 5,7-DHT infusions, several animals developed seizures and died within the first 3 post-operative days. Upon investigation of the literature (Browning et al., 1978), we modified our surgical procedure to include a single i.p. injection of 15 mg/kg pentobarbital (Nembutol; Ovation Pharmaceuticals, USA) between 5,7-DHT infusions for all animals to help prevent post-operative seizure activity.
2.4. Blood ethanol concentrations
On the last EtOH session of IA post-treatment, trunk blood was collected from rats under isoflurane anesthesia immediately prior to perfusion, 30 minutes after EtOH was presented. Blood EtOH concentrations (BEC; mg/dL) for 8 representative rats were measured with an Ethanol L3k kit (Sekisui Diagnostics, Exton, PA) according to the manufacturer's protocol. Fluorescence was measured with a Synergy 2 plate reader (Biotek, Winooski, VT) using Gen5 data analysis software (Biotek, Winooski, VT). Measurements were normalized to standards before measuring the correlation between BEC and g EtOH consumed for each rat.
2.5. Serotonin immunohistochemistry
Serotonin depletion was examined by staining for tryptophan hydroxylase-2 (TPH2), the rate limiting enzyme for the production of 5-HT, and viewing the DRN. Animals were anesthetized under isoflurane gas anesthesia (5%), trunk blood collected, and perfused with 0.1 M phosphate buffer (PB) followed by 4% paraformaldehyde in 0.1 M PB. Brains were removed, post-fixed for 24 hr and then transferred to 20% sucrose solution until they sank. Sections 20-30 μm thick were collected in 0.1 M PB serially across the DRN (120-180 μm apart). Sections were washed in 0.1 M PB saline (PBS) 5 min × 5 then in 0.1 M PBS + 0.3% Triton X (PBS-T) 5 min × 5. Sections were blocked in 10% donkey serum in PBS-T × 1 hr before being incubated in rabbit:TPH2 1:1000 (Sigma-Aldrich, USA) in PBS-T at 4 degrees Celsius × 36 – 48 hr. Following, sections were washed in 0.1 M PBS and incubated in secondary antibody donkey:rabbit Alexa Fluor 594 (red; Molecular Probes Inc., Eugene, OR) × 2 hr, washed in 0.1 M PBS 5 min × 3 and then mounted on gelatin coated slides. Slides were dried overnight and then cover slipped with SlowFade Gold mounting medium (Life Technologies, ThermoFisher Scientific, Inc., Carlsbad, CA).
2.6 Imaging and Quantification
Images were captured using a Nikon E1000 fluorescent microscope (Nikon Instruments, Melville, NY), using a Retiga EXi Fast 1394 digital camera and QCapture Suite imaging software (Quantitative Imaging Corp., Surrey, BC, Canada). Images were converted to 8-bit grayscale and inverted for comparison using CorelDraw Graphics Suite X6 Version 16.4.1.1281 (Corel Corporation, Ottawa, Canada). A sample of rats was selected for TPH2 quantification (n = 12 rats total, 3 per treatment group; Commons et al., 2003; Okere and Waterhouse, 2006; Vasudeva and Waterhouse, 2014). Positively stained cells were counted across 9 DRN sub-regions per animal according to Vasudeva and Waterhouse (2014). Briefly, two sections for each DRN level (rostral, intermediate, caudal) were quantified for each sub-region (dorsomedial, ventromedial, lateral wing) at 40X magnification (approximately 150 μm2 of tissue) and averaged per animal. It is important to note that the rostral and caudal “lateral wings” are not true sub-regions of the DRN (Vasudeva and Waterhouse, 2014). These areas were quantified as controls for negative TPH2 staining and to ensure proper localization of other DRN sub-regions.
2.7. Data Analysis
Individual EtOH consumption patterns were investigated by creating a frequency histogram of baseline drinking behavior, defined as the last 6 EtOH sessions prior to surgery. Blood EtOH concentrations (mg/dL) were correlated with EtOH consumption (g EtOH/kg/30 min) and daily EtOH preference (volume 20% EtOH / total volume) was correlated with EtOH consumption (g EtOH/kg/24 hr) using linear regression analysis (Microsoft Excel 2010, Microsoft, Redmond, WA).
Several groups were identified for statistical analysis and were designated based on the measured variable. Ethanol consumption (g EtOH/kg/24 hr) and EtOH preference (volume 20% EtOH consumed / total volume) were evaluated using one- and two-way repeated measures (RM) ANOVAS for EtOH consumption (Drinker, Non-drinker) and treatment (vehicle, 5,7-DHT) across time (baseline and post-treatment days). Serotonin depletion (number of TPH2 cells per DRN sub-region) was analyzed using two- and three-way ANOVAs for EtOH consumption behavior (Drinker, Non-drinker), treatment (vehicle, 5,7-DHT), and DRN sub-region (9 levels; see Table 1). Each dependent variable was evaluated across four possible groups based on EtOH consumption and treatment: Non-drinker+vehicle; Non-drinker+5,7-DHT; Drinker+vehicle; Drinker+5,7-DHT. Bonferroni post-hoc tests were conducted for comparison of individual time-points, cell counts, and groups when appropriate. All analyses were completed using SigmaPlot 12.5 (Systat Software Inc., San Jose, CA, USA).
Table 1.
TPH2 neuron counts per DRN sub-region at 40× magnification. Standard error is depicted below each value.
| Treatment | EtOH Consumption | Combination | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DRN Location | Vehicle |
* 5,7- DHT |
% depletion |
Drinker | Non- Drinker |
Drinker + Vehicle |
*Drinker + 5,7- DHT |
% depletion |
Non- Drinker + Vehicle |
*Non- Drinker + 5,7-DHT |
% depletion |
| rDM | 10.92 | 0.75 | 93.13 | 7.50 | 4.17 | 15.00 | 0.00 | 100.00 | 6.83 | 1.50 | 78.04 |
| 2.10 | 0.75 | 3.51 | 1.38 | 2.29 | 0.00 | 0.44 | 1.50 | ||||
| rVM | 15.83 | 6.00 | 62.10 | 11.08 | 10.75 | 20.83 | 1.33 | 93.61 | 10.83 | 10.67 | 1.48 |
| 3.10 | 2.29 | 4.67 | 1.36 | 3.72 | 0.33 | 3.03 | 2.09 | ||||
| rLAT | 0.92 | 0.00 | 100.00 | 0.92 | 0.00 | 1.83 | 0.00 | 100.00 | 0.00 | 0.00 | n/a |
| 0.58 | 0.00 | 0.58 | 0.00 | 0.93 | 0.00 | 0.00 | 0.00 | ||||
| iDM | 13.42 | 0.33 | 97.54 | 9.00 | 4.75 | 18.00 | 0.00 | 100.00 | 8.83 | 0.67 | 92.41 |
| 3.39 | 0.33 | 4.47 | 2.63 | 4.36 | 0.00 | 4.17 | 0.67 | ||||
| iVM | 38.50 | 24.08 | 37.45 | 28.58 | 34.00 | 44.67 | 12.50 | 72.02 | 32.33 | 35.67 | 0.00 |
| 4.44 | 6.28 | 8.15 | 3.25 | 6.35 | 5.75 | 4.49 | 5.46 | ||||
| iLAT | 12.25 | 1.00 | 91.84 | 7.17 | 6.08 | 14.33 | 0.00 | 100.00 | 10.17 | 2.00 | 80.33 |
| 2.72 | 1.00 | 3.41 | 3.05 | 2.60 | 0.00 | 5.09 | 2.00 | ||||
| cDM | 9.92 | 1.58 | 84.07 | 1.17 | 2.17 | 15.17 | 1.17 | 92.29 | 4.67 | 2.00 | 57.17 |
| 3.73 | 0.96 | 0.88 | 1.11 | 6.42 | 1.17 | 0.93 | 1.76 | ||||
| cVM | 20.67 | 8.67 | 58.06 | 15.86 | 15 | 26.17 | 2.50 | 90.45 | 15.17 | 14.83 | 2.24 |
| 4.34 | 4.23 | 6.48 | 3.02 | 7.96 | 2.50 | 1.26 | 6.71 | ||||
| cLAT | 2.42 | 0.92 | 61.98 | 3.33 | 8.17 | 4.33 | 0 | 100.00 | 0.50 | 1.83 | 0.00 |
| 1.02 | 0.92 | 1.07 | 4.28 | 1.2 | 0 | 0.29 | 1.83 | ||||
p < .05 versus other group(s) for the comparison condition.
r= rostral, i= intermediate, c= caudal; DM= dorsomedial, VM= ventromedial, LAT= lateral wing
SEM indicated below average cell counts.
