Abstract
We previously demonstrated that skeletal structure and strength phenotypes vary considerably in heterogeneous stock (HS) rats. These phenotypes were found to be strongly heritable, suggesting that the HS rat model represents a unique genetic resource for dissecting the complex genetic etiology underlying bone fragility. The purpose of this study was to identify and localize genes associated with bone structure and strength phenotypes using 1524 adult male and female HS rats between 17 to 20 weeks of age. Structure measures included femur length, neck width, head width; femur and lumbar spine (L3-5) areas obtained by DXA; and cross-sectional areas (CSA) at the midshaft, distal femur and femoral neck, and the 5th lumbar vertebra measured by CT. In addition, measures of strength of the whole femur and femoral neck were obtained. Approximately 70,000 polymorphic SNPs distributed throughout the rat genome were selected for genotyping, with a mean linkage disequilibrium coefficient between neighboring SNPs of 0.95. Haplotypes were estimated across the entire genome for each rat using a multipoint haplotype reconstruction method, which calculates the probability of descent at each locus from each of the 8 HS founder strains. The haplotypes were then tested for association with each structure and strength phenotype via a mixed model with covariate adjustment. We identified quantitative trait loci (QTLs) for structure phenotypes on chromosomes 3, 8, 10, 12, 17 and 20, and QTLs for strength phenotypes on chromosomes 5, 10 and 11 that met a conservative genome-wide empiric significance threshold (FDR=5%; P<3 × 10−6). Importantly, most QTLs were localized to very narrow genomic regions (as small as 0.3Mb and up to 3 Mb), each harboring a small set of candidate genes, both novel and previously shown to have roles in skeletal development and homeostasis.
Keywords: Heterogeneous stock rat, Bone structure, Bone strength, Genes, Osteoporosis
Introduction
Osteoporosis is a common, genetically complex disorder characterized by reduced bone mineral density (BMD), abnormal bone microarchitecture and compromised bone strength leading to increased susceptibility to fracture risk [1]. Bone mineral density (BMD), structure and strength are the major determinants of skeletal fracture [2-4]. As much as 80% of the variability of BMD and about one-third of the variance in the risk of fracture is due to heritable factors [5-8]. Although BMD by DXA is most often used for predicting fracture risk in humans, it is not an adequate measure to capture several important aspects of bone strength. The genetic basis of fracture susceptibility depends on coordination of bone density, morphology, structure and tissue-quality, all of which contribute to bone strength. Identification and characterization of genes underlying bone structure and strength, particularly at the most common sites of fracture, will ultimately lead to better diagnosis, prevention and treatment of osteoporosis and other high bone-fragility conditions.
Previously, we identified several quantitative trait loci (QTLs) linked to bone structure and strength phenotypes in inbred F344, LEW, COP and DA rats [9-12]. However, most of these QTLs are large (20-30 cM) and harbor hundreds of potential candidate genes. It is a formidable challenge to narrow these critical QTL regions to a small chromosomal segment containing a few genes. To address this issue, in this study we exploited a unique rat model, the heterogeneous stock (HS) rat, developed by the National Institutes of Health (NIH) in 1984 [13]. These rats were derived from eight inbred founder strains: Agouti (ACI/N), Brown Norway (BN/SsN), Buffalo (BUF/N), Fischer 344 (F344/N), M520/N, Maudsley Reactive (MR/N), Wistar-Kyoto (WKY/N) and Wistar-Nettleship (WN/N) [13-14]. Importantly, the descendants of these rats represent a unique, genetically random mosaic of the founding animals’ chromosomes due to recombination that has accumulated over 50 generations, enabling the fine mapping of QTLs to very small genomic regions. Recently, these rats have been successfully used for high-resolution mapping for diabetes and fear-related behavior phenotypes [15-16].
In a previous study, we demonstrated that bone structure and strength phenotypes vary considerably among the HS founder strains [17]. Recently, using the sequence data from these strains and genotypes for a dense SNP marker map in the HS offspring population, we identified several QTLs and underlying genetic variants for multiple bone phenotypes [18]; however, no single genetic variants explaining associations with bone phenotypes were detected, consistent with the complex genetic architecture of skeletal phenotypes observed previously both in humans and animal models [18-19]. The purpose of this study is to identify and localize QTLs for bone structure and strength phenotypes using high-resolution mapping in the HS rat offspring at the most common skeletal fracture sites. We anticipate that using this approach the bone structure and strength QTLs will be localized to much smaller genomic regions than QTLs detected using inbred rat crosses. Ultimately, this will allow us to identify a smaller set of potential candidate genes underlying these QTLs, and contribute to a better understanding of the complex genetic architecture of the fracture risk phenotypes in the rat model and in human.
Materials and Methods
Animals
We used 1524 HS rats (male n=728; female n=796) in this study. The HS rats were bred and grown at the Autonomous University of Barcelona. The rats were housed in cages in pairs (males) and trios (females) and maintained with food and water available ad libitum. The HS rats were raised over 2.5 years in batches of approximately 250 animals in accordance with the Spanish legislation on “Protection of Animals used for Experimental and Other Scientific Purposes” and the European Communities Council Directive (86/609/EEC).
Euthanasia and specimen collection
HS rats were euthanized between 17 and 20 weeks of age by ether inhalation. The lower limbs and lumbar vertebrae (L3-5) were dissected from these animals. The lower limbs on the right side were immediately frozen after harvest wrapped in saline soaked gauge in plastic Ziplock bags at −20°C for subsequent biomechanical testing. To prevent dehydration and any adverse effect on the mechanical properties, we kept the muscle attached to the limbs during the storage period until testing. The lower limbs on the left side and lumbar vertebrae (L3-5) were stripped of muscle, transferred to 70% ethyl alcohol and stored at 4°C for bone structure analyses.
Dual energy X-ray absorptiometry (DXA)
The left femur and lumbar vertebrae 3-5 (L3-5) of the HS rats were scanned using DXA (PIXImus II mouse densitometer; Lunar Corp., Madison, WI, USA) with ultra-high resolution (0.18 × 0.18 mm/pixel). The machine was calibrated prior to each DXA scanning session using a phantom supplied by the manufacturer. During scanning dissected femurs were positioned with anterior surface facing up and the distal end on left side whereas L3-5 were oriented anterior surface facing up on a standardized platform in air. After completion of the scan of each bone, mutually exclusive region of interest (ROI) boxes were drawn manually around the bones from which femur area (mm2) and lumbar area (mm2) measurements were obtained. The intra-specimen % coefficient variation for area was less than 1%.
Femur length, femoral head and neck width measurements
The femur size parameters were measured using digital calipers accurate to 0.01 mm, with a precision of ± 0.005 mm (Mitutoyo, Aurora, IL). The femur length (mm) was measured from the end of the medial condyle to the end of the greater trochanter. The maximum transverse diameter (mm) of the femoral head and the shortest transverse distance (mm) of the femoral neck were considered as the width of the femoral head and neck, respectively.
Peripheral quantitative computed tomography (pQCT)
The left femurs were placed in plastic tubes filled with 70% ethyl alcohol and centered in the gantry of a Norland Stratec XCT Research SA+pQCT (Stratec Electronics, Pforzheim, Germany) machine. Single slice measurements of 0.26 mm thickness and a voxel size of 0.07 mm were taken for the femur: one slice through femoral midshaft and one slice approximately 1 mm below the growth plate of distal femur. L5 vertebrae were scanned in cross-section at the caudo-cranial center of the vertebral body. For femoral neck, five consecutive scans perpendicular to the neck axis were obtained 0.25 mm apart from each other starting at the base of the femoral head and ending at the greater trochanter. For each slice, the X-ray source was rotated through 180° of projection. Total (trabecular and cortical) cross-sectional area (CSA; mm2) from each slice for femur and L5 spine were measured using the thresholds of 500 and 900 mg/cm3. For femoral neck, CSA were measured from the average values of all five slices.