3. Results
3.1. Sprague-Dawley rats separate into distinct EtOH consumption phenotypes
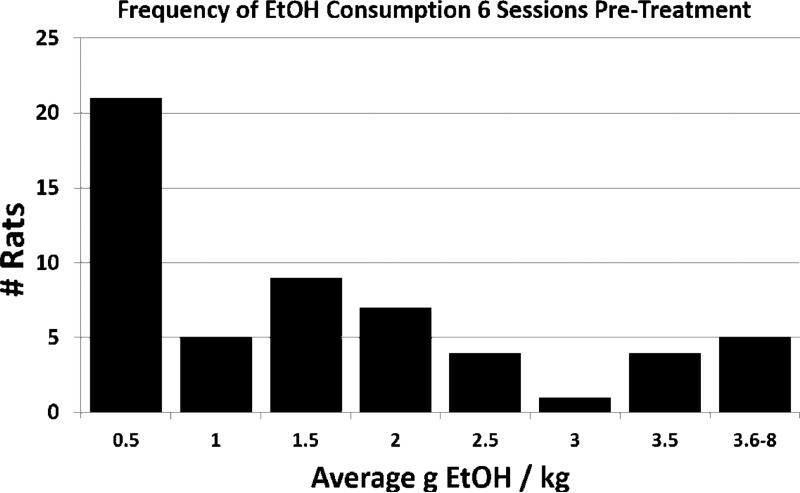
After 6-8 weeks on the IA two-bottle choice paradigm, rats demonstrated individual differences in EtOH baseline consumption levels and preferences. Across the 6 EtOH sessions prior to treatment (surgery), individual EtOH consumption patterns were investigated across all rats (n = 56) according to frequency of consumption (Fig. 1). Within 0.5 g EtOH/kg bins, two groups of rats were apparent within this timespan based on average consumption above or below 0.5 g EtOH/kg. Further investigation of individual EtOH consumption on each of the baseline days allowed for the designation of an EtOH consumption phenotype that remained consistent across time. Rats that consumed less than 0.5 g EtOH/kg/24 hr on > 50% of sessions within the baseline time period were labeled Non-drinkers (n = 23) and exhibited the most distinct separation in EtOH consumption (Fig. 1, first bin) (Li et al, 2011; Moorman and Aston-Jones, 2009). Rats that consumed at least 0.5 g EtOH/kg/24 hr on > 50% of sessions within the baseline time-period were labeled Drinkers (n = 33). Although peaks were found within this group, they did not appear consistently enough to demarcate additional drinking phenotypes within this population.
Figure 1.
The frequency of the average g EtOH/kg consumed by all rats (n = 56) during the 6 sessions prior to treatment intervention reveals a separation of rats based on EtOH consumption between 0-0.5 g EtOH/kg (n = 23) and consumption above 0.5 g EtOH/kg (n = 33). Rats were labeled as Drinkers or Non-drinkers, respectively, based on this distinction, and analyses of consumption behavior were conducted using this distinction.
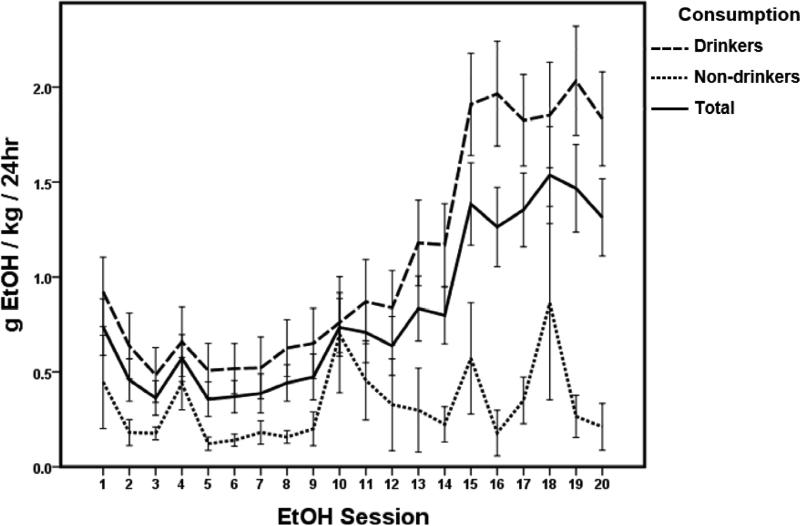
During acquisition of EtOH consumption (the first 20 EtOH sessions, Fig. 2), rats showed stable baseline EtOH consumption by session 15, with an average of 1.09+/.05 g EtOH/kg/24 hr (10.40+/−.51 % EtOH preference) across all acquisition sessions for Drinkers and an average of 0.32+/−.04 g EtOH/kg/24 hr (2.50+/−.33 % EtOH preference) for Non-Drinkers. For both groups combined (Total, Fig. 2 solid line), there was a significant effect of session (time) on EtOH consumption (one-way RM ANOVA; F(55,19)=10.007, p < .001). Post-hoc Bonferroni tests showed an increase in drinking for all rats during sessions 15-20, compared to most EtOH sessions prior (p < .05). A similar effect was seen specifically within the Drinker group (F(32,19)=12.503, p < .001; post hoc Bonferroni p < .05). No effect of time was seen within the Non-drinker group (F(22, 19)=1.435, p = 0.102)
Figure 2.
Sprague Dawley rats (n = 56) voluntarily consume ethanol (EtOH) and acquire a stable consumption level by 5 weeks (session 15) of 20% EtOH Intermittent Access (Total, solid line). Based on the percentage of days rats consumed more or less than 0.5 g EtOH/kg/24 hr during the 6 EtOH sessions prior to treatment intervention (corresponding to weeks 6-8 of EtOH acquisition), rats were assigned to one of two groups. Drinkers (n = 33, dashed line) consumed > 0.5g EtOH/kg/24 hr on > 50% of sessions, while Non-drinkers (n = 23, dotted line) consumed less than 0.5 g EtOH/kg/24hr on > 50% of sessions (see also Fig 1). Across the acquisition period (sessions 1-20), Drinkers consumed an average of 1.09+/−.05 g EtOH/kg/24 hr, while Non-Drinkers consumed an average of 0.32+/−.04 g EtOH/kg/24 hr. The average consumption for both groups was 0.80+/−.04 g EtOH/kg/24 hr. For all rats (Total, solid line), there was a significant effect of session (time) on EtOH consumption. Data are represented by mean ± SEM.
3.2. Decreases in CNS 5-HT induced by 5,7-DHT had no effect on EtOH consumption for Drinkers or Non-drinkers
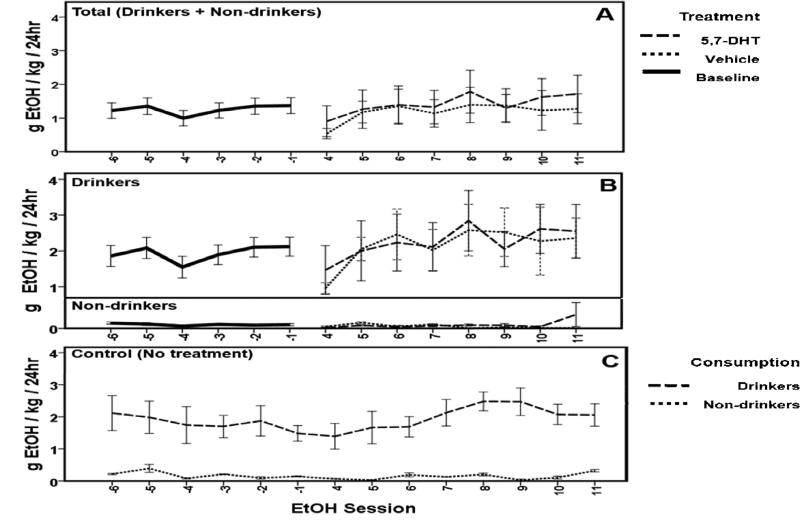
Analysis of data for pre- and post-treatment EtOH consumption in rats surviving postoperatively was completed using a two-way RM ANOVA. For all experimental rats (Total: Drinkers + Non-drinkers, n = 26; Fig. 4A), there was no effect of treatment (F(1,24)=0.123, p = 0.728) or session (time) (F(1,8)=1.498, p = 0.160) on EtOH consumption pre- versus post-treatment. In addition, there was no interaction between treatment and session (Vehicle, n =13, 5,7-DHT, n = 13; F(1,24)=0.412, p = 0.913). Within specific EtOH consumption phenotypes (Fig. 4A) there were also no significant effects seen. For Drinkers specifically (n = 15, Fig. 4B), there was no effect of treatment (F(1,13)=0.000, p = 0.999) or session (time) (F(1,8)=1.538, p = 0.153) on EtOH consumption pre- versus post-treatment. In addition, there was no interaction within Drinkers between treatment and session (F(1,8)=0.306, p = 0.962). For Non-drinkers (n = 11; Fig. 4A) there was no effect of treatment (F(1,9)=2.343, p = 0.160) or session (time) (F(1,8)=1.308, p = 0.416) on EtOH consumption pre- and post-treatment. In addition, there was no interaction within Non-drinkers between treatment and session (F(1,8)=1.562, p = 0.151). No effect of time was seen on EtOH consumption within the control group (n = 8; Fig. 4C) for Drinkers (n = 6; dashed line) or Non-drinkers (n = 2; dotted line) at time-points in the IA procedure that were equivalent to the data shown for 5,7-DHT and vehicle treated subjects in panels A and B (F(7,8)=0.754, p = 0.644). There was not an interaction between EtOH phenotype and session (time) (F(1,8)=0.656, p = 0.727).
Figure 4.
EtOH consumption was not affected by serotonin depletion. No effect of treatment was seen on EtOH consumption behavior compared to baseline for all rats (A; n = 26). Within consumption-based groups (B), there was no effect of 5,7-DHT (n = 13, dashed line) or vehicle (n = 13, dotted line). No effect of time was seen on EtOH consumption within the control group (C, n = 8) for Drinkers (n = 6; dashed line) or Non-drinkers (n = 2; dotted line). Data are represented by mean ± SEM.
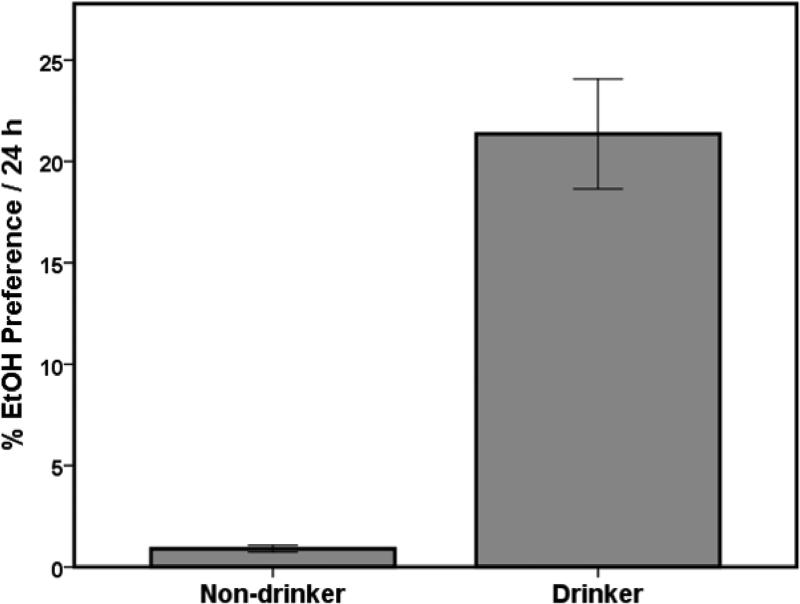
Across the last six EtOH sessions prior to surgery, the average baseline EtOH preference for Drinkers was 21.36+/−2.7 % and 0.90+/−.16 % for Non-Drinkers (Fig. 3). These preference averages were consistent with previously reported ranges (Carnicella et al., 2014; Moorman and Aston-Jones, 2009). The amount of EtOH consumed (g/kg) correlated positively with EtOH preference (R = .969, p < .01). Post-treatment analysis of EtOH preferences followed the same statistical pattern as described above (data not shown).
Figure 3.
Preference for 20% EtOH compared to total fluid consumption during the 6 baseline sessions prior to treatment is greater for Drinkers than Non-drinkers. Drinkers averaged 21.36 +/−2.7% EtOH preference while Non-Drinkers averaged 0.90 +/− .16% EtOH preference. This was consistent with prior studies (Li et al., 2011). Data are represented by mean ± SEM.
3.3 Blood EtOH concentrations correlate with g EtOH consumption
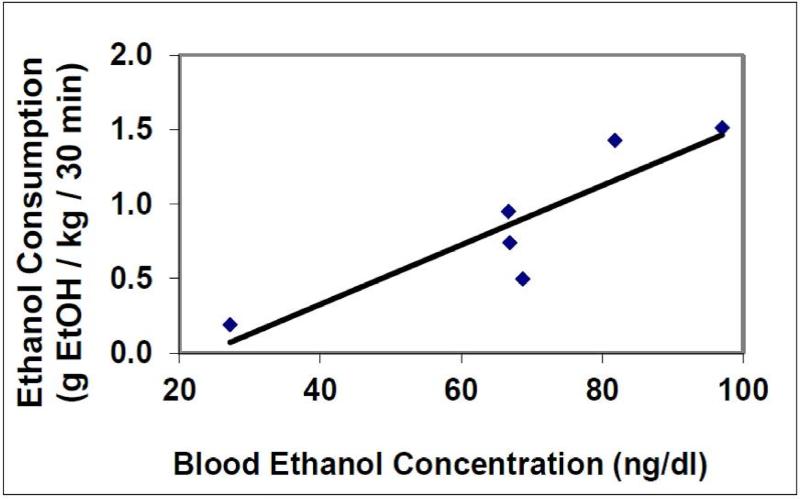
Across 8 selected rats, two did not consume EtOH during the 30 minutes of access as confirmed by serum analysis. The 6 animals that consumed EtOH had BECs ranging from 27 – 97 mg/dL (mean BEC: 68 ± 9 mg/dL) following 30 minutes of EtOH access correlated with the g EtOH consumed within that time period (0.2 – 1.6 g EtOH/kg/30 min consumption: 0.89 ± 0.21 g EtOH/kg/30 min) (R = 0.798, p < .05; Fig 5). This range was consistent with other studies using SD rats (Carnicella et al., 2014).