Biomechanical testing
The frozen right femurs were brought to room temperature slowly in a saline bath. The femurs were tested in three-point bending by positioning them with anterior surface facing up and the distal end as close to the left supporting point as possible on the lower supports (15 mm span for female and 20 mm span for male) of a three-point bending fixture and applying load at the midpoint using a material testing machine (Alliance RT/5, MTS Systems Corp., Eden Prairie, USA). For femoral neck, the proximal end of the femurs was mounted vertically in a special chuck that clamped the femoral shaft to the lower platen of the same material testing machine. The bones were held in place by a small (1N) preload, and then load was applied directly downward at a crosshead speed of 20 mm/min onto the mid-femur and femoral head at room temperature in monotonic axial compression until fracture. Force and displacement measurements were collected every 0.05 second. From the force vs. displacement curves, we measured the phenotypes that are critical for different aspects of bone fragility - ultimate force (Fu; N), stiffness (S; N/mm), work to failure (W; mJ) and ultimate displacement or elongation (E; mm) in TestWorks software, version 4.06. Fu reflects the strength of the bone or maximum load that the bone can support before failing; S is the slope of the curve represents the bone brittleness; W reflects the amount of energy the specimen can absorb prior to fracture and E is the reciprocal of brittleness. The phenotypes, together, best reflect the clinical aspect of skeletal fragility.
Genotyping
DNA was extracted from liver tissues from 8 original founders and 1524 HS rats using standard protocols. To reconstruct the genome of each HS rat, genotypes for over 900,000 SNPs for each rat were selected from an Affymetrix rat custom SNP array (www.affymetrix.com) as described previously [19]. We used only high quality informative (SNP call rate more than 0.99, polymorphic SNPs and no missing genotyping) markers. The average spacing between adjacent SNPs is 12.5 kb, with a maximum gap size of 1 Mb. The maximum density is 15 SNPs in a 10kb window. In addition, there are 19 larger gaps (1-3.8 Mb) on the autosomes (chromosome 1 to chromosome 20) and 12 larger gaps on chromosome X, with a maximum gap of 4.8 Mb. The set of SNPs were pruned to approximately 70,000 high quality SNPs which covered the HS rat genome with a mean linkage disequilibrium coefficient between neighboring SNPs of 0.95.
Measurements of intra- and inter-observer errors
The structure and strength phenotypes were measured in batches consisting approximately 250 samples involving multiple individuals, therefore, we analyzed the intra- (measurement of a phenotype across multiple samples by an individual) and inter-observer (measurement of a phenotype across multiple samples by different individuals) variations for these measurements. We found that the intra-observer % of coefficient of variations (CV) for femur length (<4%), neck width (<13%), head width (<9%), lumbar area (<18%), femur work to failure (<34%), femur elongation (<32%) and femur neck ultimate force (<23%) were comparable to inter-observer variations of these measurements (<6%, <12%, 7%, <16%, <39%, <25% and <20%, respectively), suggesting that the quality of these phenotypic measurements was consistent across all samples in this study.
Statistical genetic analysis
Haplotypes were constructed for each rat across the genome using the multipoint haplotype reconstruction method HAPPY (http://www.well.ox.ac.uk/happy) [20] as described previously [19]. A mixed model approach was employed to test for association between each haplotype and the bone phenotype of interest. Variance components to correct for pedigree relationships were estimated using the EMMA package for the R statistical software [21]. The test for association was conducted for each phenotype via a mixed model, adjusting for age, sex, body weight and batch as described previously [19]. An overall significance threshold of P<3 × 10−6 (−log10P=5.5) was used, corresponding to the most stringent of the 5% FDR levels established by permutation for each of the bone structure and strength phenotypes, and applying a Bonferonni correction for the number of traits considered. All models were fitted using the statistical language R (R-Development-Core-Team 2004) [22]. For each QTL meeting the significance threshold, the resampling-based model inclusion probability (RMIP) was obtained as a measure of robustness; QTLs with RMIP values above 0.3 were further explored for candidates of interest. A 95% confidence interval for the position of each QTL detected was obtained as described previously [9,23].
Results
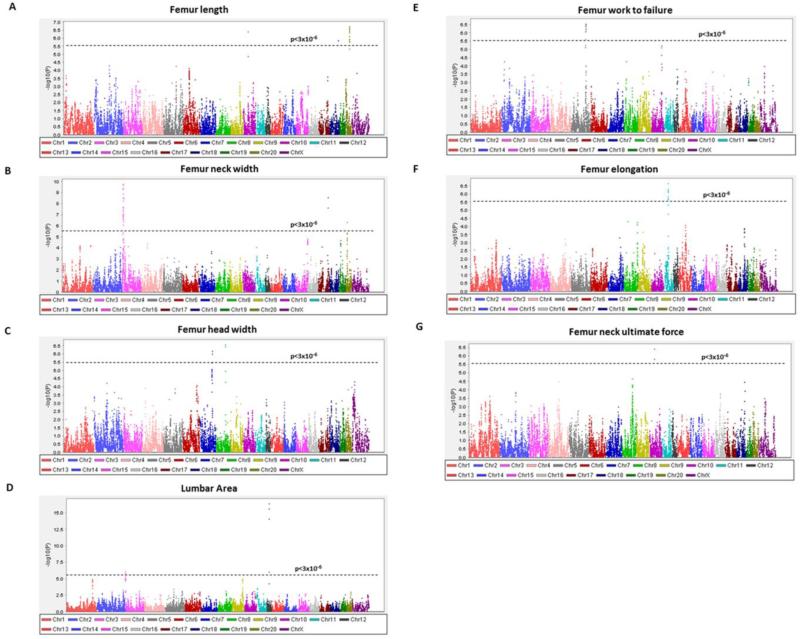
QTL mapping results were obtained throughout the genome for the structural measurements of femur length, neck width, head width and lumbar area (Figure 1A-1D). Results for femur work to failure, elongation and femur neck ultimate force are shown in Figure 1E-1G. Several QTLs reaching the genome-wide FDR and RMIP significance thresholds were observed in the HS rat sample, and are included in Table 1. Candidate genes within the 95% confidence intervals (CI) for these QTLs are listed in Table 2.
Fig. 1.
Genome-wide plots for femur length (A), femur neck width (B), femur head width (C), lumbar area (D), femur work to failure (E), femur elongation (F), and femur neck ultimate force (G). The –log10P values plotted on the Y-axis versus chromosome position on the X-axis. For comparability with other mapping studies, QTL results are shown at each position regardless of the conservative RMIP threshold (0.3) employed to select the most robust QTLs for our report. The dashed horizontal lines indicate the threshold value for genome-wide significance corresponding to FDR=5% (p<3 × 10−6).
Table 1.
Chromosomal regions associated with significant linkage (p<3 × 10−6) for bone structure and strength phenotypes
| Phenotypes | Chromosome | Position (bp) | Interval (Mb) | P-value | logP |
|---|---|---|---|---|---|
| Neck width (mm) | 3 | 13253530 | 12.2 - 15.0 | 1.75E-10 | 9.757 |
| Femur work to failure (mJ) | 5 | 131940562 | 131.0 - 133.5 | 2.87E-07 | 6.542 |
| Head width (mm) | 8 | 85352278 | 84.9 - 85.8 | 2.71E-07 | 6.567 |
| Femur length (mm) | 10 | 46530592 | 45.7 - 47.1 | 3.84E-07 | 6.416 |
| Femur neck ultimate force (N) | 10 | 46530592 | 46.4 - 46.9 | 3.89E-07 | 6.410 |
| Femur elongation (mm) | 11 | 41851716 | 41.4 - 44.3 | 2.20E-07 | 6.658 |
| Lumbar area (mm2) | 12 | 22185260 | 22.0 - 22.5 | 4.00E-17 | 16.398 |
| Neck width (mm) | 17 | 89388214 | 89.0 - 89.8 | 2.78E-09 | 8.556 |
| Femur length (mm) | 20 | 33840664 | 32.6 - 35.4 | 1.90E-07 | 6.721 |
Table 2.
Candidate genes within the chromosomal locations with significant (p<3 × 10−6) associations for bone structure and strength phenotypes
| Phenotype | Chromosome | Interval (Mb) | Gene symbol | Gene name | Human synteny |
|---|---|---|---|---|---|
| Neck width (mm) | 3 | 12.2 - 15.0 | Angptl2 | Angiopoietin-related protein 2 | 9q31-q34.11 |
| Cntr1 | Centriolin | ||||
| Dab2ip | Disabled homolog 2-interacting protein | ||||
| Fbxw2 | F-box/WD repeat-containing protein 2 | ||||
| Gapvd1 | GTPase activating protein and VPS9 domains 1 | ||||
| Ggta1 | Glycoprotein alpha-galactosyltransferase 1 | ||||
| Ggta1l1 | Glycoprotein alpha-galactosyltransferase 1-like 1 | ||||
| Gsn | Gelsolin | ||||
| Hspa5 | 78 kDa glucose-regulated protein | ||||
| Lmx1b | Homeodomain protein LMX1b | ||||
| Mapkap1 | Target of rapamycin complex 2 subunit MAPKAP1 | ||||
| Pbx3 | Pre-B-cell leukemia transcription factor 3 | ||||
| Phf19 | PHD finger protein 19 | ||||
| Psmd5 | 26S proteasome non-ATPase regulatory subunit 5 | ||||
| Rab14 | Ras-related protein Rab-14 | ||||
| Rabepk | Rab9 effector protein with kelch motifs | ||||
| Stom | Erythrocyte band 7 integral membrane protein | ||||
| Zbtb34/43 | Zinc finger and BTB domain-containing protein 34/43 | ||||
| Femur work to failure (mJ) | 5 | 131.0 - 133.5 | Agbl4 | ATP/GTP binding protein-like 4 | 1p32.2-p33 |
| Bend5 | BEN domain containing 5 | ||||
| Cdkn2c | Cyclin-dependent kinase inhibitor 2C (p18 inhibits CDK4) | ||||
| Dmrta2 | DMRT-like family A2 | ||||
| Elavl4 | ELAV (embryonic lethal abnormal vision Drosophila)-like 4 | ||||
| Faf1 | Fas (TNFRSF6) associated factor 1 | ||||
| Skint8 | Selection and upkeep of intraepithelial T cells 8 | ||||
| Slc5a9 | Solute carrier family 5 (sodium/sugar cotransporter) member 9 | ||||
| Spata6 | Spermatogenesis associated 6 | ||||
| Head width (mm) | 8 | 84.9 - 85.8 | Filip1 | Filamin A interacting protein 1 | 6q13-q14.3 |
| Impg1 | Interphotoreceptor matrix proteoglycan 1 | ||||
| Myo6 | Myosin VI | ||||
| Senp6 | SUMO1/sentrin specific peptidase 6 | ||||
| Femur length (mm) | 10 | 45.7 - 47.1 | Alkbh5 | AlkB alkylation repair homolog 5 (E. coli) | 1q42.1-q44 |
| Atpaf2 | ATP synthase mitochondrial F1 complex assembly factor 2 | 17p11.2 | |||
| Cops3 | COP9 signalosome subunit 3 | ||||
| Dhrs7b | Dehydrogenase/reductase (SDR family) member 7B | ||||
| Drg2 | Developmentally regulated GTP binding protein 2 | ||||
| Flcn | Folliculin | ||||
| Flii | Flightless I homolog (Drosophila) | ||||
| Gtlf3b | Gene trap locus F3b | ||||
| Jmjd4 | Jumonji domain containing 4 | ||||
| Llgl1 | Lethal giant larvae homolog 1 (Drosophila) | ||||
| Lrrc48 | Leucine-rich repeat-containing protein 48 | ||||
| Map2k3 | Mitogen activated protein kinase kinase 3 | ||||
| Med9 | Mediator complex subunit 9 | ||||
| Mprip | Myosin phosphatase Rho interacting protein | ||||
| Myo15a | Myosin XVA | ||||
| Nlrp3 | NLR family pyrin domain containing 3 | ||||
| Olr1462/1462/1464 | Olfactory receptor 1462/1463/1464 | ||||
| Pld6 | Phospholipase D family member 6 | ||||
| Rai1 | Retinoic acid induced 1 | ||||
| Rasd1 | RAS dexamethasone-induced 1 | ||||
| Shmt1 | Serine hydroxymethyltransferase 1 (soluble) | ||||
| Smcr7/8 | Smith-Magenis syndrome chromosome region candidate 7/8 | ||||
| Snap47 | Synaptosomal-associated protein 47 | ||||
| Srebf1 | Sterol regulatory element-binding protein 1 | ||||
| Tmem11 | Transmembrane protein 11 | ||||
| Tom1l2 | TOM1-like protein 2 | ||||
| Top3a | Topoisomerase (DNA) III alpha | ||||
| Zfp496/867 | zinc finger protein 496/867 | ||||
| Femur neck ultimate force (N) | 10 | 46.4 - 46.9 | Alkbh5 | AlkB alkylation repair homolog 5 (E. coli) | 17p11.2 |
| Atpaf2 | ATP synthase mitochondrial F1 complex assembly factor 2 | ||||
| Drg2 | Developmentally regulated GTP binding protein 2 | ||||
| Flii | Flightless I homolog (Drosophila) | ||||
| Llgl1 | Lethal giant larvae homolog 1 (Drosophila) | ||||
| Lrrc48 | Leucine-rich repeat-containing protein 48 | ||||
| Myo15a | Myosin XVA | ||||
| Rai1 | Retinoic acid induced 1 | ||||
| Smcr7/8 | Smith-Magenis syndrome chromosome region candidate 7/8 | ||||
| Srebf1 | Sterol regulatory element-binding protein 1 | ||||
| Tom1l2 | TOM1-like protein 2 | ||||
| Top3a | Topoisomerase (DNA) III alpha | ||||
| Femur elongation (mm) | 11 | 41.4 - 44.3 | Arl6 | ADP-ribosylation factor-like 6 | 3q11.2-q13.33 |