Figure 5.
Blood EtOH concentrations (BEC) correlate with the amount of EtOH consumed (g EtOH) during the first 30 minutes of EtOH presentation. (n =6; R=0.798, p < .05).
3.4 Serotonin depletion with 5,7-dihydroxytryptamine is more robust in EtOH-consuming rats
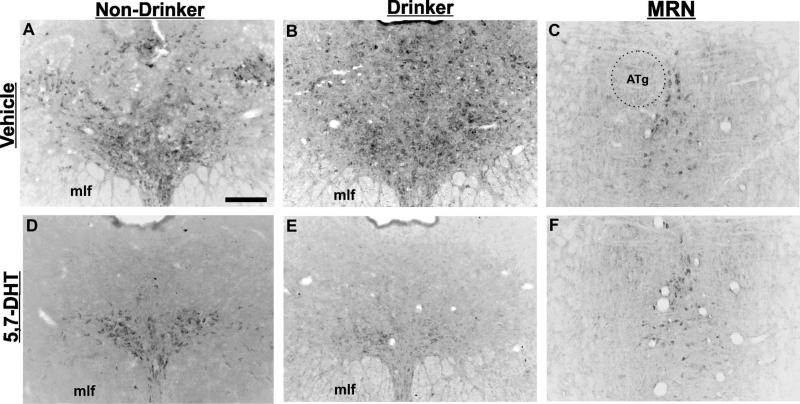
Average 5-HT depletion across all DRN sub-regions in 5,7-DHT treated animals was ~76%, consistent with studies using high pressure liquid chromatography (HPLC) measurements of forebrain 5-HT terminal fields (Table 1; Adell and Myers, 1995; Hall et al., 1999). Immunohistochemical staining for TPH2 for each treatment condition is shown in Figure 6. All cell counts for each treatment group and condition are arranged in Table 1. A 3-way ANOVA between groups was used to examine the main effects and interactions of EtOH consumption and treatment on TPH2 neuronal depletion across DRN sub-regions. A significant 3-way interaction was found (F(8,8)=2.530, p = 0.017). As previously reported (see Vasudeva and Waterhouse, 2014), expected differences in TPH2 cell counts were seen across DRN sub-regions in all vehicle treated animals, regardless of drinking preference (F(8, 3)=31.043, p < .001). There was a main effect of treatment (F(1, 10)=66.492, p < .001) but not EtOH consumption (F(1,10)=0.935, p = .337) on TPH2 cell numbers in DRN sub-regions. An interaction was seen between EtOH consumption and treatment (F(1, 10)=37.657, p < .001), treatment and DRN sub-region (F(8,10)=2.074, p = .05), but not EtOH consumption and DRN sub-region (F(8, 10)=0.845, p = .566). This suggests that EtOH consumption alone did not have a significant effect on TPH2 neuronal depletion.
Figure 6.
Intracerebroventricular administration of 5,7-DHT reduces TPH2 immunoreactivity in the dorsal raphe nucleus (DRN). 5,7-DHT treated rats in both groups (D, E) showed a decrease in TPH2 staining across the DRN compared to vehicle (A, B), with the exception of the ventromedial sub-region. In addition, the median raphe nucleus (MRN) is preserved in both vehicle and 5,7-DHT treated animals (C, F), indicating the level of depletion may be dependent upon the diffusion of the toxin through the brain. ATg = anterior tegmental nucleus; mlf = medial longitudinal fasciculus. Scale bar = 250μm.
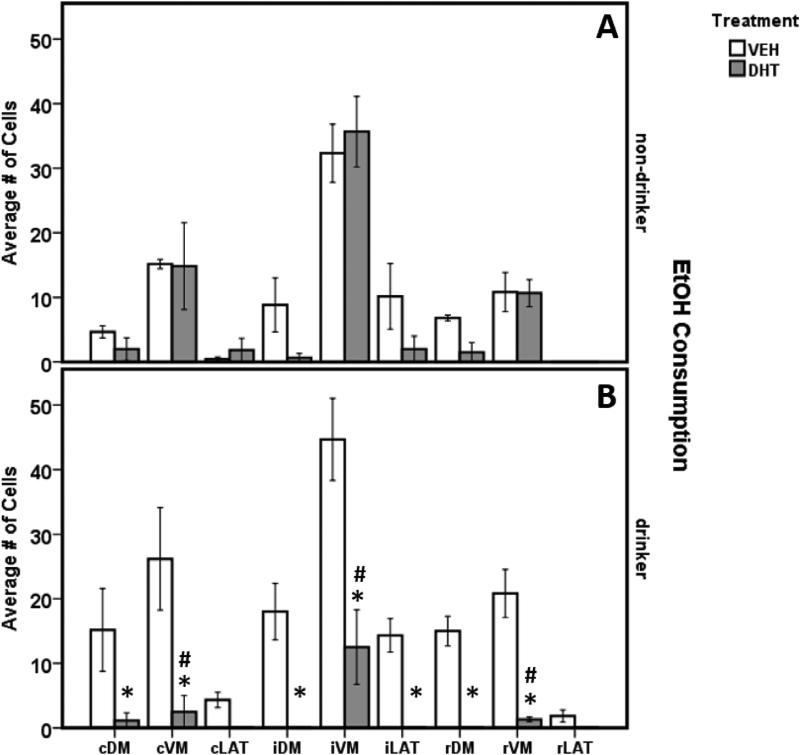
Within the 3-way comparison, TPH2 neurons were significantly lower in number across the ventromedial (VM) sub-regions in Drinkers treated with 5,7-DHT compared to all other treatment groups, most notably the Non-drinker-5,7-DHT treated animals (Fig. 7; post hoc Bonferroni, p < .005 caudal, intermediate and rostral VM subdivisions). Within Drinkers, there was a significant decrease in TPH2 cell numbers in the intermediate lateral wing division and across all dorsomedial sub-regions in the 5,7-DHT treated rats compared to vehicle (two-way ANOVA; (F(1, 6)=3.288, p = .006; post-hoc Bonferroni, p < .01). This suggests an effect of EtOH consumption on the sensitivity of DRN TPH2 neurons to 5,7-DHT, with Drinkers being more sensitive to the neurotoxin than Non-drinkers. Interestingly, the iVM sub-region was preserved to an extent in all 5,7-DHT treated animals, showing significantly higher TPH2 cell counts compared to all other sub-regions for that treatment group (Fig. 6, Fig. 7; post-hoc Bonferroni p < .012). Qualitative examination showed that the median raphe nucleus (MRN), another major source of forebrain 5-HT, also exhibited consistent sparing of 5-HT cells (Fig. 6C, F). All DRN sub-regions (except for the rostral and caudal lateral wings) showed a significant decrease in TPH2 numbers for 5,7-DHT treated animals compared to vehicle controls (Table 1, Fig. 7; post-hoc Bonferroni p < .05).
Figure 7.