| Cldnd1 | Claudin domain containing 1 | 3q12-q26 | |||
| Cmss1 | Cms1 ribosomal small subunit homolog (yeast) | ||||
| Col8a1 | Collagen type VIII alpha 1 | ||||
| Cpox | Coproporphyrinogen oxidase | ||||
| Crybg3 | Beta-gamma crystallin domain containing 3 | ||||
| Dcbld2 | Discoidin CUB and LCCL domain containing 2 | ||||
| Epha6 | Eph receptor A6 | ||||
| Filip1l | Filamin A interacting protein 1-like | ||||
| Gabrr3 | Gamma-aminobutyric acid (GABA) A receptor rho 3 | ||||
| Gpr15 | G protein-coupled receptor 15 | ||||
| Mina | Myc induced nuclear antigen | ||||
| Nit2 | Nitrilase family member 2 | ||||
| Olrl clusters | Olfactory receptors clusters | ||||
| St3gal6 | ST3 beta-galactoside alpha-23-sialyltransferase 6 | ||||
| Tbc1d23 | TBC1 domain family member 23 | ||||
| Tmem30c | Transmembrane protein 30C | ||||
| Lumbar area (mm2) | 12 | 22.0 - 22.5 | Ccl24/26 | Chemokine (C-C motif) ligand 24/26 | 7q11.23 |
| Fkbp6 | Peptidyl-prolyl cis-trans isomerase FKBP6 | ||||
| Hip1 | Huntingtin interacting protein 1 | ||||
| Mdh2 | Malate dehydrogenase, mitochondrial | ||||
| Nsun5 | NOL1/NOP2/Sun domain family, member 5 | ||||
| Pom121 | Nuclear envelope pore membrane protein POM 121 | ||||
| Por | NADPH-cytochrome P450 reductase | ||||
| Rhbdd2 | Rhomboid domain containing 2 | ||||
| Srrm3 | Serine/arginine repetitive matrix 3 | ||||
| Styxl1 | Serine/threonine/tyrosine-interacting-like protein 1 | ||||
| Tmem120a | Transmembrane protein 120A | ||||
| Trim50 | E3 ubiquitin-protein ligase TRIM50 | ||||
| Neck width (mm) | 17 | 89.0 - 89.8 | Arl5b | ADP-ribosylation factor-like 5B | 10p12.1-p13 |
| Cacnb2 | Calcium channel voltage-dependent beta 2 subunit | ||||
| Nsun6 | NOP2/Sun domain family member 6 | ||||
| Femur length (mm) | 20 | 32.6 - 35.4 | Msl3l2 | Male-specific lethal 3-like 2 | 6q21-q22.33 |
| Tbc1d32 | TBC1 domain family member 32 |
Genome-wide significant association results of femur and femoral neck structure
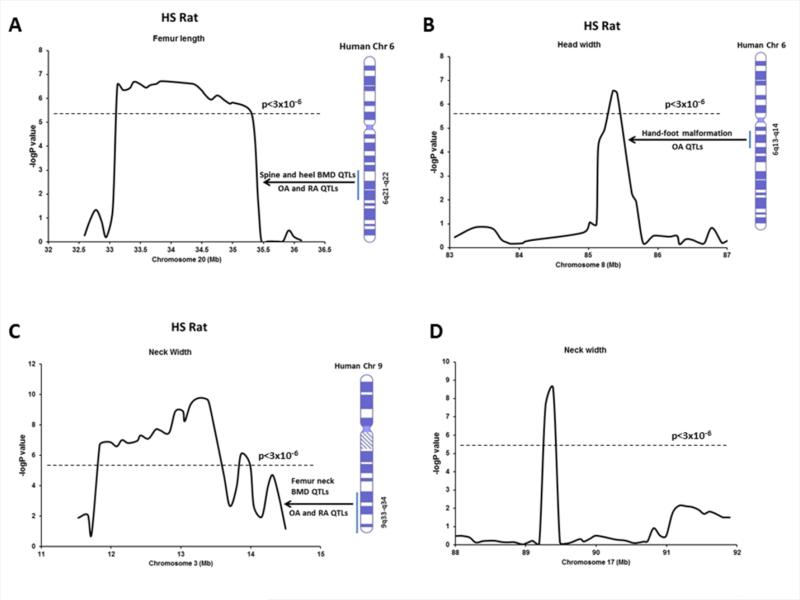
On chromosome 20 at position 34 Mb, significant linkage was detected for femur length with a –logP value of 6.72 (p=1.9 × 10−7; Figure 2A and Table 1). The CI for this QTL spanned 2.8 megabase (Mb). On chromosome 8, a QTL was identified which was linked to femur head width with a –logP value of 6.56 (p=2.7 × 10−7; Figure 2B) spanning less than one megabase. On chromosome 3, a QTL was identified which was linked to femur neck width with a –logP value of 9.75 (p=1.7 × 10−10; Figure 2C) spanning 2.8 megabase. In addition, a QTL encompassing 0.8 Mb for femur neck width with a –logP value of 8.55 (p=2.7 × 10−9; Figure 2D) was detected on chromosome 17.
Fig. 2.
Association results for femur length on chromosome 20 (A), femur head width on chromosome 8 (B), femoral neck width on chromosome 3 (C) and femoral neck width on chromosome 17 (D). The –logP values are plotted on the Y-axis vs. the chromosomal position (MB) on the X-axis. The dashed horizontal lines indicate the threshold value for genome-wide significance corresponding to FDR=5% (p<3 × 10−6). Corresponding human syntenic regions and associated QTLs for bone phenotypes are indicated.
Genome-wide significant association results of lumbar spine structure
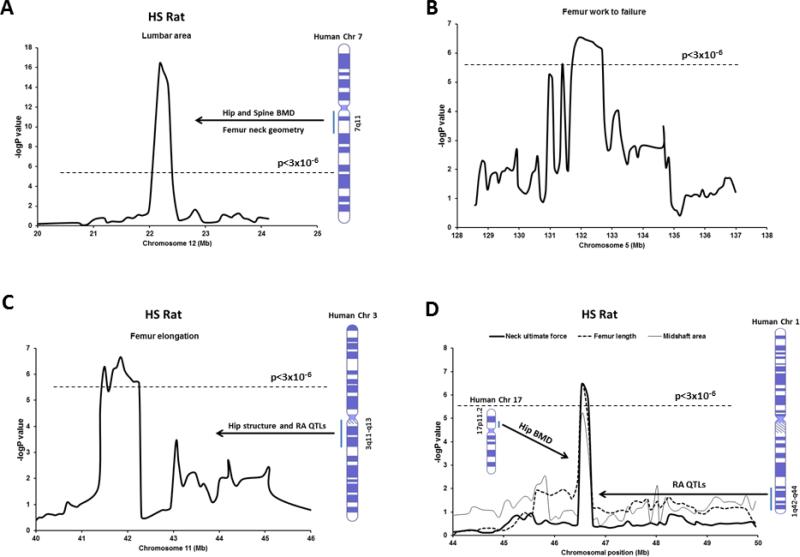
The only significant QTL for lumbar area was detected on chromosome 12 at position 22 Mb, with a –logP value of 16.39 (p=4.0 × 10−17; Figure 3A and 4B) spanning 0.5 Mb chromosomal region.
Fig. 3.
Association results for lumbar area on chromosome 12 (A), femur work to failure on chromosome 5 (B), femur elongation on chromosome 11 (C), and femoral neck ultimate force, femur length and midshaft area on chromosome 10 (D). The –logP values are plotted on the Y-axis vs. the chromosomal position (MB) on the X-axis. The dashed horizontal lines indicate the threshold value for genome-wide significance corresponding to FDR=5% (p<3 × 10−6). Corresponding human syntenic regions and associated QTLs for bone phenotypes are indicated.
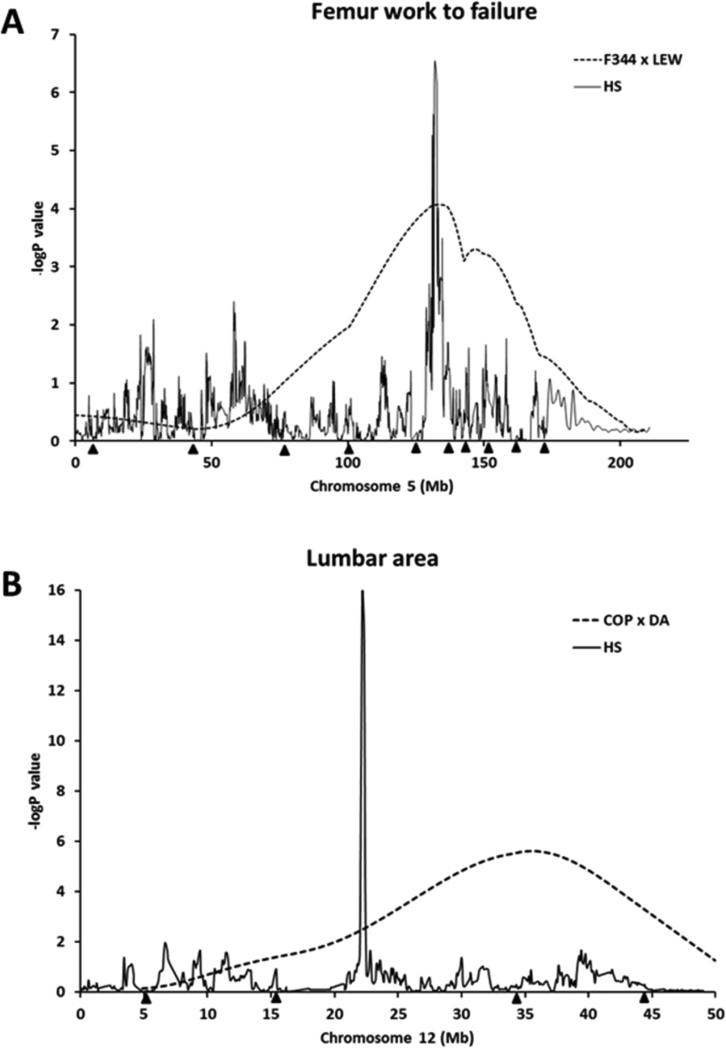
Fig. 4.
Mapping results on chromosome 5 (A) for femur work to failure and on chromosome 12 (B) for lumbar area, indicating evidence for QTLs from the HS analysis (solid line) and an F2 intercross (F344 X LEW or COP X DA) reported previously (dotted line). Black triangles along the x-axis correspond to the positions of microsatellite markers typed on each chromosome for the particular F2 intercross.
Genome-wide significant association results of femur and femoral neck strength
We observed two genome-wide significant QTLs for femur strength phenotypes, one each for femur work to failure (Figure 3B and 4A) and femur elongation (Figure 3C) on chromosomes 5 and 11, respectively. The CI for the QTL region on chromosome 5 spans approximately 2.5 Mb whereas the QTL region on chromosome 11 spans 2.9 Mb. In addition, a QTL was identified for femoral neck ultimate force between 46-47 Mb position on chromosome 10 (Figure 3D) spanning 0.3 Mb region. In the same region on chromosome 10, a QTL for femur length was also observed with a significant –logP value of 6.46 (p=3.8 × 10−7).
Discussion
In this study, we detected and localized QTLs for several key bone structure and strength phenotypes in HS rats at most common skeletal fracture sites. Importantly, most of these loci were localized to very small genomic regions, as small as 0.5 Mb up to 3 Mb, compared to the F2 design used previously for QTL mapping. This approach also allowed us to identify a narrowed list of positional candidate genes underlying each QTL, which can then be analyzed in future functional studies. Such a direct translation from gene identification to functional work is not possible in the traditional F2 design which typically identifies a QTL region harboring hundreds of potential candidate genes.
A critical factor for identification of genes underlying any complex trait such as skeletal fragility is replication of QTLs across studies. If chromosomal regions truly harbor gene/s for a trait, independent studies involving sufficiently large samples will most likely detect the same QTL for that particular trait. Importantly, the genomic resolution of replicated QTLs could be enhanced, thereby narrowing the number of positional candidate genes, by employing a genetically random mosaic model of the founder animals rather than using traditional two-strain parental crosses. Indeed, several chromosomal regions previously identified in our inbred F2 studies were replicated in HS rats. For example, we detected association with femur work to failure in the HS rats on chromosome 5 (LOD 6.54) (Figure 3B and 4A), which overlapped with multiple QTLs in our F344 X LEW and COP X DA F2 crosses for femur structure and strength phenotypes [10,12]. This QTL in HS rat is syntenic to human chromosome 1p32.2-p33 and close to the location of the tissue-nonspecific ALP gene, which is important for skeletal mineralization. In addition, lumbar area QTL identified in HS rats on chromosome 12 (LOD 16.39) (Figure 3A and 4B) overlapped the QTLs in COP X DA F2 cross for spinal BMD and trabecular area [9,10,58]. This QTL in HS rats is homologous to human chromosome 7q11 (Figure 3A), which was linked to hip and spine BMD and femoral neck geometry [55-57]. Importantly, using HS rats, we were able to fine-map these regions to 1-3 Mb resolution, enabling us to identify a much smaller number of potential candidate genes on these overlapped chromosomes (Table 2). Notably, 2 genes (Hip1 and Por) underlying the QTL on chromosome 12 have been previously reported to have important roles in skeletal development and homeostasis. Hip1, a member of Huntingtin interactin protein, plays an important role in the clathrin trafficking network. Hip1 deficient mice have developmental abnormalities and growth defects including severe spinal abnormalities and dwarfism [40,41]. Por is the primary electron donor for cytochromes P450. Mutations in Por in humans lead to severe malformations including defects in craniofacial and long bones development [42]. In addition, deletion of Por recapitulates the human skeletal defects in mouse model, indicating this gene is important for proper bone development [43].
The genes underlying QTLs identified in this study might act alone or in combination to influence bone structure and strength phenotypes in different manner. For example, a single gene might affect multiple bone phenotypes or a cluster of genes may act together to modify a single bone phenotype. Also, the pleiotropic gene/s may contribute not only to different bone phenotypes but also influence phenotypes at different skeletal sites even within a given bone. Indeed, we detected several QTLs in HS rats that overlapped the QTLs in F344 X LEW and COP X DA F2 crosses for different bone phenotypes. The head width QTL in HS rats on chromosome 8 (LOD 6.56) (Figure 2B) overlapped with femur BMD and femoral neck strength QTLs in COP X DA cross [9,10]. The femur length and femur neck ultimate force QTLs identified in HS rats on chromosome 10 (LOD 6.41) (Figure 3D) overlapped the QTLs for spine BMD in both F344 X LEW and COP X DA F2 crosses [9,44]. This region was also coincided with the position of the femur BMC QTL that we reported previously in HS rat [19]. Similarly, the femur length QTL identified in HS rats on chromosome 20 (LOD 6.72) (Figure 2A) overlapped the QTL for femur BMD in COP X DA F2 cross [9]. The QTL region for femoral head width on chromosome 8 in HS rat is syntenic to human chromosome 6q13-14 (Figure 2B). This region was previously linked to osteoarthritis QTL and hand-foot malformation [49,52]. A locus for otosclerosis, a common form of hearing impairment caused by abnormal bone homeostasis of the otic capsule, was mapped to the 6q13-16 region [59]. In addition, 6q14.2-14.3 region harbors gene for cleft lip and palate, a defect of craniofacial development in human [60]. The distal peaks of QTLs for ALP and OC in baboon were mapped close to human orthologous 6q13 region [61]. The femur length QTL on chromosome 10 in HS rat is homologous to the human chromosomes 1q42-44 and 17p11.2 which were linked to rheumatoid arthritis QTL and hip BMD, respectively (Figure 3D) [45,46]. The susceptibility loci for split-hand/foot malformation with long-bone deficiency, a rare severe limb deformity condition were detected at 1q42.2-q43 and 6q14.1 [62]. Furthermore, a locus for Kenny-Caffey syndrome, an osteosclerotic bone dysplasia was identified at 1q42-q43 [63]. Amplification and overexpression of genes in 17p11.2-p12 leads to osteosarcoma [64]. QTLs for developmental components of the craniofacial complex were mapped to baboon ortholog of human chromosome 17p12 [65]. The neck width QTL on chromosome 17 in HS rat is homologous to the human chromosomes 10p12.1-p13, where Paget's disease locus was mapped [66-68]. The QTL for the femur length on chromosome 20 in HS rats is syntenic to 6q21-22 where spine and heel BMD QTLs were detected (Figure 2A) [47,48]. In addition, this human region was linked to osteoarthritis and rheumatoid arthritis QTLs [45,49]. Mutation in a locus of 6q21 harboring OSTM1 gene was found to be linked to human malignant infantile osteopetrosis and craniometaphyseal dysplasia with severe craniofacial involvement shows hmozygosity at 6q21-q22.1 locus in human [69,70]. Among all the genes detected underlying QTL on chromosome 10, several genes have previously shown to play important functions in bone growth and remodeling (Table 2). Cops3 is an oncogene residing in the human chromosomal region 17p11.2-p12 - the copy number and expression level of Cops3 was significantly associated with the development of osteosarcoma, the most common primary malignancy of bone [29,30]. Drg2, a GTP binding protein, overexpression of which in transgenic mice leads to increased number and activity of osteoclasts and bone loss [31]. Map2k3 is increased by RANKL, which in turn aids in osteoclastogenesis from bone marrow precursor cells [39]. Nlrp3, a member of the NLR family of cytosolic receptors, mediates bone loss at sites of infection by apoptotic cell death of osteoblasts [36]. Mutations in Nlrp3 are responsible for neonatal-onset multisystem inflammatory disease, exhibiting growth retardation, osteopenia and increased osteoclastogenesis [37], suggesting that this gene is important for postnatal skeletal growth and bone remodeling. Rai1 encodes a nuclear protein containing a zinc finger homeodomain and regulates cell growth, cell cycle regulation, lipid metabolism, neurological development and behavioral functions [32-33]. Mutation of Rai leads to craniofacial and skeletal anomalies (short extremities) in Smith-Magenis syndrome [32]. Both the copy number and expression level of Rasd1 were significantly associated with the development of osteosarcoma [29]. In addition, using an integrative genetics approach, Rasd1 was identified as a strong candidate gene for a BMD QTL in mice [34]. Srebf1 activates genes that regulate lipid biosynthesis, and polymorphism in this gene was found to be associated with a higher risk of osteonecrosis of the femoral head in the Korean population [35]. Shmt1 and Top3a are oncogenes and contribute to the development of osteosarcoma [29,38].