Treatment with 5,7-DHT (grey bars) significantly reduced TPH2 cell numbers in several DRN sub-regions. EtOH-consuming rats treated with 5,7-DHT (panel B, grey bars) showed significantly more TPH2 cell depletion across all VM sub-regions compared to all other treatment groups, including non-drinkers experiencing the same treatment (panel A; # = p < .012). Within drinkers (B), 5,7-DHT treated animals had a significant decrease in TPH2 cell numbers compared to vehicle treated animals across all sub-regions except cLAT and rLAT (* = p < .05) It is important to note that the rostral and caudal “lateral wings” are not true sub-regions of the DRN (Vasudeva and Waterhouse, 2014). These areas were quantified as controls for negative TPH2 staining and to ensure proper identification of other DRN sub-regions.Interestingly, there was no difference in depletion in all VM sub-regions of non-drinkers, regardless of treatment, and this sub-region was preserved in Drinkers (B). Data are represented by mean ± SEM.
4. Discussion
The aim of our present work was to examine the individual differences in EtOH consumption in the SD rat using the IA 20% EtOH drinking paradigm. In addition, we investigated the potential interaction between the serotonergic system and EtOH consumption behavior, predicting 5-HT depletion would lead to an increase in EtOH intake. Our findings indicate that SD rats are pre-disposed for high or low EtOH consumption and can be divided into two phenotypes based on drinking behavior, Drinkers and Non-drinkers, respectively. Contrary to our prediction, depletion of central 5-HT levels with the serotonergic neurotoxin 5,7-DHT did not affect EtOH consumption for either of these phenotypes. Upon investigation of 5-HT cell depletion in the DRN, one of the main sources of 5-HT to the forebrain, we found preservation of the VM sub-region in all animals regardless of treatment. Further, animals that consumed stable baseline levels of EtOH above 0.5 g EtOH/kg showed higher levels of 5-HT depletion than those consuming less than 0.5 g EtOH/kg, indicating a potential effect of EtOH on the sensitivity of these neurons to 5,7-DHT.
In accordance with other studies (Bito-Onon et al., 2011; Melchior and Myers, 1976; Linseman, 1987; Khanna et al, 1990; Wayner et al., 1972), we showed that SD rats will voluntarily drink to stable baseline levels in the 20% EtOH IA drinking paradigm. While the level of consumption was not as robust as the out-bred Long Evans (Simms et al., 2008; Simms et al., 2010) or of rats specifically bred to prefer EtOH (Rodd et al., 2004), it was consistent across drinking sessions after 6-8 weeks of IA, with rats naturally dividing into two groups based on EtOH consumption levels. This result has been mentioned previously in the literature but not examined thoroughly (Carnicella et al., 2014; Li et al., 2011; Moorman and Aston-Jones, 2009; Richardson and Novakovski, 1978). This dichotomous split in SD populations with regard to EtOH consumption allows for measurable correlates of pharmacological manipulations that may change EtOH intake based on consumption profiles, unlike rats that have a higher preference for EtOH that potentially exhibit maximal consumption of EtOH under voluntary conditions (LeMarquand, et al., 1994a,b).
The pre-disposition for high or low EtOH intake seen in SD rats may be the result of genetic (hard-wired) or epigenetic (environmental) mechanisms. It is possible that some SD rats are genetically prone to developing addictive behaviors and have a natural preference for EtOH consumption before exposure. Alternatively, epigenetic changes may take place in the CNS following EtOH consumption, such that EtOH exposure itself induces changes in the brain that trigger higher ingestion of EtOH for some animals and not others (Vassoler et al., 2014; Zakhari, 2013). Another consideration may be SD rats that voluntarily drink EtOH have a naturally occurring (genetic) dysfunction of the 5-HT system prior to EtOH exposure compared to those that abstain from EtOH or consume trace amounts. This possibility coincides with data from human alcoholics (as considered in McBride, 2010) and further supports the notion that out-bred SD rats are representative of the human population with regard to EtOH consumption patterns. Regardless of pre-disposition factors, the IA drinking model shows sensitivity and reliability for distinguishing between these two drinking phenotypes of SD rats, thus increasing its validity as a model and measure of voluntary EtOH consumption in out-bred strains of rat.
A reduction of CNS 5-HT via 5,7-DHT failed to increase EtOH consumption in the IA drinking paradigm, contrary to findings by Wang and colleagues (1996) using a continuous-access voluntary EtOH consumption model. Interestingly, Adell and Myers (1995) also failed to show no change in EtOH consumption in the SD rat following 5-HT depletion with 5,7-DHT infusion into either the DRN or the median raphe nucleus (MRN) in the three-bottle, two-choice self-selection procedure. In this study, depletion of forebrain 5-HT levels with DRN lesions alone resulted in a 50-80% reduction of 5-HT compared to sham controls via HPLC analysis. Although Adell and Myers (1995) targeted the DRN directly with 5,7-DHT, quantification of 5-HT depletion via cell counts and examination of the distribution of any preserved cells was not completed. Without this analysis it is impossible to assess the diffusion of microinjections or if depletion was achieved across the entire extent of the DRN, or only in select locations.
Inconsistent results have been obtained in previous studies investigating the effect of 5-HT depletion on EtOH drinking behavior (Adell and Myers, 1995; Ellison et al., 1979; Geller, 1973; Hill and Goldstein, 1974; Ho et al., 1974; Melchior and Myers, 1976; Myers and Melchior, 1975; Richardson and Novakovski, 1978; Wang et al., 1996). This limitation cannot be revealed by measuring forebrain levels of 5-HT alone, given the specific topographical organization and diverse efferent projections of the DRN (O'Hearn and Molliver, 1984; Vasudeva et al., 2014; Vertes, 1991). This discrepancy in 5-HT quantification following the administration of 5,7-DHT (cellular depletion versus forebrain 5-HT levels) has not been addressed previously in the literature to our knowledge (although some dual quantification was completed in Jha et al. (2006) and may be a major contributing factor for such varied results in the EtOH literature (see also Feenstra and van der Plasse, 2010).
Depletion of 5-HT neurons via 5,7-DHT appears to be limited by the ability of the toxin to penetrate and diffuse through brain tissue from the ventricular system. Across animals that received 5,7-DHT (regardless of EtOH consumption), the furthest DRN area from the aqueduct, the ventromedial (VM) sub-region, was preserved to an extent (Table 1; Fig. 6A, B, D, E), as was the MRN (Fig. 6C, F). Previous neurochemical studies have shown ~50-75% reduction in forebrain 5-HT levels 2-4 weeks following administration of 150-300 μg 5,7-DHT (Baumgarten et al., 1974; Hall et al., 1999), and our immunohistochemical data confirmed a similar depletion of 5-HT neurons within the DRN (~76% average depletion across sub-regions; see Table 1). Although it is typical in the literature to quantify 5-HT depletion using HPLC or microdialysis to measure forebrain levels of 5-HT itself (Hall et al., 1999; Jankowska et al., 1994; Jha et al., 2006; Kirby et al., 1995; Li et al., 1996) we specifically wanted to examine the contribution of the DRN and its multiple subdivisions to drinking behavior. The DRN has a distinct topographical efferent projection pattern (Abrams et al., 2004; Lowry et al., 2005; O'Hearn and Molliver, 1984; Vasudeva et al., 2014; Vertes, 1991), and the VM specifically projects to cortical and subcortical brain regions, including the somatosensory and motor cortices, caudate putamen, and substantia nigra (Lowry et al., 2005; Vasudeva et al., 2011). Interestingly, our lesions resulted in the ablation of the dorsomedial (DM) and lateral wing (LAT) sub-regions, which project to the amygdala and anxiogenic brain regions (respectively), yet no change in EtOH consumption was induced (Lowry et al., 2005).