Two novel chromosomal regions linked to bone structure and strength phenotypes were identified in HS rats (Table 1) not found in our F2 studies. On chromosome 3, a QTL was identified for neck width (Figure 2C) and on chromosome 11 we detected a QTL for femur strength (Figure 3C). The QTL region for femoral neck width on chromosome 3 in HS rat is syntenic to human 9q33-34 (Figure 2C), where linkage to neck BMD and osteoarthritis and rheumatoid arthritis QTLs were detected previously [45,50,51]. KBG syndrome, a postnatal short stature, macrodontia, facial and hand anomalies and delayed bone age was associated with 9q31.2-q33.1 [71]. The femur elongation QTL on chromosome 11 in HS rats is syntenic to human chromosome 3q11-13 and 3q12-26 (Figure 3C), where femur and hip structural QTLs and QTL for rheumatoid arthritis were observed [45,53,54]. QTLs for developmental components of the craniofacial complex were mapped to baboon ortholog of human chromosome 3q11-13 [65]. Three genes (Gsn, Hspa5 and Lmx1b) underlying the QTL on chromosome 3 play important roles in bone and teeth development (Table 2). A haplotype in Gsn (gelsolin) was associated with the hip bone phenotypes, and mRNA and protein expressions of Gsn in peripheral blood monocytes were lower in female Caucasians with low hip BMD [28]. Hspa5 (heat shock 70kDa protein 5) or GRP-78, an endoplasmic reticulum chaperone protein localized on the plasma membrane in preosteoblasts, is responsible for cellular uptake of Dmp1 for its internalization to the nucleus during bone and tooth development [27]. Lmx1b is required for patterning and morphogenesis of the mouse calvaria and is necessary for dorsal-ventral patterning during limb development in mice [24-26].
Several novel genes underlying QTLs discovered in this study were not previously directly linked to any bone phenotype but they code for proteins for various cellular structures and trafficking pathways – such as membrane proteins (Impg1, Senp6, Lrrc48, Dcbld2, Jmjd4 and Gabrr3), membrane trafficking (Gapvd1, Llgl1 and Tom1l2), cytoskeletal proteins (Stom, Mprip and Tom1l2) and cell junction proteins (Myo6, Myo15a and Dcbld2) that might be important for overall bone homeostasis (Table 2). Also, genes that act as transcription factors or cofactors (Rhbdd2, Zbtb34/43 and Msl3l2), G-protein coupled receptors (Gpr15, Mprip, Myo6 and Myo15a), small GTPase (Arl6 and Arl5b) and calcium binding proteins (Flii and Fkbp6) were identified (Table 2). These genes might play role in connection between skeletal metabolism and other systems functions.
There are some limitations in this study. Although, rat skeleton is very similar to human bone with peak bone mass gain or bone loss due to aging, and rat models have served as a highly predictive model for fracture risk in humans, a potential drawback is rat skeleton lacks the Haversian remodeling system found in human. Also, we could not identify any specific sequence variants in the HS founder strains that fully accounted for structure and strength QTLs identified in this study. In the future, full sequence information of HS offspring will shed light on the complex genetic interactions among the different haplotype variants underlying these phenotypes in these animals. Furthermore, while QTLs for bone structure and strength phenotypes in the HS rat were localized to very small genomic regions, further functional studies are necessary to identify the causative genes from these narrowed lists of candidate genes.
In this study, we demonstrated that HS rats are a powerful resource for fine mapping of QTLs for bone structure and strength phenotypes. These phenotypes, along with BMD, are complex in nature in the rat, just as they are in humans and are likely due to multiple variants inherited from different founders as well as interactions among these variants. The number of founder rat lines used in the generation of the HS population and the number of recombination events accumulated over many generations, allowed us to more accurately detect the correct QTL position. Most importantly, this approach allows us to delineate a much smaller chromosomal QTL interval and thus generate a narrower list of potential candidate genes than the traditional F2 approach – which is a cross of only two founder rat lines. In the future, sequencing studies in the HS offspring in these narrowed regions, along with analysis of the founder strain sequence data, will enable us to dissect the complex genetic architecture underlying the structure and strength phenotypes in the HS rats.
Highlights.
We detected QTLs for bone structure and strength phenotypes in HS rats at the most common skeletal fracture sites
Several chromosomal regions previously identified in our inbred F2 cross were replicated in HS rats
Most QTLs in HS rats were localized to very narrow genomic regions
HS rat model allowed us to identify a narrower list of potential candidate genes than the traditional F2 approach
We demonstrated that HS rats are a powerful resource for fine mapping of QTLs for bone structure and strength phenotypes
Acknowledgments
This work was supported by the US National Institutes of Health through the following grants: AR047822 and AG041517. The research leading to these results has received funding from the European Union‘s Seventh Framework Programme (FP7/2007-2013) under grant agreement HEALTH-F4-2010-241504 (EURATRANS), the Wellcome Trust (090532/Z/09/Z, 083573/Z/07, 089269/Z/09/Z), the Ministerio de Ciencia e Innovación (reference PSI2009-10532) and the Fundació La Maratò TV3 (reference 092630).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: All authors have no conflicts of interest.
Authors’ roles
Study design: IA, DLK, MJE, and TF. Study conduct: IA, DLK, TC, GB, CM, RL, EM, SD, AT, AF, PS, MD, TO, MJ, and AB. Data analysis: IA, DLK, AB, and TF. Data interpretation: IA, DLK, MJE and TF. Drafting manuscript: IA and DLK. Revising manuscript content: IA, DLK, AF, MJE, and TF. Approval of final version of manuscript: IA, DLK, TC, GB, CM, RL, EM, SD, AT, AF, PS, MD, TO, MJ, AB, MJE and TF.
References
- 1.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–87. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 3.Faulkner KG, Cummings SR, Black D, Palermo L, Gluer CC, Genant HK. Simple measurement of femoral geometry predicts hip fracture: the study of osteoporotic fractures. J Bone Miner Res. 1993;8:1211–17. doi: 10.1002/jbmr.5650081008. [DOI] [PubMed] [Google Scholar]
- 4.Peacock M, Turner CH, Liu G, Manatunga AK, Timmerman L, Johnston CC., Jr. Better discrimination of hip fracture using bone density, geometry and architecture. Osteoporos Int. 1995;5:167–73. doi: 10.1007/BF02106096. [DOI] [PubMed] [Google Scholar]
- 5.Arden NK, Baker J, Hogg C, Bann K, Spector TD. The heritability of bone mineral density, ultrasound of the calcaneus and hip axis length: a study of postmenopausal twins. J Bone Miner Res. 1996;11:530–34. doi: 10.1002/jbmr.5650110414. [DOI] [PubMed] [Google Scholar]
- 6.Garnero P, Arden NK, Griffiths G, Delmas PD, Spector TD. Genetic influence on bone turnover in postmenopausal twins. J Clin Endocrinol Metab. 1996;81:140–46. doi: 10.1210/jcem.81.1.8550741. [DOI] [PubMed] [Google Scholar]
- 7.Peacock M, Turner CH, Econs MJ, Foroud T. Genetics of osteoporosis. Endocr Rev. 2002;23:378–83. doi: 10.1210/edrv.23.3.0464. [DOI] [PubMed] [Google Scholar]
- 8.Ralston SH. Genetic determinants of osteoporosis. Curr Opin Rheumatol. 2005;17:475–79. doi: 10.1097/01.bor.0000166385.62851.92. [DOI] [PubMed] [Google Scholar]
- 9.Alam I, Sun Q, Liu L, et al. Sex-specific genetic loci for femoral neck bone mass and strength identified in inbred COP and DA rats. J Bone Miner Res. 2008;23:850–59. doi: 10.1359/JBMR.080221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Q, Alam I, Liu L, et al. Genetic loci affecting bone structure and strength in inbred COP and DA rats. Bone. 2008;42:547–53. doi: 10.1016/j.bone.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alam I, Sun Q, Liu L, et al. Identification of a quantitative trait locus on rat chromosome 4 that is strongly linked to femoral neck structure and strength. Bone. 2006;39:93–99. doi: 10.1016/j.bone.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Alam I, Sun Q, Liu L, et al. Whole-genome scan for linkage to bone strength and structure in inbred Fischer 344 and Lewis rats. J Bone Miner Res. 2005;20:1589–96. doi: 10.1359/JBMR.050512. [DOI] [PubMed] [Google Scholar]
- 13.Hansen C, Spuhler K. Development of the National Institutes of Health genetically heterogeneous rat stock. Alcohol Clin Exp Res. 1984;8:477–9. doi: 10.1111/j.1530-0277.1984.tb05706.x. [DOI] [PubMed] [Google Scholar]
- 14.Boucher W, Cotterman CW. On the classification of regular systems of inbreeding. J Math Biol. 1990;28:293–305. doi: 10.1007/BF00178778. [DOI] [PubMed] [Google Scholar]
- 15.Solberg Woods LC, Holl K, Tschannen M, Valdar W. Fine-mapping a locus for glucose tolerance using heterogeneous stock rats. Physiol Genomics. 2010;41:102–8. doi: 10.1152/physiolgenomics.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johannesson M, Lopez-Aumatell R, Stridh P, et al. A resource for the simultaneous high-resolution mapping of multiple quantitative trait loci in rats: the NIH heterogeneous stock. Genome Res. 2009;19:150–8. doi: 10.1101/gr.081497.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam I, Koller DL, Sun Q, et al. Heterogeneous stock rat: A unique animal model for mapping genes influencing bone fragility. Bone. 2011;48:1169–1177. doi: 10.1016/j.bone.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baud A, Hermsen R, Guryev V, et al. Combined sequence-based and genetic mapping analysis of complex traits in outbred rats. Nat Genet. 2013;45(7):767–75. doi: 10.1038/ng.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alam I, Koller DL, Cañete T, et al. High-Resolution Genome Screen for Bone Mineral Density in Heterogeneous Stock Rat. J Bone Miner Res. 2014 Feb 12; doi: 10.1002/jbmr.2195. doi: 10.1002/jbmr.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mott R, Talbot CJ, Turri MG, Collins AC, Flint J. A method for fine mapping quantitative trait loci in outbred animal stocks. Proc Natl Acad Sci USA. 2000;97:12649–54. doi: 10.1073/pnas.230304397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang HM, Zaitlen NA, Wade CM, et al. Efficient control of population structure in model organism association mapping. Genetics. 2008;178(3):1709–23. doi: 10.1534/genetics.107.080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R. Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2004. [Google Scholar]
- 23.Visscher PM, Thompson R, Haley CS. Confidence intervals in QTL mapping by bootstrapping. Genetics. 1996;143(2):1013–20. doi: 10.1093/genetics/143.2.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H, Ovchinnikov D, Pressman CL, Aulehla A, Lun Y, Johnson RL. Multiple calvarial defects in lmx1b mutant mice. Dev Genet. 1998;22(4):314–20. doi: 10.1002/(SICI)1520-6408(1998)22:4<314::AID-DVG2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Johnson RL. Interactions between dorsal-ventral patterning genes lmx1b, engrailed-1 and wnt-7a in the vertebrate limb. Int J Dev Biol. 2002;46(7):937–41. [PubMed] [Google Scholar]
- 26.Feenstra JM, Kanaya K, Pira CU, Hoffman SE, Eppey RJ, Oberg KC. Detection of genes regulated by Lmx1b during limb dorsalization. Dev Growth Differ. 2012;54(4):451–62. doi: 10.1111/j.1440-169X.2012.01331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravindran S, Narayanan K, Eapen AS, et al. Endoplasmic reticulum chaperone protein GRP-78 mediates endocytosis of dentin matrix protein 1. J Biol Chem. 2008;283(44):29658–70. doi: 10.1074/jbc.M800786200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng FY, Zhu W, Zeng Y, et al. Is GSN significant for hip BMD in female Caucasians? Bone. 2014;63:69–75. doi: 10.1016/j.bone.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Both J, Wu T, Bras J, Schaap GR, Baas F, Hulsebos TJ. Identification of novel candidate oncogenes in chromosome region 17p11.2-p12 in human osteosarcoma. PLoS One. 2012;7(1):e30907. doi: 10.1371/journal.pone.0030907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan T, Wunder JS, Gokgoz N, et al. COPS3 amplification and clinical outcome in osteosarcoma. Cancer. 2007;109(9):1870–6. doi: 10.1002/cncr.22595. [DOI] [PubMed] [Google Scholar]
- 31.Ke K, Sul OJ, Kim WK, et al. Overexpression of developmentally regulated GTP-binding protein-2 increases bone loss. Am J Physiol Endocrinol Metab. 2013;304(7):E703–1. doi: 10.1152/ajpendo.00517.2012. [DOI] [PubMed] [Google Scholar]
- 32.Dubourg C, Bonnet-Brilhault F, Toutain A, et al. Identification of Nine New RAI1-Truncating Mutations in Smith-Magenis Syndrome Patients without 17p11.2 Deletions. Mol Syndromol. 2014;5(2):57–64. doi: 10.1159/000357359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams SR, Aldred MA, Der Kaloustian VM, et al. Haploinsufficiency of HDAC4 causes brachydactyly mental retardation syndrome, with brachydactyly type E, developmental delays, and behavioral problems. Am J Hum Genet. 2010;87(2):219–28. doi: 10.1016/j.ajhg.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farber CR, van Nas A, Ghazalpour A, et al. An integrative genetics approach to identify candidate genes regulating BMD: combining linkage, gene expression, and association. J Bone Miner Res. 2009;24(1):105–16. doi: 10.1359/JBMR.080908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HJ, Choi SJ, Hong JM, et al. Association of a polymorphism in the intron 7 of the SREBF1 gene with osteonecrosis of the femoral head in Koreans. Ann Hum Genet. 2009;73(1):34–41. doi: 10.1111/j.1469-1809.2008.00490.x. [DOI] [PubMed] [Google Scholar]
- 36.Bonar SL, Brydges SD, Mueller JL, et al. Constitutively activated NLRP3 inflammasome causes inflammation and abnormal skeletal development in mice. PLoS One. 2012;7(4):e35979. doi: 10.1371/journal.pone.0035979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCall SH, Sahraei M, Young AB, et al. Osteoblasts express NLRP3, a nucleotide-binding domain and leucine-rich repeat region containing receptor implicated in bacterially induced cell death. J Bone Miner Res. 2008;23(1):30–40. doi: 10.1359/JBMR.071002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Dartel M, Cornelissen PW, Redeker S, et al. Amplification of 17p11.2 approximately p12, including PMP22, TOP3A, and MAPK7, in high-grade osteosarcoma. Cancer Genet Cytogenet. 2002;139(2):91–6. doi: 10.1016/s0165-4608(02)00627-1. [DOI] [PubMed] [Google Scholar]
- 39.Huang H, Ryu J, Ha J, et al. Osteoclast differentiation requires TAK1 and MKK6 for NFATc1 induction and NF-kappaB transactivation by RANKL. Cell Death Differ. 2006;13(11):1879–91. doi: 10.1038/sj.cdd.4401882. [DOI] [PubMed] [Google Scholar]
- 40.Hyun TS, Li L, Oravecz-Wilson KI, et al. Hip1-related mutant mice grow and develop normally but have accelerated spinal abnormalities and dwarfism in the absence of HIP1. Mol Cell Biol. 2004;24(10):4329–40. doi: 10.1128/MCB.24.10.4329-4340.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oravecz-Wilson KI, Kiel MJ, Li L, et al. Huntingtin Interacting Protein 1 mutations lead to abnormal hematopoiesis, spinal defects and cataracts. Hum Mol Genet. 2004;13(8):851–67. doi: 10.1093/hmg/ddh102. [DOI] [PubMed] [Google Scholar]