Our findings are consistent with other studies that used a variety of EtOH-drinking models in combination with 5,7-DHT-induced 5-HT depletion in the CNS (McBride, 2010), supporting the notion that a reduction in central 5-HT via neurotoxic agents does not actually increase EtOH consumption. This is in stark contrast to studies that administered 5HT1A or GABAA agonists to reduce DRN 5-HT electrophysiological activity and 5-HT release in the forebrain, which produced an increase in EtOH consumption (Tomkins et al., 1994; Tomkins and Fletcher, 1996; for an excellent overview of this literature see McBride, 2010). This discrepancy suggests that the death, and essentially the removal, of 5-HT neurons and their axonal projections from the DRN has a different effect than the inhibition of serotonergic transmission originating from this nucleus. In both methodologies, 5-HT from the DRN is reduced or removed, however preservation of the tissue during agonist administration appears to effect EtOH behavior differently than inducing 5-HT cell death. This discrepancy indicates that the role of DRN-5-HT in EtOH intake may be mediated by the 5-HT1A receptor or via GABAergic mechanisms rather than by 5-HT directly. One possibility is that the reduction of 5-HT release from cells in the DRN (regardless of technique) may remove tonic inhibition of the dopaminergic mesolimbic reward system. It is possible that the reduction of 5-HT DRN transmission in 5,7-DHT treated animals is not robust or perhaps specific enough to affect EtOH consumption via this pathway, but the actions of 5-HT1A or GABAA agonists are. This may indicate that there is a greater disinhibition of the mesolimbic dopamine system with DRN inhibition versus DRN destruction (McBride, 2010). Alternatively, increases in EtOH intake seen following DRN 5-HT inhibition combined with no change seen following 5-HT lesions strongly supports the involvement of non-5HT DRN cells in the maintenance of addictive behaviors (McDevitt et al., 2014).
Our most striking finding was the apparent sensitization of 5-HT DRN neurons to 5,7-DHT in Drinkers but not Non-drinkers. Drinkers showed significantly higher 5-HT-neuron depletion compared to any other treatment group, including Non-drinkers receiving 5,7-DHT (Fig. 7). One possible explanation is that, via non-5-HT cytotoxic mechanisms, chronic intake of EtOH reduces the density of the brain tissue within the DRN (Kruman et al., 2012; McDevitt et al., 2014), increasing the diffusion depth of 5,7-DHT and causing greater depletion of 5-HT neurons. While there is no direct evidence of this specifically in the DRN, a significant decrease in brain volume and tissue density is present in human alcoholics (Badawy, 2002), thus this possibility should be considered. A more likely scenario regarding the integrity of brain tissue in Drinkers may be genetic/biological differences present in 5-HT functionality prior to EtOH exposure. Both human alcoholics and EtOH-preferring rat strains show decreased 5-HT system functionality and fiber density (Badawy, 2002). This anatomical difference in EtOH-preferring rats is present prior to EtOH exposure, strongly suggesting decreased 5-HT function may be a pre-disposing factor to excessive EtOH consumption (Badawy, 2002; McBride, 2010). A pre-existing 5-HT dysfunction, increasing vulnerability to both higher rates of EtOH consumption and sensitivity to neurotoxicity, could also explain the higher 5-HT depletion rates by 5,7-DHT in Drinkers compared to Non-drinkers.
A second consideration is that an interaction may be taking place between tryptophan hydroxylase metabolism and EtOH in the brain, such that the homeostatic properties of DRN 5-HT cells have been altered by CNS levels of EtOH, making this population more susceptible to neurotoxic agents and cell death (Agudelo et al., 2012; Badawy, 2002; Kruman et al., 2012). Ethanol readily perfuses the blood-brain barrier into the CNS and has varying, wide-spread effects on neuronal function and signaling. The molecule interacts with the NMDA-receptor, increasing calcium influx and the production of reactive oxygen species within cells, altering homeostasis (Kruman et al., 2012). Although the specific actions of EtOH within the DRN have not been examined at the cellular or molecular level, there is an abundance of literature that suggests EtOH impacts 5-HT metabolism both in the periphery and in the CNS (see Badawy, 2002) and also 5-HT signaling via interactions with 5-HT receptors and transporters (Agudelo et al., 2012; Babu et al., 2009). In combination, the homeostatic cellular interference of EtOH and the interaction of this molecule with serotonergic pathways makes a strong case for decreased stability of 5-HT cells in the DRN and increased cell death upon neurotoxin challenge.
5. Conclusions
The impact of our current study is several fold and reflects upon genetic and epigenetic factors that may contribute to the development of EtOH consumption in the SD rat and the potential interactions between EtOH and 5-HT in the CNS. A combination of genetic predisposition to EtOH intake and decreased 5-HT function likely increases the susceptibility of 5HT cells to perturbations. Consumption of EtOH could further reduce 5-HT functioning that may already be present in alcoholic humans and EtOH consuming rats. This may contribute to the dysfunctionality of the 5-HT system in alcoholic humans and lead to a better understanding of current pharmacological treatments for this addiction.
Highlights.
Intermittent access 2-bottle choice induces 20% EtOH intake in Sprague-Dawley rats
Individual EtOH consumption patterns separate rats into Drinkers and Non-drinkers
EtOH intake is not affected by 5-HT depletion with 5,7-DHT
The ventromedial DRN sub-region is preserved with icv administered 5,7-DHT
Drinkers are more sensitive to 5-HT depletion than Non-drinkers
Acknowledgements
This research was supported by PHS grants DA007237 and DA013429. The authors wish to thank Anthony Tuan Le, Taylor Agate, and Jonathan Palma for their assistance with the ethanol consumption studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133(4):983–997. doi: 10.1016/j.neuroscience.2005.03.025. doi: S0306-4522(05)00344-1 [pii]10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Adell A, Myers RD. Selective destruction of midbrain raphe nuclei by 5,7-DHT: is brain 5-HT involved in alcohol drinking in Sprague-Dawley rats? Brain Res. 1995;693(1-2):70–79. doi: 10.1016/0006-8993(95)00701-q. doi: 0006-8993(95)00701-Q [pii] [DOI] [PubMed] [Google Scholar]
- Agudelo M, Yoo C, Nair MP. Alcohol-induced serotonergic modulation: the role of histone deacetylases. Alcohol. 2012;46(7):635–642. doi: 10.1016/j.alcohol.2012.03.005. doi: 10.1016/j.alcohol.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadiantehrani S, Barak S, Ron D. GDNF is a novel ethanol-responsive gene in the VTA: implications for the development and persistence of excessive drinking. Addict Biol. 2014;19(4):623–633. doi: 10.1111/adb.12028. doi: 10.1111/adb.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu DK, Diaz A, Samikkannu T, Rao KV, Saiyed ZM, Rodriguez JW, Nair MP. Upregulation of serotonin transporter by alcohol in human dendritic cells: possible implication in neuroimmune deregulation. Alcohol Clin Exp Res. 2009;33(10):1731–1738. doi: 10.1111/j.1530-0277.2009.01010.x. doi: 10.1111/j.1530-0277.2009.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy AA. Tryptophan metabolism in alcoholism. Nutr Res Rev. 2002;15(1):123–152. doi: 10.1079/NRR200133. doi: 10.1079/NRR200133S0954422402000069 [pii] [DOI] [PubMed] [Google Scholar]
- Baumgarten HG, Klemm HP, Sievers J, Schlossberger HG. Dihydroxytryptamines as tools to study the neurobiology of serotonin. Brain Res Bull. 1982;9(1-6):131–150. doi: 10.1016/0361-9230(82)90128-9. [DOI] [PubMed] [Google Scholar]
- Baumgarten HG, Victor SJ, Lovenberg W. Effect of intraventricular injection of 5,7-dihydroxytryptamine on regional tryptophan hydroxylase of rat brain. J Neurochem. 1973;21(1):251–253. doi: 10.1111/j.1471-4159.1973.tb04246.x. [DOI] [PubMed] [Google Scholar]
- Bito-Onon JJ, Simms JA, Chatterjee S, Holgate J, Bartlett SE. Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addict Biol. 2011;16(3):440–449. doi: 10.1111/j.1369-1600.2010.00309.x. doi: 10.1111/j.1369-1600.2010.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning RA, Hoffmann WE, Simonton RL. Changes in seizure susceptibility after intracerebral treatment with 5,7-dihydroxytryptamine: role of serotonergic neurons. Ann N Y Acad Sci. 1978;305:437–456. doi: 10.1111/j.1749-6632.1978.tb31540.x. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009;43(1):35–43. doi: 10.1016/j.alcohol.2008.12.001. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48(3):243–252. doi: 10.1016/j.alcohol.2014.01.006. doi: 10.1016/j.alcohol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DA, Paraherakis AM, Negrete JC, Gill KJ. The impact of depression on the outcome of addictions treatment. J Subst Abuse Treat. 1998;15(2):123–130. doi: 10.1016/s0740-5472(97)00183-9. doi: S0740547297001839 [pii] [DOI] [PubMed] [Google Scholar]
- Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59(Suppl 14):11–14. [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28(2):206–215. doi: 10.1038/sj.npp.1300045. doi: 10.1038/sj.npp.13000451300045 [pii] [DOI] [PubMed] [Google Scholar]
- Connor TJ, Song C, Leonard BE, Anisman H, Merali Z. Stressor-induced alterations in serotonergic activity in an animal model of depression. Neuroreport. 1999;10(3):523–528. doi: 10.1097/00001756-199902250-00015. [DOI] [PubMed] [Google Scholar]
- Contreras S, Alvarado R, Mardones J. Effects of p-chlorophenylalanine on the voluntary consumption of ethanol, water and solid food by UChA and UChB rats. Alcohol. 1990;7(5):403–407. doi: 10.1016/0741-8329(90)90023-6. [DOI] [PubMed] [Google Scholar]
- Ellison G, Daniel F, Zoraster R. Delayed increases in alcohol consumption occur in rat colonies but not in isolated rats after injections of monoamine neurotoxins. Exp Neurol. 1979;65(3):608–615. doi: 10.1016/0014-4886(79)90047-5. [DOI] [PubMed] [Google Scholar]
- Feenstra MGP, van der Plasse G. Tryptophan Depletion and Serotonin Release- A Critical Reappraisal. In: Muller CP, Jacobs BL, editors. Handbook of Behavioral Neurobiology of Serotonin. Academic Press; New York: 2010. pp. 249–258. [Google Scholar]
- Geller I. Effects of para-chlorophenylalanine and 5-hydroxytryptophan on alcohol intake in the rat. Pharmacol Biochem Behav. 1973;1(3):361–365. doi: 10.1016/0091-3057(73)90130-5. doi: 0091-3057(73)90130-5 [pii] [DOI] [PubMed] [Google Scholar]
- Hall FS, Devries AC, Fong GW, Huang S, Pert A. Effects of 5,7-dihydroxytryptamine depletion of tissue serotonin levels on extracellular serotonin in the striatum assessed with in vivo microdialysis: relationship to behavior. Synapse. 1999;33(1):16–25. doi: 10.1002/(SICI)1098-2396(199907)33:1<16::AID-SYN2>3.0.CO;2-8. doi: 10.1002/(SICI)1098-2396(199907)33:1. [DOI] [PubMed] [Google Scholar]
- Hill SY, Goldstein R. Effect of p-chlorophenylalanine and stress on alcohol consumption by rats. Q J Stud Alcohol. 1974;35(1):34–41. [PubMed] [Google Scholar]
- Ho AK, Tsai CS, Chen RC, Begleiter H, Kissin B. Experimental studies on alcoholism. I. Increased in alcohol preference by 5.6-dihydroxytryptamine and brain acetylcholine. Psychopharmacologia. 1974;40(2):101–107. doi: 10.1007/BF00421359. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Bidzinski A, Kostowski W. Alcohol drinking in rats treated with 5,7-dihydroxytryptamine: effect of 8-OH-DPAT and tropisetron (ICS 205-930). Alcohol. 1994;11(4):283–288. doi: 10.1016/0741-8329(94)90093-0. doi: 0741-8329(94)90093-0 [pii] [DOI] [PubMed] [Google Scholar]
- Jha S, Rajendran R, Davda J, Vaidya VA. Selective serotonin depletion does not regulate hippocampal neurogenesis in the adult rat brain: differential effects of pchlorophenylalanine and 5,7-dihydroxytryptamine. Brain Res. 2006;1075(1):48–59. doi: 10.1016/j.brainres.2005.12.110. doi: 10.1016/j.brainres.2005.12.110. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Kalant H, Shah G, Sharma H. Comparison of sensitivity and alcohol consumption in four outbred strains of rats. Alcohol. 1990;7(5):429–434. doi: 10.1016/0741-8329(90)90027-a. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Allen AR, Lucki I. Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1995;682(1-2):189–196. doi: 10.1016/0006-8993(95)00349-u. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Chou-Green JM, Davis K, Lucki I. The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1997;760(1-2):218–230. doi: 10.1016/s0006-8993(97)00287-4. doi: S0006-8993(97)00287-4 [pii] [DOI] [PubMed] [Google Scholar]
- Kirby LG, Lucki I. Interaction between the forced swimming test and fluoxetine treatment on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the rat. J Pharmacol Exp Ther. 1997;282(2):967–976. [PubMed] [Google Scholar]
- Kirby LG, Pan YZ, Freeman-Daniels E, Rani S, Nunan JD, Akanwa A, Beck SG. Cellular effects of swim stress in the dorsal raphe nucleus. Psychoneuroendocrinology. 2007;32(6):712–723. doi: 10.1016/j.psyneuen.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruman II, Henderson GI, Bergeson SE. DNA damage and neurotoxicity of chronic alcohol abuse. Exp Biol Med (Maywood) 2012;237(7):740–747. doi: 10.1258/ebm.2012.011421. doi: 10.1258/ebm.2012.011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: findings of animal studies. Biol Psychiatry. 1994;36(6):395–421. doi: 10.1016/0006-3223(94)91215-7. doi: 0006-3223(94)91215-7 [pii] [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: clinical evidence. Biol Psychiatry. 1994;36(5):326–337. doi: 10.1016/0006-3223(94)90630-0. doi: 0006-3223(94)90630-0 [pii]. [DOI] [PubMed] [Google Scholar]
- Li J, Cheng Y, Bian W, Liu X, Zhang C, Ye JH. Region-specific induction of FosB/DeltaFosB by voluntary alcohol intake: effects of naltrexone. Alcohol Clin Exp Res. 2010;34(10):1742–1750. doi: 10.1111/j.1530-0277.2010.01261.x. doi: 10.1111/j.1530-0277.2010.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zou Y, Ye JH. Low frequency electroacupuncture selectively decreases voluntarily ethanol intake in rats. Brain Res Bull. 2011;86(5-6):428–434. doi: 10.1016/j.brainresbull.2011.08.013. doi: 10.1016/j.brainresbull.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MY, Yan QS, Coffey LL, Reith ME. Extracellular dopamine, norepinephrine, and serotonin in the nucleus accumbens of freely moving rats during intracerebral dialysis with cocaine and other monoamine uptake blockers. J Neurochem. 1996;66(2):559–568. doi: 10.1046/j.1471-4159.1996.66020559.x. [DOI] [PubMed] [Google Scholar]