- 42.Panda SP, Guntur AR, Polusani SR, et al. Conditional deletion of cytochrome p450 reductase in osteoprogenitor cells affects long bone and skull development in mice recapitulating antley-bixler syndrome: role of a redox enzyme in development. PLoS One. 2013;8(9):e75638. doi: 10.1371/journal.pone.0075638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wudy SA, Hartmann MF, Draper N, Stewart PM, Arlt W. A male twin infant with skull deformity and elevated neonatal 17-hydroxyprogesterone: a prismatic case of P450 oxidoreductase deficiency. Endocr Res. 2004;30(4):957–64. doi: 10.1081/erc-200044174. [DOI] [PubMed] [Google Scholar]
- 44.Koller DL, Alam I, Sun Q, et al. Genome screen for bone mineral density phenotypes in Fischer 344 and Lewis rats. Mammalian Genome. 2005;16:578–86. doi: 10.1007/s00335-004-2459-0. [DOI] [PubMed] [Google Scholar]
- 45.Jawaheer D, Seldin MF, Amos CI, et al. A genomewide screen in multiplex rheumatoid arthritis families suggests genetic overlap with other autoimmune diseases. Am J Hum Genet. 2001;68(4):927–36. doi: 10.1086/319518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng HW, Xu FH, Huang QY, et al. A whole-genome linkage scan suggests several genomic regions potentially containing quantitative trait Loci for osteoporosis. J Clin Endocrinol Metab. 2002;87(11):5151–9. doi: 10.1210/jc.2002-020474. [DOI] [PubMed] [Google Scholar]
- 47.Karasik D, Myers RH, Cupples LA, et al. Genome screen for quantitative trait loci contributing to normal variation in bone mineral density: the Framingham Study. J Bone Miner Res. 2002;17(9):1718–27. doi: 10.1359/jbmr.2002.17.9.1718. [DOI] [PubMed] [Google Scholar]
- 48.Moayyeri A, Hsu YH, Karasik D, et al. Genetic determinants of heel bone properties: genome-wide association meta-analysis and replication in the GEFOS/GENOMOS consortium. Hum Mol Genet. 2014;23(11):3054–68. doi: 10.1093/hmg/ddt675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loughlin J, Mustafa Z, Irven C, et al. Stratification analysis of an osteoarthritis genome screen-suggestive linkage to chromosomes 4, 6, and 16. Am J Hum Genet. 1999;65(6):1795–8. doi: 10.1086/302685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ioannidis JP, Ng MY, Sham PC, et al. Meta-analysis of genome-wide scans provides evidence for sex- and site-specific regulation of bone mass. J Bone Miner Res. 2007;22(2):173–83. doi: 10.1359/jbmr.060806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leppävuori J, Kujala U, Kinnunen J, et al. Genome scan for predisposing loci for distal interphalangeal joint osteoarthritis: evidence for a locus on 2q. Am J Hum Genet. 1999;65(4):1060–7. doi: 10.1086/302569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naveed M, Nath SK, Gaines M, et al. Genomewide linkage scan for split-hand/foot malformation with long-bone deficiency in a large Arab family identifies two novel susceptibility loci on chromosomes 1q42.2-q43 and 6q14.1. Am J Hum Genet. 2007;80(1):105–11. doi: 10.1086/510724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koller DL, Liu G, Econs MJ, et al. Genome screen for quantitative trait loci underlying normal variation in femoral structure. J Bone Miner Res. 2001;16(6):985–91. doi: 10.1359/jbmr.2001.16.6.985. [DOI] [PubMed] [Google Scholar]
- 54.Koller DL, White KE, Liu G, et al. Linkage of structure at the proximal femur to chromosomes 3, 7, 8, and 19. J Bone Miner Res. 2003;18(6):1057–65. doi: 10.1359/jbmr.2003.18.6.1057. [DOI] [PubMed] [Google Scholar]
- 55.Tang ZH, Xiao P, Lei SF, et al. A bivariate whole-genome linkage scan suggests several shared genomic regions for obesity and osteoporosis. J Clin Endocrinol Metab. 2007;92(7):2751–7. doi: 10.1210/jc.2006-2607. [DOI] [PubMed] [Google Scholar]
- 56.Lei S, Deng F, Xiao P, et al. Bivariate whole-genome linkage scan for bone geometry and total body fat mass. J Genet Genomics. 2009;36(2):89–97. doi: 10.1016/S1673-8527(08)60095-8. [DOI] [PubMed] [Google Scholar]
- 57.Huang QY, Xu FH, Shen H, et al. A second-stage genome scan for QTLs influencing BMD variation. Calcif Tissue Int. 2004;75(2):138–43. doi: 10.1007/s00223-004-0088-y. [DOI] [PubMed] [Google Scholar]
- 58.Koller DL, Liu L, Alam I, et al. Linkage screen for BMD phenotypes in male and female COP and DA rat strains. J Bone Miner Res. 2008;23(9):1382–8. doi: 10.1359/JBMR.080401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thys M, Van Den Bogaert K, Iliadou V, et al. A seventh locus for otosclerosis, OTSC7, maps to chromosome 6q13-16.1. Eur J Hum Genet. 2007;15(3):362–8. doi: 10.1038/sj.ejhg.5201761. [DOI] [PubMed] [Google Scholar]
- 60.Letra A, Menezes R, Fonseca RF, et al. Novel cleft susceptibility genes in chromosome 6q. J Dent Res. 2010;89(9):927–32. doi: 10.1177/0022034510370004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Havill LM, Rogers J, Cox LA, Mahaney MC. QTL with pleiotropic effects on serum levels of bone-specific alkaline phosphatase and osteocalcin maps to the baboon ortholog of human chromosome 6p23-21.3. J Bone Miner Res. 2006;21(12):1888–96. doi: 10.1359/jbmr.060812. [DOI] [PubMed] [Google Scholar]
- 62.Naveed M, Nath SK, Gaines M, et al. Genome wide linkage scan for split-hand/foot malformation with long-bone deficiency in a large Arab family identifies two novel susceptibility loci on chromosomes 1q42.2-q43 and 6q14.1. Am J Hum Genet. 2007;80(1):105–11. doi: 10.1086/510724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diaz GA, Khan KT, Gelb BD. The autosomal recessive Kenny-Caffey syndrome locus maps to chromosome 1q42-q43. Genomics. 1998;54(1):13–8. doi: 10.1006/geno.1998.5530. [DOI] [PubMed] [Google Scholar]
- 64.van Dartel M, Hulsebos TJ. Amplification and overexpression of genes in 17p11.2 ~ p12 in osteosarcoma. Cancer Genet Cytogenet. 2004;153(1):77–80. doi: 10.1016/j.cancergencyto.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 65.Sherwood RJ, Duren DL, Havill LM, et al. A genomewide linkage scan for quantitative trait loci influencing the craniofacial complex in baboons (Papio hamadryas spp.). Genetics. 2008;180(1):619–28. doi: 10.1534/genetics.108.090407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ralston SH, Albagha OM. Genetics of Paget's disease of bone. Curr Osteoporos Rep. 2014;12(3):263–71. doi: 10.1007/s11914-014-0219-y. [DOI] [PubMed] [Google Scholar]
- 67.Lucas GJ, Riches PL, Hocking LJ, et al. Identification of a major locus for Paget's disease on chromosome 10p13 in families of British descent. J Bone Miner Res. 2008;23(1):58–63. doi: 10.1359/jbmr.071004. [DOI] [PubMed] [Google Scholar]
- 68.Hocking LJ, Herbert CA, Nicholls RK, et al. Genomewide search in familial Paget disease of bone shows evidence of genetic heterogeneity with candidate loci on chromosomes 2q36, 10p13, and 5q35. Am J Hum Genet. 2001;69(5):1055–61. doi: 10.1086/323798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramírez A, Faupel J, Goebel I, et al. Identification of a novel mutation in the coding region of the grey-lethal gene OSTM1 in human malignant infantile osteopetrosis. Hum Mutat. 2004;23(5):471–6. doi: 10.1002/humu.20028. [DOI] [PubMed] [Google Scholar]
- 70.Prontera P, Rogaia D, Sobacchi C, et al. Craniometaphyseal dysplasia with severe craniofacial involvement shows homozygosity at 6q21-22.1 locus. Am J Med Genet A. 2011;155A(5):1106–8. doi: 10.1002/ajmg.a.33826. [DOI] [PubMed] [Google Scholar]
- 71.Xu M, Zhou H, Yong J, et al. A Chinese patient with KBG syndrome and a 9q31.2-33.1 microdeletion. Eur J Med Genet. 2013;56(5):245–50. doi: 10.1016/j.ejmg.2013.01.007. [DOI] [PubMed] [Google Scholar]