- Linseman MA. Alcohol consumption in free-feeding rats: procedural, genetic and pharmacokinetic factors. Psychopharmacology (Berl) 1987;92(2):254–261. doi: 10.1007/BF00177925. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8(4):233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Melchior CL, Myers RD. Genetic differences in ethanol drinking of the rat following injection of 6-OHDA, 5,6-DHT or 5,7-DHT into the cerebral ventricles. Pharmacol Biochem Behav. 1976;5(1):63–72. doi: 10.1016/0091-3057(76)90289-6. [DOI] [PubMed] [Google Scholar]
- McBride WJ. Role of Serotonin in Brain Reward and Regulation of Alcohol Drinking Behavior. In: Muller CP, Jacobs BL, editors. Handbook of Behavioral Neurobiology of Serotonin. Academic Press; New York: 2010. pp. 399–414. [Google Scholar]
- McDevitt RA, Tiran-Cappello A, Shen H, Balderas I, Britt JP, Marino RA, Bonci A. Serotonergic versus nonserotonergic dorsal raphe projection neurons: differential participation in reward circuitry. Cell Rep. 2014;8(6):1857–1869. doi: 10.1016/j.celrep.2014.08.037. doi: 10.1016/j.celrep.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol--preferring Sprague--Dawley rats. Alcohol. 2009;43(5):379–386. doi: 10.1016/j.alcohol.2009.07.002. doi: 10.1016/j.alcohol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Waller MB, Gatto GJ, McBride WJ, Lumeng L, Li TK. Monoamine uptake inhibitors attenuate ethanol intake in alcohol-preferring (P) rats. Alcohol. 1985;2(2):349–352. doi: 10.1016/0741-8329(85)90073-4. [DOI] [PubMed] [Google Scholar]
- Myers RD, Melchior CL. Alcohol drinking in the rat after destruction of serotonergic and catecholaminergic neurons in the brain. Res Commun Chem Pathol Pharmacol. 1975;10(2):363–378. [PubMed] [Google Scholar]
- Myers RD, Quarfordt SD. Alcohol drinking attenuated by sertraline in rats with 6-OHDA or 5,7-DHT lesions of N. accumbens: a caloric response? Pharmacol Biochem Behav. 1991;40(4):923–928. doi: 10.1016/0091-3057(91)90107-d. [DOI] [PubMed] [Google Scholar]
- Myers RD, W. L. Veale L. Alcohol preference in the rat: reduction following depletion of brain serotonin. Science. 1968;160(3835):1469–1471. doi: 10.1126/science.160.3835.1469. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse Comorbidity: Addiction and Other Mental Illnesses. Research Report Series. 2010:12. [Google Scholar]
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int. 1995;26(4):305–336. doi: 10.1016/0197-0186(94)00139-l. discussion 337-342. [DOI] [PubMed] [Google Scholar]
- Novier A, Diaz-Granados JL, Matthews DB. Alcohol use across the lifespan: An analysis of adolescent and aged rodents and humans. Pharmacol Biochem Behav. 2015 doi: 10.1016/j.pbb.2015.03.015. doi: 10.1016/j.pbb.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Okere CO, Waterhouse BD. Acute restraint increases NADPH-diaphorase staining in distinct subregions of the rat dorsal raphe nucleus: implications for raphe serotonergic and nitrergic transmission. Brain Res. 2006;1119(1):174–181. doi: 10.1016/j.brainres.2006.08.058. [DOI] [PubMed] [Google Scholar]
- O'Hearn E M, Molliver ME. Organization of raphe-cortical projections in the rat: a quantitative retrograde study. Brain Research Bulletin. 1984;13:709–726. doi: 10.1016/0361-9230(84)90232-6. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Rezvani AH, Djouma E, Parsian A, Lawrence AJ. Depressive-like behavior and high alcohol drinking co-occur in the FH/WJD rat but appear to be under independent genetic control. Neurosci Biobehav Rev. 2007;31(1):103–114. doi: 10.1016/j.neubiorev.2006.07.002. doi: S0149-7634(06)00071-6 [pii] 10.1016/j.neubiorev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 2005. [Google Scholar]
- Richardson JS, Novakovski DM. Brain monoamines and free choice ethanol consumption in rats. Drug Alcohol Depend. 1978;3(4):253–264. doi: 10.1016/0376-8716(78)90079-0. [DOI] [PubMed] [Google Scholar]
- Robertson DA, Beattie JE, Reid IC, Balfour DJ. Regulation of corticosteroid receptors in the rat brain: the role of serotonin and stress. Eur J Neurosci. 2005;21(6):1511–1520. doi: 10.1111/j.1460-9568.2005.03990.x. [DOI] [PubMed] [Google Scholar]
- Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci. 2003;23(3):970–977. doi: 10.1523/JNEUROSCI.23-03-00970.2003. doi: 23/3/970 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman GE, Amit Z, Brown ZW, Bourque C, Ogren SO. An investigation of the mechanisms of action of 5-hydroxytryptamine in the suppression of ethanol intake. Neuropharmacology. 1982;21(4):341–347. doi: 10.1016/0028-3908(82)90098-3. [DOI] [PubMed] [Google Scholar]
- Rockman GE, Amit Z, Carr G, Brown ZW, Ogren SO. Attenuation of ethanol intake by 5-hydroxytryptamine uptake blockade in laboratory rats. I. Involvement of brain 5-hydroxytryptamine in the mediation of the positive reinforcing properties of ethanol. Arch Int Pharmacodyn Ther. 1979;241(2):245–259. [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav. 2004;79(3):439–450. doi: 10.1016/j.pbb.2004.08.018. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE. Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology. 2010;35(7):1453–1463. doi: 10.1038/npp.2010.15. doi: 10.1038/npp.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32(10):1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. doi: ACER753 [pii] 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins DM, Fletcher PJ. Evidence that GABA(A) but not GABA(B) receptor activation in the dorsal raphe nucleus modulates ethanol intake in Wistar rats. Behav Pharmacol. 1996;7(1):85–93. [PubMed] [Google Scholar]
- Tomkins DM, Higgins GA, Sellers EM. Low doses of the 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH DPAT) increase ethanol intake. Psychopharmacology (Berl) 1994;115(1-2):173–179. doi: 10.1007/BF02244769. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, Byrnes EM, Pierce RC. The impact of exposure to addictive drugs on future generations: Physiological and behavioral effects. Neuropharmacology. 2014;76(Pt B):269–275. doi: 10.1016/j.neuropharm.2013.06.016. doi: 10.1016/j.neuropharm.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudeva RK, Waterhouse BD. Cellular profile of the dorsal raphe lateral wing sub-region: Relationship to the lateral dorsal tegmental nucleus. J Chem Neuroanat. 2014;57-58C:15–23. doi: 10.1016/j.jchemneu.2014.03.001. doi: 10.1016/j.jchemneu.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. A Pha-L analysis of ascending projections of the dorsal raphe nucleus in the rat. The Journal of Comparative Neurology. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Wang JY, Shum AY, Lin TC, Wang Y. Central serotonergic lesions increase voluntary alcohol consumption in Sprague Dawley rats: moderation by long-term ethanol administration. Alcohol Clin Exp Res. 1996;20(7):1252–1259. doi: 10.1111/j.1530-0277.1996.tb01120.x. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Greenberg I, Tartaglione R, Nolley D, Fraley S, Cott A. A new factor affecting the consumption of ethyl alcohol and other sapid fluids. Physiol Behav. 1972;8(2):345–362. doi: 10.1016/0031-9384(72)90383-6. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29(3):203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Zakhari S. Alcohol metabolism and epigenetics changes. Alcohol Res. 2013;35(1):6–16. doi: 10.35946/arcr.v35.1.02. [DOI] [PMC free article] [PubMed] [Google Scholar]